Abstract
Lipid emulsions (LEs), an integral component in parenteral nutrition (PN) feeding, have shifted from the primary aim of delivering non-protein calories and essential fatty acids to defined therapeutic outcomes such as reducing inflammation, and improving metabolic and clinical outcomes. Use of LEs in PN for surgical and critically ill patients is particularly well established, and there is enough literature assigning therapeutic and adverse effects to specific LEs. This narrative review contrarily puts into perspective the fatty acid compositional (FAC) nature of LE formulations, and discusses clinical applications and outcomes according to the biological function and structural functionality of fatty acids and co-factors such as phytosterols, α-tocopherol, emulsifiers and vitamin K. In addition to soybean oil-based LEs, this review covers clinical studies using the alternate LEs that incorporates physical mixtures combining medium- and long-chain triglycerides or structured triglycerides or the unusual olive oil or fish oil. The Jaded score was applied to assess the quality of these studies, and we report outcomes categorized as per immuno-inflammatory, nutritional, clinical, and cellular level FAC changes. It appears that the FAC nature of LEs is the primary determinant of desired clinical outcomes, and we conclude that one type of LE alone cannot be uniformly applied to patient care.
Keywords: fatty acids, lipid emulsions, triglycerides, triacylglycerols, parenteral nutrition
Introduction
Fundamental knowledge in lipid science is well elucidated in terms of the fat molecule’s contribution to total caloric needs, metabolic pathways of energy production, utilization, and storage in the human system. Further, the differentiation of fats based on chemical structure has led to an understanding on metabolic risks associated with atherogenecity in the non-critical status (Karupaiah et al., 2005). On the other hand, benefits of lipid emulsions (LEs) in parenteral therapy has been evolving since the 1960s. I interestingly, although LEs are been administered intravenously in critical care and long-term nutrition support, there is little knowledge regarding the compositional nature and metabolic outcomes related to the nature of lipids in these formulations. Lipids were a late macronutrient addition to parenteral nutrition (PN) formulation in the 1960s, with experimentation leading to formulation of a broad range of intravenous LE products from variable fat sources (Wretlind, 1981).
The first commercially available LE in 1961 was based exclusively on soybean oil, contributing ~ 50% from n-6 linoleic acid (LA) in its total fatty acid (FA) profile. But side effects related to the high LA content added to a higher oxidative stress burden in critically ill patients (Calder et al., 2010) leading to a decision to reduce the LA content in LEs, and opening the way for alternative LEs in parenteral applications (Waitzberg et al., 2006). This development offers new challenges evidenced from emerging clinical studies with aspects relating to inflammation (Calder, 2012; Miles and Calder, 2015), immunomodulation (Waitzberg et al., 2002), and clinical outcomes (Calder, 2013) been reviewed in specific patient populations. However, a collective examination of the functionality of LEs relating to the structure and composition of lipids and their properties to metabolic outcomes has not been related to parenteral therapy in literature. In contrast, core knowledge on metabolic effects of stereospecific positioning of FAs in triglyceride molecules in native and structured fats has been reviewed in the context of atherogenic and metabolic risk (Karupaiah and Sundram, 2007) in healthy humans.
Therefore, this review highlights the composition of FAs in LEs, other components in LEs, biological function of FAs, the metabolic properties of FAs in different LEs, and clinical evidence associated with the use of these LEs in surgery, critically ill, and long-term PN patients.
Fatty Acids
The functionality of the triacylglycerol (TAG) molecules is determined by the type of FA esterified to the glycerol backbone and the stereospecificity of the linkage (Karupaiah and Sundram, 2007). Although FAs share the common structural formula of CH3(CH2)XCOOH, the chain length of carbon atoms linked to the methyl and carboxyl groups varies (Gunstone et al., 2007), with 4 carbon atoms or less termed as short-chain FAs, 6–14 carbon atoms as medium chain, and above 14 carbon atoms classified as long-chain FAs. Again FAs can be reclassified according to the presence or absence of saturation between carbon bonds. The saturated FAs (SFAs) belonging to medium-chain triglycerides (MCTs) feature in some LE formulations. Longer carbon chains with the 22-carbon docosahexaenoic acid (DHA) and the 20-carbon eicosapentaenoic acid (EPA) are also known in LE formulations. Saturation and unsaturation between carbon atoms will determine the functionality and usability of the FAs (Calder, 2004; Kim et al., 2010; Calder, 2012; Calder, 2013; Miles and Calder, 2015). Thus, in discussing FAs, their classification according to carbon chain length, degree of saturation or unsaturation, and the location of double bonds need to be appreciated in the context of LEs.
The melting point of saturated oils used in LEs increase with increasing FAs chain length (Gunstone et al., 2007). Physically MCTs are liquid at room temperature (Ulrich et al., 1996) and have a higher melting point compared to unsaturated FAs. For this reason MCTs are blended with other long-chain FAs such as oleic acid, LA, α-linolenic acid, DHA, and EPA to reduce the melting point to facilitate administration via the intravenous route ( Table 1 ). Contrarily, the presence of double bonds in the carbon chain length lowers the melting point which increases the liquidity of lipid at room temperature (Gunstone et al., 2007) ( Table 1 ). Therefore the unsaturated MUFAs and polyunsaturated fatty acids (PUFAs) benefit lower melting points enabling the liquid state of LEs at room temperature compared to the more saturated MCTs.
Table 1.
Melting point of commonly used fatty acids in lipid emulsions (Gunstone et al., 2007).
| Chain length | Fatty acids | Melting point |
|---|---|---|
| C12:0 | Lauric acid | 44.8°C |
| C18:1 | Oleic acid | 13.4°C |
| C18:2 | Linoleic acid | −5.0°C |
| C18:3 | α-Linolenic acid | −11.0°C |
Other Components in LEs
Other microcomponents in LEs such as phytosterols, α-tocopherol, emulsifiers, and vitamin K further confer functional characteristics. In practice, Vanek et al. (2012) has noted information on these additives may not be declared on the LE product label depending on country availability and local regulation.
Phytosterols
Phytosterols are plant-based and include sitosterol, campesterol, and stigmasterol, sharing a similar structure with cholesterol. In normal metabolism, less than 5% of sterols are absorbed with the rest entering the colon (Ling and Jones, 1995). However in children receiving PN infusion, Clayton et al. (1993) reported occurrence of cholestatic liver disease, likely attributed to the 100% uptake of phytosterols into systemic circulation. In this situation, liver metabolism favors the conversion of sterols to bile salts, which been less soluble than cholesterol, is likely to be precipitated. The accumulation of sterol precipitates within liver cells is the hallmark of cholestasis (Clayton et al., 1993). Sterol precipitation inhibits rate-limiting cholesterol 7α-hydroxylase in the liver affecting bile acid secretion (Boberg et al., 1989). Pianese et al. (2008) reported that accumulation of phytosterols in newborns’ plasma and red blood cell membrane may induce PN-related cholestasis.
Indeed intravenous infusion of phytosterols in animal models, indicate its accumulation in the blood, liver, and bile, causing cholestasis (Iyer et al., 1998). Cholestasis emerged as an issue with long-term use of LEs when incorporating olive oil in infants (Savini et al., 2013) or soybean oil in children (Clayton et al., 1993).
α-Tocopherol
The main form of vitamin E used in LEs is tocopherol occurring as α, β, γ-, or ơ-isoforms (Gurr, 1992). Addition of α-tocopherol into LEs is to prevent peroxidation in susceptible PUFA-rich lipids (Biesalski, 2009) because of unsaturated double-bonds (Pironi et al., 2003).
Wanten et al. (2002) evaluated tocopherol isoform applications, and concluded that long-chain triglycerides (LCTs) in LEs formulated from soybean and safflower oils are predominantly γ-tocopherol, which provide little protection against lipid peroxidation compared to fish oil LE (FOLE) with the highest concentration of α-tocopherol (Wanten et al., 2002). Therefore it is reasonable to increase the α-tocopherol concentration to protect soybean oil LEs against lipid peroxidation. In order to maintain LE stability, addition of α-tocopherol requires a separate administration (Steger and Muhlebach, 1998). Thus, the tocopherol content of LEs is a critical factor in patient administration.
In animal models, some vitamin E isoforms show benefit in prevention of hepatic injury. Ng et al. (2016) reported the use of α-tocopherol in pre-term piglets causing reduction in serum markers of hepatic injury and cholestasis. However, Raphael and Duggan (2012) observed that tocopherol alone was not effective in treatment and prevention of intestinal failure associated liver disease in infants. Diamond et al. (2009) proposed several mechanisms for the reduction of intestinal failure-associated liver disease in children using fish oil-based LE, reduced phytosterol load, increased α-tocopherol and n-3 PUFAs (DHA and EPA) content.
Emulsifiers
LEs consist of two immiscible liquid phases, oil in water. Phospholipids play the role of emulsifying agent to allow the dispersion of fat droplets in the aqueous phase (Driscoll, 2015). Main sources of phospholipid are soybean oil and egg yolk. Lecithin from egg yolk has non-polar (lipophilic) and polar (hydrophilic) properties, which allow the dispersion of the fat droplets in aqueous phase of the emulsion (Ferezou and Bach, 1999; Driscoll, 2015). Fat droplets are prevented from coalescing by an electrostatic barrier generated by the anionic charge of phospholipid polar ends dissociating toward the aqueous phase, enabling stability of LEs (Driscoll, 2015).
With excess emulsifier, liposomes are generated in vesicular form termed as phospholipid-rich particles, forming a bilayer in the aqueous phase of LE. The liposome content is greater in LEs with a lower percentage of oil due to the higher phospholipid:oil ratio (Ferezou et al., 1994; Ferezou and Bach, 1999). The greater liposomes generation potentiates inhibition of lipolysis of artificial chylomicron, and also contributes to abnormal lipoproteins such as Lipoprotein-X. Lipoprotein-X causes hypercholesterolemia, cholesterol accumulation, and increase in free cholesterol:esterified cholesterol plasma ratio in human studies (Ferezou and Bach, 1999). Therefore the extent of liposome metabolism disturbance in plasma depends on the oil concentration in the LE, infusion rate, and duration of infusion (Hultin et al., 1994; Driscoll, 2015). It is noted that liposomes with diameter of <80 nm have no purpose as an energy source (Ferezou and Bach, 1999).
Vitamin K
Phylloquinone or vitamin K1 is important for normal blood clotting, and a deficiency would prolong the clotting time (Gurr, 1992). The dietary recommendation for vitamin K is 1 μg/kg for adults (Shearer, 2009) but for patients on anticoagulant therapy, the content of vitamin K1 in LEs should be considered when prescribing coumarin-based anticoagulants (Raman et al., 2017). The vitamin K content per 100 g of lipids used in LE formulation varies (Shearer, 2009; Raman et al., 2017), as indicated for olive oil (1.1–5.5 μg), soybean oil (150–300 μg), and safflower oil (6–12 μg).
Biological Function of LEs
Absorption and Transport of LEs
FAs introduced intravenously bypass the intestinal lumen and directly enter blood circulation. This means bypassing digestion by lipases, solubilization by bile, uptake into intestinal enterocytes, or packaging into chylomicrons compatible for circulatory transport to target organs. Lipids infused intravenously are already packaged into artificial chylomicrons ready for transport to target organs (Carpentier and Dupont, 2000). LE chylomicrons mimic the structural similarity of natural chylomicrons produced by intestinal enterocytes (Carpentier and Dupont, 2000) excepting the spherical size, as LE chylomicrons vary between 200 and 500 nm depending on the type of oil and its concentration in the LE (Lutz et al., 1989; Ferezou and Bach, 1999). With LCTs mean particle diameter is larger than for MCTs, and droplets in 20% emulsions are larger than with 10% emulsions (Lutz et al., 1989). Ease of passage of LE chylomicrons through the smallest of capillaries is essential to prevent vascular occlusion and is enabled with particle size within 200–500 nm (Driscoll et al., 2001).
LE chylomicrons are hydrolyzed to yield FAs by lipoprotein lipase, and then transported to the liver to provide energy substrate similar to the fate of natural chylomicrons (Carpentier and Dupont, 2000). Differentially, LCTs are transported in blood as chylomicrons while MCTs from partial uptake become free FAs which bind to albumin. The composition of the LE particle will influence the rates of hydrolysis and uptake of FAs as determined by the FA carbon chain length and the relative position of the FA on the glycerol backbone (Karupaiah and Sundram, 2007). Therefore, the shorter carbon chain length of MCTs are hydrolyzed by lipases at a faster rate compared to LCTs (Deckelbaum et al., 1990).
Metabolism of FAs in LEs
Transported long-chain FAs are activated at the outer mitochondrial membrane by adenosine tri-phosphate to form acyl-adenylates before catalysis by acyl-CoA synthase. The sulfhydryl group of CoA reacts with the acyl-adenylate to form acyl-CoA. Aided by carnitine, the activated long-chain FAs then get transported into the mitochondrial matrix. The acyl carnitine formed from the reaction of acyl-CoA with the hydroxyl group of carnitine diffuses into the inner mitochondrial membrane. The acyl group of acyl-CoA reacts with the hydroxyl group of carnitine to form acyl-carnitine which enables diffusion into the inner mitochondrial membrane. Once inside the mitochondrial membrane, the acyl group will be transferred back to acyl-CoA and carnitine is released. These transacylation reactions are catalyzed by fatty acyl-CoA:carnitine FA transferase (Berg et al., 2012).
Medium-chain FAs been saturated and having shorter carbon chains generate energy faster, as the route of its fat oxidation differs from the longer FAs. These FAs promote passive movement across the mitochondrial double membrane, independent from carnitine allowing for direct oxidation (Ulrich et al., 1996; Gunstone et al., 2007). Metabolically, single-source MCT infusion gives rise to ketone bodies, predominantly β-hydroxybutyrates, as incomplete oxidation of medium-chain FAs in the mitochondria via β-oxidation converts intermediate metabolites of acetyl-CoA to ketones (Ball, 1993; Ulrich et al., 1996).
