Abstract
Objective:
This study sought to examine the prospective effects of early adolescent marijuana use on late adolescent attentional and inhibitory control. Alcohol use, antisocial problems, and gender were included as statistical control variables.
Method:
The community sample of 387 adolescents and a caregiver was drawn from a longitudinal study of adolescent substance use that included nine annual assessments. Adolescents were eligible if they were between ages 11 and 12 at recruitment and did not have any disabilities that would preclude them from either understanding or completing the assessment. The sample was evenly split on gender (55% female) and was predominantly non-Hispanic White (83.16%) or African American (9.07%). Attentional and inhibitory control were assessed using parent and adolescent self-reports on the Early Adolescent Temperament Questionnaire-Revised and the Adult Temperament Questionnaire.
Results:
Hypotheses were tested using structural equation modeling. High levels of early marijuana use at ages 12–14 significantly predicted low levels of adolescent attentional control at ages 18–21 (β = -.20, p < .05), above and beyond early attentional control, early alcohol use, and antisocial problems.
Conclusions:
Findings suggest that marijuana use may adversely affect cognitive development, especially during the sensitive period of early adolescence. Results emphasize the need for further prospective work to investigate relationships between early adolescent marijuana use and the development of executive functioning.
Marijuana use is increasingly common among adolescents in the United States. Recent data suggest that by 12th grade, 37% of youth report past-year use (Johnston et al., 2018). Moreover, legalization of marijuana has been associated with reductions in perceived harmfulness of marijuana among adolescents (Cerdá et al., 2017). Yet, marijuana use is associated with a host of negative psychosocial outcomes, including poor academic performance and increased risk of psychotic disorders, and we know little of its effects on long-term cognitive development (Hall & Degenhardt, 2009). This is a significant gap in the literature, as the adverse effects of marijuana use on cognitive functioning have been established cross-sectionally (Solowij et al., 2002), and earlier onset of marijuana use may be especially harmful to cognitive development (Fontes et al., 2011).
Significant neurobiological development during adolescence (Blakemore & Choudhury, 2006) is accompanied by changes in executive functioning (Crone, 2009). Executive functioning comprises three main domains: working memory operations (“executive attention”; Engle, 2002), inhibition of prepotent impulses, and mental set-shifting (Miyake et al., 2000). Executive functions are critical to self-regulation (Hofmann et al., 2012). Indeed, there is empirical evidence that working memory capacities support self-regulation by enabling individuals to control attention (Hofmann et al., 2008). Response inhibition has also been closely tied to healthy self-regulation by facilitating the ability to actively override behavioral responses that are incompatible with one’s goals (Houben & Wiers, 2009). Impaired abilities to self-regulate put a person at risk for many adverse outcomes, including higher levels of negative emotionality, school dropout, and antisocial behaviors (Eisenberg et al., 2011).
Of note, early adolescence is thought to represent a window of vulnerability when exposure to substances is particularly harmful (Guttmannova et al., 2011) because it may derail healthy brain development and put those who use at risk for subsequent reduced executive functioning and self-regulatory capacities (Castellanos-Ryan et al., 2017; Squeglia et al., 2009). Evidence suggests that use of other substances, including alcohol, during early adolescence negatively affects subsequent executive functioning (Tapert et al., 2002). Understanding whether early adolescent marijuana use also negatively affects executive functioning is important because this line of work has the potential to inform prevention efforts and policy as legalization becomes more common. The current study seeks to investigate whether early adolescent marijuana use is associated with late adolescent executive functioning (self-reported attentional and inhibitory control).
Cross-sectional research on marijuana use in adults suggests that heavy use is associated with poor performance on tests of learning and memory, attention, visuospatial skills, processing speed, and executive functioning (Schweinsburg et al., 2008). However, it is hard to make strong conclusions about direction of effects from these studies. Although marijuana use may influence executive functioning, it is equally plausible that poor executive functioning may lead to marijuana use (Aytaclar et al., 1999). Moreover, findings from longitudinal research have been mixed. Some longitudinal studies report no association between adolescent marijuana use and subsequent executive functioning (Meier et al., 2018), and others suggest that marijuana use may adversely affect attention (Jacobus et al., 2015). One study reported that heavy marijuana use from ages 16 to 24 predicted poor attention at 24 years old, above and beyond the effects of baseline attention and testing experience (Tapert et al., 2002). In addition, animal models suggest that adolescent marijuana use may have particularly harmful effects on inhibition. In a longitudinal study with rats, Schneider and Koch (2003) found that exposure to marijuana during adolescence disrupted inhibition responses in adulthood. Taken together, the limited prospective work that exists in this area suggests that adolescent marijuana use may have particularly adverse effects on attention and inhibition.
