Abstract
Chikungunya fever is a major public health issue in India affecting millions of people and occurs due to Chikungunya. Chikungunya virus (CHIKV) is a single stranded RNA virus from the family of Togaviridae and genus alpha virus. It contain three structural proteins: glycosylated E1 and E2, embedded in the viral envelope, and a non-glycosylated nucleocapsid protein. Till date, researchers are working on inhibition of CHIKV but till now no cheap and effective medicine is available in the market. Therefore, the authors of this work thought of isoquinoline based noscapine to inhibit the nsP3 protease of CHIKV. The aim of the work is to understand the mechanism for the synthesis of noscapine theoretically using DFT. Further study the potential of all four isomers of noscapines {(13 (S,R), 14 (R,R), 15 (R,S) and 16 (S,S)} against nsP3 protease of CHIKV with the help of docking and MD simulation. The integrated e-pharmacophore binding affinity based virtual screening, docking and molecular dynamics simulation recognized four hits isomers as inhibition nsP3 protease of CHIKV. The docking energies of all the isomers of noscapine (13–16) with nsP3 protease CHIKV was found out to be more negative than baicalin (−8.06 kcal/mol) on selected sites. Amongst the isomers of noscapine, CMPD 13 possessed best binding affinity with four hydrogen bonding interactions. Further, ADME properties and blood-brain barrier permeability properties have been calculated. DFT studies of all the isomers of noscapine was investigated.
Keywords: Theoretical chemistry, Noscapine, Stereochemistry, nsP3 protease of CHIKV, Virtual screening, Docking, MD simulations, MM-GBSA
Theoretical Chemistry, Noscapine, stereochemistry, nsP3 protease of CHIKV, Virtual screening, Docking, MD Simulations, MM-GBSA.
1. Introductions
Fever is an abnormally high body temperature usually accompanied by shivering, headache and in severe instances delirium [1, 2]. There are different types of fever, which are transmitted by mosquitoes. The main four types of fever are dengue, malaria, chikungunya and zika fever [3]. Chikungunya is a viral infection transmitted by Aedesaegypti mosquito symptoms may include fever, joint pain, fatigue, rash, muscle pain, headache. It is spread by two types of mosquitoes Aedesalbopictus and Aedesaegypti [4, 5, 6]. Chikungunya fever is a major public health issue in India affecting billions. After 2010, the infection was in a decline stage until in 2016, when a massive outbreak affected the country. CHIKV is a single stranded RNA virus from the family of Togaviridae and genus alpha virus. CHIKV contain three structural proteins: glycosylated E1 and E2, embedded in the viral envelope, and a nonglycosylated nucleocapsid protein [7]. The non-structural polyprotein is divided into four different proteins (nsP1, nsP2, nsP3, and nsP4). Non-structural protein (nsp) are necessary for the transcription and translation of viral mRNA inside the cytoplasm of host cells [8]. Current therapies for CHIKV-infected patients with arthritis/arthralgia mainly involve management of pain and inflammation using non-steroid anti-inflammatory drugs (NSAIDs), as well with fluid intake to prevent dehydration. There is no licensed antivirals or vaccines available for CHIKF, therefore, there is a vital need for the development of novel and potent drugs against CHIKV [9, 10, 11]. Noscapine is benzylisoquinoline alkaloid belongs to plant family poppy. It possesses various functional moieties. It is used for its antitussive effect by its sigma receptor agonist activity [12, 13]. Noscapine is an anti-cancer drug and the elimination half-life of noscapine is 1.5–4 h. It is a tubulin-binding anti-angiogenic anticancer drug that causes cell cycle arrest and induces apoptosis in cancer cells both in vitro as well as in vivo [13, 14, 15]. The aim of the work is to understand the mechanism for the synthesis of noscapine using Density functional theory (DFT). Further, it aims to understand the potential of all four isomers of noscapines (R,R; R,S; S,R and S,S configuration on 10th and 15th number) as mentioned in Fig. 1 against nsP3 protease of CHIKV with the help of docking; absorption, distribution, metabolism and excretion (ADME); molecular dynamics (MD) simulations and molecular mechanics-generalized born surface area (MM-GBSA) analysis.
Fig. 1.
Structure of noscapine showing the chiral center at 10th and 15th positions.
2. Experimental
In this work, authors studied the effect of four isomers of noscapine to inhibit the activity of nsP3 protease of CHIKV. First the synthetic strategy was proved based on the DFT studies. After the successful synthetic procedure, author screen them by molecular docking, ADME and DFT to get the potent one. Finally, the screened compound was refined by MD simulation and the MM-GBSA. The work is explained in Fig. 2.
Fig. 2.
The schematic representation of the experimental methodology.
2.1. Designing of ligand
Schemes 1, 2, and 3 for the synthesis of noscapine have been taken from the literature. The molecule was chosen because of its potential in different area of medical science [14, 15]. As a chemist, the biological potency of this molecule may be due to the functional group, presence of chiral carbons etc. Noscapine has two chiral carbon atoms and therefore, it has of four isomers, 13–16 (Fig. 3). The mechanism of synthesis of the noscapines was studied using DFT.
Scheme 1.
Synthesis of 2-aeylethylamine containing blocking group.
Scheme 2.
Synthesis of phthalide-3-carboxylic acid.
Scheme 3.
Regioselective synthesis of (S,R) isomer of noscapine (13).
Fig. 3.
List of the stereoisomers of the noscapine.
2.2. DFT studies
The mechanistic proof for the synthesis was subjected to analyze by the quantum mechanical approach via the DFT. The basic preparation of all four molecule were done in Gauss View and DFT analysis was performed by the GAUSSIAN 09 package [16, 17]. A three layer function based on the Becke's as Lee, Yang, Parr was used. 6-311 basis set was used to optimize the geometries of the molecules. On the basis of studies of the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) values of the isomers were taken to calculate all the physiochemical descriptors. With the help of HOMO and LUMO values, hardness (η), softness (S), chemical potential (μ), electronegativity (χ), and global electrophilicity index (ω) were calculated as given by Eqs. (1), (2), (3), (4), and (5). For a molecule, N denotes the no. of electron and E denotes the energy states [18, 19].
