Abstract
Industrial development has increased wastewater (WW) volume; generating contamination and disturbing ecosystems, because of breeching disposal parameters. In this work, Coloured Laboratory Wastewater (CLWW), (1500.00 colour units, CU) was separately submitted to two secondary treatments. For the first one CLWW was treated for three cycles C1, C2 and C3 with P. pastoris X33/pGAPZαA-LaccPost-Stop producing rPOXA 1B laccase, immobilized in calcium alginate beads. For the second-one, rPOXA 1B enzyme concentrate was used (three processes: P1, P2, and P3). Both treatments were carried out in a 15 L reactor with 10 L effective work volume (EWV) with 72 h hydraulic retention time. C1, C2, and C3 effluents were flocculated and filtered through quartzite sand, while P1, P2, and P3 effluents were only filtered through quartzite sand. The mixture of secondary effluents was submitted to a tertiary treatment with Chlorella sp. For C1, C2, C3, P1, P2, and P3, CU removal was of 99.16, 99.58, 99.53, 96.72, 97.05 and 96.47%, respectively. Discharge parameters, total organic carbon (TOC), inorganic carbon (IC), chemical oxygen demand (COD) and biological oxygen demand (BOD5) decreased, although they reached different final values. After the tertiary treatment (144 h) effluent discharge parameters were reduced to 34 ± 4 CU, TOC to 6.6 ± 0.9 mg L−1 and COD to 155 ± 4 mg L−1. It was demonstrated that secondary treatments (immobilized recombined cells or recombinant enzyme concentrate) combined with Chlorella sp., (tertiary treatment) attained a considerable removal of discharge parameters, demonstrating a promissory alternative for CLWW sequential treatment.
Keywords: Discolouration, Pichia pastoris, rPOXA 1B, Chlorella sp., Laboratory coloured wastewater, Secondary treatment, Tertiary treatment
Introduction
Over the past years, anthropogenic contamination has increased due to industrial development, resulting in considerable environmental impact, which has prompted the scientific community to develop new bioremediation processes. Biotechnological processes to treat wastewater is a major challenge because water sources have been contaminated with hydrocarbons (Ferrera-Cerrato et al. 2006), heavy metals (mercury, arsenic, and lead), emergent pollutants (persistent organic compounds, endocrine disruptors, antibiotics), organic matter and compounds such as dyes and pigments (Deblonde et al. 2011; Barrios-Ziolo et al. 2015).
Hospitals, universities, research centers among others use different dyes, such as basic fuchsin, carbol fuchsin, crystal violet, lactophenol blue, methylene blue, Congo red, eosin, malachite green, among others for biological stains. Annually these institutions produce coloured laboratory wastewater (CLWW), which in some cases do not comply with established norms for their disposal in superficial bodies of water or public sewer system (Pedroza-Camacho et al. 2018).
Several types of treatments exist to reduce the effect of CLWW pollutant load. Among them physical treatments can reduce the pollutant load by filtering systems. Other treatments include ionic exchange resins, chemical treatment, such as ozonation, Fenton process, flocculation, and coagulation processes (Barrios-Ziolo et al. 2015). Last, biological processes are based on the capacity of microorganisms or their enzymes to degrade organic matter present in wastewater for their growth (Ferrer Polo et al. 2018).
Some microorganisms can produce laccase (E.C. 1.10.3.2) and p-diphenyl oxidases, enzymes, which are multicopper oxidases that catalyze aromatic and aliphatic compound degradation, as well as dyes and toxic pollutants by reducing molecular oxygen into water (Rivera-Hoyos et al. 2013, 2015, 2018). White-rot fungi generally produce these enzymes, such as Pleurotus ostreatus and Ganoderma lucidum, among others (Morales-Álvarez et al. 2016, 2017). Laccase production by native sources is inadequate to comply with environmental requirements. Therefore, its heterologous expression in yeast, such as Saccharomyces cerevisiae or Pichia pastoris becomes an important option as secondary wastewater treatment (Piscitelli et al. 2005; Rivera-Hoyos et al. 2015, 2018).
For wastewater, pollutant removal conventional secondary treatments are efficient. However, at the end of secondary treatment sludge is generated, which is composed of water, organic compounds and dissolved inorganic compounds (continuous phase) and microbial biomass, rich in extracellular polymeric substances (Ratkovich et al. 2013). Part of secondary treatment sludge is used as a starter culture (10–20%) to maintain the continuous operation of biological reactors; the remaining sludge is disposed of as solid waste in sanitary landfills. If sludge has undergone stabilization processes, it is selected for agricultural purposes as long as it complies with physical, chemical, and microbiological quality criteria (Environmental Protection Agency (EPA) 2005; Baily 2009).
Therefore, biological treatment alternatives are sought, such as the use of enzyme concentrates to decrease and/or eliminate secondary sludge production. Enzymes are employed free or immobilized in different supports materials to guarantee stability and activity for long periods (Ji et al. 2017; Bilal et al. 2019). For wastewater treatment polyphenol oxidase (E.C. 1.10.3.1) is distinct among a group of commercial enzymes, as well as various peroxidases (E.C. 1.11.1). These enzymes catalyze the oxidation of phenolic and non-phenolic compounds, have low specificity and are stable under different physical and chemical conditions (Chang et al. 2015; Ji et al. 2017; Kashefi et al. 2019). However, oxidation processes undertaken by these proteins can produce intermediates with less degree of complexity, colour or ions dissolved as sulphates, nitrates, nitrites, and phosphates (Abdel-Kareem 2012; Rivera-Hoyos et al. 2018). An alternative to remove all of these constituents is to implement a tertiary treatment with microalgae (Arias et al. 2018; Li et al. 2019).
Microalgae use organic/inorganic carbon (mixotrophic culture conditions), inorganic nitrogen and phosphorous, for their growth. Moreover, they eliminate heavy metals and remove dyes (Khandare and Govindwar 2015; Ditta et al. 2016). The mechanisms by which microalgae perform these bioremediation processes take place in two stages: an initial physicochemical process independent of metabolic processes, where the algae adsorb pollutant by its cell wall. Following, processes of bioaccumulation and biotransformation take place mediated by an ample gamut of enzymes (Fazal et al. 2018). Once microalgae have performed this tertiary treatment its biomass can be employed as biofertilizer, for the extraction of bioproducts, and can be thermally transformed for biochar production (Yu et al. 2017; Santos and Pires 2018). Hence, they can integrate CLWW or industrial treatment systems into low circular economy models, such as biorefinery. Consequently, improving wastewater treatment and generating higher value-added bioproducts.
The objective of this work was to implement and compare two secondary treatments [Pichia pastoris X33/pGAPZαA-LaccPost-Stop (Clone 1), producing rPOXA 1B, immobilized in calcium alginate beads (Gouzy-Olmos et al. 2018) and free enzyme concentrate rPOXA 1B concentrate (Rivera-Hoyos et al. 2015; Ardila-Leal et al. 2019)] for CLWW (1500.00 CU) discolouration, followed by a tertiary treatment with Chlorella sp., to complete removal of TOC, IC, COD and BOD5 produced in secondary treatments.
Materials and methods
Coloured laboratory wastewater (CLWW)
CLWW from lot No. 1810 from wastewater from the teaching and research laboratories at the “Departamento de Microbiología de la Facultad de Ciencias de la Pontificia Universidad Javeriana, Bogotá D.C., Colombia” was used. CLWW were characterized with regard to UV/VIS spectra to determine the wavelength where maximum absorbance was obtained.
P. pastoris immobilized cells
Recombinant P. pastorisX33/pGAPZαA-LaccPost-Stop (Clone 1), (Rivera-Hoyos et al. 2015) was immobilized in calcium alginate beads according to previously described conditions (Gouzy-Olmos et al. 2018).
Recombinant POXA 1B (rPOXA 1B) enzyme concentrate
Two enzyme concentrates produced with P. pastoris X33/pGAPZαA-LaccPost-Stop (Clone 1) were used (Ardila-Leal et al. 2019). Concentrate lot number 1 (11,713 UL−1, pH 7.40 ± 0.20) was used for process 1 (P1) and concentrate lot number 2 (41,273 UL−1, pH 7.40 ± 0.20) was used for processes 2 and 3 (P2 and P3).
Chlorella sp. cells
Chlorella sp., cells isolated from Winogradsky columns were used. Cells were cultured in Bold media [25 mg L−1 CaCl2, 25 mg L−1 NaCl, 250 mg L−1 NaNO3, 75 mg L−1 MgSO4, 105 mg L−1 KH2PO4, 75 mg L−1 K2HPO4, 3 mL L−1 trace element solution (0.194 mg L−1 FeCl3, 0.082 mg L−1 MnCl2, 0.16 mg L−1 CoCl2, 0.008 mg L−1 Na2MoO4·2H2O and 0.005 mg L−1 ZnCl2)], (Blair et al. 2014) and incubated at 19.00 ± 3.00 °C, 120 r.p.m, with artificial light cycles of 12 h.
Treatment of different CU of CLWW with P. pastoris X33/pGAPZαA-LaccPost-Stop (Clone 1) immobilized cells in calcium alginate
Assays were carried out with CLWW containing different CU to select the most favourable concentration for use in pilot-scale treatments. Solutions were prepared with 300.00, 1200.00 and 2300.00 CU from wastewater lot No. 1810. Colour units were determined from solutions by measuring absorbance at a wavelength of 614 nm and utilizing Equation (1), (Livernoche et al. 1983):
| 1 |
where A1 is the effluent’s absorbance at 614 nm and 0.312 is the ABS465n from a 500.00 CU platinum-cobalt standard solution (Livernoche et al. 1983).
Each assay was performed in 100 mL Erlenmeyer flask containing 25 mL of solution. Each assay consisted of control 1 (only CLWW solution), control 2 with calcium alginate beads (CLWW and calcium alginate beads) and treatment 1 (dye solution and Pichia pastoris X33/pGAPZαA-LaccPost-Stop (Clone 1) immobilized in calcium alginate beads). Erlenmeyer flasks were incubated at 30 °C at 150 rpm with photographic monitoring every 24 h. Assays were performed in triplicate. For CU determination, sampling was carried out at the beginning (0 h) and the end of the assay (144 h).
CLWW (1500.00 CU) secondary treatment with P. pastoris X33/pGAPZαA-LaccPost-Stop (Clone 1) immobilized cells (pilot scale)
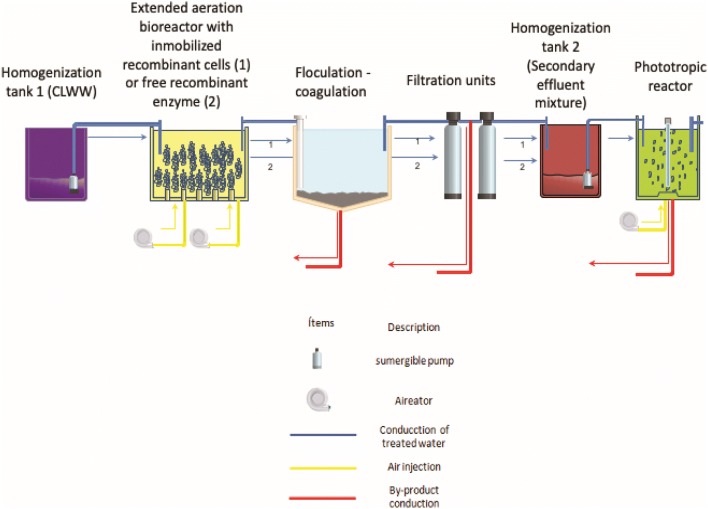
The pilot treating plant consisted of a 20 L homogenization, neutralization and mixing tank, followed by a 15 L extended aeration bioreactor (turbulent flow and dissolved oxygen > 1 mg L−1) with a 10 L effective work volume (EWV) at laboratories’ room temperature (~ 19 ± 3 °C). The reactor was inoculated with 10,000 beads containing immobilized cells (1 bead mL−1), with 72 h hydraulic retention time. To flocculate the effluent after immobilized cell treatment 340 mL Al2(SO4)3 (3% w/v) and 36 mL NaOH (60% w/v) were used. Subsequently, the system was transferred to two filtration units with 5 L quartzite sand with 2-min retention time (Fig. 1). Three cycles per treatment were performed (Batch, C1, C2, and C3) for CLWW of the same lot with sampling every 12 h, to determine chemical oxygen demand COD (mg L−1), biological oxygen demand at day five BOD5 (mg L−1), total suspended solids TSS (mg L−1), total organic carbon TOC (mg L−1), inorganic carbon IC (mg L−1), total carbon TC (mg L−1), total nitrogen TN (mg L−1), pH and UV/VIS absorption spectra changes (Pedroza-Camacho et al. 2018).
