Abstract
Metabolic associated fatty liver disease (MAFLD), formerly named non-alcoholic fatty liver disease is the most common liver disorder in many countries. The inflammatory subtype termed steatohepatitis is a driver of disease progression to cirrhosis, hepatocellular carcinoma, liver transplantation, and death, but also to extrahepatic complications including cardiovascular disease, diabetes and chronic kidney disease. The plasticity of macrophages in response to various environmental cues and the fact that they can orchestrate cross talk between different cellular players during disease development and progression render them an ideal target for drug development. This report reviews recent advances in our understanding of macrophage biology during the entire spectrum of MAFLD including steatosis, inflammation, fibrosis, and hepatocellular carcinoma, as well as for the extra-hepatic manifestations of MAFLD. We discuss the underlying molecular mechanisms of macrophage activation and polarization as well as cross talk with other cell types such as hepatocytes, hepatic stellate cells, and adipose tissue. We conclude with a discussion on the potential translational implications and challenges for macrophage based therapeutics for MAFLD.
Keywords: Metabolic associated fatty liver disease, Macrophages, Inflammation, Fibrosis, Hepatic stellate cells
Core tip: In this work, we review the recent advances in our understanding of macrophage biology during the entire spectrum of metabolic associated fatty liver disease (MAFLD) including steatosis, inflammation, fibrosis, and hepatocellular carcinoma, as well as for the extra-hepatic manifestations of MAFLD. We discuss the underlying molecular mechanisms of macrophage activation and polarization as well as cross talk with other cell types such as hepatocytes, hepatic stellate cells and adipose tissue. We conclude with a discussion on the potential translational implications and challenges for macrophage based therapeutics for MAFLD.
INTRODUCTION
Metabolic associated fatty liver disease (MAFLD), formerly named non-alcoholic fatty liver disease has now reached an “alert” level, an outcome of its high prevalence and its clinical and economic burden. It is estimated that nearly 1 billion people are afflicted globally[1-3], while projections suggest that the incidence of MAFLD and MAFLD-related complications will continue to burgeon over the next few decades[4]. By current indications, MAFLD is on trajectory to become the primary cause of hepatocellular carcinoma (HCC) and the primary indication for liver transplantation[5,6]. Of most concern, around 20 million people who have MAFLD are expected to die of their liver disease. MAFLD not only increases the risk of liver failure and HCC, but also increase the risk for extra-hepatic complications such as type 2 diabetes, cardiovascular disease, chronic kidney disease, osteoporosis, and some type of cancers. The estimated annual medical costs directly attributable to MAFLD is about $100 billion in the United States and €35 billion in four large European countries (Italy, France, Germany and The United Kingdom)[7].
The pathogenesis of MAFLD suggests that it is a heterogeneous disease with a variable clinical presentation shaped by interactions between gene and environmental cues[8,9]. In turn, the clinical presentation is affected by biological and chronological age[10]. Though MAFLD is classically linked to other metabolic dysfunction and disease such as diabetes and obesity with a shared genetic basis between the conditions[11], it is now recognised that a significant proportion of patients are non-obese[1]. The spectrum of disease varies widely ranging from simple steatosis to the presence of inflammation, through to fibrosis, cirrhosis and HCC. Only a proportion (5%-10%) of patients with MAFLD will develop the more severe subtype of steatohepatitis, and some of these will progress to advanced liver fibrosis or cirrhosis, the leading cause of liver related morbidity, and mortality[12]. While HCC typically develops in the context of cirrhosis, it is increasingly described in patients even in its absence. Hence, the transition from steatosis to steatohepatitis represents a pivotal checkpoint in the natural history of patients with the disease. The current view is that innate immune mechanisms are central to the transition to hepatic inflammation[13] with macrophages playing a critical role. In this review, we provide a detailed overview on current knowledge on the role of macrophages for liver immune homeostasis and steatohepatitis development and the translational implications and challenges arising from this knowledge.
HEPATIC MACROPHAGES
Hepatic macrophages consist of different cell populations including resident macrophages (aka Kupffer cells) which originate from the yolk sac and function as the dominant liver phagocyte. In addition, bone marrow derived monocytes in the circulation can infiltrate the liver[14].
The liver has the largest proportion of tissue macrophages among solid organs and it is estimated that for every 100 hepatocytes in a healthy rodent, there are between 20-40 macrophages[15]. This emphasizes the critical role of liver macrophages in maintaining liver homeostasis[16], but also indicates the high levels of heterogeneity of this cell population that must exist to enable homeostasis maintenance. Two recent studies employed single cell RNA sequencing to de-convolute hepatic macrophage heterogeneity. In these studies, distinct hepatic macrophage populations with inflammatory and non-inflammatory/immunoregulatory functions were demonstrated[17]. The second study demonstrated that myeloid cells in liver and bone marrow acquire a functionally distinct inflammatory phenotype in diet induced mouse models of steatohepatitis[18].
Physiologically, hepatic immune homeostasis is controlled by sinusoidal endothelial cells with liver macrophages part of the liver reticulo-endothelial system[19]. A recent report suggests that liver macrophages compromise two separate and non-overlapping niches mediating immunosurveillance and that the hepatic capsule has a cellular network of resident macrophages phenotypically distinct from KCs. These cells sense peritoneal bacteria and restricts hepatic dissemination of these bacteria by promoting neutrophil recruitment to the capsule[20]. This system creates a dynamic and complex network which therefore constitutes the first line of defence against invading pathogens with contribution of other immune cells such as neutrophils[16]. At the same time, the upkeep of tissue homeostasis is monitored by the reticuloendothelial system through the production of immunoregulatory cytokines such as interleukin-10 and programmed cell death 1 ligand 1[21]. Their function includes scavenging bacteria and bacteria-associated products which end up in the liver from the intestine through the portal vein[22]. However, alterations in this fine-tuned system can lead to different pathological disorders and is strongly implicated in MAFLD development.
MACROPHAGE POLARIZATION
Macrophages polarization is the process whereby macrophages differentiate into sub-phenotypes with specific biological functions in response to signals from their microenvironment including cytokines, growth factors, fatty acids, prostaglandins and pathogen-derived molecules. A simplified classification is the M1 and M2 based activation state[23,24]. M1 macrophages are pro-inflammatory and antimicrobial and initiate inflammatory processes by expressing high levels of proinflammatory cytokines and producing high amounts of reactive oxygen and nitrogen species[25]. In contrast, M2 macrophages have anti-inflammatory and reparative functions with high expression of different chemokines compared with M1 macrophages[24,25]. However, the true complexity of macrophage phenotypes and their regulation is greater than described above and is context dependent and dynamic[26]. Difficulties in interpreting many published reports results from insufficient characterization of macrophage polarization and function[14].
MACROPHAGES AND THEIR ROLES IN MAFLD
Macrophages, steatosis development, and the transition to steatohepatitis
Macrophages are highly versatile, while extensive experimental and clinical data indicates that they play a central role in MAFLD development with pro-inflammatory macrophages determining disease severity[14]. In human MAFLD, portal macrophage infiltration is observed at an early stage before inflammation is evident and is associated with progressive disease[27]. Another study of young adult Koreans showed an increase in the numbers of CD68+ Kupffer cells in biopsy samples from patients with steatohepatitis compared to those with steatosis[28]. Similarly, an increase of activated macrophages was found in children with MAFLD and these were located in the vicinity of damaged hepatocytes[29].
Additional evidence for a central role for macrophages in steatohepatitis comes from experimental models. In dietary mouse models, an increase in hepatic macrophages that produce pro-inflammatory cytokines was observed in high-fat diet (HFD) and methionine-choline-deficient (MCD) diet fed mice[30,31]. Moreover, the production of these inflammatory mediators was increased after 4 wk in mice fed a MCD diet and decreased subsequently, suggesting a determinant role in the transition to steatohepatitis[32]. In addition, immune responses are innately different between mouse strains; T helper 1 cell and M1 responses are observed in C57BL/6 mice whereas T helper 2 cell and M2 response are observed in BALB/c mice[33]. Feeding these two strains an MCD diet showed that steatosis and hepatic inflammation was more induced in M1-prone C57BL/6 mice compared with the M2 prone BALB/c strain[34]. Furthermore, the use of clodronate liposomes or gadolinium chloride to deplete macrophages protected mice from steatosis development[35].
Pro-inflammatory macrophages have also been found to induce hepatic insulin resistance and decrease hepatocyte responsiveness to insulin by attenuating phosphorylation of the insulin receptor substrate 1 in hepatocytes[36]. Thus, silencing of NF-κB specifically in Kupffer cells improves insulin sensitivity and decreases cytokine secretion in a HFD-fed model further suggesting a crucial role for them in hepatic insulin resistance[37]. Another report found that HFD-fed mice injected with clodronate to deplete Kupffer cells had decreased steatosis and steatohepatitis via reducing interleukin-1β-dependent suppression of peroxisome proliferator-activated receptor-α (PPARα)[38]. The latter is suggested to have an anti-inflammatory role in liver and adipose tissue[39]. Along the same line, depletion of Kupffer cells leads to reduction of inflammatory cytokine expression and decreases inflammation and liver cell death[40]. Another suggested mechanism is of p38 mitogen-activated protein kinases being upregulated in the liver of patients with MAFLD in multiple diet induced steatohepatitis mouse models. Macrophage p38 induces M1 polarization and pro-inflammatory cytokine secretion promoting the progression to steatohepatitis[41].
Macrophages and liver fibrosis
In virtually all chronic liver diseases, there is no fibrosis without preceding or concomitant inflammation. Liver macrophages play a pivotal role in fibrosis progression with these cells and hepatic stellate cells (HSCs, the major producer of extracellular matrix) exhibiting bidirectional signalling[42]. Thus, chemo-kines/cytokines from HSCs augment macrophage infiltration, while macrophages amplify inflammation, contribute to maintain the fibrogenic phenotype and promotes HSC survival. Macrophages also play a dominant role in fibrosis resolution. Alternatively activated M2 macrophages correlate with hepatic injury in MAFLD[31] orchestrating a fibrosis response favouring liver remodelling and tissue repair by producing transforming growth factor-β and platelet-derived growth factor among other proteins[43].
Distinct monocytes/macrophage populations can be found in human and mouse liver based on levels of Ly-6C (Gr1) or CD14/CD16 expression, in murine and humans respectively. In humans it includes “classical” CD14++CD16− and “non-classical” CD14+CD16+ monocytes/macrophages as well as CD16++ cells. Notably, fibrosis is associated with preferential enrichment of CD14+CD16+ cells or its functional counterpart Ly-6Chi in mouse[44,45].These cells activate HSCs in vitro, partially dependent on transforming growth factor (TGF) -β release[44,45]. Conversely, the production of soluble mediators such as CC-chemokine ligand 2 (also known as MCP1) and macrophage colony-stimulating factor by activated HSCs augments inflammatory cell infiltration to initiate and maintain myofibroblast activation[46]. Ly-6Clo cells have been identified as a feature of “restorative” hepatic macrophages in mice[47] but the exact counterpart of these in humans remains to be clarified. In mouse models, autophagy gene Atg5 in the myeloid lineage Atg5(fl/fl) LysM-Cre knockout mice have demonstrated that macrophage autophagy attenuates liver fibrosis[48]. Additionally, immune cell subset differentiation can perpetuate or restrict hepatic injury[19].
In sum, at different stages of hepatic injury, both resident Kupffer cells and freshly recruited monocyte-derived macrophages play critical roles in the regulation of inflammation, fibrosis and fibrolysis[49].
Macrophages and HCC
MAFLD can increase the risk of HCC even in the absence of cirrhosis[50]. Tumor associated macrophages secrete inflammatory cytokines such as tumour necrosis factor-α and growth factors such as vascular endothelial growth factor and TGF-β that are involved in angiogenesis and contribute to tumor development, progression, and metastasis[51]. Toll like receptor (TLR) 4 but not TLR2 on macrophages has also been demonstrated to contribute to steatohepatitis-related HCC in mice by inducing proinflammatory cytokines and the proliferation of HCC and cancer progenitor cells[52]. Another mechanistic study suggests that obesity-associated oxidative stress increases STAT-1 and STAT-3 signaling which can independently contribute to the pathogenesis of steatohepatitis, fibrosis, and HCC in mouse models[53].
