Abstract
Phosphorylation of proteins on serine/threonine residues represents an important biochemical mechanism to regulate several cellular processes. Polo-like kinases (PLKs) are a family of serine-threonine kinases that play an imminent role in cell cycle regulation in yeast to humans, and thus an important therapeutic target for cancers. The present study provides insights into the enzymatic features of Saccharomyces cerevisiae PLK, Cdc5 using in vitro casein phosphorylation assays. The recombinant yeast PLK, GST-Cdc5 showed maximum casein phosphorylation activity at 30 °C, pH 9 and 45 min of incubation period. GST-Cdc5 exhibited a KM of 1.35 μM for casein, and high affinity for ATP, since addition of non-radioactive ATP chased out casein phosphorylation by radiolabeled ATP. The recombinant enzyme showed maximum kinase activity at 2.7 μM of GST-Cdc5. Casein was found to be the best in vitro substrate of GST-Cdc5 followed by BSA (Bovine Serum Albumin) and MBP (Myelin Basic Protein). Of the metal ions tested, Mg2+ (at 20 mM) was found to enhance GST-Cdc5 kinase activity, while Ca2+ (at 5 mM) and Mn2+ (at 10 mM) inhibited the same. The presence of EDTA, SDS and PMSF inhibited phosphorylation by GST-Cdc5, while DTT had no effect. The recombinant GST-Cdc5 can be used as a tool for deciphering PLKs’ structure and functions, which are still at infancy.
Keywords: Biological sciences, Cell biology, Proteins, Biochemistry, Cancer research, Health sciences, Oncology, PLK, Cdc5, Saccharomyces cerevisiae, GST-Cdc5, In vitro kinase assay, Mg2+
Biological sciences; Cell biology; Proteins; Biochemistry; Cancer research; Health sciences; Oncology; PLK; Cdc5; Saccharomyces cerevisiae; GST-Cdc5; In vitro kinase assay; Mg2+
1. Introduction
Polo-like kinases (PLKs) are serine-threonine kinases, which regulate the cell cycle progression in eukaryotes [1]. They comprise of N-terminal kinase domain (KD) and C-terminal signature motif called polo box domain (PBD) [2, 3]. PLKs were first identified in fruit flies and subsequently in most eukaryotes, except plants. In humans, multiple PLK members are known (Plk 1, Plk 2, Plk 3, Plk 4), while there is a single PLK, Cdc5 in budding yeast Saccharomyces cerevisiae [2, 3, 4].
CDC5 is an essential gene and loss of its function directly leads to mitotic arrest [4]. CDC5 encodes a polypeptide of 705 amino acids, with an N-terminal kinase domain critical for catalytic functions, and a C-terminal polo box domain containing two polo-boxes PB1 and PB2 [1,5,6]. The PBD functions as a phosphopeptide binding motif, thereby regulating the localization of the PLK to its target substrates/locations [5]. Cdc5 exhibits high level of conservation with the human PLK1. It shows 49% identity (69–70% similarity) in kinase domain and 33–46% identity (53–61% similarity) in polo box domain [7]. Consistent with structural conservation, Cdc5 shows significant functional conservation with mammalian PLK1, since human PLK1 can complement the defects in S. cerevisiae mutants lacking functional Cdc5 [8,9].
PLKs regulate a variety of functions, including centrosome maturation [10], mitosis [11], and mitotic exit [12]. PLKs are also involved in DNA replication, DNA repair, and other post mitotic functions [1, 13, 14]. In S. cerevisiae, Cdc5 regulates G2/M phase transition [15], metaphase to anaphase transition during mitosis [7, 16], exit from mitosis and cytokinesis [7]. Cdc5 also plays an important role during meiosis in yeasts, including meiosis-specific events such as pachytene exit, and meiosis-I [17, 18].
In humans, overexpression of PLKs is associated with carcinogenesis, thereby inflicting PLK as an anti-cancer therapeutic target [19]. Thus, understanding the biochemical characteristics of PLKs will contribute significantly to advances in therapeutics development. The present study was undertaken to develop an amenable system for biochemical characterization of the yeast PLK, Cdc5. So far, there are no reports on the in vitro studies of Cdc5 or any other PLK. Studies on PLKs in genetically amenable lower eukaryotes such as budding yeast can provide valuable insights into the functions of PLKs of higher eukaryotes.