Fig. 1.
Schematic representation of the treatment plant employed. Conduction line of treated water 1: CLWW was homogenized at homogenization tank 1, treated with P. pastorisX33/pGAPZαA-LaccPost-Stop (Clone 1) immobilized in calcium alginate beads at extended aeration bioreactor, them flocculated and coagulated follow by a quartzite sand filtration; effluent was stored at homogenization tank 2. Conduction line of treated water 2: CLWW was homogenized at homogenization tank 1, treated with rPOXA 1B concentrate at extended aeration bioreactor, follow by a quartzite sand filtration; effluent was stored at homogenization tank 2. Once the secondary effluent mixture was homogenized in the homogenization tank 2, it was treated in the phototropic reactor with Chlorella sp
Only for C1 immobilized cell reactivation 1 L of media was added (0.05 mM CuSO4, 30 g L−1 glucose, 2.5 mM NH4SO4, 10 g L−1 peptone, 30 g L−1 yeast extract), (Gouzy-Olmos et al. 2018), and cells were soaked in media for 1 h. For the following two cycles (C2 and C3), cells from the previous cycle remained in the bioreactor and 1 L effluent from the previous cycle was added (before flocculation and filtration through quartzite sand). Reactivation in culture media was not performed for C2 or C3.
CLWW (1500.00 CU) secondary treatment with rPOXA 1B free enzyme concentrate (pilot scale)
As described in the previous assay the same treatment plant and conditions were used, except only free enzyme concentrate, was employed and the flocculation step was omitted (Fig. 1). At 72 h of treatment, the effluent was filtered through quartzite sand. Initial dose for concentrated rPOXA lots was ~ 400 U L−1. Three processes were evaluated (Batch, P1, P2, and P3). To determine COD (mg L−1), TSS, (mg L−1), TOC (mg L−1), IC (mg L−1), TC (mg L−1), TN (mg L−1), pH and UV/VIS absorption spectra changes samples were collected every 12 h (Pedroza-Camacho et al. 2018).
Tertiary treatment with Chlorella sp., of effluents from secondary treatment
For tertiary treatment, effluents from secondary treatments (experiments with immobilized P. pastoris and free rPOXA 1B enzyme concentrate) were mixed to generate an only lot. Treatment was performed in 15 L bioreactor with 8 L WEV, inoculated with 4% (v/v) Chlorella sp., (10 × 106 CFU mL−1) suspension, aerated with three submergible pumps to maintain a 1 L min−1 flux. A fluorescent lamp was installed within the quartz jacket, placed in the center in the bioreactor (1000 lx with 12 h light/dark cycles), (Fig. 1). Average temperature in the area where the phototrophic reactor was placed oscillated between ~ 19.00 ± 3.00 °C. To determine COD (mg L−1), TOC (mg L−1), NO3= (mg L−1), NO2− (mg L−1), TSS, (mg L−1), orthophosphates (mg L−1) and pH, samples were collected in the beginning and at 144 h (6 days). Additionally, microalgae dry biomass was determined (dry weight determination (g L−1) at 105 °C for 20 min), (American Public Health Association et al. 2005). The procedure for estimating the bacteria number was based on the 9215C spread plate method (Dichter and LeChevallier 2017).
Analytical techniques
UV/VIS spectrophotometry
UV/VIS spectra using Mecasys OPTIZEN spectrophotometer were determined for each sample before and after treatment to evaluate changes in absorption spectra in sample coloured compounds.
Colour Units (CU) determination
Colour units were determined measuring CLWW at 614 nm wavelengths, using Eq. (2), (Livernoche et al. 1983):
| 2 |
where A1 is the absorbance at which CLWW wave length was at its maximum, and 0.132 is the ABS465nm of a standard platinum-cobalt solution with 500.00 CU (Livernoche et al. 1983).
TSS determination
Total suspended solids concentration (mg L−1) was determined using the 2540 D Standard Methods for the Examination of Water and Wastewater (Rice 2017).
COD determination
The HACH commercial kit was used based on the 8000 HACH method. Reading was performed in a HACH spectrophotometer at 620 nm using as a blanc a COD tube with dH2O instead of wastewater. Technique detection ranged between 0 and 15,000 mg L−1.
BOD5 determination
The VELP SCIENTIFICA system was employed to determine this parameter.
TOC, IC, TC and TN determination
The concentration of organic matter (OM) of the effluents of each cycle and process at time 0 and post-filtration was measured using a TOC analyzer (Shimadzu TOC-L total organic carbon analyzer), this equipment uses a unique combustion catalytic oxidation and NDIR according to 5310B Standard method (Rice 2017).
For TOC determination in tertiary treatment with Chlorella sp., a HACH commercial kit for low range Total Organic Carbon (TOC), (Test’ N Tube™ 03) was used based on the 5310 C method, specifically persulphate combustion oxidation. Reading was performed in a HACH spectrophotometer at 430 nm using as a blanc one COT tube with Milli Q water instead of CLWW (American Society for Testing and Materials 1994).
Nitrates, nitrites and orthophosphate determination
NitraVer® commercial kit from HACH was used to determine nitrates, based on cadmium reduction method 8171 ranging from 0.1 to 10 mg L−1 NO3–N (Hach Company/Hach Lange GmbH 2014). Nitrite determination was performed using NitriVer® 3 commercial kit from HACH, based on method 8507 ranging from 0.002–0.300 mg L−1 NO2–N (Hach Company/Hach Lange GmbH 2007). Last, for orthophosphate determination (HPO4−3 in mg L−1) Spectroquant® for orthophosphates from Merck (MQuantTM three phosphate test, Merck) was used, based on previously reported phosphomolybdic acid colourimetric formation (Murphy and Riley 1962).
On the other hand, with algae biomass in mg L−1, COD (mg L−1) and TOC (mg L−1) consumed at 144 h after tertiary treatment yield (YX COD−1 and YX TOC−1) and biomass volumetric productivity (mg L−1 h−1) were calculated (Morales-Álvarez et al. 2016, 2017).
pH determination
Effluent’s and influent’s pH were measured using a JENWAY pH meter.
Laccase activity determination
Laccase activity (UL−1) was monitored by a change in absorbance at 420 nm (ε420 = 36.000 M−1 cm−1) based on ABTS oxidation in 0.1 M citrate buffer (pH 3.0 ± 0.2). 2 µL of the sample were added to 898 µL of 0.1 M citrate buffer, and 100 µL of 20 mM ABTS at room temperature (25 °C). Green radical formation was evaluated spectrophotometrically for 1 min. Blanc solution contained 2 µL of distilled water, 898 µL of citrate buffer solution, and 100 µL of 20 mM ABTS, Eq. (3).
| 3 |
where ΔE corresponds to the difference between final and initial absorbance after 1 min of reaction, Vt refers to the total reaction volume (mL), ε refers to the ABTS molar extinction coefficient (M−1 cm−1), d is the length of the cuvette in cm and Vs is the volume of sample (mL) contained in the reaction.
Scanning electron microscopy (SEM)
This service was contracted with Universidad de Los Andes (UNIANDES), Bogotá, D.C, Colombia. Alginate bead morphology containing P. pastoris cells and Chlorella sp. cells were observed under scanning electron microscopy (SEM). A JEOL microscope was used, model JSM 6490-LV imaging from 10 to 30 kV. Samples were covered with gold under vacuum conditions using Metalizador Desk IV. Samples were observed at 8000× magnification for Chlorella sp. and for P. pastoris at 20,000× magnification.
Results
Treatment of different CU of CLWW with P. pastoris X33/pGAPZαA-LaccPost-Stop (Clone 1) immobilized cells in calcium alginate
Following are the CU selection assays performed at Erlenmeyer flask scale (Fig. 2), which allowed establishing CLWW CU to be used at pilot scale for the plant treatment. Colour at 300.00 and 1200.00 CU notably decreased after 144 h of treatment with percentages of decolouration of 60.53 and 73.16%, respectively (Table 1).
Fig. 2.
Photographic monitoring of CLWW treatments. CLWW treatments for ~ 300.00, ~ 1200.00 and ~ 2300.00 CU, respectively, at 0 h and 144 h. The objective was to select best CU of CLWW for pilot treatment with P. pastoris X33/pGAPZαA-LaccPost-Stop (Clone 1) immobilized cells, and rPOXA 1B enzyme concentrate
Table 1.
CLWW percentage decolouration after 144 h of treatment. Treatment with calcium alginate beads and P. pastoris X33/pGAPZαA/LaccPost-Stop (Clone 1) immobilized in calcium alginate beads
| CLWW, colour unit (CU) | Treatment | % decolouration (144 h) |
|---|---|---|
| ~ 300.00 | C1a | 0.00 |
| C2b | 7.89 | |
| T1c | 60.53 | |
| ~ 1200.00 | C1 | 0.00 |
| C2 | 14.38 | |
| T1 | 73.16 | |
| ~ 2300.00 | C1 | 0.00 |
| C2 | 7.97 | |
| T1 | 1.95 |
Bold values indicate major decolouration treatments
aCLWW
bCLWW + Ca2+ alginate beads
cCLWW + Ca2+ alginate beads and P. pastoris X33/pGAPZαA-LaccPost-Stop (Clone 1)
CLWW, UV/Vis characterization
CLWW (1500.00 CU) UV/VIS spectra for influent to treat with P. pastoris X33/pGAPZαA-LaccPost-Stop (Clone 1) immobilized in calcium alginate beads and for free rPOXA 1B enzyme concentrate is presented in Fig. 3.
Fig. 3.
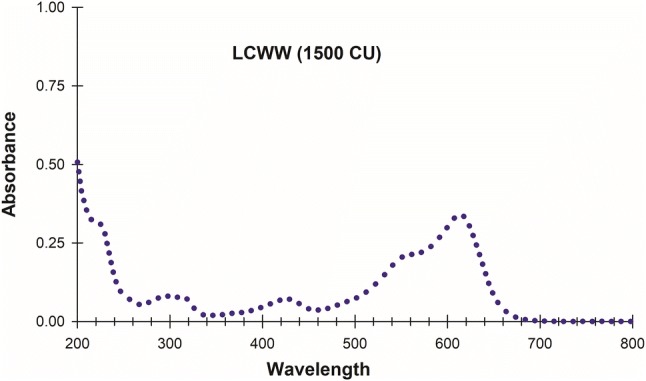
LCWW UV/VIS (200–800 nm) absorption spectra. LCWW (1500.00 CU) spectra; where 614 nm was selected as the visible wavelength for maximal absorption
CLWW (1500.00 CU) secondary treatment with P. pastoris X33/pGAPZαA-LaccPost-Stop (Clone 1) immobilized cells at pilot scale
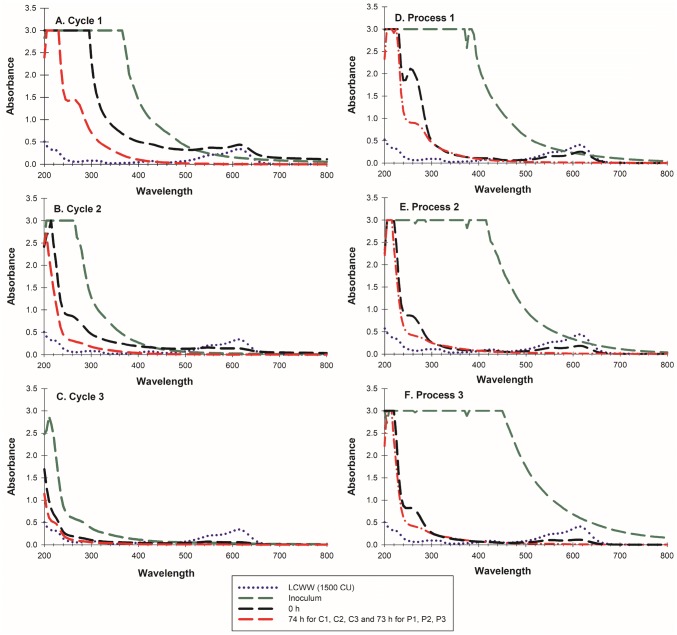
In Fig. 4a–c, the UV/VIS absorption spectra are presented for the three cycles of CLWW (1500.00 CU, influents) treatment with P. pastoris immobilized cells producing the recombinant POXA 1B enzyme, as well as removal percentages of some CLWW discharge parameters after treatment (secondary effluent).
