Abstract
The aim of the present study was to investigate the anti-inflammatory effects of glycine thymosin β4 (Gly-Tβ4) eye drops, and to compare the efficacy of topical Gly-Tβ4 with Cyclosporine A (CsA) in a mouse model of experimental dry eye (EDE). Eye drops consisting of balanced salt solution (BSS), 0.1% Gly-Tβ4 or 0.05% CsA were used for treatment of EDE. Tear volume, tear film break-up time and corneal staining scores were measured after 7 and 14 days. Periodic acid-Schiff staining for conjunctival gobleT cells, TUNEL assay for corneal apoptotic positive cells, multiplex immunobead assay for interleukin (IL)-1β, IL-6, tumor necrosis factor-α and interferon-γ levels, and flow cytometry for CD4+/CCR5+ T cells were performed after 14 days. All clinical parameters showed improvement in the Gly-Tβ4 and CsA groups (all P<0.05). Significantly increased conjunctival gobleT cells and decreased corneal TUNEL positive cells were observed in the Gly-Tβ4 and CsA groups. The Gly-Tβ4 and CsA treated groups showed significantly reduced inflammatory cytokine levels and T cells in the conjunctiva compared with the EDE and BSS groups (all P<0.05). However, there were no significant differences observed in the inflammatory and clinical parameters between the Gly-Tβ4 and CsA treatment groups. Topical application of 0.1% Gly-Tβ4 significantly reduced inflammation on the ocular surface, as well as clinical parameters of EDE, with a similar efficacy to that of 0.05% CsA emulsions, suggesting that Gly-Tβ4 eye drops may be used as a therapeutic agent for treatment of dry eye disease.
Keywords: glycine thymosin β4, experimental dry eye, inflammation, ocular surface
Introduction
Dry eye disease (DED) is a chronic and progressive ocular disorder that is frequently encountered in ophthalmic practice (1,2). According to the definition and classification proposed by the Dry Eye Workshop II, dry eye is a multifactorial disease of the ocular surface characterized by loss of homeostasis of the tear film and accompanied by ocular symptoms, such as ocular surface disease index (3). Tear film instability, hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities serve major roles in the etiology of the condition (4,5). The pathology of DED is closely associated with inflammation in the cornea and conjunctiva, which primarily involves T cells (5-8). Current treatment for DED includes artificial tears, immunomodulatory agents and corticosteroids (9-12). Cyclosporine A (CsA) 0.05% ophthalmic emulsions is one of the standard treatments for inflammatory DED; however, it does not completely relieve inflammation on the ocular surface (13).
Thymosin β4 (Tβ4) is a 43-amino acid peptide that is a major constituent protein found in platelets, macrophages and polymorphonuclear cells (14,15). Tβ4 downregulates inflammatory mediators by inhibiting activation of nuclear factor-κB (NF-κB) (16). Additionally, Tβ4 regulates pro-inflammatory signaling in microglia and controls inflammatory processes in the brain (17). In ophthalmology, application of topical Tβ4 significantly improves clinical signs and symptoms in patients with DED (18,19).
Glycine-Tβ4 (Gly-Tβ4) is a small peptide which is a single glycine terminal residue addition to Tβ4. Gly-Tβ4 promotes corneal epithelial repair by increasing the migration of corneal epithelial cells and reducing the production of inflammatory cytokines in a rabbit model of ocular alkali burn (20).
Although topical Tβ4 was shown to improve tear film parameters in clinical DED, there are no studies evaluating the effect of Tβ4 on inflammatory molecules or cells in the ocular surface of DED, to the best of our knowledge. In the present study, the effects of topical 0.1% Gly-Tβ4 on inflammation, apoptosis and conjunctival gobleT cell density, as well as tear film and ocular surface parameters were determined, and the treatment efficacy of Gly-Tβ4 was compared with 0.05% CsA in a mouse model of experimental dry eye (EDE).
Materials and methods
Animal model of EDE
The research protocol used in the present study was approved by the Chonnam National University School Research Institutional Animal Care and Use Committee. All animals were treated in accordance with ARVO Statement for the Use of Animals in Ophthalmic and Vision Research (arvo.org/About/policies/statement-for-the-use-of-animals-in-ophthalmic-and-vision-research/). Female C57BL/6 mice (n=35) aged 6-8 weeks were used in the following experiments. EDE was induced by subcutaneous injection of 0.5 mg/0.2 ml scopolamine hydrobromide (Sigma Aldrich; Merck KGaA) three times a day (9 a.m., 1:30 p.m. and 6 p.m.) with exposure to an air draft and 30% ambient humidity, as previously described (13,21). During these experiments, animal behavior, and food and water intake were not restricted. Mice were randomly divided into five groups according to the topical treatment administered as follows: i) Untreated control mice that were not exposed to desiccating stress or topical treatment (UT group); ii) EDE mice that were exposed to desiccating stress but were not administered eye drops (EDE group); iii) EDE mice treated with balanced salt solution (BSS; obtained from Alcon) (BSS group); iv) EDE mice treated with 0.05% CsA (Restasis®, Allergan, Ltd.) (CsA group); and v) EDE mice treated with 0.1% Gly-Tβ4. Gly-Tβ4 was prepared at 0.1% by Huons Co., Ltd. based on the results of preliminary experiments (Fig. S1; Gly-Tβ4 group). A total of 2 µl eye drops was applied topically to both eyes three times a day in BSS and 0.1% Gly-Tβ4 groups, and twice a day in 0.05% CsA group until they were euthanized. Clinical parameters, including tear volume, tear film break-up time (TBUT) and corneal staining scores (CSS) were measured 7 and 14 days after treatment. The clinical measurements were taken 3 h after the last scopolamine injection and application of eye drops. After measurement of the clinical parameters, the mice were euthanized, and periodic acid-Schiff (PAS) staining, TUNEL assay, multiplex immunobead assay, and flow cytometry were performed as described below. Each group consisted of six animals, and the experiments were performed on three independent sets of mice.