As regards structured triglycerides (STG), symmetrical TAGs formed by medium-chain FAs esterified at the sn-1 and sn-3 positions of the glycerol backbone would facilitate faster hydrolysis by lipoprotein lipase (Hultin et al., 1994; Karupaiah and Sundram, 2007). This facilitates faster uptake of long-chain monoglycerides into the cell, and medium-chain FAs to be transported into mitochondria for oxidation (Hultin et al., 1994).
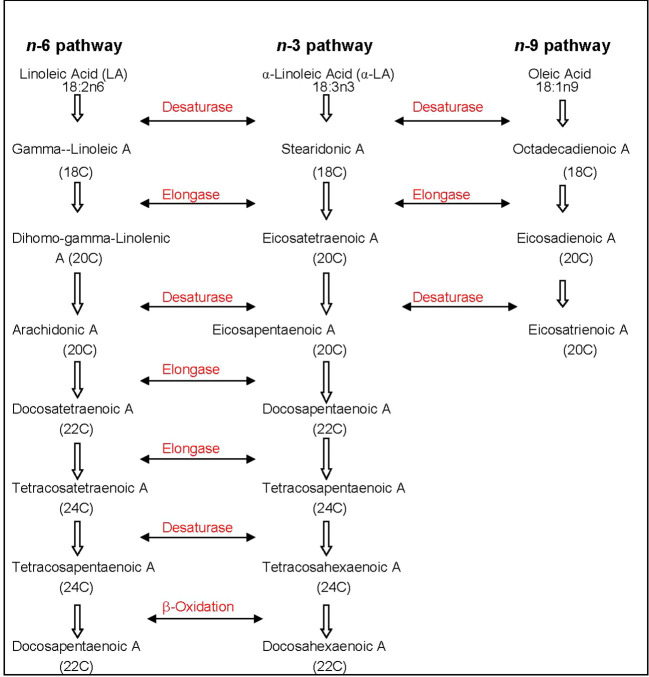
The metabolic process of assimilating n-3 or n-6 PUFAs is competitive depending on their concentration and incorporating desaturation and chain elongation (Gunstone et al., 2007). The pathways of metabolism are shown in Figure 1 . The preferential order of FA affinity for enzyme competition is in the order of α-linolenic > linoleic > oleic acid (Gurr, 1992). With essential FA deficiency, a third FA route utilizing oleic acid, becomes an option in the biological system (Vanek et al., 2012).
Figure 1.
Metabolic pathway of n-3 and n-6 PUFAs with desaturase and elongase enzymes. The affinity for n-6 PUFAs by these enzymes are higher compared to n-9; A, acid; C, carbon; PUFA, polyunsaturated fatty acid. References (Gurr, 1992; Vanek et al., 2012).
Functionality of LEs
Unlike SFAs and MUFAs, humans are unable to synthesize PUFAs endogenously, particularly α-linolenic acid and LA, due to the lack of Δ-12 and Δ-15 desaturases which enable chain elongation (Gunstone et al., 2007; Wanten and Calder, 2007). Therefore supplementation of these FAs is vital in LEs. When α-linolenic acid and LA are supplemented, they undergo systemic chain elongation and desaturation driven by liver enzymes to produce a series of longer chain FAs which acquire additional double bonds (Wanten and Calder, 2007). Both these PUFAs maintain membrane structure and fluidity, tissue permeability and cell signaling, and are precursors of important lipid mediators (Calder and Grimble, 2002; Wanten and Calder, 2007). EPA and DHA generated from the chain elongation of α-linolenic acid biologically become structural components of cell membranes, especially in the brain and the retina.
Oleic acid originating from olive oil is the major form of MUFA in LEs and even available as free FAs. When LA is scarce and oleic acid is available in excess, oleic acid will competitively inhibit enzymes involved in arachidonic acid formation (Delgado et al., 2017). Evidence from animal and human studies suggest that PUFA and MUFA increase the activity of lipoprotein lipase which enhances TAG clearance during postprandial lipemia (Williams, 1997). In contrast, MCTs in LEs sourced from coconut oil carry shorter carbon chain FAs that are saturated, allowing ready oxidization in mitochondria and utilization as fuel for energy (Ulrich et al., 1996; Gunstone et al., 2007).
With fish oil supplementation, the n-3-PUFAs outstrip the arachidonic acids competitively in the enzyme-catalyzed eicosanoid synthesis pathway to generate eicosanoids of three-series prostaglandins, thromboxanes, and five-series leukotrienes, which are less proinflammatory (Wachtler et al., 1997; Calder and Grimble, 2002; Grimm et al., 2006). Therefore, reduced n-6:n-3 PUFA ratio of FOLE results in less-proinflammatory eicosanoid derivatives. Changes in the ratio of leukotrienes C5 to C4 production by peripheral blood mononuclear cells was highest when a mixture of 2:1 of the two FAs were used, and this ratio exerts the most favorable modulation of lipid mediator synthesis (Morlion et al., 1996).
LEs in Formulation
The nature of oil used in LE formulations, depending on source and percentage contribution to the energy matrix, will determine the key functionalities between LEs. These differences account for their additional benefits or detrimental effects depending on prolonged use for critically ill or home PN patients. Table 2 describes commercially available LEs and other components in PN for intravenous application. Soybean oil, predominantly n-6 PUFAs rich was combined with saturated MCTs identified as second generation LE. The third generation of LE primarily decreased the load of n-6 PUFAs by 75% with olive oil. By the year 2000, fish oil either alone or in combination with one or more of the based vegetable oils yielded the fourth generation LEs. Details of each LE will be discussed in the following sections. Table 3 compares the concentrations of FAs from vegetable and fish oil sources. Their profile therefore varies in terms of therapeutic benefits and subsequent discussions reflect these aspects.
Table 2.
Commercially Available Lipid Emulsions in Parenteral Nutrition.
| Type of LE | 1st generation (1960s to 1970s) Soybean Oil LE | 2nd generation (since 1985) | 3rd generation (since 1990s) Olive Oil LE | 4th generation (since 2000) | |||
|---|---|---|---|---|---|---|---|
| MCT/LCT physical mixture LE | Structured triglycerides LE | Pure fish Oil LE | MCT/SO/FO LE | SO/MCT/OO/FO LE | |||
| Oil source (% by wt) | 100% SO | 50% SO,50% CO | 64% SO, 36% CO | 20% SO, 80% OO | 100% FO | 50% CO, 40% SO, 10% FO | 30% SO, 30% CO, 25% OO, 15% FO |
| Commercial name | Intralipid® 20% | Lipofundin MCT/LCT® 20% | Structolipid® 20% | ClinOleic® 20% | Omegaven® 10% | Lipiderm/Lipoplus® 20% | SMOFLipid® 20% |
| Ratio of n-6:n-3 PUFAs | 7:1b,d | 7:1b,d | 7:1b,d | 9:1b,d | 1:8b,d | 2.7:1d | 2.5:1b,d |
| Fat Content (g/L) | 200b | 200b | 200b | 200c | 100c | 200a | 200b |
| Molecular weight | 865b | 634b | 683b | 873b | 882b | NAb | 732b |
| pH | 8.0b | 6.5–8.5b | 8.0c | 7.0–8.0b | 7.5–8.7b | 6.5–8.5* | 8.0b |
| Osmolality(mOsmol/L) | 350b | 380b | 350b | 270b | 273b | 410* | 380b |
| tocopherol (mg/L) | 38d | 85 ± 20d | 6.9d | 32d | 150–296d | 190 ± 30d | 200d |
| Phytosterols (μcg/ml) | 439.07 ± 5.72e | 278.14 ± 5.09e | 345.85 ± 1.64e | 274.38 ± 2.6e | NRe | NRe | 207e |
| FAC (% by weight of | |||||||
| total FAs) | |||||||
| SFA | 15b | 59.4b | 46.3b | 14.5b | 21.2b | 49–58.3a,c | 36.9c |
| MUFA | |||||||
| OA | 24b | 11b | 14b | 62.3b | 15.1b | 7.9–13.4a,c | 30.8c |
| PUFA | |||||||
| LA | 44–62 b,d | 27–29.1b,d | 35b,d | 18.5–18.7b,d | 4.4b,d | 24.4–25.7a,d | 21.4d |
| α-LA | 4–11 b,d | 4–4.5b,d | 5b,d | 2–2.3b,d | 1.8b,d | 3.3–3.4a,d | 2.5d |
| AA | 0.1b | 0.2b | NAb | 0.5b | 2.1b | 0.5c | 0.4c |
| EPA | NAb,d | NAb,d | NAb,d | NAb,d | 19.2b,d | 3.1–3.7a,d | 3.0d |
| DHA | NAb,d | NAb,d | NAb,d | 0.0–0.5b,d | 12.1,d | 2.3–2.5a,d | 2.0d |
AA, arachidonic Acid, CO, coconut oil; DHA, docosahexanoic acid; EPA, eicosapentaenoic acid, FAC, fatty acids concentration; FO, fish oil; LCT, long-chain triglycerides; LE, lipid emulsion; MCT, medium-chain triglycerides; MUFA, monounsaturated fatty acids; NA, not available; NR, not reported; OO, olive oil; PUFA, poly unsaturated fatty acids; SMOF, soybean oil, coconut oil, olive oil and fish oil; SFA, saturated fatty acids; SO, soybean oil.
aLinseisen et al., 2000.
bWanten and Calder 2007.
cDriscoll et al., 2009.
dVanek et al., 2012.
eXu et al., 2012.
Fatty acid concentrations cited in the Table are reported as percentage by weight for the full product profiles but will not add up to 100% as only selected FAs are listed.
*Data provided is according to manufacturer monograph as per the lipid emulsion product.
Table 3.
Fatty acid composition in selected plant and fish sources used in intravenous lipid emulsions.
| FAC (% by weight) | Plant sources* | Fish species# | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Soybean | Olive | Coconut | Atlantic mackerela | Atlantic herringb | European anchoviesc | Rainbow smeltd | Atlantic salmone | Yellowfin tunaf | |
| Caprylic acid (8:0) | NR/ND | NR/ND | 8 | NR/ND | NR/ND | NR/ND | NR/ND | NR/ND | NR/ND |
| Capric acid (10:0) | NR/ND | NR/ND | 7 | NR/ND | NR/ND | NR/ND | NR/ND | NR/ND | NR/ND |
| Lauric acid (12:0) | NR/ND | NR/ND | 48 | NR/ND | NR/ND | NR/ND | NR/ND | NR/ND | NR/ND |
| Myristic acid (14:0) | NR/ND | NR/ND | 16 | 5.6 | 7.0 | 7.4 | 3.9 | 2.4 | 1.6 |
| Palmitic acid (16:0) | 11 | 10 | 9 | 17.6 | 17.1 | 17.4 | 16.6 | 11.2 | 23.2 |
| Stearic acid (18:0) | 4 | 2 | 2 | NA | NA | NA | NA | NA | NA |
| Oleic acid (18:1) | 23 | 78 | 7 | 18.9 | 19.2 | 15.2 | 20.6 | 24.0 | 16.1 |
| Linoleic acid (18:2) | 53 | 7 | 2 | 1.8 | 1.6 | 2.4 | 2.3 | 3.1 | 1.2 |
| α-LA (18:3) | 8 | 1 | NR/ND | 1.3 | 1.3 | 0 | 2.5 | 5.2 | 1.8 |
| EPA (20:5) | NR/ND | NR/ND | NR/ND | 7.4 | 8.9 | 13.1 | 13.9 | 5.7 | 5.4 |
| DPA (22:5) | NR/ND | NR/ND | NR/ND | 1.7 | 0.6 | 0.7 | 0.9 | 5.1 | 1.9 |
| DHA (22:6) | NR/ND | NR/ND | NR/ND | 11.6 | 10.8 | 22.2 | 21.1 | 19.8 | 26.8 |
α-LA, alpha-linolenic acid; DHA, docosahexanoic acid; DPA, docosapentaenoic acid; EPA, eicosapentaenoic acid; FAC, fatty acid concentration; NA; not available; ND; not detected; NR, not reported. FAC, fatty acids concentration based on percentage by weight, percentages do not add up to 100% because not all FAs are listed. Fish species from marine families of Carangidao, Clupeidae, Engraulidae, Osmeridae, Salmonidae, Scombridge (a–f).*Reference for plant sources: Iyer et al., 1998; Garnacho-Montero et al., 2002; Ma et al., 2012.#Reference for fish species: Driscoll et al., 2009.
Jadad scoring (Jadad et al., 1996) was applied to rate quality of cited randomized controlled trials evaluating various LEs formulations, and detailed in Supplementary File ( Table 1 ). Overall, 8 studies (Gogos et al., 1990; Sedman et al., 1991; Ball, 1993; Jiang et al., 1993; Smirniotis et al., 1998; Mayer et al., 2003a; Sungurtekin et al., 2011; Demirer et al., 2016) were rated with a Jadad score of 1, 19 studies (Dionigi et al., 1985; Monson et al., 1986; Morlion et al., 1996; Battistella et al., 1997; Roulet et al., 1997; Waitzberg et al., 1997; Furukawa et al., 2002; Schauder et al., 2002; Weiss et al., 2002; Grau et al., 2003; Köller et al., 2003; Mayer et al., 2003b; Chen et al., 2005; Grimm et al., 2006; Iovinelli et al., 2007; Sabater et al., 2011; Ma et al., 2012; Zhu et al., 2012; Wu et al., 2014) scored 2, 11 studies (Wachtler et al., 1997; Chambrier et al., 1999; Linseisen et al., 2000; Garnacho-Montero et al., 2002; Wichmann et al., 2007; Berger et al., 2008; Barbosa et al., 2010; Onar et al., 2011; Wang et al., 2012; Gultekin et al., 2014; Chen et al., 2017) scored 3, 6 studies (Sandstrom et al., 1995; Kruimel et al., 2001; Lindgren et al., 2001; Vahedi et al., 2005; Wang et al., 2008; Piper et al., 2009) scored 4, and 11 studies (Bohnert et al., 2018; Klek et al., 2003; Senkal et al., 2007; Liang et al., 2008; Badia-Tahull et al., 2010; Jiang et al., 2010; Han et al., 2012; de Miranda Torrinhas et al., 2013; Metry et al., 2014; Grau-Carmona et al., 2015; Ma et al., 2015) scored 5.