A large number of cross-sectional and longitudinal studies of marijuana use and executive functioning have not included alcohol use in their statistical analyses, or have excluded individuals who drink (Pope et al., 2003; Schwartz et al., 1989; Tapert et al., 2002). This is a notable limitation in the literature for several reasons. Alcohol is the most commonly used substance among adolescents (Swendsen et al., 2012), and the overwhelming majority of adolescents who use marijuana also drink alcohol, with some estimates exceeding 90% (Behrendt et al., 2012). Hence, excluding drinkers creates an unusual subsample of marijuana users. Moreover, there is a large body of prospective, longitudinal research on the adverse effects of early alcohol use on subsequent executive functioning (e.g., Brown et al., 2000). Accordingly, alcohol use is a potential confound, and failure to account for it when examining the effects of marijuana use on neurocognitive outcomes makes it difficult to draw firm conclusions about marijuana use because observed associations could be attributable to alcohol use.
Another notable limitation of studies of marijuana use and executive functioning is the lack of attention to the broader constellation of antisocial behaviors that are also associated with substance use and executive functioning development. Indeed, a considerable body of research has suggested that early substance use is related to a wide range of antisocial problems (Hussong et al., 2004). Thus, antisocial problems may be more strongly related to deficits in executive functioning and self-regulation than substance use (Giancola et al., 1998). Indeed, antisocial problems may lead to negative feedback from the environment, including poor parenting, which in turn may negatively affect executive functioning development (Eisenberg et al., 2015). Here again, failure to account for antisocial problems leaves observed associations between marijuana use and executive functioning open to the possibility that this relationship is attributable to antisocial behavior rather than marijuana use.
The current study used a longitudinal sample spanning early to late adolescence, which includes a sensitive period of brain development, to test the prospective long-term effects of marijuana use on subsequent attentional and inhibitory control. Alcohol use and antisocial problems are included as statistical control variables. Gender is also included as a statistical control variable given prior work suggesting associations with adolescent substance use, antisocial problems, and executive functioning (Karreman et al., 2009; Windle, 1990).
Hypotheses
Marijuana use in early adolescence (ages 11–14) was hypothesized to be related to low levels of attentional and inhibitory control in late adolescence (ages 18–21). It is also expected that early alcohol use (ages 11–14) will be related to low levels of attentional and inhibitory control in late adolescence (ages 18–21).
Method
Procedure and protection of human subjects
The study was approved at inception and annually thereafter by the Institutional Review Board at the University at Buffalo, State University of New York. Interviews at Waves 1–3 were conducted annually in university research offices. Assessments included laboratory tasks and questionnaires, and data for this study were taken from the questionnaires. Families were compensated $75, $85, and $125 at Waves 1–3, respectively. Research assistants obtained written consent from caregivers and assent from adolescents at Waves 1–3. A federal Certificate of Confidentiality protected all dyads’ data from subpoena.
Annual assessments at Waves 4–6 involved a brief, telephone-administered, audio Computer-Assisted Self-Interview of substance use. The interview took between 10 and 15 minutes to complete. Parents provided consent over the phone and were given a phone number and PIN for their adolescent to use. Assent from the adolescent was obtained at the initiation of the audio Computer-Assisted Self-Interview survey.
The procedures for annual assessments at Waves 7–9 were similar to those used in Waves 1–3. Adolescents provided written informed consent after age 18 and were compensated $125 for completing the full assessment (lab tasks and questionnaires) or $50 for completing only the questionnaires. There were 17 (4.80%), 38 (10.83%), and 83 (23.31%) participants who completed the questionnaires outside of the lab across Waves 7–9, respectively. Caregivers were compensated $40.
Participants
The community sample of 387 adolescents and a caregiver was drawn from a longitudinal study of adolescent substance use that included nine annual assessments. Adolescents were eligible for the study if they were between ages 11 and 12 at recruitment and did not have any disabilities that would preclude them from either understanding or completing the assessment. Data collection began in 2007.