MACROPHAGES AND MAFLD EXTRA-HEPATIC MANIFESTATIONS
The consequences and complications of MAFLD are not limited to liver, but also extend to include various extra-hepatic organ involvement including type 2 diabetes mellitus, chronic kidney disease, osteoporosis, hypothyroidism, some type of cancers, and cardiovascular disease[54]. The mechanisms that contribute to this heightened risk for cardiovascular disease and type 2 diabetes mellitus risk are poorly understood.
Disordered myelopoiesis and macrophage-mediated inflammation was recently suggested as a plausible overarching mechanism linking MAFLD to cardiovascular diseases[55]. Soluble CD163, a macrophage activation marker correlates with liver injury[56] with similar finding reported for CVD risk[57]. As discussed above, apart from their central role in progression to steatohepatitis and fibrosis, macrophages are known to enter to plaques and promote lesion progression, instability and rupture[58].
FACTORS REGULATING MACROPHAGE PHENOTYPE AND PLORAIZATION IN MAFLD
Dietary factors
Nutrition and the intracellular metabolism of macrophages are a key regulator of their function and can determine the skew of macrophages towards a pro or anti-inflammatory phenotype[59]. For example, dietary cholestrol differentially shapes the transcriptome of Kupffer cells and infiltrating macrophages during steatohepatitis progression tworads a pro-inflammatotry phenotype[60]. Similarly, a HFD induces macrophage polarization and aggravates the liver inflammatory microenvironment and cancer progression in a zebrafish model of MAFLD-associated HCC; this effect was reversed by metformin[61]. Conversely, exercise training and weight loss suppress macrophage activation as assessed by soluble CD163 (sCD163)[62,63]. Similarly, a reduction in sCD163 was noticed following bariatric surgery which was accompanied by improvements in insulin sensitivity and liver enzymes[64]. Functionally, endolysosomal lipid trapping and accumulation in Kupffer cells in response to high fat diet feeding upregulates hepatic inflammatory gene expression[65,66] while fatty acids can incresae mitochondrial DNA release causing activation of NOD-like receptor family pyrin domain containing 3 (NLRP3) inflammasomes in Kupffer cells[67]. In contrast, vitamin D receptor activation in hepatic macrophages improves insulin resistance, steatosis and hepatic inflammation through the induction of an anti-inflammatory phenotype[68].
Hematopoiesis in bone marrow is influenced by environmental cues including diet. Myelopoiesis is tightly regulated in bone marrow[69], governing the generation of mature cells via sequential progression from HSCs to differentiated cells including monocytes and macrophages that share a distinct committed progenitor[70,71]. Dysregulation of hematopoiesis switches the protective response to pro-inflammatory myelopoiesis in the marrow and governs ongoing and future inflammatory responses[72-74]. A recent study showed that bone marrow derived macropahges from western diet fed mice have an inflammatory activation profile compared to those from normal chow fed mice[18].
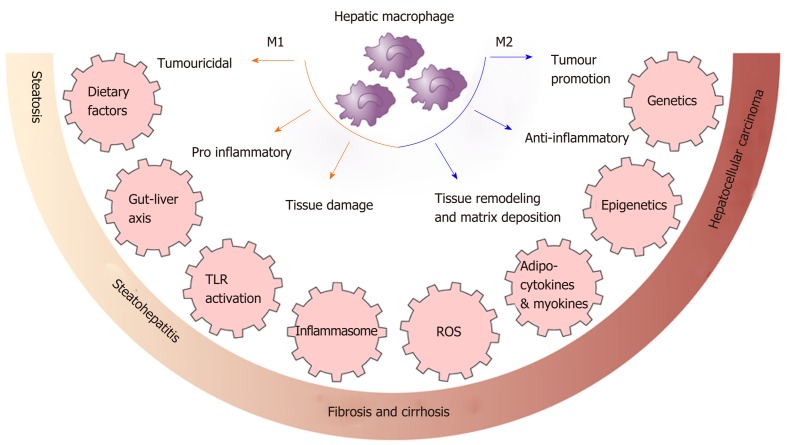
Thus, dietary factors play a role in determining macrophage polarisation and activation as well as in shaping myelopiesis. Macrophage polarisation and activation is shown in Figure 1.
Figure 1.
Macrophage polarisation and activation. Macrophages polarize to sub-phenotypes with specific biological functions in response to signals from their microenvironment. These include signals from adipocytokines and myokines, the gut liver axis and toll-like receptor activation, damaged hepatocytes, reactive oxygen species, genetic and epigenetic factors. The process of macrophage activation is a critical determinant of disease progression and is a target for potential treatment. ROS: Reactive oxygen species; TLR: Toll-like receptor.
The Gut–liver axis
Dysbiosis of the gut microbiota is a factor contributing to the development and progression of fatty liver disease[75]. Dysbiosis affects hepatic macrophage activation and polarization through multiple potential mechanisms. For example, the gut microbiome plays a central regulatory role in host metabolism whereby alterations in the metabolic outputs of intestinal microbiota affect macrophage polarization[75]. A recent study using metabolomics analysis of cecal and fecal material from germ-free and conventionally raised HFD fed mice identified a gut microbiota-derived metabolite indole-3-acetate that directly modulates inflammatory responses in macrophages and dampens the release of pro-inflammatory cytokines[76]. Mechanistically, these effects were mediated through the aryl-hydrocarbon receptor, a transcription factor that regulates responses to multiple environmental signals including dietary factors[76]. In the same vein, ARNT mRNA expression is downregulated in liver tissues from MAFLD patients, while deletion of ARNT in mouse myeloid cells leads to steatohepatitis[77]. Similarly, the dysregulation of gut microbiota of infants of obese mothers leads to impaired macrophage function including cytokine production, phagocytosis and adaptation to various stimuli that ultimately increases susceptibility to MAFLD[78,79].
Another mechanism exists whereby alterations in the intestinal permeability in patients and murine models of MAFLD that is mediated by a western diet, leads to increases in the circulating levels of bacterial products including lipopolysaccharide (LPS). This leads to activation of TLRs, a sensor for these products[80] and results in macrophage activation and the initiation of injury[81-85]. Serum LPS and hepatic TLR4+ macrophages are higher in steatohepatitis patients compared to those with MAFLD or control subjects[86]. Similarly hepatic TLR 2, TLR4 and TLR9 expression was upregulated in human and murine steatohepatitis but not in simple steatosis; expression was localized to inflammatory cells, particularly macrophages[87]. Finally, Tlr4 and Tlr9 KO mice are protected from steatohepatitis induced by different diet models of MAFLD such as MCD feeding or a cholesterol rich diet[88,89]. Notably, the TLR effects also involves interaction and activation with other intersecting pathways with a role in inflammation. In a recent study, LPS treatment induced the accumulation of yes-associated protein (YAP) in Kupffer cells, a transcription coactivator that plays a role in the Hippo-YAP pathway and is implicated in the immune response[90]. YAP accumulation in Kupffer cells in steatohepatitis enhances the production of pro-inflammatory cytokines[90]. Furthermore, the elevated LPS in MAFLD[84,85] provides a critical hit for sustained inflammasome activation in macrophages[91].
TLR4 stimulation also induces alterations in lipid homeostasis[92] and macrophage activation as assessed by sCD163 as was found in a small trial that included 8 healthy male subjects[93]. In that study, sCD163 correlated with accelerated lipolysis following LPS exposure and enhanced mitochondrial reactive oxygen species (ROS) generation, a trigger for inflammasome activation[94].
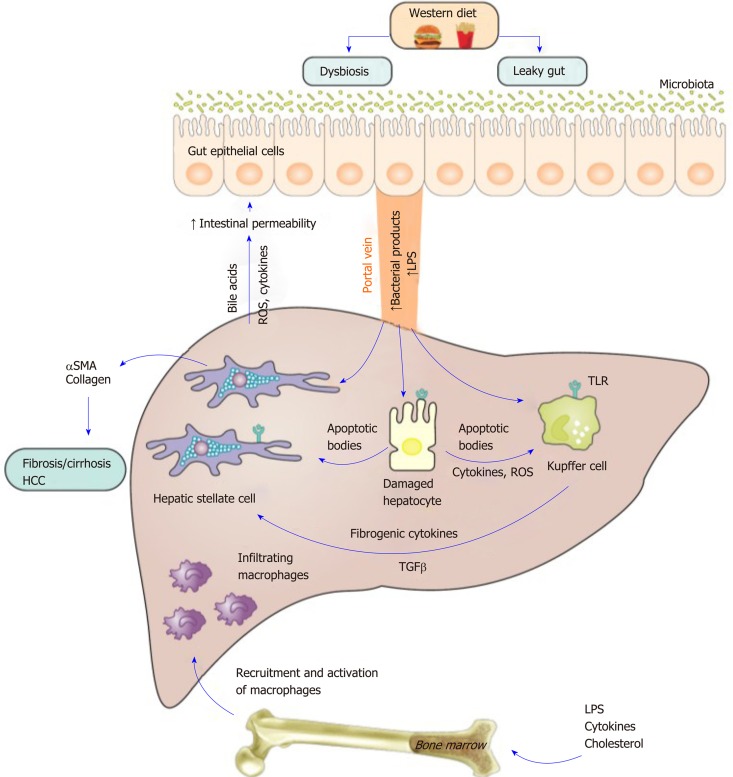
Collectively, gut dysbiosis is implicated in macrophage activation and hepatic inflammation through multiple mechanisms including by alterations in the metabolic outputs of microbiota, increased bacterial products generation and the activation of TLRs and inflammasomes. The gut liver axis in metabolic associated fatty liver disease is shown in Figure 2.
Figure 2.
The gut liver axis in metabolic associated fatty liver disease and the central role of macrophages. A western diet alters gut permeability and causes dysbioisis. This increases hepatic exposure to lipopolysaccharide and bacterial products and leads to toll-like receptor activation in hepatocytes, hepatic stellate cells and macrophages; the latter are the main responsive cells. Hepatocyte injury leads to the generation of apoptotic bodies, reactive oxygen species and cytokines. Engulfment of apoptotic bodies by macrophages increases expression of death ligands such as tumor-necrosis-factor-related apoptosis-inducing ligand, Fas, and tumour necrosis factor-α leading to a feed-forward loop that promotes further hepatocyte apoptosis. Engulfment of apoptotic bodies by hepatic stellate cells promotes their activation and the secretion of transforming growth factor β1 and extracellular matrix, promoting fibrosis and subsequently cirrhosis. Activated macrophages also lead to increased activation of stellate cells. Cholesterol, bacterial products and cytokines can stimulate myelopoiesis in bone marrow. Infiltrating monocytes lead to amplification of the inflammatory response. Increased bile acid and reactive oxygen species production by the injured liver also contributes to damage to gut epithelial cells and to detrimental alterations in microbiota setting up a vicious cycle of injury. LPS: Lipopolysaccharide; TLR: Toll-like receptor; TGF-β: Transforming growth factor β; HCC: Hepatocellular carcinoma.
Adipocytokines and myokines
Both adipocytokines and myokines play an important regulatory role in macrophage activation and polarisation. The anti-inflammatory effects of adipocyte-derived adiponectin can partially be attributed to promoting the polarization of macrophages toward an anti-inflammatory phenotype[95] as well as promoting macrophage tolerance to pro-inflammatory stimuli[96]. Adiponectin KO mice are more prone to steatohepatitis, while adiponectin administration attenuates steatohepatitis progression by reducing macrophage infiltration and skewing polarization towards an anti-inflammatory phenotype[97]. Levels of leptin are increased in MAFLD and this regulates macrophage phenotype including Kupffer cell activation, inflammatory phenotype and sensitivity to LPS-induced induced inflammatory cytokine secretion and acquisition of a fibrogenic phenotype[98-100]. A previous study showed that Kupffer cells likely mediate leptin-induced liver fibrosis effects by inducing the upregulation of fibrogenic gene expression such as that of TGFβ1 and connective tissue growth factor[100]
Levels of fibronectin type III domain-containing 5 (FNDC5) a myokine with favourable metabolic effects is decreased in MAFLD and correlates with the severity of hepatic steatosis[101]. FNDC5 attenuates adipose tissue insulin resistance and inflammation via AMP-activated protein kinase-induced macrophage polarization in a high fat diet mice model[102]. Another member of the fibronectin type III domain family of proteins is FNDC4 a secreted factor with a high homology to FNDC5. This was demonstrated to have anti-inflammatory effects on macrophages by reducing proinflammatory chemokine expression and dampening macrophage activity[103]. The role of FNDC4 in MAFLD is unknown. In sum, adipocytokines and myokines represent another crucial regulator of macrophage biology in the context of metabolic disorders.