Evaluation of tear film and ocular surface parameters
Tear volume was measured using phenol red-impregnated cotton threads (Zone-Quick; Oasis Medical, Inc.) 3 h after the last scopolamine injection, as previously described (22,23). The thread was placed on the lower conjunctival fornix at approximately one-third of the lower eyelid distance from the lateral canthus for 20 seconds. The length of the wet red thread was measured in millimeters under a photomicroscope (magnification, x1; SMZ 1500; Nikon Corporation). A standard curve was plotted to convert distance into volume.
A total of 1 µl 1% sodium fluorescein was instilled into the inferior conjunctival sac using a micropipette. After three blinks, TBUT was recorded in sec using slit lamp biomicroscopy (magnification, x16; BQ-900; Haag-Streit) under cobalt blue light. After a total of 90 sec, punctate staining of the corneal surface was evaluated by a researcher who was blinded to the therapeutic conditions. Each cornea was divided into four quadrants, which were scored individually. CSS was calculated using a 4-point scale, based on a previous study (24): 0, absent; 1, slightly punctate staining <30 spots; 2, punctate staining >30 spots, but not diffuse; 3, severe diffuse staining but no positive plaque; and 4, positive fluorescein plaque. The four scores were added to generate a final grade; the maximum possible score was 16 points.
Histology
The eye and the adnexa were surgically excised, fixed in 4% paraformaldehyde overnight at 4˚C, dehydrated in a gradient concentration of ethanol (70-100%), and embedded in paraffin. Serial sections were cut from the lateral and medial borders of each paraffin block (6 µm thick slices) and stained with PAS reagent (cat. no. 395B-1 KT; Sigma-Aldrich Corporation) for 15 min at room temperature. Sections obtained from four animals in each group were examined and imaged with a light microscope (magnification, x10; Olympus Corporation) equipped with a digital camera. GobleT cell density in the superior and inferior conjunctiva was measured in three sections from each eye using Image-Pro version 10.0.5; Medial Cybernetics, Inc.) and was expressed as the number of goble T cells per 100 µm.
TUNEL staining
A TUNEL assay was used to detect the 3' hydroxyl ends of fragmented DNA, an early event in the apoptotic cascade, and used to identify apoptotic cells. The eye and the adnexa were surgically excised, fixed in 4% paraformaldehyde overnight at 4˚C and embedded in paraffin. Staining was performed using a DeadEnd™ Fluorometric TUNEL system (Promega Corporation), according to the manufacturer's protocol. Stained tissues were mounted on slides, the nuclei were visualized with DAPI present in the ProLong Gold Antifade Mounting Medium (Invitrogen; Thermo Fisher Scientific, Inc.) and the tissues were observed using a Leica TCS SP5 AOBS laser scanning confocal microscope (Leica Microsystems, GmbH) under a Leica x63 (N.A. 1.4) oil objective. Cell images were obtained separately with the following fluorescence excitation and emission settings: Excitation at 405 and 488 nm and emission between 424-472 and 502-550 nm for TUNEL assay and DAPI, respectively. TUNEL positive cells and nuclear staining with DAPI in the cornea were viewed under a fluorescent microscope (magnification, x20).
Multiplex immunobead assay
A multiplex immunobead assay (Luminex 200; Luminex Corporation) was used to measure the concentrations of interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF)-α and interferon (IFN)-γ (all from Milliplex®, EMD Millipore; cat. no. MCYTOMAG-70K) in the conjunctiva, as previously described (21). The tissues were collected and pooled in TissueLyser lysis buffer (Qiagen, Inc.) containing protease inhibitors for 30 min. The cell extracts were centrifuged at 14,000 x g for 15 min at 4˚C, and the supernatants were stored at -70˚C until use. After centrifugation, each sample (10 µg/25 µl/well) was added to a 96-well plate and incubated overnight at 4˚C in the dark, with 25 µl 1x beads coupled to mouse cytokine/chemokine-specific antibodies. Serial dilutions of each cytokine/chemokine were also added to wells in the same plate, to generate a standard curve. The following day, the beads were washed and mixed with 25 µl 1x biotinylated secondary cytokine/chemokine antibody mixture for 1 h at room temperature, followed by washing and subsequent incubation with 25 µl streptavidin-phycoerythrin for 30 min at room temperature (both steps performed in the dark). After a final wash, the wells were resuspended with 100 µl assay buffer. The reactions were detected after addition of streptavidin-phycoerythrin using an analysis system (xPONENT; Luminex Corporation). Concentrations of the cytokines in the tissues were calculated from standard curves of known concentrations of recombinant mouse cytokines.