Fig. 4.
The behaviour of the 3 cycles and the 3 treatment processes of CLWW (1500.00 CU) treatment with P. pastoris X33/pGAPZαA-LaccPost-Stop (Clone 1) immobilized cells, and with rPOXA 1B enzyme concentrate, respectively. a–c UV/VIS absorption spectra of C1, C2 and C3 respectively. d–f UV/VIS absorption spectra of P1, P2 and P3 respectively
In the three treatment cycles, the UV/VIS spectrum revealed a decrease in absorbance (approximately 0) in the VIS region (400–800 nm) as a consequence of rPOXA 1B action, produced by immobilized cells (Fig. 4a–c). In addition, in the three cycles at 74 h of treatment an increase in absorbance in the UV region (200–400 nm) was observed in comparison with the same region of CLWW (1500.00 CU). However, the signal was gradually decreasing with cycle change (C1 > C2 > C3). Table 2 shows CLWW (1500.00 CU, influents) discharge parameters of the effluent of the different treatment cycles.
Table 2.
Discharge parameters behaviour of different cycles of CLWW treatment (1500.00 CU) with P. pastoris X33/pGAPZαA-LaccPost-Stop (Clone 1) immobilized of the cells
| Discharge parameter | 0 h | 74 h | Removal (%) |
|---|---|---|---|
| Cycle 1 | |||
| TOC (mg L−1) | 2214.26 ± 46.84 | 591.97 ± 3.63 | 73.26 ± 0.40 |
| TC (mg L−1) | 2290.00 ± 48.08 | 608.50 ± 4.38 | 73.42 ± 0.37 |
| IC (mg L−1) | 75.74 ± 1.24 | 16.53 ± 0.75 | 78.18 ± 0.64 |
| TN (mg L−1) | 411.49 ± 20.53 | 264.40 ± 4.81 | 36.49 ± 5.00 |
| CU | 1500.00 ± 0.00 | 12.63 ± 2.19 | 99.16 ± 0.15a |
| COD (mg L−1) | 6045.00 ± 35.36 | 1660.00 ± 0.00 | 72.43 ± 0.00 |
| BOD5 (mg L−1) | 999.00 ± 0.00 | 999.00 ± 0.00 | 0.00 ± 0.00 |
| TSS (mg L−1) | 275.00 ± 35.36 | 50.00 ± 0.00 | 80.00 ± 0.00 |
| BOD5/COD | 0.16 | 0.60 | N/A |
| TOC/TN | 5.38 | 2.24 | N/A |
| COD/TOC | 2.73 | 2.80 | N/A |
| pH | 4.16 ± 0.20 | 5.05 ± 0.20 | N/A |
| Cycle 2 | |||
| TOC (mg L−1) | 135.86 ± 6.70 | 74.01 ± 0.50 | 45.45 ± 3.06 |
| TC (mg L−1) | 165.09 ± 3.97 | 93.22 ± 1.22 | 43.36 ± 2.65 |
| IC (mg L−1) | 29.11 ± 0.93 | 19.36 ± 0.57 | 33.49 ± 0.19 |
| TN (mg L−1) | 94.76 ± 1.63 | 93.20 ± 0.62 | 2.17 ± 1.32 |
| CU | 1500.00 ± 0.00 | 6.31 ± 4.37 | 99.58 ± 0.29a |
| COD (mg L−1) | 760.00 ± 155.56 | 280.00 ± 14.14 | 67.82 ± 1.63 |
| BOD5 (mg L−1) | 999.00 ± 0.00 | 250.00 ± 0.00 | 74.98 ± 0.00 |
| TSS (mg L−1) | 66.67 ± 28.87 | 66.67 ± 28.87 | 0.00 ± 0.00 |
| BOD5/COD | 1.30 | 0.80 | N/A |
| TOC/TN | 1.43 | 0.79 | N/A |
| COD/TOC | 5.59 | 3.78 | N/A |
| pH | 7.21 ± 0.20 | 8.01 ± 0.20 | N/A |
| Cycle 3 | |||
| TOC (mg L−1) | 47.08 ± 1.41 | 20.60 ± 3.13 | 56.13 ± 7.96 |
| TC (mg L−1) | 54.22 ± 2.17 | 27.81 ± 1.54 | 48.61 ± 4.59 |
| IC (mg L−1) | 6.33 ± 0.73 | 7.34 ± 0.85 | 0.00 ± 0.00 |
| TN (mg L−1) | 60.07 ± 0.66 | 60.60 ± 0.00 | 0.00 ± 0.00 |
| CU | 1500.00 ± 0.00 | 10.10 ± 2.19 | 99.33 ± 0.15a |
| COD (mg L−1) | 370.00 ± 0.00 | 295.00 ± 21.21 | 15.71 ± 6.06 |
| BOD5 (mg L−1) | 250.00 ± 0.00 | 250.00 ± 0.00 | 0.00 ± 0.00 |
| TSS (mg L−1) | 166.67 ± 28.87 | 33.33 ± 28.87 | 83.33 ± 14.43 |
| BOD5/COD | 0.60 | 0.80 | N/A |
| TOC/TN | 0.78 | 0.34 | N/A |
| COD/TOC | 7.86 | 14.32 | N/A |
| pH | 7.28 ± 0.20 | 7.50 ± 0.20 | N/A |
N/A does not apply, N/C unable to calculate
aPercentage calculated from 1500.00 CU at the beginning of treatment
CLWW (1500.00 CU) secondary treatment with rPOXA 1B free enzyme concentrate (pilot scale)
UV/VIS absorption spectra for CLWW (1500.00 CU, influents) for the three treatment processes with rPOXA 1B concentrate, as well as removal percentages of certain CLWW discharge parameters after treatment (effluent) are illustrated in Fig. 4e, f.
For the three processes, UV/VIS spectra exhibited a decrease in absorbance signal, almost 0 in the VIS region (400–800 nm) as a consequence of the presence of rPOXA 1B enzyme (Fig. 4d–f). At 73 h of treatment an increase in absorbance for the three processes in the UV region (200–400 nm) was observed, in comparison with the same region of CLWW (1500.00 CU), however, the signal decreased when the process was changed from P1 to P2 (P1 > P2 = P3).
Average enzyme activity at the beginning of treatment was 401.23 ± 6.83 U L−1 and after 73 h decreased to 296.60 ± 12.29 U L−1, with oscillations during treatment. Table 3 summarizes CLWW (1500.00 CU, influents) parameter quality and parameters of effluents after different treatments.
Table 3.
Discharge parameters for different CLWW (1500.00 CU) treatment processes with rPOXA 1B enzyme concentrate
| Discharge parameter | 0 h | 73 h | Removal (%) |
|---|---|---|---|
| Process 1 | |||
| TOC (mg L−1) | 420.06 ± 9.53 | 273.71 ± 5.08 | 34.84 ± 0.27 |
| TC (mg L−1) | 481.20 ± 11.03 | 332.80 ± 6.79 | 30.84 ± 0.18 |
| IC (mg L−1) | 61.14 ± 1.50 | 59.09 ± 1.71 | 3.36 ± 0.43 |
| TN (mg L−1) | 205.37 ± 6.71 | 198.53 ± 6.11 | 3.32 ± 1.48 |
| CU | 1500.00 ± 0.00 | 49.24 ± 3.79 | 96.72 ± 0.25a |
| COD (mg L−1) | 1865.00 ± 49.49 | 840.00 ± 84.85 | 55.00 ± 3.36 |
| TOC/TN | 2.05 | 1.38 | N/A |
| COD/TOC | 4.44 | 3.07 | N/A |
| pH | 6.96 ± 0.20 | 7.97 ± 0.20 | N/A |
| Process 2 | |||
| TOC (mg L−1) | 172.53 ± 2.81 | 112.16 ± 0.68 | 34.98 ± 1.45 |
| TC (mg L−1) | 210.70 ± 4.10 | 134.64 ± 0.08 | 36.09 ± 1.20 |
| IC (mg L−1) | 38.49 ± 1.07 | 22.48 ± 0.76 | 41.11 ± 0.02 |
| TN (mg L−1) | 112.94 ± 0.59 | 104.25 ± 5.08 | 8.85 ± 5.23 |
| CU | 1500.00 ± 0.00 | 44.19 ± 35.36 | 97.05 ± 0.15a |
| COD (mg L−1) | 885.00 ± 77.78 | 345.00 ± 35.36 | 60.69 ± 7.45 |
| TOC/TN | 1.53 | 1.08 | N/A |
| COD/TOC | 5.13 | 3.08 | N/A |
| pH | 7.16 ± 0.20 | 7.65 ± 0.20 | N/A |
| Process 3 | |||
| TOC (mg L−1) | 155.81 ± 0.41 | 101.61 ± 2.70 | 34.79 ± 1.56 |
| TC (mg L−1) | 191.70 ± 2.21 | 129.64 ± 3.71 | 32.89 ± 1.67 |
| IC (mg L−1) | 36.29 ± 1.45 | 27.61 ± 1.69 | 23.98 ± 1.69 |
| TN (mg L−1) | 105.12 ± 1.07 | 103.35 ± 1.43 | 1.77 ± 0.91 |
| CU | 1500.00 ± 0.00 | 53.03 ± 0.00 | 96.47 ± 0.00a |
| COD (mg L−1) | 785.00 ± 35.36 | 360.00 ± 14.14 | 54.05 ± 3.87 |
| TOC/TN | 1.48 | 0.98 | N/A |
| COD/TOC | 5.04 | 3.54 | N/A |
| pH | 7.19 ± 0.20 | 7.90 ± 0.20 | N/A |
N/A does not apply, N/C unable to calculate
aPercentage calculated from 1500.00 CU at the beginning of treatment
Tertiary treatment with con Chlorella sp. of a mixture of secondary effluents treated with P. pastoris X33/pGAPZαA-LaccPost-Stop (Clone 1) immobilized cells and enzyme extract
The mix of six secondary effluents presented the following initial characteristics: CU (90 ± 3 CU614 nm), COD (300 ± 9 mg L−1), TOC (10 ± 2 mg L−1), TSS (66.7 ± 1.2 mg L−1), NO3 (6.25 ± 0.98 mg L−1), NO2 (0.065 ± 0.002 mg L−1), orthophosphates (15.7 ± 0.57 mg L−1) and pH (7.06 ± 1.1). UV/VIS absorption spectra of secondary effluents mixture at the beginning of tertiary treatment and at 144 h is presented in Fig. 5. Signal associated with initial CU decreased to end at 34 ± 4 CU. In the UV region two changes were observed, one at signal 289 nm (decrease in absorbance from 0.263 to 0.211), the second change consisted of the appearance of a signal at 253 nm (0.183 units of absorbance), which could be associated with an intermediate produced by Chlorella sp., or to a pigment produced by the algae.
Fig. 5.
