Abstract
Gastric cancer is one of the most common cancers in modern societies. Previous studies have shown that the use of nanoparticle complexes is effective in the treatment of cancer. The aim of this study was to investigate the cytotoxicity and anticancer properties of cobalt oxide (Co3O4) nanoparticles (NPs) functionalized by glutamic acid (Glu) and conjugated with thiosemicarbazide (TSC) on gastric cancer (AGS) cell line. First, the Co3O4@Glu/TSC nanoparticles were synthesized via co-condensation reaction. Fourier-transform infrared (FTIR), X-ray diffraction (XRD), scanning electron microscopy (SEM) and energy dispersive X-ray (EDX) tests were performed for identifying the morphology, structure, size and functional groups of produced nanoparticles. MTT assay was also performed to evaluate cytotoxicity effect. Moreover, Annexin V/PI staining with flow cytometry analysis, caspase-3 activation assay, and Hoechst 33258 staining was carried out for evaluating apoptosis. The FTIR results showed that the components of Co3O4@Glu/TSC NPs complex were successfully fabricated. Crystallographic structure of Co3O4@Glu/TSC NPs was confirmed by XRD patterns. SEM results indicated that the size of the nanoparticles was in the range of 16–40 nm. An EDX spectrum was determined and data explained the existence of cobalt as the prominent element. MTT test results showed that AGS cell life was significantly decreased compared to the control group with increasing concentration of nanoparticles (dose-dependent) (P < 0.05), IC50 = 107.5 μg/mL. The results of flow cytometry assay and caspase-3 activity showed that fabricated Co3O4@Glu/TSC NPs induced apoptosis in the treated group. Moreover, Co3O4@Glu/TSC NPs treated AGS cells indicate an increase in the apoptotic characteristics including nuclear fragmentation. In the current work, the promising cytotoxicity and anti-cancer activities of Co3O4@Glu/TSC NPs complex toward gastric cancer (AGS) cell line were showed and it can be suggested for the drug delivery system.
Keywords: AGS, Cobalt oxide, Gastric cancer, Glutamic acid, Thiosemicarbazide
Introduction
After cardiovascular disease, cancer is the second leading cause of death in developed countries. Mortality due to the disease has increased gradually in recent years and is considered a serious threat to human health. Gastric cancer is the fourth most prevalent cancer in modern societies and the second leading cause of cancer death (Heise et al. 2009). About 90–95% of gastric cancers are gastric adenocarcinoma. In this case, the cancer spreads to the mucosa-producing cells (the cells that cover the inner lining of the stomach). Gastric cancer is a multifactorial disease that results from a combination of environmental and genetic factors. In this case, helicobacter infection, malnutrition, mutations and polymorphisms in key genes can be mentioned (Ma et al. 2017; Conteduca et al. 2012). The balance between proliferation and cell death plays a critical role in physiological homeostasis in tissues. Disruption of this balance will enable the cell to escape the apoptotic stage and lead it to uncontrolled cell proliferation and become cancer-causing. Chromatin condensation, nucleus fragmentation, cell contraction, and ultimately cell death are events that occur during apoptosis (Gonzalez et al. 2002, 2013). Nowadays, nanotechnology using nanoparticles helps to diagnose and treat cancer. In a way, it recognizes and destroys cancer cells (Boyle et al. 2008). Researchers use nanoparticles as a drug to treat malignant cancer cells because they do not have a negative impact on healthy cells and tissues in the body (Mokwena et al. 2018).
Cobalt oxide nanoparticles with Co3O4 formula are a mineral compound that has various applications in magnetic and catalytic activities (Ito et al. 2005). It also has antiviral activity and is used for drug delivery into cells and magnetic resonance imaging (MRI) (Liu et al. 2003). Research results have shown that the cellular uptake of Co3O4-NP is high and these nanoparticles can enter cells rapidly and settle into vesicles inside the cytoplasm. Because of these properties, cobalt oxide nanoparticles have attracted researchers' attention for cancer treatment (Papis et al. 2007a, b). Glutamic acid is one of the unnecessary amino acids that is biodegradable and non-toxic to humans and plays an important role in cellular metabolism. Glutamic acid is used as a cancer-fighting compound because it enhances the effect of the anticancer drug and reduces its toxicity against normal cells (Shih et al. 2004). Thiosemicarbazide is one of the most important organic compounds which is very important due to its unique chemical structure and specific biological activities (Kasuga et al. 2001). Thiosemicarbazide has antibacterial, antiviral and antitumor properties (Li et al. 2000; Finch et al. 1999). Thiosemicarbazide can interact with many metal ions and increase their chemical activity. Research has shown that the complexion of ion nanoparticles with thiosemicarbazide can inhibit cancer cells. It also increases the pharmacological effect of metallic ions with thiosemicarbazide (Yildirim et al. 2014; Yang et al. 2004). Our aim in this study was to investigate the anti-apoptotic effect of Co3O4@Glu/TSC NP on gastric cancer cells (AGS).