2. Methods
2.1. Chemicals
Chemicals and reagents were purchased from GE Biosciences and SIGMA. Dephosphorylated casein (cat no. M4032; Sigma, USA) was used as a substrate for kinase assays. Radioactive ATP (γ-P32-ATP 3000 Ci/mMol) was obtained from BARC, Mumbai and used in radioactive facility, NIPGR, New Delhi. Myelin-basic protein (MBP), histone type IIIS, and Bovine serum albumin (BSA) were procured from Sigma, USA.
2.2. Biochemical characterization of GST-Cdc5 kinase
GST-Cdc5 was purified as described [20]. The kinase activity of recombinant Cdc5 was assayed in vitro and quantified. The kinase assay was performed according to Mortenson et al. [21], with slight modifications. Dephosphorylated casein (Sigma cat no. M4032) was used as a substrate for the in vitro kinase assays. β- Glycerol phosphate and sodium ortho-vanadate (Na3VO4) served as phosphatase inhibitors. Kinase reactions were performed by incubating purified GST-Cdc5 kinase (2.5 μg) in a 20 μl reaction containing 50 mM Tris-Cl (pH 7.5), 10 mM MgCl2, 5 mM MnCl2, 1 mM dithiothreitol (DTT), 1 mM β-glycerol phosphate, 1 mM Na3VO4 supplemented with 0.25 mM ATP, 0.1 μl of 10 mCi/ml γ-32P ATP (1 μCi; 3000 Ci/mmol) and 5 μg casein. The kinase reactions were incubated for 30 min at 30 °C followed by addition of 4 μl of 5X Laemmli SDS-PAGE sample buffer. Samples were resolved on 10% SDS-PAGE gel. After the run, the radioactive gel was wrapped in plastic wrap and exposed to the phosphor screen in a cassette for 12–16 h. The screen was scanned and developed using Typhoon ImageQ.
3. Results and discussion
PLKs regulate multiple events of cell division. However, there are no biochemical studies on the kinase activity of any of the PLKs. Previously, we reported the purification of recombinant yeast PLK as GST-Cdc5, which was found to be exhibit kinase activity in vitro [20]. In the present study, we report the characterization of in vitro kinase activity of recombinant Cdc5.
3.1. Recombinant Cdc5 is a robust kinase optimally active at 30 °C and alkaline pH
To study the kinetics of GST-Cdc5 activity, reaction mixtures were incubated at 30 °C for varying time, ranging for 0–90 min. Quantification of the intensity of casein phosphorylation showed that the kinetics of phosphorylation was linear until ~45 min, after which saturation in casein phosphorylation was observed (Figure 1A). Thus, GST-Cdc5 kinase is a fast and robust enzyme, with maximum substrate (casein) phosphorylation at 45 min of incubation.
Figure 1.
Effect of reaction parameters (time, temperature and pH) on GST-Cdc5 casein phosphorylation activity: A) Autoradiogram showing kinetics of casein phosphorylation by GST-Cdc5 kinase at different time intervals as indicated. Graph represents the quantitation of casein phosphorylation. The intensity of phosphorylated casein band was quantified and plotted in arbitrary units against indicated time of incubation (minutes). B) and C) represent the casein phosphorylation by GST-Cdc5 at varying temperatures and pH, respectively. The data represent an average of two independent experiments. Error bars denote range of upper and lower values. The full images of the blots in A, B, and C are provided in supplementary material.
The effect of temperature on GST-Cdc5 kinase activity was studied by incubating reaction mixtures at 15–40 °C for 45 min. Quantification of casein phosphorylation showed that GST-Cdc5 exhibits a typical bell shaped profile of kinase activity, with maximum activity at 30 °C (Figure 1B). The optimum temperature of GST-Cdc5 kinase, i.e., 30 °C, is consistent with the optimum temperature of growth of Saccharomyces cerevisiae.
