Abstract
Background
Tuberculosis (TB) remains the leading cause of infectious disease-related death. Recently, a trial of BCG revaccination and vaccination with H4:IC31, a recombinant protein vaccine, in South African adolescents (Aeras C-040-404) showed efficacy in preventing sustained QuantiFERON (QFT) conversion, a proxy for Mycobacterium tuberculosis (M.tb) infection. A phase 1b trial of 84 South African adolescents was conducted, concurrent with Aeras C-040-404, to assess the safety and immunogenicity of H4:IC31, H56:IC31 and BCG revaccination, and to identify and optimize immune assays for identification of candidate correlates of protection in efficacy trials.
Methods
Two doses of H4:IC31 and H56:IC31 vaccines were administered intramuscularly (IM) 56 days apart, and a single dose of BCG (2–8 × 105 CFU) was administered intradermally (ID). T-cell and antibody responses were measured using intracellular cytokine staining and binding antibody assays, respectively. Binding antibodies and CD4+/CD8+ T-cell responses to H4- and H56-matched antigens were measured in samples from all participants. The study was designed to characterize safety and immunogenicity and was not powered for group comparisons. (Clinicaltrials.gov NCT02378207).
Findings
In total, 481 adolescents (mean age 13·9 years) were screened; 84 were enrolled (54% female). The vaccines were generally safe and well-tolerated, with no reported severe adverse events related to the study vaccines. H4:IC31 and H56:IC31 elicited CD4+ T cells recognizing vaccine-matched antigens and H4- and H56-specific IgG binding antibodies. The highest vaccine-induced CD4+ T-cell response rates were for those recognizing Ag85B in the H4:IC31 and H56:IC31 vaccinated groups. BCG revaccination elicited robust, polyfunctional BCG-specific CD4+ T cells, with no increase in H4- or H56-specific IgG binding antibodies. There were few antigen-specific CD8+ T-cell responses detected in any group.
Interpretation
BCG revaccination administered as a single dose ID and both H4:IC31 and H56:IC31 administered as 2 doses IM had acceptable safety profiles in healthy, QFT-negative, previously BCG-vaccinated adolescents. Characterization of the assays and the immunogenicity of these vaccines may help to identify valuable markers of protection for upcoming immune correlates analyses of C-040-404 and future TB vaccine efficacy trials.
Funding
NIAID and Aeras.
Keywords: Mycobacterium tuberculosis, H4:IC31, H56:IC31, BCG
Research in context.
Evidence before this study
The Aeras C-040-404 study (NCT02075203) assessed the efficacy of H4:IC31 and BCG revaccination in preventing initial or sustained QuantiFERON (QFT) conversion as a marker of initial or sustained Mycobacterium tuberculosis (M.tb) infection, respectively. The H56:IC31 vaccine was planned for a similar prevention of infection efficacy trial but was withdrawn (NCT03265977). Each of these vaccines had been studied in several phase 1 or 2 safety and immunogenicity trials, but no direct comparisons of these vaccines in a single trial had been conducted.
Added value of this study
This study provided a unique opportunity to compare immune responses elicited by H4:IC31 and H56:IC31 to BCG and to evaluate and optimize immunogenicity assays for use in future correlates analyses.
Implications of all the available evidence
BCG revaccination administered as a single dose intradermally and each of H4:IC31 and H56:IC31 administered as 2 doses intramuscularly were associated with acceptable safety profiles in healthy, QFT-negative, previously BCG-vaccinated adolescents. BCG revaccination, H4:IC31, and H56:IC31 all induced CD4+ T-cell responses measurable by intracellular cytokine staining, with H4:IC31 and H56:IC31 inducing serum IgG to their matched antigens as measured by ELISA. These immunological methods using archived specimens may be valuable for identifying correlates of protection in current and future M.tb vaccine trials where evidence of efficacy warrants correlates of protection analyses.
Alt-text: Unlabelled box
1. Introduction
Tuberculosis (TB) remains the leading cause of infectious disease-related death due to a single pathogen worldwide, with the highest burden in low- to middle-income countries. An effective vaccine is crucial for controlling and ultimately eliminating TB as a global public health problem [1]. To date, the bacillus Calmette-Guérin (BCG) vaccine is the only available TB vaccine and provides only partial and variable protection against Mycobacterium tuberculosis (M.tb) [2], [3], [4].
Aeras C-040-404, a phase 2 Prevention of Infection (POI) trial of H4:IC31 and BCG regimens, was conducted among HIV-uninfected, BCG-vaccinated healthy adolescents in high tuberculosis transmission settings and showed that the rate of sustained QuantiFERON-TB Gold In-tube (QFT-GIT) conversion, a secondary endpoint thought to be a marker of sustained M.tb infection, could be reduced by vaccination [5]. Specifically, BCG revaccination reduced the rate of sustained QFT-GIT conversion with an efficacy of 45·4% (p = 0·03); the primary endpoint, QFT-GIT conversion, was not significantly reduced by BCG revaccination (VE = 20%, p = 0·14). The efficacy against sustained QFT-GIT conversion of the H4:IC31 vaccine was 30·5% but was not statistically significant (p = 0·16). This was the first evidence that sustained M.tb infection could be prevented by vaccination, assuming that sustained QFT conversion is a surrogate of sustained M.tb infection, even in a high-transmission setting such as Cape Town, South Africa [5].
The lack of known correlates of protection is a major impediment for the rational and expeditious development of TB vaccines. A study to identify immunological correlates of protection in the POI trial is underway; however, sample volumes and resources are limited, making the prioritization of assays and vaccine response biomarkers essential. For example, with limited peripheral blood mononuclear cells (PBMC) for intracellular cytokine staining, one might select only a limited subset of antigens for use in the case-control immune correlates analysis. For this reason, conducting pilot studies in parallel to efficacy trials can provide opportunities to characterize vaccine responses and optimize immunogenicity assays that will facilitate correlates analyses . To this end, we conducted a concurrent phase 1b trial, known as HVTN 602/Aeras A-042, to characterize the immunogenicity of H4:IC31 and BCG revaccination, in addition to a third vaccine candidate, H56:IC31. The objective was to help identify candidate vaccine response biomarkers and to optimize immune assays that could be evaluated as correlates of protection against M.tb infection in the completed C-040-404 efficacy trial and anticipated efficacy trial of H56:IC31. HVTN 602/Aeras A-042 was conducted in QFT-GIT-negative, HIV-uninfected, healthy adolescents (aged 12–17 years) in Cape Town who had been BCG vaccinated at birth – mirroring the C-040-404 population – to investigate safety, tolerability and primary immunogenicity.
2. Methods
2.1. Study setting and participant characteristics
The HVTN 602/Aeras A-042 study was conducted at the Emavundleni Clinical Research Site (CRS) in Crossroads, Cape Town, South Africa. This CRS is situated in a high TB transmission area, where rates of TB among the general population are >1000/100,000 persons [6], [7], [8]. Adolescents in this trial had received BCG vaccination at birth per the South African vaccination policy, and vaccine coverage is high [9,10]. Deltoid scarification or clinical immunization card verification were used to confirm prior immunization with BCG. The study aimed to enroll 84 QFT-GIT-negative, BCG-vaccinated at birth, healthy, HIV-uninfected volunteers aged 12 to 17 years. Adolescents with previously-treated or current TB, a household TB contact, substance use, or pregnancy were excluded. Adolescents provided written informed assent and legal guardians/parents provided written informed consent.
2.2. Study design
HVTN 602/Aeras A-042 (Clinicaltrials.gov NCT02378207) was a randomized, placebo-controlled, four arm, partially blinded phase 1b clinical trial to evaluate the safety, tolerability and cellular immune responses of BCG revaccination, H4:IC31, and H56:IC31 vaccination. The number of participants per group was sufficient to have a >90% chance of observing at least one serious adverse event if the true rate of serious adverse events was 9%. Secondary objectives included measuring humoral, innate and adaptive immune responses to the vaccines. The study was not designed nor powered to make comparisons between the immune responses to each vaccine or placebo. Participants were randomized to 1 of 3 treatment groups or the control group in a 2:2:2:1 ratio (Table 1). Participants received 2 doses of H4:IC31, H56:IC31, or placebo, or a single dose of BCG and were followed through study day 224 (8 months).
Table 1.