Materials and methods
Synthesis of Co3O4 NPs
300 mg of cobalt salt (Co (NO3)2 6H2O) was dissolved in 150 mL distilled water and the pH of the solution was increased to 11 by NaOH and then placed at 80 °C for 2 h. After centrifugation, the precipitate was removed from the solution and washed with ethanol and kept at 70 °C to dry (Yang et al. 2004).
Synthesis of Co3O4@Glu NPs
Co3O4-NPs with 152 mg of glutamic acid was dissolved in 150 mL of distilled water and reached 50 °C and its pH increased to about 11 by adding NaOH. Then the steps continued similar to Co3O4-NP synthesis.
Synthesis of Co3O4@Glu/TSC NPs
Co3O4-NPs were functionalized by glutamic acid (Glu) via co-condensation reaction. In brief, 500 mg of Co3O4@GA-NP was mixed with 200 mg of thiosemiccarbazide in 150 ml of ethanol 96% and incubated in an ultrasonic bath for 40 min at 45 °C. The solution was placed on a heater at 40 °C. After 24 h, the precipitate was separated by centrifugation and washed with ethanol at 70 °C to dry.
Characterization of Co3O4@Glu/TSC NPs
Several tests were performed to check the accuracy of the synthesized compounds. In FTIR spectra, the samples of Co3O4@Glu NP and Co3O4@Glu/TSC NP were examined. FTIR spectra of samples in the form of KBr pellets were recorded using a FTIR spectroscopy device (Shimadzu, Japan). X-ray diffraction (XRD) test was performed to investigate the crystal structure of Co3O4@Glu/TSC NP. X-ray powder diffraction (PXRD) data were collected using a Philips X’Pert MPD diffractometer (Co-Ka X-radiation, λ = 1.79 A°). The morphology and elemental composition of the Co3O4@Glu/TSC NPs were analyzed using scanning electron microscopy (SEM) image was taken using an electron microscope and Energy Dispersive X-ray Spectroscopy EDX (FE-SEM, Model: Hitachi S-4500).
Cell line and culture medium
The Gastric adenocarcinoma cell line (AGS) was purchased from the cell bank of Pasteur Institute of Iran. The cells were grown in the DMEM medium supplemented with penicillin/streptomycin at a final concentration of 100 units per ml and 10% fetal bovine serum (FBS) (Gibco, Netherland). Cells were cultured on cell culture plates and incubated in a humidified atmosphere of 5% CO2 at 37 °C.
MTT assay and cell viability
MTT assay was performed to investigate the effect of Co3O4@Glu/TSC nanoparticles on AGS cells and normal fibroblast cells (L929). By approximate growing cell count, 104 cells/100 µL were transferred from cell suspension to 96-well plates and incubated with cell culture conditions. After 24 h, the culture medium was evacuated and Co3O4@Glu/TSC NPs were added to each well at concentrations of 62.5, 125, 250 and 500 μg/mL. A well was considered only with cells treated as the control group. After incubation, 10 µL of MTT solution (3-(4,5-dimethyl thiazol-2-yl)-2,5-diphenyl tetrazolium Bromide)) was added to each well at a concentration of 5 mg/mL and incubated for 4 h. 100 µL of DMSO was added to each well. Finally, optical absorption at 570 nm was recorded by ELISA and IC50 was calculated using the following formula:
Analysis of apoptosis/necrosis by flow cytometry
Flow cytometry was used to evaluate apoptosis and necrosis in AGS cells. At this stage, cells were treated with a concentration of 107.5 μg/mL of Co3O4@Glu/TSC NPs in 6-well plates and incubated. After 24 h, cells were separated by trypsin and centrifuged and washed with PBS and added to them 10 µL Annexin-V FLUOS and 5 µL propidium iodide (PI) according to the manufacturer’s protocol (Roche, Germany) and then, the numbers of apoptotic and/or necrotic cells were measured by flow cytometry device using PAS machine (Partec, Germany).