Casein phosphorylation by the GST-Cdc5 kinase was determined over the pH range of 6.0–10. GST-Cdc5 exhibited a narrow pH activity profile with maximum kinase activity at pH 9.0 (Figure 1C). In most cases, pH determines the ionization state of the active site residues and cofactors/coenzymes involved in catalysis. Thus, Cdc5 requires alkaline pH for optimal transfer of phosphate group to its substrate.
3.2. Recombinant Cdc5 exhibits hyperbolic substrate kinetics and high affinity for its substrates
To optimize the GST-Cdc5 concentration on its activity, kinase assay was performed with a fixed amount of casein (5 μg) at different concentrations of GST-Cdc5 (0.5–3.15 μM) Quantification of phosphorylated casein showed that kinase activity was maximum at 2.7 μM of GST-Cdc5 kinase (Figure 2A).
Figure 2.
Characterization of enzyme and substrate kinetics of GST-Cdc5. A) Effect of GST-Cdc5 concentration on GST-Cdc5 kinase activity. Autoradiogram showing the kinase assay reactions with indicated GST-Cdc5 concentrations and graph showing the quantitation of casein phosphorylation by GST-Cdc5. B) Kinase activity of GST-Cdc5 with varying concentrations of casein as indicated and graph showing the quantitation of casein phosphorylation. C) Autoradiogram showing the kinase assays reactions with varying ATP concentrations as indicated and graph represents the quantitation of casein phosphorylation by GST-Cdc5. The data represents an average of two independent experiments. Error bars denote range of upper and lower values. The full images of the blots in A, B, and C are provided in supplementary material.
The reaction catalyzed by protein kinases are bi-substrate reactions using ATP (phosphoryl donor) and protein substrates (phosphoryl acceptor). Hence, the kinetics of GST-Cdc5 activity was analyzed for the substrates, casein and ATP. To study the effect of casein concentration on GST-Cdc5 kinase activity, casein concentration was varied from 1.66- 29 μM. Phosphorylation activity was measured as described in methods. A linear increase in casein phosphorylation was observed with increasing concentrations of casein (Figure 2B). Quantification of casein phosphorylation showed that GST-Cdc5 kinase exhibits a typical hyperbolic pattern of enzyme catalysis, and thus behaves in accordance with Michaelis Menten's enzyme kinetics (Figure 2B). Casein phosphorylation was maximum at ~ 16.6 μM of casein (Figure 2B). From these data, the KM of GST-Cdc5 kinase for casein was determined to be 1.35 μM, which is indicative of good affinity of GST-Cdc5 for casein. There are no other reports on KM for other PLKs, including Cdc5.
The effect of ATP concentration on GST-Cdc5 kinase activity was studied with varying concentrations of unlabeled ATP (0.25–2.5 mM). An exponential decrease in casein phosphorylation was observed with increasing concentrations of non-radioactive ATP (Figure 2C). Quantification of casein phosphorylation indicated that GST-Cdc5 exhibited high specificity for ATP, since unlabeled ATP competed out the radiolabeled ATP. These findings indicate high affinity of recombinant Cdc5 kinase for ATP.
3.3. Screening of in vitro substrates of GST-Cdc5 kinase
In the present study, casein was used as a generic substrate for assaying kinase activity of PLKs [21]. To determine the possibility that GST-Cdc5 can phosphorylate other generic substrates, histone type IIIS, BSA and MBP were used in the kinase assay (5 μg each). Casein was found to be maximally phosphorylated by GST-Cdc5 followed by MBP and BSA, although the latter two showed very weak phosphorylation (Figure 3). However, GST-Cdc5 did not phosphorylate histone type III S (Figure 3). The presence of active GST-Cdc5 in all the reactions was confirmed by the detection of robust auto-phosphorylation of GST-Cdc5 (indicated as GST-Cdc5-P) in all the samples (Figure 3; [20]). These results indicate that casein is the best substrate for GST-Cdc5, which is consistent with the studies of Mortenson and coworkers [21].
Figure 3.
Kinase activity of GST-Cdc5 on different substrates in vitro. Autoradiogram showing the phosphorylation of different substrates, casein, BSA, Histone type IIIS and MBP by GST-Cdc5 kinase. The full image of the blot is provided in supplementary material.
