Abstract
Lysosomal acid lipase (LAL) deficiency (LAL-D) is a lysosomal lipid storage disorder in which the accumulation of cholesteryl esters and triglycerides predominantly in hepatocytes and cells of the macrophage-monocyte system is observed. The disturbance in the synthesis and trafficking of cholesterol and other lipids (triglycerides as well as phospholipids) as well as the systemic lipoprotein dysregulation, reflects the pathophysiology of LAL-D.
The aim of this study was to present the occurrence of macrophage derived structures in LAL-D patient, and to provide an overview on underlying mechanisms, as the literature about the presence of such cluster cells in LAL deficiency is sparse.
We describe the case of LAL-D patient diagnosed at 3 years of age, in whom the massive macrophage accumulation resulting in the abdominal lymphadenopathy, subcutaneous papules and hepatosplenomegaly, have been observed within 4 years since diagnosis. Histopathological examination of the excised lymph nodes and subcutaneous papules revealed them to be diffusely infiltrated by lipid-overloaded histiocytes. The immunohistochemistry revealed the macrophages to be CD68-positive.
This study comprises one of the first reports of accumulation of lipid-laden macrophages throughout the body in the course of LAL-D.
Keywords: Lysosomal acid lipase, Lysosomal acid lipase deficiency, Macrophage, Lymphadenitis, CD68
1. Background
Lysosomal acid lipase (LAL) deficiency (LAL-D) is a lysosomal lipid storage disorder caused by mutations of the LIPA gene encoding the lysosomal acid lipase which catalyzes the hydrolysis of cholesteryl esters and triglycerides. Depending on the residual enzyme activity, LAL-D results in an early-onset, severe and lethal phenotype, known as Wolman disease, or a late-onset, attenuated phenotype, cholesteryl ester storage disease (CESD) [1].
A reduced hydrolysis of cholesteryl esters and triglycerides and their accumulation predominantly in hepatocytes and macrophage-monocyte system cells is observed in LAL-D [2,3]. Subsequently, a reduced release of free cholesterol and fatty acids in the lysosome, with reduced abundance of the oxidized and other derivatives is observed leading to a paradoxical stimulation of lipogenesis with decreased free intracellular cholesterol [4].
The aim of this study was to present the occurrence of macrophage derived structures in LAL deficiency patient, and to provide an overview on underlying mechanisms, as the literature about the presence of such cluster cells in LAL deficiency is sparse.
2. Case report
The patient was the third child of nonconsanguineous Polish parents born at 40 weeks of gestation with a birth mass of 3650 g. At the age of 2 years she was accidentally found to present with a mild hepatomegaly and elevated serum transaminases. Dyslipidemia defined by elevated levels of TC, LDL-C and TG, and decreased HDL-C level, was noted as well (Table 1).
Table 1.
Detailed characteristics of the patient's phenotype.
| Parameter and reference values | Age [years] |
|||||
|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | 6 | 7 | |
| AST [<59 U/l] | 100 | 128 | 82 | 72 | 121 | 63 |
| ALT [<39 U/l] | 125 | 193 | 69 | 68 | 71 | 68 |
| TC [<170 mg/dl] | 330 | 366 | 339 | 423 | 380 | 344 |
| LDL-C [<110 mg/dl] | 263 | 298 | 266 | 349 | 292 | 265 |
| TG [<75 mg/dl] | 274 | 298 | 324 | 301 | 383 | 338 |
| HDL-C [>45 mg/dl] | 10 | 8 | 8 | 14 | 11 | 11 |
| Liver measurement on ultrasound [cm below right costal margin] | 2 | 3 | 4 | 4 | 5 | 6 |
| Spleen length on ultrasound [cm] | 7,5 | 8,5 | 9,5 | 10 | 11 | 12 |
| Plasma chitotriosidase activity [<150 nmol/ml/h] | n.a. | 784 | n.a. | n.a. | n.a. | 760 |
At the age of 3 years, the diagnosis of LAL-D was established by demonstration of reduced LAL activity in peripheral blood leukocytes (2,7 nmol/mg protein/h; reference range 213,7 ± 94,2) and LIPA gene mutations in sequence analysis. The patient was found to be heterozygous for two missense variants: c.894G > A (p.delS275_Q298) and c.309C > A (p.S103R). Enzyme replacement therapy was not available.