Soybean Oil-Based LEs
Soybean oil-based LEs (SOLEs) were the primary LEs in PN therapy, formulated with the objective of preventing the development of essential FA deficiency (Gramlich et al., 2015), as well as to offset the glucose load to prevent hyperglycemia (Schloerb, 2004). The total FA composition profile of soybean oil includes ~53% LA (18:2n-6), ~23% oleic acid, and ~8% α-linolenic acid (Gunstone et al., 2007). About 84% of soybean oil carries three 18-carbon, long-chain unsaturated FAs with the remaining ~15% from SFAs such as stearic acid ~4% and palmitic acid ~11% (Vanek et al., 2012; Kagawa et al., 2013). SOLEs have high n-6 PUFA compared to n-3 PUFA with ratio of 7:1 (Waitzberg et al., 2006). Commercially available LEs with 100% soybean oil is listed in Table 2 .
Soybean oil is naturally rich in phytosterols, mainly ~60% β-sitosterol followed by ~20% each of campesterol and stigmasterol (Gunstone et al., 2007). Phytosterol concentration in soybean oil is ~320 mg/100 g oil (Verleyen et al., 2002). The abundance of phytosterols in SOLE is associated with the severity of intestinal failure associated liver disease in home PN patients (Ellegard et al., 2005; Llop et al., 2008). The concentration of α-tocopherol in soybean oil is ~7.5 mg/100 g oil but γ-tocopherol content ~80 mg/100 g oil (Gunstone et al., 2007) been greater, contributes to 10% of vitamin E’s biological activity (Biesalski, 2009).
Comparative Studies of SOLEs vs Lipid Free Glucose Infusions
Table 4 reports on immuno-inflammatory and clinical outcomes of trials comparing the use of SOLE in PN with lipid free PN admixtures. Some studies reported either no change or immunostimulatory effects when SOLE was used compared to lipid-free PN admixtures in surgical patients (Dionigi et al., 1985; Monson et al., 1986; Li et al., 2007; Kagawa et al., 2013). Li et al. (2007) and Kagawa et al. (2012) suggested LE infusion is dose-dependent and the infusion rate at 0.09–0.12 g/kg/h does not alter human immune function (Li et al., 2007; Kagawa et al., 2013).
Table 4.
Clinical outcomes of randomized controlled trials categorized as per lipid emulsion formulation type.
| No. | Type of LE | Patient subgroups | Significant outcomes | References |
|---|---|---|---|---|
| 1. | SOLE | Surgery | Nutrition | |
| ↑ glucagon, cumulative NB negative | (Furukawa et al., 2002) | |||
| Immune and inflammatory | ||||
| ↑ PMN cells | (Dionigi et al., 1985) | |||
| ↑ IL-2 | (Monson et al., 1986; Sedman et al., 1991) | |||
| ↓ LAK cell activity | (Sedman et al., 1991) | |||
| ↑ IL-6, ↑ CRP, ↓ lymphocyte proliferation | (Furukawa et al., 2002) | |||
| ↑ T cells and helper T cells, ↑ ADCC function | (Monson et al., 1986) | |||
| ↓ Bacteria killing mediated by neutrophils | (Waitzberg et al., 1997) | |||
| Clinical | ||||
| ↓ Sepsis score | (Dionigi et al., 1985) | |||
| ICU | Immune and inflammatory | |||
| ↓ Helper/suppressor T cells ratio Clinical |
(Gogos et al., 1990) | |||
| ↑ Infection, ↑ ICU stay, ↑ hospital stay, ↑ duration MV | (Battistella et al., 1997) | |||
| 2. | Physical | Surgery | Nutrition | |
| MCT/LCT | ↓ Body weight, ↓ TG, ↑ β hydroxybutyrate | (Jiang et al., 1993) | ||
| ↑ Pre-albumin | (Chen et al., 2005) | |||
| ↑ Insulin | (Jiang et al., 1993; Chen et al., 2005) | |||
| Immune and inflammatory | ||||
| ↑ NK and ↑ LAK cell activity Clinical | (Sedman et al., 1991) | |||
| ↓ Intra-abdominal infection | (Grau et al., 2003) | |||
| ↓ Mortality | (Grau et al., 2003) | |||
| ICU | Nutrition | |||
| ↓ Negative NB | (Ball, 1993; Garnacho-Montero et al., 2002) | |||
| ↑ Retinol binding protein, ↑ insulin | (Garnacho-Montero et al., 2002) | |||
| Immune and inflammatory | ||||
| No change in immune markers | (Iovinelli et al., 2007) | |||
| Clinical | ||||
| ↑ Oxygen consumption, VO2 | (Smirniotis et al., 1998) | |||
| ↓ Duration of MV | (Iovinelli et al., 2007) | |||
| 3. | STG-LE | Surgery | Nutrition | |
| No ↑ TG, No ↑ ASAT, No ↑ ALAT, NB positive | (Chambrier et al., 1999) | |||
| Improved cumulative NB | (Chambrier et al., 1999; Kruimel et al., 2001) | |||
| Less ↑ TG | (Kruimel et al., 2001) | |||
| ↑ Carbon dioxide production, ↑ whole-body fat oxidation, ↑ free fatty acid, ↑ plasma glycerol, ↑ 3-hydroxybutyric acid | (Sandstrom et al., 1995) | |||
| ICU | Nutrition | |||
| Improved cumulative NB | (Lindgren et al., 2001) | |||
| 4. | OOLE | HPN | Cellular fatty acid changes | |
| ↑ γ-LA, ↑ oleic acid, ↑ mead acid | (Vahedi et al., 2005) | |||
| Surgery | Nutrition | |||
| ↑ Body weight | (Demirer et al., 2016) | |||
| ↓ TBARS | (Demirer et al., 2016) | |||
| ↑ ALP, ↑ GGT, ↑ total protein, ↑ albumin, ↓ total bilirubin | (Onar et al., 2011) | |||
| Clinical | ||||
| No changes on catheter infections | (Onar et al., 2011) | |||
| 5. | FOLE | Surgery | Nutrition | |
| ↑ α-Tocopherol | (Linseisen et al., 2000; Klek et al., 2003; Grimm et al., 2006; Wichmann et al., 2007) | |||
| ↓ AST, ↓ ALT | (Klek et al., 2003; Piper et al., 2009) | |||
| ↓ α-GST, ↓TG | (Piper et al., 2009) | |||
| ↓ LDL | (Ma et al., 2012) | |||
| ↑ APTT, | (Wang et al., 2012) | |||
| ↓ Total bilirubin, | (Klek et al., 2003; Wang et al., 2012) | |||
| ↓ Glucose | (Wu et al., 2014) | |||
| Less ↓ HDL, less ↓ free fatty acid | (Ma et al., 2015) | |||
| ↑ Body weight, ↓ TBARS | (Demirer et al., 2016) | |||
| Cellular fatty acid changes | ||||
| ↑ EPA or DHA | (Morlion et al., 1996; Roulet et al., 1997; Linseisen et al., 2000; Mayer et al., 2003b; Klek et al., 2003; Grimm et al., 2006; Senkal et al., 2007; Berger et al., 2008) | |||
| ↑ ALA | (Morlion et al., 1996) | |||
| ↑ EPA/AA ratio | (Roulet et al., 1997; Grimm et al., 2006; Senkal et al., 2007) | |||
| ↓ LA | (Linseisen et al., 2000) | |||
| ↑ Total n-3 PUFA, ↑ n-3:n-6 PUFA ratio | (Klek et al., 2003; Grimm et al., 2006; Senkal et al., 2007) | |||
| Immune and inflammatory | ||||
| ↓ IL-6 | (Clayton et al., 1993; Mayer et al., 2003b; Liang et al., 2008; Zhu et al., 2012; de Miranda Torrinhas et al., 2013) | |||
| ↓ LTB4 | (Wachtler et al., 1997) | |||
| ↓ TNF-α | (Wachtler et al., 1997; Mayer et al., 2003b; Zhu et al., 2012) | |||
| ↑ TNF-α | (Schauder et al., 2002; Wang et al., 2012) | |||
| ↑ HLA-DR | (Clayton et al., 1993) | |||
| ↑ IL-2 | (Schauder et al., 2002) | |||
| ↑ LTB5, ↑ LTB5/LTB4 ratio | (Wachtler et al., 1997; Köller et al., 2003; Grimm et al., 2006; Wichmann et al., 2007; Wang et al., 2012) | |||
| ↓ IL-1β, and ↓ IL-8 | (Mayer et al., 2003b) | |||
| ↑ CD4+/CD8+ | (Schauder et al., 2002; Liang et al., 2008) | |||
| ↓ CD4+/CD8+ | (Zhu et al., 2012) | |||
| ↑ NF-Kβ | (Wang et al., 2012) | |||
| ↓ IL-10 | (de Miranda Torrinhas et al., 2013) | |||
| Clinical | ||||
| ↓ Post-operative stay on medical ward | (Clayton et al., 1993) | |||
| ↓ Length of hospital stay | (Grimm et al., 2006; Jiang et al., 2010; Zhu et al., 2012) | |||
| ↓ Infection rates | (Badia-Tahull et al., 2010) | |||
| ↓ Duration of SIRS | (Jiang et al., 2010; Zhu et al., 2012) | |||
| ICU | Nutrition | |||
| ↑ Albumin | (Gultekin et al., 2014) | |||
| Cellular fatty acid changes | ||||
| ↑ EPA and DHA | (Mayer et al., 2003a; Mayer et al., 2003b; Wang et al., 2008; Barbosa et al., 2010) | |||
| Immune and inflammatory | ||||
| ↓ IL-6 | (Mayer et al., 2003b; Barbosa et al., 2010; Sungurtekin et al., 2011; Han et al., 2012; Metry et al., 2014) | |||
| ↓ TNF-α | (Mayer et al., 2003b; Barbosa et al., 2010; Sungurtekin et al., 2011; Han et al., 2012) | |||
| ↓ IL-10 | (Barbosa et al., 2010; Sungurtekin et al., 2011) | |||
| ↑ Neutrophil inositol phosphate, ↑ PAF, ↑LTB5 | (Mayer et al., 2003a) | |||
| ↑ Respiratory burst | (Mayer et al., 2003a) | |||
| ↓ IL-8 | (Mayer et al., 2003b; Han et al., 2012) | |||
| ↓ CRP | (Wang et al., 2008; Gultekin et al., 2014) | |||
| ↓ IL-1β | (Mayer et al., 2003b; Barbosa et al., 2010) | |||
| ↓ IL-1 | (Sungurtekin et al., 2011; Han et al., 2012) | |||
| ↓ LTB4 | (Sabater et al., 2011; Gultekin et al., 2014) | |||
| ↓ IFN-γ | (Han et al., 2012) | |||
| Clinical | ||||
| Improvement in oxygenation index | (Wang et al., 2008; Barbosa et al., 2010) | |||
| ↓ CRRT days | (Wang et al., 2008) | |||
| ↓ Hospital stay | (Barbosa et al., 2010) | |||
| ↓ Nosocomial infections, ↑ predicted time free of infection | (Grau-Carmona et al., 2015) | |||
| ↓ 60-day mortality | (Chen et al., 2017) | |||
| HPN | Cellular fatty acid changes | |||
| ↑EPA,↑ DHA, ↑DPA in erythrocytes,platelets, serum phospholipids | (Bohnert et al., 2018) |
AA, arachidonic acid; ADCC, antibody-dependent cellular cytotoxicity; ALA, α-linolenic acid; ALAT, alanine aminotransferase; ALP, alkaline phosphatase; ALT, alanine transaminase; APTT, activated partial thromboplastin time; ASAT/AST, aspartate aminotransferase; CD4/CD8+, T4 helper cells/T8 suppressor cells; CRP, C-reactive protein; CRRT, continuous renal replacement therapy; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; DPA, docosapentaenoic acid; FOLE, fish oil lipid emulsion; GGT, gamma-glutamyl transferase; GST, glutathione S-transferase; HDL, high-density lipoprotein; HLA-DR, human leukocyte antigen-antigen D related; HPN, home parenteral nutrition; ICU, intensive care unit; IFN, interferon; Ig, immunoglobulin; IL, interleukin; LA, linoleic acid; LAK, lymphokine activated killer; LDL, low-density lipoprotein; LTB4, leukotriene B4; LTB5, leukotriene B5; MV, mechanical ventilation; NB, nitrogen balance; NK, natural killer; NF-Kβ, nuclear factor kappa β; OOLE, olive oil lipid emulsion, PAF, platelet activating factor; PMN, polymorphonuclear; PN, parenteral nutrition; PO2/FiO2, partial pressure of oxygen/fraction of inspired oxygen; PUFA, polyunsaturated fatty acid; SIRS, systemic inflammatory response syndrome; SOLE, soybean oil-based lipid emulsion; STG-LE, structured triglyceride lipid emulsion; TBARS, thiobarbituric acid-reactive substances; TG, triglyceride; TNF, tumor necrosis factor; VO2, oxygen consumption. For more details, please refer to Supplementary File ( Table 1 ).
Evidence is conflicting associating SOLE use with clinical risk ( Table 4 ) (Gogos et al., 1990; Sedman et al., 1991; Battistella et al., 1997; Waitzberg et al., 1997; Furukawa et al., 2002). Reductions in the ratio of helper to suppressor T-cells (Gogos et al., 1990), lymphokine-activated killer cell activity (Sedman et al., 1991), lymphocyte function (Battistella et al., 1997), lymphocyte proliferation (Furukawa et al., 2002), and bacteria killing by neutrophils (Waitzberg et al., 1997) have been reported. Post-surgical patients with severe stress, experienced increased interleukin-6 (IL-6) and C-reactive protein (CRP) levels after receiving SOLE compared to lipid-free PN. The high ratio of n–6:n–3 PUFAs in SOLE poses greater risk toward inflammatory response (Furukawa et al., 2002). LA metabolism promotes eicosanoid formation via enzymatic chain elongation, known to be proinflammatory mediators affecting macrophage, neutrophil and lymphocyte function (Wanten and Calder, 2007).