Flow cytometry
Flow cytometry was performed to measure the proportion of CD4+/CCR5+ T cells from conjunctiva using a previously described method (25). Tissues from each group were harvested, dipped in PBS, teased apart with scissors, and shaken at 37˚C for 60 min in the presence of 0.5 mg/ml collagenase type D (Roche Applied Science). After incubation, the tissues were homogenized by grinding with a syringe plunger and passed through a cell strainer with a pore size of 100 µm. Cells were centrifuged at 450 x g at 4˚C for 7 min and re-suspended in PBS with 1% BSA. The samples were incubated with monoclonal antibodies. For detecting CD4+/CCR5+ double-stained cells, the samples were incubated with fluorescein-conjugated anti-mouse CD4 antibody (0.5 mg/ml; cat. no. 553651; BD Biosciences) and phycoerythrin-conjugated anti-mouse CCR5 antibody (0.5 mg/ml; cat. no. 559923; BD Biosciences), or isotype control antibody (cat. nos. 553929 and 559841; BD Biosciences) at 4˚C for 30 min. After 30 min of incubation, the cells were washed in PBS. The cells were then centrifuged three times in 1 ml PBS and resuspended. The number of CD4+/CCR5+ T cells were counted using a FACSCalibur flow cytometer (BD Biosciences) with CellQuest software (version 5.2.1; BD Biosciences).
Statistical analysis
SPSS version 18.0; SPSS, Inc.) was used for all statistical analyses. Results are presented as the mean ± standard deviation. Statistical differences in tear volume, TBUT and CSS among the groups were determined using a one-way ANOVA with a post-hoc Tukey's test. A Kruskal-Wallis test followed by a Dunn's multiple comparisons post-hoc test was used to compare the cytokine levels, flow cytometry, goblet cell density and apoptotic cell density between the groups. P<0.05 was considered to indicate a statistically significant difference.
Results
Clinical parameters in the ocular surface
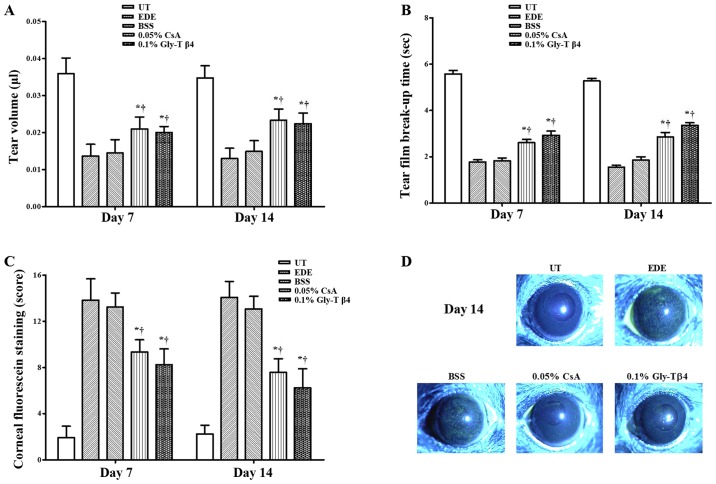
Mean tear volumes were 0.036±0.004 µl in the UT group, 0.014±0.013 µl in the EDE group, 0.015±0.004 µl in the BSS group, 0.020±0.001 µl in the CsA group and 0.021±0.003 µl in the Gly-Tβ4 group after 7 days. The mean tear volume after 14 days was 0.035±0.003 µl (UT group), 0.013±0.003 µl (EDE group), 0.015±0.003 µl (BSS group), 0.023±0.003 µl (CsA group) and 0.022±0.003 µl (Gly-Tβ4 group), respectively. The Gly-Tβ4 and CsA groups showed a significant improvement in the tear volume compared with the EDE and BSS groups after 7 and 14 days (EDE vs. Gly-Tβ4 and CsA, P<0.05; BSS vs. Gly-Tβ4 and CsA, P<0.05; Fig. 1A).
Figure 1.
Tear film and ocular surface parameters. (A) Mean tear volumes, (B) tear film break-up time, (C) corneal staining scores and (D) representative images of corneal staining in the UT, EDE, BSS, 0.05% CsA and 0.1% Gly-Tβ4 groups. The Gly-Tβ4 and CsA groups showed an improvement in all clinical parameters compared with the EDE and PSS groups. There were no significant differences between the two treatment groups after 7 and 14 days. *P<0.05 vs. EDE group; †P<0.05 vs. BSS group. UT, untreated control; EDE, experimental dry eye; BSS, balanced salt solution; CsA, Cyclosporine A; Gly-Tβ4, glycine-thymosin β4.
Mean TBUTs were 5.58±0.52 sec in the UT group, 1.79±0.34 sec in the EDE group, 1.83±0.41 sec in the BSS group, 2.57±0.52 sec in the CsA group and 2.91±0.66 sec in the Gly-Tβ4 group after 7 days. Mean TBUTs after 14 days were 5.28±0.39 sec (UT group), 1.56±0.24 sec (EDE group), 1.85±0.51 sec (BSS group), 2.86±0.66 sec (CsA group) and 3.36±0.39 sec (Gly-Tβ4 group). The Gly-Tβ4 and CsA groups showed a significantly higher TBUT compared with the EDE and BSS groups after 7 and 14 days (EDE vs. Gly-Tβ4 and CsA, P<0.05; BSS vs. Gly-Tβ4 and CsA, P<0.05; Fig. 1B).