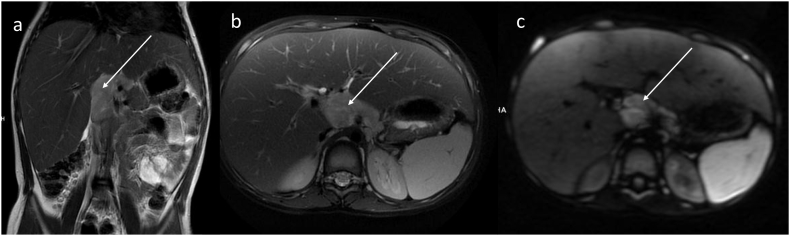
During four years of follow-up, an increasing volume of the liver and spleen was observed (Table 1). Elevated serum transaminases persisted at similar levels as found at the baseline but dyslipidemia worsened reflecting elevated serum TC and TG. At the age of 7 years, the presence of pathologic mass (dimension 50 × 27 × 55 mm) in the hepatic hilum, was found in a routinely perform abdominal ultrasound. Two papules in the subcutaneous tissue of the forearms, additionally have been found. In magnetic resonance imaging (MRI), conglomerated enlarged lymph nodes surrounding, but not occluding coeliac trunk and abdominal aorta, were observed (Fig. 1).
Fig. 1.
Abdominal MR in (a) coronal T2-WI, (b) axial T2-WI with fat saturation and (c) axial DWI images demonstrates an irregular mass of lymph nodes (arrows) surrounding, but not occluding coeliac trunk and abdominal aorta.
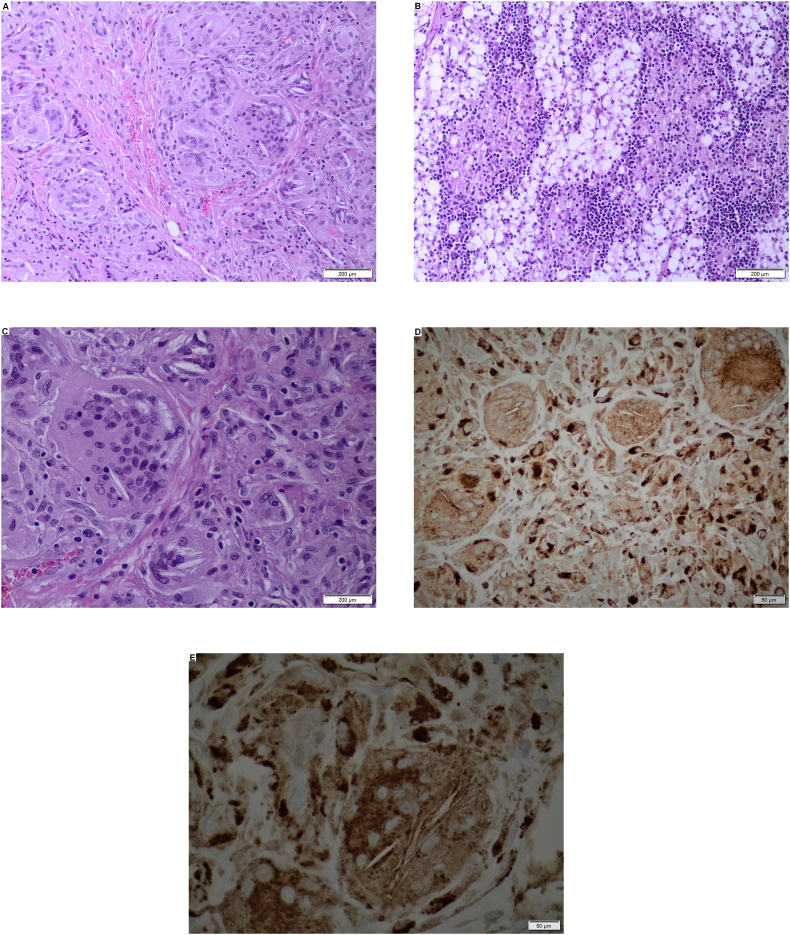
Several subpleural nodules in the lungs have been noted, as well. The surgical biopsy of abdominal lymph nodes and subcutaneous papules revealed them to be diffusely infiltrated by lipid-overloaded histiocytes (Fig. 2). Light cytoplasm as a result of leached lipid material and needles of cholesteryl esters have been also observed in both biopsy specimens. The immunohistochemical staining by means of monoclonal antibody Dako CD68 demonstrated the clusters of multinucleated macrophages with cholesteryl esters needles located in their cytoplasm (Fig. 2).
Fig. 2.
abc – Diffuse infiltration of lipid-overloaded macrophages; hematoxylin and eosine stain. de – clusters of multinucleated macrophages with cholesterol esters needles located in their cytoplasm; CD68 antibody stain.