Damaged hepatocytes and ROS and macrophage activation
Damaged hepatocytes are a trigger for macrophage activation. Data from mice fed a HFD suggest that steatotic hepatocytes promote the release of pro-inflammatory cytokines by macrophages, indicating that hepatocyte damage elicits macrophage activation[104]. In turn, apoptotic body engulfment by Kupffer cells is a potent trigger for inflammation and fibrosis and for activation of macrophages via pattern recognition receptors such as TLRs[105]. Trying to elucidate the link between damaged hepatocytes and macrophage activation and inflammation, a recent study has suggested that proapoptotic lipotoxic signaling by death receptor 5 (also known as tumor necrosis factor receptor superfamily member 10b) induces the release of extracellular vesicles from hepatocytes. These in turn activate macrophages and promotes an inflammatory phenotype[106]. Similarly, another study showed that hepatocytes release ceramide-enriched pro-inflammatory extracellular vesicles under lipotoxic conditions that promote macrophage recruitment to the liver[107]. Recent reports have also demonstrated increased expression of the G protein‐coupled transmembrane receptor Smoothened and glioma‐associated oncogene (Gli) transcription factors (components of the hedgehog signaling pathway) in primary hepatocytes from damaged livers[108]. Pharmacological and genetic liver specific inhibition of Smoothened prevented hepatic inflammation in MAFLD models through decreased macrophage recruitment and activation[109].
ROS are another potential mechanism for macrophage activation. ROS impacts Kupffer cells both directly and indirectly. ROS prompts tumour necrosis factor-α generation in Kupffer cells and they can increase the susceptibility of these cells to endotoxin[110,111].
Genetic variants that influence macrophage function and thereby liver inflammation
There is strong evidence that MAFLD has high heritability with a shared genetic basis between MAFLD and other liver diseases as well as with other metabolic disorders[11]. The genetic basis of macrophage activation is still not known, however several genetic variants implicated in MAFLD have been demonstrated to regulate macrophage phenotype. For example, the interferon lambda 3/4 (IFNL3/IFNL4) genotype is strongly associated with hepatic inflammation and fibrosis in MAFLD as well as hepatitis C and B[112,113] and scores incorporating these variants with other clinical variables can predict liver fibrosis in patients[114]. A correlation between IFNL3/IFNL4 genotype and hepatic macrophage infiltration as well as macrophage activation as assessed by the activation marker sCD163 has been reported[115-119]. Recently, increases in hepatic IFNL3 expression in liver tissues from patients with MAFLD compared to controls was also reported[120]. IFNL3 skews macrophages toward a proinflammatory phenotype orchestrating their interaction with other immune cells including T-cells to mediate hepatic inflammation[120].
Another example is of variants in the Membrane Bound O-Acyltransferase Domain Containing 7 (MBOAT7) gene that encodes an acyltransferase enzyme and catalyses the transfer of an acyl-CoA to lysophosphatidylinositol. This regulates the availability of arachidonic acid for pro-inflammatory eicosanoids production[121-124]. A variant (rs641738) in the MBOAT7 gene is associated with liver injury in MAFLD as well as in viral hepatitis[121-124]. MBOAT7 is robustly expressed by inflammatory cells including macrophages and this variant associates with macrophage activation as assessed by sCD163; the detailed mechanisms for this association are unknown[121-124]. A recent study showed that Mboat7 loss of function promotes the progression of MAFLD and hepatic inflammatory and fibrotic phenotypes in response to a high fat diet[125].
Another example is a variant (rs4374383) in the MER Proto-Oncogene, Tyrosine Kinase (MERTK) gene, a member of the Tyro-Axl-MerTK family of receptor tyrosine kinases. By a genome-wide association study this polymorphism has been associated with fibrosis in patients with hepatitis C[126]. Similar findings were later observed in patients with MAFLD[127]. A recent study has shown that this variant regulates MERTK expression in circulating and hepatic macrophages and that total and myeloid specific MERTK deficiency decreased liver fibrosis in mice[128].
Epigenetic regulation
Epigenetic modifications include DNA methylation, histone modification and non-coding RNA[8]. Growing evidence suggests an important role for epigenetic mechanisms in the regulation of macrophage phenotype, polarization, inflammatory and profibrotic gene production. A recent work used reduced representation bisulfite sequencing in a mouse carbon tetrachloride (CCL4) fibrosis model and identified that hypermethylation of proline–serine–threonine–phosphatase-interacting protein2 links it to hepatic fibrosis[129]. In another study, the histone methyltransferase Suv39h2 was found to contribute to steatohepatitis in mouse models through suppression of PPARγ. This leads to macrophage polarisation towards a proinflammatory M1 over an anti-inflammatory M2 phenotype[130]. miRNAs are also implicated in macrophage activation and polarisation. For example, lipotoxic hepatocyte-derived exosomal miR-192-5p plays a pivotal role in the activation of proinflammatory macrophages and in disease progression of MAFLD via Rictor/Akt/FoxO1 signaling[131].
ADIPOSE TISSUE AND FATTY LIVER CROSS TALK: ROLE OF MACROPHAGES
A pivotal role for macrophages in the crosstalk between adipose tissue and liver in fatty liver disease has been suggested. Thus, adipose tissue insulin resistance and free fatty acid flux to liver activates hepatic macrophages in MAFLD independent of obesity and diabetes[132]. It has also been suggested that adipose tissue inflammation might precede hepatic inflammation since in high fat and cholesterol diet fed mice, the upregulation of genes associated with macrophage recruitment and inflammation occurred earlier in adipose tissue compared to the liver[133]. During obesity, both adipose tissue macrophage (ATM) numbers and activation are enhanced[134]. In humans, in a cohort of obese patients undergoing bariatric surgery, ATMs from patients with MAFLD and steatohepatitis produced higher levels of inflammatory cytokines compared to controls[135]. In animal models, ablation of these ATMs led to normalization of whole-body insulin sensitivity[136] while ATMs from obese visceral adipose tissue amplified hepatic inflammation through augmentation of hepatic macrophage infiltration[137].
TRANSLATIONAL IMPLICATIONS
Although MAFLD is a major clinical problem, to date there are no approved treatments. Recently, obeticholic acid was reported to improve MAFLD related fibrosis and will likely be the first drug to be approved for its treatment[138,139]. With multiple other investigational drugs in Phase 3 clinical trials, it can be expected that other drugs will follow and be approved. However, the efficacy of these drugs appears modest suggesting that more novel approaches to drug development are required[140]. Given the central role of macrophages in the transition to steatohepatitis and to fibrosis, macrophages might be a suitable target for therapy and as a biomarker of disease severity, as discussed below. The central role of macrophages is shown in Figure 2.
Macrophage based diagnostic biomarkers
Soluble CD163, a macrophage activation marker was reported to correlate with liver injury and demonstrated good predictive ability for advanced fibrosis (≥ F3) with an area under the receiver operating curve (AUROC) of 0.77 and 0.80 in two independent cohorts. This was further increased in combination with the MAFLD fibrosis score (AUROC of 0.83 for both cohorts)[56]. However, macrophage activation markers such as sCD163 and soluble mannose receptor demonstrated poor associations with liver histology in two cross-sectional paediatric MAFLD cohorts (n = 155 and 36) suggesting a differential role for macrophage activation in adult and paediatric disease or perhaps indicates that disease severity is different between adult and paediatric patients at least in the cohorts examined[141].
A recent study also suggested that the circulating activity of adenosine deaminase (a macrophage-derived deaminase that converts adenosine or deoxyadenosine to inosine and derivatives) can predict MAFLD cirrhosis, advanced fibrosis (≥ F3), and significant fibrosis (≥ F2) with AUROCs of 0.94, 0.82, and 0.84, respectively[142]. However, the molecular mechanisms for this association are yet to be determined.
Macrophage based therapeutic targets
Some macrophage-targeted therapies for liver diseases have been investigated in the clinic[143]. One of these is limiting monocyte and macrophage recruitment. Many of the pathways characterized in mice for monocyte recruitment are also strongly regulated in patients with liver diseases suggesting well conserved mechanisms across species. For instance, cenicriviroc, an oral dual inhibitor of the chemokine receptor C-C ligand/ receptor 2 and 5 pathway (inhibits hepatic monocyte infiltration)[144] was evaluated in a phase 2b clinical trial in patients with MAFLD and fibrosis. Early results suggest that patients receiving cenicriviroc oral therapy were twice as likely to have improvement of fibrosis (without worsening of steatohepatitis) after 1 year of follow up[141]. However, the differences did not appear to be significant by the second year of follow up.
Another approach for treatment is to promote anti-inflammatory macrophage polarization. The PPAR family that includes α, β/δ, and γ is a member of the nuclear receptor superfamily and polarizes macrophages towards an anti-inflammatory state. This can explain at least partially their role in MAFLD[145,146]. For example, among modulators of the PPARs, PPARγ agonists such as pioglitazone have insulin sensitizing effects but also induces polarization of macrophages towards an anti-inflammatory phenotype thereby improving hepatic steatosis[145,146]. Similarly, the PPARα/PPARγ agonist saroglitazar reverses fibrosis in MAFLD[147] and elafibranor, an agonist of PPARα and δ attenuates hepatic inflammation without worsening fibrosis[148]. PPARδ induces polarization of macrophages towards an anti-inflammatory M2 phenotype[145] while elafibranor decreases hepatic macrophage infiltration in animal models of MAFLD[149].
An alternative approach is targeting activating signals for macrophages. For example, NLRP3 inflammasome pharmacological blockade using MCC950 attenuates hepatic inflammation and fibrosis in mouse models and reduces the numbers of macrophages in liver[150]. Currently, a clinical trial (NCT03676231) using an inflammasome inhibitor SGM-1019 is recruiting. TLR4 is a key regulator of MAFLD[151] however the results of a phase-2 study failed to discern a beneficial effect of JKB-121, a small molecule TLR-4 receptor antagonist in human MAFLD[152].
The growing body of knowledge on immunometabolism and epigenetic regulation indicates that metabolic reprogramming and epigenetic regulation may be a target for regulating macrophage responses such as polarization and activation. Two recent reports demonstrate that digoxin improves steatohepatitis in mice models through regulation of the pyruvate kinase muscle isozyme M2-hypoxia-inducible factors axis in hepatocytes and macrophages[153,154]. Pyruvate kinase muscle isozyme M2 is a rate limiting glycolytic enzyme that catalyzes the final step in glycolysis[155] and promotes NLRP3 and AIM2 inflammasome activation in macrophages[156].
In a recent study of mice fed a MCD diet, miR-141 and miR-200c deficiency attenuated hepatic steatosis and inflammation via multiple mechanisms including reprogramming of macrophages toward anti-inflammatory phenotype[157]. Although several epigenetic drugs are currently approved for treating different type of cancers, data are still limited on effects of these drugs on macrophage activation and polarisation. Histone deacetylase inhibition was demonstrated to improve post- myocardial infraction healing through modulating macrophage polarization[158]. Another study showed that targeting histone deacetylases in myeloid cells using CHR-4487 (ESM-HDAC528) a small molecule that can inhibits their inflammatory phenotype has a limited impact in atherosclerosis[159]; there is no data on MAFLD.
CHALLENGES AND OPEN QUESTIONS
Although targeting macrophages is attractive, a major obstacle for the development of therapies is that of macrophage heterogeneity and differences between mice and humans[143,160]. Macrophages exhibit remarkable plasticity and phenotypic heterogeneity and different subsets have distinct and sometimes opposite properties (pro- vs anti-inflammatory, pro- vs antifibrotic). Further, environmental factors including cytokines control the polarization of macrophages but are not well defined. It is very likely that the functionality of hepatic macrophage subsets is also influenced by the nature of the underlying liver disease and this will hinder their use in an etiology independent manner. Another challenge for translating findings from murine models to humans is the fact that murine and human monocyte/macrophage subpopulations do not share the same surface-marker profiles. However new technologies including single cell RNA-Seq can help to de-convolute macrophage heterogeneity and may enable the development of specific therapies. Further, clarifying the basis of modulation of epigenetic and metabolic programs in macrophages can guide therapeutics. Delivery modalities represents another challenge, with multiple delivery platforms having been explored including nanoparticles, liposomes and oligopeptide complexes. These methods can be used to target macrophages for delivery of agents such as gene-silencing siRNA and miRNA inhibitors. While CRISPR-Cas9 technology might revolutionize medicine, their application for modulating macrophage biology is not well explored.
