Abstract
High-grade gliomas are still characterized by a poor prognosis, despite recent advances in surgical treatment. Chemotherapy is currently practiced after surgery, but its efficacy is limited by aspecific toxicity on healthy cells, tumour cell chemoresistance, poor selectivity, and especially by the blood–brain barrier (BBB). Thus, despite the large number of potential drug candidates, the choice of effective chemotherapeutics is still limited to few compounds. Malignant gliomas are characterized by high infiltration and neovascularization, and leaky BBB (the so-called blood–brain tumour barrier); surgical resection is often incomplete, leaving residual cells that are able to migrate and proliferate. Nanocarriers can favour delivery of chemotherapeutics to brain tumours owing to different strategies, including chemical stabilization of the drug in the bloodstream; passive targeting (because of the leaky vascularization at the tumour site); inhibition of drug efflux mechanisms in endothelial and cancer cells; and active targeting by exploiting carriers and receptors overexpressed at the blood–brain tumour barrier. Within this concern, a suitable nanomedicine-based therapy for gliomas should not be limited to cytotoxic agents, but also target the most important pathogenetic mechanisms, including cell differentiation pathways and angiogenesis. Moreover, the combinatorial approach of cell therapy plus nanomedicine strategies can open new therapeutical opportunities. The major part of attempted preclinical approaches on animal models involves active targeting with protein ligands, but, despite encouraging results, a few number of nanomedicines reached clinical trials, and most of them include drug-loaded nanocarriers free of targeting ligands, also because of safety and scalability concerns.
Keywords: glioma, blood–brain barrier, blood–brain tumour barrier, nanoparticles, targeting
Classification of Brain Tumours
The most frequent brain tumours (gliomas) originate from glial cells, and range from low infiltrating to highly aggressive. In the 2007 World Health Organization (WHO) classified gliomas within four grades, basing on histopathologic features, such as mitotic index, anaplasia, cytological atypia, microvascular proliferation, and necrosis: grade I (ie pilocytic astrocytoma), grade II (ie astrocytomas and oligodendrogliomas), grade III (ie anaplastic astrocytomas and oligodendrogliomas), and grade IV (ie glioblastoma multiforme). In 2016 WHO included in the classification also molecular diagnostic criteria for infiltrating gliomas, including mutation of isocitrate dehydrogenase, deletion of 1p/19q chromosome, and histone mutations.1 However, malignant or high grade (III and IV) gliomas are characterized by very poor prognosis. Furthermore, 8–10% of the adult patients with cancer develop brain metastases, with considerably variable incidence among different primary cancers. Lung, breast, colon, kidney cancer or melanoma can lead to brain metastases, 70% of which originating from lung and breast cancer.2
Current Therapy of Gliomas
Surgery is the first-line treatment both in low and high-grade gliomas3 and the extent of resection has demonstrated a positive prognostic effect.4 Several techniques have been designed to refine tumour resection: neuronavigation, use of 5-aminolevulinic acid,5 and intra-operative magnetic resonance imaging (MRI). There is evidence that the combined use of these techniques improves the rate of gross total resection. The choice and the timeframe of subsequent adjuvant chemotherapy and radiation therapy (alone or as combined treatments) is still considered controversial. A survey within the European Low-Grade Glioma Network showed a relevant heterogeneity in the usage of chemotherapy. Generally, oral temozolomide (TMZ) is the first-line treatment after surgery for high-risk low-grade gliomas, or at progression, although, according to the Radiation Therapy Oncology Group, combination of radiotherapy with procarbazine, lomustine and vincristine regimen has been indicated as the gold-standard treatment.6 While investigations are currently underway to evaluate the potential role of chemotherapy in low-grade gliomas, combined chemotherapy/radiotherapy approaches are currently practiced after surgery in high-grade gliomas. Radiotherapy is related to important side effects, such as post-radiation leuko-encephalopathy, nerve damage, hair loss, vomiting, infertility, and skin rash. Moreover, the effectiveness of chemotherapy is limited by toxic effects on healthy cells, tumour cell chemoresistance, and poor selectivity of anticancer drugs. Finally, the blood–brain barrier (BBB) is the major limit for the delivery of chemotherapeutic agents.7 Thus, the chemotherapeutics currently employed for high-grade gliomas are still limited to few chemical compounds. Currently, owing to the Food and Drug Administration (FDA), oral TMZ is the standard chemotherapy for glioblastoma and anaplastic astrocytoma. Bevacizumab (Avastin®) is a monoclonal antibody that specifically binds vascular endothelial growth factor (VEGF). Despite FDA accelerated approval for bevacizumab for brain tumours, basing on its efficacy towards recurrent glioblastoma, its use has been involved with many controversies. Indeed, this anti-angiogenic therapy failed to improve patient overall survival, despite showing efficacy in shrinking or halting tumour growth.8 In 1996, FDA approved biodegradable polyanhydride wafers loaded with carmustine (Gliadel®) for chemotherapy of recurrent high-grade gliomas. Patients with recurrent tumours benefit of an 8 weeks survival increase, when wafers were placed at the second surgery. Instead, the survival increase was 2.3 months in patients with early diagnosed tumours, undergoing primary resection followed by wafer placement.9
Experimental Drugs for Gliomas
Apart from currently approved chemotherapy, several drugs belonging to various therapeutic categories are currently under investigation for high-grade glioma treatment: the main mechanisms underlying their activity towards glioma are summarized in Figure 1. Advantages and disadvantages of such therapeutic drugs are listed in Table 1. In the following sections, the most important attempts and findings at preclinical and clinical level concerning such drugs are briefly described.
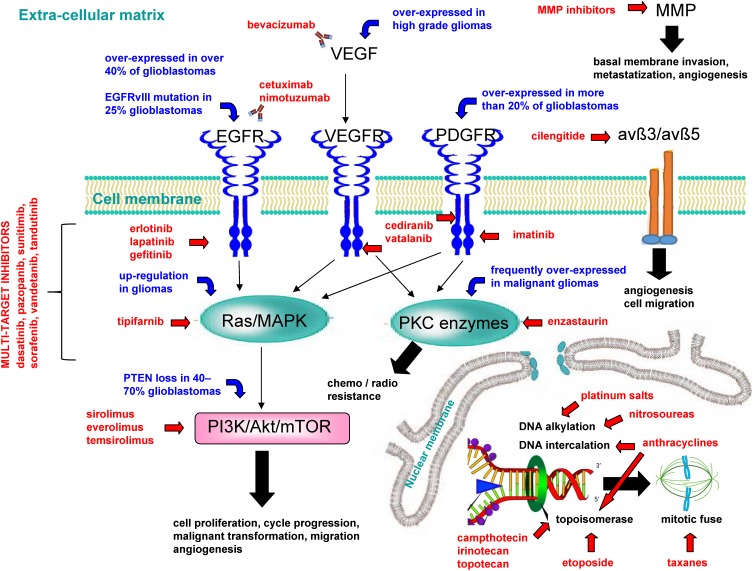
Figure 1.
Main mechanisms of experimental drugs used against high-grade gliomas.
Abbreviations: avß3/avß5, avß3/avß5 heterodimers; EGFR, epidermal growth factor receptor; EGFRvIII, mutant EGFR; MMP, matrix metalloproteinase; PDGFR, platelet-derived growth factor receptor; PI3K/Akt/mTOR, phosphoinositide 3-kinase/protein kinase B/mammalian target of rapamycin; PKC, protein kinase C; PTEN, phosphatase and tensin homolog; Ras/MAPK, Ras mitogen-activated protein kinase; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
Table 1.
Advantages and Disadvantages of Experimental Drugs Against High-Grade Gliomas
| Advantages | Disadvantages | |
|---|---|---|
| Cytotoxic agents |
|
|
| VEGFR inhibitors |
|
|
| Anti-VEGF antibodies |
|
|
| EGFR inhibitors |
|
|
| Anti-EGFR antibodies |
|
|
| PDGFR inhibitors |
|
|
| Ras/MAPK inhibitors |
|
|
| PKC inhibitors |
|
|
| PI3K/Akt/mTOR inhibitors |
|
|
| Multi target inhibitors |
|
|
| MMP inhibitors |
|
|
| Integrin inhibitors |
|
|
Abbreviations: Akt, protein kinase B; BBB, blood–brain barrier; DOX, doxorubicin; EGFR, epidermal growth factor receptor; EGFRvIII, mutant EGFR; MMP, matrix metalloproteinase; PDGFR, platelet-derived growth factor receptor; PI3K/Akt/mTOR, phosphoinositide 3-kinase/protein kinase B/mammalian target of rapamycin; PKC, protein kinase C; Ras/MAPK, Ras mitogen-activated protein kinase; TMZ, temozolomide; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
Cytotoxic Agents
Different marketed cytotoxic drugs have been tested off-label in preclinical glioma models and clinical trials, including nitrosoureas (alkylating agents), platinum salts, inhibitors of topoisomerase I (etoposide) and II (camptothecin, irinotecan, topotecan), mitotic inhibitors that is taxanes derivatives (paclitaxel, docetaxel), anthracyclines such as doxorubicin (DNA intercalation and topoisomerase II inhibition), and paclitaxel–cisplatin–vincristine (PCV) combination.10
Prodrugs of Cytotoxic Agents
Lipophilic prodrugs with molecular weight (MW) lower than 500 Da, and capable of forming less than 8 hydrogen bonds, should be able to overcome the BBB:11 the cytotoxic drug chlorambucil was modified accordingly, with improved brain delivery.12 However, recent interest has been growing concerning higher MW compounds, such as fatty acid – paclitaxel (PTX) prodrugs. In particular cis-linoleic acid conjugate with PTX (CLA-PTX) resulted in much higher plasmatic half-life and brain accumulation than free PTX, with encouraging therapeutic effect on brain tumour bearing rats.13
Angiogenesis Inhibitors
The expression level of VEGF directly correlates with tumour grade, with a nearly 10-fold gap between high and low-grade gliomas.14 Thus, endothelial cells are a suitable target for high-grade glioma treatment. Apart from monoclonal antibody bevacizumab, that selectively binds VEGF, potential therapeutic agents include thalidomide and VEGF receptor (VEGFR) inhibitors, belonging to receptor tyrosine kinase (RTK) inhibitors category.15 Cediranib and vatalanib are orally bioavailable VEGFR inhibitors, with simultaneous inhibitory activity on tyrosine-protein kinase KIT (c-Kit) and platelet-derived growth factor receptor (PDGFR), that, currently, are undergoing clinical trials for high-grade gliomas.16
Epidermal Growth Factor Receptor (EGFR) Inhibitors
EGFR, together with downstream signalling pathways (such as phosphoinositide 3-kinase/protein kinase B/mammalian target of rapamycin - PI3K/Akt/mTOR and Ras mitogen-activated protein kinase - Ras/MAPK), regulates cell survival, proliferation, angiogenesis and migration. EGFR is overexpressed in over 40% of glioblastomas.17 Furthermore, 25% of glioblastomas are characterized by the expression of an EGFR mutant (EGFRvIII), which is defective of the extracellular ligand-binding domain.18 Erlotinib, lapatinib, and gefitinib, three RTK inhibitors acting on EGFR, have been tested at preclinical and clinical stage for malignant gliomas treatment, with limited efficacy.15 Also, cetuximab and nimotuzumab, two mouse anti-human EGFR antibodies, were employed in clinical trials for recurrent high-grade glioma patients.9,19
PDGFR Inhibitors
PDGFR is an RTK, which is overexpressed in more than 20% of glioblastomas,20 and, like EGFR, it is upstream of Ras/MAPK and PI3K/Akt/mTOR signalling pathways.21 Imatinib, an inhibitor of PDGFR, c-Kit, Abelson murine leukemia viral oncogene (Abl), and arginase (ARG), was tested in several clinical studies involving high-grade glioma patients.15,22
Ras/MAPK Inhibitors
The Ras/MAPK signalling pathway is downstream to several RTK inhibitors, such as fibroblast growth factor receptor (FGFR), insulin-like growth factor (IGFR), and the aforementioned EGFR, VEGFR, PDGFR. Therefore, it is responsible for cell proliferation and migration processes, as well as for cell cycle progression, and malignant transformation. Ras/MAPK is also upstream to PI3K/Akt/mTOR signalling pathway.15,21,23 Furthermore, malignant gliomas are characterized by Ras/MAPK up-regulation.23 Oral tipifarnib, a farnesyl transferase inhibitor with demonstrated radio-sensitizing effect is currently undergoing several clinical trials for high-grade gliomas.24
Protein Kinase C (PKC) Inhibitors
PKC enzymes belong to a serine/threonine kinases family, and it is downstream to different RTKs, including VEGFR and PDGFR.25 PKC is involved in chemoresistance and radioresistance in malignant gliomas .26 Furthermore, PKC is frequently overexpressed in malignant gliomas.26–28 Enzastaurin, a lipophilic, orally administered PKC inhibitor, underwent different clinical trials as mono or combination therapy, but with limited clinical benefit.9
PI3K/Akt/mTOR Pathway Inhibitors
PI3K/Akt/mTOR is down-regulated by phosphatase and tensin homolog (PTEN), which, in turn, is altered (deleted, inactivated, or mutated) in nearly 40–70% glioblastomas.29,30 Sirolimus, an orally bioavailable peptide macrolide, which is able to overcome the BBB and acts as mammalian target of rapamycin (mTOR) inhibitor, is currently employed in clinical trials for malignant gliomas.31 Also, everolimus and temsirolimus, two analogs of sirolimus, respectively, orally and i.v administered, have been tested in clinical trials.32,33 However, mTOR inhibitors induce significant activation of protein kinase B (Akt) in 50% of the patients, potentially causing a reduced time to progression: simultaneous inhibition of mTOR and Akt could overcome this limitation.34
Multi-Target Inhibitors
The simultaneous inhibition of different aberrant signalling pathways should result in great clinical benefit for targeted therapies. Apart from the combination of different drug therapies, multiple-target inhibition can be achieved through employment of small molecule inhibitors of newer-generation.15 Dasatinib, pazopanib, sunitinib, sorafenib, vandetanib, tandutinib are currently in evaluation in clinical trials for glioblastoma, alone or in combination therapy.9
Matrix Metalloproteinase (MMP) Inhibitors
MMPs are proteolytic enzymes active also in physiological conditions. In tumours, they promote basal membrane invasion, metastatization and angiogenesis, being directly secreted by tumour cells or by the surrounding stroma, under tumoural stimulus. Marimastat, the most studied MMP inhibitor, showed promising in vitro inhibition of malignant glioma, but no advantage in clinical trials with glioblastoma patients.35,36
Integrin Inhibitors
The expression of avß3 and avß5 heterodimers is increased in malignant gliomas, and it has been hypothesized that they contribute to regulate angiogenesis and migration.37 Cilengitide is a potent antagonist of both avß3 and avß5. Several clinical trials testing cilengitide in combination therapy regimens are currently ongoing.9
Rationale for Employment of Nanomedicines in Glioma Therapy
The main mechanisms underlying the rationale of employing nanomedicines for glioma treatment are summarized below (Figure 2).