Dosage, injection schedule and group assignment.
| Injection schedule |
|||||
|---|---|---|---|---|---|
| Group | N | Dose | Volume | Day 0 | Day 56 |
| 1 | 24 | 15 μg H4/500 nmol IC31 | 0·5 mL | H4:IC31 | H4:IC31 |
| 2 | 24 | 5 μg H56/500 nmol IC31 | 0·5 mL | H56:IC31 | H56:IC31 |
| 3 | 24 | 2–8 × 105 CFU | 0·1 mL | BCG | – |
| 4 | 12 | Placebo | 0·5 mL | Control | Control |
| Total | 84 | ||||
CFU = colony forming units.
2.3. Vaccines and vaccine administration
H4:IC31 (AERAS-404) is a field-reconstituted vaccine with H4 antigen (Sanofi Pasteur) and IC31® proprietary adjuvant (Valneva, formerly Intercell) supplied in different vials. H56:IC31 is a vaccine with the H56 antigen (Statens Serum Institut; SSI) formulated in IC31® adjuvant. The BCG vaccine (Danish strain) was manufactured and supplied by SSI.
The H4:IC31 and H56:IC31 vaccines each had two components: a recombinant fusion protein of M.tb antigens (antigens Ag85B and TB10.4 in H4; antigens Ag85B, ESAT-6, and Rv2660c in H56); and the adjuvant IC31® (contains KLK peptide and TLR9 agonist ODN1a, synthetic oligonucleotide) that has been demonstrated to augment both cellular and humoral immune responses [11].
Vaccines were administered as follows: Group 1: H4:IC31 (15 μg H4/500 nmol IC31) administered intramuscularly (IM) as 0·5 mL in alternating deltoid at days 0 and 56. Group 2: H56:IC31 (5 μg H56/500 nmol IC31) administered IM as 0·5 mL in alternating deltoid at days 0 and 56. Group 3: BCG (2–8 × 105 CFU) administered intradermally (ID), using the standard Mantoux technique, as 0·1 mL over the upper left deltoid at day 0. Group 4: Placebo; sterile sodium chloride 0·9% for injection administered IM as 0·5 mL in alternating deltoid at days 0 and 56.
2.4. Randomization and blinding
Participants were randomized to 1 of 3 treatment groups or the control group in a 2:2:2:1 ratio. The randomization sequence was computer-generated and provided to the CRS through an interactive web response system (IWRS) developed and managed by Almac (https://www.almacgroup.com/). The randomization schedule was prepared by a statistician who was not involved in the analysis of the study in order to maintain blinding of the study team. The randomization was done in blocks to ensure balance across arms over time. The CRS pharmacist with primary responsibility for dispensing study products was charged with maintaining security of the treatment assignments. This was a partially blinded trial, as H4:IC31, H56:IC31 and placebo were administered in a blinded fashion; given that BCG is administered by a different route and is associated with well-characterized vaccination site reactions, BCG was administered in an unblinded fashion by a staff member not otherwise involved in the study. The day of enrolment for each participant was study day 0, and participants who discontinued from the trial were not replaced.
3. Outcomes
3.1. Safety assessments
Serum chemistry, full blood count with differential, and urinalysis (dipstick) were conducted at screening, seven days after each vaccination, and, except for urinalysis, at study day 168. All adverse and serious adverse events (AE and SAE) reported post-vaccination were collected. Evaluation was performed through 28 days after each study vaccination for unsolicited AEs; seven days after each vaccination for solicited systemic AEs; 28 days after each vaccination for solicited injection site reactions in the placebo, H4:IC31, and H56:IC31 groups; and 84 days after vaccination for solicited injection site reactions in the BCG group. SAEs and AEs of special interest were collected throughout the entire study period. Solicited AEs included injection site reactions of pain, erythema, swelling, and axillary lymphadenopathy; and systemic AEs of pyrexia, myalgia, arthralgia, fatigue, headache, nausea, diarrhea, and chills. Vital signs (blood pressure, pulse, and temperature [axillary or by infrared thermometry]) were measured at every clinic visit (pre-vaccination and at least 30 min post-vaccination on each vaccination day).
3.2. Immunogenicity assessments
Blood samples for longitudinal cellular and humoral immunogenicity assays were collected on study days 0, 70, and 168 for all treatment groups and additionally at study day 14 for the H4:IC31, H56:IC31 and placebo groups; and at study day 28 for the BCG group. Blood samples for the QFT-GIT assay were collected at screening and study days 70, 168, and 224.
3.3. Regulatory and study oversight
The study was approved by the Health Science Human Research Ethics Committee of the University of Cape Town and the Medicines Control Council of South Africa. The study was overseen by a Collaboration Oversight Group (COG), composed of representatives from Aeras, Sanofi Pasteur, SSI, the National Institutes of Health (NIH), and the HIV Vaccine Trials Network (HVTN); members were not involved with conducting the study but responsible for overseeing the collaboration.
3.4. Laboratory assessments
3.4.1. QFT-GIT assay
The QFT-GIT in vitro diagnostic test (Qiagen, Hilden, Germany) was used to assess IFN-γ responses to peptide antigens that represent mycobacterial proteins (ESAT-6, CFP-10 and TB7.7), as a measure of prior exposure to M.tb. The test was run at baseline and repeated at study days 70, 168 and 224. Assay procedures were standardized according to the recommendations in the Aeras C-040-404 trial [12]. Supernatants were run in the QFT-GIT ELISA and positivity was determined as per the manufacturer's instructions (Qiagen, QuantiFERON-TB Gold). Response rates and corresponding 95% confidence intervals were calculated using Wilson's score method [13].
3.4.2. Intracellular cytokine staining (ICS) assay
PBMC were isolated and cryopreserved from whole blood collected in acid citrate dextrose (ACD)-anticoagulant as previously described [14]. T-cell responses to M.tb antigens were measured by ICS using multiparameter flow cytometry as previously described [15,16]. Briefly, cryopreserved PBMC were thawed, incubated overnight and stimulated on day 2 for six hours at 37 °C with either peptide pools (peptides of 15 amino acids overlapping in sequence by 11 amino acids) for the vaccine-matched proteins (Ag85B, ESAT-6, Rv2660c and TB10.4), BCG (Pasteur strain grown from glycerol stocks and provided by Aeras, Rockville, MD), dimethyl sulfoxide (DMSO, 0·5%, Sigma Aldrich, Saint Louis, MO; negative control) or staphylococcal enterotoxin B (SEB, 0·25 µg/mL, Sigma Aldrich; positive control) in the presence of costimulatory antibodies CD28 and CD49d (1 µg/mL, BD Biosciences, San Jose, CA) and brefeldin A (BFA, 10 µg/mL, Sigma Aldrich, Saint Louis, MO). Cells were incubated with ethylenediaminetetraacetic acid (EDTA, 2 mM, Life Technologies) overnight at 4 °C, then stained with a 26-color antibody staining panel (modified version of [17]) and acquired on a BD FACSymphony A5 flow cytometer (BD Biosciences, San Jose, CA), and analyzed using FlowJo version 9·9·6 (BD, Franklin Lakes, NJ). Data would have been excluded from analyses if fewer than 1000 CD4+ and/or CD8+ T cells were counted; however, no samples were excluded based on this criterion.
To assess the response of CD4+ and CD8+ T cells to each ex vivo antigen stimulation, cell frequencies were measured based on their expression of IL-2, IFN-γ and/or TNF-α. The magnitude of the response to an antigen was computed as the fraction of CD4+ or CD8+ T cells expressing ≥2 of the cytokines minus the same fraction measured in the DMSO negative control condition. To assess positivity for an antigen stimulation within the CD4+ or CD8+ T-cell subset, a two-by-two contingency table was constructed to compare the antigen-stimulated and DMSO negative control data. The four entries in each table were the number of cells expressing ≥2 of the cytokines, and the number of cells expressing <2 of the cytokines, for both the stimulated and the negative control data. The probability of response was estimated for each sample using a Bayesian hierarchical mixture model approach (MIMOSA [18]); responses with a posterior probability of response greater than 99·9% were considered positive. The response probability at post-vaccination timepoints did not adjust for responses measured at baseline. Therefore, positive responses measured after vaccination indicate a response to the antigen stimulation but may not reflect an increase in response due to vaccination. Treatment group response rates and the corresponding 95% confidence intervals were calculated by Wilson's score method [13].
Longitudinal comparisons: for comparisons between two timepoints within a group, positive response rates were compared using McNemar's test [19], and response magnitudes were compared using the Wilcoxon signed rank test [20]. Unlike Fisher's exact test and the Wilcoxon rank sum test, the McNemar's and Wilcoxon signed rank tests account for the paired nature of these comparisons (i.e., the repeated measurements on each individual at each timepoint). Longitudinal comparisons were adjusted for multiple comparisons using the method of Holm-Bonferroni to compute FWER-adjusted p-values. Adjustment was performed across antigens, timepoints, and treatment groups, with significance based on FWER-p < 0·05. Adjustment was performed independently for response rate and magnitude comparisons.