Caspase-3 activation assay
Caspase 3 activity was evaluated in AGS cells treated with Co3O4@Glu/TSC NPs compared to untreated cells (control group). ApoTarget TM kit was used in this test. 3 × 106 cells/well were cultured in 6 well plates and incubated for 24 h at 37 °C, then treated with 107.5 μg/mL Co3O4@Glu/TSC NPs. One group of untreated cells was considered as the control group. After 24 h, the cells were re-suspended in 50 μL of Cell lysis buffer and incubated on ice for 10 min and centrifuged at 10,000 rpm for 60 s. Then, the dilution of supernatant to a concentration of 100 μL proteins with 50 μL Cell Lysis Buffer was performed. After measuring the protein concentration, the DEVD-pNA (Caspase-3) substrate was added and the samples were read in 400 or 405 nm using a microplate reader.
Hoechst staining
The evaluations of nuclear damage were determined by Hoechst staining. The density of cells 4 × 105 cells/well was grown and treated with IC50 concentration of Co3O4@Glu/TSC nanoparticles followed by incubation for 24 h. After that, untreated and treated AGS cell line were stained with Hoechst 33258 dye and examined by a fluorescent microscope (Incell Analyser 2000, USA).
Statistical analysis
All steps were repeated three times and the results were reported as mean ± SD. Data were analyzed using SPSS software. P < 0.05 was considered as the level of significance. For comparing the means of control and treated groups, one-way ANOVA test (University Park, USA) used.
Results and discussion
Gastric cancer is one of the most common cancers in modern societies with high mortality rates (Shen et al. 2016). Nowadays, scientists are investigating the effects of different types of nanoparticles on cancer cells with the goal of inhibiting cancer cell growth and treating cancer. Metal nanoparticles have unique properties, including optical and magnetic properties and electrical activity (Riddhipratim et al. 2018; Mandal et al. 2005). Iron, nickel and cobalt nanoparticles were considered in medical biotechnology because of their magnetic properties and new studies have been done to identify their properties. These nanoparticles have been used in the drug delivery and treatment of hyperthermic cancer (Pankhurst et al. 2003). Binding of nanoparticles to the plasma membrane and their uptake by the cell are required for their toxicity process (Papis et al. 2007b). In the present study, we synthesized Co3O4@Glu/TSC NPs complex to find novel anticancer compounds and the results of FTIR, XRD, SEM and EDX tests showed that the nanoparticle complex was successfully synthesized. A schematic representation for the fabrication procedure of Co3O4@Glu and Co3O4@Glu/TSC NPs is indicated in Scheme 1.
Scheme 1.
A schematic representation for the synthesis procedure of Co@Glu and Co@Glu/TSC NPs is illustrated
The FTIR spectrum results showed that the produced materials were correctly synthesized (Fig. 1a, b). Figure 1a shows the FT-IR spectrum for the Co3O4@Glu NPs. The peaks of 3632 cm−1 and 2929 cm−1 are related to the N–H vibrations and the C–H stretching band. Figure 1b shows the FT-IR spectrum for the Co3O4@Glu/TSC NPs. There is a band in area 1340 cm−1 that belongs to the C–N functional group. The C=O amide strong band was formed at region 1611 cm−1 confirmed that thiosemicarbazide was stabilized on cobalt oxide with glutamic acid. The band between cobalt, glutamic acid and thiosemicarbazide is amide.
Fig. 1.
FT-IR spectra of a Co3O4@Glu and b Co3O4@Glu/TSC nanoparticles. The FTIR spectrum result showed that the surface adsorption of functional groups on nanoparticles. The XRD patterns of the synthesized Co3O4@Glu/TSC NPs (c). In the XRD spectrum, the peaks at 2θ values of 85.34°, 82.77°, 81.04°, 72.96°, 68.48°, 60.39°, 44.33°, 37.92°, and 22.07° belong to the crystal structure of Co3O4@Glu/TSC NPs
The crystal structure of Co3O4@Glu/TSC NPs was investigated using the X-ray power diffraction (XRD) method (Fig. 1c). The XRD patterns of the samples were recorded from 2θ = 10°–95°. The XRD sharp peaks at 2θ values of 85.34°, 82.77°, 81.04°, 72.96°, 68.48°, 60.39°, 44.33°, 37.92°, and 22.07° might be attributed to the structure of Co3O4@Glu/TSC nanoparticle.
SEM imaging was performed to investigate the morphology and size of Co3O4@Glu/TSC NPs. These nanoparticles were between 16.66 and 40.39 nm (Fig. 2a, b).
Fig. 2.