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
2.3. Docking preparation
Preparation of receptor and ligand are the important step before the docking. The preparation of ligand is performed with the help of Marvin Chemsketch. Herein, the geometry optimization and addition of explicit solvent molecules were checked. Receptor is prepared with UCSF Chimera 1.11.2 in dock prep module [20]. Removal of solvents, adding hydrogen, replacing incomplete residues using Dunbrack rotamer library, etc. Finally, the prepared receptor and ligand were used for docking.
2.4. Binding site prediction
The PDB of nsP3 protease of CHIKV has been taken from Research Collaboratory for Structural Bioinformatics (RCSB) and the code is 3GPO [21]. It is a tetramer and one chain was taken for the binding site prediction. The automated version of active site prediction (AADS) of Supercomputing Facility for Bioinformatics & Computational Biology Centre (SCFBio) of the Indian Institute of Delhi was used to determine the number of active sites in the chain of nsp3 protease of CHIKV [22].
2.5. Docking
Molecular docking is a computational technique based on the atomic level interaction of the ligand into the active binding site of the receptor [23]. Molecular docking between the optimized ligand and prepared nsP3 protease of CHIKV was performed by the ParDOCK server provided by the Supercomputing Facility for Bioinformatics & Computational Biology Centre of the Indian Institute of Delhi [24]. All the four isomers of noscapine were docked on the all sites determined by AADS. ParDOCK is a computational tool used for rigid docking of a molecules against a receptor. The post screening of the potent molecule was also performed with the help of ParDOCK. ParDOCK performed the rigid docking of the ligand in binding cavity of protein. Herein, author studies the conformations of four Noscapines via the rigid docking. The scoring function of ParDOCK can be understood by Eq. (6) [24].
| E = ∑ Eel + Evdw + Ehpb | (6) |
where, E represents total non-bonded energy, Eel represents electrostatic contribution, Evdw represents van der Waals interaction and Ehpb represents hydrophobic term.
The post dock modelling was performed through the Discovery Studio visualizer client 2019 [25].
2.6. ADME properties
The pharmacokinetic behavior of molecule is important to consider it as the drug. Bioavailability score in term of lipophilicity and aqueous solubility for molecule to be the promising drug is more important [26]. The ADME properties of the all four ligand molecule were calculated through although the online server http://swissadme.ch/ [27].
2.7. Molecular dynamics
MD Simulations is a best method for studying the physical movements of atoms and molecules in a system. The trajectories of atoms and molecules are resolved by numerically solved Newton's equations of motion (F = ma) for a system of interacting particles, where forces within the particles and their potential energies are often calculated by using interatomic potentials or molecular mechanics (MM) force fields. [28, 29] MD simulations of 13–16 selected binding site of nsP3 protease of CHIKV were performed by using AMBER18 with the ff14SB force field and TIP3P 8.0 model were employed to produce the force field paramete15 of nsP3 protease and water molecules [30]. The molecular structures of 13-16 were optimized at the AM1 level and BBC charges were assigned to atoms of four inhibitors by using the Antechember module in AMBER18 suit. Generally Amber force field (GAFF) was applied to produce the force field parameters of 13–16 (46). MD simulation in solvent system were minimized by the steepest descent minimization of 2000 steps followed by the conjugate gradient minimization of 5000 steps to remove those unfavorable factors. The system was heated from 0 to 300 K under a softly heating process of 1 ns at constant volume and subsequently equilibrated for another 1 ns at 300 K. Finally, MD production is consist of 20000000 steps (nstlim) with a 2fs time step (dt) giving for 40ns. The reference temperature 300K (temp0 = 300.0) using Berendsen coupling algorithm to maintain constant temperature and pressure 1 bar was maintained with a temperature coupling time of 2ps (tautp = 2.0) is required. The time step was set to 2fs, print energy output every 500 steps (ntwr = 500) and save coordinates every 500 (ntwx = 500) in amber input files and were used for 40ns MD Simulations. Trajectories analyses were performed using the CPPTRAJ modules [31].
2.8. MM/GBSA studies
Molecular mechanics Generalized born surface area (MM-GBSA) is methods for fast calculations of binding free energies. MM-GBSA method was applied for the estimation binding free energies of protein-ligand complex (pl_complex) and evaluates the influence of structural difference on binding ability of ligand to protein. Binding free energies are determined by the given equation [32, 33].
| ΔGbind = ΔGpl_complex–wt – ΔGp–wt – ΔGl–wt – ΔGgas |
where ΔGbind is binding free energy between ligands and protein; ΔGpl_complex–wt, ΔGp–wt and ΔGl–wt represent the change in free energies of pl_complex, protein and ligand in water while ΔGgas describe a change of free energy induced by binding of ligand to protein in gas phase. AMBER18 suit was used to perform the MM-GBSA analysis [30].
3. Result & discussion
3.1. Design of scheme for the synthesis of noscapine
Schemes 1, 2, and 3 for the synthesis of noscapine have been taken from the literature [13, 14, 15]. The molecule was chosen because of its potential in different area of medical science [34]. As a chemist, the biological potency of this molecule may be due to the functional group, parent moiety stereoisomer 13 or presence of chiral carbons. Noscapine has two chiral carbons, therefore, it has of four isomers, 13–16. Literature discussed the biological potency of one of the isomer of noscapine, 15 against the cancer. In the present work, all four isomers have been taken and studied their potential against nsP3 protease of CHIKV. Till date, no one has studied the mechanism for the synthesis of noscapine and all its isomers using computational tools.