Statistical analyses were done using SAS (version 9·4; SAS Institute, Cary, NC, USA) and R statistical software (version 3·3·2; R Foundation for Statistical Computing, Vienna, Austria). Boxplots are used to show the distribution of all available data by group, timepoint or antigen. The mid-line of the box denotes the median and the ends of the box denote the 25th and 75th percentiles. The whiskers that extend from the top and bottom of the box extend to the most extreme data points that are no more than 1·5 times the interquartile range beyond the 25th and 75th percentiles. Descriptive tables of the ICS data and longitudinal comparisons can be found in the Supplemental Materials (Supplemental ICS Tables).
3.4.3. Binding antibody multiplex assay (BAMA)
Total serum IgG and IgG subclass binding antibodies were measured at dilutions of 1:50 (total IgG binding to H4, total IgG, IgG1, IgG2, IgG3, and IgG4 binding to H56) and 1:40 (IgG1, IgG2, IgG3, and IgG4 binding to H4) using a binding antibody multiplex assay [21]. The readout was mean fluorescence intensity (MFI) after background subtraction measured on a Bio-Plex instrument (Bio-Rad, Hercules, CA). Samples from post-enrolment visits were designated as positive responses if they met three conditions: (1) the net-MFI values were ≥ antigen-specific cut-off (based on the 95th percentile of the baseline visit serum samples and at least 100 MFI), (2) the net-MFI values were > 3 times the baseline (day 0) net-MFI values, and (3) the MFI values were > 3 times the baseline MFI values. Response rates and corresponding 95% confidence intervals were calculated by the Wilson score method [13]. Response rates between treatment arms were compared using Fisher's exact test. No formal comparisons of response rates were made between the active treatment groups, as comparing the vaccines to each other was not the aim of the study, and the study was not powered for this comparison. Each group was compared to the placebo group.
3.4.4. Role of the funding source
The study funders, NIAID and Aeras, participated in data collection, data analysis, data interpretation, and writing of the report. The authors had full access to all the data in the study. The decision to submit for publication was joint among all co-authors.
4. Results
4.1. Participant disposition at enrolment
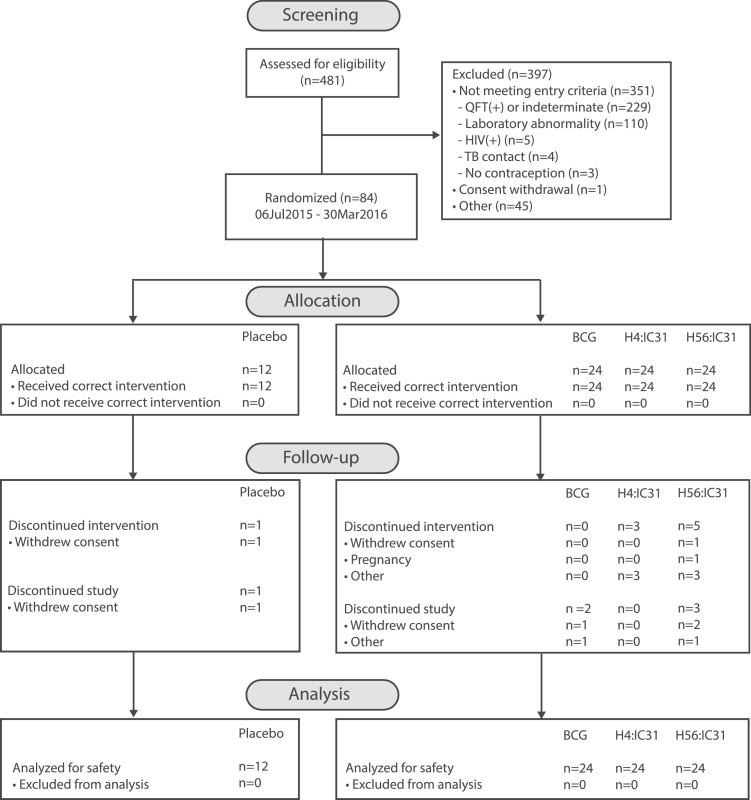
A total of 481 participants were screened (Fig. 1), and of these, 84 were randomized into the study between July 2015 and March 2016. The most common reason for exclusion (229 of the 397 [58%]) was a positive QFT-GIT response. Mean age across the study was 13·9 years and 98·8% of the participants were Black. The treatment groups were comparable for age, race, weight, and height. Participants in the H4:IC31 group were predominantly female (62·5%), while in each of the remaining three groups approximately half the participants were female (Table 2).
Fig. 1.
HVTN 602/AERAS A-042 CONSORT flow diagram.
Table 2.
Baseline characteristics total and by arm.
| Variable | Placebo (n = 12) | BCG (n = 24) | H4:IC31 (n = 24) | H56:IC31 (n = 24) | Total (N = 84) |
|---|---|---|---|---|---|
| Age (years) | |||||
| n | 12 | 24 | 24 | 24 | 84 |
| Mean (SD) | 13·2 (1·1) | 14·5 (1·5) | 13·8 (1·4) | 13·7 (1·5) | 13·9 (1·5) |
| Median | 13·0 | 15·0 | 14·0 | 13·5 | 14·0 |
| Min, Max | 12,15 | 12,17 | 12,17 | 12,17 | 12,17 |
| Sex | |||||
| Male | 6 (50) | 13 (54) | 9 (38) | 11 (46) | 39 (46) |
| Female | 6 (50) | 11 (46) | 15 (63) | 13 (54) | 45 (54) |
| Race | |||||
| Asian | 0 | 0 | 0 | 0 | 0 |
| Black | 12 (100) | 24 (100) | 24 (100) | 23 (96) | 83 (99) |
| White | 0 | 0 | 0 | 0 | 0 |
| Coloreda | 0 | 0 | 0 | 1 (4·2) | 1 (1·2) |
| Other | 0 | 0 | 0 | 0 | 0 |
| Height (cm) | |||||
| n | 12 | 24 | 24 | 24 | 84 |
| Mean (SD) | 156·3 (7·4) | 159·6 (7·4) | 155·7 (9·8) | 157·6 (8·7) | 157·5 (8·5) |
| Median | 154·0 | 160·0 | 155·0 | 157·5 | 158·0 |
| Min, Max | 148,173 | 145,174 | 138,183 | 141,175 | 138,183 |
| Weight (kg) | |||||
| n | 12 | 24 | 24 | 24 | 84 |
| Mean (SD) | 55·9 (13·2) | 57·7 (12·8) | 54·2 (10·2) | 54·5 (9·5) | 55·5 (11·1) |
| Median | 51·0 | 55·5 | 52·0 | 52·5 | 52·5 |
| Min, Max | 44,85 | 40,84 | 41,74 | 42,78 | 40,85 |
Colored refers to persons of multiracial backgrounds within southern Africa, primarily within the western part of South Africa.
Max = maximum; Min = minimum; SD = standard deviation.
All randomized participants received their first vaccination per protocol. One individual in the placebo group, five in the H56:IC31 group and three in the H4:IC31 group did not receive their second vaccination. Of these participants, three in the H56:IC31 group and one in the H4:IC31 group contributed immunogenicity data at day 70 and 163 and one in the H56:IC31and H4:IC31 group contributed data at day 163 only. A total of 78 (93%) participants completed the study (Fig. 1) and 75 (89%) participants completed all study vaccinations. Analyses of safety and immunological data were conducted on all available data, without exclusions for participants that missed a study vaccination.
4.2. Safety
Most AEs were mild or moderate in severity, and no severe AEs deemed related to study vaccine were reported. No AEs resulted in discontinuation of vaccination in any study group. No apparent increased incidence or severity of AEs overall was observed after the second vaccination of H4:IC31 or H56:IC31. AEs reported as related to vaccination are shown in Table 3 and by grade in Supplemental Table 1. The incidence of moderate AEs was higher (≥20 percentage points difference) in the H56:IC31 group vs. placebo after the first vaccination, largely due to an increased incidence of moderate injection site pain and headache. One normal term pregnancy occurred in a young woman in the H4:IC31 arm. Mild to moderate injection site induration and ulcer formation were most commonly seen with BCG revaccination, and these events resolved without sequelae.
Table 3.