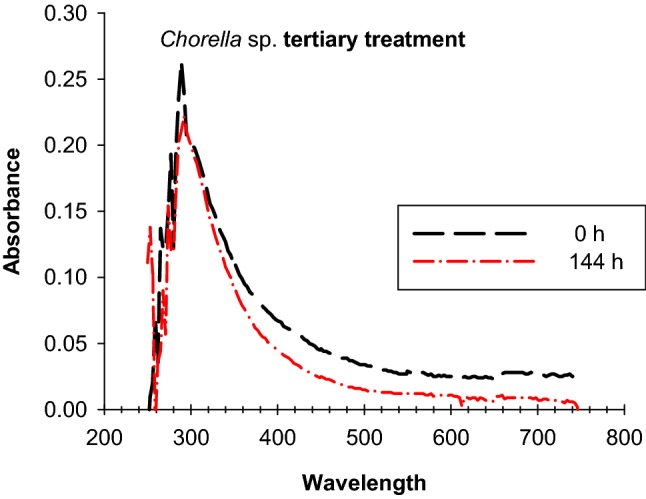
Chorella sp. tertiary treatment. UV/VIS absorption spectra of mix of secondary effluents treated for 144 h
Concerning to quantify discharge parameters for the tertiary treatment, initial and final values are presented in Table 4. It is noteworthy a decrease in COD (155 ± 4 mg L−1), TOC (6.6 ± 0.87 mg L−1), NO3 (4.25 ± 1.11 mg L−1) and orthophosphates (9.2 ± 2.3 mg L−1) concentration. On the contrary, NO2 and TSS values increased with values of 0.156 ± 0.07 mg L−1 and 149 ± 7 mg L−1, respectively, at 144 h.
Table 4.
Discharge parameters for tertiary treatment with Chorella sp., at 144 h
| Tertiary treatment | |||
|---|---|---|---|
| Discharge parameter | 0 h | 144 h | Removal (%) |
| CU | 90 ± 3 | 34 ± 4 | 62.2 ± 1.3 |
| TOC (mgL−1) | 10 ± 2 | 6.6 ± 0.87 | 34 ± 3 |
| COD (mgL−1) | 300 ± 9 | 155 ± 4 | 48.3 ± 2.4 |
| NO3= | 6.25 ± 0.98 | 4.25 ± 1.11 | 32 ± 2 |
| NO2− | 0.065 ± 0.002 | 0.156 ± 0.07 | 0 |
| Ortophososphates | 15.7 ± 0.57 | 9.2 ± 2.3 | 41.4 ± 3.5 |
| TSS (mgL−1) | 66.7 ± 1.2 | 149 ± 7 | 0 |
| Algae dry weight | 500 ± 10 | 1400 ± 80 | N/A |
| Total bacteria (CFU mL−1) | 1 × 104 ± 1 × 101 | 1 × 106 ± 1 × 102 | N/A |
| P(biomass) (mg L−1 h−1) | N/C | 9.7 ± 1.3 | N/A |
| Y(biomass/COD) (mg mg−1) | N/C | 9.65 ± 1.24 | N/A |
| Y(biomasa/TOC) (mg mg−1) | N/C | 411 ± 35 | N/A |
| pH | 7.06 ± 0.20 | 7.49 ± 0.20 | N/A |
N/A does not apply, N/C unable to calculate
Tertiary treatment efficiency and Chlorella sp., growth under mixotrophic conditions without sterility conditions were confirmed with a dry weight of algae biomass (initial: 500 ± 10 mg L−1 and final 1400 ± 80 mg L−1) and total bacteria count (initial: 1 × 104 ± 1 × 101 CFU mL−1 and final: 1 × 106 ± 1 × 102 CFU mL−1). Additionally, when biomass yield was calculated in terms of consumed COD (YX/DQO) and TOC (YX/COT) the values obtained were 9.65 ± 1.24 mg mg−1 and 411 ± 35 mg mg−1, respectively. Last, biomass volumetric productivity was 9.7 ± 1.3 mg L−1 h−1.
P. pastoris immobilized in alginate beads after secondary treatment (74 h) and Chlorella sp., after tertiary treatment (144 h) were characterized under SEM (Fig. 6a, b). P. pastoris were oval shaped with an approximate size of 1.6 ± 0.5 µm and were distributed in different zones of the alginate matrix (Fig. 6a). Chorella sp., cells were spherical with an approximate size of 4.9 ± 0.9 µm (Fig. 6b). Groups of variable sizes were observed, which could be associated with sample preparation for microscopic analysis.
Fig. 6.
Scanning electron micrographs (SEM). aP. pastoris cells immobilized in calcium alginate after the secondary (74 h). bChlorella sp. cells after tertiary treatment (144 h)
Regarding EDS analysis, it was observed both cell types contained carbon, oxygen, nitrogen, phosphorus, aluminium and potassium (Table 5). However, atomic percentages varied among them, such that the higher contents were observed in Chorella sp., for carbon, oxygen, nitrogen and phosphorus, which could be associated with the capacity of microalgae to assimilate carbon by mixotrophy (fix atmospheric CO2 and employ organic carbon present in water) and efficiently remove nutrients such as phosphorus and nitrogen.
Table 5.
Atomic composition for EDS from P. pastoris X33/pGAPZαA-LaccPost-Stop (Clone 1) and Chorella sp.
| Element | Atomic (%) | |
|---|---|---|
| P. pastoris | Chlorella sp. | |
| C | 51.40 | 80.75 |
| O | 40.32 | 12.14 |
| N | 0.10 | 1.83 |
| P | 0.04 | 1.20 |
| Na | ND | 1.30 |
| Al | 0.13 | 1.27 |
| K | 0.07 | 1.51 |
| Ca | 7.94 | ND |
ND not determined
Discussion
Treatment of different CU of CLWW with P. pastoris X33/pGAPZαA-LaccPost-Stop (Clone 1) immobilized cells in calcium alginate
Data illustrated in Fig. 2 and Table 1 demonstrate that up to 1200.00 CU ~ 73% color removal was obtained. Decolouration obtained in these assay was supported by three factors, (1) high rPOXA 1B redox potential (Roman et al. 2010; Mendoza et al. 2011; Drumond Chequer et al. 2013; Rivera-Hoyos et al. 2013; El-Batal et al. 2015; Morales-Álvarez et al. 2016), (2) adsorption generated by the presence of calcium alginate beads (Fig. 1) and (3) P. pastoris X33/pGAPZαA-LaccPost-Stop (Clone 1) planktonic cells (Gouzy-Olmos et al. 2018).
Modest colour elimination at 2300.00 CU could be due to aspects, such as (1) calcium alginate matrix saturation, (2) extended treatment time requirement, and (3) possible water toxicity on P. pastoris X33/pGAPZαA-LaccPost-Stop (Clone 1) immobilized cells. Experimentally the effect of time on % decolouration could have been studied, however, after 144 h the assay becomes unfeasible for industrial purposes since it would be too extensive and costly, therefore less attractive; particularly if it is an industrial batch process with a high liquid waste flow.
Considering the gap between 1200.00 and 2300.00 CU is wide, as well as the possible toxic effect on the recombinant strain, it was decided to perform the entire pilot-scale study at 1500.00 CU, an intermediate CLWW dilution, for the cycle treatments with immobilized cells in calcium alginate beads, as well as for the free rPOXA 1B enzyme concentrate. This decision suggests that in practice it will require influent dilution, to adjust the CU to 1500.00. However, it is also clear the possibility of reusing the effluent in the dilution of the influent to be treated, which would significantly reduce water expenditure.
The limitation in enzyme activity determination could have been due to low enzyme diffusion, an effect attributed to alginate concentration (≥ 4% w/v), (Tanaka et al. 1984; Won et al. 2005). Decolouration percentages are presumed to have resulted from enzyme activity, because of the difference between dye adsorption (absorption control C2, alginate beads without cells) and T1 treatment for the different CU (Table 1).
CLWW UV/VIS characterization
CLWW lots generated every semester in teaching and research laboratories at the Department of Microbiology School of Sciences, Pontificia Universidad Javeriana are heterogenous, due to different concentrations and types of colourants and/or additives (oils, alcohols and acids, etc.), depending on the time of collection. Thus, to identify the wavelength where maximum absorption was obtained it was necessary to characterize CLWW, UV/VIS spectra and determine the corresponding CU. The CLWW lot used in this work had 25,750.00 CU and was diluted into 1500.00 CU with a maximum VIS absorption at 614 nm (Fig. 3a).
CLWW (1500.00 CU) secondary treatment with P. pastoris X33/pGAPZαA-LaccPost-Stop (Clone 1) immobilized cells at pilot scale
Absorption spectra tendencies for the three cycles were similar (Fig. 4a–c). At 0 h, when compared with CLWW (1500.00 CU) an increase in absorbance in the UV region (200–400 nm) was observed. This increase resulted from reactivation media supplementation to reactivate immobilized cells in cycle 1 (Gouzy-Olmos et al. 2018), although for cycles 2 and 3 a lesser increase in the UV region was observed (0 h), because for both cycles no reactivation media was added, and only immobilized cells and 1 L effluents from the previous cycle was used (before flocculation and quartzite sand filtration). In general, an increase in UV region absorbance at 0 h could be due to supplementation with organic matter (reactivation media components, effluent from the previous cycle, cells and extracellular metabolites) and to the colour provided by the reactivation media, specifically for cycle 1.
For the VIS region between 0 and 72 h absorbance considerably decreased and flocculation and quartzite sand filtration also reduced absorbance in the UV region (200–400 nm). However, at the end of the cycles, especially cycle 1 and 2, absorbance and spectral profile for UV regions were higher in comparison with CLWW (1500.00 CU) Fig. 5a–c. These results could be due to two aspects, (1) transformation of complex compounds and/or aromatic compounds (with absorbance in the VIS region) that changed their conformation into simple or aliphatic compounds (Fernández et al. 2016; Morales-Álvarez et al. 2016; Blanco-Vargas et al. 2018; Pedroza-Camacho et al. 2018) and (2) remains of the organic load added to the system with the reactivation media.
For cycle 1 at 0 h no important decolouration was observed, varying between 1500.00 to 1436.87 CU (Table 2, Fig. 4a–c), however, at 2 h CU decreased to 273.99 CU (data not shown). Nevertheless, for cycles 2 and 3 decolouration was immediate (0 h) decreasing CU from 1500.00 a 598.48 and 209.59 CU, respectively, (Table 2, Fig. 4a–c). Once effluents from cycles 1, 2 and 3 were flocculated and filtered they demonstrated colour removal of 99.1, 99.5 and 99.3%, respectively (Table 2).
For the different cycle’s behavior of discharge parameters (BOD5, COD, TOC, TC, IC and TN) revealed an evolution in organic charge (Table 2). COD reduction was 72.43, 67.82 and 15.71% for C1, C2 y C3, respectively; suggesting the implemented system decreased organic load supplemented during alginate bead reactivation (reactivation media). However, low COD reduction in C3 indicated it reached its limit. For TOC reduction, this parameter presented removal percentages between 45 and 73%.
TOC/TN ratio in all treatment cycles decreased (Table 2), suggesting an organic matter oxidation, in addition to a greater elimination of carbon compounds in comparison with nitrogenous compound elimination (Malvis et al. 2019). In C1, C2 and C3 TOC/TN ratio decreased between 0 and 74 h of treatment (Table 2). Furthermore, for all cycles COD/TOC ratio was not below 2.5, indicating a decrease in complex sub-products (Bilinska et al. 2016). COD/TOC ratios below 2.5 indicate residual organic carbon is due to the presence of recalcitrant organic compound presence (Guedes et al. 2003); an association that can change due to non-biodegradable organic compounds that remain at the end of wastewater treatment (Mara and Horan 2003). Moreover, (BOD5/COD) treatability ratio demonstrated values higher than 0.5 for all treatment cycles, suggesting that influents (0 h) as well as effluents (74 h) were susceptible to biodegradation (Tchobanoglous et al. 2013; Bilinska et al. 2016).
In C1, pH changed to neutral (0 at 72 h), (data not shown). A change in pH could be due to the generation of aromatic intermediates and amines resulting from the transformation of the dyes through enzymes (Rani et al. 2014). However, because of the flocculation process used Al2(SO4)3 and NaOH to separate part of the TSS and free biomass in the effluent before filtration, the pH acidified. Although these chemical compounds allow for particle separation, they also generate changes in the pH (Shon et al. 2009; Zhao et al. 2011). The pH in C2 and C3 was neutral throughout the treatment, nevertheless in cycle 2 flocculation slightly modified the pH, the effect caused by the used of the aforementioned chemicals.