CONCLUSION
Macrophages play a central role at all stages of MAFLD and contributes to the pathology in extrahepatic sites that are simultaneously affected. Thus, macrophage directed therapeutics have unique potential in MAFLD, but their heterogeneity represents a challenge. Metabolic and epigenetic programming and gene-based therapies are attractive approaches to manipulate macrophage function for clinical benefit, though the optimal delivery methods are still not defined. In the future, combined approaches consisting of multiple drugs that target different key pathways are likely to provide a better strategy to treat MAFLD. It remains to be elucidated which combinations should to be used, but macrophage targeted therapies are one viable option.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Australia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: The authors declare no competing interests for this manuscript.
Peer-review started: December 23, 2019
First decision: February 18, 2020
Article in press: April 17, 2020
P-Reviewer: Andreuzzi E, Hua J, Trovato GM S-Editor: Tang JZ L-Editor: A E-Editor: Zhang YL
Contributor Information
Jawaher Alharthi, Storr Liver Centre, Westmead Institute for Medical Research, Westmead Hospital and University of Sydney, Sydney 2145, NSW, Australia.
Olivier Latchoumanin, Storr Liver Centre, Westmead Institute for Medical Research, Westmead Hospital and University of Sydney, Sydney 2145, NSW, Australia.
Jacob George, Storr Liver Centre, Westmead Institute for Medical Research, Westmead Hospital and University of Sydney, Sydney 2145, NSW, Australia.
Mohammed Eslam, Storr Liver Centre, Westmead Institute for Medical Research, Westmead Hospital and University of Sydney, Sydney 2145, NSW, Australia. mohammed.eslam@sydney.edu.au.
References
- 1.Eslam M, Newsome PN, Anstee QM, Targher G, Gomez MR, Zelber-Sagi S, Wong VW, Dufour JF, Schattenberg J, Arrese M, Valenti L, Shiha G, Tiribelli C, Yki-Järvinen H, Fan JG, Gronbaek H, Yilmaz Y, Cortez-Pinto H, Oliveira CP, Bedossa P, Adams LA, Zheng MH, Fouad Y, Chan WK, Mendez-Sanchez N, Ahn SH, Castera L, Bugianesi E, Ratziu V, George J. New definition for metabolic associated fatty liver disease: an international expert consensus. J Hepatol. 2020 doi: 10.1016/j.jhep.2020.03.039. [DOI] [PubMed] [Google Scholar]
- 2.Eslam M, Sanyal AJ, George J International Consensus Panel. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology. 2020 doi: 10.1053/j.gastro.2019.11.312. [DOI] [PubMed] [Google Scholar]
- 3.Sarin SK, Kumar M, Eslam M, George J, Al Mahtab M, Akbar SMF, Jia J, Tian Q, Aggarwal R, Muljono DH, Omata M, Ooka Y, Han KH, Lee HW, Jafri W, Butt AS, Chong CH, Lim SG, Pwu RF, Chen DS. Liver diseases in the Asia-Pacific region: a Lancet Gastroenterology & Hepatology Commission. Lancet Gastroenterol Hepatol. 2020;5:167–228. doi: 10.1016/S2468-1253(19)30342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Estes C, Anstee QM, Arias-Loste MT, Bantel H, Bellentani S, Caballeria J, Colombo M, Craxi A, Crespo J, Day CP, Eguchi Y, Geier A, Kondili LA, Kroy DC, Lazarus JV, Loomba R, Manns MP, Marchesini G, Nakajima A, Negro F, Petta S, Ratziu V, Romero-Gomez M, Sanyal A, Schattenberg JM, Tacke F, Tanaka J, Trautwein C, Wei L, Zeuzem S, Razavi H. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016-2030. J Hepatol. 2018;69:896–904. doi: 10.1016/j.jhep.2018.05.036. [DOI] [PubMed] [Google Scholar]
- 5.Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, Ahmed A. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148:547–555. doi: 10.1053/j.gastro.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 6.Dyson J, Jaques B, Chattopadyhay D, Lochan R, Graham J, Das D, Aslam T, Patanwala I, Gaggar S, Cole M, Sumpter K, Stewart S, Rose J, Hudson M, Manas D, Reeves HL. Hepatocellular cancer: the impact of obesity, type 2 diabetes and a multidisciplinary team. J Hepatol. 2014;60:110–117. doi: 10.1016/j.jhep.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 7.Younossi ZM, Blissett D, Blissett R, Henry L, Stepanova M, Younossi Y, Racila A, Hunt S, Beckerman R. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. 2016;64:1577–1586. doi: 10.1002/hep.28785. [DOI] [PubMed] [Google Scholar]
- 8.Eslam M, Valenti L, Romeo S. Genetics and epigenetics of NAFLD and NASH: Clinical impact. J Hepatol. 2018;68:268–279. doi: 10.1016/j.jhep.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Eslam M, George J. Genetic and epigenetic mechanisms of NASH. Hepatol Int. 2016;10:394–406. doi: 10.1007/s12072-015-9689-y. [DOI] [PubMed] [Google Scholar]
- 10.Petta S, Eslam M, Valenti L, Bugianesi E, Barbara M, Cammà C, Porzio M, Rosso C, Fargion S, George J, Craxì A. Metabolic syndrome and severity of fibrosis in nonalcoholic fatty liver disease: An age-dependent risk profiling study. Liver Int. 2017;37:1389–1396. doi: 10.1111/liv.13397. [DOI] [PubMed] [Google Scholar]
- 11.Eslam M, George J. Genetic contributions to NAFLD: leveraging shared genetics to uncover systems biology. Nat Rev Gastroenterol Hepatol. 2020;17:40–52. doi: 10.1038/s41575-019-0212-0. [DOI] [PubMed] [Google Scholar]
- 12.Vilar-Gomez E, Calzadilla-Bertot L, Wai-Sun Wong V, Castellanos M, Aller-de la Fuente R, Metwally M, Eslam M, Gonzalez-Fabian L, Alvarez-Quiñones Sanz M, Conde-Martin AF, De Boer B, McLeod D, Hung Chan AW, Chalasani N, George J, Adams LA, Romero-Gomez M. Fibrosis Severity as a Determinant of Cause-Specific Mortality in Patients With Advanced Nonalcoholic Fatty Liver Disease: A Multi-National Cohort Study. Gastroenterology. 2018;155:443–457.e17. doi: 10.1053/j.gastro.2018.04.034. [DOI] [PubMed] [Google Scholar]
- 13.Maher JJ, Leon P, Ryan JC. Beyond insulin resistance: Innate immunity in nonalcoholic steatohepatitis. Hepatology. 2008;48:670–678. doi: 10.1002/hep.22399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kazankov K, Jørgensen SMD, Thomsen KL, Møller HJ, Vilstrup H, George J, Schuppan D, Grønbæk H. The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nat Rev Gastroenterol Hepatol. 2019;16:145–159. doi: 10.1038/s41575-018-0082-x. [DOI] [PubMed] [Google Scholar]
- 15.Lopez BG, Tsai MS, Baratta JL, Longmuir KJ, Robertson RT. Characterization of Kupffer cells in livers of developing mice. Comp Hepatol. 2011;10:2. doi: 10.1186/1476-5926-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krenkel O, Tacke F. Liver macrophages in tissue homeostasis and disease. Nat Rev Immunol. 2017;17:306–321. doi: 10.1038/nri.2017.11. [DOI] [PubMed] [Google Scholar]
- 17.MacParland SA, Liu JC, Ma XZ, Innes BT, Bartczak AM, Gage BK, Manuel J, Khuu N, Echeverri J, Linares I, Gupta R, Cheng ML, Liu LY, Camat D, Chung SW, Seliga RK, Shao Z, Lee E, Ogawa S, Ogawa M, Wilson MD, Fish JE, Selzner M, Ghanekar A, Grant D, Greig P, Sapisochin G, Selzner N, Winegarden N, Adeyi O, Keller G, Bader GD, McGilvray ID. Single cell RNA sequencing of human liver reveals distinct intrahepatic macrophage populations. Nat Commun. 2018;9:4383. doi: 10.1038/s41467-018-06318-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krenkel O, Hundertmark J, Abdallah AT, Kohlhepp M, Puengel T, Roth T, Branco DPP, Mossanen JC, Luedde T, Trautwein C, Costa IG, Tacke F. Myeloid cells in liver and bone marrow acquire a functionally distinct inflammatory phenotype during obesity-related steatohepatitis. Gut. 2020;69:551–563. doi: 10.1136/gutjnl-2019-318382. [DOI] [PubMed] [Google Scholar]
- 19.Heymann F, Tacke F. Immunology in the liver--from homeostasis to disease. Nat Rev Gastroenterol Hepatol. 2016;13:88–110. doi: 10.1038/nrgastro.2015.200. [DOI] [PubMed] [Google Scholar]
- 20.Sierro F, Evrard M, Rizzetto S, Melino M, Mitchell AJ, Florido M, Beattie L, Walters SB, Tay SS, Lu B, Holz LE, Roediger B, Wong YC, Warren A, Ritchie W, McGuffog C, Weninger W, Le Couteur DG, Ginhoux F, Britton WJ, Heath WR, Saunders BM, McCaughan GW, Luciani F, MacDonald KPA, Ng LG, Bowen DG, Bertolino P. A Liver Capsular Network of Monocyte-Derived Macrophages Restricts Hepatic Dissemination of Intraperitoneal Bacteria by Neutrophil Recruitment. Immunity. 2017;47:374–388.e6. doi: 10.1016/j.immuni.2017.07.018. [DOI] [PubMed] [Google Scholar]
- 21.Heymann F, Peusquens J, Ludwig-Portugall I, Kohlhepp M, Ergen C, Niemietz P, Martin C, van Rooijen N, Ochando JC, Randolph GJ, Luedde T, Ginhoux F, Kurts C, Trautwein C, Tacke F. Liver inflammation abrogates immunological tolerance induced by Kupffer cells. Hepatology. 2015;62:279–291. doi: 10.1002/hep.27793. [DOI] [PubMed] [Google Scholar]
- 22.Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol. 2013;14:986–995. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yao Y, Xu XH, Jin L. Macrophage Polarization in Physiological and Pathological Pregnancy. Front Immunol. 2019;10:792. doi: 10.3389/fimmu.2019.00792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atri C, Guerfali FZ, Laouini D. Role of Human Macrophage Polarization in Inflammation during Infectious Diseases. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19061801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lichtnekert J, Kawakami T, Parks WC, Duffield JS. Changes in macrophage phenotype as the immune response evolves. Curr Opin Pharmacol. 2013;13:555–564. doi: 10.1016/j.coph.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tacke F, Zimmermann HW. Macrophage heterogeneity in liver injury and fibrosis. J Hepatol. 2014;60:1090–1096. doi: 10.1016/j.jhep.2013.12.025. [DOI] [PubMed] [Google Scholar]
- 27.Gadd VL, Skoien R, Powell EE, Fagan KJ, Winterford C, Horsfall L, Irvine K, Clouston AD. The portal inflammatory infiltrate and ductular reaction in human nonalcoholic fatty liver disease. Hepatology. 2014;59:1393–1405. doi: 10.1002/hep.26937. [DOI] [PubMed] [Google Scholar]
- 28.Park JW, Jeong G, Kim SJ, Kim MK, Park SM. Predictors reflecting the pathological severity of non-alcoholic fatty liver disease: comprehensive study of clinical and immunohistochemical findings in younger Asian patients. J Gastroenterol Hepatol. 2007;22:491–497. doi: 10.1111/j.1440-1746.2006.04758.x. [DOI] [PubMed] [Google Scholar]
- 29.Lotowska JM, Sobaniec-Lotowska ME, Lebensztejn DM. The role of Kupffer cells in the morphogenesis of nonalcoholic steatohepatitis - ultrastructural findings. The first report in pediatric patients. Scand J Gastroenterol. 2013;48:352–357. doi: 10.3109/00365521.2012.746390. [DOI] [PubMed] [Google Scholar]