SOLE use is also associated with risk of adverse clinical outcomes. A meta-analysis reported higher infectious complication rates in surgical and intensive care patients on SOLE compared to lipid-free PN (Heyland et al., 1998). Polytrauma patients on SOLE for 10 days had increased number of days on mechanical ventilation, and longer stay in intensive care and hospital stay (Battistella et al., 1997). However one study reported reduced severity of infections in gastrointestinal surgery patients on SOLE compared to lipid-free PN (Dionigi et al., 1985). In contrast, patients undergoing bone marrow transplantation did not experience increased incidence of bacterial or fungal infections when infused with SOLE providing less than 30% energy/day (Lenssen et al., 1998).
Issues Associated With Use of SOLEs
The reticuloendothelial system participates in clearance of lipid particles associated with infusion of LEs. However, long-term administration of SOLE may deleteriously affect the reticuloendothelial system function (Seidner et al., 1989). Another risk of long-term use of SOLE is hepatotoxicity attributed to phytosterol content (Saubion et al., 1998; Llop et al., 2008).
Physical Mixture MCT/LCT LEs
Concern about adverse effects from SOLE related to immuno-inflammatory and clinical risks led to the development of the physical mixture MCT/LCT. Physical mixtures combining MCT with LCT were initially formulated with coconut oil providing 50% of the MCT substrate with the remaining 50% from soybean oil. The MCT/LCT physical mixture formulation with effective manipulation of the percentage proportion of long-chain soybean oil with a shorter-chain FA, enabled the required reduction in the n-6 PUFA content in SOLEs. Coconut oil is the principal source of medium-chain FAs with lauric acid contributing 48% FA concentration, 8% caprylic acid, and 7% capric acid. Other fractions of FAs in coconut oil include 16% myristic acid, 9% palmitic acid, 2% stearic acid, 7% oleic acid and 2% LA (Gunstone et al., 2007). Typical commercial MCT/LCT LE formulations in use are detailed in Table 2 .
Total phytosterol concentration in coconut oil (67.8 mg/100 g) is lower compared to soybean oil (~320 mg/100 g) with β-sitosterol contributing ~70%, followed by stigmasterol ~18% and campesterol ~11% (Verleyen et al., 2002). The α-tocopherol concentration of coconut oil is very low at 0.5 mg/100 g oil compared to soybean oil ~7.5 mg/100 g oil (Gunstone et al., 2007) which implies a lower phytosterol and α-tocopherol content in the physical mixture of MCT/LCT LEs, compared to SOLE.
Comparative Studies of MCT/LCT LEs With SOLEs
Table 4 summarizes randomized controlled trials and crossover studies that have compared the effects of MCT/LCT LE and SOLE in abdominal surgery, critically ill, and long-term PN patients. Most of these studies concentrated on metabolic outcomes followed by clinical benefits. Two studies showed no influence of MCT/LCT LE on immune function in surgery and critically ill patients (Gogos et al., 1990; Sedman et al., 1991). In terms of immunomodulation there was no change in the ratio of helper to suppressor T-cells after MCT/LCT infusion, in contrast to a significant decrease in the ratio observed with SOLE (Gogos et al., 1990). In patients after gastrointestinal cancer surgery, MCT/LCT infusion increased natural killer cell activity and lymphokine activated killer activity compared to SOLE (Sedman et al., 1991). These findings are suggestive of improved immune function in the patient group receiving MCT/LCT LE. Indeed, MCTs do not depress the reticuloendothelial system function as evidenced from animal and human studies (Hamawy et al., 1985; Jensen et al., 1990). MCTs, being saturated cannot participate in eicosanoid synthesis and are not precursors for prostaglandins. Therefore they do not affect the production of inflammatory mediators during stress and illness in relation to n-6 PUFA (Radermacher et al., 1992).
Significant metabolic outcomes observed in intensive care and abdominal surgery patients administered with MCT/LCT LE infusion compared to SOLE are related to increase in plasma ketones (Ball and White, 1989; Jiang et al., 1993), non-esterified FAs (Ball and White, 1989), insulin (Jiang et al., 1993; Garnacho-Montero et al., 2002; Chen et al., 2005), prealbumin (Chen et al., 2005), retinol binding protein (Garnacho-Montero et al., 2002), and improved nitrogen balance (Ball, 1993; Garnacho-Montero et al., 2002). Contrarily one study did not observe any of these effects (Nijveldt et al., 1998).
Increased oxygen consumption in acute respiratory distress syndrome patients (Smirniotis et al., 1998), shorter weaning time from ventilator in chronic obstructive pulmonary disease patients (Iovinelli et al., 2007), and significant reduction in postoperative complications and mortality in preoperative surgery patients (Grau et al., 2003) are some clinical benefits attributed to MCT/LCT LE administration compared to SOLE.
Issues Associated With Use of MCT/LCT LEs
Since MCT oils are significantly low in essential FAs, dependency as a sole substrate of fat would be an issue in clinical applications unless combined with an n-6 PUFA-rich fat substrate to make up this deficit (Naber and Kruimel, 2002). A combination ratio of 1:1 for MCT and LCT is sufficient to prevent essential FA deficiency in long-term PN administration with an optimal dose of 0.41 ± 0.22 g/kg/day (Chambrier et al., 2004). Hypertriglyceridemia with initial infusion of MCTs is noted with MCT/LCT LE administration (Ball and White, 1989) and this is attributed to faster hydrolysis of MCTs. Additionally, generation of ketone metabolites, predominantly β-hydroxybutyrates (Ball, 1993) are associated with MCT/LCT LE and may induce increased insulin production (Naber and Kruimel, 2002).
Structured Triglyceride LEs
The physical mixture of MCT/LCT LEs evolved further with the introduction of STGs as a new substrate option (Chambrier et al., 2006). The predominant commercial LE is a structured mix of 64% LCT from soybean oil and 36% of MCT from coconut oil ( Table 2 ). STGs are used as a lipid source in 3-in-1 commercial PN Bag.
Unlike physical mixtures, an STG-LE is formulated by first hydrolyzing soybean and coconut oils to free FAs, before interesterification in the presence of chemical or enzyme catalysts. Essentially medium-chain FAs (from coconut oil) and long-chain FAs (from soybean oil) are randomly reassigned on the glycerol backbone, generating new TAG molecular species (Karupaiah and Sundram, 2007). In STG-LEs, medium-chain FAs are preferentially positioned at the sn-1 and sn-3 positions of the TAG molecule with a long-chain FA occupying the sn-2 position (Chambrier et al., 2006). The choice of preferential placement of medium-chain FAs at the sn-1 or sn-3 position enables faster oxidation of energy substrates. An immunomodulating effect may be associated with STG if arachidonic acid, EPA, or DHA is incorporated at the sn-2 position (Chambrier et al., 2006). Additionally, favorable stereospecific positioning of medium-chain FAs in the molecular structure of STG facilitate faster triglyceride clearance and utilization as noted by Karupaiah and Sundram (2007). MCT/LCT LE carries more MCT compared to STG-LE (50% vs 36%) which fulfills the desired functionality of rapid hydrolysis compared to LCTs (Ball and White, 1989).
Comparative Studies of STG-LEs With SOLEs and Physical Mixture MCT/LCT LEs
Table 4 reports on metabolic outcomes associated with STG-LE use compared to SOLE and MCT/LCT LE in surgery and critically ill patients. There is improved nitrogen retention in post-surgical patients receiving STG-LE compared to SOLEs and MCT/LCT LEs (Kruimel et al., 2001; Lindgren et al., 2001). Fat oxidation rate and 3-hydroxybutyric acid concentrations were found to be higher in the post-surgical STG-LE group, who received STG-LE for one day and SOLE the next day or vice versa for 6 days with lipid dose-dependence while maintaining amino acids and dextrose concentrations (Sandstrom et al., 1995). Conflicting findings related to differences in lipid infusion rates, switching of LE regimens daily, and different glucose:lipid ratios prescribed across study groups have been reported (Sandstrom et al., 1995; Bellantone et al., 1999; Chambrier et al., 1999).
The reduced risk of hypertriglyceridemia was noted in patients administered with STG-LE in post-surgical patients for 5 days (Chambrier et al., 1999; Kruimel et al., 2001) compared to MCT/LCT LEs. This effect could be attributed to favorable stereospecific positioning of FAs in the molecular structure of STG enabling faster triglyceride clearance and utilization (Karupaiah and Sundram, 2007).
Meta-analysis of 21 clinical trials with critically ill patients (n = 4) and surgical patients (n = 17) concluded short-term administration of STG-LE (5 to 7 days) was significantly associated with improved nitrogen balance, increased plasma proteins, reduced plasma triglycerides, improved liver function parameters, and reduced adverse events and length of hospital stay compared to physical mixture MCT/LCT LEs (Wu et al., 2017).
STG-LEs do not show any influence on the reticuloendothelial system, unlike the inhibition of reticuloendothelial system function shown by LCT (Chambrier et al., 2006).
Issues Associated With Use of STG-LEs
A lower α-tocopherol (6.9 mg/L) content adds to greater lipid peroxidation risk and higher phytosterols (~350 μg/ml) compared to MCT/LCT LE (~280 μg/ml), therefore adding to liver toxicity risk (Vanek et al., 2012; Xu et al., 2012). Naber and Krumel, (2002) observed STG-LE administration generated greater production of the ketone metabolite, β-hydroxybutyrate indicating a faster oxidation rate compared to physical mixture MCT/LCT (Naber and Kruimel, 2002). The impact of this metabolite on insulin and glycemic status of patients in therapy remains unreported.
Olive Oil LEs
Another option to reduce n-6 PUFAs is with olive oil as a principal component by 80% replacement of soybean oil. The FA composition of olive oil reflects ~80% of the nonessential n–9 oleic acid with lesser proportions of the essential n–6 LA ~7% and the saturated palmitic acid ~10% (Gunstone et al., 2007). The n-3 PUFA in olive oil is negligible ( Table 2 ). The small content of LA in olive oil explains the need to include an n-6 PUFA-rich source such as soybean oil to prevent essential FA deficiency. A formulation of olive oil LE (OOLE) blending ~20% soybean oil with ~80% olive oil in volume is commercially available ( Table 2 ). Olthof et al. (2015) who studied 30 home PN patients receiving a combination of olive oil (80%) and soybean oil (20%) for 3 months at five times a week, reported their patients did not develop essential FA deficiency (Olthof et al., 2015).
Phytosterol concentration in olive oil is ~190 mg/100 g oil with 70% in the form of β-sitosterol (Verleyen et al., 2002). Olive oil also contains significant amounts of α-tocopherol (Sala-Vila et al., 2007) at ~12 mg/100 g oil (Gunstone et al., 2007), and thus provides sufficient protection against lipid peroxidation (Sala-Vila et al., 2007; Cai et al., 2018).
Comparative Studies of OOLEs With SOLEs and Physical Mixture MCT/LCT LEs
Table 4 summarizes trials on OOLE compared to SOLE and physical mixture MCT/LCT LE in abdominal surgery, critically ill, and long-term PN patients. In terms of immuno-inflammatory outcomes, there was no difference in inflammatory, immune function markers, and pro-inflammatory cytokines (TNF-α and IL-6) in both critically ill (Umpierrez et al., 2012) and abdominal surgery (Demirer et al., 2016) patients randomized to receive either SOLE or MCT/LCT physical mixture vs OOLE. Similarly, inflammatory markers in home-PN patients on OOLE for 3 months were not different from baseline status (Reimund et al., 2005). Oleic acid predominant in olive oil, is hypothesized to offset immune system impairment when in combination with n-6 PUFA (Moussa et al., 2000; Cury-Boaventura et al., 2006). Metabolically, oleic acid does not influence the arachidonic eicosanoid pathway and is not a precursor for eicosanoids (Cai et al., 2018). Therefore a lesser proinflammatory response compared to soybean oil is likely.
Benefits on metabolic outcomes were reported by Onar et al. (2011). Significant increases in total protein and albumin followed by reduction in total bilirubin levels were reported in abdominal cancer surgery patients infused with OOLE for 7 days compared to SOLE. Weight gain has also been observed in patients receiving OOLE (Demirer et al., 2016). Home-PN patients with OOLE infusion over 2 months (Osowska et al., 2018), or 3 months (Reimund et al., 2005; Puiggros et al., 2009), post-abdominal surgery (Puiggros et al., 2009; Onar et al., 2011), and severely ill burns patients (Garcıa-de-Lorenzo et al., 2005) infused with OOLE did not have abnormal liver function tests. However evidence on clinical benefit of OOLE compared to MCT/LCT LE and STG-LE is still scarce.
The single unsaturated double-bond of oleic acid which characterizes OOLE likely mediates less lipid peroxidation and oxidative stress compared to LEs with n-6 and n-3 PUFAs (Sala-Vila et al., 2007). Animal studies (Dutot and Melin, 1991) and human studies with children (Goulet et al., 1999) and adults (Demirer et al., 2016) have reported reduction in thiobarbituric acid–reactive substances implying lowest risk of oxidative stress when given OOLE.
Issues Associated With Use of OOLEs
The impact of optimizing n-9 MUFA from OOLE is unknown in terms of whether there is competitive inhibition on the endogenous synthesis of n-3 PUFAs. This is because generation of gondoic acid and nervonic acid from oleic acid elongation have been reported to add on cardiovascular mortality risk (Delgado et al., 2017).