SEM a low and b high magnification images of Co@Glu/TSC NPs. These images show that the NPs sizes are between 16.66 and 40.39 nm. The EDX spectrum of Co3O4@Glu/TSC NPs (c). The presence of C, N, O, S and Co, as the major constituents, was confirmed by EDX analysis
Elemental composition of Co3O4@Glu/TSC-NPs EDX spectra was performed by EDX spectroscopy. The results showed the presence of cobalt, oxygen, nitrogen and sulfur in Co3O4@Glu/TSC NPs (Table 1). These elements correspond to the synthesized Co3O4@Glu/TSC NPs complex. In the spectrum, each peak represents an element (Fig. 2c). A higher altitude of peak means the greater concentration of the element in the sample.
Table 1.
Results of EDS analysis of Co3O4@Glu/TSC
| Elements | K | Kr | Weight% | Atomic% | ZAF |
|---|---|---|---|---|---|
| C | 0.0663 | 0.0323 | 13.49 | 22.43 | 0.2396 |
| N | 0.1012 | 0.0494 | 18.27 | 26.03 | 0.2703 |
| O | 0.1646 | 0.0803 | 28.69 | 35.80 | 0.2798 |
| S | 0.1444 | 0.0704 | 8.23 | 5.13 | 0.8550 |
| Co | 0.5235 | 0.2552 | 31.31 | 10.61 | 0.8150 |
| Total | 1.0000 | 0.4876 | 100.00 | 100.00 |
The percentage of C, N, O, S and Co in the final product (Co3O4@Glu/TSC) is displayed
Subsequently, studies were performed to identify the properties and effects of cobalt oxide nanoparticles on cancer cell lines. For example, Co3O4 NPs have toxicity properties and affect on cell viability in hepatocytes (HepG2) and endothelial cells (ECV-304) with depending on concentration and time (Papis et al. 2007b).
Thiosemicarbazide is Schiff based ligands which have gained importance over the decades as potential drug candidates. When coordinated to metals, they have proved as good anticancer agents. Studies have been performed to identify the function of a complex of thiosemicarbazide and nanoparticles and to evaluate their effect on cancer cells. For example, the complex of palladium II (PdCl2) and thiosemicarbazide has cytotoxic effects on the breast (MCF7) and lung (A594) cancer cell lines (Muralisankar et al. 2017). In a research, it was shown that the conjugated thiosemicarbazide with copper induced the apoptosis in HCT116 cells. The copper complex with thiosemicarbazide caused distinct DNA cleavage and Topo IIα inhibition unlike that for copper alone. In vivo administration of copper complex with thiosemicarbazide significantly inhibited tumor growth in HCT116 xenografts in nude mice (Palanimuthu et al. 2013).
Anticancer effect of copper (II) complex with a derivative of thiosemicarbazide on colorectal and breast cancer cells was confirmed. Also this complex was a poison inhibitor for human topoisomerase IIα (Sandhaus et al. 2016).
The interactions of Fe (II), Fe (III) and Ga (III) with thiosemicarbazone are currently undergoing phase II clinical trials as a chemotherapeutic antitumor agent (Enyedy et al. 2011).
In other study, the zinc (II) and Cd (II) complexes with thiosemicarbazone have been shown to exhibit specific cytotoxic activity against Pam-ras cancer cell line, probably due to cell killing by apoptosis (Sandhaus et al. 1999).
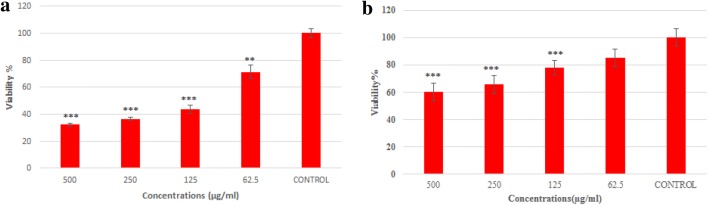
In the present study, MTT assays were performed to investigate cell viability, flow cytometry to investigate percent of apoptosis and necrosis, and caspase-3 activity assay for assessing the potential of Co3O4@Glu/TSC NPs in inducing the apoptosis in AGS cancer cell line. The MTT test results for AGS cells and normal fibroblast cells (L929) treated with 0, 62.5, 125, 250, 500 μg/mL concentrations of Co3O4@ Glu/TSC NPs are presented in Fig. 3a, b, respectively. The IC50 for AGS cells was 107.5 µg/mL and for normal cells 350 µg/mL over 24 h. According to different IC50 values of Co3O4@Glu/TSC NP on cancer and normal cell lines, seems that the lethal dose of Co3O4@Glu/TSC NP for AGS cancer cell line have a less toxic effect on the normal cell line. Results for survival, compared with control samples, are presented as mean ± SD (P > 0.05).