- 30.Kiki I, Altunkaynak BZ, Altunkaynak ME, Vuraler O, Unal D, Kaplan S. Effect of high fat diet on the volume of liver and quantitative feature of Kupffer cells in the female rat: a stereological and ultrastructural study. Obes Surg. 2007;17:1381–1388. doi: 10.1007/s11695-007-9219-7. [DOI] [PubMed] [Google Scholar]
- 31.Wan J, Benkdane M, Teixeira-Clerc F, Bonnafous S, Louvet A, Lafdil F, Pecker F, Tran A, Gual P, Mallat A, Lotersztajn S, Pavoine C. M2 Kupffer cells promote M1 Kupffer cell apoptosis: a protective mechanism against alcoholic and nonalcoholic fatty liver disease. Hepatology. 2014;59:130–142. doi: 10.1002/hep.26607. [DOI] [PubMed] [Google Scholar]
- 32.Jindal A, Bruzzì S, Sutti S, Locatelli I, Bozzola C, Paternostro C, Parola M, Albano E. Fat-laden macrophages modulate lobular inflammation in nonalcoholic steatohepatitis (NASH) Exp Mol Pathol. 2015;99:155–162. doi: 10.1016/j.yexmp.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 33.Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164:6166–6173. doi: 10.4049/jimmunol.1701141. [DOI] [PubMed] [Google Scholar]
- 34.Maina V, Sutti S, Locatelli I, Vidali M, Mombello C, Bozzola C, Albano E. Bias in macrophage activation pattern influences non-alcoholic steatohepatitis (NASH) in mice. Clin Sci (Lond) 2012;122:545–553. doi: 10.1042/CS20110366. [DOI] [PubMed] [Google Scholar]
- 35.Neyrinck AM, Cani PD, Dewulf EM, De Backer F, Bindels LB, Delzenne NM. Critical role of Kupffer cells in the management of diet-induced diabetes and obesity. Biochem Biophys Res Commun. 2009;385:351–356. doi: 10.1016/j.bbrc.2009.05.070. [DOI] [PubMed] [Google Scholar]
- 36.Huang W, Metlakunta A, Dedousis N, Zhang P, Sipula I, Dube JJ, Scott DK, O'Doherty RM. Depletion of liver Kupffer cells prevents the development of diet-induced hepatic steatosis and insulin resistance. Diabetes. 2010;59:347–357. doi: 10.2337/db09-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tencerova M, Aouadi M, Vangala P, Nicoloro SM, Yawe JC, Cohen JL, Shen Y, Garcia-Menendez L, Pedersen DJ, Gallagher-Dorval K, Perugini RA, Gupta OT, Czech MP. Activated Kupffer cells inhibit insulin sensitivity in obese mice. FASEB J. 2015;29:2959–2969. doi: 10.1096/fj.15-270496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stienstra R, Saudale F, Duval C, Keshtkar S, Groener JE, van Rooijen N, Staels B, Kersten S, Müller M. Kupffer cells promote hepatic steatosis via interleukin-1beta-dependent suppression of peroxisome proliferator-activated receptor alpha activity. Hepatology. 2010;51:511–522. doi: 10.1002/hep.23337. [DOI] [PubMed] [Google Scholar]
- 39.Stienstra R, Mandard S, Patsouris D, Maass C, Kersten S, Müller M. Peroxisome proliferator-activated receptor alpha protects against obesity-induced hepatic inflammation. Endocrinology. 2007;148:2753–2763. doi: 10.1210/en.2007-0014. [DOI] [PubMed] [Google Scholar]
- 40.Lanthier N, Molendi-Coste O, Horsmans Y, van Rooijen N, Cani PD, Leclercq IA. Kupffer cell activation is a causal factor for hepatic insulin resistance. Am J Physiol Gastrointest Liver Physiol. 2010;298:G107–G116. doi: 10.1152/ajpgi.00391.2009. [DOI] [PubMed] [Google Scholar]
- 41.Zhang X, Fan L, Wu J, Xu H, Leung WY, Fu K, Wu J, Liu K, Man K, Yang X, Han J, Ren J, Yu J. Macrophage p38α promotes nutritional steatohepatitis through M1 polarization. J Hepatol. 2019;71:163–174. doi: 10.1016/j.jhep.2019.03.014. [DOI] [PubMed] [Google Scholar]
- 42.Wynn TA, Barron L. Macrophages: master regulators of inflammation and fibrosis. Semin Liver Dis. 2010;30:245–257. doi: 10.1055/s-0030-1255354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rensen SS, Slaats Y, Nijhuis J, Jans A, Bieghs V, Driessen A, Malle E, Greve JW, Buurman WA. Increased hepatic myeloperoxidase activity in obese subjects with nonalcoholic steatohepatitis. Am J Pathol. 2009;175:1473–1482. doi: 10.2353/ajpath.2009.080999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liaskou E, Zimmermann HW, Li KK, Oo YH, Suresh S, Stamataki Z, Qureshi O, Lalor PF, Shaw J, Syn WK, Curbishley SM, Adams DH. Monocyte subsets in human liver disease show distinct phenotypic and functional characteristics. Hepatology. 2013;57:385–398. doi: 10.1002/hep.26016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zimmermann HW, Seidler S, Nattermann J, Gassler N, Hellerbrand C, Zernecke A, Tischendorf JJ, Luedde T, Weiskirchen R, Trautwein C, Tacke F. Functional contribution of elevated circulating and hepatic non-classical CD14CD16 monocytes to inflammation and human liver fibrosis. PLoS One. 2010;5:e11049. doi: 10.1371/journal.pone.0011049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Preisser L, Miot C, Le Guillou-Guillemette H, Beaumont E, Foucher ED, Garo E, Blanchard S, Frémaux I, Croué A, Fouchard I, Lunel-Fabiani F, Boursier J, Roingeard P, Calès P, Delneste Y, Jeannin P. IL-34 and macrophage colony-stimulating factor are overexpressed in hepatitis C virus fibrosis and induce profibrotic macrophages that promote collagen synthesis by hepatic stellate cells. Hepatology. 2014;60:1879–1890. doi: 10.1002/hep.27328. [DOI] [PubMed] [Google Scholar]
- 47.Ramachandran P, Pellicoro A, Vernon MA, Boulter L, Aucott RL, Ali A, Hartland SN, Snowdon VK, Cappon A, Gordon-Walker TT, Williams MJ, Dunbar DR, Manning JR, van Rooijen N, Fallowfield JA, Forbes SJ, Iredale JP. Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc Natl Acad Sci USA. 2012;109:E3186–E3195. doi: 10.1073/pnas.1119964109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lodder J, Denaës T, Chobert MN, Wan J, El-Benna J, Pawlotsky JM, Lotersztajn S, Teixeira-Clerc F. Macrophage autophagy protects against liver fibrosis in mice. Autophagy. 2015;11:1280–1292. doi: 10.1080/15548627.2015.1058473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schuppan D, Surabattula R, Wang XY. Determinants of fibrosis progression and regression in NASH. J Hepatol. 2018;68:238–250. doi: 10.1016/j.jhep.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 50.Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology. 2010;51:1820–1832. doi: 10.1002/hep.23594. [DOI] [PubMed] [Google Scholar]
- 51.Mantovani A, Schioppa T, Porta C, Allavena P, Sica A. Role of tumor-associated macrophages in tumor progression and invasion. Cancer Metastasis Rev. 2006;25:315–322. doi: 10.1007/s10555-006-9001-7. [DOI] [PubMed] [Google Scholar]
- 52.Miura K, Ishioka M, Minami S, Horie Y, Ohshima S, Goto T, Ohnishi H. Toll-like Receptor 4 on Macrophage Promotes the Development of Steatohepatitis-related Hepatocellular Carcinoma in Mice. J Biol Chem. 2016;291:11504–11517. doi: 10.1074/jbc.M115.709048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grohmann M, Wiede F, Dodd GT, Gurzov EN, Ooi GJ, Butt T, Rasmiena AA, Kaur S, Gulati T, Goh PK, Treloar AE, Archer S, Brown WA, Muller M, Watt MJ, Ohara O, McLean CA, Tiganis T. Obesity Drives STAT-1-Dependent NASH and STAT-3-Dependent HCC. Cell. 2018;175:1289–1306.e20. doi: 10.1016/j.cell.2018.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Armstrong MJ, Adams LA, Canbay A, Syn WK. Extrahepatic complications of nonalcoholic fatty liver disease. Hepatology. 2014;59:1174–1197. doi: 10.1002/hep.26717. [DOI] [PubMed] [Google Scholar]
- 55.Lefere S, Tacke F. Macrophages in obesity and non-alcoholic fatty liver disease: Crosstalk with metabolism. JHEP Rep. 2019;1:30–43. doi: 10.1016/j.jhepr.2019.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kazankov K, Barrera F, Møller HJ, Rosso C, Bugianesi E, David E, Younes R, Esmaili S, Eslam M, McLeod D, Bibby BM, Vilstrup H, George J, Grønbaek H. The macrophage activation marker sCD163 is associated with morphological disease stages in patients with non-alcoholic fatty liver disease. Liver Int. 2016;36:1549–1557. doi: 10.1111/liv.13150. [DOI] [PubMed] [Google Scholar]
- 57.Durda JP. Abstract P363: Circulating Soluble CD163 and Risk of Cardiovascular Disease and All-Cause Mortality in Older Persons: The Cardiovascular Heart Study (CHS) Circulation. 2015;131:AP363. [Google Scholar]
- 58.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alwarawrah Y, Kiernan K, MacIver NJ. Changes in Nutritional Status Impact Immune Cell Metabolism and Function. Front Immunol. 2018;9:1055. doi: 10.3389/fimmu.2018.01055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McGettigan B, McMahan R, Orlicky D, Burchill M, Danhorn T, Francis P, Cheng LL, Golden-Mason L, Jakubzick CV, Rosen HR. Dietary Lipids Differentially Shape Nonalcoholic Steatohepatitis Progression and the Transcriptome of Kupffer Cells and Infiltrating Macrophages. Hepatology. 2019;70:67–83. doi: 10.1002/hep.30401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.de Oliveira S, Houseright RA, Graves AL, Golenberg N, Korte BG, Miskolci V, Huttenlocher A. Metformin modulates innate immune-mediated inflammation and early progression of NAFLD-associated hepatocellular carcinoma in zebrafish. J Hepatol. 2019;70:710–721. doi: 10.1016/j.jhep.2018.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kawanishi N, Mizokami T, Yada K, Suzuki K. Exercise training suppresses scavenger receptor CD36 expression in kupffer cells of nonalcoholic steatohepatitis model mice. Physiol Rep. 2018;6:e13902. doi: 10.14814/phy2.13902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fjeldborg K, Christiansen T, Bennetzen M, J Møller H, Pedersen SB, Richelsen B. The macrophage-specific serum marker, soluble CD163, is increased in obesity and reduced after dietary-induced weight loss. Obesity (Silver Spring) 2013;21:2437–2443. doi: 10.1002/oby.20376. [DOI] [PubMed] [Google Scholar]
- 64.Kazankov K, Tordjman J, Møller HJ, Vilstrup H, Poitou C, Bedossa P, Bouillot JL, Clement K, Grønbaek H. Macrophage activation marker soluble CD163 and non-alcoholic fatty liver disease in morbidly obese patients undergoing bariatric surgery. J Gastroenterol Hepatol. 2015;30:1293–1300. doi: 10.1111/jgh.12943. [DOI] [PubMed] [Google Scholar]