A total of 7 days after induction, the mean CSS was 1.92±1.00 in the UT group, 13.83±1.85 in the EDE group, 13.25±1.22 in the BSS group, 9.33±1.07 in the CsA group and 8.25±1.35 in the Gly-Tβ4 group. The mean CSS after 14 days was 2.25±0.75 (UT group), 14.08±1.38 (EDE group), 13.08±1.08 (BSS group), 7.58±1.16 (CsA group) and 6.25±0.65 (Gly-Tβ4 group). The Gly-Tβ4- and CsA-treated groups showed a significant improvement in the CSS after 7 and 14 days (EDE vs. Gly-Tβ4 and CsA, P<0.05; BSS vs. Gly-Tβ4 and CsA, P<0.05; Fig. 1C).
There were no significant differences in all the clinical parameters measured between the two treatment groups (Gly-Tβ4 vs. CsA, all P>0.05).
Conjunctival goblet cell density
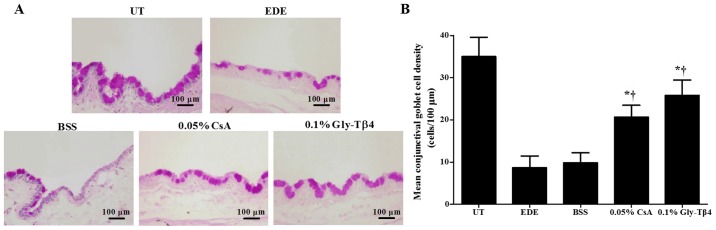
Mean goblet cell densities were 35.00±4.56 cells/100 µm in the UT group, 8.67±2.80 cells/100 µm in the EDE group, 9.83±2.40 cells/100 µm in the BSS group, 20.67±2.80 cells/100 µm in the CsA group and 25.83±3.60 cells/100 µm in the Gly-Tβ4 group. Mice in the two treatment groups exhibited significantly higher conjunctival gobleT cell densities compared with the EDE and BSS groups (EDE vs. Gly-Tβ4 and CsA, P<0.05; BSS vs. Gly-Tβ4 and CsA, P<0.05; Fig. 2). There was no significant difference in goblet cell density between the Gly-Tβ4 and CsA treatment groups.
Figure 2.
Conjunctival goblet cell density. (A) Representative images of PAS staining and (B) mean gobleT cell densities in the UT, EDE, BSS, 0.05% CsA and 0.1% Gly-Tβ4 groups at day 14. Conjunctival goblet cell density was significantly increased in the Gly-Tβ4 and CsA treatment groups. *P<0.05 vs. EDE group; †P<0.05 vs. BSS group. PAS, periodic acid-Schiff; UT, untreated control; EDE, experimental dry eye; BSS, balanced salt solution; CsA, Cyclosporine A; Gly-Tβ4, glycine-thymosin β4.
TUNEL staining
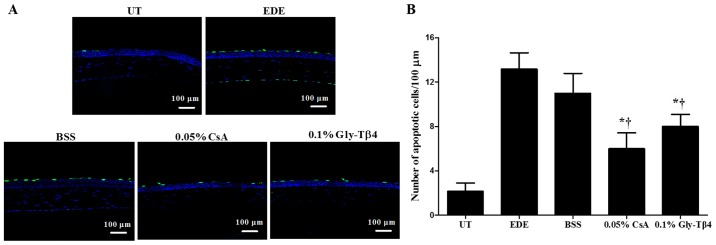
Mean apoptotic cell counts in the corneal epithelium were 2.17±0.75 cells/100 µm in the UT group, 13.17±1.47 cells/100 µm in the EDE group, 11.00±1.79 cells/100 µm in the BSS group, 6.00±1.41 cells/100 µm in the CsA group and 8.00±1.10 cells/100 µm in the Gly-Tβ4 group. Representative magnified images of the corneal sections stained with TUNEL are presented in Fig. 3. There was a significant decrease in the number of apoptotic cells observed in the Gly-Tβ4 and CsA groups when compared with both the EDE and BSS groups (EDE vs. Gly-Tβ4 and CsA, P<0.05; BSS vs. Gly-Tβ4 and CsA, P<0.05), but there was no significant difference between the Gly-Tβ4 and CsA treatment groups.
Figure 3.
Apoptotic positive cells in the corneal epithelium. (A) Representative images of TUNEL staining and (B) mean number of TUNEL positive cells in the UT, EDE, BSS, 0.05% CsA and 0.1% Gly-Tβ4 groups after 14 days. The Gly-Tβ4 and CsA groups had a lower number of TUNEL positive cells in the cornea compared with the EDE and BSS groups. *P<0.05 vs. EDE group; †P<0.05 vs. BSS group. UT, untreated control; EDE, experimental dry eye; BSS, balanced salt solution; CsA, Cyclosporine A; Gly-Tβ4, glycine-thymosin β4.