3. Discussion
In this study we present the case of LAL-deficient patient diagnosed at 3 years of age, in whom the massive macrophages accumulation resulting in the abdominal lymphadenopathy, subcutaneous papules and hepatosplenomegaly, have been observed within 4 years since diagnosis.
Vom Dahl et al. reported a similar case of 36-year-old LAL-deficient patient presenting with hepatosplenomegaly, dyslipidemia and increased mesenteric fat deposits [5]. As the patient refused a biopsy, the intraabdominal masses were considered to be lipomas or enlarged abdominal lymph nodes. Together with our case report, this is the second one found in the literature, regarding lipid-laden lymphadenitis in the course of LAL-D.
Paralelly to Gaucher disease, the accumulation of foamy macrophages (known as Gaucher cells), giving rise to gaucheroma or lymphadenitis, have been reported [6]. However, LAL-D is not simply storage disorder. The disturbance in the synthesis and trafficking of cholesterol and other lipids (triglycerides as well as phospholipids) as well as the systemic lipoprotein dysregulation, reflects the pathophysiology of LAL-D. Sterol regulatory element-binding protein (SREBP)-mediated up-regulation of endogenous cholesterol production by hydroxymethylglutaryl-coenzyme A (HMG-CoA) reductase and of endocytosis via LDL receptors, as well as increased synthesis of apolipoprotein B and very-low-density lipoproteins [4]. The dyslipidemia is compounded by a reduced formation of high-density lipoproteins (HDL-C).
In presented case, the immunohistochemistry revealed the macrophages to be CD68-positive which is routinely used as a histochemical marker associated with the involvement of endosomal/lysosomal compartment. CD68/macrosialin has been also shown to bind and internalize oxidized LDL (oxLDL) suggesting its role in the intracellular lipid accumulation [7].
4. Conclusions
The paper presents an evidence of a widespread lipid accumulation in macrophages resulting in lymphadenopathy and subcutaneous papules formation in LAL-D, which comprise not seen before feature of the condition.
Funding sources
None.
Declaration of Competing Interest
All authors declare no conflict of interest.
Acknowledgements
None.
References
- 1.Ługowska A., Tylki-Szymańska A. Lysosomal acid lipase deficiency: Wolman disease and cholesteryl ester storage disease. CML-Lysosomal Storage Dis. 2012;10:1–8. [PubMed] [Google Scholar]
- 2.Lipiński P., Ługowska A., Zakharova E.Y., Socha P., Tylki-Szymańska A. Diagnostic algorithm for cholesteryl Ester storage disease: clinical presentation in 19 polish patients. J. Pediatr. Gastroenterol. Nutr. 2018;67:452–457. doi: 10.1097/MPG.0000000000002084. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein D.L., Hülkova H., Bialer M.G., Desnick R.J. Cholesteryl ester storage disease: review of the findings in 135 reported patients with an underdiagnosed disease. J. Hepatol. 2013;58:1230–1243. doi: 10.1016/j.jhep.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 4.Reiner Ž., Guardamagna O., Nair D., Soran H., Hovingh K., Bertolini S., Jones S., Ćorić M., Calandra S., Hamilton J., Eagleton T., Ros E. Lysosomal acid lipase deficiency--an under-recognized cause of dyslipidaemia and liver dysfunction. Atherosclerosis. 2014;235:21–30. doi: 10.1016/j.atherosclerosis.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 5.vom Dahl S., Harzer K., Rolfs A., Albrecht B., Niederau C., Vogt C., van Weely S., Aerts J., Müller G., Häussinger D. Hepatosplenomegalic lipidosis: what unless Gaucher? Adult cholesteryl ester storage disease (CESD) with anemia, mesenteric lipodystrophy, increased plasma chitotriosidase activity and a homozygous lysosomal acid lipase −1 exon 8 splice junction mutation. J. Hepatol. 1999 Oct;31(4):741–746. doi: 10.1016/s0168-8278(99)80356-0. [DOI] [PubMed] [Google Scholar]
- 6.Ivanova M., Limgala R.P., Changsila E., Kamath R., Ioanou C., Goker-Alpan O. Gaucheromas: when macrophages promote tumor formation and dissemination. Blood Cells Mol. Dis. 2018;68:100–105. doi: 10.1016/j.bcmd.2016.10.018. [DOI] [PubMed] [Google Scholar]
- 7.Chistiakov D.A., Killingsworth M.C., Myasoedova V.A., Orekhov A.N., Bobryshev Y.V. CD68/macrosialin: not just a histochemical marker. Lab. Investig. 2017;97:4–13. doi: 10.1038/labinvest.2016.116. [DOI] [PubMed] [Google Scholar]