3.2. Study the mechanism for synthesis of noscapine through DFT
In modern days, computational techniques were frequently applied to solve the various problems in drug designing. Such a computational technique is the density function theory based on the quantum mechanical approach to do the same [35, 36]. The mechanism of synthesis of noscapine is studied through DFT. The energies of reactants, intermediates and product were calculated through DFT. The energies are given in Fig. 3 for the graphical correlations of the molecules 1–13 as in Schemes 1, 2, and 3. The energy of 1 is positive, shows the highly reactive nature. CMPD1 on reduction converted to alcohol 2 seems to be quite stable. 2 further converted to the chloro-derivatives 3 by the SN2 mechanism. Further, the substitution of chlorine by cyanide ion gave CMPD4. Then, 4 undergo for reduction of cyanide into the amino group to give 5. The Phthalic acid derivative 6 undergo dehydration followed by the oxidation to form 7. The acid group of 7 gets converted into acyl chloride 8 by reacting POCl3. Then 5 and 8 serve as the starting material for the synthesis of Noscapine. 5 and 8 undergo elimination reaction by releasing one molecule of HCl. The energy of 5 and 8 are 0.0027 and -0.0841 AU respectively while the energy of 9 is -0.074 AU. It indicates the feasibility of formation of intermediate 9. CMPD 9 undergo cyclization in presence of POCl3 in toluene indicate the most stability as chelation increases stability which is also supported by the huge decrease in energy having value -0.219 AU. 10 converted to 11 by the reduction and the energy of 11 is -0.0477 AU. Further, the asymmetric methylation of 11 gave 12 having energy -0.0468 AU and it is comparable to 11. Finally, 12 undergo reduction in presence of dihydrogen and raney nickel to get the desired product 13 having energy -0.0545 AU. From the energy value of 11, 12 and 13, it is clear that 13 is most stable.
Based on Fig. 4, wherein the energy was plotted against the compound and it clearly shows the feasibility of the synthesis of compound 13.
Fig. 4.
Understanding the pattern of the energy of the molecules as in Schemes 1, 2, and 3 to understand the feasibility of the synthesis of 13.
3.3. DFT studies of the isomers of noscapine
Herein, the authors tried to study the behavior of isomers of noscapine towards the chemical synthesis on the aspect of theoretical evaluation [37]. Author's proposal was to study the last steps of synthesis in the term of stability of product where actually chirality play a key role. As per expectation, authors found that the synthesis of isomer (R,R) is difficult as its energy is higher than the energy of others. The energies of product is given in Fig. 5. It is clearly evident that R,S, S,R, and S,S have almost similar energy value.
Fig. 5.
Showing the relative energy of the isomers.
3.4. UV-visible spectra of CMPD 13-16
The UV-Visible spectra of the isomers of noscapine (13–16) were determined using td-DFT calculation. It was found that they give different λmax for the 13–16 and λmax values of the compound 13, 14, 15 and 16 are 323, 311, 271; 340, 276 and 271 nm respectively as in Fig. 6. The oscillator strength for the 13 is 0.0491, 0.0437 and 0.0844; for 14 is 0.0283 and 0.0689, for 15 is 0.107 and for 16 is 0.1031 respectively.
Fig. 6.
(i) UV-Visible spectra and (ii) 1st derivative UV-Visible spectra of compounds 13-16.
3.5. Docking
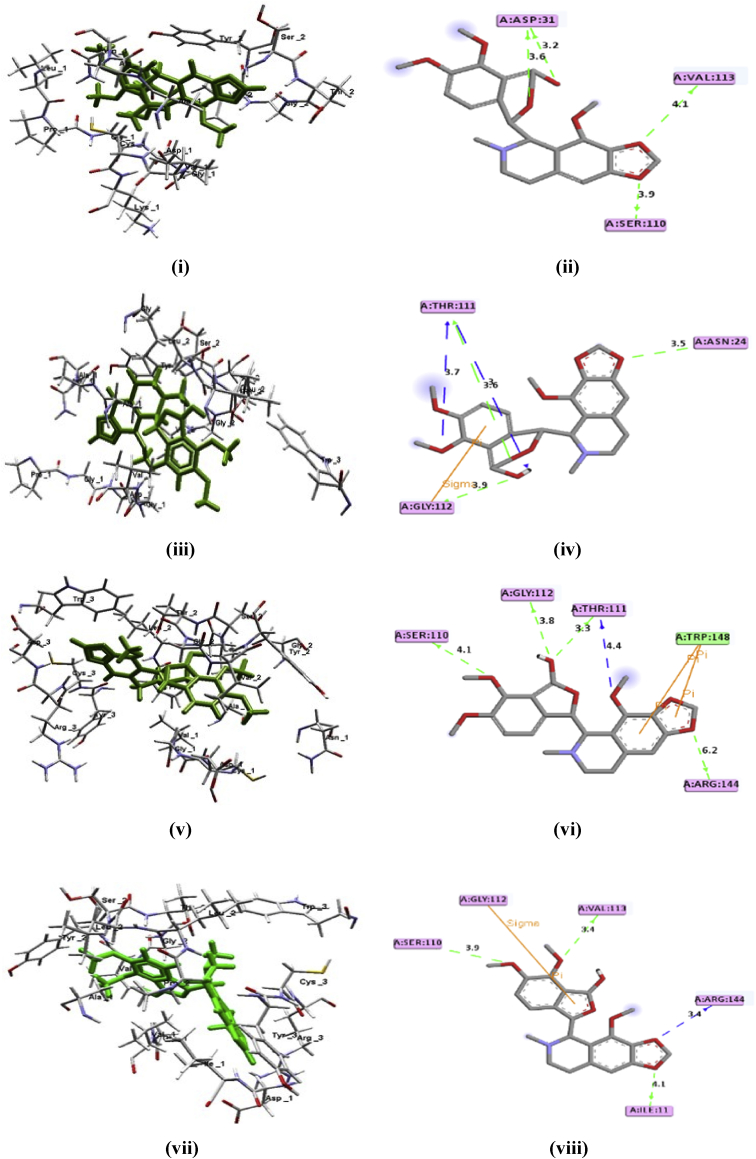
Nowadays, computer added drug designing (CADD) plays the key role in development of the new potent drug molecule. CADD uses the different tools to screen a library of molecule within the limited time frame [38, 39]. Herein, authors used the rigid docking to retain the original conformation of the ligand. Before the docking of noscapine, authors were interested to find the all binding pocket in nsP3 protease of CHIKV. Fig. 7 shows the total four available binding pocket. All four noscapines were docked against the all four binding pocket of the nsP3 protease of CHIKV. The results of docking are mentioned in Table 1. The docked view of all the isomers in the most active sites in 2D- and 3D-are given in Fig. 8. The interaction of the isomers of noscapine, 13–16 with nsP3 protease is given in Table 3. Noscapine, 13–16 shows hydrogen bonding, pi-pi and pi-σ interactions with nsP3 protease of CHIKV as in Fig. 8.
Fig. 7.
Number of binding sites in the ns3 protease of CHIKV.
Table 1.