The current study utilized substance use and executive functioning data from Waves 1–9 of the longitudinal project. The average age of participants was 11.6, 12.6, 13.6, 14.6, 15.5, 16.6, 17.9, 18.9, and 19.9 at Waves 1–9, respectively. The sample was evenly split on gender (55% female) and was predominantly non-Hispanic White (83.16%) or African American (9.07%). Median family income at Wave 1 was $70,000, and 6% of the families received public assistance income. Sample demographics compared well to those within our sampling frame, which was Erie County, NY (for more detail about sample recruitment and characteristics, see Trucco et al., 2014). Overall retention across waves was strong, with sample size varying between 350 (90%) and 373 (96%).
Materials
Measures.
(A) Substance use: Items taken from the National Youth Survey were used to assess past-year substance use at Waves 1–4 (Elliot & Huizinga, 1983). Alcohol use was assessed with two fill-in-the-blank questions: (a) how many times in the past year they had used alcohol (frequency) and (b) how many drinks were typically consumed on days when alcohol was used (quantity). A drink was defined as a 12-oz. beer, a 4-oz. glass of wine, a 12-oz. wine cooler, or a 1.25-oz. shot of distilled spirits. A Quantity × Frequency index was created to represent total drinks consumed in the past year at each assessment.
The number of times adolescents used marijuana (including hashish, hash, tetrahydrocannabinol [THC], pot, grass, weed, and reefer) in the past year was also assessed using a fill-in-the-blank item at Waves 1–4. Several studies support the reliability and validity of self-reports of adolescent substance use, like the National Youth Survey (Del Boca & Darkes, 2003; Winters et al., 1990).
Given that rates of use were very low at Wave 1 for alcohol (2.60% of the sample endorsed drinking in the past year) and marijuana (0% of the sample endorsed using marijuana in the past year), we constructed the latent variables for early alcohol and marijuana use by using data from Waves 2, 3, and 4.
(B) Attentional and inhibitory control: At Waves 1–3, attentional and inhibitory control were assessed using caregiver reports on the Early Adolescent Temperament Questionnaire–Revised (EATQ-R; Ellis & Rothbart, 1999). The attentional control scale included six items, and the inhibitory control scale included five items. The items were rated on a 5-point response scale (1 = almost always untrue of your child, 5 = almost always true of your child). Items were averaged to form scale scores that were used to control for levels of attentional and inhibitory control at Waves 1–3. The EATQ-R has demonstrated good validity with theoretically similar scales of temperament and adjustment outcomes (Muris & Meesters, 2009). Internal consistencies were .80, .82, and .81 for attentional control and .52, .59, and .59 for inhibitory control.
At Waves 7–9, attentional and inhibitory control were assessed using adolescent self-reports on the Adult Temperament Questionnaire (ATQ; Evans & Rothbart, 2007). ATQ items were written to be developmentally appropriate for describing adult functioning and to parallel EATQ-R items. Sample items for the EATQ-R and the ATQ are provided in the supplemental material. (Supplemental material appears as an online-only addendum to the article on the journal’s website.) Attentional control was assessed with five items, and inhibitory control was assessed with seven items. Items were rated on a 7-point response scale (1 = extremely untrue, 7 = extremely true) and averaged to form scale scores at Waves 7–9. Validity of the ATQ has been supported by correlations with widely used measures of temperament, executive functioning, and adjustment outcomes (Rothbart et al., 2000; Wei et al., 2019). At Waves 7–9, internal consistencies were .68, .73, and .76 for attentional control and .56, .50, and .55 for inhibitory control.
(C) Antisocial problems: Conduct disorder items from the youth self-report form of the Achenbach System of Empirically Based Assessment (Achenbach & Rescorla, 2001; Achenbach et al., 2003) were administered to measure antisocial problems. Substance use items were removed from the scale to avoid confounding with substance use variables measured by the National Youth Survey. Items were averaged to form scale scores and were used as a control variable at Waves 1–3. Internal consistencies ranged from .83 to .85.
Analytic strategy.