Adverse events by vaccination group.
| Preferred term | Placebo (N = 12) n (%) | BCG (N = 24) n (%) | H4:IC31 (N = 24) n (%) | H56:IC31 (N = 24) n (%) |
|---|---|---|---|---|
| Participants with at least 1 AE | 6 (50) | 23 (96) | 17 (71) | 15 (63) |
| Participants with at least 1 solicited AE | 6 (50) | 21 (88) | 17 (71) | 15 (63) |
| Participants with at least 1 unsolicited AE | 2 (17) | 15 (62) | 2 (8) | 1 (4) |
| Fatigue | 1 (8) | 7 (29) | 6 (25) | 7 (29) |
| Headache | 2 (17) | 7 (29) | 6 (25) | 6 (25) |
| Chills | 2 (177) | 2 (8) | 5 (21) | 6 (25) |
| Myalgia | 1 (8) | 4 (17) | 2 (8) | 5 (21) |
| Nausea | 1 (8) | 2 (8) | 3 (13) | 5 (21) |
| Arthralgia | 1 (8) | 3 (13) | 1 (4) | 3 (13) |
| Diarrhea | 1 (8) | 5 (21) | 1 (4) | 1 (4) |
| Tachycardia | 1 (8) | 0 | 0 | 0 |
| Vomiting | 0 | 1 (4) | 0 | 0 |
| Pyrexia | 1 (8) | 0 | 1 (4) | 2 (8) |
| Abdominal pain | 0 | 2 (8) | 0 | 0 |
| Feeling cold | 0 | 1 (4) | 1 (4) | 0 |
| Injection site scar | 0 | 2 (8) | 0 | 0 |
| Injection site pain | 3 (25) | 14 (58) | 14 (58) | 13 (54) |
| Injection site abscess | 0 | 1 (4) | 0 | 0 |
| Injection site discoloration | 0 | 1 (4) | 0 | 0 |
| Injection site exfoliation | 0 | 1 (4) | 0 | 0 |
| Injection site rash | 0 | 1 (4) | 0 | 0 |
| Injection site scab | 0 | 1 (4) | 0 | 0 |
4.3. M.tb exposure and QFT-GIT conversion
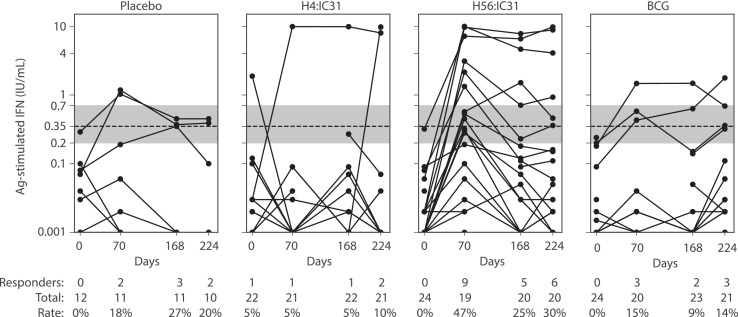
QFT-GIT was used to identify participants who may have been exposed to M.tb while enrolled; exposure was evaluated in all groups and at all visits. Because the H56:IC31 vaccine contains ESAT-6, one of the antigens included in the QFT-GIT stimulation, positive results in the QFT-GIT could either be due to recent exposure or H56:IC31 vaccination. The quantitative readouts and positivity rate within each group at each visit are presented (Fig. 2). One participant (1 of 84) was detected as being QFT-GIT positive at baseline, despite the enrolment requirement of being QFT-negative. In the placebo, H4:IC31, H56:IC31 and BCG groups there were 2/11 (18%), 1/21 (5%), 9/19 (47%) and 3/20 (15%) QFT-GIT conversions by day 70, respectively (Fig. 2). These denominators reflect the number of evaluated samples at each time point by arm, and the percentages represent those with a positive QFT-GIT test result at that time point. Since these occurred within the 84-day “wash out” period that was defined in the Aeras C-040-404 POI study, these would not have been considered conversion endpoints in that study and may indicate M.tb exposure prior to enrolment that was not yet detectable at baseline. This is with the exception of the H56:IC31 group, where the nine QFT-GIT conversions could indicate vaccine-induced responses due to the presence of ESAT-6 in the vaccine and/or to M.tb exposure. By the final visit on day 224, there were 2/10 (20%), 2/21 (10%), 6/20 (30%), and 3/21 (14%) QFT-GIT positive participants in the placebo, H4:IC31, H56:IC31 and BCG groups, respectively. Individual responses can be tracked over the three sampling intervals during the 224-day trial period in Fig. 2. There was one individual in the placebo arm with a QFT reversion between day 168 and 224; there were no occurrences of this in the H4:IC31 arm. Four participants in the H56:IC31 group reverted between day 70 and day 168, with one of these participants having a positive reading again at day 224. One participant in the BCG arm also reverted between day 70 and day 168 and was QFT-GIT positive again at day 224.
Fig. 2.
Background-subtracted readouts from the QFT-GIT. Assay magnitudes (IFN-γ ELISA readout) are plotted on a log-scale, by treatment group and visit. Each participant is represented as a single line with one symbol per visit/sample. A dashed line indicates the manufacturer's recommended positivity threshold (≥0.35 IU/mL). The shaded gray area indicates the “uncertainty” area as defined in (12) in which they showed changes in response magnitude from below the shaded region (<0.2 IU/mL) to a level above the region (>0·7 IU/mL) were more strongly associated with increased risk of TB disease. The post-vaccine responses among participants in the H56:IC31 group may indicate vaccine-induced responses as H56 contains one of the antigens in the QFT-GIT assay.
4.4. T-cell response magnitudes and rates
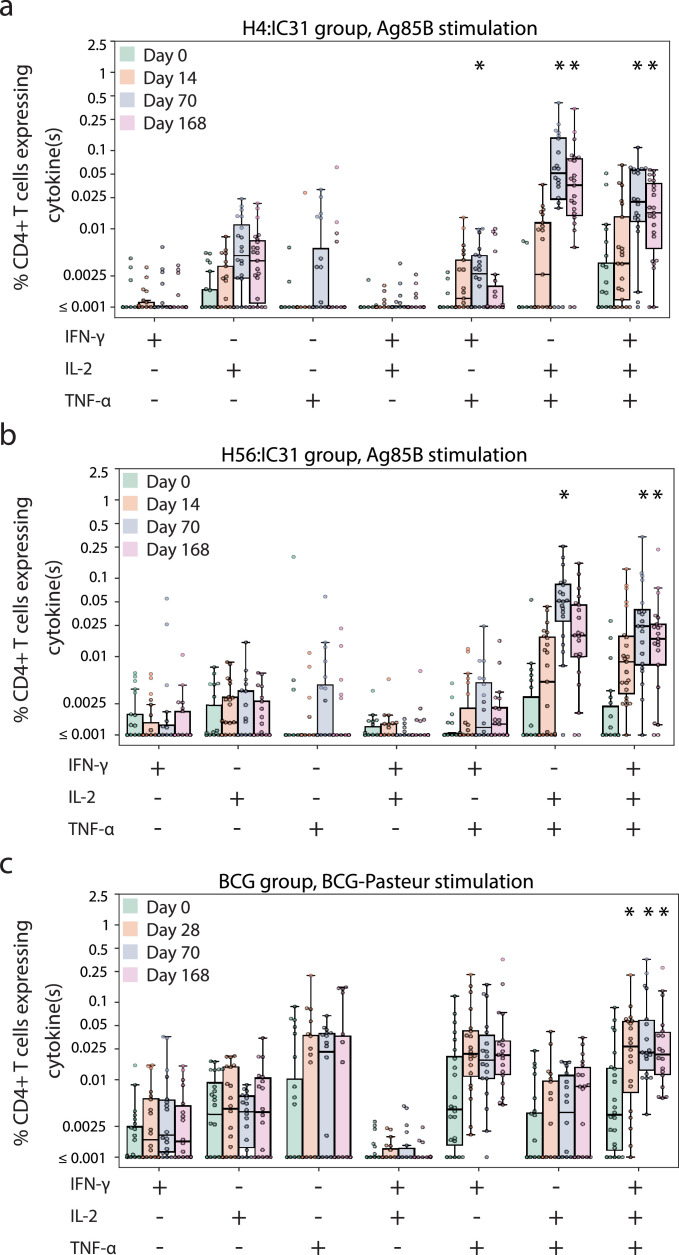
We assessed the frequencies of CD4+ and CD8+ T-cells expressing at least two of three cytokines (IL-2, IFN-γ, TNF-α) to vaccine-matched antigens or to BCG at baseline and at study days 14 (or 28 in the BCG arm), 70 (peak immunogenicity timepoint) and 168 (durability timepoint). T-cell responses were assessed at day 28 in the BCG arm to allow the adaptive responses to BCG, known for slow replication, to more fully develop. All participants provided samples at day 70 (two weeks post the second H4:IC31, H56:IC31 and placebo injections or 10 weeks post BCG revaccination), to allow for a direct comparison across all arms. In addition, we also applied a positivity call method used in our HIV vaccine studies [18] to identify the level of vaccine ‘take’ in participants. Positive responses were determined using the MIMOSA, as described in the methods. We examined T cells recognizing four M.tb peptide pools representing the proteins Ag85B, TB10.4, ESAT-6 and Rv2660c. The protein Ag85B is present in all three vaccines; it is also known to be present in environmental mycobacteria [22]. The protein TB10.4 is present in the H4:IC31 vaccine and BCG, while ESAT-6 and Rv2660c are only present in the H56:IC31 vaccine [23].