Fig. 3.
The survival rate of gastric cancer (a) and normal fibroblast cells lines (b) against serial concentrations of Co@Glu/TSC NPs after 24 h. In concentration of 500 μg/mL, the percentage of cell viability was lowest in AGS and normal cells. In addition, Co3O4@Glu/TSC NPs decreased the viability of cells by dose-dependent manner. The IC50 for AGS cells is 107.5 µg/mL and for normal cells 350 µg/mL. The results are reported compared with control samples (n = 3, *P < 0.05, **P < 0.01, ***P < 0.001)
The concentration of 500 μg/mL had a maximum inhibition of cell proliferation and viability compared to the control group. It can be concluded that Co3O4@Glu/TSC NPs decrease the viability of AGS cells by dose-dependent manner.
In the MTT assay, Co3O4@Glu/TSC NPs affected the viability of AGS cells. Cell viability decreased and apoptosis increased compared to the control group with increasing concentration of nanoparticles.
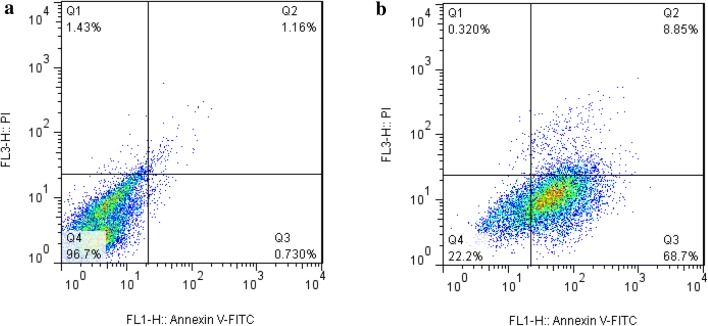
Flow cytometry with annexin V/PI staining was performed to investigate the occurrence of apoptosis and necrosis in AGS cells treated with Co3O4@Glu/TSC NPs compared to untreated cells. Nuclear fragmentation and cancer cell death by nanoparticles are shown in Fig. 4a, b. Data analysis was divided into four regions using software. Q1 region showed necrotic cells, Q2 indicate late apoptotic cells. Q3 showed early apoptotic cells and Q4 region showed healthy cells. Herein, dot plots of flow cytometry indicate 68.7% and 8.85% increases in early and late apoptotic AGS cell line treated with Co3O4@Glu/TSC NPs, respectively.
Fig. 4.
Detection of apoptosis in AGS cells by flow cytometry. a Untreated cells and b cells treated with Co3O4 @Glu/TSC NPs. Dot plots of flow cytometry indicate 68.7% and 8.85% increases in early and late apoptosis in AGS cell line, respectively
To investigate caspase-3 activity, AGS cells were treated with Co3O4@Glu/TSC nanoparticles for 24 h. Caspases play an important role in programmed cell death and apoptosis. Caspase-3 activity test results showed that Co3O4@Glu/TSC NPs induced apoptosis by increasing caspase 3 activity compared to the cells of the control group and leaded cancer cells to death (Fig. 5). Caspase-3 test results on tumor cells treated with silver nanoparticles showed that this nanoparticle was able to inhibit the proliferation of MCF-7 cancer cells (Shandiz et al. 2018). Flow cytometry is a very powerful method for investigating nuclear changes and the occurrence of cell apoptosis. In this study, flow cytometry results showed that the nanoparticles caused the fragmentation of the nucleus in cancer cells and played a key role in apoptosis. In accordance to results of this study, Mahey et al., checked out the anti-proliferative and apoptosis-inducing mechanism of three different forms of cobalt i.e., cobaltous (CoCl2·6H2O), macro-Co (II, III) oxide and nano-Co (II, III) oxide for anti-proliferative activity in PC-3 (prostate cancer) cell line. Annexin V/propidium iodide staining confirmed the apoptotic mode of cell death in PC-3 cells and also a dose-dependent increase in caspase activity was observed in PC-3 cells treated with CoCl2·6H2O (Mahey et al. 2016).
Fig. 5.

Caspase-3 activity in cells treated with Co3O4@Glu/TSC NPs and untreated cells (control group). Different letters for each column shows a significant difference (P value < 0.05)
In a research, cobalt-containing cobalamin was capable of killing leukemia cells (HL-60). Also this complex exhibited nano-molar toxicity towards breast, brain, and melanoma cancer cells, however, the IC50 values were tenfold higher than free colchicine (Munteanu and Suntharalingam 2015).