3.4. Casein phosphorylation by GST-Cdc5 was enhanced by Mg2+ ions
To study the effects of different metal ions, the kinase assay mixtures were supplemented with 2 fold excess of either MgCl2 (20 mM), MnCl2 (10 mM) or CaCl2 (5 mM). The addition of Mg2+ greatly enhanced the kinase activity, while Ca2+ and Mn2+ inhibited casein phosphorylation as compared to the standard reaction (Figure 4A). These results indicate that Mg2+ serves as an activator of GST-Cdc5 kinase, while Ca2+ and Mn2+ are inhibitors of kinase activity. Consistent with the activating role of Mg2+, addition of EDTA (5 and 10 mM) completely inhibited the kinase activity of GST-Cdc5 kinase (Figure 4B; lanes EDTA). Further studies are required to decipher the role of metal ions in the catalysis of recombinant PLK.
Figure 4.
Effect of metal ions and other agents on GST-Cdc5 kinase activity. A) Effect of metal ions on GST-Cdc5 kinase activity. Lane 1, Standard reaction; lane 2, standard reaction + MgCl2 (20 mM); lane 3, standard reaction + MnCl2 (10 mM); lane 4, standard reaction + CaCl2 (5 mM). Quantitation of casein phosphorylation in a standard reaction or in the presence of indicated metal ions is indicated in graph. The data represents an average of two independent experiments. Error bars denote range of upper and lower values. B) Effect of inhibitors on GST-Cdc5 kinase activity. The standard indicates the kinase assay reaction without any inhibitor. The full images of the blots in A, and B are provided in supplementary material.
Similar to the effect of EDTA, addition of either SDS, a denaturant or PMSF inhibited both phosphorylation by GST-Cdc5 (Figure 4B). On the other hand, DTT had no effect on kinase activity in comparison to the standard reaction (Figure 4B).
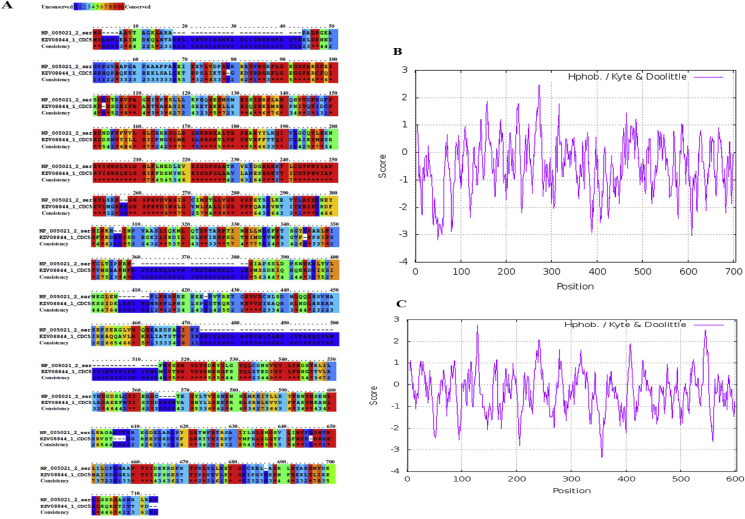
3.5. Sequence alignment and hydropathy analysis of budding yeast Cdc5 and human PLK1
Towards application of recombinant Cdc5 as a tool for cancer drug discovery, the protein sequences of yeast Cdc5 and human PLK1 were analyzed for sequence conservation and hydrophobicity. Multiple sequence alignment of Cdc5 and PLK1 protein sequences showed a limited degree of conservation (35 % sequence identity) between the two sequences (Figure 5A). Further analysis revealed a high degree of conservation between Cdc5 and PLK1 in the regions corresponding to the kinase domain (KD) towards amino-termini and PBDs towards C-termini (Figure 5A, red colored regions). These results are consistent with earlier reports by Lee et al. [7]. Hydropathy plots revealed the presence of interspersed regions of hydrophobic and hydrophilic residues in both Cdc5 and PLK1 (Figure 5B). The overall hydropathy scores (-3.2 for human PLK1 and -2.6 for budding yeast Cdc5) indicate that both Cdc5 and PLK1 are hydrophilic proteins, and thus amenable as tools for cancer drug discovery.
Figure 5.