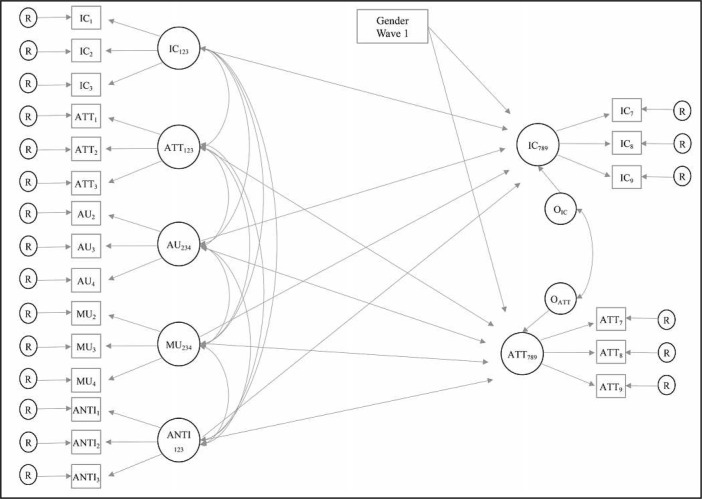
Hypotheses were tested using structural equation modeling estimated in Mplus version 8.2 (Muthén & Muthén, 1998–2017) using robust maximum likelihood estimation. A measurement model was estimated and then respecified to include prospective paths (rather than covariances) and gender as a covariate. The full structural regression model is depicted in Figure 1. Global and local fit were assessed using conventional fit statistics (e.g., model chisquare, comparative fit index [CFI], root mean square error approximation [RMSEA], and standardized root mean square residual [SRMR]).
Figure 1.
Conceptual figure of a structural regression model for the effects of early substance use on subsequent attentional and inhibitory control. IC = inhibitory control; ATT = attentional control; AU = alcohol use; MU = marijuana use; ANTI = antisocial problems; R = residual term; O = disturbance term. Numbers denote annual Waves 1–9.
Results
Descriptive analysis
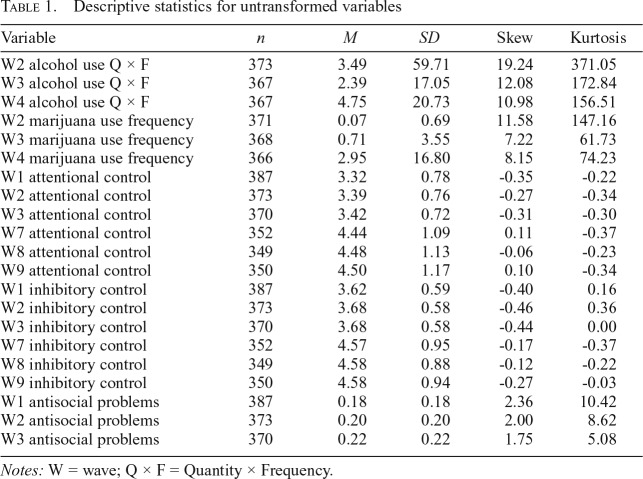
As expected, given the age of the sample, the observed Quantity × Frequency indexes of alcohol use variables and the frequency variables of marijuana use were nonnormal (Johnston et al., 2018). In addition, the observed variables for antisocial problems were nonnormal. Data for marijuana use, alcohol use, and antisocial problems were Winsorized at three standard deviations above the mean and log-transformed for analysis. However, considerable skewness and kurtosis remained after data transformation, especially among the substance use variables (kurtosis of alcohol use latent variables ranged from 2.56 to 36.18, and kurtosis of marijuana use latent variables ranged from 13.62 to 83.04). Therefore, robust maximum likelihood estimation was used to handle remaining nonnormality.
Descriptive statistics for study variables can be found in Table 1. The total sample size for the final model was 387. The number of participants who endorsed alcohol use in the past year was 83 (22.25%), 124 (33.78%), and 105 (28.61%) across Waves 2, 3, and 4, respectively. The mean Quantity × Frequency indexes of drinks in the past year were M = 3.49, 2.39, and 4.75 across Waves 2, 3, and 4, respectively. The number of participants who endorsed marijuana use in the past year started out low at Wave 2 (1.62%, n = 6) and increased to 8.42% (n = 31) and 17.21% (n = 63) at Waves 3 and 4, respectively.
Table 1.