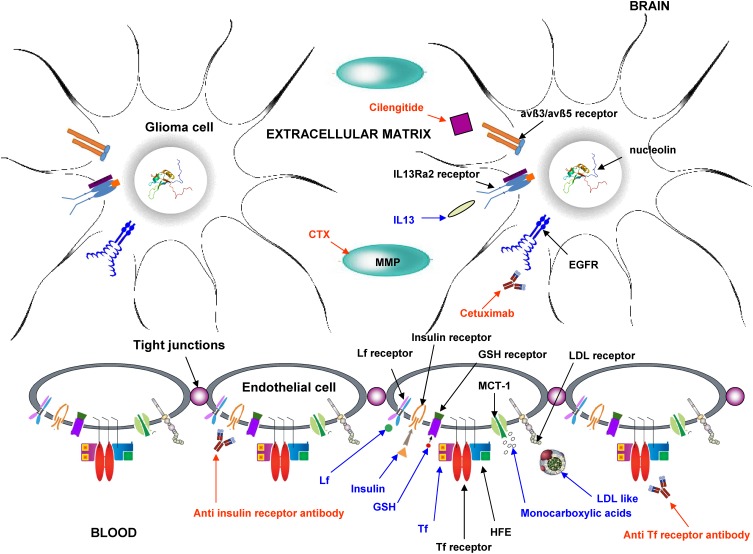
Figure 2.
Rationale of employing nanomedicines for glioma treatment.
Abbreviations: BBB, blood–brain barrier; BBTB, blood–brain tumour barrier; CMT, carrier-mediated transport; EPR, enhanced permeation and retention; HIF, hypoxia-induced factors; P-gp, P-glycoprotein; RMT, receptor-mediated transport.
Passive Targeting
The permeability of adjacent brain vasculature changes during the growth of brain tumours. At their early stage, the growth of tumour cells depends only on normal brain vessels and the BBB is intact. With tumour progression, glioma cells invade the surrounding healthy tissues. When a large enough volume (>0.2 mm3) is reached by the tumour cell cluster, a structural damage will affect the BBB, and blood–brain tumour barrier (BBTB) will be formed.38 Within this concern, claudin-1, a protein specifically expressed in the tight junctions of endothelial cells, is down-regulated in vessels surrounding high-grade gliomas, thus increasing the permeability of the BBB.39,40 This “leaky” BBTB is a common feature of high-grade gliomas, because of their increased metabolic requirements. Furthermore, it has been demonstrated that VEGF, associated with the high angiogenic nature of high-grade gliomas, increases the BBB permeability, by stimulating angiogenesis in response to hypoxia.41,42 The “leaky” BBTB can be targeted for drug delivery purposes, by exploiting the so-called enhanced permeability and retention (EPR) effect. Since the pores cut-off size at the BBTB is highly variable, the size of nanocarriers largely influences the extent and efficacy of drug delivery. In fact, the vascular leakage is significantly reduced in the tumours within the brain microenvironment compared with other districts, and it is affected by the tumour stage and/or to the tumour model employed.43 For instance, the critical cut-off size of the BBTB for intracranial U87MG xenografts ranges between 7 and 100 nm.44 Despite the reduced size of vessel fenestrations may limit the potential of passive targeting strategy to address brain cancer, drug-loaded nanocarriers free of targeting ligands showed promising efficacy in orthotopic brain glioma models, thus allowing a translation to clinical therapy.45
Active Targeting
However, high-grade gliomas rapidly infiltrate the surrounding healthy tissue, where the BBB is not altered and the EPR effect cannot be achieved.46 Indeed, in human patients, contrarily to animal tumour models, chemotherapy is only adjuvant, and it should mainly be addressed towards the eradication of residual tumour cells after surgery or radiotherapy. This cell population includes cells migrating from the tumour into healthy tissue or that feed the tumour from distant sites still active. Thus, the most relevant area for chemotherapy is the one surrounding the glioma, the so-called BAT (Brain Adjacent to Tumour), including cells in the invasion phase, that do not still affect the integrity of the BBB. Since BAT should be the main target of chemotherapy, the development of formulations easily crossing the intact BBB is essential.47 In this case, suitable active targeting of nanocarriers is needed to reach the target tissue, by exploiting carrier and/or receptors overexpressed at the BBB. Carrier-mediated transporters (CMTs) are entailed in the transport of essential small molecules into the brain. Receptor-mediated transporters (RMTs) are abundantly expressed at the BBB, being exploited by large endogenous biomolecules.2,48 Specific peptide receptors are included in RMT, eg low-density lipoprotein (LDL) receptor, transferrin (Tf) receptor, lactoferrin (Lf) receptor, insulin receptor, and receptors for insulin-like growth factors (IGF-1 and IGF-2).49 CMT and RMT ligands have been frequently exploited for drug delivery as molecular “Trojan horses” for nanocarriers.50–55
Overcoming Extrusion Mechanisms at the BBB
Additionally, in the BBB endothelial cells (on the luminal side), as well as in glioma cells, the P-glycoprotein (P-gp) is present. P-gp is a 170-kDa glycoprotein associated to the cell membrane, belonging to the superfamily of ATP binding cassette (ABC) transporters, that actively extrudes from cells chemically different substrates. P-gp can hamper BBB overcoming by chemotherapeutic drugs, and it is responsible for chemoresistance in glioma cells.56,57 Thus, P-gp inhibition can be considered as a potential dual strategy, suitable both to enhance drug penetration in the brain, and to reduce glioma chemoresistance. Thus, specific inhibitors of ABC subfamily member 1, such as elacridar and tariquidar, and anti-estrogen tamoxifen, were investigated for glioma treatment, owing to their anti-extrusion effect at the BBB.58,59 Apart from chemical modulators, also nanoparticulate systems can inhibit the P-gp drug efflux mechanism: indeed they can deliver the drug within the cells by endocytosis, therefore making it less susceptible to the membrane-bound drug efflux mechanisms, since it is in the form of drug-matrix aggregates. Also, some ingredients employed in the formulation of nanocarriers, such as surfactants, can contribute to the P-gp inhibition.60 Furthermore, co-delivery in the same nanocarriers of cytotoxic drugs and P-gp inhibitors (such as ketoconazole) has also been attempted in preclinical models.61
Inhibition of Tumour Differentiation, Migration and Neo-Vascularization
Lastly, from a histological point of view, in malignant gliomas, areas of necrosis, hypoxia and microvascular hyperplasia are present, with pseudo-palisades of cells migrating from the original necrosis area.62,63 Accordingly, it has been recently shown that cancer stem cells (CSC) are responsible for the initiation step of human glioblastoma and medulloblastoma in intracranial mouse xenografts.64 Unfortunately, the CSC location has yet to be precisely disclosed, but it has been demonstrated that hypoxia-induced factors (HIF) are responsible for CSC phenotype and can even shift normal cells to CSC.65,66 Since HIF are involved both in angiogenesis and in maintaining CSC, a suitable treatment for high-grade gliomas should be able to down-regulate their expression. This can occur by scavenging of reactive oxygen species (ROS). Indeed, during hypoxia, rising intracellular ROS concentration overcomes glutathione (GSH) guard levels. ROS can stabilize HIF, allowing VEGF transcription and consequent angiogenesis.67–70 This mechanism underlies the rationale for employing anti-oxidant and anti-VEGF agents in glioma therapy. Indeed, the combination of anti-angiogenic bevacizumab and cytotoxic irinotecan is approved for recurrent glioblastoma.71,72 Furthermore, in mice bearing intracerebral glioblastoma, pre-treatment with Tempol, a ROS scavenger, followed by TMZ chemotherapy, suppressed tumour growth and increased survival rate.73 Within this concern, nanocarriers should be able to achieve co-delivery of anti-VEGF drugs and antioxidants, together to cytotoxic agents within the tumour tissue, owing to the aforementioned mechanisms. A recent experimental study showed an increase in bevacizumab activity and permeation through the BBB, when loaded within solid lipid nanoparticles (SLN).74 Also treatment cerium oxide nanoparticles (nanoceria), with ROS scavenging properties, caused decreased expression levels of VEGF in a human astrocytoma cell line, associated with reduced motility and capacity of endothelial cells to form new capillaries.75 Recently, camptothecin (CPT), a topoisomerase inhibitor, was employed in a co-delivery nanoparticulate system, that could stop the stabilization of HIF in brain tumours. For this, a suitable CPT prodrug was synthesized, by linkage with a tetraethylene glycol (TEG) spacer and α-lipoic acid (ALA). The obtained CPT-TEG-ALA prodrug can be cleaved by oxidation, thus acting as a ROS scavenger, and release CPT in its active form within glioblastoma cells. CPT-TEG-ALA was loaded in nanoemulsion along with α-tocopherol, an additional ROS scavenger, thus preventing HIF production.76
Preclinical Nanomedicines
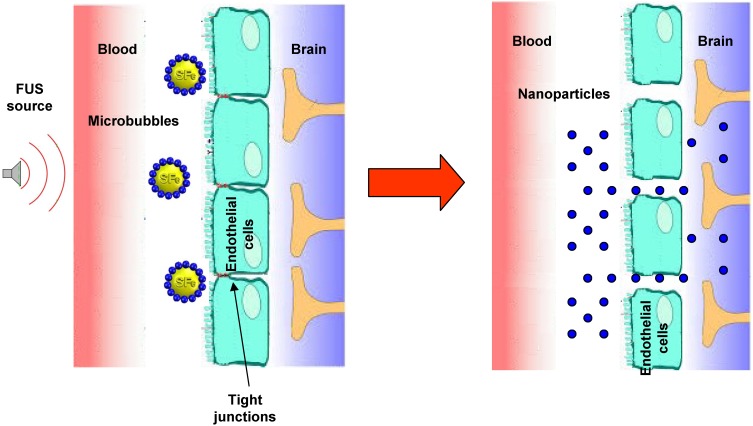
Several approaches involve the alteration of the BBB, in order to increase brain penetration of nanocarriers loaded with chemotherapeutic drugs against glioma tumours. They include both invasive methods,77 such as convection-enhanced delivery78,79 and post-surgical implantation,80–82 and noninvasive BBB opening through focused ultrasound (FUS).83 Indeed, ultrasound with a frequency below 1 MHz can induce reversible and temporary BBB opening with the aid of microbubbles (Figure 3). This technique can be employed to deliver theranostic agents for the detection and treatment of various brain diseases, and it has been already subject of clinical trials.84 In particular, Cu-Se ultrasmall nanoparticles and rare earth nanoparticles, labelled with near-infrared (NIR) dyes, have been employed, at preclinical level, to monitor FUS-induced temporary opening of the BBB and following recovery,85 as well as to detect glioma in orthotopic animal models.86 In further studies, the aforementioned Cu-Se ultrasmall nanoparticles, loaded with doxorubicin (DOX) and labelled with NIR dyes, were used concurrently with FUS and photodynamic therapy, and demonstrated good efficacy against orthotopic tumour models.87
Figure 3.
Scheme of brain delivery of nanoparticles with focused ultrasound (FUS) technique.
Abbreviation: SF6, sulfur hexafluoride.
Anyway, the nanocarriers discussed here below include only those used to overcome the BBB/BBTB without disruption, aiming to deliver anti-cancer drugs to the brain tumours. They can be made of different matrixes, including metals (ie calcium phosphate, iron oxide), lipids (SLN), phospholipids (liposomes), proteins (bovine serum albumin – BSA), synthetic polymers (ie poly-lactide – PLA; poly-lactide-glycolide – PLGA; PEG: polyethyleneglycol), natural polymers (ie chitosan) and polymer-lipid hybrid nanoparticles (PLN).88
Requested features of such nanomedicines are the following:46
They should stabilize the drug from physicochemical or biological standpoints;
They should avoid opsonization and, therefore, clearance by the reticuloendothelial system (RES), prolonging plasma circulation time and allowing EPR effect;
They should be endowed with selective targeting strategies to the brain;
They should not stimulate immune response.
However, the fact that almost 98% of drugs are unable to overcome the BBB is the main reason for employment of nanocarriers in high-grade glioma treatment. Two approaches have been documented in literature: the first employs plain nanocarriers, the second exploits active targeting, in order to enhance uptake of the nanocarriers by the endothelial cells at the BBB/BBTB.89
Within this concern, a huge number of nanocarriers aiming to glioma treatment have been engineered in the last decades. However, the following discussion will be focused only on the ones that underwent preclinical in vivo testing. Indeed, despite the presence of several in vitro investigation methods,90 only preclinical animal models can be predictive of the real potential of administered nanomedicines, because of the simultaneous presence of biological barriers and metabolism/distribution. Pharmacokinetic and biodistribution experiments are relevant in order to foresee the metabolic fate of nanocarriers and loaded drugs, while glioma models allow to predict in vivo efficacy. Different mouse and rat glioma models are described in literature:91 ethyl-nitrosourea induced orthotopic models in allogeneic or syngeneic healthy animals, orthotopic xenografts, or genetically engineered models. Within this concern, it should be pointed out that the glioma model employed can affect in a relevant manner the measured efficacy of the therapeutic drug delivery system under investigation, with the immune system playing a key role. In fact, today the so-called “immune privilege” of the brain is no more a retained concept.92,93 Thus, if the absence of the immune system could cause an under-estimation of the therapeutic effect in xenografts, on the other side, the presence of the immunity in immuno-competent models can hamper the reproducibility of the glioma model itself. Indeed, the superior reproducibility of syngeneic models over allogeneic has been documented, owing to a lower graft rejection.94 Furthermore, despite surgical resection is the primary approach for high-grade gliomas, the majority of preclinical models focus solely on drug treatment of solid intracranial tumours. Within this concern, recently a resection and recurrence orthotopic model has been developed, with potential for the investigation of tumour ablation combined with local and systemic chemotherapy.95
Plain Nanocarriers
Plain nanocarriers can improve drug delivery to gliomas by stabilizing the drug in the bloodstream, such as in the case of TMZ, which suffer from pH-dependent nonenzymatic chemo-degradation at the neutral pH, despite being the most employed drug for the treatment of brain cancers. Published reports demonstrated the therapeutic advantage of loading TMZ inside nanostructured lipid carriers (NLC), which is an optimized form of SLN with higher drug loading capacity.96,97 Naked nucleic acid delivery is also associated with fast degradation, aspecific biodistribution and poor cell internalization. In fact, small interfering RNA (siRNA) and microRNA (miRNA) are promising tools to treat various diseases, but, due to their instability and poor delivery within target tissues, naked RNA is not robustly used. Thus, entrapment in positively charged nanocarriers can prevent from degradation/metabolism, and favour cell internalization, both at the BBTB and in glioma cells.98
Nanocarriers can also increase drug half-life (for EPR effect), and/or favour endocytosis across BBTB endothelial cells, also owing to P-gp inhibition. In particular, this mechanism has been well documented for lipid nanocarriers, such as liposomes and SLN.99 The physiological nature of the lipid matrix employed should improve natural brain uptake, and the employment of surfactants, such as Brij 78 or Polysorbate 80, to coat SLN can improve the nanoparticle brain uptake and drug activity, being the two molecules associated to P-gp inhibition.100,101 However, SLN mechanism of action excludes any significant toxic effect on the BBB, as assessed by the absence of alterations to cell integrity and permeability, vessel blood flow, and choline active transport. Indeed, Western blot analyses of occludin and claudin-1 in the BBB cells, run following SLN administration, showed no modifications in protein expression.102 Interestingly, in the case of the insoluble prototype anticancer drug named edelfosine (EDF) and of ion-paired idarubicin, also oral administration of drug-loaded SLN was effective against glioma subcutaneous models101 and in enhancing drug concentration in the brain,103 respectively.