Protein Sequence Alignment and hydropathy analysis of the PLKs from budding yeast Saccharomyces cerevisiae (Cdc5) and humans (PLK1). A) Alignment of budding yeast Cdc5 and human PLK1 protein sequences. The amino acid sequences of human PLK1 (NP_005021_2_ser) and Saccharomyces cerevisiae Cdc5 (KZV08844_1_CDC5) were subjected to multiple sequence alignment using PRALINE. The conservation scale is indicated on the top, with 0 score for the least conserved position to 10 for the most conserved alignment position. B & C) Hydropathy analysis of budding yeast Cdc5 and human PLK1. The amino acid sequences of human PLK1 (NP_005021_2_ser) and Cdc5 (KZV08844_1_CDC5) were subjected to Expasy-Protscale analysis and the Kyte and Doolittle hydropathy plots of budding yeast Cdc5 (panel B) and human PLK1 (panel C) were generated. Hydropathy plots represent the hydrophobicity score plotted against the position of amino acid residues of the respective proteins.
4. Conclusions
The present study provides insights into the enzymatic features of S. cerevisiae PLK, Cdc5. The recombinant Cdc5 protein was found to be functional despite the presence of GST tag. Although the GST by itself was not found to be active in kinase assays as reported in our previous study [20], the possibility that the GST tag downregulates the kinase activity of GST-Cdc5 fusion protein needs to be eliminated by studying the kinase activity post thrombin cleavage of the GST tag. Subsequent studies can shed light into the catalytic active site of Cdc5 kinase. The recombinant functional Cdc5 can be used as a versatile tool for characterization of enzymatic features of Cdc5 as a kinase, pull down of substrates of Cdc5 from yeast extracts, mutational analysis for deciphering structural-function correlation, and analysis of consensus sequence for PLK phosphorylation, which is still an enigma. Since PLKs exhibit a remarkable structural and functional homology, studies from yeast PLK can aid in understanding the mammalian Plk1 and its spectrum for anticancer targets can be expanded.
Declarations
Author contribution statement
A. Sourirajan and K. Dev: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
S. Chauhan: Performed the experiments; Wrote the paper.
S. Samanta: Performed the experiments.
J. Thakur: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
N. Sharma: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by Shoolini University, Solan.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors would like to acknowledge Shoolini University, Solan, for the infrastructural support and NIPGR, New Delhi for the assistance provided in this study. The authors also thank Divyanshi Sharma for assistance in hydropathy analysis.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Vaid R., Sharma N., Chauhan S., Deshta A., Dev K., Sourirajan A. Functions of Polo- like kinases: a journey from yeast to humans. Protein Pept. Lett. 2016;23:185–197. doi: 10.2174/092986652302160105143348. [DOI] [PubMed] [Google Scholar]
- 2.Nigg E.A. Polo-like kinases: positive regulators of cell division from start to finish. Curr. Opin. Cell Biol. 1998;10:776–783. doi: 10.1016/s0955-0674(98)80121-x. [DOI] [PubMed] [Google Scholar]
- 3.Zitouni S., Nabais C., Jana S.C. Polo-like kinases: structural variations lead to multiple functions. Nat. Rev. Mol. Cell Biol. 2014;15:433–452. doi: 10.1038/nrm3819. [DOI] [PubMed] [Google Scholar]
- 4.Kitada K., Johnson A.L., Johnston L.H. A multicopy suppressor gene of Saccharomyces cerevisiae G1 cell cycle mutant gene dbf4 encodes a protein kinase and is identified as CDC5.Mol. Cell Biol. 1993;13:4445–4457. doi: 10.1128/mcb.13.7.4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elia A.E., Rellos P., Haire L.F., Chao J.W., Ivins F.J., Hoepker K., Mohammad D., Cantley L.C., Smerdon S.J., Yaffe M.B. The molecular basis for phosphodependent substrate targeting and regulation of plks by polo-box domain. Cell. 2003;115:83–95. doi: 10.1016/s0092-8674(03)00725-6. [DOI] [PubMed] [Google Scholar]