During CLWW treatment cycles (C1, C2 and C3), no enzyme activity was detected (U L−1). Enzyme activity arrest difficulties were observed since UC selection analyses (Fig. 2, Table 1). Probably, activity was below detection values by the used methodology, given the low diffusion of the enzyme from the calcium alginate bead. During the first hours of the cycles, it was possible to observe dye adsorption to the alginate beads and subsequently the gradual discolouration of beads, a similar effect observed in Fig. 2. Other authors reported limited enzyme diffusion with increased alginate concentration (≥ 4% w/v), (Tanaka et al. 1984; Won et al. 2005), as was employed in this study. Hence, alginate beads should be dissolved in phosphate buffer to detect activity (Hung et al. 2008). Additionally, our group demonstrated in a computational model laccase does not rupture Ca2+ alginate matrix (Gouzy-Olmos et al. 2018).
CLWW (1500.00 CU) rPOXA 1B free enzyme concentrate treatment at pilot scale
For the three treatment processes absorption spectra tendencies are shown in Fig. 4d–f. At 0 h an increase in the absorption pattern in the UV region (200–400 nm) was observed, as a consequence of supplementation with enzyme concentrate. Process 1 had the highest increase since 330 mL of rPOXA 1B concentrate (lot number 1) was used for initial enzyme activity of ~ 400 U L−1 in comparison with processes 2 and 3, where only 88 mL (lot number 2) were employed to attain the same initial activity (~ 400 U L−1). In general, an increase in UV region absorbance (0 h) could be due to organic matters incorporated with the addition of enzyme concentrate.
At the end of each process, absorbance and UV spectral profile (200–400 nm) was higher in comparison with CLWW (1500.00 CU), as was observed for treatment cycles with immobilized cells in calcium alginate. This could be related to complex and/or aromatic compound transformation (absorbencies in the VIS region) with changes in configuration into simple or aliphatic compounds displaying maximum absorbencies in the UV region (200–400 nm), (Langergraber et al. 2004; Fernández et al. 2016; Morales-Álvarez et al. 2016; Blanco-Vargas et al. 2018; Pedroza-Camacho et al. 2018). Another possibility is to the organic matter in the bioreactor since at 72 h of treatment it was impossible to reduce to 100% the incorporated organic load with rPOXA 1B, likely caused by low microbial load within the treatment.
In all processes with rPOXA 1B, as well as the three cycles developed with immobilized cells an instantaneous decolouration effect was observed at 0 h decreasing 1500.00 CU into 1041.66, 652.77 and 597.22 CU for processes 1, 2 and 3, respectively (Fig. 4d–f). In all treatment processes (P1, P2 and P3), it was noticed absorption in the UV region considerably decreased after 72 h, with 96.71, 97.05 and 96.46% dye removal, respectively (Table 3).
Discharge parameters for the different processes (COD, TOC, TC, IC and TN), (Table 3) demonstrated that enzyme concentrate contributed with organic matter to the media (concentrate residual components), because they presented values greater than 0 h in all the processes, compared to those obtained in the CLWW (1500.00 CU), (Fig. 4d–f). However, organic matter contribution was less in comparison with reactivation media supplementation in cycle 1, which could explain why COD and TOC removal percentages were similar for the three processes.
TOC/TN ratio in all processes (P1, P2 and P3), (Table 3) decreased between 0 and 73 h. Additionally COD/TOC ratio also decreased with final rations greater than 2.5 at 73 h (Guedes et al. 2003), which could demonstrate a decrease in complex sub-products after treatment (Bilinska et al. 2016), caused by dye mineralization and/or residual components of the enzyme concentrate.
The pH in all three processes maintained a relatively neutral behaviour because it was not necessary to perform flocculation procedures. Hence, no variations in pH were observed as took place in the cycles.
During the three processes (P1, P2 and P3) enzyme activity ranged between 289.81 ± 3.21 and 415.33 ± 5.86 U L−1, after quartzite sand filtration enzyme activity decreased in all processes, ranging between 282.40 ± 3.21 and 303.70 ± 8.49, as a result of enzyme adsorption by the quartzite sand (Jada et al. 2006).
Collectively, results demonstrated the capacity to eliminate more than 90% of the CU with both treatments. CLWW treatment with immobilized cells revealed they could be re-used (at least for 216 h) without losing their removal capacity, which presents a technological advantage since it is not necessary to prepare immobilized cells for each treatment and opens new possibilities to implement a continuous system for treatment of this type of CLWW. Moreover, CLWW (1500.00 CU) treatment with 400 U L−1 de rPOXA 1B was also successful in decolouration, obtaining > 96% removal regardless of the volume of enzyme concentrate incorporated.
Although both treatments (cycles and processes) removed > 95% of the colour (which was the objective of the first part in this work), removal of organic matter only reached between 15.71 and 72.43% in the cycles and the processes between 54.05 and 60.69%. Therefore, residual wastewater obtained from the treatments may contain nutrients (sources of carbon, nitrogen and phosphorus) necessary for a tertiary treatment, which in this case propose microalgae use (Malvis et al. 2019).
Chlorella sp. tertiary treatment of effluents from secondary treatment
The initial characterization of six lots of secondary effluents mixture (C1, C2, C3, P1, P2 and P3), changed substantially concerning the individual values obtained for each secondary treatment (CU 90 ± 3 UC614nm, COD 300 ± 9 mg L−1, TOC 10 ± 2 mg L−1, TSS 66.7 ± 1.2 mg L−1, NO3 6.25 ± 0.98 mg L−1, NO2 0.065 ± 0.002 mg L−1, orthophosphates 15.7 ± 0.57 mg L−1 and pH 7.06 ± 1.1 (Table 4). This was due to a dilution effect when both secondary effluents were mixed. Even so, initial tertiary treatment values had concentrations that had to decrease before discharging of the tertiary treatment or re-use as irrigation water (Ministerio de Ambiente y Desarrollo Sostenible 2014).
Regarding CU decrease in the VIS region, Chorella sp. efficiently eliminated 62.2 ± 1.3% of the initial 90 CU. The decrease was observed from the first hours of contact and increased as a function of time (data not shown), (Table 4); demonstrating that initial removal was carried out by dye adsorption to the microalgae’s cell wall, a process that depended on biomass quantity, pH, temperature and time of contact (Dirbaz and Roosta 2018; Furuhashi et al. 2019). The increase in microalgae biomass and their size (Fig. 6b), was a factor that favoured dye removal (from 500 to 1400 mg L−1 at 144 h) since contact surface area was increased and more functional groups were made available to adsorb the dyes, where the most representative were those close to pH 7.0 ± 0.2 and –OH, –NH, and the chelant C=O present in amides, and C–O, methyl (–CH3) and methylene (–CH2) groups (Khataee et al. 2013; Dirbaz and Roosta 2018).
Another factor that favoured dye adsorption was the pH since during the entire experiment it remained between 7.06 and 7.49 ± 0.2. Under this condition microalgae, cells wall charges negatively due to OH- adsorption and proton loss, allowing for a more efficient electrostatic attraction adsorption (Table 4). In particular, cationic dyes, such as crystal violet, malachite green and methylene blue (Vyavahare et al. 2019).
Even though it was not verified, microalgae could have also produced enzymes that participated in dye biotransformation process, since in the UV region a decrease in the 289 nm signal was observed with an increase in the 253 nm signal (Fig. 5). This UV region (200–400 nm) absorption changes could be attributed to transformations through enzyme activity. In this sense, it has been reported azoic dyes (such as Congo red) can be degraded by Chlorella Vulgaris, Chlorella pyrenoidosa and Oscillatoria tenuisin, converting them into simple aromatic rings and amines (Forgacs et al. 2004). This type of degradation can be carried out by two types of enzymes azoreductases (E.C. 1.7.1.6) and oxidases (E.C. 1.1.3), (Solís et al. 2012; Otto and Schlosser 2014).
COD and TOC removal were 48.3 ± 2.4 and 34 ± 3, respectively. Reduction in these parameters could be accounted by two aspects (Table 4). The first makes reference to Chlorella sp. cells adaptation to this type of wastewaters since to keep them under laboratory conditions, secondary effluents from another treatment plant that operates continuously in the laboratory, in which the present investigation was conducted, was used as a culture medium (Pedroza-Camacho et al. 2018). Some authors report microalgae bioremediation potential can increase if a prior adaptation process is carried out by culturing in media similar to wastewater be treated (Hu et al. 2019). The second reason was related to the capacity Chlorella sp. has to grow under mixotrophy conditions, alternating between heterotrophic and autotrophic metabolism. Heterotrophic metabolites were obtained from secondary effluent organic compounds and autotrophic from produced CO2 by bacterial metabolism present during tertiary treatment (initial: 1 × 104 ± 1 × 101 and final: 1 × 106 ± 1 × 102), (Table 4).
Combination of both types of metabolism allows Chorella sp., to grow more rapidly and to produce greater quantities of biomass in comparison with only autotrophic conditions, which was evident by biomass volumetric productivity (9.7 ± 1.3 mg L−1 h−1) and biomass yield in terms of COD and TOC (9.65 ± 1.24 and 411 ± 35 mg mg−1), respectively (Table 4). TOC yield was higher than COD since dissolved organic carbon is easier to employ than total organic matter present, with a mix of carbon, nitrogen and sulphur compounds with different states of oxidation (Nirmalakhandan et al. 2019).
Nitrate (32 ± 2%) and orthophosphate (41.4 ± 3.5%) removal demonstrated Chlorella sp. eliminated part of the nutrients produced by partial mineralization of the dyes and were accumulated within the microalgae (Table 4). Nitrate removal was not particularly high, which could be due to microalgae preference for ammonium as the inorganic source of nitrogen. This process does not require previous reductions steps and is actively incorporated into the cells by the glutamine synthetase-glutamate synthase (E.C. 6.3.1.2)—(E.C. 1.4.7.1) metabolism for direct amino acid conversion, where glutamine synthase catalyzes glutamine formation from glutamate and adenosine triphosphate (ATP), (Gonçalves et al. 2017). Another factor that might have determined low nitrate removal is associated with nitrites, which increased in concentration. These ions could have been generated by nitrate reduction to nitrite, due to bacteria present during tertiary treatment under non-sterile conditions. Nitrite at various concentrations can exert an inhibitory effect on microalgae.
Regarding orthophosphates, their removal was higher in comparison with nitrates, but it might have been affected by the amount of light on Chorella sp., cells. Within the bioreactor when cell density is high, the same algae generate a blocking effect where light cannot go through, and not all cells receive the same luminosity, which determines that ATP and NADPH decreases and orthophosphate ions present in the secondary effluent adsorption becomes rather inefficient (Khalid et al. 2019).
Last, when tertiary effluent results were analyzed it was determined post-treated water was suitable for Lolium perennial seed germination (90% germination at 5 days), (data not shown) since its final composition had low organic carbonaceous organic matter, decreased colour, neutral pH and nutrients that were easily assimilated by the plant, such as nitrates, nitrites and orthophosphates. Results obtained in this work were similar to those reported by Pedroza-Camacho et al. (2018); the same type of wastewater was used; however, a fungal/bacterial consortium carried out biological treatment. Moreover, sedimentation and filtration were used to remove activated sludge, and no microalgae were employed. In that study, final effluent was classified as Class two water suitable for irrigation favoring the growth of Lolium perennial on a greenhouse scale (Pedroza-Camacho et al. 2018).
Table 6 shows differences of control parameters for pollutants removal; differentiating between biological and physicochemical treatments. It is observed that removal parameters vary depending on the treatment applied and that removal of pollutants such as NO3–N, is difficult to eliminate in some physicochemical treatments, however, using microalgae this drawback can be overcome since the algae uses the nitrogen source as a nutrient (Wang et al. 2010).
Table 6.