Inflammatory cytokine levels in conjunctival tissues
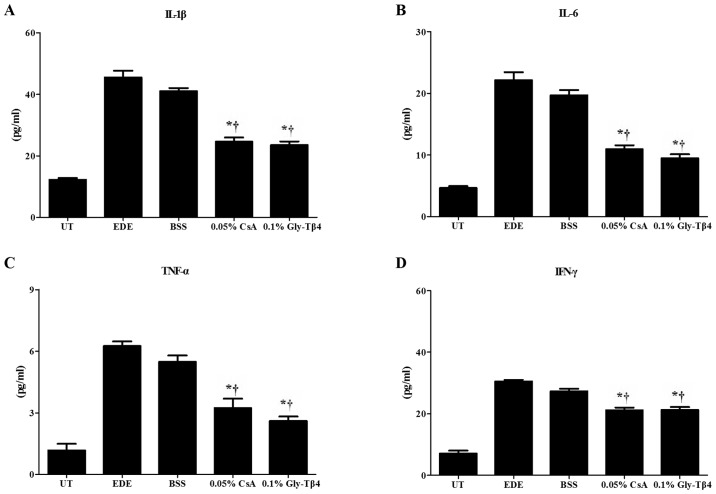
There was a significant decrease in the levels of IL-1β, IL-6, TNF-α and IFN-γ observed in the conjunctiva of mice in the Gly-Tβ4 and CsA treatment groups when compared with the EDE and BSS groups (EDE vs. Gly-Tβ4 and CsA, P<0.05; BSS vs. Gly-Tβ4 and CsA, P<0.05). There was no significant difference observed between the two treatment groups (Fig. 4).
Figure 4.
Inflammatory molecular levels in the conjunctiva. Levels of (A) IL-1β, (B) IL-6, (C) TNF-α and (D) IFN-γ in the UT, EDE, BSS, 0.05% CsA and 0.1% Gly-Tβ4 groups after 14 days. Significantly decreased levels of IL-1β, IL-6, TNF-α, and IFN-γ were observed in the Gly-Tβ4- and CsA-treated groups when compared with the EDE and BSS groups. There were no significant differences found between the two treatment groups. *P<0.05 vs. EDE group; †P<0.05 vs. BSS group. Il, interleukin; TNF, tumor necrosis factor; IFN, interferon; UT, untreated control; EDE, experimental dry eye; BSS, balanced salt solution; CsA, Cyclosporine A; Gly-Tβ4, glycine-thymosin β4.
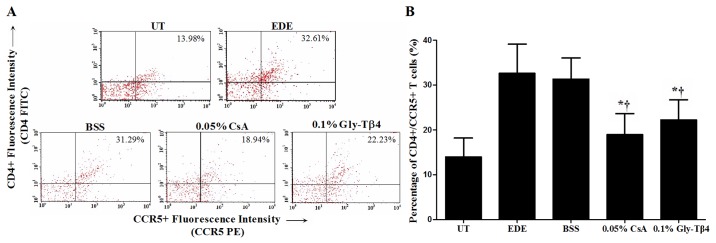
Flow cytometry analysis
The percentage of CD4+/CCR5+ T cells in the conjunctiva was 13.98±4.18% in the UT group, 32.61±6.54% in the EDE group, 31.29±4.77% in the BSS group, 18.94±4.77% in the CsA group and 22.23±4.47% in the Gly-Tβ4 group. The Gly-Tβ4- and CsA-treated groups both showed a significantly lower percentage of CD4+/CCR5+ T cells in the conjunctiva compared with the EDE and BSS groups (EDE vs. Gly-Tβ4 and CsA, P<0.05; BSS vs. Gly-Tβ4 and CsA, P<0.05), with no differences between the two treatment groups (Fig. 5).
Figure 5.
CD4+/CCR5+ T cell percentage in the conjunctiva. (A) Representative images of CD4+/CCR5+ T cells and (B) mean percentage of CD4+/CCR5+ T cells in the UT, EDE, BSS, 0.05% CsA and 0.1% Gly-Tβ4 groups after 14 days. The Gly-Tβ4- and CsA-treated groups had a significantly lower percentage of CD4+/CCR5+ T cells in the conjunctiva compared with the EDE and BSS groups. There were no notable differences between the two treatment groups. *P<0.05 vs. EDE group; †P<0.05 vs. BSS group. UT, untreated control; EDE, experimental dry eye; BSS, balanced salt solution; CsA, Cyclosporine A; Gly-Tβ4, glycine-thymosin β4.
Discussion
DED is a chronic and progressive ocular surface disorder in which ocular surface inflammation and damage serve an important role in the pathogenesis of the disease. In addition, severe DED may result in epithelial defects of the ocular surface, corneal ulcers and serious loss of vision, which result in discomfort to patients, and reduce their quality of life. Various treatments have proven to be useful for the treatment of DED, and topical CsA has become one of the standard treatments for inflammatory DED (1,13). However, no single agent or combination of agents successfully results in the resolution of DED and ocular surface healing.