- 65.Galassi TV, Jena PV, Shah J, Ao G, Molitor E, Bram Y, Frankel A, Park J, Jessurun J, Ory DS, Haimovitz-Friedman A, Roxbury D, Mittal J, Zheng M, Schwartz RE, Heller DA. An optical nanoreporter of endolysosomal lipid accumulation reveals enduring effects of diet on hepatic macrophages in vivo. Sci Transl Med. 2018;10 doi: 10.1126/scitranslmed.aar2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bieghs V, Walenbergh SM, Hendrikx T, van Gorp PJ, Verheyen F, Olde Damink SW, Masclee AA, Koek GH, Hofker MH, Binder CJ, Shiri-Sverdlov R. Trapping of oxidized LDL in lysosomes of Kupffer cells is a trigger for hepatic inflammation. Liver Int. 2013;33:1056–1061. doi: 10.1111/liv.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pan J, Ou Z, Cai C, Li P, Gong J, Ruan XZ, He K. Fatty acid activates NLRP3 inflammasomes in mouse Kupffer cells through mitochondrial DNA release. Cell Immunol. 2018;332:111–120. doi: 10.1016/j.cellimm.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 68.Dong B, Zhou Y, Wang W, Scott J, Kim K, Sun Z, Guo Q, Lu Y, Gonzales NM, Wu H, Hartig SM, York RB, Yang F, Moore DD. Vitamin D Receptor Activation in Liver Macrophages Ameliorates Hepatic Inflammation, Steatosis, and Insulin Resistance in Mice. Hepatology. 2019 doi: 10.1002/hep.30937. [DOI] [PubMed] [Google Scholar]
- 69.Weissman IL. Stem cells: Units of development, units of regeneration, and units in evolution. Cell. 2000;100:157–168. doi: 10.1016/s0092-8674(00)81692-x. [DOI] [PubMed] [Google Scholar]
- 70.Frame JM, McGrath KE, Palis J. Erythro-myeloid progenitors: "definitive" hematopoiesis in the conceptus prior to the emergence of hematopoietic stem cells. Blood Cells Mol Dis. 2013;51:220–225. doi: 10.1016/j.bcmd.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Plein A, Fantin A, Denti L, Pollard JW, Ruhrberg C. Erythro-myeloid progenitors contribute endothelial cells to blood vessels. Nature. 2018;562:223–228. doi: 10.1038/s41586-018-0552-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.O'Neill LA, Kishton RJ, Rathmell J. A guide to immunometabolism for immunologists. Nat Rev Immunol. 2016;16:553–565. doi: 10.1038/nri.2016.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Riksen NP, Stienstra R. Metabolism of innate immune cells: impact on atherosclerosis. Curr Opin Lipidol. 2018;29:359–367. doi: 10.1097/MOL.0000000000000539. [DOI] [PubMed] [Google Scholar]
- 74.Wang YH, Israelsen WJ, Lee D, Yu VWC, Jeanson NT, Clish CB, Cantley LC, Vander Heiden MG, Scadden DT. Cell-state-specific metabolic dependency in hematopoiesis and leukemogenesis. Cell. 2014;158:1309–1323. doi: 10.1016/j.cell.2014.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Campo L, Eiseler S, Apfel T, Pyrsopoulos N. Fatty Liver Disease and Gut Microbiota: A Comprehensive Update. J Clin Transl Hepatol. 2019;7:56–60. doi: 10.14218/JCTH.2018.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Krishnan S, Ding Y, Saedi N, Choi M, Sridharan GV, Sherr DH, Yarmush ML, Alaniz RC, Jayaraman A, Lee K. Gut Microbiota-Derived Tryptophan Metabolites Modulate Inflammatory Response in Hepatocytes and Macrophages. Cell Rep. 2018;23:1099–1111. doi: 10.1016/j.celrep.2018.03.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Scott C, Stokes R, Cha KM, Clouston A, Eslam M, Metwally M, Swarbrick MM, George J, Gunton JE. Myeloid cell deletion of Aryl hydrocarbon Receptor Nuclear Translocator (ARNT) induces non-alcoholic steatohepatitis. PLoS One. 2019;14:e0225332. doi: 10.1371/journal.pone.0225332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Soderborg TK, Clark SE, Mulligan CE, Janssen RC, Babcock L, Ir D, Young B, Krebs N, Lemas DJ, Johnson LK, Weir T, Lenz LL, Frank DN, Hernandez TL, Kuhn KA, D'Alessandro A, Barbour LA, El Kasmi KC, Friedman JE. The gut microbiota in infants of obese mothers increases inflammation and susceptibility to NAFLD. Nat Commun. 2018;9:4462. doi: 10.1038/s41467-018-06929-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Soderborg TK, Friedman JE. Imbalance in gut microbes from babies born to obese mothers increases gut permeability and myeloid cell adaptations that provoke obesity and NAFLD. Microb Cell. 2018;6:102–104. doi: 10.15698/mic2019.01.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ye D, Li FY, Lam KS, Li H, Jia W, Wang Y, Man K, Lo CM, Li X, Xu A. Toll-like receptor-4 mediates obesity-induced non-alcoholic steatohepatitis through activation of X-box binding protein-1 in mice. Gut. 2012;61:1058–1067. doi: 10.1136/gutjnl-2011-300269. [DOI] [PubMed] [Google Scholar]
- 81.Miura K, Yang L, van Rooijen N, Brenner DA, Ohnishi H, Seki E. Toll-like receptor 2 and palmitic acid cooperatively contribute to the development of nonalcoholic steatohepatitis through inflammasome activation in mice. Hepatology. 2013;57:577–589. doi: 10.1002/hep.26081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kawai T, Akira S. TLR signaling. Semin Immunol. 2007;19:24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 83.Jialal I, Kaur H, Devaraj S. Toll-like receptor status in obesity and metabolic syndrome: a translational perspective. J Clin Endocrinol Metab. 2014;99:39–48. doi: 10.1210/jc.2013-3092. [DOI] [PubMed] [Google Scholar]
- 84.Ilan Y. Leaky gut and the liver: a role for bacterial translocation in nonalcoholic steatohepatitis. World J Gastroenterol. 2012;18:2609–2618. doi: 10.3748/wjg.v18.i21.2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Enomoto N, Ikejima K, Yamashina S, Hirose M, Shimizu H, Kitamura T, Takei Y, Sato And N, Thurman RG. Kupffer cell sensitization by alcohol involves increased permeability to gut-derived endotoxin. Alcohol Clin Exp Res. 2001;25:51S–54S. doi: 10.1097/00000374-200106001-00012. [DOI] [PubMed] [Google Scholar]
- 86.Carpino G, Del Ben M, Pastori D, Carnevale R, Baratta F, Overi D, Francis H, Cardinale V, Onori P, Safarikia S, Cammisotto V, Alvaro D, Svegliati-Baroni G, Angelico F, Gaudio E, Violi F. Increased liver localization of lipopolysaccharides in human and experimental non-alcoholic fatty liver disease. Hepatology. 2019 doi: 10.1002/hep.31056. [DOI] [PubMed] [Google Scholar]
- 87.Mridha AR, Haczeyni F, Yeh MM, Haigh WG, Ioannou GN, Barn V, Ajamieh H, Adams L, Hamdorf JM, Teoh NC, Farrell GC. TLR9 is up-regulated in human and murine NASH: pivotal role in inflammatory recruitment and cell survival. Clin Sci (Lond) 2017;131:2145–2159. doi: 10.1042/CS20160838. [DOI] [PubMed] [Google Scholar]
- 88.Rivera CA, Adegboyega P, van Rooijen N, Tagalicud A, Allman M, Wallace M. Toll-like receptor-4 signaling and Kupffer cells play pivotal roles in the pathogenesis of non-alcoholic steatohepatitis. J Hepatol. 2007;47:571–579. doi: 10.1016/j.jhep.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Miura K, Kodama Y, Inokuchi S, Schnabl B, Aoyama T, Ohnishi H, Olefsky JM, Brenner DA, Seki E. Toll-like receptor 9 promotes steatohepatitis by induction of interleukin-1beta in mice. Gastroenterology. 2010;139:323–34.e7. doi: 10.1053/j.gastro.2010.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Song K, Kwon H, Han C, Chen W, Zhang J, Ma W, Dash S, Gandhi CR, Wu T. Yes-Associated Protein in Kupffer Cells Enhances the Production of Proinflammatory Cytokines and Promotes the Development of Nonalcoholic Steatohepatitis. Hepatology. 2019 doi: 10.1002/hep.30990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21:677–687. doi: 10.1038/nm.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.West AP, Brodsky IE, Rahner C, Woo DK, Erdjument-Bromage H, Tempst P, Walsh MC, Choi Y, Shadel GS, Ghosh S. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature. 2011;472:476–480. doi: 10.1038/nature09973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rittig N, Svart M, Jessen N, Møller N, Møller HJ, Grønbæk H. Macrophage activation marker sCD163 correlates with accelerated lipolysis following LPS exposure: a human-randomised clinical trial. Endocr Connect. 2018;7:107–114. doi: 10.1530/EC-17-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 95.Ohashi K, Parker JL, Ouchi N, Higuchi A, Vita JA, Gokce N, Pedersen AA, Kalthoff C, Tullin S, Sams A, Summer R, Walsh K. Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype. J Biol Chem. 2010;285:6153–6160. doi: 10.1074/jbc.M109.088708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tsatsanis C, Zacharioudaki V, Androulidaki A, Dermitzaki E, Charalampopoulos I, Minas V, Gravanis A, Margioris AN. Adiponectin induces TNF-alpha and IL-6 in macrophages and promotes tolerance to itself and other pro-inflammatory stimuli. Biochem Biophys Res Commun. 2005;335:1254–1263. doi: 10.1016/j.bbrc.2005.07.197. [DOI] [PubMed] [Google Scholar]
- 97.Fukushima J, Kamada Y, Matsumoto H, Yoshida Y, Ezaki H, Takemura T, Saji Y, Igura T, Tsutsui S, Kihara S, Funahashi T, Shimomura I, Tamura S, Kiso S, Hayashi N. Adiponectin prevents progression of steatohepatitis in mice by regulating oxidative stress and Kupffer cell phenotype polarization. Hepatol Res. 2009;39:724–738. doi: 10.1111/j.1872-034X.2009.00509.x. [DOI] [PubMed] [Google Scholar]
- 98.Chatterjee S, Ganini D, Tokar EJ, Kumar A, Das S, Corbett J, Kadiiska MB, Waalkes MP, Diehl AM, Mason RP. Leptin is key to peroxynitrite-mediated oxidative stress and Kupffer cell activation in experimental non-alcoholic steatohepatitis. J Hepatol. 2013;58:778–784. doi: 10.1016/j.jhep.2012.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Imajo K, Fujita K, Yoneda M, Nozaki Y, Ogawa Y, Shinohara Y, Kato S, Mawatari H, Shibata W, Kitani H, Ikejima K, Kirikoshi H, Nakajima N, Saito S, Maeyama S, Watanabe S, Wada K, Nakajima A. Hyperresponsivity to low-dose endotoxin during progression to nonalcoholic steatohepatitis is regulated by leptin-mediated signaling. Cell Metab. 2012;16:44–54. doi: 10.1016/j.cmet.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 100.Wang J, Leclercq I, Brymora JM, Xu N, Ramezani-Moghadam M, London RM, Brigstock D, George J. Kupffer cells mediate leptin-induced liver fibrosis. Gastroenterology. 2009;137:713–723. doi: 10.1053/j.gastro.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Metwally M, Bayoumi A, Romero-Gomez M, Thabet K, John M, Adams LA, Huo X, Aller R, García-Monzón C, Teresa Arias-Loste M, Bugianesi E, Miele L, Gallego-Durán R, Fischer J, Berg T, Liddle C, Qiao L, George J, Eslam M. A polymorphism in the Irisin-encoding gene (FNDC5) associates with hepatic steatosis by differential miRNA binding to the 3'UTR. J Hepatol. 2019;70:494–500. doi: 10.1016/j.jhep.2018.10.021. [DOI] [PubMed] [Google Scholar]