Comparison of tertiary treatments
| Tertiarty treatment | Removal | Operational parameters affecting process | Negative effect/problems | Costs | Reference | |
|---|---|---|---|---|---|---|
| Biological process | Microalgae |
UC 62.2% COT 48.3% TOC 34% NO3= 32% Ortophososphates 41.4% |
Biomass, pH | Nitrate removal was not particularly high | No calculated | This study |
| Dye Removal 30% | Effect of dye concentration, contact time, pH, nutrients | High dye concentration | High production cost under autotrophic laboratory conditions | Behl et al. (2019) | ||
|
NO3–N: 62.5% COD: 4.7% |
pH, nutrients concentrations | Type of pre-treatments | Dual roles of nutrient reduction and valuable biofuel feedstock production | Wang et al. (2010) | ||
| TDN > 79.0% Phosphorous > 98.0% TN 88.6–96.4% TP 99.7–99.9 | Temperature, light, pH, biomass productivity, CO2 solubility in the culture medium, CO2 consumption rates | There are sometimes allelochemicals production | Microalgal production in open systems is less expensive (construction and operation) | Arbib et al. (2014) and Gonçalves et al. (2017) | ||
| Physiochemical process | Ozonation (AOP) | COD 54.9% Micropollutants > 90.0% | Temperature, pH, Effluent organic matter concentration levels, alkalinity scavengers, ozone reactive inorganic | Increase of toxicity; low reactivity in dyes aromatic, heterocyclic and nitrogen-containing; low solubility at acidic pH | High production cost, and high investment and operational costs | de Arruda Guelli Ulson de Souza et al. (2010), Chys et al. (2018) and Arzate et al. (2019) |
| Electrochemical advanced oxidation (AOP) | Color 55.0% (BDD anode) color 14.9% (Pt anode) TOC 17.8% (BDD anode) TOC < 2.0% (Pt anode) | Time, anode type, current density | Production of carboxylic acids (highly recalcitrant organic compound) | Technology impractical and costly (long operational times and increases in current density) | Jhones dos Santos et al. (2018) | |
| Heterogeneous photocatalysis (AOP) | COD of 10% (only photolysis) COD ~ 45.0–77.0% (TiO2) COD ~ 19.0–50.0% (TVA) COD ~ 5.0–40.0% (Volcanic ashes) | Adsorption capacity and type of the materials, amounts of materials, turbidity, light intensity | Some effluent final has got a high level of genotoxicity or that grow inhibitor of green algae | Increased costs occasioned by separation of photocatalyst from water after treatment | Borges et al. (2014) and Rueda-Marquez et al. (2020) | |
| Photocatalytic ozonation (AOP) | TOC 9.0–25.0% COD ~ 70.0% | Pollutant concentrations, ozone dose, photocatalytic load and properties,pH, temperature, irradiation wavelength | An initial increase in the concentration of phenolics compound and toxicity of effluent | Process considered expensive for wastewater treatment (UV generation with conventional lamps) | Mehrjouei et al. (2015) and Quiñones et al. (2015) | |
| Sonolysis (AOP) | TOC 28.4% COD 16.2% BOD5 8.3% TN 39.6% NH3-N TP 14.5% | Temperature, ultrasound range, power density, power input, pollutant concentration | Process has a low mineralizing ability that it is not enough to comply with an acceptable water quality; the increased temperature by the ultrasound affecting the disinfection efficiency | When sonolysis is combined with processes like ozonation, demands high economical costs. The economic cost t is in function of energy consumption | Torres-Palma and Serna-Galvis (2018) and Vázquez-López et al. (2019) | |
| Photo-fenton (AOP) |
Total polyphenols ~ 90.0% COD ~ 90.0% DOC 4.0–36.0% Micropollutants > 90.0% |
pH, sales (FeSO4), UV radiation, H2SO4, H2O2 and KOH conectration | Waste generated are low biodegradabilit (ratio rbCOD/COD 0.45); Required a strongly acidic pH to avoid precipitation of iron. CO2 liberation during acidification stage | High cost associated with its installation and the energy demand | Lucas et al. (2012) and Arzate et al. (2019) | |
| Ultrafiltration (membrane processes) | COD 78.8% BOD 87.5.4% | Cross-flow velocity, trans-membrane pressure and backflushing methods | Operative problems in wastewater with high solids | High cost | Tchobanoglous et al. (1998) | |
| Coagulation/filtration (Membrane processes) | COD 24.5–35.2% TS 50.0–74.0% TN 3.8–19.2% TP 55.0–80.0% | TSS, Temperature, pH, Filtration rate of D/V filter (m/h), hydraulic retention time of coagulation tank | Increase of 61% of total GHG emissions generated from electricity use; coagulation/filtration processes had no significant effect on TN removal causing eutrophication by NO3-N permanence | High consumption of electricity increased cost of technology; depending on filter type the costs fluctuate between 324.6 and 84.8 USD/t COD eq removed | Wang et al. (2018) | |
| Filtration (Sand) | Turbidity 80.0% SS 72.0% TSS 83.0–98.0% COD 43% BOD 47.0–74.0% | Filtration rates (m/h), size of suspended solids, filters with an effective size filters and uniformity coefficient filters | Operative problem with particles less than 20 microm because are not removed efficiently | The technology is more economical using local sand | AI-Jadhai (2003) and Zahid (2003) | |
On the other hand, the emission of greenhouse gases (CO2) generated during physicochemical treatments harms the environment; however, microalgae have the advantage, to consume CO2, being an important operational parameter.
Finally, the use of an open system such as Chlorella sp. (tertiary treatment), stands out as a viable option for wastewater treatment; since some physicochemical treatments, in addition to having a higher cost than microalgae, are waste generators that generate negative impacts on the environment (Quiñones et al. 2015; Wang et al. 2018).
Conclusions
This study presented the successful use of recombinant yeast and a recombinant enzyme for the secondary treatment at a pilot-scale of coloured CLWW. Secondary treatment with P. pastoris X33/pGAPZαA-LaccPost-Stop (Clone 1) immobilized in calcium alginate beads presented a decolouration percentage > 99%. In addition, it demonstrates immobilized cells can be re-used and maintain their colour removal capacity for at least 216 h, which opens new possibilities for a continuous treating system. For secondary treatment with rPOXA 1B free enzyme concentrate, decolouration was > 96%; demonstrating the system has the advantage to incorporate less organic matter, due to the absence of cells and initial reactivation media. Chlorella sp., complemented dye removal (62.2%), COD (48.3%), TOC (34%), nitrates (32%) and orthophosphates (41.4%); becoming a valuable option for CLWW tertiary treatment. Data presented demonstrate a promissory alternative for coloured CLWW sequential treatment.
Acknowledgements
This research was funded by Grant No: 00007885 (Estudio de la estabilidad a tiempo real del concentrado de la lacasa rPOXA 1B de Pleurotus ostreatus producida en Pichia pastoris) and Grant ID: 00007135 (Diseño, implementación y evaluación a escala de laboratorio de un sistema secuencial para la remoción de color y carga orgánica presente en los subproductos líquidos derivados de las tinciones de microbiología, con fines de re uso en zonas verdes.) from Pontificia Universidad Javeriana. Financing entity had no role in the study design, data collection, or analysis, decision to publish, or preparation of the manuscript. Authors thank to María Lucía Gutiérrez, Ph.D., for English editing.
Abbreviations
- WW
Wastewater
- CLWW
Coloured Laboratory Wastewater
- CU
Colour units
- C1
Cycle 1
- C2
Cycle 2
- C3
Cycle 3
- P1
Process 1
- P2
Process 2
- P3
Process 3
- EWV
Effective work volume
- TOC
Total organic carbon
- IC
Inorganic carbon
- COD
Chemical oxygen demand
- BOD5
Biological oxygen demand
- POXA 1B
Laccase from P. ostreatus
- rPOXA 1B
Recombinant laccase from P. ostreatus
- TSS
Total suspended solids
- TN
Total nitrogen
- CFU
Colony forming units
- OM
Organic matter
- Y
Yield
- ABTS
2, 2-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)
- SEM
Scaning electron microscopy
- EDS
Energy dispersive X-ray spectroscopy
- TDN
Total dissolved nitrogen
- MP
Micropollutants
- AOP
Advanced oxidation processes
- TVA
Titania supported over volcanic ashes
Author contributions
Conceived and designed the analyses: CMR-H, RAP-P, AMP-R, AP-F, BEQ-H. Acquired funding: AMP-R, RAP-P. Collected the data and performed the analyses: LDA-L, VH-R, DNC-B, JFM-M, LDP-C. Administrated resources for the project: AMP-R, RAP-P. Wrote the paper: LDA-L, AMP-R, RAP-P. Performed critical review of the manuscript with editions: AMP-R, CMR-H, RAP-P, AP-F, BEQ-H.
Compliance with ethical standards
Conflict of interests
The authors declare they have no competing interests.
Footnotes
Leidy D. Ardila-Leal, Valentina Hernández-Rojas and Diana N. Céspedes-Bernal contributed equally to this work.