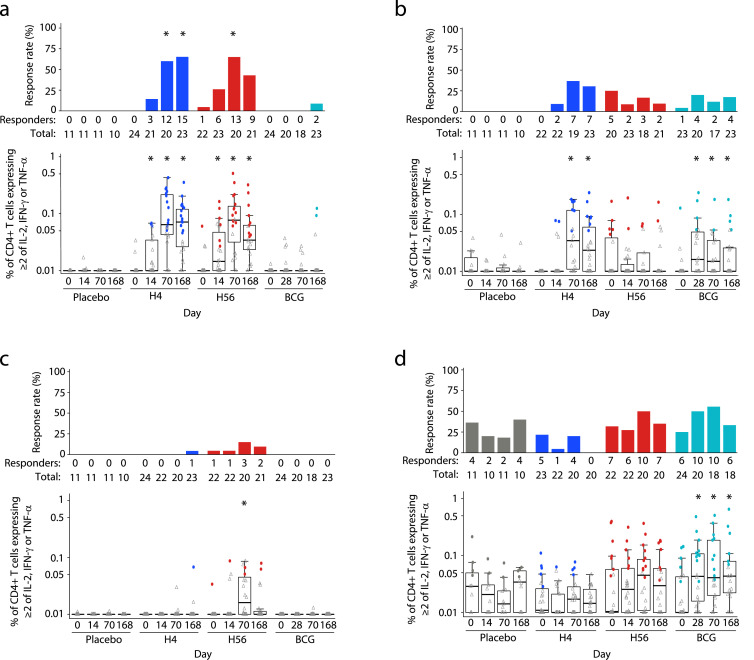
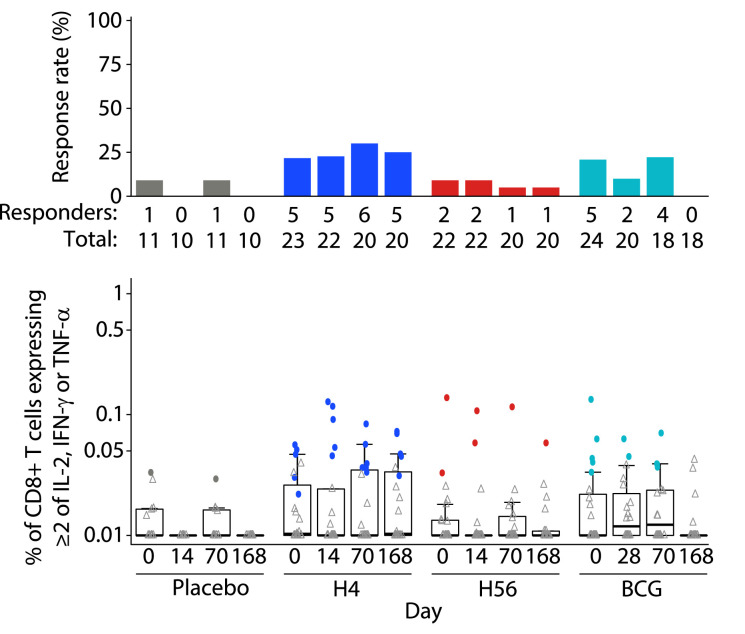
As seen in other clinical trials of these vaccines [5,[24], [25], [26]], vaccine antigen-specific CD4+ T cells were prevalent and few CD8+ T-cell responses were detected. No antigen-specific CD4+ or CD8+ T-cell responses to Ag85B, ESAT-6, Rv2660c or TB10.4 were detected in the placebo group at any timepoint (Figs. 3, 4, Suppl. Figures 1 & 2). CD4+ or CD8+ T-cell responses to the peptide pools at baseline in the vaccine groups were also rare. The H4 group showed no positive responses to any peptide pools at baseline, but the H56 group had one participant who had positive responses to Ag85B, TB10.4 and ESAT-6 as well as four additional participants who showed a baseline positive response to just the TB10.4 peptide pool (Fig. 3A–C). As expected, since all volunteers received BCG vaccine at birth, BCG-specific responses were detectable in the placebo group with the highest response rate in CD4+ T cells of 40% at day 168 (Fig. 3D) and in CD8+ T cells of 9% at days 0 and 70 (Fig. 4). Additionally, all vaccine groups had responses to BCG at baseline; the H56 group had the highest CD4+ T-cell response rates at 32% (Fig. 3D) and the H4 group had the highest CD8+ T-cell response rates at 22% (Fig. 4).
Fig. 3.
Antigen-specific CD4+ T-cell responses after re-stimulation with vaccine-matched peptide pools or BCG. Frequency of CD4+ T cells expressing at least 2 of the cytokines IL-2, TNF-α and IFN-γ in response to (a) Ag85B, (b) TB10.4, (c) ESAT-6 and (d) BCG, at study days 0, 14/28, 70 and 168 after vaccination (day 28 for BCG group and day 14 for all other groups). Respective response rates are indicated above each box plot. Comparisons to baseline response rates were made using McNemar's test (top of each panel) and to baseline magnitudes with the Wilcoxon Signed rank test (bottom of each panel) (* indicates FWER-p < 0·05). The mid-line of the box denotes the median and the ends of the box denote the 25th and 75th percentiles. Whiskers extend to the most extreme data points, no more than 1·5 times the interquartile range.
Fig. 4.
Antigen-specific CD8+ T-cell responses after re-stimulation with BCG. Frequency of CD8+ T cells expressing at least 2 of the cytokines IL-2, TNF-α and IFN-γ in response BCG at study days 0, 14/28, 70 and 168 after vaccination (day 28 for BCG group and day 14 for all other groups). Respective response rates are indicated above each box plot. Comparisons to baseline response rates were made using McNemar's test and to baseline magnitudes with the Wilcoxon Signed rank test; no responses met the significance criteria FWER-p < 0·05. The mid-line of the box denotes the median and the ends of the box denote the 25th and 75th percentiles. Whiskers extend to the most extreme data points, no more than 1·5 times the interquartile range.
H4 vaccination induced CD4+ T-cell responses to both Ag85B and TB10.4 peptide pools, with significant increases in the response magnitudes over baseline seen for Ag85B two weeks after the first dose (day 14) and to both peptide pools after the second dose (day 70; Fig. 3A, B). The response contracted by day 168 but the magnitudes remained elevated relative to baseline. H56 vaccination similarly increased response magnitudes to Ag85B on days 14 and 70 and these responses also contracted at day 168, remaining elevated relative to baseline (Fig. 3A). The only significant increase in the magnitude of the response to ESAT-6 or Rv2660c peptide pools was to ESAT-6 at day 70 for the H56 vaccine (Fig. 3C, Suppl. Fig. 1). Neither H4 nor H56 vaccination induced significant increases in the magnitude of the response to BCG (Fig. 3D).
Vaccination with H4 or H56 induced significant increases in the proportion of positive CD4+ T-cell responders to Ag85B after 2 doses of vaccine at day 70, with H4 achieving a 60% and H56 achieving a 65% response rate (Fig. 3A). The response rate in the H4 group to Ag85B remained significantly higher than baseline of 65% at day 168.
BCG re-vaccination only induced significant increases in the CD4+ T-cell response magnitude to the TB10.4 peptide pool with increases in the magnitude of the response over baseline observed at days 14, 70 and 168 (Fig. 3B). Re-stimulation with BCG also revealed increases in the magnitude of the response at all three timepoints (Fig. 3D) in the BCG group, however no significant increases in the CD4+ T-cell response rate were observed (Fig. 3D). CD8+ T-cell response magnitudes and rates to BCG were not significantly altered post-vaccination with BCG (Fig. 4).