Docking energy score of the four sites for the isomers of noscapine, 13-16.
| S. No. | Isomers | Drug sites | Binding Affinity |
|---|---|---|---|
| 1. | 14 | 1 | -8.94 |
| 2. | 14 | 2 | -8.90 |
| 3. | 14 | 3 | -8.93 |
| 4. | 14 | 4 | -8.24 |
| 5. | 15 | 1 | -9.27 |
| 6. | 15 | 2 | -9.02 |
| 7. | 15 | 3 | -8.02 |
| 8. | 15 | 4 | -7.66 |
| 9. | 13 | 1 | -8.91 |
| 10. | 13 | 2 | -9.57 |
| 11. | 13 | 3 | -8.73 |
| 12. | 13 | 4 | -9.64 |
| 13. | 16 | 1 | -8.09 |
| 14. | 16 | 2 | -8.64 |
| 15. | 16 | 3 | -9.15 |
| 16. | 16 | 4 | -8.79 |
Fig. 8.
(i), (iii), (v), (vii) for the 3D-poses and (ii), (iv), (vi), (viii) for 2D-poses for the interaction of 13–16 with the amino acids of nsP3 protease of CHIKV.
Table 3.
ADME properties of all the isomers of noscapine (13–16).
| Physiochemical descriptors | Isomers |
|||
|---|---|---|---|---|
| 13 | 14 | 15 | 16 | |
| Log S | -2.61 | -4.14 | -4.14 | -4.14 |
| Heavy atoms | 30 | 30 | 30 | 30 |
| MW (g/mol) | 413.42 | 413.42 | 413.42 | 413.42 |
| No. of rotational bonds | 4 | 4 | 4 | 4 |
| No. H-bond accepto15 | 8 | 8 | 8 | 8 |
| Num. H-bond donors | 0 | 0 | 0 | 0 |
| Log Po/w (iLOGP) | 0 | 3.56 | 3.29 | 3.53 |
| Lipinski | Yes; 0 violation | Yes; 0 violation | Yes; 0 violation | Yes; 0 violation |
| Log Kp in cm/s | -8.29 | -6.90 | -6.90 | -6.90 |
| tPSA(Å2) | 58.62 | 75.69 | 75.69 | 75.69 |
| Bioavailability Score | 0.55 | 0.55 | 0.55 | 0.55 |
| Synthetic accessibility | 5.36 | 4.31 | 4.31 | 4.31 |
Based on the docking score of 13-16 against the all four binding pockets of the nsP3 protease of CHIKV, the isomer 13 shows the best binding affinity at the fourth drug site of nsP3 protease of CHIKV. The binding energy value of the fourth site for 13 is -9.64 kJ/mol (Table 1). The binding energy values for top score for the other three isomers 14, 15 and 16 are -8.94, -9.27 and -9.15 kJ/mol respectively. The docking score reveals the potency of 13 over the others. As per the literature, several research groups choose the 1st/2nd binding site. But in this work, it was found that different sites can show stronger interactions. 14 and 15 showed best binding on site 1st while 13 and 16 showed best binding with the site 3rd and site 4th respectively.
The docked pose analysis of the top scorer of isomers, 13–16 are given in Fig. 8. From the dock pose, it is found that 13 form Hydrogen (H) bonding with ASP-31 (3.2, 3.6), VAL-113 (4.1) and SER-110 (3.9). 14 form H-bonding with THR-111 (3.7, 3.6, 3.0), GLY-112 (3.8), THR-111 (3.3, 4.4) and ARG-144 (6.2) (Table 2).
Table 2.
Interaction of 13–16 with different amino-acids of nsP3 protease of CHIKV.
| CMPD | Π-σ Interactions |
Π- Π Interactions |
H-Bond Interactions |
|||
|---|---|---|---|---|---|---|
| Amino acid | Distance (Å) | Amino acid | Distance (Å) | Amino acid | Distance (Å) | |
| 13 | - | - | - | - | ASP-31 VAL-113 SER-110 |
3.2, 3.6 4.1 3.9 |
| 14 | GLY-112 | 2.73 | - | - | THR-111 GLY-112 ASN-24 |
3.7, 3.6, 3.0 3.9 3.5 |
| 15 | - | - | TRP-148 | 5.20, 4.27 | SER-110 GLY-112 THR-111 ARG-144 |
4.1 3.8 3.3, 4.4 6.2 |
| 16 | GLY-112 | 2.80 | - | - | SER-110 VAL-113 ARG-144 ILE-11 |
3.9 3.4 3.4 4.1 |
CMPD13 forms H-bonding interactions with SER-110 (3.9), ASP-31 (3.2, 3.6), VAL-113 (4.1). 15 forms H-bonding interactions with SER-110 (4.1), GLY-112 (3.8), THR-111 (3.3, 4.4) and ARG-144 (6.2). 16 forms H-bonding interactions with SER-110 (3.9), VAL-113 (3.4), ARG-144 (3.4) and ILE-11 (4.1). Beside this 14 and 16 forms Π-σ interactions with GLY-112 (2.73) and GLY-112 (2.80) respectively, while only 15 forms the Π- Π Interactions with TRP-148 (5.20, 4.27).
3.6. ADME properties
The bioavailability of the molecule is most important. The biological properties have been calculated and discussed on the basis of TPSA, chemical structure, milogP (partition coefficient) and Lipinski's “Rule of Five” states that most of the molecules with good membrane permeability will have LogP ≤5, molecular weight ≤500, the number of hydrogen bond acceptors ≤10, and the number of hydrogen bond donors ≤5 [40, 41, 42]. All the parameters 13–16 were reported in Table 3. From the data, it is understood that except Log P value and synthetic accessibility all the remaining data is same. It means on changing the absolute configuration on the chiral center, a change in the hydrophobicity is found/observed. It is also found that 13–16 follow Lipinski's rule with no isolation.