Contributor Information
Raúl A. Poutou-Piñales, Email: rpoutou@javeriana.edu.co
Aura M. Pedroza-Rodríguez, Email: apedroza@javeriana.edu.co
References
- Abdel-Kareem O. History of dyes used in different historical periods of Egypt. Res J Text Appar. 2012;16(4):79–92. doi: 10.1108/RJTA-16-04-2012-B009. [DOI] [Google Scholar]
- AI-Jadhai IS. Pilot-plant study of the tertiary filtration of wastewater using local sand. J King Saud Univ Eng Sci. 2003;16:83–96. [Google Scholar]
- American Public Health Association A, American Water Works Association A, Water Environment Federation W (2005) Standard Methods for the Examination of Water and Wastewater, 21st edn. Washington DC., USA
- American Society for Testing and Materials A (1994) Standard test method for total carbon in water by ultraviolet, or persulfate oxidation, or both, and infrared detection. D4839-88. American Soc. Testing & Materials, Philadelphia, USA
- Arbib Z, Ruiz J, Álvarez-Díaz P, Garrido-Pérez C, Perales JA. Capability of different microalgae species for phytoremediation processes: wastewater tertiary treatment, CO2 bio-fixation and low cost biofuels production. Water Res. 2014;49:465–474. doi: 10.1016/j.watres.2013.10.036. [DOI] [PubMed] [Google Scholar]
- Ardila-Leal LD, Albarracín-Pardo DA, Rivera-Hoyos CM, Morales ED, Poutou-Piñales RA, Cardozo-Bernal AM, Quevedo-Hidalgo BE, Pedroza-Rodríguez AM, Díaz-Rincón DJ, Rodríguez-Lopez A, Alméciga-Díaz CJ, Cuervo-Patiño CL. Media improvement for 10 L bioreactor production of rPOXA 1B laccase by P. pastoris. 3 Biotech. 2019;9(12):447. doi: 10.1007/s13205-019-1979-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias DM, Solé-Bundó M, Garfí M, Ferrer I, García J, Uggetti E. Integrating microalgae tertiary treatment into activated sludge systems for energy and nutrients recovery from wastewater. Bioresour Technol. 2018;247:513–519. doi: 10.1016/j.biortech.2017.09.123. [DOI] [PubMed] [Google Scholar]
- Arzate S, Pfister S, Oberschelp C, Sánchez-Pérez JA. Environmental impacts of an advanced oxidation process as tertiary treatment in a wastewater treatment plant. Sci Total Environ. 2019;694:133572. doi: 10.1016/j.scitotenv.2019.07.378. [DOI] [PubMed] [Google Scholar]
- Baily RE. Sludge: types, treatment processes and disposal. Hauppauge: Nova Science Publishers Inc; 2009. [Google Scholar]
- Barrios-Ziolo LF, Gaviria-Restrepo LF, Agudelo EA, Cardona-Gallo SA. Technologies for the removal of dyes and pigments present in wastewater. A review. Dyna. 2015;82(191):118–126. doi: 10.15446/dyna.v82n191.42924. [DOI] [Google Scholar]
- Behl K, Sinha S, Sharma M, Singh R, Joshi M, Bhatnagar A, Nigam S. One-time cultivation of Chlorella pyrenoidosa in aqueous dye solution supplemented with biochar for microalgal growth, dye decolorization and lipid production. Chem Eng J. 2019;364:552–561. doi: 10.1016/j.cej.2019.01.180. [DOI] [Google Scholar]
- Bilal M, Ashraf SS, Barceló D, Iqbal HMN. Biocatalytic degradation/redefining “removal” fate of pharmaceutically active compounds and antibiotics in the aquatic environment. Sci Total Environ. 2019;691:1190–1211. doi: 10.1016/j.scitotenv.2019.07.224. [DOI] [PubMed] [Google Scholar]
- Bilinska L, Gmurek M, Ledakowicz S. Comparison between industrial and simulated textile wastewater treatment by AOPs—biodegradability, toxicity and cost assessment. Chem Eng J. 2016;306:550–559. doi: 10.1016/j.cej.2016.07.100. [DOI] [Google Scholar]
- Blair MF, Kokabian B, Gude VG. Light and growth medium effect on Chlorella vulgaris biomass production. J Environ Chem Eng. 2014;2:665–674. doi: 10.1016/j.jece.2013.11.005. [DOI] [Google Scholar]
- Blanco-Vargas A, Ramírez-Sierra CF, Duarte-Castañeda M, Beltrán-Villarraga M, Medina LK, Florido-Cuellar A-E, Cardona-Bedoya JA, Campos-Pinilla C, Pedroza-Rodríguez AM. A novel textile wastewater treatment using ligninolytic co-culture and photocatalysis with TiO2. Univ Sci. 2018;23(3):437–464. doi: 10.11144/Javeriana.SC23-3.antw. [DOI] [Google Scholar]
- Borges ME, Hernández T, Esparza P. Photocatalysis as a potential tertiary treatment of urban wastewater: new photocatalytic materials. Clean Technol Environ Policy. 2014;16:431–436. doi: 10.1007/s10098-013-0637-z. [DOI] [Google Scholar]
- Chang Q, Jiang G, Tang H, Li N, Huang J, Wu L. Enzymatic removal of chlorophenols using horseradish peroxidase immobilized on superparamagnetic Fe3O4/graphene oxide nanocomposite. Chin J Catal. 2015;36:961–968. doi: 10.1016/S1872-2067(15)60856-7. [DOI] [Google Scholar]
- Chys M, Demeestere K, Nopens I, Audenaert WTM, Van Hulle SWH. Municipal wastewater effluent characterization and variability analysis in view of an ozone dose control strategy during tertiary treatment: the status in Belgium. Sci Total Environ. 2018;625:1198–1207. doi: 10.1016/j.scitotenv.2018.01.032. [DOI] [PubMed] [Google Scholar]
- de Arruda Guelli Ulson de Souza SM, Santos Bonilla KA, Ulson de Souza AA. Removal of COD and color from hydrolyzed textile azo dye by combined ozonation and biological treatment. J Hazard Mater. 2010;179:35–42. doi: 10.1016/j.jhazmat.2010.02.053. [DOI] [PubMed] [Google Scholar]
- Deblonde T, Cossu-Leguille C, Hartemann P. Emerging pollutants in wastewater: a review of the literature. Int J Hyg Environ Health. 2011;214:442–448. doi: 10.1016/j.ijheh.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Dichter G, LeChevallier MW. 9215 heterotrophic plate count. In: Baird RB, Eaton AD, Rice EW, Bridgewater LL, editors. Standard methods for examination of water and wastewater. 23. Washington DC: American Public Health Association, American Water Works Association, Water Environment Federation; 2017. pp. 53–59. [Google Scholar]
- Dirbaz M, Roosta A. Adsorption, kinetic and thermodynamic studies for the biosorption of cadmium onto microalgae Parachlorella sp. J Environ Chem Eng. 2018;6:2302–2309. doi: 10.1016/j.jece.2018.03.039. [DOI] [Google Scholar]
- Ditta A, Bibi R, Hussain A, Noureen S, Khalid A, Aziz I. Production of algal biomass using different dilutions of textile effluent wastewater. Sci Lett. 2016;4(1):71–77. [Google Scholar]
- Drumond Chequer FM, Rodrigues de Oliveira GA, Anastácio Ferraz ER, Carvalho Cardoso J, Boldrin Zanoni MV, Palma de Oliveira D (2013) Textile dyes: dyeing process and environmental impact. In: Gunay M (ed) Eco-friendly textile dyeing and finishing. IntechOpen, pp 151–176. 10.5772/3436
- El-Batal AI, ElKenawy NM, Yassin AS, Amin MA. Laccase production by Pleurotus ostreatus and its application in synthesis of gold nanoparticles. Biotechnol Rep. 2015;5:31–39. doi: 10.1016/j.btre.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Environmental Protection Agency (EPA) Biosolids generation, use, and disposal in the United States. Washington, DC: EPA; 2005. [Google Scholar]
- Fazal T, Mushtaq A, Rehman F, Khan AU, Rashid N, Farooq W, Rehman MSU, Xu J. Bioremediation of textile wastewater and successive biodiesel production using microalgae. Renew Sustain Energy Rev. 2018;82:3107–3126. doi: 10.1016/j.rser.2017.10.029. [DOI] [Google Scholar]
- Fernández JA, Suan A, Ramírez JC, Robles J, Salcedo JC, Pedroza AM, Daza CE. Treatment of real wastewater with TiO2-films sensitized by a natural-dye obtained from Picramnia sellowii. J Environ Chem Eng. 2016;4:2848–2856. doi: 10.1016/j.jece.2016.05.037. [DOI] [Google Scholar]
- Ferrer Polo J, Seco Torrecillas A, Robles Martínez A. Tratamientos biológicos de las aguas residuales. 3. Valencia: Editorial UPV; 2018. [Google Scholar]
- Ferrera-Cerrato R, Rojas-Avelizapa NG, Poggi-Varaldo HM, Alarcón A, Cañizares-Villanueva RO. Procesos de biorremediación de suelo y agua contaminados por hidrocarburos del petróleo y otros compuestos orgánicos. Revista Latinoamericana de Microbiología. 2006;48(2):179–187. [PubMed] [Google Scholar]
- Forgacs E, Cserháti T, Oros G. Removal of synthetic dyes from wastewaters: a review. Environ Int. 2004;30:953–971. doi: 10.1016/j.envint.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Furuhashi Y, Honda R, Noguchi M, Hara-Yamamura H, Kobayashi S, Higashimine K, Hasegawa H. Optimum conditions of pH, temperature and preculture for biosorption of europium by microalgae Acutodesmus acuminatus. Biochem Eng J. 2019;143:58–64. doi: 10.1016/j.bej.2018.12.007. [DOI] [Google Scholar]
- Gonçalves AL, Pires JCM, Simões M. A review on the use of microalgal consortia for wastewater treatment. Algal Res. 2017;24:403–415. doi: 10.1016/j.algal.2016.11.008. [DOI] [Google Scholar]
- Gouzy-Olmos M, Cháves-Tequia LM, Rojas-Fajardo MF, Morales-Álvarez ED, Rivera-Hoyos CM, Poutou-Piñales RA, González-Neira EM, Reyes-Montaño EA, Cardozo-Bernal ÁM, Gómez-Méndez LD, Pedroza-Rodríguez AM. Statistical improvement of batch culture with immobilized Pichia pastoris cells for rPOXA 1B laccase production. Am J Biochem Biotechnol. 2018;14(2):88–107. doi: 10.3844/ajbbsp.2018.88.107. [DOI] [Google Scholar]
- Guedes AMFM, Madeira LMP, Boaventura RAR, Costa CAV. Fenton oxidation of cork cooking wastewater—overall kinetic analysis. Water Res. 2003;37:3061–3069. doi: 10.1016/S0043-1354(03)00178-7. [DOI] [PubMed] [Google Scholar]
- Hach Company/Hach Lange GmbH (2007) HACH 8507: nitrogen nitrite-low range, diazotization method for water and wastewater
- Hach Company/Hach Lange GmbH (2014) Nitrate, cadmium reduction method (Method 8171)
- Hu X, Meneses YE, Stratton J, Wang B. Acclimation of consortium of micro-algae help removal of organic pollutants from meat processing wastewater. J Clean Prod. 2019;214:95–102. doi: 10.1016/j.jclepro.2018.12.255. [DOI] [Google Scholar]
- Hung C-P, Lo H-F, Hsu W-H, Chen S-C, Lin L-L. Immobilization of Escherichia coli novablue γ-glutamyltranspeptidase in Ca-alginate-k-carrageenan beads. Appl Biochem Biotechnol. 2008;150:157–170. doi: 10.1007/s12010-008-8244-x. [DOI] [PubMed] [Google Scholar]
- Jada A, Ait Akbour R, Douch J. Surface charge and adsorption from water onto quartz sand of humic acid. Chemosphere. 2006;64:1287–1295. doi: 10.1016/j.chemosphere.2005.12.063. [DOI] [PubMed] [Google Scholar]
- Jhones dos Santos A, de Araújo T, Costa EC, Ribeiro da Silva D, Garcia-Segura S, Martínez-Huitle CA. Electrochemical advanced oxidation processes as decentralized water treatment technologies to remediate domestic washing machine effluents. Environ Sci Pollut Res. 2018;25:7002–7011. doi: 10.1007/s11356-017-1039-2. [DOI] [PubMed] [Google Scholar]
- Ji C, Nguyen LN, Hou J, Hai FI, Chen V. Direct immobilization of laccase on titania nanoparticles from crude enzyme extracts of P. ostreatus culture for micro-pollutant degradation. Sep Purif Technol. 2017;178:215–223. doi: 10.1016/j.seppur.2017.01.043. [DOI] [Google Scholar]
- Kashefi S, Borghei SM, Mahmoodi NM. Covalently immobilized laccase onto graphene oxide nanosheets: preparation, characterization, and biodegradation of azo dyes in colored wastewater. J Mol Liq. 2019;276:153–162. doi: 10.1016/j.molliq.2018.11.156. [DOI] [Google Scholar]