To better understand how the quality of the responses differed by vaccine arm, we then examined the frequency of single, dual or triple cytokine-expressing CD4+ T-cell subsets in the populations of cells that showed changes after vaccination (Fig. 5; Suppl. Fig. 3 shows only the H4:IC31 [n = 3] and H56:IC31 [n = 5] that received the first of two scheduled injections). As shown in Fig. 5A, H4 vaccine-induced responses to Ag85B that were boosted at day 70 post-vaccination consisted primarily of IL-2/TNF-α dual-expressing and polyfunctional triple cytokine-expressing cells. This pattern was also observed in responses to the H56 vaccine (Fig. 5B). In contrast, BCG vaccination only significantly boosted triple cytokine-expressing CD4+ T cells and this subset increased as early as day 28 post-vaccination (Fig. 5C). No CD8+ T-cell subsets showed changes after vaccination (Suppl. Fig. 2).
Fig. 5.
Cytokine expression of stimulated CD4+ T cells. Boxplots show the median and interquartile (IQR) of the percentage of CD4+ T cells expressing the combination of cytokines indicated on the x-axis. The magnitude is background-subtracted. Panels show Ag85B-specific responses among (a) H4:IC31 and (b) H56:IC31 recipients and (c) BCG-specific responses among BCG recipients. Comparisons to baseline were made within each functional subset using a Wilcoxon signed-rank test (*FWER-p < 0·05).
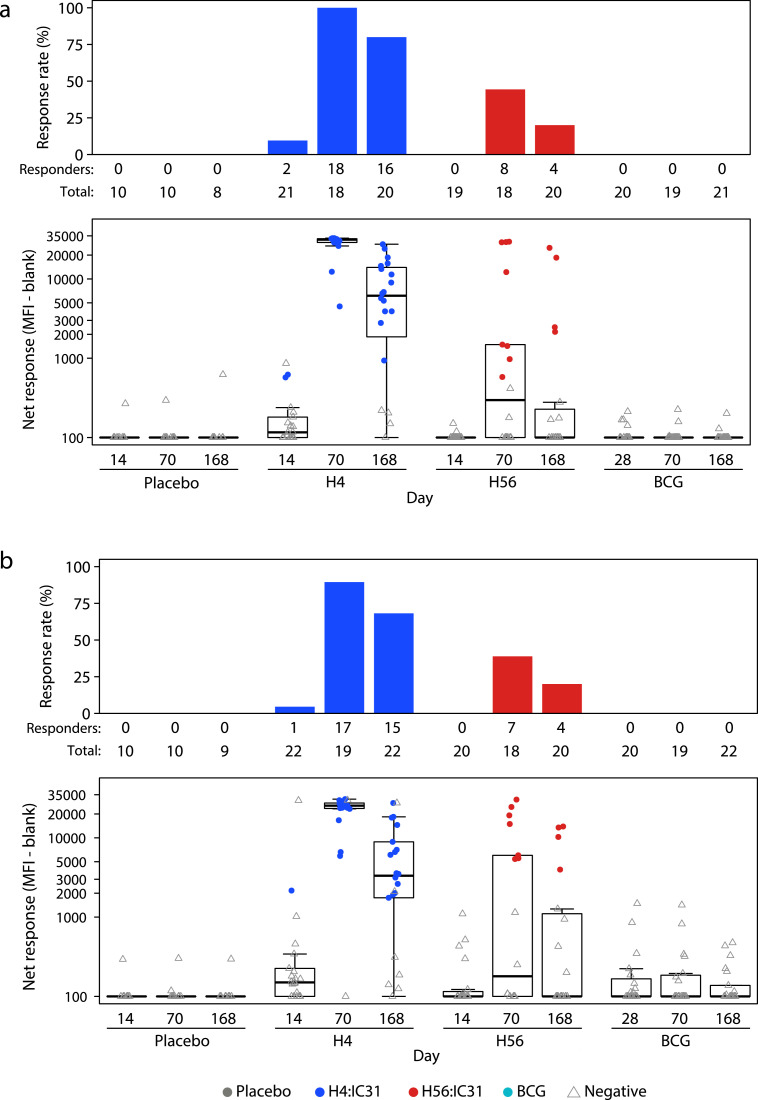
4.5. Vaccine-specific binding antibody responses
Levels of H4- and H56-specific binding antibodies were measured in serum samples provided on days 0, 28, 70, and 168 in the BCG revaccination group and days 0, 14, 70 and 168 in all other groups (Fig. 6). On study day 14 in the H4:IC31 group, there were detectable levels of H4-specific IgG antibodies in 2/21 recipients and H56-specific IgG antibodies in 1/22 recipients; the response rates were significantly higher at day 70 (H4, 18/18; H56, 17/19). Responses were also seen among H56:IC31 recipients, but only at day 70 (H4, 8/18; H56, 7/18). In both the H4:IC31 and H56:IC31 groups, the IgG responses were predominantly comprised of the cytophilic subclasses of IgG1 (Suppl. Fig. 4) and IgG3 (Suppl. Fig. 5). Fewer antigen-specific IgG4 responses (Suppl. Fig. 6) and no substantial IgG2 responses were detected (Suppl. Fig. 7). No responses to H4 and H56 were detected among BCG or placebo recipients.
Fig. 6.
H4- and H56-specific IgG binding antibody. Levels of binding antibody were measured using a binding antibody multiplex assay. Boxplots show the median and IQR of the net response on a log scale. Panels show response rates (upper graphs) and net response magnitude (lower graphs) to the H4 recombinant fusion protein (a) and the H56 recombinant fusion protein (b).
5. Discussion
This study is the first to assess immune responses induced by BCG revaccination, H4:IC31 and H56:IC31 vaccination in QFT-negative adolescents who received BCG vaccination at birth in a single trial. The notable M.tb transmission rate in this setting was indicated by the numerous participants who were ineligible to participate in this study due to QFT-GIT positivity (229 of 481 screened), even among adolescents as young as 12 years. This was also observed in the companion efficacy study and has been previously described in the Cape Town region [5,27,28]. The single positive QFT-GIT result observed in an enrolled participant at baseline may be explained by the short delay between screening and enrolment or fluctuations in QFT positivity, which have been previously observed [29]. These data suggest that in such settings, should a vaccine candidate be successful in preventing M.tb infection, it would best be deployed to children younger than 12 years to ensure maximum impact.
All three vaccinations, including BCG revaccination, were generally safe and well tolerated in this population of adolescents in Cape Town. Most adverse events were mild or moderate in severity, and no severe vaccine-related events were reported. In the mild-to-moderate category, injection site pain was twice as frequent in the active vaccine arms compared with placebo, and fatigue was three times more common. The tolerability profiles of H4:IC31 and H56:IC31 were generally similar. The adverse events seen after BCG revaccination were most commonly those expected after BCG vaccination, including induration and vaccine site abscess, which occurred in one participant with full resolution.
While this trial was not designed to measure efficacy, by the final visit on day 224 there were 3/21 (14%), 2/21 (10%), 6/20 (30%), and 2/10 (20%) QFT-GIT positive participants in the BCG, H4:IC31, H56:IC31, and placebo groups, respectively; at day 70, nearly half of H56:IC31 recipients (9/19, 47%) had positive QFT-GIT results. The greater percentage of QFT-GIT conversions among H56:IC31 recipients is consistent with previous observations that vaccinations with the ESAT-6 antigen result in interferon gamma release assay (IGRA) conversion; an ESAT-6 free IGRA has been developed to monitor M.tb infection following administration of such vaccines [30]. In the C-040-404 efficacy trial, BCG did not prevent primary QFT-GIT conversion but did reduce sustained QFT-GIT conversion by 45·4% [5]; H4:IC31 reduced sustained conversion by 30%.
All three vaccines were immunogenic, and the CD4+ T-cell response profiles differed by antigen specificity. The highest vaccine-induced CD4+ T-cell response rates were for those recognizing Ag85B in both the H4:IC31 and H56:IC31 vaccinated groups, which were statistically different at day 70 in comparison to baseline and placebo group responses. This was consistent with its presence in both the H4 and H56 immunogens and also with previous studies that have documented its immunodominance [26]. Unsurprisingly, the cytokine co-expression frequencies and patterns of the Ag85B-specific responses were also similar between the two arms. Responses to ESAT-6 in the H56:IC31 group and TB10.4 in the H4:IC31 group were also significantly increased by vaccination.