Thamilarasan et al. (2016) showed that the Cobalt (III) complexes including [Co(acetylacetone)(2,2′-bipyridine)(N3)2·H2O] and [Co(acetylacetone)( ethylenediamine)(N3)2] had in vitro cytotoxicity on breast cancer cell line (MCF-7) and suggested that these complexes have the potential to act as effective anticancer drugs.
In another study, cobalt oxide nanoparticles coated with Phosphonomethyl iminodiacetic acid (PMIDA-CoO) increased reactive oxygen species (ROS) in leukemia cells (Jurkat, K562 and KG1A). PMIDA-CoO caused DNA damage and eventually induced apoptosis in leukemic cells by increasing inflammatory cytokines (Chattopadhyay et al. 2015).
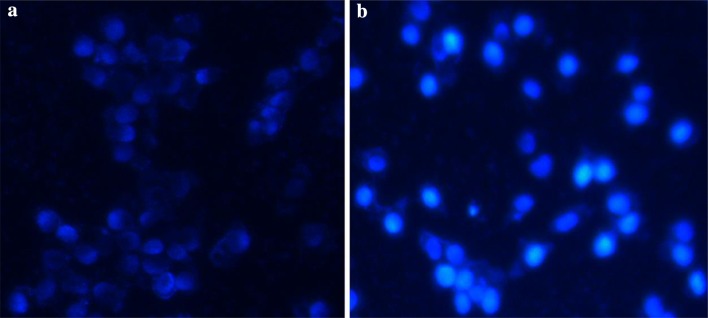
Hoechst 33258 staining was explained to evaluate the occurrence of nuclear fragmentation in AGS cells treated with Co3O4@Glu/TSC NPs compared to untreated cells. After exposure to Co3O4@Glu/TSC NPs, it can be indicated that the cell apoptotic morphological changes involved in apoptotic bodies and clumping were observed owning to cell death by Co3O4@Glu/TSC nanoparticles treatment, While, the untreated cells were viable and healthy (Fig. 6).
Fig. 6.
Fluorescence images of AGS cells which were treated with 107 μg/ml of Co3O4@Glu/TSC NPs and recognized with Hoechst 33258 staining. a Control, b the cells treated with Co3O4@Glu/TSC NPs. Control cells indicate normal morphology but treated cells show condensed chromatin and brightly stain
Conclusion
Results of previous studies have shown that metal nanoparticles with magnetic and fast cellular uptake properties are successful in medical biotechnology and cancer therapy. Separately, each of the Co3O4, thiosemicarbazide and glutamic acid has a little anticancer effect. We synthesized the Co3O4@Glu/TSC nanoparticles complex and investigated its effect on AGS cells and found that this complex inhibits the growth of AGS cancer cells and induces apoptosis in them with their anticancer activity.
Acknowledgements
The authors would like to thanks the microbiolgy lab of Islamic Azad University, Rasht Branch.
Author contributions
Conceptualization: MJ and KK, methodology: SASS and SB formal analysis: MH and AS, investigation: AM, resources: SASS, data curation: AP, writing—original draft preparation: AS, visualization: FA, supervision: AS.
Compliance with ethical standards
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical statements
This article does not contain any studies with human participants or animals performed by any of the authors.