- 102.Xiong XQ, Geng Z, Zhou B, Zhang F, Han Y, Zhou YB, Wang JJ, Gao XY, Chen Q, Li YH, Kang YM, Zhu GQ. FNDC5 attenuates adipose tissue inflammation and insulin resistance via AMPK-mediated macrophage polarization in obesity. Metabolism. 2018;83:31–41. doi: 10.1016/j.metabol.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 103.Bosma M, Gerling M, Pasto J, Georgiadi A, Graham E, Shilkova O, Iwata Y, Almer S, Söderman J, Toftgård R, Wermeling F, Boström EA, Boström PA. FNDC4 acts as an anti-inflammatory factor on macrophages and improves colitis in mice. Nat Commun. 2016;7:11314. doi: 10.1038/ncomms11314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pan X, Wang P, Luo J, Wang Z, Song Y, Ye J, Hou X. Adipogenic changes of hepatocytes in a high-fat diet-induced fatty liver mice model and non-alcoholic fatty liver disease patients. Endocrine. 2015;48:834–847. doi: 10.1007/s12020-014-0384-x. [DOI] [PubMed] [Google Scholar]
- 105.Canbay A, Feldstein AE, Higuchi H, Werneburg N, Grambihler A, Bronk SF, Gores GJ. Kupffer cell engulfment of apoptotic bodies stimulates death ligand and cytokine expression. Hepatology. 2003;38:1188–1198. doi: 10.1053/jhep.2003.50472. [DOI] [PubMed] [Google Scholar]
- 106.Hirsova P, Ibrahim SH, Krishnan A, Verma VK, Bronk SF, Werneburg NW, Charlton MR, Shah VH, Malhi H, Gores GJ. Lipid-Induced Signaling Causes Release of Inflammatory Extracellular Vesicles From Hepatocytes. Gastroenterology. 2016;150:956–967. doi: 10.1053/j.gastro.2015.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kakazu E, Mauer AS, Yin M, Malhi H. Hepatocytes release ceramide-enriched pro-inflammatory extracellular vesicles in an IRE1α-dependent manner. J Lipid Res. 2016;57:233–245. doi: 10.1194/jlr.M063412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhao L, Yu Y, Deng C. Protein and mRNA expression of Shh, Smo and Gli1 and inhibition by cyclopamine in hepatocytes of rats with chronic fluorosis. Toxicol Lett. 2014;225:318–324. doi: 10.1016/j.toxlet.2013.12.022. [DOI] [PubMed] [Google Scholar]
- 109.Kwon H, Song K, Han C, Chen W, Wang Y, Dash S, Lim K, Wu T. Inhibition of hedgehog signaling ameliorates hepatic inflammation in mice with nonalcoholic fatty liver disease. Hepatology. 2016;63:1155–1169. doi: 10.1002/hep.28289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kudo H, Takahara T, Yata Y, Kawai K, Zhang W, Sugiyama T. Lipopolysaccharide triggered TNF-alpha-induced hepatocyte apoptosis in a murine non-alcoholic steatohepatitis model. J Hepatol. 2009;51:168–175. doi: 10.1016/j.jhep.2009.02.032. [DOI] [PubMed] [Google Scholar]
- 111.Di Naso FC, Porto RR, Fillmann HS, Maggioni L, Padoin AV, Ramos RJ, Mottin CC, Bittencourt A, Marroni NA, de Bittencourt PI., Jr Obesity depresses the anti-inflammatory HSP70 pathway, contributing to NAFLD progression. Obesity (Silver Spring) 2015;23:120–129. doi: 10.1002/oby.20919. [DOI] [PubMed] [Google Scholar]
- 112.Eslam M, Hashem AM, Leung R, Romero-Gomez M, Berg T, Dore GJ, Chan HL, Irving WL, Sheridan D, Abate ML, Adams LA, Mangia A, Weltman M, Bugianesi E, Spengler U, Shaker O, Fischer J, Mollison L, Cheng W, Powell E, Nattermann J, Riordan S, McLeod D, Armstrong NJ, Douglas MW, Liddle C, Booth DR, George J, Ahlenstiel G International Hepatitis C Genetics Consortium (IHCGC) Interferon-λ rs12979860 genotype and liver fibrosis in viral and non-viral chronic liver disease. Nat Commun. 2015;6:6422. doi: 10.1038/ncomms7422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Eslam M, Ahlenstiel G, George J. Interferon Lambda and Liver Fibrosis. J Interferon Cytokine Res. 2019;39:627–635. doi: 10.1089/jir.2018.0175. [DOI] [PubMed] [Google Scholar]
- 114.Eslam M, Hashem AM, Romero-Gomez M, Berg T, Dore GJ, Mangia A, Chan HLY, Irving WL, Sheridan D, Abate ML, Adams LA, Weltman M, Bugianesi E, Spengler U, Shaker O, Fischer J, Mollison L, Cheng W, Nattermann J, Riordan S, Miele L, Kelaeng KS, Ampuero J, Ahlenstiel G, McLeod D, Powell E, Liddle C, Douglas MW, Booth DR, George J International Liver Disease Genetics Consortium (ILDGC) FibroGENE: A gene-based model for staging liver fibrosis. J Hepatol. 2016;64:390–398. doi: 10.1016/j.jhep.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 115.O'Connor KS, Read SA, Wang M, Schibeci S, Eslam M, Ong A, Weltman MD, Douglas MW, Mazzola A, Craxì A, Petta S, Stewart GJ, Liddle C, George J, Ahlenstiel G, Booth DR. IFNL3/4 genotype is associated with altered immune cell populations in peripheral blood in chronic hepatitis C infection. Genes Immun. 2016;17:328–334. doi: 10.1038/gene.2016.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Eslam M, George J. Genome-Wide Association Studies and Hepatitis C: Harvesting the Benefits of the Genomic Revolution. Semin Liver Dis. 2015;35:402–420. doi: 10.1055/s-0035-1567830. [DOI] [PubMed] [Google Scholar]
- 117.Dultz G, Gerber L, Zeuzem S, Sarrazin C, Waidmann O. The macrophage activation marker CD163 is associated with IL28B genotype and hepatic inflammation in chronic hepatitis C virus infected patients. J Viral Hepat. 2016;23:267–273. doi: 10.1111/jvh.12488. [DOI] [PubMed] [Google Scholar]
- 118.Eslam M, McLeod D, Kelaeng KS, Mangia A, Berg T, Thabet K, Irving WL, Dore GJ, Sheridan D, Grønbæk H, Abate ML, Hartmann R, Bugianesi E, Spengler U, Rojas A, Booth DR, Weltman M, Mollison L, Cheng W, Riordan S, Mahajan H, Fischer J, Nattermann J, Douglas MW, Liddle C, Powell E, Romero-Gomez M, George J International Liver Disease Genetics Consortium (ILDGC) IFN-λ3, not IFN-λ4, likely mediates IFNL3-IFNL4 haplotype-dependent hepatic inflammation and fibrosis. Nat Genet. 2017;49:795–800. doi: 10.1038/ng.3836. [DOI] [PubMed] [Google Scholar]
- 119.Honda M, Shirasaki T, Shimakami T, Sakai A, Horii R, Arai K, Yamashita T, Sakai Y, Yamashita T, Okada H, Murai K, Nakamura M, Mizukoshi E, Kaneko S. Hepatic interferon-stimulated genes are differentially regulated in the liver of chronic hepatitis C patients with different interleukin-28B genotypes. Hepatology. 2014;59:828–838. doi: 10.1002/hep.26788. [DOI] [PubMed] [Google Scholar]
- 120.Read SA, Wijaya R, Ramezani-Moghadam M, Tay E, Schibeci S, Liddle C, Lam VWT, Yuen L, Douglas MW, Booth D, George J, Ahlenstiel G. Macrophage Coordination of the Interferon Lambda Immune Response. Front Immunol. 2019;10:2674. doi: 10.3389/fimmu.2019.02674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Buch S, Stickel F, Trépo E, Way M, Herrmann A, Nischalke HD, Brosch M, Rosendahl J, Berg T, Ridinger M, Rietschel M, McQuillin A, Frank J, Kiefer F, Schreiber S, Lieb W, Soyka M, Semmo N, Aigner E, Datz C, Schmelz R, Brückner S, Zeissig S, Stephan AM, Wodarz N, Devière J, Clumeck N, Sarrazin C, Lammert F, Gustot T, Deltenre P, Völzke H, Lerch MM, Mayerle J, Eyer F, Schafmayer C, Cichon S, Nöthen MM, Nothnagel M, Ellinghaus D, Huse K, Franke A, Zopf S, Hellerbrand C, Moreno C, Franchimont D, Morgan MY, Hampe J. A genome-wide association study confirms PNPLA3 and identifies TM6SF2 and MBOAT7 as risk loci for alcohol-related cirrhosis. Nat Genet. 2015;47:1443–1448. doi: 10.1038/ng.3417. [DOI] [PubMed] [Google Scholar]
- 122.Mancina RM, Dongiovanni P, Petta S, Pingitore P, Meroni M, Rametta R, Borén J, Montalcini T, Pujia A, Wiklund O, Hindy G, Spagnuolo R, Motta BM, Pipitone RM, Craxì A, Fargion S, Nobili V, Käkelä P, Kärjä V, Männistö V, Pihlajamäki J, Reilly DF, Castro-Perez J, Kozlitina J, Valenti L, Romeo S. The MBOAT7-TMC4 Variant rs641738 Increases Risk of Nonalcoholic Fatty Liver Disease in Individuals of European Descent. Gastroenterology. 2016;150:1219–1230.e6. doi: 10.1053/j.gastro.2016.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Thabet K, Asimakopoulos A, Shojaei M, Romero-Gomez M, Mangia A, Irving WL, Berg T, Dore GJ, Grønbæk H, Sheridan D, Abate ML, Bugianesi E, Weltman M, Mollison L, Cheng W, Riordan S, Fischer J, Spengler U, Nattermann J, Wahid A, Rojas A, White R, Douglas MW, McLeod D, Powell E, Liddle C, van der Poorten D, George J, Eslam M International Liver Disease Genetics Consortium. MBOAT7 rs641738 increases risk of liver inflammation and transition to fibrosis in chronic hepatitis C. Nat Commun. 2016;7:12757. doi: 10.1038/ncomms12757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Thabet K, Chan HLY, Petta S, Mangia A, Berg T, Boonstra A, Brouwer WP, Abate ML, Wong VW, Nazmy M, Fischer J, Liddle C, George J, Eslam M. The membrane-bound O-acyltransferase domain-containing 7 variant rs641738 increases inflammation and fibrosis in chronic hepatitis B. Hepatology. 2017;65:1840–1850. doi: 10.1002/hep.29064. [DOI] [PubMed] [Google Scholar]
- 125.Helsley RN, Varadharajan V, Brown AL, Gromovsky AD, Schugar RC, Ramachandiran I, Fung K, Kabbany MN, Banerjee R, Neumann CK, Finney C, Pathak P, Orabi D, Osborn LJ, Massey W, Zhang R, Kadam A, Sansbury BE, Pan C, Sacks J, Lee RG, Crooke RM, Graham MJ, Lemieux ME, Gogonea V, Kirwan JP, Allende DS, Civelek M, Fox PL, Rudel LL, Lusis AJ, Spite M, Brown JM. Obesity-linked suppression of membrane-bound O-acyltransferase 7 (MBOAT7) drives non-alcoholic fatty liver disease. Elife. 2019:8. doi: 10.7554/eLife.49882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Patin E, Kutalik Z, Guergnon J, Bibert S, Nalpas B, Jouanguy E, Munteanu M, Bousquet L, Argiro L, Halfon P, Boland A, Müllhaupt B, Semela D, Dufour JF, Heim MH, Moradpour D, Cerny A, Malinverni R, Hirsch H, Martinetti G, Suppiah V, Stewart G, Booth DR, George J, Casanova JL, Bréchot C, Rice CM, Talal AH, Jacobson IM, Bourlière M, Theodorou I, Poynard T, Negro F, Pol S, Bochud PY, Abel L Swiss Hepatitis C Cohort Study Group, International Hepatitis C Genetics Consortium; French ANRS HC EP 26 Genoscan Study Group. Genome-wide association study identifies variants associated with progression of liver fibrosis from HCV infection. Gastroenterology. 2012;143:1244–1252.e12. doi: 10.1053/j.gastro.2012.07.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Petta S, Valenti L, Marra F, Grimaudo S, Tripodo C, Bugianesi E, Cammà C, Cappon A, Di Marco V, Di Maira G, Dongiovanni P, Rametta R, Gulino A, Mozzi E, Orlando E, Maggioni M, Pipitone RM, Fargion S, Craxì A. MERTK rs4374383 polymorphism affects the severity of fibrosis in non-alcoholic fatty liver disease. J Hepatol. 2016;64:682–690. doi: 10.1016/j.jhep.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 128.Cai B, Dongiovanni P, Corey KE, Wang X, Shmarakov IO, Zheng Z, Kasikara C, Davra V, Meroni M, Chung RT, Rothlin CV, Schwabe RF, Blaner WS, Birge RB, Valenti L, Tabas I. Macrophage MerTK Promotes Liver Fibrosis in Nonalcoholic Steatohepatitis. Cell Metab. 2020;31:406–421.e7. doi: 10.1016/j.cmet.2019.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yang Y, Wu XQ, Li WX, Huang HM, Li HD, Pan XY, Li XF, Huang C, Meng XM, Zhang L, Lv XW, Wang H, Li J. PSTPIP2 connects DNA methylation to macrophage polarization in CCL4-induced mouse model of hepatic fibrosis. Oncogene. 2018;37:6119–6135. doi: 10.1038/s41388-018-0383-0. [DOI] [PubMed] [Google Scholar]