Finally, plain nanocarriers can also be employed for stimuli-responsive formulations. Indeed, the combination of silver nanoparticles (AgNPs) with magnetic nanoparticle hyperthermia (MNPH) was used as a treatment in the glioma model.104
The most important plain nanocarriers, employed in in vivo preclinical studies for glioma non-invasive treatment, are shown in Table 2.
Table 2.
Plain Nanocarriers Aiming to Glioma Therapy Employed in Preclinical Studies
| Nanocarriers | Drug | Experimental in vivo Model | Achievements in vivo | References |
|---|---|---|---|---|
| AgNPs combined with MNPH | Ag+ | Efficacy in glioma rat model | Enhanced Bcl-2-associated X protein expression | [104] |
| Cationic SLN | PEGylated c-Met siRNA | Efficacy in U87 xenografts | Enhanced accumulation in brain tumour and down-regulation of c-Met levels | [93] |
| Liposomes | Oxaliplatin | Biodistribution and survival analysis in F98/Fischer glioma model | Increased brain oxaliplatin concentration and median survival time of glioma models | [167] |
| NLC | TMZ; GFP encoding pDNA | Efficacy in U87 xenografts | Gene transfection and enhanced antitumor activity | [96] |
| PLGA nanoparticles, SLN, NLC | TMZ | Efficacy in U87 xenografts | Best efficacy obtained with NLC formulation | [168] |
| Polymer nanogel | miRNA miR.34a | Efficacy in U87 xenografts | Significant tumor growth inhibition | [169] |
| Polysorbate 80 coated PBCA nanoparticles | DOX | Biodistribution in glioma models | Increased accumulation of DOX in brain tumour | [170] |
| Polysorbate 80 coated PBCA nanoparticles | TMZ | Biodistribution in healthy rats | Increased brain uptake of TMZ | [171] |
| Polysorbate 80 coated PBCA nanoparticles | Gemcitabine | Survival analysis in C6/Sprague Dawley rat glioma models | Prolonged survival of glioma models | [172] |
| Polysorbate 80 coated PLA nanoparticles | TMZ | Pharmacokinetic and biodistribution in rats | Enhancement in half-life of TMZ with higher deposition in the brain | [173] |
| Polysorbate 80 coated SLN | CPT | Pharmacokinetic and biodistribution in rats | Increased brain accumulation of CPT | [108] |
| Polysorbate 80 coated SPION | DOX | Biodistribution and efficacy in C6/Sprague Dawley glioma model | Enhanced brain accumulation of SPION and increased anti- tumour efficacy under magnetic field | [174] |
| SLN | PTX | Rat brain perfusion experiment after intra-carotid administration | Enhanced PTX accumulation in brain; P-gp overcoming | [100] |
| SLN | EDF | Biodistribution and efficacy in subcutaneous mouse model | Good drug accumulation in brain after oral administration; P-gp overcoming | [101] |
| SLN | DOX | Pharmacokinetic and biodistribution in rats and rabbits | Enhanced DOX accumulation in brain | [175,176] |
| SLN | Idarubicin | Pharmacokinetic and biodistribution in rats | Enhanced idarubicin accumulation in brain after SLN oral administration | [103] |
| SLN, NLC | TMZ; vincristine | Efficacy in U87 xenografts | Improved glioma inhibition with NLC and drug co-delivery | [97] |
Abbreviations: AgNPs, silver nanoparticles; Bcl-2, B-cell lymphoma 2; c-Met, tyrosine-protein kinase Met; C6, C6 cells; CPT, camptothecin; DOX, doxorubicin; EDF, edelfosine; GFP, green fluorescent protein; miRNA, microRNA; MNPH, magnetic nanoparticle hyperthermia; NLC, nanostructured lipid carriers, PBCA, poly(butyl cyanoacrylate); pDNA, plasmid DNA; PEG, polyethylenglycol; P-gp, P-glycoprotein; PLA, poly-lactide; PLGA, poly-lactide-glycolide; PTX, paclitaxel; siRNA, small interfering RNA; SLN, solid lipid nanoparticles; SPION, superparamagnetic iron oxide nanoparticles; U87, U87 cells; TMZ, temozolomide.
Functionalized Nanocarriers
Nanocarriers can be functionalized on their surface with suitable ligands, in order to exploit RMT and CMT at the BBB. As previously mentioned, several specific receptors, in particular for endogenous proteins and peptides, were employed as molecular “Trojan horse” for nanocarrier systems.49 LDL receptor is overexpressed both at the BBB and in glioma cells and, therefore, it is a potential target to be exploited for the delivery of therapeutic agents. Apolipoproteins (Apo) are usually employed to target LDL receptors, but Apo being a high MW protein, usually shorter chimera peptides including the receptor-binding domain are employed.50,105 Angiopep-2 (TFFYGGSRGKRNNFKTEEY, molecular weight 2.4 kDa), a peptide belonging to the Kunitz domain-derived family, is a potent ligand of LDL on the BBB employed for nanocarriers functionalization.106 Within this concern, it should be pointed out that Polysorbate 80 is frequently employed as suspending agent for plain nanocarriers aiming to drug brain delivery. Indeed, it has been hypothesized that this nonionic surfactant can adsorb endogenous Apo E present in serum on the surface of nanoparticles.107,108 Mammalian Lf is a cationic iron transporting glycoprotein (80 kDa), whose receptor is expressed on the endothelial side of the BBB. Studies with membrane preparations of mouse brain have shown that the Lf receptor at the BBB has two classes of binding sites: a high-affinity, with a dissociation constant Kd of 10.61 nM, and a low-affinity with a Kd of 2228 nM. Plasmatic concentration of endogenous Lf (~5 nM) is lower than the Kd of Lf receptors at the BBB. Thus, the competitive inhibition with endogenous Lf is avoided, allowing employment of Lf functionalized nanocarriers for drug delivery in glioma models.109 Finally, apart from protein ligands, nanocarriers can be functionalized also with small molecules, recognized at the BBB from specific receptors/carriers, such as GSH receptor,110 folate receptor and monocarboxylic acid transporter (MCT-1).61,111
However, different critical issues are associated with active targeting to the BBB. First of all, most of the RMT are not present exclusively at the BBB. For example, Tf receptor is expressed in monocytes, red blood cells, lungs, hepatocytes, and in the gut, along with the BBB. Within this concern, it is reported that active targeting of nanocarriers should benefit from employment of a “spacer” between the nanoparticles’ surface and the grafted protein, and that grafting a low amount of protein should enhance selectivity for the BBB rather than nontarget tissues.51 Moreover, nanocarriers grafted with physiological ligands (ie Tf) undergo binding competition with the corresponding endogenous protein, which can decrease targeting efficacy;46 then, most of the available targeting proteins can cause immunogenic reactions; finally, some receptors on the BBB, such as insulin and Tf, control homeostasis of iron and glucose within the brain, and nanocarriers grafted with monoclonal antibodies against these receptors may down-regulate their activity and raise safety concerns.112
Functionalization of nanocarriers can be exploited also to target specific receptors overexpressed in glioma and/or at the BBTB. Previously mentioned overexpression of EGFR and EGFRvIII mutant at the BBTB allows a selective targeting.113 Also, integrins play important roles in tumour invasion and angiogenesis: in glioblastoma avß3 and avß5 integrin receptors are overexpressed on brain tumour cells and neo-vessels, favouring interaction with the extracellular matrix. Integrins can be specifically targeted by employing arginine-glycine-aspartic acid (RGD) peptides, such as cilengitide (cyclo [RGDfV]), a cyclic RGD peptide.114 Nucleolin, instead, exists only in the nucleus of cells, thus offering an attractive target for cells characterized from a high internalization rate. F3 peptide, that specifically binds to nucleolin, was utilized to decorate nanocarriers, in order to realize glioma cell and neo-vasculature dual-cellular targeting.115 Furthermore, as previously mentioned, MMPs are a group of zinc-dependent proteins acting as key modulators of tumour invasion and metastatization, due to their degrading capacity of the extracellular matrix. In addition, MMP-2/9 is also required for the tumoural angiogenic switch. Thus, MMP-2/9 conjugated low-molecular-weight protamine (ALMWP) was employed for glioma targeting of nanocarriers.116 Also chlorotoxin (CTX), a small peptide (36-amino acid) derived from Leiurus quinquestriatus (scorpion) venom, can bind to MMP-2, and it was employed for nanocarriers functionalization.117 Finally, IL-13Ra2 receptor is overexpressed in pilocytic astrocytomas and glioblastoma. Therefore, interleukin-13 (IL-13) can be used to target nanocarriers to glioblastoma tissue.
A summary of the most important functionalized nanocarriers employed in in vivo preclinical studies is shown in Table 3, and a scheme of the main active targeting strategies is displayed in Figure 4.
Table 3.
Functionalized Nanocarriers Aiming to Glioma Therapy Employed in Preclinical Studies
| Nanocarriers | Drug | Functionalization | Experimental in vivo Model | Achievements in vivo | References |
|---|---|---|---|---|---|
| Polymeric nanoparticles | TMZ | Angiopep-2 | Biodistribution of liposomes and efficacy in C6/ICR mouse glioma models | Enhanced brain distribution of DOX and promising efficacy in glioma models | [177] |
| Dendrimers | TRAIL DNA | Angiopep-2 | Biodistribution of dendrimers and survival analysis in C6 mouse xenografts | Increased survival of xenografts | [106] |
| PCL nanoparticles | DOX | Angiopep-2 | Pharmacokinetics, biodistribution and survival analysis in C6/Wistar rat glioma models | Enhanced brain uptake of DOX, prolonged survival of glioma models | [178] |
| PLGA nanoparticles | IP10 | Angiopep-2; EGFRvIII scFv | Efficacy in U87-EGFRvIII cells xenografts | Reduced tumour growth, prolonged survival of glioma models | [179] |
| Liposomes | Daunomycin | Anti Tf Receptor antibody | Pharmacokinetic & biodistribution in rats | Increased daunomycin accumulation in brain | [180] |
| Calcium phosphate nanoparticles | ATF5 siRNA | Apo E | Glioma distribution, ATF5 expression and survival analysis in C6 mouse xenografts | Efficient tumour targeting and increased survival of xenografts | [181] |
| Polymersomes | Saporin | Apo E | Biodistribution and efficacy in U87 mouse xenografts | Specific brain accumulation of polymersomes, encouraging efficacy towards brain tumours | [182] |
| SLN | MTX prodrug | Apo E chimera peptide | Biodistribution and survival analysis in F98/Fischer rat glioma model | Increased brain accumulation of MTX; encouraging efficacy | [50] |
| Lipid nanoparticles | Porphyrin | Apo E3 | Pharmacokinetics and biodistribution in mice, efficacy in U87 mouse xenografts | Selective drug accumulation in brain tumour compared to healthy parenchyma | [183] |
| Liposomes | – | Cetuximab | Biodistribution in U87 mouse xenografts | Increased brain accumulation of liposomes | [113] |
| Liposomes | DOX | Chlorotoxin | Biodistribution of liposomes and efficacy in U87 mouse xenografts | Brain accumulation of liposomes, reduced tumour growth | [117] |
| Polyionic micelles | Cilengitide | Cilengitide | Survival analysis in C6/Wistar rat glioma models | Prolonged survival of glioma models | [184] |
| Polymeric micelles | DACHPt | Cilengitide | Efficacy in U87 mouse xenografts | Reduced tumour growth | [185] |
| Liposomes | DOX | Cilengitide and Peptide 22 (LDL receptor) | Biodistribution and survival analysis in intracranial glioma-bearing mice | Prolonged survival time of glioma models | [114] |
| PEG−PLA micelles | PTX | EGFR/EGFRvIII targeting peptide | Pharmacokinetics in healthy rats, biodistribution and efficacy in U87 mouse xenografts | Specific micelles distribution to the brain, reduced tumour growth in glioma models | [186] |
| PEG-PLA nanoparticles | PTX | F3 peptide (targeting nucleolin) and tLyp-1 peptide (targeting neuropilin) | Pharmacokinetics in rats; biodistribution and survival analysis in C6 mouse xenografts | Enhanced PTX accumulation and deep penetration at the tumour location; prolonged survival in xenografts | [115] |
| PEGilated liposomes | DOX | GSH | Pharmacokinetics, biodistribution and efficacy in U87 mouse xenografts | Enhanced brain retention of DOX; strong inhibition of brain tumour growth | [110] |
| Liposomes | DOX | IL-13 | Efficacy in U251 mouse xenografts | Reduced tumour growth in glioma models | [187] |
| BSA nanoparticles | DOX | Lf | Pharmacokinetics in rats, biodistribution in C6/Wistar rat glioma model | Increased brain uptake of DOX | [188] |
| Cationic liposomes | DOX | Lf | Biodistribution and survival analysis in C6/Wistar rat glioma models | Increased accumulation of DOX in brain and prolonged survival time of glioma models | [109] |
| Liposomes | 99mTc-BMEDA | Lf | Pharmacokinetic & biodistribution in mice | Increased brain accumulation | [189] |
| Olive oil nanoparticles | TMZ | Lf | Pharmacokinetic & biodistribution in healthy mice; biodistribution and efficacy in GL261/C57BL/6 mouse models | Enhanced brain distribution of TMZ and promising efficacy towards glioma | [190] |
| Olive oil nanoparticles | Aurora Kinase B siRNA | Lf | Gene silencing and survival analysis in GL261/C57BL/6 mouse models | Survival improvement of glioma models treated with nanoparticles and TMZ simultaneously | [191] |
| PEG-PCL polymersomes | DOX and tetrandrine | Lf | Pharmacokinetics, biodistribution and efficacy in C6/Wistar rat glioma model | Improved DOX distribution in brain, reduced tumour growth and increased survival in glioma models | [192] |
| PEG-PLA nanoparticles | PTX | Lf and tLyp-1 peptide (targeting neuropilin) | Pharmacokinetics in healthy rats, biodistribution and efficacy in C6 mouse xenografts | Enhanced tumour accumulation of PTX and increased survival of glioma models | [53] |
| NLC | TMZ and vincristine | Lf, RGD | Biodistribution and efficacy in U87 xenografts | Specific brain distribution of the drugs, promising efficacy in glioma models | [193] |
| Liposomes | DOX and vincristine | T7 and DA7R | Biodistribution of liposomes and efficacy in C6/ICR mouse glioma models | Enhanced brain distribution of liposomes and promising efficacy in glioma models | [194] |
| Liposomes | 5-fluorouracil | Tf | Biodistribution of radiolabelled liposomes in healthy rats | Increased brain uptake of liposomes | [195] |
| Liposomes | TMZ and bromodomain inhibitor | Tf | Biodistribution in mice and efficacy in U87 mouse xenografts and C57BL/6 mouse models | Improved liposomes distribution to the brain; promising efficacy in glioma models | [196] |
| SLN | MTX prodrug | Tf, Insulin | Biodistribution in healthy rats | Increased brain accumulation of MTX | [51] |
| Liposomes | DOX | Tf, Octaarginin | Biodistribution of liposomes and efficacy in U87 mouse xenografts | Prolonged survival of glioma models | [197] |
| SLN | Docetaxel | HBA | Pharmacokinetics and biodistribution in healthy rats | Enhanced drug brain uptake | [111] |
| SLN | Docetaxel ketoconazole | Folic acid | Pharmacokinetics and biodistribution in healthy rats | Enhanced drug brain uptake; P-gp overcoming | [61] |
| PEG-co-PCL nanoparticles | PTX | Activatable LMWP coupled to a MMP-2/9-cleavable peptide sequence | Pharmacokinetics in rats; biodistribution and efficacy in C6 mouse xenografts | Specific PTX accumulation in glioma; enhanced efficacy in xenografts | [116] |
Abbreviations: 99mTc-labeled N,N-bis(2-mercaptoethyl)-N’,N’-diethylethylenediamine (99mTc-BMEDA); Apo, apolipoprotein; ATF5, activating transcription factor-5; C6, C6 cells; DA7R, DADTDWDLDPDPDR sequence, which has a high affinity for VEGFR 2; DACHPt, (1,2-diaminocyclohexane)platinum(II); DOX, doxorubicin; EGFR, epidermal growth factor receptor; EGFRvIII, mutant EGFR; F98, F98 cells; GL261, GL261 cells; GSH, glutathione; HBA, β-hydroxybutyric acid; IP10, Interferon-γ-inducible protein; LDL, low density lipoprotein; Lf, lactoferrin; LMWP, low-molecular-weight protein; MMP, matrix metalloproteinase; MTX, methotrexate; NLC, nanostructured lipid carriers; PCL, poly(ε-caprolactone); PEG, polyethylenglycol; P-gp, P-glycoprotein; PLA, poly-lactide; PLGA, poly-lactide-glycolide; PTX, paclitaxel; RGD, arginine-glycine-aspartic acid; scFv, single-chain Fv fragments; siRNA, small interfering RNA; SLN, solid lipid nanoparticles; T7, HAIYPRH sequence, which can bind to Tf receptors; Tf, transferrin; TMZ, temozolomide; TRAIL, tumor necrosis factor (TNF) related apoptosis-inducing ligand; U251, U251 cells; U87, U87 cells.