Fish Oil LEs
Another option to reduce n-6 PUFA was to increase n-3 PUFAs with FOLE. Fish oil carries the characteristic longer chain n-3 PUFAs, EPA, and DHA in greater proportion compared to n–6 PUFAs. The FA concentration of fish oil comprises 1%–4% LA; 10%–30% of α-linolenic acid, EPA, and DHA; 5%–30% of oleic acid; and 18%–35% of SFAs (Gunstone et al., 2007). However, the nature of FA concentration depends on fish species used, as indicated in Table 3 (Gunstone et al., 2007; Driscoll et al., 2009). Three different formulations of FOLEs have been commercialized as detailed in Table 2 .
Phytosterol content is not an issue associated with fish oil use in LEs as fish oil is not plant based (Burrin et al., 2014). But the n-3 PUFAs been more prone to lipid peroxidation compared to n-6 PUFAs with more double bonds carried in their carbon chain (Linseisen et al., 2000). However the oxidative risk of FOLE is minimized by addition of α-tocopherol (Goulet et al., 2010). In fact, FOLEs compared to other LEs characteristically carry the highest α-tocopherol content ranging from 150 to 296 mg/L ( Table 2 ).
FOLE administration after abdominal surgery is reported to modulate increased EPA content of plasma FA concentration (Morlion et al., 1996; Linseisen et al., 2000; Klek et al., 2003; Grimm et al., 2006; Senkal et al., 2007; Wichmann et al., 2007; Berger et al., 2008), platelets (Roulet et al., 1997; Bohnert et al., 2018), and erythrocytes (Klek et al., 2003; Senkal et al., 2007; Bohnert et al., 2018). Similarly, infusion of FOLE in sepsis patients reflected in increased n-3 FAs in plasma (Mayer et al., 2003a; Mayer et al., 2003b; Wang et al., 2008; Barbosa et al., 2010) and in mononuclear leukocyte membrane (Wachtler et al., 1997). This modulation of membrane lipids may favorably affect eicosanoids’ profile and other lipid mediators generated from arachidonic acid and EPA.
Comparative Studies of FOLE With SOLE, Physical Mixture MCT/LCT, and OOLE
Table 4 summarizes trials related to FOLE for PN administration compared to SOLE, physical mixture MCT/LCT LE and OOLE in abdominal surgery and critically ill patients. Abdominal surgery patients receiving fish oil in the PN regimen demonstrated decreased production of inflammatory eicosanoids (Morlion et al., 1996; Wachtler et al., 1997; Köller et al., 2003; Grimm et al., 2006; Wichmann et al., 2007; Wang et al., 2012) and cytokines (Wachtler et al., 1997; Weiss et al., 2002; Liang et al., 2008; Zhu et al., 2012; de Miranda Torrinhas et al., 2013). This may offset the surgery-induced decline in antigen presenting cell activity (Weiss et al., 2002) and production of cytokines (Schauder et al., 2002). Modulation of inflammatory mechanisms and inflammatory mediator production appears to be attenuated in septic and acute respiratory distress syndrome patients (Mayer et al., 2003b; Barbosa et al., 2010; Sabater et al., 2011; Sungurtekin et al., 2011; Han et al., 2012; Gultekin et al., 2014; Metry et al., 2014). Pro-inflammatory cytokines TNF-α, IL-β, IL-6, and IL-8 secretion were shown to be significantly reduced by endotoxin-stimulated mononuclear cells in the FOLE group whereas an increase occurred 2 days after SOLE infusion (Mayer et al., 2003b). Significant reduced levels of inflammatory mediators IL-6 and TNF-α were noted in critically ill patients on FOLE compared to SOLE (161) or MCT/LCT LE (Barbosa et al., 2010; Sungurtekin et al., 2011; Han et al., 2012). Acute respiratory distress syndrome patients infused with FOLE in combination with MCT/LCT for 12 h had reduced leukotriene B4 production compared to SOLE alone (Sabater et al., 2011). Reduced leukotriene B4 production was also observed in septic patients receiving FOLE in combination with OOLE (160). In contrast, fish oil provided at varied doses before and after surgery in malignant large bowel surgical patients did not show any immunosuppressive benefit (Schauder et al., 2002). In patients undergoing abdominal aorta aneurysm repair (Berger et al., 2008) on MCT/LCT physical mixture or FOLE, no significant difference in the inflammatory marker CRP, in both groups of patients were noted.
Surgery patients administered with FOLE had higher plasma α-tocopherol concentration compared to SOLE, physical mixture MCT/LCT, or OOLE (Linseisen et al., 2000; Grimm et al., 2006; Wichmann et al., 2007). Other metabolic outcomes reported were significant improvement in liver enzymes (Klek et al., 2003; Piper et al., 2009), reduced triglycerides (Wu et al., 2014; Ma et al., 2015), total bilirubin (Wang et al., 2012), low-density lipoprotein-cholesterol (Ma et al., 2012), and weight gain (Demirer et al., 2016). However, only one study showed increased albumin levels in critically ill patients receiving FOLE infusion compared to OOLE (Gultekin et al., 2014).
Studies evaluating clinical benefit from FOLE administration compared to other LEs in surgical and critically ill patients report reduced mortality (Tsekos et al., 2004; Heller et al., 2006; Chen et al., 2017), shorter length of intensive care unit (ICU) (Heller et al., 2006) and hospital stay (Weiss et al., 2002; Tsekos et al., 2004; Grimm et al., 2006; Heller et al., 2006; Zhu et al., 2012), lesser mechanical ventilation (Tsekos et al., 2004), reduced infections (Badia-Tahull et al., 2010; Grau-Carmona et al., 2015), improved oxygenation index and reduction in renal replacement therapy days (Wang et al., 2008), reduced antibiotic demand (Heller et al., 2006), and decreased incidence and duration of systemic inflammatory response syndrome (Jiang et al., 2010; Zhu et al., 2012). Significant patient clinical outcomes were observed when fish oil was provided before surgery (Tsekos et al., 2004). However there are studies reporting no superior benefits for their critically ill and surgical patients (Mertes et al., 2006; Berger et al., 2008; Friesecke et al., 2008; Liang et al., 2008; Makay et al., 2011; Ma et al., 2012) when FOLE was prescribed with PN admixtures. Improvement in clinical outcomes is likely associated with dose of fish oil in PN (Tsekos et al., 2004; Heller et al., 2006). Heller et al. (2006) observed reduced mortality with FOLE dosed at 0.1 g/kg/day, shorter length of ICU and hospital stays with FOLE doses between 0.1 and 0.2 g/kg/day, and antibiotic demand reduced by 23% with infusion doses of 0.15–0.2 g/kg/day (Heller et al., 2006).
Issues Associated With Use of FOLEs
Since pure FOLE carries n-6 PUFA minimally, there is potential risk for essentially FA deficiency in patients administered with this emulsion as monotherapy. However some studies demonstrate patient success with single-therapy pure FOLE in pediatrics (Gura et al., 2005; De Meijer et al., 2009; Le et al., 2011). It is suggested that essential FA deficiency prevention is possible with pure FOLE dosed at 1 g/kg/day (Gura et al., 2005; Le et al., 2011).
Conclusions
This review relates fundamental knowledge on FA composition, functionality, and clinical evidence of lipids used in PN, which should be appreciated for future LE formulations. Clearly, LEs have evolved from just been a primary source of energy for PN to becoming therapeutic substrates capable of targeted outcomes in critically ill patients. It appears FA composition of LEs is the primary determinant of desired clinical outcomes, matched to its biological function. Options for LE choice becomes more specific based on performance outcomes as indicated for immuno-inflammatory, metabolic and clinical outcomes. Additionally the final composition of LE formulations must take into consideration the potential for essential FA deficiency, lipid peroxidation, phytosterol toxicity, hypertriglyceridemia, and hyperglycemia. It appears therefore, one type of LE alone cannot be uniformly applied to patients undergoing medical interventions.
We note that at this point, most clinical trials evaluating LEs describe only limited clinical outcomes. More studies reported the physical mixture MCT/LCT, STG-LE, and FOLE administrations benefit metabolic outcomes whereas studies on SOLE indicate greater risk on immuno-inflammatory outcomes. Current evidence is insufficient to support clinical benefits associated with FOLE use compared to OOLEs but some studies do report that fish oil included in PN may be beneficial for critically ill patients. More studies adequately powered, with standardized dosage and longer duration of supplementation are needed to indicate clear benefit of FOLE in hyperinflammatory states.
Author Contributions
BS, SN, and TK were involved in all processes of the review from literature search, study selection, and data extraction. BS and BK performed quality assessment. BS, SN, and BK finalized tables. BS, SN, SS, BK, and TK drafted the manuscript. AA, EF, and KS contributed to the writing and critical revision of the manuscript. All authors read, revised, and approved the final manuscript.
Funding
This work is fully supported by the Fundamental Research Grant Scheme (FRGS/1/2016/SKK03/UKM/01/1) from the Ministry of Higher Education, (MOHE) Malaysia. BS is a recipient of a PhD study scholarship from Universiti Kebangsaan Malaysia and BK is a PhD Zamalah scholar under the Vice Chancellor of UKM. SS is a recipient of mybrain scholarship from MOHE.
Conflict of Interest
Co-author KS is employed at the Malaysian Palm Oil Council. He has published widely in lipid science.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2020.00506/full#supplementary-material
References
- Badia-Tahull M. B., Llop-Talaveron J. M., Leiva-Badosa E. (2010). A randomized study on the clinical progress of high-risk elective major gastrointestinal surgery patients treated with olive oil-based parenteral nutrition with or without a fish oil supplement. Br. J. Nutr. 104, 737–741. 10.1017/S0007114510001066 [DOI] [PubMed] [Google Scholar]
- Ball M., White K. (1989). Comparison of medium and long chain triglyceride metabolism in intensive care patients on parenteral nutrition. Intensive Care Med. 15, 250–254. 10.1007/BF00271061 [DOI] [PubMed] [Google Scholar]
- Ball M. J. (1993). Parenteral nutrition in the critically ill: use of a medium chain triglyceride emulsion. Intensive Care Med. 19, 89–95. 10.1007/BF01708368 [DOI] [PubMed] [Google Scholar]
- Barbosa V. M., Miles E. A., Calhau C., Lafuente E., Calder P. C. (2010). Effects of a fish oil containing lipid emulsion on plasma phospholipid fatty acids, inflammatory markers, and clinical outcomes in septic patients: a randomized, controlled clinical trial. Crit. Care 14, R5. 10.1186/cc8844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battistella F. D., Widergren J. T., Anderson J. T., Siepler J. K., Weber J. C., MacColl K. (1997). A prospective, randomized trial of intravenous fat emulsion administration in trauma victims requiring total parenteral nutrition. J. Trauma 43, 52–58. 10.1097/00005373-199707000-00013 [DOI] [PubMed] [Google Scholar]
- Bellantone R., Bossola M., Carriero C., Malerba M., Nucera P., Ratto C., et al. (1999). Structured versus long-chain triglycerides: a safety, tolerance, and efficacy-randomized study in colorectal surgical patients. JPEN J. Parenter Enteral Nutr. 23, 123–127. 10.1177/0148607199023003123 [DOI] [PubMed] [Google Scholar]
- Berg J. M., Tymoczko J. L., Stryer L. (2012). Biochemistry. 7th ed. (New York: W.H. Freeman & Company; ). [Google Scholar]
- Berger M. M., Tappy L., Revelly J. P., Koletzko B. V., Gepert J., Corpataux J. M., et al. (2008). Fish oil after abdominal aorta aneurysm surgery. Eur. J. Clin. Nutr. 62, 1116–1122. 10.1038/sj.ejcn.1602817 [DOI] [PubMed] [Google Scholar]
- Biesalski H. K. (2009). Vitamin E requirements in parenteral nutrition. Gastroenterology 137, s92–s104. 10.1053/j.gastro.2009.07.073 [DOI] [PubMed] [Google Scholar]
- Boberg K. M., Akerlundb J. E., Bjorkhem I. (1989). Effect of sitosterol on the rate-limiting enzymes in cholesterol synthesis and degradation. Lipids 24, 9–12. 10.1007/BF02535257 [DOI] [PubMed] [Google Scholar]