3.7. DFT study of the isomers of noscapine
On changing absolute configuration on the chiral carbon, the chemical properties changes mainly due to the ΔE value. ΔE comes from the difference of energy of LUMO and HOMO [43]. Further, the chemical potential, affinity, softness have been studied which comes from the EHOMO and ELUMO. DFT method was used for the calculations of HOMO-LUMO gap (ΔE), EHOMO, ELUMO and total energy (E) [44]. The HOMO-LUMO energy gap are quantum mechanical descriptors which play a major role in chemical interactions. The energy gaps between HOMO and LUMO helps to characterize the chemical reactivity and kinetic stability of molecules [45]. If any molecule have small energy gap that is more polarizable and is generally associated with a high chemical reactivity, low kinetic stability and is also termed as soft molecule. The HOMO orbital is primarily acts as an electron donor and the LUMO orbitals is largely acts as the electron acceptor [46]. The energy of all the isomers calculated from the DFT calculation are found similar except 14. But the value of ΔE of all the isomers differs significantly and 15 has lowest change in energy while the 14 has the highest ΔE value as in Fig. 9. Plot for energy and change in energy explained the significance of absolute configuration. Further, ionization, affinity, softness and other parameters were calculated as in Table 5.
Fig. 9.
Plot for the ΔE of isomers of noscapine (13–16).
Table 5.
Calculated binding free energies drug-target complex, target, drug and differences of drug-target complex in kcal/mol of the 13-16.
| Energy Component | Differences of 14 |
Differences of 15 |
Differences of 13 |
Differences of 16 |
||||
|---|---|---|---|---|---|---|---|---|
| Average | Std. Err. of Mean | Average | Std. Err. of Mean | Average | Std. Err. of Mean | Average | Std. Err. of Mean | |
| BOND | 0.00 | 0.00 | 0.00 | 0.00 | -0.00 | 0.00 | 0.00 | 0.00 |
| ANGLE | 0.00 | 0.00 | -0.00 | 0.00 | -0.00 | 0.00 | 0.00 | 0.00 |
| DIHED | -0.00 | 0.00 | -0.00 | 0.00 | 0.00 | 0.00 | -0.00 | 0.00 |
| VDWAALS | -49.36 | 0.02 | -48.53 | 0.02 | -58.93 | 0.02 | -54.34 | 0.015 |
| EEL | -12.21 | 0.02 | -17.92 | 0.03 | -22.66 | 0.03 | -8.19 | 0.05 |
| 1-4 VDW | 0.00 | 0.00 | -0.00 | 0.00 | -0.00 | 0.00 | 0.00 | 0.00 |
| 1-4 EEL | -0.00 | 0.00 | 0.00 | 0.00 | -0.00 | 0.00 | -0.00 | 0.00 |
| EGB | 32.05 | 0.02 | 37.11 | 0.03 | 41.22 | 0.02 | 28.44 | 0.04 |
| ESURF | -4.95 | 0.001 | -4.89 | 0.001 | -5.81 | 0.001 | -5.76 | 0.001 |
| Ggas/ΔGgas | -61.57 | 0.03 | -66.44 | 0.04 | -81.59 | 0.03 | -62.54 | 0.05 |
| Gsolv/ΔGsolv | 27.10 | 0.02 | 32.22 | 0.03 | 35.41 | 0.02 | 22.68 | 0.04 |
| TOTAL/ΔGtotal | -34.47 | 0.02 | -34.22 | 0.02 | -46.18 | 0.01 | -39.85 | 0.02 |
The physiochemical descriptors for 13, 14, 15 and 16 were calculated from the DFT result. The values for chemical potential (μ), global softness (S), global hardness (η), electronegativity (χ), and electrophilicity index (ω) are given in Table 4. Hardness defines the polarizability of the compound or the disturbance of electronic cloud in an electric field. Hardness describes the resistance towards the mechanical deformation and the softness is the reciprocal of the hardness [47]. Chemical potential is defined as the escaping tendency of the electrons from a system. Literature reported that the electron flow from high chemical potential to the low chemical potential [48]. Electronegativity may be defined as to attract the electron by the molecule. The electronegativity of the molecule may be defined as the drop in energy of molecule when some amount of electronic charge is added to the system [49]. Electrophilicity index is defined as the ability of molecule to accept electron. It also measures the decrease in energy of the molecule due to transfer of electron from donor to acceptor [50].
Table 4.
Physio-chemical descriptors of the isomers of noscapine, 13-16.
| S. No. | A | I | μ | S | ɳ | Χ | ω |
|---|---|---|---|---|---|---|---|
| 13 | 0.05454 | 0.21375 | -0.13415 | 6.28101 | 0.07961 | 0.13415 | 0.11301 |
| 14 | 0.07800 | 0.16954 | -0.12377 | 10.92418 | 0.04577 | 0.12377 | 0.16734 |
| 15 | -0.00093 | 0.22771 | -0.11339 | 4.37368 | 0.11432 | 0.11339 | 0.05623 |
| 16 | 0.04579 | 0.20568 | -0.12574 | 6.25429 | 0.07995 | 0.12574 | 0.09887 |
The electronic distribution of frontier molecular orbitals were also performed to understand the effect of configuration, whether it changes or not. It is very interesting that only in case of 15, HOMO is centered on nitrogen of isoquinoline ring, while for the rest of the isomers it centered on the methoxy part of the benzofuran ring. The pattern of LUMO is observed different for 13–16. For 13, it is centered on the methoxy group of isoquinoline part, for 14 it is centered on methoxy group of benzofuran ring, for 15 it is centered on core benzofuran ring and for 16 it is centered on core of isoquinoline ring. The frontier orbital pictures are given in Fig. 10.
Fig. 10.
The EHOMO, ELUMO, E & ΔE of isomers of noscapine (13–16) using DFT.
3.8. MD simulation
MD simulations analysis of macromolecular system is useful to study the interaction between ligand and receptor [51]. The inhibition of protein, folding unfolding and stability can be explain very well with the help of MD simulations. Root mean square deviation (RMSD) measures the deviation of mean atomic position by square rooting it. RMSD of backbone of protein play key role to provide the inhibition/stability [52]. MD simulation analysis of 13–16 with nsP3 protease of CHIKV was performed. MD simulations of 13, 14, 15 and 16 on binding sites 3, 1, 1, and 4 respectively were performed and given in Fig. 11. The RMSD values was plotted versus the simulation time in nanoseconds (ns) to distinguish whether the simulations had reached to minimum deviations. Based on the results obtained in docking of isomers of noscapine on all four binding sites, MD simulation was performed to study the inhibition of nsP3 protease of CHIKV using 13–16. The value of RMSD for SS isomer is found lowest with little deviation while 13 isomers showed maximum deviation having higher RMSD value than that of 16.