Previous trials with H56:IC31 have demonstrated CD4+ T-cell responses to Rv2660c, and the lack of responses in this study may be attributed to differences in the specimens tested (whole blood versus cryopreserved PBMC), antigen stimulation times, the lower antigen dose (5 µg H56 vs. 15 µg H56), and positivity criteria of the assays that were used [24,25]. There were CD4+ T-cell responses to BCG-Pasteur detected at baseline in all the groups; the response magnitude was significantly boosted at day 28 by BCG revaccination and remained significantly higher than baseline at days 70 and 168. The Ag85B and TB10.4-specific responses induced by H4:IC31 vaccination and the BCG-specific responses induced by BCG revaccination were consistent with those observed in the concurrent efficacy trial. The TNFα-expressing CD4+ T cells contributed to a large proportion of the responding cell subsets in the polyfunctional analyses, and more comprehensive comparative studies of other functional markers will be important in the full assessment of the vaccine-induced responses of these three immunogens.
We present the primary immunogenicity endpoints of multiple vaccines in a single clinical trial. Characterization of the vaccine-induced immune response is the first step toward identifying a correlate of protection; in fact, presence of a significant post-vaccine response is one of the criteria for establishing a Prentice surrogate endpoint [31]. The endpoints measured constitute vaccine response biomarkers that may be associated with prevention of M.tb infection and/or reversion and their characterization will help inform the ongoing design of immune correlates studies nested in the Aeras C-040-404 study. The recent BCG revaccination efficacy of 45·4% reported by Nemes, et al. provides a unique opportunity to investigate immunological correlates of vaccine protection against sustained QFT-GIT conversion [5]. These data may help to identify biomarkers with high reproducibility and robust vaccine-induced changes, two criteria that are necessary – though not sufficient – for establishing an association with protection. The increased reversions that contributed to vaccine efficacy occurred most frequently within months from the initial QFT-GIT conversion, which may be explained by BCG-induced immune clearance of these infections, whether by trained immunity [32] or antigen-specific adaptive responses. The recently reported efficacy of the GSK M72/AS01E vaccine to prevent tuberculosis disease will also provide an opportunity to identify correlates of protection [33]. Together, results of these investigations could shape the future of TB vaccine development.
BCG revaccination administered as a single dose ID (2–8 × 105 CFU) and both H4:IC31 (15 μg H4/500 nmol IC31) and H56:IC31 (5 μg H56/500 nmol IC31) administered as 2 doses IM were associated with acceptable safety profiles in healthy, QFT-GIT-negative, previously BCG-vaccinated adolescents. BCG revaccination, H4:IC31, and H56:IC31 all induced CD4+ T-cell responses with H4:IC31 and H56:IC31 inducing serum IgG. Characterization of these responses may help to identify valuable markers of protection in current and future TB vaccine trials.
6. Data availability
Upon journal acceptance, a copy of the study protocol and the data underlying the findings of this manuscript (participant data de-identified) will be available via the following publicly accessible data portal: https://atlas.scharp.org/cpas/project/HVTN%20Public%20Data/begin.view?
Declaration of Competing Interest
The HVTN 602 clinical trial was funded by the National Institutes of Health (NIH) National Institute of Allergy and Infectious Diseases (NIAID). Within the terms of the Grant Award of the Cooperative Agreement with the HVTN, JH served as the NIAID medical officer of the trial, and contributed to the study design, safety reviews and monitoring, and review of the data and manuscript. JH is a full-time paid employee of NIAID. No pharmaceutical company or other agency paid JH for the contributions to this study or manuscript. LGB reports grants from NIH/NIAID, during the conduct of the study. OD reports rants from NIH/NIAID, during the conduct of the study. AFG reports grants from NIH/NIAID, during the conduct of the study. KM reports grants from NIH/NIAID, during the conduct of the study. AW reports grants from NIH/NIAID, during the conduct of the study. AKR reports grants from NIH/NIAID, during the conduct of the study. IK reports non-financial support from Valneva, during the conduct of the study. PLA has a patent WO2006013612 issued, and a patent WO2010006607 issued. All rights have been assigned to Statens Serum Institut, a Danish non-profit governmental institute. CAD reports being a full-time employee and shareholder for Sanofi Pasteur. KTR reports grants from Bill & Melinda Gates Foundation, other from Sanofi Pasteur, grants from UK DFID, other from NIAID, from Statens Serum Institut, during the conduct of the study; other from GSK, outside the submitted work. DT reports other from Aeras, during the conduct of the study. MDM reports grants from NIH/NIAID, during the conduct of the study. EAN reports grants from NIH/NIAID, during the conduct of the study. SCD reports grants from NIH/NIAID during the conduct of the study. KES reports grants from NIH/NIAID, during the conduct of the study. GDT reports grants from NIH/NIAID, during the conduct of the study. MJM reports grants from NIH/NIAID, during the conduct of the study. AG reports grants from Bill & Melinda Gates Foundation, other from Sanofi Pasteur, grants from UK DFID, other from NIAID and from Statens Serum Institut, during the conduct of the study; other from GSK, outside the submitted work. JGK reports grants from NIH/NIAID, during the conduct of the study. MR has nothing to disclose.
Acknowledgments
We thank the participants and the clinical site staff for their commitment to this study and TB prevention. We thank Dalene de Swardt, Margaret Mazyambe, Zinhle Mgaga, Saleha Omarjee, Shamiska Rohith, Ellen Shrontz, Stephany Wilcox and Nicolette Schuller at CHIL/Fred Hutch for assistance with lab assays and QA oversight of the cellular immunogenicity assays. We thank Kristy Long, Jack Heptinstall, Caroline Brackett, Lu Zhang, David Beaumont, Yong Lin, Sheetal Sawant, Marcella Sarzotti-Kelsoe, and the Duke QAU for assistance with lab assays and Lauren Young, Lisa Bunts, and Sara Thiebaud for assistance with lab data management. We thank Ashley Clayton for assistance with manuscript preparation. This study was funded by Aeras, Sanofi Pasteur, National Institute of Allergy and Infectious Diseases (NIAID, https://www.niaid.nih.gov/) U.S. Public Health Service Grants UM1 AI068614 [LOC: HIV Vaccine Trials Network], UM1 AI068618 [LC: HIV Vaccine Trials Network], and UM1 AI068635 [SDMC: HIV Vaccine Trials Network], UM1 AI069519 [Cape Town – Emavundleni Clinical Research Site], and the NIH/NIAID Duke Center for AIDS Research award P30 AI064518. Aeras funders for this study are the Bill & Melinda Gates Foundation (https://www.gatesfoundation.org/) award OPP1018930, and the United Kingdom Department for International Development Grant 204136-101 (https://www.gov.uk/government/organisations/department-for-international-development). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIAID, the National Institutes of Health (NIH), the Bill & Melinda Gates Foundation, or the United Kingdom Department for International Development. Role of the funding source: The study funders, NIAID and Aeras, participated in data collection, data analysis, data interpretation, and writing of the report. The authors had full access to all the data in the study. The decision to submit for publication was joint among all co-authors.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2020.100313.