- Bohnert H., Maurer M., Calder P. C., Pratschke J., Thul P., Müller V. (2018). Efficacy of a long-term home parenteral nutrition regimen containing fish oil derived n-3 polyunsaturated fatty acids: a single-centre, randomized, double blind study. Nutr. J. 17, 113. 10.1186/s12937-018-0419-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrin D. G., Ng K., Stoll B., Sáenz De Pipaón M. (2014). Impact of new-generation lipid emulsions on cellular mechanisms of parenteral nutrition–associated liver disease. Adv. Nutr. 5, 82–91. 10.3945/an.113.004796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W., Calder P. C., Cury-Boaventura M. E., De Waele E., Jakubowski J., Zaloga G. (2018). Biological and clinical aspects of an olive oil-based lipid emulsion – a review. Nutrients 10, 1–35. 10.3390/nu10060776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder P. C., Grimble R. F. (2002). Polyunsaturated fatty acids, inflammation and immunity. Eur. J. Clin. Nutr. 56, S14–S19. 10.1038/sj.ejcn.1601478 [DOI] [PubMed] [Google Scholar]
- Calder P. C., Jensen G. L., Koletzko B. V., Singer P., Wanten G. J. (2010). Lipid emulsions in parenteral nutrition of intensive care patients: current thinking and future directions. Intensive Care Med. 36, 735–749. 10.1007/s00134-009-1744-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder P. C. (2004). n–3 Fatty acids and cardiovascular disease: evidence explained and mechanisms explored. Clin. Sci. (Lond) 107, 1–11. 10.1042/CS20040119 [DOI] [PubMed] [Google Scholar]
- Calder P. C. (2012). Fatty acids, long-chain fatty acids and inflammation. Proc. Nutr. Soc 71, 284–289. 10.1017/S0029665112000067 [DOI] [PubMed] [Google Scholar]
- Calder P. C. (2013). Lipids for intravenous nutrition in hospitalised adult patients: a multiple choice of options. Proc. Nutr. Soc 72, 263–276. 10.1017/S0029665113001250 [DOI] [PubMed] [Google Scholar]
- Carpentier Y. A., Dupont I. E. (2000). Advances in intravenous lipid emulsions. World J. Surg. 24, 1493–1497. 10.1007/s002680010267 [DOI] [PubMed] [Google Scholar]
- Chambrier C., Guiraud M., Gibault J. P., Labrosse H., Bouletreau P. (1999). Medium- and long-chain triacylglycerols in postoperative patients: structured lipids versus a physical mixture. Nutrition 15, 274–277. 10.1016/S0899-9007(99)00006-4 [DOI] [PubMed] [Google Scholar]
- Chambrier C., Bannier E., Lauverjat M., Drai J., Bryssine S., Boulétreau P. (2004). Replacement of long chain triglyceride with medium chain triglyceride/long chain triglyceride lipid emulsion in patients receiving long term parenteral nutrition: effects on essential fatty acid status and plasma vitamin K1 levels. JPEN J. Parenter Enter Nutr. 28, 7–12. 10.1177/014860710402800107 [DOI] [PubMed] [Google Scholar]
- Chambrier C., Lauverjat M., Bouletreau P. (2006). Structured triglyceride emulsions in parenteral nutrition. Nutr. Clin. Pract. 21, 342–350. 10.1177/0115426506021004342 [DOI] [PubMed] [Google Scholar]
- Chen F. M., Wang J. Y., Huang T. J., Juang R. F., Huang T. J., Hsieh J. S. (2005). Efficacy of medium-chain triglycerides compared with long-chain triglycerides in total parenteral nutrition in patients with digestive tract cancer undergoing surgery. Kaohsiung J. Med. Sci. 21, 487–494. 10.1016/S1607-551X(09)70156-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Wang W., Hong C., Zhang M., Hong Y., Wang S., et al. (2017). Omega-3 fish oil reduces mortality due to severe sepsis with acute gastrointestinal injury grade III. Pharmacogn. Mag. 13, 407–412. 10.4103/pm.pm_418_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton P. T., Bowron A., Mills K. A., Massoud A., Casteels M., Milla P. J. (1993). Phytosterolemia in children with parenteral nutrition-associated cholestatic liver Disease. Gastroenterology 105, 1806–1813. 10.1016/0016-5085(93)91079-W [DOI] [PubMed] [Google Scholar]
- Cury-Boaventura M. F., Gorjao R., de Lima T. M., Newsholme P., Curi R. (2006). Comparative toxicity of oleic and linoleic acid on human lymphocytes. Life Sci. 78, 1448–1456. 10.1016/j.lfs.2005.07.038 [DOI] [PubMed] [Google Scholar]
- De Meijer V. E., Gura K. M., Le H. D., Meisel J. A., Puder M. (2009). Fish oil-based lipid emulsions prevent and reverse parenteral nutrition-associated liver disease: the Boston experience. JPEN J. Parenter Enteral Nutr. 33, 541–547. 10.1177/0148607109332773 [DOI] [PubMed] [Google Scholar]
- de Miranda Torrinhas R. S., Santana R., Garcia T., Cury-Boaventura M. F., Sales M. M., Curi R., et al. (2013). Parenteral fish oil as a pharmacological agent to modulate post-operative immune response: a randomized, double-blind, and controlled clinical trial in patients with gastrointestinal cancer. Clin. Nutr. 32, 503–510. 10.1016/j.clnu.2012.12.008 [DOI] [PubMed] [Google Scholar]
- Deckelbaum R. J., Hamilton J. A., Moser A., Bengtsson-Olivecrona G., Butbul E., Carpentier Y. A., et al. (1990). Medium-chain vs. long-chain triacylglycerol emulsion hydrolysis by lipoprotein lipase and hepatic lipase: implications for the mechanism of lipase action. Biochemisty 29, 1136–1142. 10.1021/bi00457a006 [DOI] [PubMed] [Google Scholar]
- Delgado G. E., Kramer B. K., Lorkowski S., März W., von Schacky C., Kleber M. E. (2017). Individual omega-9 monounsaturated fatty acids and mortality-the Ludwigshafen risk and cardiovascular health study. J. Clin. Lipidol. 11, 126–135. 10.1016/j.jacl.2016.10.015 [DOI] [PubMed] [Google Scholar]
- Demirer S., Sapmaz A., Karaca A. S., Kepenekci I., Aydintug S., Balci D., et al. (2016). Effects of postoperative parenteral nutrition with different lipid emulsions in patients undergoing major abdominal surgery. Ann. Surg. Treat Res. 91, 309–315. 10.4174/astr.2016.91.6.309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond I. R., Pencharz P. B., Wales P. W. (2009). Omega-3 lipids for intestinal failure associated liver disease. Semin. Pediatr. Surg. 18, 239–245. 10.1053/j.sempedsurg.2009.07.005 [DOI] [PubMed] [Google Scholar]
- Dionigi P., Dionigi R., Prati U., Pavesi F., Jemos V., Nazari S. (1985). Effect of Intralipid® on some immunological parameters and leukocyte functions in patients with esophageal and gastric cancer. Clin. Nutr. 4, 229–234. 10.1016/0261-5614(85)90008-1 [DOI] [PubMed] [Google Scholar]
- Driscoll D. F., Giampietro K., Wichelhaus D. P., Peterss H., Nehne J., Niemann W., et al. (2001). Physicochemical stability assessments of lipid emulsions of varying oil composition. Clin. Nutr. 20, 151–157. 10.1054/clnu.2001.0375 [DOI] [PubMed] [Google Scholar]
- Driscoll D. F., Ling P. R., Bistrian B. R. (2009). Pharmacopeial compliance of fish oil–containing parenteral lipid emulsion mixtures: Globule size distribution (GSD) and fatty acid analyses. Int. J. Pharm. 379, 125–130. 10.1016/j.ijpharm.2009.06.021 [DOI] [PubMed] [Google Scholar]
- Driscoll D. F. (2015). Commercial lipid emulsions and all-in-one mixtures for intravenous infusion – composition and physicochemical properties. World Rev. Nutr. Diet. 112, 150–162. 10.1159/000365430 [DOI] [PubMed] [Google Scholar]
- Dutot G., Melin C. (1991). Assessment of lipid peroxidation during lipid infusion: influence of fatty acid composition of fat emulsion. Clin. Nutr. 10, 51. 10.1016/0261-5614(91)90271-D [DOI] [Google Scholar]
- Ellegard L., Sunesson A., Bosaeus I. (2005). High serum phytosterol levels in short bowel patients on parenteral nutrition support. Clin. Nutr. Edinb Scotl. 24, 415–420. 10.1016/j.clnu.2005.01.001 [DOI] [PubMed] [Google Scholar]
- Ferezou J., Bach A. C. (1999). Structure and metabolic fate of triacylglycerol-and phospholipid-rich particles of commercial parenteral fat emulsions. Nutrition 15, 44–50. 10.1016/S0899-9007(98)00130-0 [DOI] [PubMed] [Google Scholar]
- Ferezou J., Nguyen T. L., Leray C., Hajri T., Frey A., Cabaret Y. (1994). Lipid composition and structure of commercial parenteral emulsions. Biochim. Biophys. Acta 1213, 149–158. 10.1016/0005-2760(94)90021-3 [DOI] [PubMed] [Google Scholar]
- Friesecke S., Lotze C., Kohler J., Heinrich A., Felix S. B., Abel P. (2008). Fish oil supplementation in the parenteral nutrition of critically ill medical patients: a randomized controlled trial. Intensive Care Med. 34, 1411–1420. 10.1007/s00134-008-1072-1 [DOI] [PubMed] [Google Scholar]
- Furukawa K., Yamamori H., Takagi K., Hayashi N., Suzuki R., Nakajima N., et al. (2002). Influences of soybean oil emulsion on stress response and cell mediated immune function in moderately or severely stressed patients. Nutrition 18, 235–240. 10.1016/S0899-9007(01)00784-5 [DOI] [PubMed] [Google Scholar]
- Garcıa-de-Lorenzo A., Denia R., Atlan P., Martinez-Ratero S., Le Brun A., Evard D., et al. (2005). Parenteral nutrition providing a restricted amount of linoleic acid in severely burned patients: a randomised double- blind study of an olive oil-based lipid emulsion v.medium/long chain triacylglycerols. Br. J. Nutr. 94, 221–230. 10.1079/BJN20051467 [DOI] [PubMed] [Google Scholar]
- Garnacho-Montero J., Ortiz-Leyba C., Jimenez-Jimenez F., Garcia-Garmendia J. L., Jiménez-Jiménez L. M., Garnacho-Montero M. C., et al. (2002). Clinical and metabolic effects of two lipid emulsions on the parenteral nutrition of septic patients. Nutrition 18, 134–138. 10.1016/S0899-9007(01)00716-X [DOI] [PubMed] [Google Scholar]
- Gogos C. A., Kalfarentzos F. E., Zoumbos N. C. (1990). Effect of different types of total parenteral nutrition on T lymphocyte subpopulations and NK cells. Am. J. Clin. Nutr. 51, 119–122. 10.1093/ajcn/51.1.119 [DOI] [PubMed] [Google Scholar]
- Goulet O., Antébi H., Wolf C., Talbotec C., Alcindor L. G., Corriol O., et al. (2010). A new intravenous fat emulsion containing soybean oil, medium-chain triglycerides, olive oil, and fish oil: a single-center, double- blind randomized study on efficacy and safety in pediatric patients receiving home parenteral nutrition. JPEN J. Parenter Enteral Nutr. 34, 485–495. 10.1177/0148607110363614 [DOI] [PubMed] [Google Scholar]
- Goulet O., de Potter S., Antebi H., Driss F., Colomb V., Béréziat G., et al. (1999). Long-term efficacy and safety of a new olive oil–based intravenous fat emulsion in pediatric patients: a double-blind randomized study. Am. J. Clin. Nutr. 70, 338–345. 10.1093/ajcn/70.3.338 [DOI] [PubMed] [Google Scholar]
- Gramlich L., Meddings L., Alberda C., Wichansawakun S., Robbins S., Driscoll D., et al. (2015). Essential fatty acid deficiency in 2015: the impact of novel intravenous lipid emulsions. J. Parenter Enter Nutr. 39, 61S–66S. 10.1177/0148607115595977 [DOI] [PubMed] [Google Scholar]
- Grau T., Ruiz de Adana J. C., Zubillaga S., Fuerte S., Girón C. (2003). Randomized study of two different fat emulsions in total parenteral nutrition of malnourished surgical patients: effect of infectious morbidity and mortality. Nutr. Hosp. 18, 159–166. [PubMed] [Google Scholar]
- Grau-Carmona T., Bonet-Saris A., Garcia-de-Lorenzo A., Sánchez-Alvarez C., Rodríguez-Pozo A., Acosta-Escribano J., et al. (2015). Influence of n-3 polyunsaturated fatty acids enriched lipid emulsions on nosocomial infections and clinical outcomes in critically ill patients: ICU lipids study. Crit. Care Med. 43, 31–39. 10.1097/CCM.0000000000000612 [DOI] [PubMed] [Google Scholar]
- Grimm H., Mertes N., Goeters C., Schlotzer E., Mayer K., Grimminger F. (2006). Improved fatty acid and leukotriene pattern with a novel lipid emulsion in surgical patients. Eur. J. Nutr. 45, 55–60. 10.1007/s00394-005-0573-8 [DOI] [PubMed] [Google Scholar]
- Gultekin G., Sahin H., Inanc N., Uyanik F., Ok E. (2014). Impact of omega-3 and omega-9 fatty acids enriched total parenteral nutrition on blood chemistry and inflammatory markers in septic patients. Pak J. Med. Sci. 30, 299–304. 10.12669/pjms.302.3957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunstone F. D., Harwood J. L., Dijkstra A. J. (2007). The Lipid Handbook. 3rd ed. (Florida: CRC Press; ). [Google Scholar]
- Gura K. M., Parsons S. K., Bechard L. J., Henderson T., Dorsey M., Phipatanakul W., et al. (2005). Use of a fish oil-based lipid emulsion to treat essential fatty acid deficiency in a soy allergic patient receiving parenteral nutrition. Clin. Nutr. 24, 839–847. 10.1016/j.clnu.2005.05.020 [DOI] [PubMed] [Google Scholar]
- Gurr M. I. (1992). Role of Fats In Food and Nutrition. 2nd ed. (New York: Elsevier Science Publishers Ltd.). [Google Scholar]
- Hamawy K. J., Moldawer L. L., Georgieff M., Valicenti A. J., Babayan V. K., Bistrian B. R., et al. (1985). The Henry M. Vars Award: the effect of lipid emulsions on reticuloendothelial system function in the injured animal. JPEN J. Parenter Enteral Nutr. 9, 559–565. 10.1177/0148607185009005559 [DOI] [PubMed] [Google Scholar]