Fig. 11.
MD Simulation of nsP3 protease of CHIKV and with 13–16.
3.9. Binding energy for the complex in nsP3 protease of CHIKV with 13–16 through MM-GBSA calculations
Generalized born surface area methods is more prominent and accurate compare to the docking algorithms. MM-GBSA analysis of the all MD output was performed to get the precise values of solvation, binding energy, etc. [53] Binding energy analysis for the inhibition of nsP3 protease of CHIKV on selected sites using 13–16 is given in Fig. 12. For all isomers, the binding energy values is negative. These negative values corroborate the docking result. Although the binding energy is recorded for the 40 ns at the interval of 2 fs and an average value is given in Table 5. The average binding energy for 13–16 with nsP3 protease of CHIV are -46.18, -34.47, -34.22 and -39.85 kcal/mol respectively.
Fig. 12.
(i)-(iv) Plot for the binding energy between the nsP3 protease of CHIKV with 13–16 respectively.
Results shows an interesting thing that 13 showed the best binding with nsP3 protease of CHIKV although longer deviation were seen in the RMSD pattern. Even, 16 isomer also showed good binding energy indicates that 13 and 16 have the strong ability to inhibit the nsP3 protease of CHIKV.
4. Conclusion
In this work, the mechanism for the synthesis of noscapine was studied using DFT. Then, in silico biological activity was performed to check the biological potency of noscapine isomers against the nsP3 protease of CHIKV. Docking results indicate the supremacy of 13 over the other isomers. Physiochemical descriptors from DFT result shows the acceptable result in the context of flow. Further. MD simulations were performed for the nsP3 protease of CHIKV with and without isomers of noscapine, 13–16 on selected sites based by docking. MD result reveals more deviation in case of 13 but for 16, it is in the acceptable range. Further, the binding was calculated using MM-GBSA to study the potency of 13 and 16 for the inhibition of nsP3 protease of CHIKV. MM-GBSA result again shows the highest value of binding energy for the 13.
Declarations
Author contribution statement
Prashant Singh, Kamlesh Kumari: Conceived and designed the experiments; Wrote the paper.
Durgesh Kumar, Vijay Kumar Vishvakarma, Parul Yadav: Performed the experiments.
Abhilash Jayarak: Analyzed and interpreted the data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
Durgesh Kumar (DK) is thankful to Prof. B. Jayaram, Incharge, SCFBio, Indian Institute of Technology, Delhi, India for accessing the facilities and training at SCFBio. DK convey thanks to the Department of Chemistry, University of Delhi, India for the facilities to carry out research work. Prashant Singh (PS) dedicates his contribution in this work to his guide, Late Dr. N. N. Ghosh.
Contributor Information
Prashant Singh, Email: psingh@arsd.du.ac.in.
Kamlesh Kumari, Email: biotechnano@gmail.com.
References
- 1.Pialoux G., Gauzere B.A., Jaureguiberry S., Strobel Chikungunya M. An epidemic arbovirosis. Lancet Infect. Dis. 2007;7:319–327. doi: 10.1016/S1473-3099(07)70107-X. [DOI] [PubMed] [Google Scholar]
- 2.Powers A.M., Brault A.C., Tesh R.B., Weaver S.C. Re-emergence of chikungunya and o’nyong-nyong viruses: evidence for distinct geographical lineages and distant evolutionary relationships. J. Gen. Virol. 2000;81:471–479. doi: 10.1099/0022-1317-81-2-471. [DOI] [PubMed] [Google Scholar]
- 3.Yergolkar P.N., Tandale B.V., Arankalle V.A., Sathe P.S., Sudeep A.B., Gandhe S.S., Gokhle M.D., Jacob G.P., Hundekar S.L., Mishra A.C. Chikungunya outbreaks caused by African genotype. India Emerging Infect. Dis. 2006;12:1580–1583. doi: 10.3201/eid1210.060529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Charrel R.N., Brouqui P., Foucauet C., de Lamballerie X. Concurrent dengue and malaria Emerging. Inf. Disp. 2005;11:1153–1154. doi: 10.3201/eid1107.041352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Appassakij H., Khuntikij P., Kemapunamanus M., Wutthanarungsan R., Silpapojakul K. Viremic profiles in asymptomatic and symptomatic Chikungunya fever: a blood transfusion threat. Transfusion. 2013;53:2567–2574. doi: 10.1111/j.1537-2995.2012.03960.x. [DOI] [PubMed] [Google Scholar]
- 6.Gibney K.B., Fischer M., Prince H.E., Kramer L.D., St George K., Kosoy O.L., Laven J.J., Staples J.E. Chikungunya fever in the United States: a fifteen year review of cases. Clin. Infect. Dis. 2011;52:121–126. doi: 10.1093/cid/ciq214. [DOI] [PubMed] [Google Scholar]
- 7.Abu Bakar F., Ng L.F.P. NSP of alpha virus potential targets for drug development. Viruses. 2018;10:1–15. doi: 10.3390/v10020071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neogi D.K., Bhattacharya N., Mukherjee K.K. Serosurvey of chikungunya Antibody in Calcutta metropolis. J. Commun. Dis. 1995;37:19–22. [PubMed] [Google Scholar]
- 9.Ganu M.A., Ganu A.S. Post chikungunya chronic arthritis - our experience with DMARD over two yea15 follow up. J. Assoc. Phys. India. 2011;9:83–86. [PubMed] [Google Scholar]
- 10.Bickerman H.A., Barach A.L. The experimental production of cough in human subjects induced by citric acid aerosols. Preliminary studies on the evaluation of antitussive agents. Am. J. Med. Sci. 1954;228:156–163. doi: 10.1097/00000441-195408000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Kumar D., Singh P., Jayaraj A., Kumar V., Kumari K., Patel A R. Theoretical model to study the interaction of erythro-noscapines with nsP3 protease of chikungunya virus. Chemistryselect. 2019;4:4892–4900. [Google Scholar]
- 12.Landen J.W., Lang R., McMahon S.J., Rusan N.M., Yvon A.M., Adams A.W., Sorcinelli M.D., Campbell R., Bonaccorsi P., Ansel J.C., Archer D.R., Wadsworth P., Armstrong C.A., Joshi H.C. Noscapine alters microtubule dynamics in living cells and inhibits the progression of melanoma. Cancer Res. 2002;62:4109–4114. [PubMed] [Google Scholar]
- 13.Mahmoudian M., Mojaverian N. Effect of noscapine, the antitussive opioid alkaloid, on bradykinin-induced smooth muscle contraction in the isolated ileum of the Guinea-pig. Acta Physiol. Hung. 2001;88:231–237. doi: 10.1556/APhysiol.88.2001.3-4.5. [DOI] [PubMed] [Google Scholar]
- 14.Mourey R.J., Dawson T.M., Barrow R.K., Enna A.E., Snyder S.H. [3H]Noscapine binding sites in brain: relationship to indoleamines and the phosphoinositide and adenylyl cyclase messenger systems. Mol. Pharmacol. 1992;42:619–626. [PubMed] [Google Scholar]
- 15.Xu Y., Ni J., Xiao P., Lu Q., Zhang Q., Cong J., Cao X., Wen L., Sun X. 2014. Method for Selective Synthesis of Alpha-Narcotine with Participation of Blockage Group Patent Publication of CN101775021B. [Google Scholar]
- 16.Dennington Roy; Keith, T. A. M., John M. GaussView Version 6.