- 130.Fan Z, Li L, Li M, Zhang X, Hao C, Yu L, Zeng S, Xu H, Fang M, Shen A, Jenuwein T, Xu Y. The histone methyltransferase Suv39h2 contributes to nonalcoholic steatohepatitis in mice. Hepatology. 2017;65:1904–1919. doi: 10.1002/hep.29127. [DOI] [PubMed] [Google Scholar]
- 131.Liu XL, Pan Q, Cao HX, Xin FZ, Zhao ZH, Yang RX, Zeng J, Zhou H, Fan JG. Lipotoxic Hepatocyte-Derived Exosomal miR-192-5p Activates Macrophages via Rictor/Akt/FoxO1 Signaling in NAFLD. Hepatology. 2019 doi: 10.1002/hep.31050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rosso C, Kazankov K, Younes R, Esmaili S, Marietti M, Sacco M, Carli F, Gaggini M, Salomone F, Møller HJ, Abate ML, Vilstrup H, Gastaldelli A, George J, Grønbæk H, Bugianesi E. Crosstalk between adipose tissue insulin resistance and liver macrophages in non-alcoholic fatty liver disease. J Hepatol. 2019;71:1012–1021. doi: 10.1016/j.jhep.2019.06.031. [DOI] [PubMed] [Google Scholar]
- 133.Stanton MC, Chen SC, Jackson JV, Rojas-Triana A, Kinsley D, Cui L, Fine JS, Greenfeder S, Bober LA, Jenh CH. Inflammatory Signals shift from adipose to liver during high fat feeding and influence the development of steatohepatitis in mice. J Inflamm (Lond) 2011;8:8. doi: 10.1186/1476-9255-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Boutens L, Stienstra R. Adipose tissue macrophages: going off track during obesity. Diabetologia. 2016;59:879–894. doi: 10.1007/s00125-016-3904-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.du Plessis J, van Pelt J, Korf H, Mathieu C, van der Schueren B, Lannoo M, Oyen T, Topal B, Fetter G, Nayler S, van der Merwe T, Windmolders P, Van Gaal L, Verrijken A, Hubens G, Gericke M, Cassiman D, Francque S, Nevens F, van der Merwe S. Association of Adipose Tissue Inflammation With Histologic Severity of Nonalcoholic Fatty Liver Disease. Gastroenterology. 2015;149:635–48.e14. doi: 10.1053/j.gastro.2015.05.044. [DOI] [PubMed] [Google Scholar]
- 136.Patsouris D, Li PP, Thapar D, Chapman J, Olefsky JM, Neels JG. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab. 2008;8:301–309. doi: 10.1016/j.cmet.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bijnen M, Josefs T, Cuijpers I, Maalsen CJ, van de Gaar J, Vroomen M, Wijnands E, Rensen SS, Greve JWM, Hofker MH, Biessen EAL, Stehouwer CDA, Schalkwijk CG, Wouters K. Adipose tissue macrophages induce hepatic neutrophil recruitment and macrophage accumulation in mice. Gut. 2018;67:1317–1327. doi: 10.1136/gutjnl-2016-313654. [DOI] [PubMed] [Google Scholar]
- 138.Younossi ZM, Ratziu V, Loomba R, Rinella M, Anstee QM, Goodman Z, Bedossa P, Geier A, Beckebaum S, Newsome PN, Sheridan D, Sheikh MY, Trotter J, Knapple W, Lawitz E, Abdelmalek MF, Kowdley KV, Montano-Loza AJ, Boursier J, Mathurin P, Bugianesi E, Mazzella G, Olveira A, Cortez-Pinto H, Graupera I, Orr D, Gluud LL, Dufour JF, Shapiro D, Campagna J, Zaru L, MacConell L, Shringarpure R, Harrison S, Sanyal AJ REGENERATE Study Investigators. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2019;394:2184–2196. doi: 10.1016/S0140-6736(19)33041-7. [DOI] [PubMed] [Google Scholar]
- 139.Eslam M, Alvani R, Shiha G. Obeticholic acid: towards first approval for NASH. Lancet. 2019;394:2131–2133. doi: 10.1016/S0140-6736(19)32963-0. [DOI] [PubMed] [Google Scholar]
- 140.Eslam M, George J. Genetic Insights for Drug Development in NAFLD. Trends Pharmacol Sci. 2019;40:506–516. doi: 10.1016/j.tips.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 141.Kazankov K, Alisi A, Møller HJ, De Vito R, Rittig S, Mahler B, Nobili V, Grønbæk H. Macrophage Markers Are Poorly Associated With Liver Histology in Children With Nonalcoholic Fatty Liver Disease. J Pediatr Gastroenterol Nutr. 2018;67:635–642. doi: 10.1097/MPG.0000000000002111. [DOI] [PubMed] [Google Scholar]
- 142.Jiang ZG, Sandhu B, Feldbrügge L, Yee EU, Csizmadia E, Mitsuhashi S, Huang J, Afdhal NH, Robson SC, Lai M. Serum Activity of Macrophage-Derived Adenosine Deaminase 2 Is Associated With Liver Fibrosis in Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol. 2018;16:1170–1172. doi: 10.1016/j.cgh.2017.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tacke F. Targeting hepatic macrophages to treat liver diseases. J Hepatol. 2017;66:1300–1312. doi: 10.1016/j.jhep.2017.02.026. [DOI] [PubMed] [Google Scholar]
- 144.Krenkel O, Puengel T, Govaere O, Abdallah AT, Mossanen JC, Kohlhepp M, Liepelt A, Lefebvre E, Luedde T, Hellerbrand C, Weiskirchen R, Longerich T, Costa IG, Anstee QM, Trautwein C, Tacke F. Therapeutic inhibition of inflammatory monocyte recruitment reduces steatohepatitis and liver fibrosis. Hepatology. 2018;67:1270–1283. doi: 10.1002/hep.29544. [DOI] [PubMed] [Google Scholar]
- 145.Odegaard JI, Ricardo-Gonzalez RR, Red Eagle A, Vats D, Morel CR, Goforth MH, Subramanian V, Mukundan L, Ferrante AW, Chawla A. Alternative M2 activation of Kupffer cells by PPARdelta ameliorates obesity-induced insulin resistance. Cell Metab. 2008;7:496–507. doi: 10.1016/j.cmet.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Luo W, Xu Q, Wang Q, Wu H, Hua J. Effect of modulation of PPAR-γ activity on Kupffer cells M1/M2 polarization in the development of non-alcoholic fatty liver disease. Sci Rep. 2017;7:44612. doi: 10.1038/srep44612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jain MR, Giri SR, Bhoi B, Trivedi C, Rath A, Rathod R, Ranvir R, Kadam S, Patel H, Swain P, Roy SS, Das N, Karmakar E, Wahli W, Patel PR. Dual PPARα/γ agonist saroglitazar improves liver histopathology and biochemistry in experimental NASH models. Liver Int. 2018;38:1084–1094. doi: 10.1111/liv.13634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ratziu V, Harrison SA, Francque S, Bedossa P, Lehert P, Serfaty L, Romero-Gomez M, Boursier J, Abdelmalek M, Caldwell S, Drenth J, Anstee QM, Hum D, Hanf R, Roudot A, Megnien S, Staels B, Sanyal A GOLDEN-505 Investigator Study Group. Elafibranor, an Agonist of the Peroxisome Proliferator-Activated Receptor-α and -δ, Induces Resolution of Nonalcoholic Steatohepatitis Without Fibrosis Worsening. Gastroenterology. 2016;150:1147–1159.e5. doi: 10.1053/j.gastro.2016.01.038. [DOI] [PubMed] [Google Scholar]
- 149.Staels B, Rubenstrunk A, Noel B, Rigou G, Delataille P, Millatt LJ, Baron M, Lucas A, Tailleux A, Hum DW, Ratziu V, Cariou B, Hanf R. Hepatoprotective effects of the dual peroxisome proliferator-activated receptor alpha/delta agonist, GFT505, in rodent models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology. 2013;58:1941–1952. doi: 10.1002/hep.26461. [DOI] [PubMed] [Google Scholar]
- 150.Mridha AR, Wree A, Robertson AAB, Yeh MM, Johnson CD, Van Rooyen DM, Haczeyni F, Teoh NC, Savard C, Ioannou GN, Masters SL, Schroder K, Cooper MA, Feldstein AE, Farrell GC. NLRP3 inflammasome blockade reduces liver inflammation and fibrosis in experimental NASH in mice. J Hepatol. 2017;66:1037–1046. doi: 10.1016/j.jhep.2017.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhao GN, Zhang P, Gong J, Zhang XJ, Wang PX, Yin M, Jiang Z, Shen LJ, Ji YX, Tong J, Wang Y, Wei QF, Wang Y, Zhu XY, Zhang X, Fang J, Xie Q, She ZG, Wang Z, Huang Z, Li H. Tmbim1 is a multivesicular body regulator that protects against non-alcoholic fatty liver disease in mice and monkeys by targeting the lysosomal degradation of Tlr4. Nat Med. 2017;23:742–752. doi: 10.1038/nm.4334. [DOI] [PubMed] [Google Scholar]
- 152.Diehl AM, Harrison S, Caldwell S, Rinella M, Paredes A, Moylan C, Guy C, Bashir M, Wang Y, Miller L, Chang A, Wu ES, Abdelmalek M. JKB-121 in patients with nonalcoholic steatohepatitis: A phase 2 double blind randomized placebo control study. J Hepatol. 2018;68:S103. [Google Scholar]
- 153.Ouyang X, Han SN, Zhang JY, Dioletis E, Nemeth BT, Pacher P, Feng D, Bataller R, Cabezas J, Stärkel P, Caballeria J, Pongratz RL, Cai SY, Schnabl B, Hoque R, Chen Y, Yang WH, Garcia-Martinez I, Wang FS, Gao B, Torok NJ, Kibbey RG, Mehal WZ. Digoxin Suppresses Pyruvate Kinase M2-Promoted HIF-1α Transactivation in Steatohepatitis. Cell Metab. 2018;27:339–350.e3. doi: 10.1016/j.cmet.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhao P, Han SN, Arumugam S, Yousaf MN, Qin Y, Jiang JX, Torok NJ, Chen Y, Mankash MS, Liu J, Li J, Iwakiri Y, Ouyang X. Digoxin improves steatohepatitis with differential involvement of liver cell subsets in mice through inhibition of PKM2 transactivation. Am J Physiol Gastrointest Liver Physiol. 2019;317:G387–G397. doi: 10.1152/ajpgi.00054.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chen L, Shi Y, Liu S, Cao Y, Wang X, Tao Y. PKM2: the thread linking energy metabolism reprogramming with epigenetics in cancer. Int J Mol Sci. 2014;15:11435–11445. doi: 10.3390/ijms150711435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Xie M, Yu Y, Kang R, Zhu S, Yang L, Zeng L, Sun X, Yang M, Billiar TR, Wang H, Cao L, Jiang J, Tang D. PKM2-dependent glycolysis promotes NLRP3 and AIM2 inflammasome activation. Nat Commun. 2016;7:13280. doi: 10.1038/ncomms13280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tran M, Lee SM, Shin DJ, Wang L. Loss of miR-141/200c ameliorates hepatic steatosis and inflammation by reprogramming multiple signaling pathways in NASH. JCI Insight. 2017;2 doi: 10.1172/jci.insight.96094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kimbrough D, Wang SH, Wright LH, Mani SK, Kasiganesan H, LaRue AC, Cheng Q, Nadig SN, Atkinson C, Menick DR. HDAC inhibition helps post-MI healing by modulating macrophage polarization. J Mol Cell Cardiol. 2018;119:51–63. doi: 10.1016/j.yjmcc.2018.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Luque-Martin R, Van den Bossche J, Furze RC, Neele AE, van der Velden S, Gijbels MJJ, van Roomen CPPA, Bernard SG, de Jonge WJ, Rioja I, Prinjha RK, Lewis HD, Mander PK, de Winther MPJ. Targeting Histone Deacetylases in Myeloid Cells Inhibits Their Maturation and Inflammatory Function With Limited Effects on Atherosclerosis. Front Pharmacol. 2019;10:1242. doi: 10.3389/fphar.2019.01242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol. 2013;13:709–721. doi: 10.1038/nri3520. [DOI] [PMC free article] [PubMed] [Google Scholar]