Tβ4 is a natural peptide found in high concentrations in the majority of tissues and cells, such as blood platelets, macrophages and other lymphoid tissues (14). Tβ4 effectively downregulates the levels of inflammatory mediators by inhibiting NF-κB activity and promoting cell migration (16). Gly-Tβ4 is a small 44-amino acid peptide with a single glycine terminal residue added to Tβ4 that can bind to G-actin, and consequently affects cell migration (14,15). Topical application of Gly-Tβ4 results in reduced release of inflammatory molecules and improved corneal epithelial recovery in an animal model of alkali burn injury (20).
Previous studies have reported that Tβ4 regulated the inflammatory process by reducing the production of inflammatory cytokines and chemokines in acute myocardial infarction, alcoholic liver disease and neurodegenerative diseases (17,26-28). In ophthalmology, several studies have reported that topical Tβ4 application improved clinical dry eye parameters, including tear volume, TBUT and corneal staining scores in human and experimental DED (18,19). However, the effects of topical Tβ4 on inflammatory or apoptotic changes in DED have not been investigated.
Ocular surface inflammation, which is characterized by increased expression of inflammatory cytokines and T cells, serves a critical role in the pathogenesis of DED. It has demonstrated that increased levels of inflammatory molecules and increased number of Th1 cells on the ocular surface may specifically induce the expression of chemokine receptors, such as CCR5 and CXCR3 (4,29,30). In the present study, the effects of topical 0.1% Gly-Tβ4 on inflammatory cytokines, Th1 cells, apoptotic cells and conjunctival gobleT cells, as well as on tear film and ocular surface parameters were investigated using a mouse model of EDE. Regarding Th1 cells, the percentage of CD4+/CCR5+ cells in the conjunctiva was measured using flow cytometry, similar to previously reported studies (31,32). The anti-inflammatory and anti-apoptotic characteristics of Gly-Tβ4 were clearly shown in our results. Topical instillation of 0.1% Gly-Tβ4 significantly decreased the levels of inflammatory cytokines (IL-1β, IL-6, TNF-α and IFN-γ) and the percentage of Th1 cells in the conjunctiva, to a similar degree as 0.05% CsA. In addition, a decrease in the number of TUNEL positive cells in the cornea and increased conjunctival gobleT cell density were observed with treatment of 0.1% Gly-Tβ4 equivalent to that observed with 0.05% CsA.
Anti-inflammatory medicines for the treatment of DED, such as steroids and CsA, primarily focus on the improvement of ocular surface inflammation and tear secretion (33,34). However, ocular surface injury is a risk factor for severe DED that intensifies the ocular surface inflammatory response, and results in corneal ulcers and a serious impairment to vision (35). Tβ4 has been shown to improve cellular epithelium repair by enhancing the expression of laminin-5, promoting cell migration, and consequently downregulating inflammatory responses (36). Additionally, Gly-Tβ4 eye drops have been shown to inhibit corneal neovascularization and improve epithelial wound healing in a rabbit model of alkali burn (20). In the present study, topical 0.1% Gly-Tβ4 treatment resulted in a significant reversal of corneal epithelial damage, which was indicated by the reduction in CSS. Thus, it is hypothesized that the protective effects of topical Gly-Tβ4 on corneal epithelial healing may be more effective in improving tear film parameters and ocular surface damage, including tear volume, TBUT and CSS.
Based on the results of the present and previous studies, topical 0.1% Gly-Tβ4 therapy significantly improves tear film parameters, ocular surface damage and corneal epithelial apoptosis in DED, by reducing ocular surface inflammation and promoting corneal epithelial repair and shows a similar therapeutic efficacy as 0.05% CsA emulsions. Therefore, Gly-Tβ4 may be used as a supplementary agent for effective treatment of DED, particularly for patients with ocular surface defects.
Supplementary Material
Acknowledgements
Not applicable.
Funding
This study was supported by Huons Co., Ltd., the Basic Research Program through the National Research Foundation of Korea and funded by the Ministry of Science, ICT & Future Planning (grant no. 2017R1A2B4003367), and the Chonnam National University Hospital Biomedical Research Institute (grant nos. CRI18093-1 and BCRI 19038).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