- 17.Frisch M.J.T.G.W., Schlegel H.B., Scuseria G.E., Robb M.A., Cheeseman J.R., Scalmani G., Barone V., Petersson G.A., Nakatsuji H., Li X., Caricato M., Marenich A.V., Bloino J., Janesko B.G., Gomperts R., Mennucci B., Hratchian H.P., Ortiz J.V., Izmaylov A.F., Sonnenberg J.L., Williams-Young D., Ding F., Lipparini F., Egidi F., Goings J., Peng B., Petrone A., Henderson T., Ranasinghe D., Zakrzewski V.G., Gao J., Rega N., Zheng G., Liang W., Hada M., Ehara M., Toyota K., Fukuda R., Hasegawa J., Ishida M., Nakajima T., Honda Y., Kitao O., Nakai H., Vreven T., Throssell K., Montgomery J.A., Jr., Peralta J.E., Ogliaro F., Bearpark M.J., Heyd J.J., Brothers E.N., Kudin K.N., Staroverov V.N., Keith T.A., Kobayashi R., Normand J., Raghavachari K., Rendell A.P., Burant J.C., Iyengar S.S., Tomasi J., Cossi M., Millam J.M., Klene M., Adamo C., Cammi R., Ochterski J.W., Martin R.L., Morokuma K., Farkas O., Foresman J.B., Fox D.J. 2016. Gaussian 16, Revision C.01. [Google Scholar]
- 18.Bourass M., Benjelloun A.T., Benzakour M., Mcharfi M., Hamidi M., Bouzzine S.M., Bouachrine M. DFT and TD-DFT calculation of new thienopyrazine-based small molecules for organic solar cells. Chem. Cent. J. 2016;10(67):1–11. doi: 10.1186/s13065-016-0216-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bavadi M., Niknam K., Shahraki O. Novel pyrrole derivatives bearing sulfonamide groups: synthesis invitro cytotoxicity evaluation, molecular docking and DFT study. J. Mol. Struct. 2017;1146:242–253. [Google Scholar]
- 20.Pettersen E.F., Goddard T.D., Huang C.C., Couch G.S., Greenblatt D.M. UCSF Chimera-a visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 21.Malet H., Coutard B., Jamal S., Dutartre H., Papageorgiou N., Neuvonen M., Ahola T., Forrester N., Gould E.A., Lafitte D., Ferron F., Lescar J., Gorbalenya A.E., de Lamballerie X., Canard B. The crystal structures of Chikungunya and Venezuelan equine encephalitis virus nsP3 macro domains define a conserved adenosine binding pocket. J. Virol. 2009;83:6534–6545. doi: 10.1128/JVI.00189-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh T., Biswas D., Jayaram B. Aads - an automated active site identification, docking, and scoring protocol for protein targets based on physicochemical descriptors. J. Chem. Inf. Model. 2011;51(10):2515–2527. doi: 10.1021/ci200193z. [DOI] [PubMed] [Google Scholar]
- 23.Singh P., Vishvakarma V.K., Pant B.N., Yadav S., Aslam M., Yadav J., Yadav A., Kumari K., Patel R., Chandra R. Computational docking studies of Noscapines: a potential bioactive agent. Am. J. Pharmacol. Pharmacother. 2017;4:9–14. [Google Scholar]
- 24.Gupta A., Gandhimathi A., Sharma P., Jayaram B. ParDOCK: an all atom energy based Monte Carlo docking protocol for protein-ligand complexes protein pept. Lettres. 2007;14:632–646. doi: 10.2174/092986607781483831. [DOI] [PubMed] [Google Scholar]
- 25.BIOVIA, D. S. Release; 2017. Discovery Studio Modelling Environment. 2017. [Google Scholar]
- 26.Vishvakarma V.K., Shukla N., Reetu, Kumari K., Patel R., Singh P. A model to study the inhibition of nsP2B-nsP3 protease of dengue virus with imidazole, oxazole, triazole thiadiazole, and thiazolidine based scaffolds. Heliyon. 2019;5 doi: 10.1016/j.heliyon.2019.e02124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daina A., Michielin O., Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017;7:42717. doi: 10.1038/srep42717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vishvakarma V.K., Singh P., Kumar V., Kumari K., Patel P., Chandra R. Pyrrolothiazolones as potential inhibitors for the nsP2B-nsP3 protease of dengue virus and their mechanism of synthesis. Chemistryselect. 2019;4(32):9410–9419. [Google Scholar]
- 29.Vishvakarma V.K., Patel R., Kumari K., Singh P. Interaction between bovine serum albumin and gemini surfactants molecular docking characterization Infor. Sci. Lett. 2017;6:33–38. [Google Scholar]
- 30.Case D.A., Ben-Shalom I.Y., Brozell S.R., Cerutti D.S., Cheatham T.E., III, Cruzeiro V.W.D., Darden T.A., Duke R.E., Ghoreishi D., Gilson M.K., Gohlke H., Goetz A.W., Greene D., Harris R., Homeyer N., Izadi S., Kovalenko A., Kurtzman T., Lee T.S., LeGrand S., Li P., Lin C., Liu J., Luchko T., Luo R., Mermelstein D.J., Merz K.M., Miao Y., Monard G., Nguyen C., Nguyen H., Omelyan I., Onufriev A., Pan F., Qi R., Roe D.R., Roitberg A., Sagui C., Schott-Verdugo S., Shen J., Simmerling C.L., Smith J., Salomon-Ferrer R., Swails J., Walker R.C., Wang J., Wei H., Wolf R.M., Wu X., Xiao L., York D.M., Kollman P.A. University of California; San Francisco: 2018. AMBER 2018. [Google Scholar]