- Khalid AAH, Yaakob Z, Sheikh Abdullah SR, Takriff MS. Analysis of the elemental composition and uptake mechanism of Chlorella sorokiniana for nutrient removal in agricultural wastewater under optimized response surface methodology (RSM) conditions. J Clean Prod. 2019;210:673–686. doi: 10.1016/j.jclepro.2018.11.095. [DOI] [Google Scholar]
- Khandare RV, Govindwar SP. Phytoremediation of textile dyes and effluents: current scenario and future prospects. Biotechnol Adv. 2015;33:1697–1714. doi: 10.1016/j.biotechadv.2015.09.003. [DOI] [PubMed] [Google Scholar]
- Khataee A, Dehghan G, Zarei M, Fallah S, Niaei G, Atazadeh I. Degradation of an azo dye using the green macroalga Enteromorpha sp. Chem Ecol. 2013;29(3):221–233. doi: 10.1080/02757540.2012.744831. [DOI] [Google Scholar]
- Langergraber G, Fleischmann N, Hofstaedter F, Weingartner A. Monitoring of a paper mill wastewater treatment plant using UV/VIS spectroscopy. Water Sci Technol. 2004;49(1):9–14. doi: 10.2166/wst.2004.0004. [DOI] [PubMed] [Google Scholar]
- Li K, Liu Q, Fang F, Luo R, Lu Q, Zhou W, Huo S, Cheng P, Liu J, Addy M, Chen P, Chen D, Ruan R. Microalgae-based wastewater treatment for nutrients recovery: a review. Biores Technol. 2019;291:121934. doi: 10.1016/j.biortech.2019.121934. [DOI] [PubMed] [Google Scholar]
- Livernoche D, Jurasek L, Desrochers M, Dorica J. Removal of color from Kraft mill wastewater with cultures of white rot fungi and with immobilized mycelium of Coliorus versicolor. Biotechnol Bioeng. 1983;24(8):2055–2065. doi: 10.1002/bit.260250814. [DOI] [PubMed] [Google Scholar]
- Lucas MS, Peres JA, Amor C, Prieto-Rodríguez L, Maldonado MI, Malato S. Tertiary treatment of pulp mill wastewater by solar photo-Fenton. J Hazard Mater. 2012;225–226:173–181. doi: 10.1016/j.jhazmat.2012.05.013. [DOI] [PubMed] [Google Scholar]
- Malvis A, Hodaifa G, Halioui M, Seyedsalehi M, Sa S. Integrated process for olive oil mill wastewater treatment and its revalorization through the generation of high added value algal biomass. Water Res. 2019;151:332–342. doi: 10.1016/j.watres.2018.12.026. [DOI] [PubMed] [Google Scholar]
- Mara D, Horan N. Handbook of water and wastewater microbiology. California: Academic Press; 2003. [Google Scholar]
- Mehrjouei M, Müller S, Möller D. A review on photocatalytic ozonation used for the treatment of water and wastewater. Chem Eng J. 2015;263:209–219. doi: 10.1016/j.cej.2014.10.112. [DOI] [Google Scholar]
- Mendoza L, Jonstrup M, Hatti-Kaul R, Mattiasson B. Azo dye decolorization by a laccase/mediator system in a membrane reactor: enzyme and mediator reusability. Enzyme Microb Technol. 2011;49:478–484. doi: 10.1016/j.enzmictec.2011.08.006. [DOI] [PubMed] [Google Scholar]
- Ministerio de Ambiente y Desarrollo Sostenible (2014) Resolución 1207 de 2014. Por la cual se adoptan disposiciones relacionadas con el uso de aguas residuales tratadas. Bogotá, DC, Colombia
- Morales-Álvarez ED, Rivera-Hoyos CM, González-Ogliastri N, Rodríguez-Vázquez R, Poutou-Piñales RA, Daza CE, Pedroza-Rodríguez AM. Partial removal and detoxification of Malachite Green and Crystal Violet from laboratory artificially contaminated water by Pleurotus ostreatus. Univ Sci. 2016;21(3):259–285. doi: 10.11144/Javeriana.SC21-3.prad. [DOI] [Google Scholar]
- Morales-Álvarez ED, Rivera-Hoyos CM, Chaparro-Núnez LE, Daza CE, Poutou-Piñales RA, Pedroza-Rodríguez AM. Decolorization and detoxification of Malachite Green by Ganoderma lucidum: key operating parameters and adsorption studies. J Environ Eng. 2017;143(4):04016093. doi: 10.1061/(ASCE)EE.1943-7870.0001180. [DOI] [Google Scholar]
- Murphy J, Riley JP. A modified singles solution method for the determination of phosphate in natural waters. Anal Chim Acta. 1962;27:31–36. doi: 10.1016/S0003-2670(00)88444-5. [DOI] [Google Scholar]
- Nirmalakhandan N, Selvaratnam T, Henkanatte-Gedera SM, Tchinda D, Abeysiriwardana-Arachchige ISA, Delanka-Pedige HMK, Munasinghe-Arachchige SP, Zhang Y, Holguin FO, Lammers PJ. Algal wastewater treatment: photoautotrophic vs. mixotrophic processes. Algal Res. 2019;41:101569. doi: 10.1016/j.algal.2019.101569. [DOI] [Google Scholar]
- Otto B, Schlosser D. First laccase in green algae: purification and characterization of an extracellular phenol oxidase from Tetracystis aeria. Planta. 2014;240:1225–1236. doi: 10.1007/s00425-014-2144-9. [DOI] [PubMed] [Google Scholar]
- Pedroza-Camacho LD, Lores-Acosta JC, Rojas-Enríquez JF, Mateus-Maldonado JF, Puentes CS, Ramírez-Rodríguez J, Mendez-Casallas FJ, Salcedo-Reyes JC, Díaz-Ariza LA, Lozano-Puentes HS, Pedroza-Rodríguez AM. Effect of domestic wastewater as co-substrate on biological stain wastewater treatment using fungal/Bacterial consortia in pilot plant and greenhouse reuse. J Water Resour Protect. 2018;10:369–393. doi: 10.4236/jwarp.2018.103020. [DOI] [Google Scholar]
- Piscitelli A, Giardina P, Mazzoni C, Sannia G. Recombinant expression of Pleurotus ostreatus laccases in Kluyveromyces lactis and Saccharomyces cerevisiae. Appl Microbiol Biotechnol. 2005;69:428–439. doi: 10.1007/s00253-005-0004-z. [DOI] [PubMed] [Google Scholar]
- Quiñones DH, Álvarez PM, Rey A, Contreras S, Beltrán FJ. Application of solar photocatalytic ozonation for the degradation of emerging contaminants in water in a pilot plant. Chem Eng J. 2015;260:399–410. doi: 10.1016/j.cej.2014.08.067. [DOI] [Google Scholar]
- Rani B, Kumar V, Singh J, Bisht S, Teotia P, Sharma S, Kela R. Bioremediation of dyes by fungi isolated from contaminated dye effluent sites for bio-usability. Braz J Microbiol. 2014;45(3):1055–1063. doi: 10.1590/S1517-83822014000300039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratkovich N, Horn W, Helmus FP, Rosenberger S, Naessens W, Nopens I, Bentzen TR. Activated sludge rheology: a critical review on data collection and modelling. Water Res. 2013;47:463–482. doi: 10.1016/j.watres.2012.11.021. [DOI] [PubMed] [Google Scholar]
- Rice EW. Standard methods for the examination of water and wastewater 2540 A. 23. Alexandria: Water Environment Federation; 2017. [Google Scholar]
- Rivera-Hoyos CM, Morales-Álvarez ED, Poutou-Piñales RA, Pedroza-Rodríguez AM, Rodríguez-Vázquez R, Delgado-Boada JM. Fungal laccases. Fungal Biol Rev. 2013;27(3–4):67–82. doi: 10.1016/j.fbr.2013.07.001. [DOI] [Google Scholar]
- Rivera-Hoyos CM, Morales-Álvarez ED, Poveda-Cuevas SA, Reyes-Guzmán EA, Poutou-Piñales RA, Reyes-Montaño EA, Pedroza-Rodríguez AM, Rodríguez-Vázquez R, Cardozo-Bernal ÁM. Computational analysis and low-scale constitutive expression of laccases synthetic genes GlLCC1 from Ganoderma lucidum and POXA 1B from Pleurotus ostreatus in Pichia pastoris. PLoS One. 2015;10(1):e0116524. doi: 10.1371/journal.pone.0116524.g002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Hoyos CM, Morales-Álvarez ED, Abelló-Esparza J, Buitrago-Pérez DF, Martínez-Aldana N, Salcedo-Reyes JC, Poutou-Piñales RA, Pedroza-Rodríguez AM. Detoxification of pulping black liquor with Pleurotus ostreatus or recombinant Pichia pastoris followed by CuO/TiO2/visible photocatalysis. Sci Rep. 2018;8:3503. doi: 10.1038/s41598-018-21597-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman R, Torres Duarte C, Ayala M, Vazquez Duhalt R. Producción a escala piloto de lacasa de Coriolopsis gállica. Revista Mexicana de Micología. 2010;32:19–27. [Google Scholar]
- Rueda-Marquez JJ, Levchuk I, Fernández Ibañez P, Sillanpää M. A critical review on application of photocatalysis for toxicity reduction of real wastewaters. J Clean Prod. 2020;258:120694. doi: 10.1016/j.jclepro.2020.120694. [DOI] [Google Scholar]
- Santos FM, Pires JCM. Nutrient recovery from wastewaters by microalgae and its potential application as bio-char. Biores Technol. 2018;267:725–731. doi: 10.1016/j.biortech.2018.07.119. [DOI] [PubMed] [Google Scholar]
- Shon H, Vigneswaran S, Kandasamy J, Zareie M, Kim J, Cho D, Kim JH. Preparation and characterization of titanium dioxide (TiO2) from sludge produced by TiCl4 flocculation with FeCl3, Al2 (SO4)3 and Ca(OH)2 coagulant aids in wastewater. Sep Sci Technol. 2009;44:1525–1543. doi: 10.1021/es062062g. [DOI] [Google Scholar]
- Solís M, Solís A, Pérez HI, Manjarrez N, Floresa M. Microbial decolouration of azo dyes: a review. Process Biochem. 2012;47:1723–1748. doi: 10.1016/j.procbio.2012.08.014. [DOI] [Google Scholar]
- Tanaka H, Matsumura M, Veliky IA. Diffusion characteristics of substrates in Ca-alginate gel beads. Biotechnol Bioeng. 1984;XXVI:053–058. doi: 10.1002/bit.260260111. [DOI] [PubMed] [Google Scholar]
- Tchobanoglous G, Darby J, Bourgeous K, McArdle J, Genest P, Tylla M. Ultrafiltration as an advanced tertiary treatment process for municipal wastewater. Desalination. 1998;119:315–322. doi: 10.1016/S0011-9164(98)00175-1. [DOI] [Google Scholar]
- Tchobanoglous G, Stensel HD, Tsuchihashi R, Burton FL, Abu-Orf M, Bowden G, Pfrang W. Wastewater engineering: treatment and resource recovery. New York: MC Graw Hill Education; 2013. [Google Scholar]
- Torres-Palma RA, Serna-Galvis EA. Sonolysis. In: Ameta SC, Ameta R, editors. Advanced oxidation processes for wastewater treatment: emerging green chemical technology. Cambridge: Academic Press; 2018. pp. 177–213. [Google Scholar]
- Vázquez-López M, Amabilis-Sosa LE, Moeller-Chávez EG, Roé-Sosa A, Neumann P, Vidal G. Evaluation of the ultrasound effect on treated municipal wastewater. Environ Technol Innov. 2019;40(27):3568–3577. doi: 10.1080/09593330.2018.1481889. [DOI] [PubMed] [Google Scholar]
- Vyavahare G, Jadhav P, Jadhav J, Patil R, Aware C, Patil D, Gophane A, Yang Y-H, Gurav R. Strategies for crystal violet dye sorption on biochar derived from mango leaves and evaluation of residual dye toxicity. J Clean Prod. 2019;2017:296–305. doi: 10.1016/j.jclepro.2018.09.193. [DOI] [Google Scholar]
- Wang L, Min M, Li Y, Chen P, Chen Y, Liu Y, Wang Y, Ruan R. Cultivation of green algae Chlorella sp. in different wastewaters from municipal wastewater treatment plant. Appl Biochem Biotechnol. 2010;162:1174–1186. doi: 10.1007/s12010-009-8866-7. [DOI] [PubMed] [Google Scholar]
- Wang D, Guo F, Wu Y, Li Z, Wu G. Technical, economic and environmental assessment of coagulation/filtration tertiary treatment processes in full-scale wastewater treatment plants. J Clean Prod. 2018;170:1185–1194. doi: 10.1016/j.jclepro.2017.09.231. [DOI] [Google Scholar]
- Won K, Kim S, Kim K-J, Park HW, Moon S-J. Optimization of lipase entrapment in Ca-alginate gel beads. Process Biochem. 2005;40:2149–2154. doi: 10.1016/j.procbio.2004.08.014. [DOI] [Google Scholar]
- Yu KL, Show PL, Ong HC, Ling TC, Lan JC-W, Chen W-H, Chang J-S. Microalgae from wastewater treatment to biochar—feedstock preparation and conversion technologies. Energy Convers Manag. 2017;150:1–13. doi: 10.1016/j.enconman.2017.07.060. [DOI] [Google Scholar]
- Zahid WMK. Tertiary filtration of wastewater using local sand. J King Saud Univ Eng Sci. 2003;16:23–35. doi: 10.1016/S1018-3639(18)30778-5. [DOI] [Google Scholar]
- Zhao YX, Gao BY, Shon HK, Cao BC, Kim J-H. Coagulation characteristics of titanium (Ti) salt coagulant compared with aluminum (Al) and iron (Fe) salts. J Hazard Mater. 2011;185:1536–1542. doi: 10.1016/j.jhazmat.2010.10.084. [DOI] [PubMed] [Google Scholar]