- 6.Lowery D.M., Lim D., Yaffe M.B. Structure and function of polo like kinases. Oncogene. 2005;24:248–259. doi: 10.1038/sj.onc.1208280. [DOI] [PubMed] [Google Scholar]
- 7.Lee K.S., Park J.E., Asano S., Park C.J. Yeast polo-like kinases: functionally conserved multitask mitotic regulators. Oncogene. 2005;24:217–229. doi: 10.1038/sj.onc.1208271. [DOI] [PubMed] [Google Scholar]
- 8.Lee K.S., Erikson R.L. Plk1 is a functional homolog of Saccharomyces cerevisiae Cdc5 and elevated Plk activity induces multiple septation structures. Mol. Cell. Biol. 1997;17:3408–3417. doi: 10.1128/mcb.17.6.3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ouyang B., Pan H., Lu L., Li J., Stambrook P., Li B., Dai W. Human prk is a conserved protein serine/threonine kinase involved in regulating M phase functions. J. Biol. Chem. 1997;272:28646–28651. doi: 10.1074/jbc.272.45.28646. [DOI] [PubMed] [Google Scholar]
- 10.Lane H.A., Nigg E.A. Antibody microinjection reveals an essential role for human polo-like kinase 1 (Plk1) in the functional maturation of mitotic centrosomes. J. Cell Biol. 1996;135:1701–1713. doi: 10.1083/jcb.135.6.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lenart P., Petronczki M., Steegmaier M. The small-molecule inhibitor BI 2536 reveals novel insights into mitotic roles of polo-like kinase 1. Curr. Biol. 2007;17:304–315. doi: 10.1016/j.cub.2006.12.046. [DOI] [PubMed] [Google Scholar]
- 12.Schuyler S.C., Liu J.Y., Pellman D. The molecular function of Ase1p: evidence for a MAP-dependent mid zone-specific spindle matrix. J. Cell Biol. 2003;160:517–528. doi: 10.1083/jcb.200210021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hardy C.F., Pautz A. A novel role for Cdc5p in DNA replication. Mol. Cell. Biol. 1996;16:6775–6782. doi: 10.1128/mcb.16.12.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song B., Liu X.S., Davis K. Plk1 phosphorylation of Orc2 promotes DNA replication under conditions of stress. Mol. Cell. Biol. 2011;31:4844–4856. doi: 10.1128/MCB.06110-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakchaisri K., Asano S., Yu L.R. Coupling morphogenesis to mitotic entry. Proc. Natl. Acad. Sci. U.S.A. 2004;101:4124–4129. doi: 10.1073/pnas.0400641101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alexandru G., Uhlmann F., Mechtler K. Phosphorylation of the cohesin subunit Scc1 by Polo/Cdc5 kinase regulates sister chromatid separation in yeast. Cell. 2001;105:459–472. doi: 10.1016/s0092-8674(01)00362-2. [DOI] [PubMed] [Google Scholar]
- 17.Lee B.H., Amon A. Polo kinase–meiotic cell cycle coordinator. Cell Cycle. 2003;2:400–402. [PubMed] [Google Scholar]
- 18.Sourirajan A., Lichten M. Polo-like kinase Cdc5 drives exit from pachytene during budding yeast meiosis. Genes Dev. 2008;22:2627–2632. doi: 10.1101/gad.1711408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Cárcer G., Venkateswaran S.V., Salgueiro L., El Bakkali A., Somogyi K., Rowald K., Montañés P., Sanclemente M., Escobar B., de Martino A., McGranahan N., Malumbres M., Sotillo R. Plk1 overexpression induces chromosomal instability and suppresses tumor development. Nat. Commun. 2018;9:3012–3026. doi: 10.1038/s41467-018-05429-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chauhan S., Samanta S., Thakur J.K., Sourirajan A. Cloning, expression and purification of functionally active Saccharomyces cerevisiae Polo-like Kinase, Cdc5 in E. coli. J. Appl. Biol. Biotechnol. 2015;3:20–24. [Google Scholar]
- 21.Mortensen E.M., Haas W., Gygi M., Gygi S.P., Kellogg D.R. Cdc28-Dependent Regulation of the cdc5/polo kinase. Curr. Biol. 2005;15:2033–2037. doi: 10.1016/j.cub.2005.10.046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.