- 31.Roe R.D., Cheatham T.E., III PTRAJ and CPPTRAJ: software for processing and analysis of molecular dynamics trajectory data. J. Chem. Theory Comput. 2013;9(7):3084–3095. doi: 10.1021/ct400341p. [DOI] [PubMed] [Google Scholar]
- 32.Genheden S., Ryde U.l. f. The MM/PBSA and MM/GBSA methods to estimate ligand-binding affinities. Expert Opin. Drug Discov. 2015;10(5):449–461. doi: 10.1517/17460441.2015.1032936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Homeyer N., Gohlke H. Free energy calculations by the molecular mechanics Poisson–Boltzmann surface area method. Mol. Inf. 2012;31:114–122. doi: 10.1002/minf.201100135. [DOI] [PubMed] [Google Scholar]
- 34.Singh H., Singh P., Kumari K., Chandra A., Dass S.K., Chandra R. A review on noscapine, and its impact on heme metabolism. Curr. Drug Metabol. 2013;14(3):351–360. doi: 10.2174/1389200211314030010. [DOI] [PubMed] [Google Scholar]
- 35.Miehlich B., Savin a., Stroll h., Preuss h. Results obtained with the correlation energy density functionals of becke and lee, yang and parr chem. Phys. Lett. 1989;157:1–7. [Google Scholar]
- 36.Pirnau A., Chis V., Oniga O., Leopold N., Szabo L., Baias M., Cozar O. Vibrational and DFT study of 5-(3-pyridyl-methylidene)-thiazolidine-2-thione-4-one. Vib. Spectrosc. 2008;48:289–296. [Google Scholar]
- 37.Schatz G.C., Van Duyne R.P., Chalmers J.M., Griffiths P.R. Handbook of Vibrational Spectroscopy. Wiley; New York: 2002. Electromagnetic mechanism of surface-enhanced spectroscopy; pp. 759–774. [Google Scholar]
- 38.Singh P., Kumari K., Awasthi S.K., Chandra R. Virtual screening and docking studies of synthesized chalcones: potent anti-malarial drug. Int. J. Drug Dev. Res. 2016;8:49–56. [Google Scholar]
- 39.Vishvakarma V.K., Kumari K., Dixit V.S., Patel R., Singh P., Malhotra G.K., Chandra R., Chakrawarty A.K. Theoretical model to investigate the alkyl chain and anion dependent interaction of Gemini surfactant with bovine serum albumin. Spectrochim. Acta. 2015;143:319–323. doi: 10.1016/j.saa.2015.01.068. [DOI] [PubMed] [Google Scholar]
- 40.Lipinski C.A. Lead- and drug-like compounds: the rule-of-five revolution. Drug Discov. Today Technol. 2004;1:337–341. doi: 10.1016/j.ddtec.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 41.Zhao Y., Abraham M.H., Lee J., Hersey A., Luscombe N.C., Beck G. Rate-limited steps of human oral absorption and QSAR studies. Pharm. Res. 2002;19:1446–1457. doi: 10.1023/a:1020444330011. [DOI] [PubMed] [Google Scholar]
- 42.Ertl P., Rohde B., Selzer P. Fast calculation of molecular polar surface area as a sum of fragment-based contributions and its application to the prediction of drug transport properties. J. Med. Chem. 2000;43:3714–3717. doi: 10.1021/jm000942e. [DOI] [PubMed] [Google Scholar]
- 43.Domingo L.R., Rios-Gutierrez M., Perez P. Applications of the conceptual density functional theory indices to organic chemistry. React. Mol. 2016;21:1–22. doi: 10.3390/molecules21060748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mourik V., Tanja Gdanitz, Robert J. A critical note on density functional theory studies on rare-gas dimers. J. Chem. Phys. 2002;116:9620–9623. [Google Scholar]
- 45.Huang Y., Rong C., Zhang R., Liu S. Evaluating frontier orbital energy and HOMO/LUMO gap with descriptors from density functional reactivity theory. J. Mol. Model. 2017;23(3) doi: 10.1007/s00894-016-3175-x. [DOI] [PubMed] [Google Scholar]
- 46.Bavadi M., Niknam K., Shahraki O. Novel pyrrole derivatives bearing sulfonamide groups: synthesis invitro cytotoxicity evaluation, molecular docking and DFT study. J. Mol. Struct. 2017;1146:242–253. [Google Scholar]
- 47.Makov G. Chemical hardness in density functional theory. J. Phys. Chem. 1995;99(23):9337–9339. [Google Scholar]
- 48.Schneider W.B., Auer A.A., Behm R.J. Constant chemical potential approach for quantum chemical calculations in electrocatalysis. Beilstein J. Nanotechnol. 2014;5:668–676. doi: 10.3762/bjnano.5.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Putz M.V., Russo N., Sicilia E. About the mulliken electronegativity in DFT theor. Chem. Acc. 2005;114:38–45. [Google Scholar]
- 50.Parr R.G., Szentpály L.V., Liu S. Electrophilicity index. J. Am. Chem. Soc. 1999;121(9):1922–1924. [Google Scholar]
- 51.Gibson J.B., Goland A.N., Milgram M., Vineyard G.H. Dynamics of radiation damage. Phys. Rev. 1960;120(4):1229–1253. [Google Scholar]
- 52.Alder B.J., Wainwright T.E. Studies in molecular dynamics. I. General method. J. Chem. Phys. 1959;31:459–466. [Google Scholar]
- 53.Tsui V., Case D. Theory and applications of the generalized Born solvation model in macromolecular simulations Biopolymers. Nucl. Acid. Sci. 2001;56:275–291. doi: 10.1002/1097-0282(2000)56:4<275::AID-BIP10024>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]