References
- Boyle P, Levin B. World cancer report 2008. Beijing: IARC Press, International Agency for Research on Cancer; 2008. [Google Scholar]
- Chattopadhyay S, Dash SK, Tripathy S, Pramanik P, Roy S. Phosphonomethyliminodiacetic acid-conjugated cobalt oxide nanoparticles liberate Co++ ion-induced stress associated activation of TNF-α/p38 MAPK/caspase 8-caspase 3 signaling in human leukemia cells. J Biol Inorg Chem. 2015;20(1):123–141. doi: 10.1007/s00775-014-1221-7. [DOI] [PubMed] [Google Scholar]
- Conteduca V, Sansonno D, Lauletta G, Russi S, Ingravallo G, Dammacco F. H pylori infection and gastric cancer: state of the art (review) Int J Oncolog. 2012;42(1):5–18. doi: 10.3892/ijo.2012.1701. [DOI] [PubMed] [Google Scholar]
- Enyedy EA, Primik MF, Kowol CR, Arion VB, Kiss T, Keppler BK. Interaction of Triapine and related thiosemicarbazones with iron(iii)/(ii) and gallium(iii): a comparative solution equilibrium study. Dalton Trans. 2011;40(22):5895–5905. doi: 10.1039/c0dt01835j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch RA, Liu MC, Cory AH, Cory JG, Sartorelli AC. Riapine (3-aminopyridine-2-carboxaldehyde thiosemicarbazone; 3-AP): an inhibitor of ribonucleotide reductase with antineoplastic activity. Adv Enzym Regul. 1999;39:3–12. doi: 10.1016/s0065-2571(98)00017-x. [DOI] [PubMed] [Google Scholar]
- Gonzalez CA, Sala N, Capella G. Genetic susceptibility and gastric cancer risk. Int J Cancer. 2002;100(3):249–260. doi: 10.1002/ijc.10466. [DOI] [PubMed] [Google Scholar]
- Gonzalez CA, Sala N, Rokkas T. Gastric cancer: epidemiologic aspects. Helicobacter. 2013;1:34–38. doi: 10.1111/hel.12082. [DOI] [PubMed] [Google Scholar]
- Heise K, Bertran E, Andia ME, Ferreccio C. Incidence and survival of stomach cancer in a high-risk population of Chile. World J Gastroenterol. 2009;15(15):1854–1862. doi: 10.3748/wjg.15.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito A, Shinkai M, Honda H, Kobayashi T. Medical application of functionalized magnetic nanoparticles. J Biosci Bioeng. 2005;100(1):1–11. doi: 10.1263/jbb.100.1. [DOI] [PubMed] [Google Scholar]
- Kasuga NK, Sekino K, Koumo K, Shimada N, Ishikawa M, Nomiya K. Synthesis, structural characterization and antimicrobial activities of 4-and 6-coordinate nickel (II) complexes with three thiosemicarbazones and semicarbazone ligands. J Inorg Biochem. 2001;84(1):55–65. doi: 10.1016/S0162-0134(00)00221-x. [DOI] [PubMed] [Google Scholar]
- Li QX, Tang HA, Li YZ, Wang M, Wang LF, Xia CG. Synthesis, characterization, and antibacterial activity of novel Mn(II), Co(II), Ni(II), Cu(II), and Zn(II) complexes with vitamin K3-thiosemicarbazone. J Inorg Biochem. 2000;78(2):167–174. doi: 10.1016/s0162-0134(99)00226-3. [DOI] [PubMed] [Google Scholar]
- Liu ZS, Tang SL, Ai ZL. Effects of hydroxyapatite nanoparticles on proliferation and apoptosis of human hepatoma BEL-7402 cells. World J Gastroenterol. 2003;9(9):1968–1971. doi: 10.3748/wjg.v9.i9.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Wu D, Hu X, Li J, Cao M, Dong W. Associations between cytokine gene polymorphisms and susceptibility to Helicobacter pylori infection and Helicobacter pylori related gastric cancer, peptic ulcer disease: a meta-analysis. PLoS ONE. 2017;12(4):e0176463. doi: 10.1371/journal.pone.0176463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahey S, Kumar R, Arora R, Mahajan J, Arora S, Bhardwaj R, Thukral AK. Effect of cobalt (II) chloride hexahydrate on some human cancer cell lines. Springerplus. 2016;5(1):930. doi: 10.1186/s40064-016-2405-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal S, Phadtare S, Sastry M. Interfacing biology with nanoparticles. Curr Appl Phys. 2005;5(2):118–127. doi: 10.1016/j.cap.2004.06.006. [DOI] [Google Scholar]
- Mokwena MG, Kruger CA, Ivan MT, Heidi A. A review of nanoparticle photosensitizer drug delivery uptake systems for photodynamic treatment of lung cancer. Photodiagn Photodyn Ther. 2018;22:147–154. doi: 10.1016/j.pdpdt.2018.03.006. [DOI] [PubMed] [Google Scholar]
- Munteanu CR, Suntharalingam K. Advances in cobalt complexes as anticancer agents. Dalton Trans. 2015;44(31):13796–13808. doi: 10.1039/C5DT02101D. [DOI] [PubMed] [Google Scholar]