KCY designed the experiment and revised the manuscript. RJ, YL, LL, JHC and DHK performed the experiments. RJ, YL, HJY, CDY and HSS analyzed and interpreted the data. RJ, YL, DHK, CDY and HSS drafted the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The research protocol used in the present study was approved by the Chonnam National University Medical School Research Institutional Animal Care and Use Committee. Maintenance of animals and all in vivo experiments were performed in accordance with the Association for Research in Vision and Ophthalmology statement for the Use of Animals in Ophthalmic and Vision Research.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1. doi: 10.1016/s1542-0124(12)70082-4. The epidemiology of dry eye disease: Report of the epidemiology subcommittee of the international dry eye workshop (2007). Ocul Surf 5: 93-107, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Farrand KF, Fridman M, Stillman IÖ, Stillman IO. Prevalence of diagnosed dry eye disease in the united states among adults aged 18 years and older. Am J Ophthalmol. 2017;182:90–98. doi: 10.1016/j.ajo.2017.06.033. [DOI] [PubMed] [Google Scholar]
- 3.Wolffsohn JS, Arita R, Chalmers R, Djalilian A, Dogru M, Dumbleton K, Gupta PK, Karpecki P, Lazreg S, Pult H, et al. TFOS DEWS II diagnostic methodology report. Ocul Surf. 2017;15:539–574. doi: 10.1016/j.jtos.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Yoon KC, De Paiva CS, Qi H, Chen Z, Farley WJ, Li DQ, Pflugfelder SC. Expression of Th-1 chemokines and chemokine receptors on the ocular surface of C57BL/6 mice: Effects of desiccating stress. Invest Ophthalmol Vis Sci. 2007;48:2561–2569. doi: 10.1167/iovs.07-0002. [DOI] [PubMed] [Google Scholar]
- 5.Craig JP, Nichols KK, Akpek EK, Caffery B, Dua HS, Joo CK, Liu Z, Nelson JD, Nichols JJ, Tsubota K, Stapleton F. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15:276–283. doi: 10.1016/j.jtos.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Stern ME, Pflugfelder SC. Inflammation in dry eye. Ocul Surf. 2004;2:124–130. doi: 10.1016/s1542-0124(12)70148-9. [DOI] [PubMed] [Google Scholar]
- 7.Pflugfelder SC, De Paiva CS, Li DQ, Stem ME. Epithelial-immune cell interaction in dry eye. Cornea. 2008;27:S9–S11. doi: 10.1097/ICO.0b013e31817f4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bucolo C, Musumeci N, Musumeci S, Drago F. Acidic mammalian chitinase and the eye: Implications for ocular inflammatory diseases. Front Pharmacol. 2011;2(43) doi: 10.3389/fphar.2011.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bucolo C, Fidilio A, Fresta CG, Lazzara F, Platania CBM, Cantarella G, Di Benedetto G, Burgaletto C, Bernardini R, Pizza C, et al. Ocular pharmacological profle of hydrocortisone in dry eye disease. Front Pharmacol. 2019;10(1240) doi: 10.3389/fphar.2019.01240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y, Cui L, Lee HS, Kang YS, Choi W, Yoon KC. Comparison of 0.3% hypotonic and isotonic sodium hyaluronate eye drops in the treatment of experimental dry eye. Curr Eye Res. 2017;42:1108–1114. doi: 10.1080/02713683.2017.1297462. [DOI] [PubMed] [Google Scholar]
- 11.Yang JM, Choi W, Kim N, Yoon KC. Comparison of topical cyclosporine and diquafosol treatment in dry eye. Optom Vis Sci. 2015;92:e296–e302. doi: 10.1097/OPX.0000000000000657. [DOI] [PubMed] [Google Scholar]
- 12.Kim HS, Kim TI, Kim JH, Yoon KC, Hyon JY, Shin KU, Choi CY. Evaluation of clinical efficacy and safety of a novel cyclosporine A nanoemulsion in the treatment of dry eye syndrome. J Ocul Phamacol Ther. 2017;33:530–538. doi: 10.1089/jop.2016.0164. [DOI] [PubMed] [Google Scholar]
- 13.De Oliveira, Wilson SE. Practical guidance for the use of cyclosporine ophthalmicsolutions in the management of dry eye disease. Clin Ophthalmol. 2019;13:1115–1122. doi: 10.2147/OPTH.S184412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crockford D, Turjman N, Allan C, Angel J. Thymosin beta4: Structure, function, and biological properties supporting current and future clinical applications. Ann NY Acad Sci. 2010;1194:179–189. doi: 10.1111/j.1749-6632.2010.05492.x. [DOI] [PubMed] [Google Scholar]
- 15.Huff T, Müller CS, Otto AM, Netzker R, Hannappel E. beta-Thymosins, small acidic peptides with multiple functions. Int J Biochem Cell Biol. 2001;33:205–220. doi: 10.1016/s1357-2725(00)00087-x. [DOI] [PubMed] [Google Scholar]
- 16.Sosne G, Qiu P, Christopherson PL, Wheater MK. Thymosin beta 4 suppression of corneal NFkappaB: A potential anti-inflammatory pathway. Exp Eye Res. 2007;84:663–669. doi: 10.1016/j.exer.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pardon MC. Anti-inflammatory potential of thymosin β4 in the central nervous system: Implications for progressive neurodegenerative diseases. Expert Opin Biol Ther. 2018;18 (Suppl 1):S165–S169. doi: 10.1080/14712598.2018.1486817. [DOI] [PubMed] [Google Scholar]