Figure 4.
Scheme of the main mechanism used for active targeting of nanocarriers in glioma therapy. Blue: endogenous ligands; red: exogenous ligands.
Abbreviations: avß3/avß5, avß3/avß5 heterodimers; CTX, chlorotoxin; EGFR, epidermal growth factor receptor; GSH, glutathione; HFE, homeostatic iron regulator protein; IL 13, interleukin 13; LDL, low-density lipoprotein; Lf, lactoferrin; MCT-1, monocarboxylic acid transporter 1; MMP, matrix metalloproteinase; Tf, transferrin.
Nanocarrier-Mediated Cell Therapy
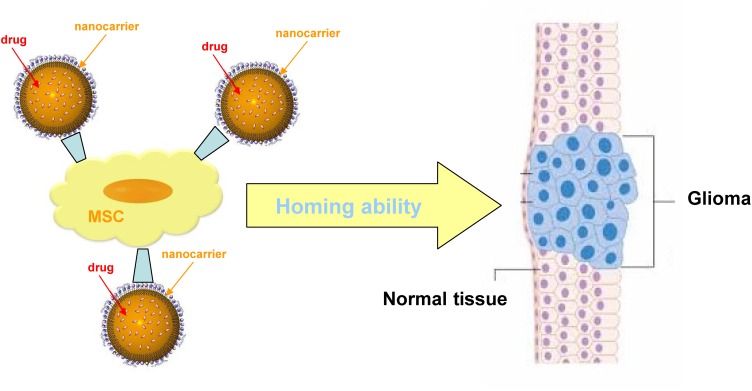
In addition to the selective BBB, glioma chemotherapy can be hampered from the fact that it is difficult to obtain a sufficiently high drug concentration in the tumour, in order to kill infiltrative malignant cells, without hampering healthy brain tissue. Thus, even if a relatively high drug accumulation within brain tissue is obtained, chemotherapeutics may undergo unwanted distribution in the extracellular matrix, or within intratumoral necrotic pockets, but without exerting the desired effects on target glioma cells. These limitations may be overcome by using stem cells as carriers of nanoparticulate delivery systems.118 Indeed, cell-based therapies represent an innovative and valuable tool for cancer treatment, and recent interest is growing also for high-grade glioma therapy. A number of cells have been recently studied, either in preclinical or clinical trials for brain tumour targeting, and can be exploited also in combination with nanocarrier systems.
In particular, adult mesenchymal stem cells (MSC) show a tumour tropic ability, that can be exploited simultaneously to target tumour and deliver therapeutic agents.119 With genetic modification, MSC are able to home cancer tissues and affect the tumor growth by the secretion of cytotoxic molecules. Moreover, MSC are able to trespass the BBB in physiological and pathological conditions, and preclinical studies showed that MSC engineered to express suicide gene enhance the antitumor response in glioblastoma animal models. Within this concern, the combinatorial approach of cell therapy, small-molecule chemotherapy and nanomedicine strategies can open new opportunities for glioma treatment (Figure 5). Internalization/binding of drug-loaded nanoparticles into MSC can be exploited to increase the antitumor efficacy by targeted delivery to the tumour microenvironment.120 Indeed, PTX loaded PLGA nanoparticles showed an enhanced activity in brain tumor, which is ascribed to the sustained release of the drug.121 In a recent work, a hybrid system consisting of MSC spheroids and methotrexate (MTX)-loaded nanoparticles was engineered, in order to increase retention at tumour site: this system improved tumour inhibition in a heterotopic glioblastoma murine model.122 Even though drug-loaded MSC are a promising strategy, some major issues should be considered, such as the fact that the conjugation of nanodrugs to MSC surface could affect the tumour homing ability, as demonstrated by a lot of studies carried out on brain tumour xenografts.
Figure 5.
Scheme of nanocarrier mesenchymal stem cells (MSC) combined therapy of gliomas.
Interestingly, also adipose-derived stem cells (ADSC) show tumour homing ability. This behaviour can be exploited to design drug delivery systems for brain tumours, such as cell-based carriers for nanoparticulate systems. Moreover, it is possible to endow such nanoparticles with stimuli-responsive properties for targeted drug delivery. Indeed, superparamagnetic iron oxide nanoparticles (SPION) were loaded with PTX and subsequently taken up by ADSC via endocytosis.123 Drug release was then activated by high-frequency magnetic field in a glioblastoma murine model. The dual-modality therapeutic strategy including ADSC and smart nanoparticles may be further investigated in the near future for clinical translation.
Given that the “immune privilege” of the brain is no more a retained concept, also the recent advances in immunotherapy strategies offer new opportunities for a synergistic combination of cell therapy with chemotherapeutic drug delivery systems, in order to target brain tumours.124 For example, the combination of dendritic cell-targeted vaccines with nanoparticles loaded with anticancer drugs should lead to a superior anticancer activity.125 Combination with nanocarrier loaded with checkpoint inhibitors could be another valuable therapeutic approach.126 Furthermore, neutrophils (NE) have a native ability to traverse BBB/BBTB and penetrate the glioma site, where the tumour associated NE favour the continuous recruitment of circulating NE; in addition, local brain inflammation, following surgical tumour removal, activates NE migration towards to the inflamed brain. This amplification of inflammatory signals supports an enhanced brain tumour targeting of nanocarriers loaded in NE, such as PTX loaded liposomes127 and DOX loaded magnetic mesoporous silica nanoparticles, which is evident also in resection and recurrence orthotopic models.128 Finally, chimeric antigen receptor (CAR) T cells should be considered, too. They are T cells, generally autologous, ex vivo artificially engineered on their surfaces in order to recognize tumour-associated antigens. The CAR shows both an antigen-binding and a T cell activating function. These modified CAR T cells can produce the lysis of the cells presenting the associated tumor antigen when administered intravenously in patients. Currently, CAR-T cell therapy has been approved in B-cell lymphoma and leukemia, but clinical trials are ongoing for glioblastoma treatment.129,130 Very recently CAR T cell administration showed to improve anti-glioma response.131 Also in this case, CAR-T cells could be associated with nanocarriers in order to improve the therapeutic outcome, and CAR-T cell surface can also be modified to targeting purposes. Indeed, human epidermal growth factor receptor 2 (HER-2) functionalized CAR T cells demonstrated increased persistence over time.
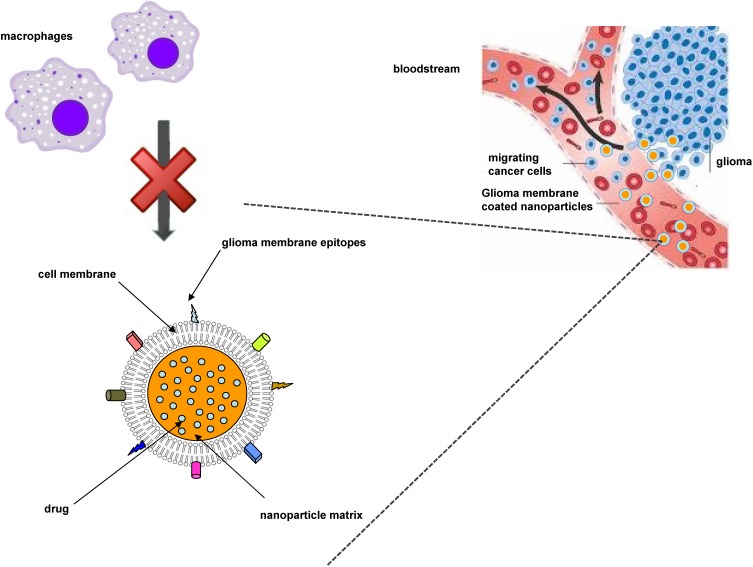
Within this context, suitable attention should be devoted also to cell membrane-coated nanoparticulate systems. Wrapping nanoparticles with cell-derived membranes provide nanocarriers of a natural surface coating with complex biological entities, which are nearly impossible to synthetically replicate via ligand attachment. This allows to overcome some of the previously mentioned shortcomings of actively targeted nanocarriers, such as opsonization and incorrect ligand recognition. Cell membrane coating technology was firstly introduced by employing red blood cell membranes, in order to provide “stealth” properties to synthetic nanoparticles. Currently, it has been applied to many cell types, including cancer cells.132 Indeed, cancer cells possess the unique ability to self-target homologous cells (the so-called homotypic targeting), which can be translated to wrapped nanocarriers, showing also reduced immune clearance compared to uncoated ones (Figure 6). Recently, in a study involving PLGA nanoparticles coated with U87 glioma cell membrane fractions, an induction of cancer cell-specific immune response was demonstrated.133
Figure 6.
Scheme of homotypic targeting of glioma cell membrane-coated nanoparticles.