- Muralisankar M, Basheer SM, Haribabu J, Bhuvanesh NS, Karvembu R, Sreekanth A. An investigation on the DNA/protein binding, DNA cleavage and in vitro anticancer properties of SNO pincer type palladium (II) complexes with N-substituted isatinthiosemicarbazone ligands. Inorg Chim Acta. 2017;466:61–70. doi: 10.1016/j.ica.2017.05.044. [DOI] [Google Scholar]
- Palanimuthu D, Shinde SV, Somasundaram K, Samuelson AG. In vitro and in vivo anticancer activity of copper bis (thiosemicarbazone) complexes. J Med Chem. 2013;56(3):722–734. doi: 10.1021/jm300938r. [DOI] [PubMed] [Google Scholar]
- Pankhurst QA, Connolly J, Jones SK, Dobson J. Applications of magnetic nanoparticles in biomedicine. J Phys D Appl Phys. 2003;36(13):R167. doi: 10.1088/0022-3727/36/13/201. [DOI] [Google Scholar]
- Papis E, Gornati R, Prati M, Ponti J, Sabbioni E, Bernardini G. Gene expression in nanotoxicology research: analysis by differential display in BALB3T3 fibroblasts exposed to cobalt particles and ions. Toxicol Lett. 2007;170(3):185–192. doi: 10.1016/j.toxlet.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Papis E, Gornati R, Ponti J, Prati M, Sabbioni E, Bernardini G. Gene expression in nanotoxicology: a search for biomarkers of exposure to cobalt particles and ions. Nanotoxicology. 2007;170(3):185–192. doi: 10.1080/17435390701644399. [DOI] [PubMed] [Google Scholar]
- Riddhipratim M, Baranwal A, Srivastava A, Chandra P. Evolving trends in bio/chemical sensors fabrication incorporating bimetallic nanoparticles. Biosens Bioelectron. 2018;117:546–561. doi: 10.1016/j.bios.2018.06.039. [DOI] [PubMed] [Google Scholar]
- Sandhaus S, Taylor R, Edwards T, Huddleston A, Wooten Y, Venkatraman R, Ralph T, et al. Synthesis and characterization of complexes of p-isopropyl benzaldehyde and methyl 2-pyridyl ketone thiosemicarbazones with Zn(II) and Cd(II) metallic centers. Cytotoxic activity and induction of apoptosis in Pam-ras cells. J Inorg Biochem. 1999;75(4):255–261. doi: 10.1016/S0162-0134(99),00096-3. [DOI] [PubMed] [Google Scholar]
- Sandhaus S, Taylor R, Edwards T, Huddleston A, Wooten Y, Venkatraman R, Weber T, et al. A novel copper (II) complex identified as a potent drug against colorectal and breast cancer cells and as a poison inhibitor for human topoisomerase Iiα. Inorg Chem Commun. 2016;64:45–49. doi: 10.1016/j.inoche.2015.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shandiz SAS, Montazeri A, Abdolhosseini M, Shahrestani SH, Hedayati M, Moradi-Shoeili Z, Salehzadeh A. Functionalization of Ag nanoparticles by glutamic acid and conjugation of Ag@ Glu by thiosemicarbazide enhances the apoptosis of human breast cancer MCF-7 cells. J Clust Sci. 2018;29:1107–1114. doi: 10.1007/s10876-018-1424-0. [DOI] [Google Scholar]
- Shen YH, Xie ZB, Yue AM, Wei QD, Zhao HF, Yin HD, Mai W, et al. Expression level of microRNA-195 in the serum of patients with gastric cancer and its relationship with the clinicopathological staging of the cancer. Eur Rev Med Pharmacol Sci. 2016;20(7):1283–1287. [PubMed] [Google Scholar]
- Shih IL, Van YT, Shen MH. Biomedical applications of chemically and microbiologically synthesized poly (glutamic acid) and poly (lysine) Mini Rev Med Chem. 2004;4(2):179–188. doi: 10.2174/1389557043487420. [DOI] [PubMed] [Google Scholar]
- Thamilarasan V, Sengottuvelan N, Sudha A, Srinivasan P, Chakkaravarthi G. Cobalt(III) complexes as potential anticancer agents: physicochemical, structural, cytotoxic activity and DNA/protein interactions. J Photochem Photobiol B Biol. 2016;162:558–569. doi: 10.1016/j.jphotobiol.2016.06.024. [DOI] [PubMed] [Google Scholar]
- Yang H, Hu Y, Zhang X, Qiu G. Mechanochemical synthesis of cobalt oxide nanoparticles. Mater Lett. 2004;58:387–389. doi: 10.1016/S0167-577X(03)00507-x. [DOI] [Google Scholar]
- Yildirim Y, Guler E, Yavuz M, Ozturk N, Yaman PK, Subasi E, Sahin E, Timur S. Ruthenium (II) complexes of thiosemicarbazone: synthesis, biosensor applications and evaluation as antimicrobial agents. Mater Sci Eng C Mater Biol Appl. 2014;44:1–8. doi: 10.1016/j.msec.2014.08.007. [DOI] [PubMed] [Google Scholar]