- 18.Sosne G, Ousler GW. Thymosin beta 4 ophthalmic solution for dry eye: A randomized, placebo-controlled, phase II clinical trial conducted using the controlled adverse environment (CAE™) model. Clin Ophthalmol. 2015;9:877–884. doi: 10.2147/OPTH.S80954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sosne G, Dunn SP, Kim C. Thymosin β4 significantly improves signs and symptoms of severe dry eye in a phase 2 randomized trial. Cornea. 2015;34:491–496. doi: 10.1097/ICO.0000000000000379. [DOI] [PubMed] [Google Scholar]
- 20.Zhang W, Nie L, Du L, Chen W, Wu Z, Jin Y. Topical treatment of corneal alkali burns with Gly-thymosin β4 solutions and in situ hydrogels via inhibiting corneal neovascularization and improving corneal epidermal recovery in experimental rabbits. Burns. 2017;43:1742–1747. doi: 10.1016/j.burns.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 21.Yoon KC, De Paiva CS, Qi H, Chen Z, Farley WJ, Li DQ, Sterm ME, Pflugfelder SC. Desiccating environmental stress exacerbates autoimmune lacrimal keratoconjunctivitis in non-obese diabetic mice. J Autoimmun. 2008;30:212–221. doi: 10.1016/j.jaut.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoon KC, Ahn KY, Choi W, Li Z, Choi JS, Lee SH, Park SH. Tear production and ocular surface changes in experimental dry eye after elimination of desiccating stress. Invest Ophthalmol Vis Sci. 2011;52:7267–7273. doi: 10.1167/iovs.11-7231. [DOI] [PubMed] [Google Scholar]
- 23.Villareal AL, Farley W, Pflugfelder SC. Effect of topical ophthalmic epinastine and olopatadine on tear volume in mice. Eye Contact Lens. 2006;32:272–276. doi: 10.1097/01.icl.0000224360.10319.b1. [DOI] [PubMed] [Google Scholar]
- 24.Pauly A, Brignole-Baudouin F, Labbė A, Liang H, Warnet JM, Baudouin C. New tools for the evaluation of toxic ocular surface changes in the rat. Invest Ophthalmol Vis Sci. 2007;48:5473–5483. doi: 10.1167/iovs.06-0728. [DOI] [PubMed] [Google Scholar]
- 25.Yoon KC, Park CS, You IC, Choi HJ, Lee KH, Im SK, Park HY, Pflugfelder SC. Expression of CXCL9, -10, -11, and CXCR3 in the tear film and ocular surface of patients with dry eye syndrome. Invest Ophthalmol Vis Sci. 2010;51:643–650. doi: 10.1167/iovs.09-3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shah R, Reyes-Gordillo K, Cheng Y, Varatharajalu R, lbrahin J, Lakshman MR. Thymosin β4 prevents oxidative stress, inflammation, and fibrosis in ethanol- and LPS-induced liver injury in mice. Oxid Med Cell Longev. 2018;2018(9630175) doi: 10.1155/2018/9630175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cacasin MA. Therapeutic potential of thymosin-beta4 and its derivative N-acetyl-seryl-aspartyl-lysyl-proline (Ac-SDKP) in cardiac healing after infarction. Am J Cardiovasc Drugs. 2006;6:305–311. doi: 10.2165/00129784-200606050-00003. [DOI] [PubMed] [Google Scholar]
- 28.Goldstein AL, Hannappel E, Sosne G, Kleinman HK. Thymosin β4: A multi-functional regenerative peptide. Basic properties and clinical applications. Expert Opin Biol Ther. 2012;12:37–51. doi: 10.1517/14712598.2012.634793. [DOI] [PubMed] [Google Scholar]
- 29.Gulati A, Sacchetti M, Bonini S, Dana R. Chemokine receptor CCR5 expression in conjunctival epithelium of patients with dry eye syndrome. Arch Ophthalmol. 2006;124:710–716. doi: 10.1001/archopht.124.5.710. [DOI] [PubMed] [Google Scholar]
- 30.EI Annan J, Chauhan SK, Ecoiffier T, Zhang Q, Saban DR, Dana R. Characterization of effector T cells in dry eye disease. Invest Ophthalmol Vis Sci. 2009;50:3802–3807. doi: 10.1167/iovs.08-2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y, Jin R, Li L, Hsu HH, You IC, Yoon HJ, Yoon KC. Therapeutic effect of topical adiponectin-derived short peptides compared with globular adiponectin in experimental dry eye and alkali burn. J Ocul Phamacol Ther. 2020;36:88–96. doi: 10.1089/jop.2018.0131. [DOI] [PubMed] [Google Scholar]
- 32.Choi W, Li Z, Oh HJ, Im SK, Lee SH, Park SH, You IC, Yoon KC. Expression of CCR5 and its ligands CCL3, -4, and -5 in the tear film and ocular surface of patients with dry eye disease. Curr Eye Res. 2012;37:12–17. doi: 10.3109/02713683.2011.622852. [DOI] [PubMed] [Google Scholar]
- 33.Boboridis KG, Konstas AGP. Evaluating the novel application of cyclosporine 0.1% in ocular surface disease. Expert Opin Pharmacother. 2018;19:1027–1039. doi: 10.1080/14656566.2018.1479742. [DOI] [PubMed] [Google Scholar]
- 34.Yin J, Kheirkhah A, Dohiman T, Saboo U, Dana R. Reduced efficacy of low-dose topical steroids in dry eye disease associated with graft-versus-host disease. Am J Ophthalmol. 2018;190:17–23. doi: 10.1016/j.ajo.2018.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pflugfelder SC. Integrating restasis into the management of dry eye. Int Ophthlamol Clin. 2006;46:101–103. doi: 10.1097/01.iio.0000212137.85298.98. [DOI] [PubMed] [Google Scholar]
- 36.Sosne G, Xu L, Prach L, Mrock LK, Kleinman HK, Letterio JJ, Hazlett LD, Kurpakus-Wheater M. Thymosin beta 4 stimulates laminin-5 production independent of TGF-beta. Exp Cel Res. 2004;293:175–183. doi: 10.1016/j.yexcr.2003.09.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.