Clinical Nanomedicines
Despite relevant evidences at the preclinical level, and several clinical studies addressing the employment of nanocarriers for brain tumours chemotherapy, particularly for glioblastoma, till now no new nano-drug has yet approved for brain tumour therapy. In general, the approval rate for novel nanomedicines is below 10%, mainly because of safety and efficacy profile failures during preclinical and clinical studies.134 Indeed, regulatory agencies require manufacturers to perform accurate preauthorization studies to assess the quality, safety, and efficacy profiles of a new nanomedicine.135,136 Furthermore, the difficulty to find out suitable preclinical models that truly represent what happens in the humans is a major flaw, that hampers clinical translation of nanomedicines.95
Within this concern, according to the European Commission Recommendation (2011/696/EU), 100 nm is the demarcating upper size limit where the properties of materials can change significantly from conventional equivalents, and the European Medicines Agency (EMA) also included an official definition of nanomedicine as being up to a size of 100 nm.137 However, frequently nanomedicines have broader size range than the proposed definition, inducing the EMA to include all “structures” with sizes of less than 1000 nm, that are designed to have specific properties,138 can improve site-specific drug delivery and significantly alter toxicological profiles, thus allowing to perform a case-by-case evaluation.139 Nonetheless, despite the numerous guidelines existing for the validation methods of chemical parameters in such matrixes, suitable implementation of this legislation should require validated analytical methods for nanoparticles’ characterization, that, so far, do not exist.140
Some of the most important clinical trials with new emerging nanomedicines are here reported. Few of them involve active targeting mechanisms. In fact, despite the existence of good manufacturing practices for nanodrug delivery systems, that can be employed for translation to clinical trials,141 actively targeted nanomedicines are associated with high costs and scale-up issues. A Phase I clinical study involving patients with recurrent glioma (grade 2–4) has been carried out by administering PTX-Angiopep-2 peptide–drug conjugate (GRN1005). Even if GRN1005 improved PTX permeation into tumour tissue, Phase II trial interim analysis did not show therapeutic response.142,143 Tf conjugated diphtheria toxin (Tf-CRM107) showed in vitro toxicity towards glioma cells and it was effective in xenografts after local administration. Moreover, in phase I and II clinical trials, local administration of Tf-CRM107 resulted in low toxicity, encouraging response rate (35%) and promising overall survival (74 weeks) in patients with recurrent high-grade brain tumours. However, early Phase III clinical trials employing this approach were terminated due to disappointing preliminary results.9,144 In another interesting phase I study, EnGeneIC delivery vehicle loaded with DOX was tested in patients with recurrent glioblastoma. The anti-EGFR monoclonal antibody Vectibix was used to target EGFR on cancer cells, thus leading to DOX release.145
However, the most important clinical evidences were obtained with off-label employment of already marketed liposomal DOX, acting mainly owing to the EPR effect. In a clinical study liposomal DOX administered in patients with high-grade gliomas improved overall survival.146 Moreover, DOX loaded in pegylated liposomes (PEG-DOX) was efficacious and well tolerated in patients with recurrent high-grade glioma.147 Indeed, encouraging results were obtained with PEG-DOX alone (mOS 26 weeks) or in combination with TMZ (median overall survival 32 weeks, 6-months progression-free survival 32%). Owing to the available studies, PEG-DOX, alone or in combination regimens, can actually be considered as a treatment option for recurrent high-grade gliomas, but only if no further chemotherapy is available; however, it should be further evaluated in larger clinical trials.148
Conclusions
Malignant gliomas are still associated with a poor prognosis, despite recent advances in surgical treatment. Despite the large number of potential drug candidates, the efficacy of adjuvant chemotherapy remains unsatisfactory, primarily because of the BBB. Nanocarriers can favour delivery of chemotherapeutics to malignant gliomas owing to different mechanisms, including chemical stabilization of the drug in the bloodstream, EPR effect (because of the leaky BBTB), P-gp inhibition, active targeting through CMT and RMT, inhibition of cell differentiation and angiogenesis, cell-mediated targeting, or stimuli-responsive delivery. In particular, different and efficient active targeting approaches have been attempted in preclinical studies on animal models, mainly by employing protein targeting moieties. Nevertheless, a few number of nanomedicines reached the clinical trials, and most of them include drug-loaded nanocarriers free of targeting ligands, probably because of safety and scalability concerns.
Acknowledgment
The authors acknowledge Università degli Studi di Torino (Ricerca Locale 2018-2019) for funding.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Chen R, Smith-Cohn M, Cohen AL, Colman H. Glioma subclassifications and their clinical significance. Neurotherapeutics. 2017;14:284–297. doi: 10.1007/s13311-017-0519-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cerna T, Stiborova M, Adam V, Kizek R, Eckschlager T. Nanocarrier drugs in the treatment of brain tumors. J Cancer Metastasis Treat. 2016;2(10):407. doi: 10.20517/2394-4722.2015.95 [DOI] [Google Scholar]
- 3.Forst DA, Nahed BV, Loeffler JS, Batchelor TT. Low-grade gliomas. Oncologist. 2014;19:403–413. doi: 10.1634/theoncologist.2013-0345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jakola AS, Skjulsvik AJ, Myrmel KS, et al. Surgical resection versus watchful waiting in low-grade gliomas. Ann Oncol. 2017;28(8):1942–1948. doi: 10.1093/annonc/mdx230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Panciani PP, Fontanella M, Schatlo B, et al. Fluorescence and image guided resection in high grade glioma. Clin Neurol Neurosurg. 2012;114(1):37–41. doi: 10.1016/j.clineuro.2011.09.001 [DOI] [PubMed] [Google Scholar]
- 6.Darlix A, Mandonnet E, Freyschlag CF, et al. Chemotherapy and diffuse low-grade gliomas: a survey within the European Low-Grade Glioma Network. Neurooncol Pract. 2019;6(4):264–273. doi: 10.1093/nop/npy051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caffo M, Cardali S, Fazzari E, Barresi V, Caruso G. Nanoparticles drug-delivery systems and antiangiogenic approaches in the treatment of gliomas. Glioma. 2018;1(6):183. doi: 10.4103/glioma.glioma_43_18 [DOI] [Google Scholar]
- 8.Ozdemir-Kaynak E, Qutub AA, Yesil-Celiktas O. Advances in glioblastoma multiforme treatment: new models for nanoparticle therapy. Front Physiol. 2018;9:1–14. doi: 10.3389/fphys.2018.00170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arko L, Katsyv I, Park GE, Luan WP, Park JK. Experimental approaches for the treatment of malignant gliomas. Pharmacol Ther. 2010;128:1–36. doi: 10.1016/j.pharmthera.2010.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathieu D, Fortin D. The role of chemotherapy in the treatment of malignant astrocytomas. Can J Neurol Sci. 2006;33(2):127–140. doi: 10.1017/S0317167100004881 [DOI] [PubMed] [Google Scholar]
- 11.Pardridge WM. Drug transport across the blood–brain barrier. Physicochemical and pharmacokinetic parameters of seven lipophilic chlorambucil esters designed for brain penetration. J Cereb Blood Flow Metab. 2012;32:1959–1972. doi: 10.1007/bf00686229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greig NH, Genka S, Daly EM, Sweeney DJ, Rapoport SI. Physicochemical and pharmacokinetic parameters of seven lipophilic chlorambucil esters designed for brain penetration. Cancer Chemother Pharmacol. 1990;25:311–319. doi: 10.1007/BF00686229 [DOI] [PubMed] [Google Scholar]
- 13.Ke XY, Zhao BJ, Zhao X, et al. The therapeutic efficacy of conjugated linoleic acid - paclitaxel on glioma in the rat. Biomaterials. 2010;31(22):5855–5864. doi: 10.1016/j.biomaterials.2010.03.079 [DOI] [PubMed] [Google Scholar]
- 14.Norden AD, Young GS, Setayesh K, et al. Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology. 2008;70(10):779. doi: 10.1212/01.wnl.0000304121.57857.38 [DOI] [PubMed] [Google Scholar]
- 15.Rich JN, Bigner DD. Development of novel targeted therapies in the treatment of malignant glioma. Nat Rev Drug Discov. 2004;3(5):430–446. doi: 10.1038/nrd1380 [DOI] [PubMed] [Google Scholar]
- 16.Dietrich J, Wang D, Batchelor TT. Cediranib: profile of a novel anti-angiogenic agent in patients with glioblastoma. Expert Opin Investig Drugs. 2009;18(10):1549–1557. doi: 10.1517/13543780903183528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maher EA, Furnari FB, Bachoo RM, et al. Malignant glioma: genetics and biology of a grave matter. Genes Dev. 2001;15(11):1311–1333. doi: 10.1101/gad.891601 [DOI] [PubMed] [Google Scholar]
- 18.Fukai J, Nishio K, Itakura T, Koizumi F. Antitumor activity of cetuximab against malignant glioma cells overexpressing EGFR deletion mutant variant III. Cancer Sci. 2008;99(10):2062–2069. doi: 10.1111/j.1349-7006.2008.00945.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crombet T, Torres O, Rodríguez V, et al. Phase I clinical evaluation of a neutralizing monoclonal antibody against epidermal growth factor receptor in advanced brain tumor patients: preliminary study. Hybridoma. 2001;20(2):131–136. doi: 10.1089/02724570152057634 [DOI] [PubMed] [Google Scholar]
- 20.Joensuu H, Puputti M, Sihto H, Tynninen O, Nupponen NN. Amplification of genes encoding KIT, PDGFRalpha and VEGFR2 receptor tyrosine kinases is frequent in glioblastoma multiforme. J Pathol. 2005;207(2):224–231. doi: 10.1002/path.1823 [DOI] [PubMed] [Google Scholar]
- 21.Holmen SL, Williams BO. Essential role for Ras signaling in glioblastoma maintenance. Cancer Res. 2005;65(18):8250–8255. doi: 10.1158/0008-5472.CAN-05-1173 [DOI] [PubMed] [Google Scholar]
- 22.Dresemann G, Weller M, Rosenthal MA, et al. Imatinib in combination with hydroxyurea versus hydroxyurea alone as oral therapy in patients with progressive pretreated glioblastoma resistant to standard dose temozolomide. J Neurooncol. 2010;96(3):393–402. doi: 10.1007/s11060-009-9976-3 [DOI] [PubMed] [Google Scholar]
- 23.Argyriou AA, Kalofonos HP. Molecularly targeted therapies for malignant gliomas. Mol Med. 2009;15(3–4):115–122. doi: 10.2119/molmed.2008.00123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Newton HB. Molecular neuro-oncology and development of targeted therapeutic strategies for brain tumors. part 1: growth factor and Ras signaling pathways. Expert Rev Anticancer Ther. 2003;3(5):595–614. doi: 10.1586/14737140.3.5.595 [DOI] [PubMed] [Google Scholar]
- 25.Graff JR, McNulty AM, Hanna KR, et al. The protein kinase Cbeta-selective inhibitor, Enzastaurin (LY317615.HCl), suppresses signaling through the AKT pathway, induces apoptosis, and suppresses growth of human colon cancer and glioblastoma xenografts. Cancer Res. 2005;65(16):7462–7469. doi: 10.1158/0008-5472.CAN-05-0071 [DOI] [PubMed] [Google Scholar]
- 26.Da Rocha AB, Mans DR, Regner A, Schwartsmann G. Targeting protein kinase C: new therapeutic opportunities against high-grade malignant gliomas? Oncologist. 2002;7(1):17–33. doi: 10.1634/theoncologist.7-1-17 [DOI] [PubMed] [Google Scholar]
- 27.Bredel M, Pollack IF. The role of protein kinase C (PKC) in the evolution and proliferation of malignant gliomas, and the application of PKC inhibition as a novel approach to anti-glioma therapy. Acta Neurochir (Wien). 1997;139(11):1000–1013. doi: 10.1007/bf01411552 [DOI] [PubMed] [Google Scholar]
- 28.Sharif TR, Sharif M. Overexpression of protein kinase C epsilon in astroglial brain tumor derived cell lines and primary tumor samples. Int J Oncol. 1999;15(2):237–243. [PubMed] [Google Scholar]
- 29.Hu X, Pandolfi PP, Li Y, Koutcher JA, Rosenblum M, Holland EC. mTOR promotes survival and astrocytic characteristics induced by Pten/AKT signaling in glioblastoma. Neoplasia. 2005;7(4):356–368. doi: 10.1593/neo.04595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Minniti G, Muni R, Lanzetta G, Marchetti P, Enrici RM. Chemotherapy for glioblastoma: current treatment and future perspectives for cytotoxic and targeted agents. Anticancer Res. 2009;29(12):5171–5184. [PubMed] [Google Scholar]
- 31.Dancey J. mTOR signaling and drug development in cancer. Nat Rev Clin Oncol. 2010;7(4):209–219. doi: 10.1038/nrclinonc.2010.21 [DOI] [PubMed] [Google Scholar]
- 32.Yuan R, Kay A, Berg W, Lebwohl D. Targeting tumorigenesis: development and use of mTOR inhibitors in cancer therapy. J Hematol Oncol. 2009;2(1):45. doi: 10.1186/1756-8722-2-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galanis E, Buckner JC, Maurer MJ, et al. Phase II trial of temsirolimus (CCI-779) in recurrent glioblastoma multiforme: a North Central Cancer Treatment Group Study. J Clin Oncol. 2005;23(23):5294–5304. doi: 10.1200/JCO.2005.23.622 [DOI] [PubMed] [Google Scholar]