- Han Y. Y., Lai S. L., Ko W. J., Chou C. H., Lai H. S. (2012). Effects of fish oil on inflammatory modulation in surgical intensive care unit patients. Nutr. Clin. Pract. 27, 91–98. 10.1177/0884533611429796 [DOI] [PubMed] [Google Scholar]
- Heller A. R., Rossler S., Litz R. J., Stehr S. N., Heller S. C., Koch R., et al. (2006). Omega-3 fatty acids improve the diagnosis-related clinical outcome. Crit. Care Med. 34, 972–979. 10.1097/01.CCM.0000206309.83570.45 [DOI] [PubMed] [Google Scholar]
- Heyland D. K., MacDonald S., Keefe L., Drover J. W. (1998). Total parenteral nutrition in the critically ill patient: a meta-analysis. JAMA 280, 2013–2019. 10.1001/jama.280.23.2013 [DOI] [PubMed] [Google Scholar]
- Hultin M., Mullertz A., Magali A. (1994). Metabolism of emulsions containing medium- and long-chain triglycerides or interesterified trigIycerdes. J. Lipid Res. 35, 1850–1860. [PubMed] [Google Scholar]
- Iovinelli G., Marinangeli F., Ciccone A., Ciccozzi A., Leonardis M., Paladini A., et al. (2007). Parenteral nutrition in ventilated patients with chronic obstructive pulmonary disease: long chain vs medium chain triglycerides. Minerva Anestesiol. 73, 65–76. [PubMed] [Google Scholar]
- Iyer K. R., Spitz L., Clayton P. (1998). BAPS prize lecture: new insight into mechanisms of parenteral nutrition–associated cholestasis: role of plant sterols. J. Pediatr. Surg. 33, 1–6. 10.1016/S0022-3468(98)90349-9 [DOI] [PubMed] [Google Scholar]
- Jadad A. R., Moore R. A., Carroll D., Jenkinson C., Reynolds D. J., Gavaghan D. J., et al. (1996). Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin. Trials. 17, 1–12. 10.1016/0197-2456(95)00134-4 [DOI] [PubMed] [Google Scholar]
- Jensen G. L., Mascioli E. A., Seidner D. L., Istfan N. W., Domnitch A. M., Selleck K., et al. (1990). Parenteral infusion of long- and medium-chain triglycerides and reticuloendothelial system function in man. JPEN J. Parenter Enteral Nutr. 14, 467–471. 10.1177/0148607190014005467 [DOI] [PubMed] [Google Scholar]
- Jiang Z. M., Zhang S. Y., Wang X. R., Yang N. F., Zhu Y., Wilmore D. A. (1993). Comparison of medium-chain and long-chain triglycerides in surgical patients. Ann. Surg. 217, 175–184. 10.1097/00000658-199302000-00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z. M., Wilmore D. W., Wang X. R., Wei J. M., Zhang Z. T., Gu Z. Y., et al. (2010). Randomized clinical trial of intravenous soybean oil alone versus soybean oil plus fish oil emulsion after gastrointestinal cancer surgery. Br. J. Surg. 97, 804–809. 10.1002/bjs.6999 [DOI] [PubMed] [Google Scholar]
- Köller M., Senkal M., Kemen M., König W., Zumtobel V., Muhr G. (2003). Impact of omega-3 fatty acid enriched TPN on leukotriene synthesis by leukocytes after major surgery. Clin. Nutr. 22, 59–64. 10.1054/clnu.2002.0592 [DOI] [PubMed] [Google Scholar]
- Kagawa Y., Maeda T., Kato Y., Ueda I., Kudo T., Watanabe N., et al. (2013). Influence of the slow infusion of a soybean oil emulsion on plasma cytokines and ex vivo T cell proliferation after an esophagectomy. JPEN J. Parenter Enteral Nutr. 37, 123–128. 10.1177/0148607112442216 [DOI] [PubMed] [Google Scholar]
- Karupaiah T., Sundram K. (2007). Effects of stereospecific positioning of fatty acids in triacylglycerol structures in native and randomized fats: a review of their nutritional implications. Nutr. Metab. (Lond). 4, 16. 10.1186/1743-7075-4-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karupaiah T., Ismail M. N., Sundram K. (2005). ““Dietary fatty acids and their influence on blood lipids and lipoproteins,”,” in Healthful Lipids. Eds. Akoh C. C., Lai O. M. ((Champaign, Illinois: AOCS Press; ), 171–203. [Google Scholar]
- Kim W., Khan N. A., McMurray D. N., Prior I. A., Wang N., Chapkin R. S. (2010). Regulatory activity of polyunsaturated fatty acids in T-cell signalling. Prog. Lipid Res. 49, 250–261. 10.1016/j.plipres.2010.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klek S., Chambrier C., Singer P., Rubin M., Bowling T., Staun M., et al. (2003). Four-week parenteral nutrition using a third generation lipid emulsion (SMOFlipid) – A double blind, randomised, multicentre study in adults. Clin. Nutr. 32, 224–231. 10.1016/j.clnu.2012.06.011 [DOI] [PubMed] [Google Scholar]
- Kruimel J. W., Naber T. H., van der Vliet J. A., Carneheim C., Katan M. B., Jansen J. B. (2001). Parenteral structured triglyceride emulsion improves nitrogen balance and is cleared faster from the blood in moderately catabolic patients. JPEN J. Parenter Enteral Nutr. 25, 237–244. 10.1177/0148607101025005237 [DOI] [PubMed] [Google Scholar]
- Le H. D., De Meijer V. E., Robinson E. M., Zurakowski D., Potemkin A. K., Arsenault D. A., et al. (2011). Parenteral fish-oil-based lipid emulsion improves fatty acid profiles and lipids in parenteral nutrition-dependent children. Am. J. Clin. Nutr. 94, 749–758. 10.3945/ajcn.110.008557 [DOI] [PubMed] [Google Scholar]
- Lenssen P., Bruemmer B. A., Bowden R. A., Gooley T., Aker S. N., Mattson D. (1998). Intravenous lipid dose and incidence of bacteremia and fungemia in patients undergoing bone marrow transplantation. Am. J. Clin. Nutr. 67, 927–933. 10.1093/ajcn/67.5.927 [DOI] [PubMed] [Google Scholar]
- Li X., Ying J., Zeng S., Shen L., Wan X., Li X., et al. (2007). A Short-term long-chain triglycerides infusion has no influence on immune function of adult patients undergoing gastrointestinal surgery. J. Parenter. Enter. Nutr. 31, 167–172. 10.1177/0148607107031003167 [DOI] [PubMed] [Google Scholar]
- Liang B., Wang S., Ye Y. J., Yang X. D., Wang Y. L., Qu J., et al. (2008). Impact of postoperative omega-3 fatty acid-supplemented parenteral nutrition on clinical outcomes and immunomodulations in colorectal cancer patients. World J. Gastroenterol. 14, 2434–2439. 10.3748/wjg.14.2434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren B. F., Ruokonen E., Magnusson-Borg K., Takala J. (2001). Nitrogen sparing effect of structured triglycerides containing both medium-and long-chain fatty acids in critically ill patients: a double blind randomized controlled trial. Clin. Nutr. 20, 43–48. 10.1054/clnu.2000.0156 [DOI] [PubMed] [Google Scholar]
- Ling W. H., Jones P. J. H. (1995). Dietary phytosterols: a review of metabolism, benefits and side effects. Life Sci. 57, 195–206. 10.1016/0024-3205(95)00263-6 [DOI] [PubMed] [Google Scholar]
- Linseisen J., Hoffmann J., Lienhard S., Jauch K. W., Wolfram G. (2000). Antioxidant status of surgical patients receiving TPN with an omega-3-fatty acid-containing lipid emulsion supplemented with alpha-tocopherol. Clin. Nutr. 19, 177–184. 10.1054/clnu.1999.0096 [DOI] [PubMed] [Google Scholar]
- Llop J. M., Virgili N., Moreno-Villares J. M., García-Peris P., Serrano T., Forga M., et al. (2008). Phytosterolemia in parenteral nutrition patients: implications for liver disease development. Nutrition 24, 1145–1152. 10.1016/j.nut.2008.06.017 [DOI] [PubMed] [Google Scholar]
- Lutz O., Meraıhi Z., Mura J. L., Frey A., Riess G. H., Bach A. C. (1989). Fat emulsion particle size: influence on the clearance rate and the tissue lipolytic activity. Am. J. Clin. Nutr. 50, 1370–1381. 10.1093/ajcn/50.6.1370 [DOI] [PubMed] [Google Scholar]
- Ma C. J., Sun L. C., Chen F. M., Lu C. Y., Shih Y. L., Tsai H. L., et al. (2012). A double blind randomized study comparing the efficacy and safety of a composite vs a conventional intravenous fat emulsion in postsurgical gastrointestinal tumor patients. Nutr. Clin. Pract. 27, 410–415. 10.1177/0884533611436115 [DOI] [PubMed] [Google Scholar]
- Ma C. J., Wu J. M., Tsai H. L., Huang C. W., Lu C. Y., Sun L. C., et al. (2015). Prospective double-blind randomized study on the efficacy and safety of an n-3 fatty acid enriched intravenous fat emulsion in postsurgical gastric and colorectal cancer patients. Nutr. J. 14, 9. 10.1186/1475-2891-14-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makay O., Kaya T., Firat O., Sozbilen M., Caliskan C., Gezer G., et al. (2011). ω-3 fatty acids have no impact on serum lactate levels after major gastric cancer surgery. JPEN J. Parenter Enteral Nutr. 35, 488–492. 10.1177/0148607110386611 [DOI] [PubMed] [Google Scholar]
- Mayer K., Fegbeutel C., Hattar K., Sibelius U., Krämer H. J., Heuer K. U., et al. (2003. a). Omega-3 vs. omega-6 lipid emulsions exert differential influence on neutrophils in septic shock patients: impact on plasma fatty acids and lipid mediator generation. Intensive Care Med. 29, 1472–1481. 10.1007/s00134-003-1900-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer K., Gokorsch S., Fegbeutel C., Hattar K., Rosseau S., Walmrath D., et al. (2003. b). Parenteral nutrition with fish oil modulates cytokine response in patients with sepsis. Am. J. Respir. Crit. Care Med. 167, 1321–1328. 10.1164/rccm.200207-674OC [DOI] [PubMed] [Google Scholar]
- Mertes N., Grimm H., Furst P., Stehle P. (2006). Safety and efficacy of a new parenteral lipid emulsion (SMOFlipid) in surgical patients: a randomized, double-blind, multicenter study. Ann. Nutr. Metab. 50, 253–259. 10.1159/000091683 [DOI] [PubMed] [Google Scholar]
- Metry A. A., Abdelaal W., Ragaei M., Refaat M., Nakhla G. (2014). SMOFlipid versus intralipid in postoperative ISU patients. J. Anesth Crit. Care Med. 1, 1–8. [Google Scholar]
- Miles E. A., Calder P. C. (2015). Fatty Acids, lipid emulsions and the immune and inflammatory systems. World Rev. Nutr. Diet 112, 17–30. 10.1159/000365426 [DOI] [PubMed] [Google Scholar]
- Monson J. R. T., Ramsden C. W., MacFie J., Brennan T. G., Guilloul P. J. (1986). Immunorestorative effect of lipid emulsions during total parenteral nutrition. Br. J. Surg. 73, 843–846. 10.1002/bjs.1800731027 [DOI] [PubMed] [Google Scholar]
- Morlion B. J., Torwesten E., Lessire H., Sturm G., Peskar B. M., Fürst P., et al. (1996). The effect of parenteral fish oil on leukocyte membrane fatty acid composition and leukotriene-synthesizing capacity in patients with postoperative trauma. Metabolism 45, 1208–1213. 10.1016/S0026-0495(96)90237-1 [DOI] [PubMed] [Google Scholar]
- Morlion B. J., Torwesten E., Wrenger K., Puchstein C., Furst P. (1997). What is the optimum n-3 to n-6 fatty acid (FA) ratio of parenteral lipid emulsions in postoperative trauma? Clin. Nutr. 16, 49S. [Google Scholar]
- Moussa M., Le B. J., Garcia J., Tkaczuk J., Ragab J., Dutot G., et al. (2000). In vivo effects of olive oil-based lipid emulsion on lymphocyte activation in rats. Clin. Nutr. 19, 49–54. 10.1054/clnu.1999.0076 [DOI] [PubMed] [Google Scholar]
- Naber A. H. J., Kruimel J. W. (2002). Structured triglycerides in human metabolism. Clin. Nutr. 21, 67–72.11884015 [Google Scholar]
- Ng K., Stoll B., Chacko S., Saenz de Pipaon M., Lauridsen C., Gray M., et al. (2016). Vitamin E in new-generation lipid emulsions protects against parenteral nutrition–associated liver disease in parenteral nutrition–fed preterm pigs. JPEN J. Parenter Enteral Nutr. 40, 656–671. 10.1177/0148607114567900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijveldt R. J., Tan A. M., Prins H. A., de Jong D., van Rij G. L., Wesdorp R. I., et al. (1998). Use of a mixture of medium-chain triglycerides and long-chain triglycerides versus long-chain triglycerides in critically ill surgical patients: a randomized prospective double-blind study. Clin. Nutr. 17, 23–29. 10.1016/S0261-5614(98)80039-3 [DOI] [PubMed] [Google Scholar]
- Olthof E. D., Roelofs H. M., Fisk H. L., Calder P. C., Wanten G. J. (2015). No clinical or biochemical evidence for essential fatty acid deficiency in home patients who depend on long-term mixed olive oil- and soybean oil-based parenteral nutrition. JPEN J. Parenter Enteral Nutr. 40, 982–988. 10.1177/0148607115581375 [DOI] [PubMed] [Google Scholar]
- Onar P., Yildiz B. D., Yildiz E. A., Besler T., Abbasoglu O. (2011). Olive oil–based fat emulsion versus soy oil–based fat emulsion in abdominal oncologic surgery. Nutr. Clin. Pract. 26, 61–65. 10.1177/0884533610392920 [DOI] [PubMed] [Google Scholar]
- Osowska S., Kunecki M., Sobocki J., Tokarczyk J., Majewska K., Omidi M., et al. (2018). Effect of changing the lipid component of home parenteral nutrition in adults. Clin. Nutr. 2018, 1355–1361. 10.1016/j.clnu.2018.05.028 [DOI] [PubMed] [Google Scholar]