Appendix. Supplementary materials
References
- 1.Organization WH . 2014. The end TB strategy. [Google Scholar]
- 2.Colditz G.A., Brewer T.F., Berkey C.S., Wilson M.E., Burdick E., Fineberg H.V. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA. 1994;271(9):698–702. [PubMed] [Google Scholar]
- 3.Roy A., Eisenhut M., Harris R.J., Rodrigues L.C., Sridhar S., Habermann S. Effect of BCG vaccination against Mycobacterium tuberculosis infection in children: systematic review and meta-analysis. BMJ. 2014;349:g4643. doi: 10.1136/bmj.g4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soysal A., Millington K.A., Bakir M., Dosanjh D., Aslan Y., Deeks J.J. Effect of BCG vaccination on risk of Mycobacterium tuberculosis infection in children with household tuberculosis contact: a prospective community-based study. Lancet. 2005;366(9495):1443–1451. doi: 10.1016/S0140-6736(05)67534-4. [DOI] [PubMed] [Google Scholar]
- 5.Nemes E., Geldenhuys H., Rozot V., Rutkowski K.T., Ratangee F., Bilek N. Prevention of M. tuberculosis infection with H4:IC31 vaccine or BCG revaccination. N Engl J Med. 2018;379(2):138–149. doi: 10.1056/NEJMoa1714021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andrews J.R., Hatherill M., Mahomed H., Hanekom W.A., Campo M., Hawn T.R. The dynamics of QuantiFERON-TB gold in-tube conversion and reversion in a cohort of South African adolescents. Am J Respir Crit Care Med. 2015;191(5):584–591. doi: 10.1164/rccm.201409-1704OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahomed H., Hawkridge T., Verver S., Abrahams D., Geiter L., Hatherill M. The tuberculin skin test versus QuantiFERON TB Gold(R) in predicting tuberculosis disease in an adolescent cohort study in South Africa. PLoS ONE. 2011;6(3):e17984. doi: 10.1371/journal.pone.0017984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nanoo A., Izu A., Ismail N.A., Ihekweazu C., Abubakar I., Mametja D. Nationwide and regional incidence of microbiologically confirmed pulmonary tuberculosis in South Africa, 2004-12: a time series analysis. Lancet Infect Dis. 2015;15(9):1066–1076. doi: 10.1016/S1473-3099(15)00147-4. [DOI] [PubMed] [Google Scholar]
- 9.Moore D.P., Schaaf H.S., Nuttall J., Marais B.J. Childhood tuberculosis guidelines of the Southern African Society for Paediatric Infectious Diseases. South Afr J Epidemiol Infect. 2009;24(3):57–68. [Google Scholar]
- 10.WHO, UNICEF . 2018. South Africa: WHO and UNICEF estimates of immunization coverage: 2017 revision. 2018 July 7. [Google Scholar]
- 11.Szabo A., Gogolak P., Pazmandi K., Kis-Toth K., Riedl K., Wizel B. The two-component adjuvant IC31(R) boosts type i interferon production of human monocyte-derived dendritic cells via ligation of endosomal TLRs. PLoS ONE. 2013;8(2):e55264. doi: 10.1371/journal.pone.0055264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nemes E., Rozot V., Geldenhuys H., Bilek N., Mabwe S., Abrahams D. Optimization and interpretation of serial QuantiFERON testing to measure acquisition of Mycobacterium tuberculosis infection. Am J Respir Crit Care Med. 2017;196(5):638–648. doi: 10.1164/rccm.201704-0817OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agresti A., Coull B.A. Approximate is better than "Exact" for interval estimation of binomial proportions. Am Stat. 1998;52(2):119–126. [Google Scholar]
- 14.Bull M., Lee D., Stucky J., Chiu Y.L., Rubin A., Horton H. Defining blood processing parameters for optimal detection of cryopreserved antigen-specific responses for HIV vaccine trials. J Immunol Methods. 2007;322(1–2):57–69. doi: 10.1016/j.jim.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Rosa S.C., Carter D.K., McElrath M.J. OMIP-014: validated multifunctional characterization of antigen-specific human T cells by intracellular cytokine staining. Cytometry A. 2012;81(12):1019–1021. doi: 10.1002/cyto.a.22218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horton H., Thomas E.P., Stucky J.A., Frank I., Moodie Z., Huang Y. Optimization and validation of an 8-color intracellular cytokine staining (ICS) assay to quantify antigen-specific t cells induced by vaccination. J Immunol Methods. 2007;323(1):39–54. doi: 10.1016/j.jim.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dintwe O., Rohith S., Schwedhelm K.V., McElrath M.J., Andersen-Nissen E., De Rosa S.C. OMIP-056: evaluation of human conventional T cells, donor-unrestricted T cells, and NK cells including memory phenotype by intracellular cytokine staining. Cytometry A. 2019;95(7):722–725. doi: 10.1002/cyto.a.23753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Finak G., McDavid A., Chattopadhyay P., Dominguez M., De Rosa S., Roederer M. Mixture models for single-cell assays with applications to vaccine studies. Biostatistics. 2014;15(1):87–101. doi: 10.1093/biostatistics/kxt024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mc N.Q. Note on the sampling error of the difference between correlated proportions or percentages. Psychometrika. 1947;12(2):153–157. doi: 10.1007/BF02295996. [DOI] [PubMed] [Google Scholar]
- 20.Wilcoxon F. Individual comparisons of grouped data by ranking methods. J Econ Entomol. 1946;39:269. doi: 10.1093/jee/39.2.269. [DOI] [PubMed] [Google Scholar]
- 21.Yates N.L., Liao H.X., Fong Y., deCamp A., Vandergrift N.A., Williams W.T. Vaccine-induced ENV V1-V2 IGG3 correlates with lower HIV-1 infection risk and declines soon after vaccination. Sci Transl Med. 2014;6(228) doi: 10.1126/scitranslmed.3007730. 228ra39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drowart A., De Bruyn J., Huygen K., Damiani G., Godfrey H.P., Stelandre M. Isoelectrophoretic characterization of protein antigens present in mycobacterial culture filtrates and recognized by monoclonal antibodies directed against the Mycobacterium bovis BCG antigen 85 complex. Scand J Immunol. 1992;36(5):697–702. doi: 10.1111/j.1365-3083.1992.tb03130.x. [DOI] [PubMed] [Google Scholar]
- 23.Skjot R.L., Brock I., Arend S.M., Munk M.E., Theisen M., Ottenhoff T.H. Epitope mapping of the immunodominant antigen TB10.4 and the two homologous proteins TB10.3 and TB12.9, which constitute a subfamily of the esat-6 gene family. Infect Immun. 2002;70(10):5446–5453. doi: 10.1128/IAI.70.10.5446-5453.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geldenhuys H., Mearns H., Miles D.J., Tameris M., Hokey D., Shi Z. The tuberculosis vaccine H4:IC31 is safe and induces a persistent polyfunctional CD4 T cell response in South African adults: a randomized controlled trial. Vaccine. 2015;33(30):3592–3599. doi: 10.1016/j.vaccine.2015.05.036. [DOI] [PubMed] [Google Scholar]
- 25.Luabeya A.K., Kagina B.M., Tameris M.D., Geldenhuys H., Hoff S.T., Shi Z. First-in-human trial of the post-exposure tuberculosis vaccine H56:IC31 in Mycobacterium tuberculosis infected and non-infected healthy adults. Vaccine. 2015;33(33):4130–4140. doi: 10.1016/j.vaccine.2015.06.051. [DOI] [PubMed] [Google Scholar]
- 26.Norrby M., Vesikari T., Lindqvist L., Maeurer M., Ahmed R., Mahdavifar S. Safety and immunogenicity of the novel H4:IC31 tuberculosis vaccine candidate in BCG-vaccinated adults: two phase I dose escalation trials. Vaccine. 2017;35(12):1652–1661. doi: 10.1016/j.vaccine.2017.01.055. [DOI] [PubMed] [Google Scholar]
- 27.Middelkoop K., Bekker L.G., Liang H., Aquino L.D., Sebastian E., Myer L. Force of tuberculosis infection among adolescents in a high HIV and TB prevalence community: a cross-sectional observation study. BMC Infect Dis. 2011;11:156. doi: 10.1186/1471-2334-11-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahomed H., Hawkridge T., Verver S., Geiter L., Hatherill M., Abrahams D.A. Predictive factors for latent tuberculosis infection among adolescents in a high-burden area in South Africa. Int J Tuberc Lung Dis. 2011;15(3):331–336. [PubMed] [Google Scholar]
- 29.Andrews J.R., Nemes E., Tameris M., Landry B.S., Mahomed H., McClain J.B. Serial QuantiFERON testing and tuberculosis disease risk among young children: an observational cohort study. Lancet Respir Med. 2017;5(4):282–290. doi: 10.1016/S2213-2600(17)30060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruhwald M., de Thurah L., Kuchaka D., Zaher M.R., Salman A.M., Abdel-Ghaffar A.R. Introducing the ESAT-6 free IGRA, a companion diagnostic for TB vaccines based on ESAT-6. Sci Rep. 2017;7:45969. doi: 10.1038/srep45969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prentice R.L. Surrogate endpoints in clinical trials: definition and operational criteria. Stat Med. 1989;8(4):431–440. doi: 10.1002/sim.4780080407. [DOI] [PubMed] [Google Scholar]
- 32.Arts R.J.W., Moorlag S., Novakovic B., Li Y., Wang S.Y., Oosting M. BCG vaccination protects against experimental viral infection in humans through the induction of cytokines associated with trained immunity. Cell Host Microbe. 2018;23(1):89–100. doi: 10.1016/j.chom.2017.12.010. e5. [DOI] [PubMed] [Google Scholar]
- 33.Van Der Meeren O., Hatherill M., Nduba V., Wilkinson R.J., Muyoyeta M., Van Brakel E. Phase 2b controlled trial of M72/AS01E vaccine to prevent tuberculosis. N Engl J Med. 2018;379(17):1621–1634. doi: 10.1056/NEJMoa1803484. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Upon journal acceptance, a copy of the study protocol and the data underlying the findings of this manuscript (participant data de-identified) will be available via the following publicly accessible data portal: https://atlas.scharp.org/cpas/project/HVTN%20Public%20Data/begin.view?