- 34.Cloughesy TF, Yoshimoto K, Nghiemphu P, et al. Antitumor activity of rapamycin in a Phase I trial for patients with recurrent PTEN-deficient glioblastoma. PLoS Med. 2008;5(1):e8. doi: 10.1371/journal.pmed.0050008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tonn JC, Kerkau S, Hanke A, et al. Effect of synthetic matrix-metalloproteinase inhibitors on invasive capacity and proliferation of human malignant gliomas in vitro. Int J Cancer. 1999;80:764–772. doi: [DOI] [PubMed] [Google Scholar]
- 36.Brown PD. Ongoing trials with matrix metalloproteinase inhibitors. Exp Opin Invest Drugs. 2000;9:2167–2177. doi: 10.1517/13543784.9.9.2167 [DOI] [PubMed] [Google Scholar]
- 37.Bello L, Francolini M, Marthyn P, et al. Alpha(v)beta3 and alpha(v)beta5 integrin expression in glioma periphery. Neurosurgery. 2001;49(2):380–389. doi: 10.1097/00006123-200108000-00022 [DOI] [PubMed] [Google Scholar]
- 38.Groothuis DR. The blood-brain and blood-tumor barriers: a review of strategies for increasing drug delivery. Neuro Oncol. 2000;2:45–59. doi: 10.1093/neuonc/2.1.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liebner S, Fischmann A, Rascher G, et al. Claudin-1 and claudin-5 expression and tight junction morphology are altered in blood vessels of human glioblastoma multiforme. Acta Neuropathol. 2000;100:323–331. doi: 10.1007/s004010000180 [DOI] [PubMed] [Google Scholar]
- 40.Li M, Deng H, Peng H, Wang Q. Functional nanoparticles in targeting glioma diagnosis and therapies. J Nanosci Nanotechnol. 2014;14(1):415–432. doi: 10.1166/jnn.2014.8757 [DOI] [PubMed] [Google Scholar]
- 41.Argaw AT, Zhang Y, Snyder BJ, et al. IL-1beta regulates blood-brain barrier permeability via reactivation of the hypoxia-angiogenesis program. J Immunol. 2006;177:5574–5584. doi: 10.4049/jimmunol.177.8.5574 [DOI] [PubMed] [Google Scholar]
- 42.Zhang ZG, Zhang L, Jiang Q, et al. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest. 2000;106:829–838. doi: 10.1172/JCI9369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schlageter KE, Molnar P, Lapin GD, et al. Microvessel organization and structure in experimental brain tumors: microvessel populations with distinctive structural and functional properties. Microvasc Res. 1999;58:312–328. doi: 10.1006/mvre.1999.2188 [DOI] [PubMed] [Google Scholar]
- 44.Liu Y, Lu W. Recent advances in brain tumor-targeted nano-drug delivery systems. Expert Opin Drug Deliv. 2012;6:671–676. doi: 10.1517/17425247.2012.682726 [DOI] [PubMed] [Google Scholar]
- 45.Gaoe H, Pang Z, Pan S, et al. Anti-glioma effect and safety of docetaxel-loaded nanoemulsion. Arch Pharm Res. 2012;35(2):333–341. doi: 10.1007/s12272-012-0214-8 [DOI] [PubMed] [Google Scholar]
- 46.Karim R, Palazzo C, Evrard B, Piel G. Nanocarriers for the treatment of glioblastoma multiforme: current state-of-the-art. J Control Release. 2016;227:23–37. doi: 10.1016/j.jconrel.2016.02.026 [DOI] [PubMed] [Google Scholar]
- 47.Schiffer D, Annovazzi L, Caldera V, Mellai M. On the origin and growth of gliomas. Anticancer Res. 2010;30:1977–1998. [PubMed] [Google Scholar]
- 48.Allhenn D, Boushehri MAS, Lamprecht A. Drug delivery strategies for the treatment of malignant gliomas. Int J Pharm. 2012;436:299–310. doi: 10.1016/j.ijpharm [DOI] [PubMed] [Google Scholar]
- 49.Zhang TT, Li W, Meng G, Wang P, Liao W. Strategies for transporting nanoparticles across the blood‑brain barrier. Biomater Sci. 2016;4:219–229. doi: 10.1039/c5bm00383k [DOI] [PubMed] [Google Scholar]
- 50.Battaglia L, Muntoni E, Chirio D, et al. Solid lipid nanoparticles by coacervation loaded with a methotrexate prodrug: preliminary study for glioma treatment. Nanomedicine. 2017;12(6):639–656. doi: 10.2217/nnm-2016-0380 [DOI] [PubMed] [Google Scholar]
- 51.Muntoni E, Martina K, Marini E, et al. Methotrexate-loaded solid lipid nanoparticles: protein functionalization to improve brain biodistribution. Pharmaceutics. 2019;11:2. doi: 10.3390/pharmaceutics11020065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tortorella S, Karagiannis TC. Transferrin receptor-mediated endocytosis: a useful target for cancer therapy. J Membr Biol. 2014;247:291–307. doi: 10.1007/s00232-014-9637-0 [DOI] [PubMed] [Google Scholar]
- 53.Miao D, Jiang M, Liu Z, et al. Co-administration of dual-targeting nanoparticles with penetration enhancement peptide for antiglioblastoma therapy. Mol Pharm. 2014;11:90–101. doi: 10.1021/mp400189j [DOI] [PubMed] [Google Scholar]
- 54.Kuo YC, Shih-Huang CY. Solid lipid nanoparticles carrying chemotherapeutic drug across the blood-brain barrier through insulin receptor-mediated pathway. J Drug Target. 2013;21:730–738. doi: 10.3109/1061186X.2013.812094 [DOI] [PubMed] [Google Scholar]
- 55.Shilo M, Motiei M, Hana P, Popovtzer R. Transport of nanoparticles through the blood-brain barrier for imaging and therapeutic applications. Nanoscale. 2014;6:2146–2152. doi: 10.1039/c3nr04878k [DOI] [PubMed] [Google Scholar]
- 56.Tsuji A, Tamai I. Blood–brain barrier function of P-glycoprotein. Adv Drug Deliv Rev. 1997;25:287–298. doi: 10.1016/S0169-409X(97)00504-8 [DOI] [Google Scholar]
- 57.Schinkel AH. P-glycoprotein, a gatekeeper in the blood–brain barrier. Adv Drug Deliv Rev. 1999;36:179–194. doi: 10.1016/s0169-409x(98)00085-4 [DOI] [PubMed] [Google Scholar]
- 58.Kuntner C, Bankstahl JP, Bankstahl M, et al. Dose–response assessment of tariquidar and elacridar and regional quantification of P-glycoprotein inhibition at the rat blood–brain barrier using (R)-[(11)C]verapamil PET. Eur J Nucl Med Mol Imaging. 2010;37:942–953. doi: 10.1007/s00259-009-1332-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mastronardi L, Puzzilli F, Ruggeri A. Tamoxifen as a potential treatment of glioma. Anticancer Drugs. 1998;9:581–586. doi: 10.1097/00001813-199808000-00001 [DOI] [PubMed] [Google Scholar]
- 60.Miller DS, Bauer B, Hartz AMS. Modulation of P-glycoprotein at the blood–brain barrier: opportunities to improve central nervous system pharmacotherapy. Pharmacol Rev. 2008;60:196–209. doi: 10.1124/pr.107.07109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Venishetty VK, Komuravelli R, Kuncha M, Sistla R, Diwan PV. Increased brain uptake of docetaxel and ketoconazole loaded folate-grafted solid lipid nanoparticles. Nanomedicine. 2013;9:111–121. doi: 10.1016/j.nano.2012.03.003 [DOI] [PubMed] [Google Scholar]
- 62.Brat DJ, Castellano-Sanchez AA, Hunter SB, et al. Pseudopalisades in glioblastoma are hypoxic, express extracellular matrix proteases, and are formed by an actively migrating cell population. Cancer Res. 2004;64:920–927. doi: 10.1158/0008-5472.can-03-2073 [DOI] [PubMed] [Google Scholar]
- 63.Michael JS, Lee B, Zhang M, Yu JS. Nanotechnology for treatment of glioblastoma multiforme. J Transl Intern Med. 2018;6(3):128–133. doi: 10.2478/jtim-2018-0025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128 [DOI] [PubMed] [Google Scholar]
- 65.Seidel S, Garvalov BK, Wirta V, et al. A hypoxic niche regulates glioblastoma stem cells through hypoxia inducible factor 2 alpha. Brain. 2010;133:983–995. doi: 10.1093/brain/awq042 [DOI] [PubMed] [Google Scholar]
- 66.Heddleston JM, Li Z, McLendon RE, Hjelmeland AB, Rich JN. The hypoxic microenvironment maintains glioblastoma stem cells and promotes reprogramming towards a cancer stem cell phenotype. Cell Cycle. 2009;8:3274–3284. doi: 10.4161/cc.8.20.9701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–845. doi: 10.1038/359843a0 [DOI] [PubMed] [Google Scholar]
- 68.Wang GL, Semenza GL. Characterization of hypoxia-inducible factor 1 and regulation of DNA binding activity by hypoxia. J Biol Chem. 1993;268:21513–21518. [PubMed] [Google Scholar]
- 69.Semenza GL, Nejfelt MK, Chi SM, Antonarakis SE. Hypoxia-inducible nuclear factors bind to an enhancer element located 3ʹ to the human erythropoietin gene. Proc Natl Acad Sci USA. 1991;88:5680–5684. doi: 10.1073/pnas.88.13.5680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chandel NS, McClintock DS, Feliciano CE, et al. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1 alpha during hypoxia: a mechanism of O2 sensing. J Biol Chem. 2000;275:25130–25138. doi: 10.1074/jbc.M001914200 [DOI] [PubMed] [Google Scholar]
- 71.Vredenburgh JJ, Desjardins A, Herndon JE 2nd, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13:1253–1259. doi: 10.1158/1078-0432.CCR-06-2309 [DOI] [PubMed] [Google Scholar]
- 72.Vredenburgh JJ, Desjardins A, Herndon JE 2nd, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25:4722–4729. doi: 10.1200/JCO.2007.12.2440 [DOI] [PubMed] [Google Scholar]
- 73.Chen WL, Wang CC, Lin YJ, Wu CP, Hsieh CH. Cycling hypoxia induces chemoresistance through the activation of reactive oxygen species-mediated B-cell lymphoma extra-long pathway in glioblastoma multiforme. J Transl Med. 2015;13:389. doi: 10.1186/s12967-015-0758-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Battaglia L, Gallarate M, Peira E, et al. Bevacizumab loaded solid lipid nanoparticles prepared by the coacervation technique: preliminary in vitro studies. Nanotechnology. 2015;26(25):255102. doi: 10.1088/0957-4484/26/25/255102 [DOI] [PubMed] [Google Scholar]
- 75.Sack‑Zschauer M, Bader S, Brenneisen P. Cerium oxide nanoparticles as novel tool in glioma treatment: an in vitro study. J Nanomed Nanotechnol. 2017;8:474. doi: 10.4172/2157-7439.1000474 [DOI] [Google Scholar]
- 76.Pizzolato JF, Saltz LB. The camptothecins. Lancet. 2003;361:2235–2242. doi: 10.1016/S0140-6736(03)13780-4 [DOI] [PubMed] [Google Scholar]
- 77.Guiot C, Zullino S, Priano L, Cavalli R. The physics of drug delivery across the blood brain barrier. Ther Deliv. 2016;7(3):153–155. doi: 10.4155/tde-2016-0001 [DOI] [PubMed] [Google Scholar]
- 78.Lollo G, Vincent M, Ullio-Gamboa G, et al. Development of multifunctional lipid nanocapsules for the co-delivery of paclitaxel and CpG-ODN in the treatment of glioblastoma. Int J Pharm. 2015;495(2):972–980. doi: 10.1016/j.ijpharm.2015.09.062 [DOI] [PubMed] [Google Scholar]
- 79.Lopez-Bertoni H, Kozielski KL, Rui Y, et al. Bioreducible polymeric nanoparticles containing multiplexed cancer stem cell regulating miRNAs inhibit glioblastoma growth and prolong survival. Nano Lett. 2018;18(7):4086–4094. doi: 10.1021/acs.nanolett.8b00390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bastiancich C, Vanvarenberg K, Ucakar B, et al. Lauroyl-gemcitabine-loaded lipid nanocapsule hydrogel for the treatment of glioblastoma. J Control Release. 2016;225:283–293. doi: 10.1016/j.jconrel.2016.01.054 [DOI] [PubMed] [Google Scholar]
- 81.Bastiancich C, Lemaire L, Bianco J, et al. Evaluation of lauroyl-gemcitabine-loaded hydrogel efficacy in glioblastoma rat models. Nanomedicine. 2018;13:1999–2013. doi: 10.2217/nnm-2018-0057 [DOI] [PubMed] [Google Scholar]
- 82.Bastiancich C, Bozzato E, Luyten U, et al. Drug combination using an injectable nanomedicine hydrogel for glioblastoma treatment. Int J Pharm. 2019;559:220–227. doi: 10.1016/j.ijpharm.2019.01.042 [DOI] [PubMed] [Google Scholar]
- 83.Landhuis E. Ultrasound for the brain. Nature. 2017;551:257–259. doi: 10.1038/d41586-017-05479-7 [DOI] [PubMed] [Google Scholar]
- 84.Carpentier A, Canney M, Vignot A, et al. Clinical trial of blood-brain barrier disruption by pulsed ultrasound. Sci Transl Med. 2016;8:343re22016. doi: 10.1126/scitranslmed.aaf6086 [DOI] [PubMed] [Google Scholar]
- 85.Zhang H, Wang T, Qiu W, et al. Monitoring the opening and recovery of the blood − brain barrier with noninvasive molecular imaging by biodegradable ultrasmall Cu2−xSe nanoparticles. Nano Lett. 2018;18(8):4985–4992. doi: 10.1021/acs.nanolett.8b01818 [DOI] [PubMed] [Google Scholar]
- 86.Liu Z, Ren F, Zhang H, et al. Biomaterials boosting often overlooked long wavelength emissions of rare-earth nanoparticles for NIR-II fluorescence imaging of orthotopic glioblastoma. Biomaterials. 2019;219:119364. doi: 10.1016/j.biomaterials.2019.119364 [DOI] [PubMed] [Google Scholar]
- 87.Zhang H, Wang T, Liu H, et al. Second near-infrared photodynamic therapy and chemotherapy of orthotopic malignant glioblastoma with ultra-small Cu2−xSe nanoparticles. Nanoscale. 2019:7600–7608. doi: 10.1039/c9nr01789e. [DOI] [PubMed] [Google Scholar]
- 88.Frosina G. Nanoparticle-mediated drug delivery to high-grade gliomas. Nanomed Nanotechnol Biol Med. 2016;12(4):1083–1093. doi: 10.1016/j.nano.2015.12.375 [DOI] [PubMed] [Google Scholar]
- 89.Gastaldi L, Battaglia L, Peira E, et al. Solid lipid nanoparticles as vehicles of drugs to the brain: current state of the art. Eur J Pharm Biopharm. 2014;87(3):433–444. doi: 10.1016/j.ejpb.2014.05.004 [DOI] [PubMed] [Google Scholar]
- 90.Grant GA, Abbott NJ, Janigro D. Understanding the physiology of the blood-brain barrier: in vitro models. News Physiol Sci. 1998;13:287–293. doi: 10.1152/physiologyonline.1998.13.6.287 [DOI] [PubMed] [Google Scholar]
- 91.Lenting K, Verhaak R, Ter Laan M, Wesseling P, Leenders W. Glioma: experimental models and reality. Acta Neuropathol. 2017;133(2):263–282. doi: 10.1007/s00401-017-1671-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Becher B, Prat A, Antel JP. Brain–immune connection: immunoregulatory properties of CNS-resident cells. Glia. 2000;29:293–304. [PubMed] [Google Scholar]