- Pianese P., Salvia G., Campanozzi A., D’Apolito O., Dello Russo A., Pettoello-Mantovani M., et al. (2008). Sterol profiling in red blood cell membranes and plasma of newborns receiving total parenteral nutrition. J. Pediatr. Gastroenterol. Nutr. 47, 645–651. 10.1097/MPG.0b013e318170956a [DOI] [PubMed] [Google Scholar]
- Piper S. N., Schadeb I., Beschmanna R. B., Maleck W. H., Boldt J., Röhm K. D. (2009). Hepatocellular integrity after parenteral nutrition: comparison of a fish-oil-containing lipid emulsion with an olive-soybean oil-based lipid emulsion. Eur. J. Anaesthesiol. 26, 1076–1082. 10.1097/EJA.0b013e32832e08e0 [DOI] [PubMed] [Google Scholar]
- Pironi L., Guidetti M., Zolezzi C., Fasano M. C., Paganelli F., Merli C., et al. (2003). Peroxidation potential of lipid emulsions after compounding in all-in-one solutions. Nutrition 19, 784–788. 10.1016/S0899-9007(03)00099-6 [DOI] [PubMed] [Google Scholar]
- Puiggros C., Sanchez J., Chacon P., Sabín P., Roselló J., Bou R., et al. (2009). Evolution of lipid profile, liver function, and pattern of plasma fatty acids according to the type of lipid emulsion administered in parenteral nutrition in the early postoperative period after digestive surgery. J. Parenter Enteral Nutr. 33, 501–512. 10.1177/0148607109333001 [DOI] [PubMed] [Google Scholar]
- Radermacher P., Santak B., Strobach H., Schrör K., Tarnow J. (1992). Fat emulsions containing medium chain triglycerides in patients with sepsis syndrome: effects on pulmonary hemodynamics and gas exchange. Intensive Care Med. 18, 231–234. 10.1007/BF01709838 [DOI] [PubMed] [Google Scholar]
- Raman M., Almutairdi A., Mulesa L., Alberda C., Beattie C., Gramlich L. (2017). Parenteral nutrition and lipids. Nutrients 9, E388. 10.3390/nu9040388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raphael B. P., Duggan C. (2012). Prevention and treatment of intestinal failure–associated liver disease in children. Semin. Liver Dis. 32, 341–347. 10.1055/s-0032-1329903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimund J. M., Rahmi G., Escalin G., Pinna G., Finck G., Muller C. D., et al. (2005). Efficacy and safety of an olive oil-based intravenous fat emulsion in adult patients on home parenteral nutrition. Aliment Pharmacol. Ther. 21, 445–454. 10.1111/j.1365-2036.2005.02354.x [DOI] [PubMed] [Google Scholar]
- Roulet M., Frascarolo P., Pilet M., Chapuis G. (1997). Effects of intravenously infused fish oil on platelet fatty acid phospholipids composition and on platelet function in postoperative trauma. JPEN J. Parenter Enteral Nutr. 21, 296–301. 10.1177/0148607197021005296 [DOI] [PubMed] [Google Scholar]
- Sabater J., Masclans J. R., Sacanell J., Chacon P., Sabin P., Planas M. (2011). Effects of an omega-3 fatty acid-enriched lipid emulsion on eicosanoid synthesis in acute respiratory distress syndrome (ARDS): a prospective, randomized, doubleblind, parallel group study. Nutr. Metab. 8, 22. 10.1186/1743-7075-8-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala-Vila A., Barbosa V. M., Calder P. C. (2007). Olive oil in parenteral nutrition. Curr. Opin. Clin. Nutr. Metab. Care 10, 165–174. 10.1097/MCO.0b013e32802bf787 [DOI] [PubMed] [Google Scholar]
- Sandstrom R., Hyltander A., Korner U., Lundholm K. (1995). Structured triglycerides were well tolerated and induced increased whole body fat oxidation compared with long-chain triglycerides in postoperative patients. JPEN J. Parenter Enteral Nutr. 19, 381–386. 10.1177/0148607195019005381 [DOI] [PubMed] [Google Scholar]
- Saubion J. L., Hazane C., Jalabert M. (1998). The role of sterols in lipid emulsions for parenteral nutrition. Nutrition 14, 477–478. 10.1016/S0899-9007(98)00025-2 [DOI] [PubMed] [Google Scholar]
- Savini S., D’Ascenzo R., Biagetti C., Serpentini G., Pompilio A., Bartoli A. (2013). The effect of five intravenous lipid emulsions on plasma phytosterols in preterm infants receiving parenteral nutrition: a randomized clinical trial. Am. J. Clin. Nutr. 98, 312–318. 10.3945/ajcn.112.056556 [DOI] [PubMed] [Google Scholar]
- Schauder P., Rohn U., Schafer G., Korff G., Schenk H. D. (2002). Impact of fish oil enriched total parenteral nutrition on DNA synthesis, cytokine release and receptor expression by lymphocytes in the postoperative period. Br. J. Nutr. 87, S103–S110. 10.1079/BJN2001463 [DOI] [PubMed] [Google Scholar]
- Schloerb P. R. (2004). Glucose in parenteral nutrition: a survey of us medical centers. JPEN J. Parenter Enteral Nutr. 28, 447–452. 10.1177/0148607104028006447 [DOI] [PubMed] [Google Scholar]
- Sedman P. C., Somers S. S., Ramsden C. W., Brennan T. G., Guillou P. J. (1991). Effects of different lipid emulsions on lymphocyte function during total parenteral nutrition. Br. J. Surg. 78, 1396–1399. 10.1002/bjs.1800781142 [DOI] [PubMed] [Google Scholar]
- Seidner D. L., Mascioli E. A., Istfan N. W., Porter K. A., Selleck K., Blackburn G. L., et al. (1989). Effects of long-chain triglyceride emulsions on reticuloendothelial system function in humans. JPEN J. Parenter Enteral Nutr. 13, 614–619. 10.1177/0148607189013006614 [DOI] [PubMed] [Google Scholar]
- Senkal M., Geier B., Hannemann M., Deska T., Linseisen J., Wolfram G., et al. (2007). Supplementation of w-3 fatty acids in parenteral nutrition beneficially alters phospholipid fatty acid pattern. JPEN J. Parenter Enteral Nutr. 31, 12–17. 10.1177/014860710703100112 [DOI] [PubMed] [Google Scholar]
- Shearer M. J. (2009). Vitamin K in parenteral nutrition. Gastroenterology 137, S105–S118. 10.1053/j.gastro.2009.08.046 [DOI] [PubMed] [Google Scholar]
- Smirniotis V., Kostopanagiotou G., Vassiliou J., Arkadopoulos N., Vassiliou P., Datsis A., et al. (1998). Long chain versus medium chain lipids in patients with ARDS: effects on pulmonary haemodynamics and gas exchange. Intensive Care Med. 24, 1029–1033. 10.1007/s001340050711 [DOI] [PubMed] [Google Scholar]
- Steger P. J. K., Muhlebach S. F. (1998). Lipid peroxidation of IV lipid emulsions in TPN bags: the influence of tocopherols. Nutrition 14, 179–185. 10.1016/S0899-9007(97)00438-3 [DOI] [PubMed] [Google Scholar]
- Sungurtekin H., Degirmenci S., Sungurtekin U., Oguz B. E., Sabir N., Kaptanoglu B. (2011). Comparison of the effects of different intravenous fat emulsions in patients with systemic inflammatory response syndrome and sepsis. Nutr. Clin. Pract. 26, 665–671. 10.1177/0884533611418783 [DOI] [PubMed] [Google Scholar]
- Tsekos E., Reuter C., Stehle P., Boeden G. (2004). Perioperative administration of parenteral fish oil supplements in a routine clinical setting improves patient outcome after major abdominal surgery. Clin. Nutr. 23, 325–330. 10.1016/j.clnu.2003.07.008 [DOI] [PubMed] [Google Scholar]
- Ulrich H., Pastores S. M., Kvetan V. (1996). Parenteral use of medium-chain triglycerides: A reappraisal. Nutrition 12, 231–238. 10.1016/S0899-9007(96)00089-6 [DOI] [PubMed] [Google Scholar]
- Umpierrez G. E., Spiegelman R., Zhao V., Smiley D. D., Pinzon I., Griffith D. P., et al. (2012). A double-blind, randomized clinical trial comparing soybean oil–based versus olive oil–based lipid emulsions in adult medical–surgical intensive care unit patients requiring parenteral nutrition. Crit. Care Med. 40, 1792–1798. 10.1097/CCM.0b013e3182474bf9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahedi K., Atlan P., Joly F., Le Brun A., Evard D., Perennec V., et al. (2005). A 3-month double-blind randomised study comparing an olive oil with a soybean oil based intravenous lipid emulsion in home parenteral nutrition patients. Br. J. Nutr. 94, 909–916. 10.1079/BJN20051550 [DOI] [PubMed] [Google Scholar]
- Vanek V. W., Seidner D. L., Allen P., Bistrian B., Collier S., Gura K., et al. (2012). A.S.P.E.N. position paper: clinical role or alternative intravenous fat emulsions. Nutr. Clin. Pract. 27, 150–192. 10.1177/0884533612439896 [DOI] [PubMed] [Google Scholar]
- Verleyen T., Forcades M., Verhe R., Dewettinck K., Huyghebaert A., De Greytet W. (2002). Analysis of free and esterified sterols in vegetable oils. J. Am. Oil Chem. Soc 79, 117–122. 10.1007/s11746-002-0444-3 [DOI] [Google Scholar]
- Wachtler P., Konig W., Senkal M., Kemen M., Köller M. (1997). Influence of a total parenteral nutrition enriched with n-3 fatty acids on leukotriene synthesis of peripheral leukocytes and systemic cytokine levels in patients with major surgery. J. Trauma 42, 191–198. 10.1097/00005373-199702000-00004 [DOI] [PubMed] [Google Scholar]
- Waitzberg D. L., Bellinati-Pires R., Salgado M. M., Hypolito I. P., Colleto G. M., Yagi O., et al. (1997). Effect of total parenteral nutrition with different lipid emulsions of human monocyte and neutrophil functions. Nutrition 13, 128–132. 10.1016/S0899-9007(96)00386-3 [DOI] [PubMed] [Google Scholar]
- Waitzberg D. L., Lotierzol P. H., Logullo A. F., Torrinhas R. S., Pereira C. C., Meier R. (2002). Parenteral lipid emulsions and phagocytic systems. Br. J. Nutr. 87, S49–S57. 10.1079/BJN2001456 [DOI] [PubMed] [Google Scholar]
- Waitzberg D. L., Torrinhas R. S., Jacintho T. M. (2006). New parenteral lipid emulsions for clinical use. JPEN J. Parenteral Enteral Nutr. 30, 351–367. 10.1177/0148607106030004351 [DOI] [PubMed] [Google Scholar]
- Wang X., Li W., Li N., Li J. (2008). Omega-3 fatty acids-supplemented parenteral nutrition decreases hyper inflammatory response and attenuates systemic disease sequelae in severe acute pancreatitis: a randomized and controlled study. JPEN J. Parenter Enteral Nutr. 32, 236–241. 10.1177/0148607108316189 [DOI] [PubMed] [Google Scholar]
- Wang J., Yu J. C., Kang W. M., Ma Z. Q. (2012). Superiority of a fish oil-enriched emulsion to medium-chain triacylglycerols/long-chain triacylglycerols in gastrointestinal surgery patients: a randomized clinical trial. Nutrition 28, 623–629. 10.1016/j.nut.2011.08.004 [DOI] [PubMed] [Google Scholar]
- Wanten G. J. A., Calder P. C. (2007). Immune modulation by parenteral lipid emulsions. Am. J. Clin. Nutr. 85, 1171–1184. 10.1093/ajcn/85.5.1171 [DOI] [PubMed] [Google Scholar]
- Wanten G., Beunk J., Naber A., Swinkels D. (2002). Tocopherol isoforms in parenteral lipid emulsions and neutrophil activation. Clin. Nutr. 21, 417–422. 10.1054/clnu.2002.0570 [DOI] [PubMed] [Google Scholar]
- Weiss G., Meyer F., Matthies B., Pross M., Koenig W., Lippert H. (2002). Immunomodulation by perioperative administration of n-3 fatty acids. Br. J. Nutr. 87, S89–S94. 10.1079/BJN2001461 [DOI] [PubMed] [Google Scholar]
- Wichmann M. W., Thul P., Czarnetzki H. D., Morlion B. J., Kemen M., Jauch K. W. (2007). Evaluation of clinical safety and beneficial effects of a fish oil containing lipid emulsion (Lipoplus, MLF541): data from a prospective, randomized, multicenter trial. Crit. Care Med. 35, 700–706. 10.1097/01.CCM.0000257465.60287.AC [DOI] [PubMed] [Google Scholar]
- Williams C. M. (1997). Postprandial lipid metabolism: effects of dietary fatty acids. Proc. Nutr. Soc 6, 679–692. 10.1079/PNS19970068 [DOI] [PubMed] [Google Scholar]
- Wretlind A. (1981). Development of fat emulsions. JPEN J. Parenteral Enteral Nutr. 5, 230–235. 10.1016/S0899-9007(99)00102-1 [DOI] [PubMed] [Google Scholar]
- Wu M. H., Wang M. Y., Yang C. Y., Kuo M. L., Lin M. T. (2014). Randomized clinical trial of new intravenous lipid (SMOFlipid 20%) versus medium-chain triglycerides/long-chain triglycerides in adult patients undergoing gastrointestinal surgery. JPEN J. Parenter Enteral Nutr. 38, 800–808. 10.1177/0148607113512869 [DOI] [PubMed] [Google Scholar]
- Wu G. H., Zaniolo O., Schuster H., Schlotzer E., Pradelli L. (2017). Structured triglycerides versus physical mixtures of medium- and long-chain triglycerides for parenteral nutrition in surgical or critically ill adult patients: systematic review and meta-analysis. Clin. Nutrition. 36, 150–161. 10.1016/j.clnu.2016.01.004 [DOI] [PubMed] [Google Scholar]
- Xu Z., Harvey K. A., Pavlina T., Dutot G., Hise M., Zaloga G. P., et al. (2012). Steroidal compounds in commercial parenteral lipid emulsions. Nutrients 4, 904–921. 10.3390/nu4080904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M. W., Tang D. N., Hou J., Wei J. M., Hua B., Sun J. H., et al. (2012). Impact of fish oil enriched total parenteral nutrition on elderly patients after colorectal cancer surgery. Chin. Med. J. (Engl) 125, 178–181. 10.3760/cma.j.issn.0366-6999.2012.02.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.