- 93.Hickey WF. Basic principles of immunologic surveillance of the normal central nervous system. Glia. 2001;36:118–124. doi: 10.1002/glia.1101 [DOI] [PubMed] [Google Scholar]
- 94.Biasibetti E, Valazza A, Capucchio MT, et al. Comparison of allogeneic and syngeneic rat glioma models by using MRI and histopathologic evaluation. Comp Med. 2017;67(2):147–156. [PMC free article] [PubMed] [Google Scholar]
- 95.Bianco J, Bastiancich C, Joudiou N, et al. Novel model of orthotopic U-87 MG glioblastoma resection in athymic nude mice. J Neurosci Methods. 2017;284:96–102. doi: 10.1016/j.jneumeth.2017.04.019 [DOI] [PubMed] [Google Scholar]
- 96.Chen Z, Lai X, Song S, Zhu X, Zhu J. Nanostructured lipid carriers based temozolomide and gene co-encapsulated nanomedicine for gliomatosis cerebri combination therapy. Drug Deliv. 2015;23:1369–1373. doi: 10.3109/10717544.2015.1038857 [DOI] [PubMed] [Google Scholar]
- 97.Wu M, Fan Y, Lv S, Xiao B, Ye M, Zhu X. Vincristine and temozolomide combined chemotherapy for the treatment of glioma: a comparison of solid lipid nanoparticles and nanostructured lipid carriers for dual drugs delivery. Drug Deliv. 2016;23:2720–2725. doi: 10.3109/10717544.2015.1058434 [DOI] [PubMed] [Google Scholar]
- 98.Jin J, Bae KH, Yang H, et al. In vivo specific delivery of c-Met siRNA to glioblastoma using cationic solid lipid nanoparticles. Bioconjug Chem. 2011;22:2568–2572. doi: 10.1021/bc200406n [DOI] [PubMed] [Google Scholar]
- 99.Tapeinos C, Battaglini M, Ciofani G. Advances in the design of solid lipid nanoparticles and nanostructured lipid carriers for targeting brain diseases. J Control Release. 2017;264:306–332. doi: 10.1016/j.jconrel.2017.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Koziara JM, Lockman PR, Allen DD, Mumper RJ. Paclitaxel nanoparticles for the potential treatment of brain tumors. J Controlled Release. 2004;99:259–269. doi: 10.1016/j.jconrel.2004.07.006 [DOI] [PubMed] [Google Scholar]
- 101.Estella-hermoso De Mendoza A, Préat V, Mollinedo F, Blanco-Prieto MJ. In vitro and in vivo efficacy of edelfosine-loaded lipid nanoparticles against glioma. J Control Release. 2011;156(3):421–426. doi: 10.1016/j.jconrel.2011.07.030 [DOI] [PubMed] [Google Scholar]
- 102.Lockman PR, Koziara J, Roder KE, et al. In vivo and in vitro assessment of baseline blood–brain barrier parameters in the presence of novel nanoparticles. Pharm Res. 2003;20:705–713. doi: 10.1023/a:1023492015851 [DOI] [PubMed] [Google Scholar]
- 103.Zara GP, Bargoni A, Cavalli R, et al. Pharmacokinetics and tissue distribution of idarubicin-loaded solid lipid nanoparticles after duodenal administration to rats. J Pharm Sci. 2002;91(5):1324–1333. doi: 10.1002/jps.10129 [DOI] [PubMed] [Google Scholar]
- 104.Liu L, Ni F, Zhang J, et al. Silver nanocrystals sensitize magnetic‑nanoparticle‑mediated thermo‑induced killing of cancer cells. Acta Biochim Biophys Sin (Shanghai). 2011;43:316–323. doi: 10.1093/abbs/gmr015 [DOI] [PubMed] [Google Scholar]
- 105.Nikanjam M, Gibbs AR, Hunt CA, Budinger TF, Forte TM. Synthetic nano-LDL with paclitaxel oleate as a targeted drug delivery vehicle for glioblastoma multiforme. J Control Release. 2007;124:163. doi: 10.1016/j.jconrel.2007.09.007 [DOI] [PubMed] [Google Scholar]
- 106.Huang S, Li J, Han L, et al. Dual targeting effect of Angiopep-2-modified, DNA loaded nanoparticles for glioma. Biomaterials. 2011;32:6832. doi: 10.1016/j.biomaterials.2011.05.064 [DOI] [PubMed] [Google Scholar]
- 107.Dal Magro R, Albertini B, Beretta S, et al. Artificial apolipoprotein corona enables nanoparticle brain targeting. Nanomed. 2017;14(2):429–438. doi: 10.1016/j.nano.2017.11.008 [DOI] [PubMed] [Google Scholar]
- 108.Martins SM, Sarmento B, Nunes C, Lúcio M, Reis S, Ferreira DC. Brain targeting effect of camptothecin-loaded solid lipid nanoparticles in rat after intravenous administration. Eur J Pharm Biopharm. 2013;85:488–502. doi: 10.1016/j.ejpb.2013.08.011 [DOI] [PubMed] [Google Scholar]
- 109.Chen H, Qin Y, Zhang Q, et al. Lactoferrin modified doxorubicin-loaded procationic liposomes for the treatment of gliomas. Eur J Pharm Sci. 2011;44:164. doi: 10.1016/j.ejps.2011.07.007 [DOI] [PubMed] [Google Scholar]
- 110.Gaillard PJ, Appeldoorn CCM, Dorland R, et al. Pharmacokinetics, brain delivery, and efficacy in brain tumor-bearing mice of glutathione pegylated liposomal doxorubicin (2B3-101). PLoS One. 2014;9(1):e82331. doi: 10.1371/journal.pone.0082331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Venishetty VK, Samala R, Komuravelli R, Kuncha M, Sistla R, Diwan PV. β-hydroxybutyric acid grafted solid lipid nanoparticles: a novel strategy to improve drug delivery to brain. Nanomedicine. 2013;9:388–397. doi: 10.1016/j.nano.2012.08.004 [DOI] [PubMed] [Google Scholar]
- 112.De Boer AG, Gaillard PJ. Drug targeting to the brain. Annu Rev Pharmacol Toxicol. 2007;47:323–355. doi: 10.1146/annurev.pharmtox.47.120505.105237 [DOI] [PubMed] [Google Scholar]
- 113.Mortensen JH, Jeppesen M, Pilgaard L, et al. Targeted antiepidermal growth factor receptor (cetuximab) immunoliposomes enhance cellular uptake in vitro and exhibit increased accumulation in an intracranial model of glioblastoma multiforme. J Drug Deliv. 2013;2013:209205. doi: 10.1155/2013/209205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen C, Duan Z, Yuan Y, et al. Peptide‑22 and cyclic RGD functionalized liposomes for glioma targeting drug delivery overcoming BBB and BBTB. ACS Appl Mater Interfaces. 2017;9:5864–5873. doi: 10.1021/acsami.6b15831 [DOI] [PubMed] [Google Scholar]
- 115.Hu Q, Gu G, Liu Z, et al. F3 peptide-functionalized PEG-PLA nanoparticles co-administrated with tLyp-1 peptide for anti-glioma drug delivery. Biomaterials. 2013;34:1135. doi: 10.1016/j.biomaterials.2012.10.048 [DOI] [PubMed] [Google Scholar]
- 116.Gu G, Xia H, Hu Q, et al. PEG-co-PCL nanoparticles modified with MMP-2/9 activatable low molecular weight protamine for enhanced targeted glioblastoma therapy. Biomaterials. 2013;34:196. doi: 10.1016/j.biomaterials.2012.09.044 [DOI] [PubMed] [Google Scholar]
- 117.Xiang Y, Liang L, Wang X, Wang J, Zhang X, Zhang Q. Chloride channel-mediated brain glioma targeting of chlorotoxin-modified doxorubicin-loaded liposomes. J Control Release. 2011;152:402. doi: 10.1016/j.jconrel.2011.03.014 [DOI] [PubMed] [Google Scholar]
- 118.Long W, Yi Y, Chen S, et al. Potential new therapies for pediatric diffuse intrinsic pontine glioma. Front Pharmacol. 2017;8:1–13. doi: 10.3389/fphar.2017.00495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Altaner C, Altanerova C. Stem cell based glioblastoma cell therapy. Neoplasma. 2012;59(6):756–760. doi: 10.4149/neo_2012_95 [DOI] [PubMed] [Google Scholar]
- 120.Kwon S, Yoo KH, Sym SJ, Khang D. Mesenchymal stem cell therapy assisted by nanotechnology: a possible combinational treatment for brain tumor and central nerve regeneration. Int J Nanomedicine. 2019;14:5925–5942. doi: 10.2147/IJN.S217923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang X, Gao J, Ouyang X, et al. Mesenchymal stem cells loaded with paclitaxel-poly(lactic-co-glycolic acid) nanoparticles for glioma-targeting therapy. Int J Nanomedicine. 2018;13:5231–5248. doi: 10.2147/IJN.S167142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Suryaprakash S, Lao YH, Cho HY, et al. Engineered mesenchymal stem cell/nanomedicine spheroid as an active drug delivery platform for combinational glioblastoma therapy. Nano Lett. 2019;19(3):1701–1705. doi: 10.1021/acs.nanolett.8b04697 [DOI] [PubMed] [Google Scholar]
- 123.Huang W-C, Lin L, Chiang W-H, et al. Tumortropic adipose-derived stem cells carrying smart nanotherapeutics for targeted delivery and dual-modality therapy of orthotopic glioblastoma. J Control Release. 2017;254:119–130. doi: 10.1016/j.jconrel.2017.03.035 [DOI] [PubMed] [Google Scholar]
- 124.McGranahan T, Therkelsen KE, Ahmad S, Nagpal S. Current state of immunotherapy for treatment of glioblastoma. Curr Treat Options Oncol. 2019;20(3):24. doi: 10.1007/s11864-019-0619-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Eagles ME, Nassiri F, Badhiwala JH, et al. Dendritic cell vaccines for high-grade gliomas. Ther Clin Risk Manag. 2018;14:1299–1313. doi: 10.2147/TCRM.S135865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Huang J, Liu F, Liu Z, et al. Immune checkpoint in glioblastoma: promising and challenging. Front Pharmacol. 2017;8:242. doi: 10.3389/fphar.2017.00242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Xue J, Zhao Z, Zhang L, et al. Neutrophil-mediated anticancer drug delivery for suppression of postoperative malignant glioma recurrence. Nat Nanotechnol. 2017;12:692–700. doi: 10.1038/nnano.2017.54 [DOI] [PubMed] [Google Scholar]
- 128.Wu M, Zhang H, Tie C, et al. MR imaging tracking of inflammation-activatable engineered neutrophils for targeted therapy of surgically treated glioma. Nat Commun. 2018;9:4777. doi: 10.1038/s41467-018-07250-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bagley SJ, Desai AS, Linette GP, et al. CAR T-cell therapy for glioblastoma: recent clinical advances and future challenges. Neuro Oncol. 2018;20(11):1429–1438. doi: 10.1093/neuonc/noy032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Migliorini D, Dietrich PY, Stupp R, et al. CAR T-cell therapies in glioblastoma: a first look. Clin Cancer Res. 2017;24(3):535–540. doi: 10.1158/1078-0432.CCR-17-2871 [DOI] [PubMed] [Google Scholar]
- 131.Zhu H, You Y, Shen Z, et al. EGFRvIII-CAR-T cells with PD-1 knockout have improved anti-glioma activity. Pathol Oncol Res. 2020. doi: 10.1007/s12253-019-00759-1 [DOI] [PubMed] [Google Scholar]
- 132.Harris JC, Scully MA, Day ES. Cancer cell membrane-coated nanoparticles for cancer management. Cancers. 2019;11:1836. doi: 10.3390/cancers11121836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jin J, Krishnamachary B, Barnett JD, et al. Human cancer cell membrane-coated biomimetic nanoparticles reduce fibroblast-mediated invasion and metastasis and induce T-cells. ACS Appl Mater Interfaces. 2019;11(8):7850–7861. doi: 10.1021/acsami.8b22309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hay M, Thomas DW, Craighead JL, et al. Clinical development success rates for investigational drugs. Nat Biotechnol. 2014;32(1):40–51. doi: 10.1038/nbt.2786 [DOI] [PubMed] [Google Scholar]
- 135.Musazzi UM, Marini V, Casiraghi A, Minghetti P. Is the European regulatory framework sufficient to assure the safety of citizens using health products containing nanomaterials? Drug Discov Today. 2017;22(6):870–882. doi: 10.1016/j.drudis.2017.01.016 [DOI] [PubMed] [Google Scholar]
- 136.Hafner A, Lovric J, Lakos GP, Pepić I. Nanotherapeutics in the EU: an overview on current state and future directions. Int J Nanomedicine. 2014;9:1005–1023. doi: 10.2147/IJN.S55359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.EMA. Reflection Paper on Nanotechnology-Based Medicinal Products for Human Use; 2006. [Google Scholar]
- 138.Etheridge ML, Campbell SA, Erdman AG, et al. The big picture on nanomedicine: the state of investigational and approved nanomedicine products. Nanomedicine. 2013;9(1):1–14. doi: 10.1016/j.nano.2012.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Battaglia L, Ugazio E. Lipid nano- and microparticles: an overview of patent-related research. J Nanomater. 2019;2834941. doi: 10.1155/2019/2834941 [DOI] [Google Scholar]
- 140.Linsinger TP, Chaudhry Q, Dehalu V, et al. Validation of methods for the detection and quantification of engineered nanoparticles in food. Food Chem. 2013;138(2–3):1959–1966. doi: 10.1016/j.foodchem.2012.11.074 [DOI] [PubMed] [Google Scholar]
- 141.Corner J, Owen A, Kwade A, Van de Voorde M. Scale‐up and cGMP manufacturing of nanodrug delivery systems for clinical investigations. Pharm Nanotechnol. 2016. doi: 10.1002/9783527800681.ch12 [DOI] [Google Scholar]
- 142.Drappatz J, Brenner A, Wong ET, et al. Phase I study of GRN1005 in recurrent malignant glioma. Clin Cancer Res. 2013;19:1567–1576. doi: 10.1158/1078-0432 [DOI] [PubMed] [Google Scholar]
- 143.Owonikoko TK, Arbiser J, Zelnak A, et al. Current approaches to the treatment of metastatic brain tumours. Nat Rev Clin Oncol. 2014;11:203–222. doi: 10.1038/nrclinonc.2014.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Weaver M, Laske DW. Transferrin receptor ligand-targeted toxin conjugate (Tf-CRM107) for therapy of malignant gliomas. J Neurooncol. 2003;65:3–13. doi: 10.1023/a:1026246500788 [DOI] [PubMed] [Google Scholar]
- 145.Whittle JR, Lickliter JD, Gan HK, et al. First in human nanotechnology doxorubicin delivery system to target epidermal growth factor receptors in recurrent glioblastoma. J Clin Neurosci. 2015;22:1889‑1894. doi: 10.1016/j.jocn.2015.06.005 [DOI] [PubMed] [Google Scholar]
- 146.Fabel K, Dietrich J, Hau P, et al. Long-term stabilization in patients with malignant glioma after treatment with liposomal doxorubicin. Cancer. 2001;92:1936–1942. doi: [DOI] [PubMed] [Google Scholar]
- 147.Hau P, Fabel K, Baumgart U, et al. Pegylated liposomal doxorubicin-efficacy in patients with recurrent high-grade glioma. Cancer. 2004;100:1199–1207. doi: 10.1002/cncr.20073 [DOI] [PubMed] [Google Scholar]
- 148.Glas M, Koch H, Hirschmann B, et al. Pegylated liposomal doxorubicin in recurrent malignant glioma: analysis of a case series. Oncology. 2008;72(5–6):302–307. doi: 10.1159/000113052 [DOI] [PubMed] [Google Scholar]
- 149.Huncharek M, Kupelnick B, Bishop D. Platinum analogues in the treatment of recurrent high grade astrocytoma. Cancer Treat Rev. 1998;24:307–316. doi: 10.1016/s0305-7372(98)90054-8 [DOI] [PubMed] [Google Scholar]
- 150.Stewart LA. Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet. 2002;359:1011–1118. doi: 10.1016/s0140-6736(02)08091-1 [DOI] [PubMed] [Google Scholar]
- 151.Batchelor TT, Sorensen AG, Di Tomaso E, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11(1):83–95. doi: 10.1016/j.ccr.2006.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kamoun WS, Ley CD, Farrar CT, et al. Edema control by cediranib, a vascular endothelial growth factor receptor-targeted kinase inhibitor, prolongs survival despite persistent brain tumor growth in mice. J Clin Oncol. 2009;27(15):2542–2552. doi: 10.1200/JCO.2008.19.9356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Stefanik DF, Fellows WK, Rizkalla LR, et al. Monoclonal antibodies to vascular endothelial growth factor (VEGF) and the VEGF receptor, FLT-1, inhibit the growth of C6 glioma in a mouse xenograft. J Neurooncol. 2001;55(2):91–100. doi: 10.1023/a:1013329832067 [DOI] [PubMed] [Google Scholar]
- 154.Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2008;27(5):740–745. doi: 10.1200/JCO.2008.16.3055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Prados MD, Chang SM, Butowski N, et al. Phase II study of erlotinib plus temozolomide during and after radiation therapy in patients with newly diagnosed glioblastoma multiforme or gliosarcoma. J Clin Oncol. 2009;27(4):579–584. doi: 10.1200/JCO.2008.18.9639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Eller JL, Longo SL, Kyle MM, Bassano D, Hicklin DJ, Canute GW. Anti-epidermal growth factor receptor monoclonal antibody cetuximab augments radiation effects in glioblastoma multiforme in vitro and in vivo. Neurosurgery. 2005;56(1):155–162. doi: 10.1227/01.neu.0000145865.25689.55 [DOI] [PubMed] [Google Scholar]
- 157.Belda-Iniesta C, JdC C, Saenz EC, Gutiérrez M, Perona R, Barón MG. Long term responses with cetuximab therapy in glioblastoma multiforme. Cancer Biol Ther. 2006;5(8):912–914. doi: 10.4161/cbt.5.8.3118 [DOI] [PubMed] [Google Scholar]
- 158.Neyns B, Sadones J, Joosens E, et al. Stratified phase II trial of cetuximab in patients with recurrent high-grade glioma. Ann Oncol. 2009;20(9):1596–1603. doi: 10.1093/annonc/mdp032 [DOI] [PubMed] [Google Scholar]
- 159.Holdhoff M, Kreuzer KA, Appelt C, et al. Imatinib mesylate radiosensitizes human glioblastoma cells through inhibition of platelet-derived growth factor receptor. Blood Cells Mol Dis. 2005;34(2):181–185. doi: 10.1016/j.bcmd.2004.11.006 [DOI] [PubMed] [Google Scholar]
- 160.Cloughesy TF, Kuhn J, Robins HI, et al. Phase I trial of tipifarnib in patients with recurrent malignant glioma taking enzyme-inducing antiepileptic drugs: a North American Brain Tumor Consortium Study. J Clin Oncol. 2005;23(27):6647–6656. doi: 10.1200/JCO.2005.10.068 [DOI] [PubMed] [Google Scholar]
- 161.Fine HA, Kim L, Royce C, et al. Results from phase II trial of enzastaurin (LY317615) in patients with recurrent high grade gliomas. J Clin Oncol. 2005;23(16_suppl):1504. doi: 10.1200/jco.2005.23.16suppl.1504 [DOI] [Google Scholar]
- 162.Kreisl TN, Kim L, Moore K, et al. A phase I trial of enzastaurin in patients with recurrent gliomas. Clin Cancer Res. 2009;15(10):3617–3623. doi: 10.1093/neuonc/nop042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chang SM, Wen P, Cloughesy T, et al. Phase II study of CCI-779 in patients with recurrent glioblastoma multiforme. Invest New Drugs. 2005;23(4):357–361. doi: 10.1007/s10637-005-1444-0 [DOI] [PubMed] [Google Scholar]
- 164.Kane RC, Farrell AT, Saber H, et al. Sorafenib for the treatment of advanced renal cell carcinoma. Clin Cancer Res. 2006;12(24):7271–7278. doi: 10.1158/1078-0432.CCR-06-1249 [DOI] [PubMed] [Google Scholar]
- 165.Levin VA, Phuphanich S, Yung WKA, et al. Randomized, double-blind, placebo-controlled trial of marimastat in glioblastoma multiforme patients following surgery and irradiation. J Neurooncol. 2006;78(3):295–302. doi: 10.1007/s11060-005-9098-5 [DOI] [PubMed] [Google Scholar]
- 166.Reardon DA, Fink KL, Mikkelsen T, et al. Randomized Phase II study of cilengitide, an integrin-targeting arginine-glycine-aspartic acid peptide, in recurrent glioblastoma multiforme. J Clin Oncol. 2008;26(34):5610–5617. doi: 10.1200/JCO.2008.16.7510 [DOI] [PubMed] [Google Scholar]
- 167.Charest G, Sanche L, Fortin D, Mathieu D, Paquette B. Glioblastoma treatment: bypassing the toxicity of platinum compounds by using liposomal formulation and increasing treatment efficiency with concomitant radiotherapy. Int J Radiat Oncol Biol Phys. 2012;84:244–249. doi: 10.1016/j.ijrobp.2011.10.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Qu J, Zhang L, Chen Z, et al. Nanostructured lipid carriers, solid lipid nanoparticles, and polymeric nanoparticles: which kind of drug delivery system is better for glioblastoma chemotherapy? Drug Deliv. 2016;23:3408–3416. doi: 10.1080/10717544.2016.1189465 [DOI] [PubMed] [Google Scholar]
- 169.Shatsberg Z, Zhang X, Ofek P, et al. Functionalized nanogels carrying an anticancer microRNA for glioblastoma therapy. J Control Release. 2016;239:159–168. doi: 10.1016/j.jconrel.2016.08.029 [DOI] [PubMed] [Google Scholar]
- 170.Ambruosi A, Khalansky AS, Yamamoto H, Gelperina SE, Begley DJ, Kreuter J. Biodistribution of polysorbate 80-coated doxorubicin-loaded 14C]-poly(butyl cyanoacrylate) nanoparticles after intravenous administration to glioblastoma-bearing rats. J Drug Target. 2006;14(2):97–105. doi: 10.1080/10611860600636135 [DOI] [PubMed] [Google Scholar]
- 171.Tian XH, Lin XN, Wei F, et al. Enhanced brain targeting of temozolomide in polysorbate-80 coated polybutylcyanoacrylate nanoparticles. Int J Nanomedicine. 2011;6:445–452. doi: 10.2147/ijn.s16570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wang CX, Huang LS, Hou LB, et al. Antitumor effects of polysorbate-80 coated gemcitabine polybutylcyanoacrylate nanoparticles in vitro and its pharmacodynamics in vivo on C6 glioma cells of a brain tumor model. Brain Res. 2009;1261:91–99. doi: 10.1016/j.brainres.2009.01.011 [DOI] [PubMed] [Google Scholar]
- 173.Jain D, Bajaj A, Athawale R, et al. Surface-coated PLA nanoparticles loaded with temozolomide for improved brain deposition and potential treatment of gliomas: development, characterization and in vivo studies. Drug Deliv. 2016;23(3):999–1016. doi: 10.3109/10717544.2014.926574 [DOI] [PubMed] [Google Scholar]
- 174.Xu HL, Mao KL, Huang YP, et al. Glioma-targeted superparamagnetic iron oxide nanoparticles as drug-carrying vehicles for theranostic effects. Nanoscale. 2016;8(29):14222–14236. doi: 10.1039/c6nr02448c [DOI] [PubMed] [Google Scholar]
- 175.Fundarò A, Cavalli R, Bargoni A, et al. Non-stealth and stealth solid lipid nanoparticles (SLN) carrying doxorubicin: pharmacokinetics and tissue distribution after i.v. administration to rats. Pharmacol Res. 2000;42(4):337–343. [DOI] [PubMed] [Google Scholar]
- 176.Zara GP, Cavalli R, Bargoni A, et al. Intravenous administration to rabbits of non-stealth and stealth doxorubicin-loaded solid lipid nanoparticles at increasing concentrations of stealth agent: pharmacokinetics and distribution of doxorubicin in brain and other tissues. J Drug Target. 2002;10(4):327–335. doi: 10.1080/10611860290031868 [DOI] [PubMed] [Google Scholar]
- 177.Zong Z, Hua L, Wang Z, et al. Self-assembled angiopep-2 modified lipid-poly (hypoxic radiosensitized polyprodrug) nanoparticles delivery TMZ for glioma synergistic TMZ and RT therapy. Drug Deliv. 2019;26(1):34–44. doi: 10.1080/10717544.2018.1534897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lu F, Pang Z, Zhao J, et al. Poly (ε-Caprolactone) polymersomes for dual-targeting drug delivery to glioma in rats. Int J Nanomedicine. 2017;12:2117–2127. doi: 10.2147/IJN.S123422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wang X, Xiong Z, Liu Z, Huang X, Jiang X. Angiopep-2/IP10-EGFRvIIIscFv modified nanoparticles and CTL synergistically inhibit malignant glioblastoma. Sci Rep. 2018;8(1):1–11. doi: 10.1038/s41598-018-30072-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Huwyler J, Wu D, Pardridge WM. Brain drug delivery of small molecules using immunoliposomes. Proc Natl Acad Sci USA. 1996;93:14164–14169. doi: 10.1073/pnas.93.24.14164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Huang JL, Jiang G, Song QX, et al. Lipoprotein-biomimetic nanostructure enables efficient targeting delivery of siRNA to Ras-activated glioblastoma cells via macropinocytosis. Nat Commun. 2017;8:1–18. doi: 10.1038/ncomms15144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Jiang Y, Zhang J, Meng F, Zhong Z. Apolipoprotein e peptide-directed chimeric polymersomes mediate an ultrahigh-efficiency targeted protein therapy for glioblastoma. ACS Nano. 2018;12(11):11070–11079. doi: 10.1021/acsnano.8b05265 [DOI] [PubMed] [Google Scholar]
- 183.Rajora MA, Ding L, Valic M, et al. Tailored theranostic apolipoprotein E3 porphyrin-lipid nanoparticles target glioblastoma. Chem Sci. 2017;8(8):5371–5384. doi: 10.1039/c7sc00732a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Liu X, Cui W, Li B, Hong Z. Targeted therapy for glioma using cyclic RGD-entrapped polyionic complex nanomicelles. Int J Nanomedicine. 2012;7:2853. doi: 10.2147/IJN.S29788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Miura Y, Takenaka T, Toh K, et al. Cyclic RGD-linked polymeric micelles for targeted delivery of platinum anticancer drugs to glioblastoma through the blood-brain tumor barrier. ACS Nano. 2013;7:8583–8592. doi: 10.1021/nn402662d [DOI] [PubMed] [Google Scholar]
- 186.Mao J, Ran D, Xie C, Shen Q, Wang S, Lu W. EGFR/EGFRvIII dual-targeting peptide-mediated drug delivery for enhanced glioma therapy. ACS Appl Mater Interfaces. 2017;9(29):24462–24475. doi: 10.1021/acsami.7b05617 [DOI] [PubMed] [Google Scholar]
- 187.Madhankumar AB, Slagle-Webb B, Mintz A, Sheehan JM, Connor JR. Interleukin-13 receptor-targeted nanovesicles are a potential therapy for glioblastoma multiforme. Mol Cancer Ther. 2006;5:3162. doi: 10.1158/1535-7163.MCT-06-0480 [DOI] [PubMed] [Google Scholar]
- 188.Su Z, Xing L, Chen Y, et al. Lactoferrin-modified poly(ethylene glycol)-grafted BSA nanoparticles as a dual-targeting carrier for treating brain gliomas. Mol Pharm. 2014;11(6):1823–1834. doi: 10.1021/mp500238m [DOI] [PubMed] [Google Scholar]
- 189.Huang FYJ, Chen WJ, Lee WY, Lo ST, Lee TW, Lo JM. In vitro and in vivo evaluation of lactoferrin-conjugated liposomes as a novel carrier to improve the brain delivery. Int J Mol Sci. 2013;14(2):2862–2874. doi: 10.3390/ijms14022862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kumari S, Ahsan SM, Kumar JM, Kondapi AK, Rao NM. Overcoming blood brain barrier with a dual purpose Temozolomide loaded Lactoferrin nanoparticles for combating glioma (SERP-17-12433). Sci Rep. 2017;7(1):1–13. doi: 10.1038/s41598-017-06888-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kumari S, Bhattacharya D, Rangaraj N, Chakarvarty S, Kondapi AK, Rao NM. Aurora kinase B siRNA-loaded lactoferrin nanoparticles potentiate the efficacy of temozolomide in treating glioblastoma. Nanomedicine. 2018;13(20):2579–2596. doi: 10.2217/nnm-2018-0110 [DOI] [PubMed] [Google Scholar]
- 192.Pang Z, Feng L, Hua R, et al. Lactoferrin-conjugated biodegradable polymersome holding doxorubicin and tetrandrine for chemotherapy of glioma rats. Mol Pharm. 2010;7(6):1995–2005. doi: 10.1021/mp100277h [DOI] [PubMed] [Google Scholar]
- 193.Zhang J, Xiao X, Zhu J, et al. Lactoferrin- and RGD-comodified, temozolomide and vincristine-coloaded nanostructured lipid carriers for gliomatosis cerebri combination therapy. Int J Nanomedicine. 2018;13:3039–3051. doi: 10.2147/IJN.S161163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zhang Y, Zhai M, Chen Z, et al. Dual-modified liposome codelivery of doxorubicin and vincristine improve targeting and therapeutic efficacy of glioma. Drug Deliv. 2017;24(1):1045–1055. doi: 10.1080/10717544.2017.1344334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Soni V, Kohli D, Jain S. Transferrin coupled liposomes as drug delivery carriers for brain targeting of 5-fluorouracil. J Drug Target. 2005;13:245–250. doi: 10.1080/10611860500107401 [DOI] [PubMed] [Google Scholar]
- 196.Lam FC, Morton SW, Wyckoff J, et al. Enhanced efficacy of combined temozolomide and bromodomain inhibitor therapy for gliomas using targeted nanoparticles. Nat Commun. 2018;9(1):1991. doi: 10.1038/s41467-018-04315-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Wang X, Zhao Y, Dong S, et al. Cell-penetrating peptide and transferrin co-modified liposomes for targeted therapy of glioma. Molecules. 2019;24:3540. doi: 10.3390/molecules24193540 [DOI] [PMC free article] [PubMed] [Google Scholar]