Descriptive statistics for untransformed variables
| Variable | n | M | SD | Skew | Kurtosis |
| W2 alcohol use Q × F | 373 | 3.49 | 59.71 | 19.24 | 371.05 |
| W3 alcohol use Q × F | 367 | 2.39 | 17.05 | 12.08 | 172.84 |
| W4 alcohol use Q × F | 367 | 4.75 | 20.73 | 10.98 | 156.51 |
| W2 marijuana use frequency | 371 | 0.07 | 0.69 | 11.58 | 147.16 |
| W3 marijuana use frequency | 368 | 0.71 | 3.55 | 7.22 | 61.73 |
| W4 marijuana use frequency | 366 | 2.95 | 16.80 | 8.15 | 74.23 |
| W1 attentional control | 387 | 3.32 | 0.78 | -0.35 | -0.22 |
| W2 attentional control | 373 | 3.39 | 0.76 | -0.27 | -0.34 |
| W3 attentional control | 370 | 3.42 | 0.72 | -0.31 | -0.30 |
| W7 attentional control | 352 | 4.44 | 1.09 | 0.11 | -0.37 |
| W8 attentional control | 349 | 4.48 | 1.13 | -0.06 | -0.23 |
| W9 attentional control | 350 | 4.50 | 1.17 | 0.10 | -0.34 |
| W1 inhibitory control | 387 | 3.62 | 0.59 | -0.40 | 0.16 |
| W2 inhibitory control | 373 | 3.68 | 0.58 | -0.46 | 0.36 |
| W3 inhibitory control | 370 | 3.68 | 0.58 | -0.44 | 0.00 |
| W7 inhibitory control | 352 | 4.57 | 0.95 | -0.17 | -0.37 |
| W8 inhibitory control | 349 | 4.58 | 0.88 | -0.12 | -0.22 |
| W9 inhibitory control | 350 | 4.58 | 0.94 | -0.27 | -0.03 |
| W1 antisocial problems | 387 | 0.18 | 0.18 | 2.36 | 10.42 |
| W2 antisocial problems | 373 | 0.20 | 0.20 | 2.00 | 8.62 |
| W3 antisocial problems | 370 | 0.22 | 0.22 | 1.75 | 5.08 |
Notes: W = wave; Q × F = Quantity × Frequency.
Measurement model
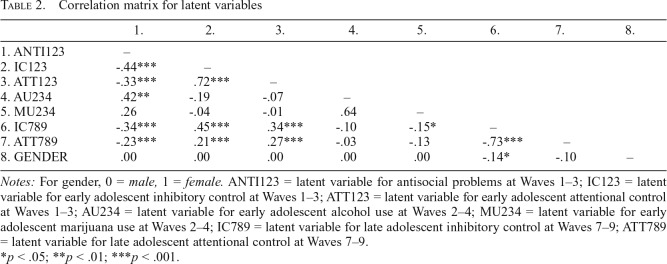
The initial measurement model produced a negative (but nonsignificant) residual for the Wave 3 indicator of marijuana use. After constraining this residual to 0, the newly estimated model did not produce any negative residual variances. The measurement model provided an acceptable fit to the data, χ2(169) = 370.93, p < .001; CFI = .93; RMSEA = .056; 90% CI [.048, .063]; SRMR = .050. Factor correlations are in Table 2, and standardized factor loadings and R2 values for the combined measurement model were strong and can be found in Table 3.
Table 2.
Correlation matrix for latent variables
| 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | |
| 1. ANTI123 | – | |||||||
| 2. IC123 | -.44*** | – | ||||||
| 3. ATT123 | -.33*** | .72*** | – | |||||
| 4. AU234 | .42** | -.19 | -.07 | – | ||||
| 5. MU234 | .26 | -.04 | -.01 | .64 | – | |||
| 6. IC789 | -.34*** | .45*** | .34*** | -.10 | -.15* | – | ||
| 7. ATT789 | -.23*** | .21*** | .27*** | -.03 | -.13 | -.73*** | – | |
| 8. GENDER | .00 | .00 | .00 | .00 | .00 | -.14* | -.10 | – |
Notes: For gender, 0 = male, 1 = female. ANTI123 = latent variable for antisocial problems at Waves 1–3; IC123 = latent variable for early adolescent inhibitory control at Waves 1–3; ATT123 = latent variable for early adolescent attentional control at Waves 1–3; AU234 = latent variable for early adolescent alcohol use at Waves 2–4; MU234 = latent variable for early adolescent marijuana use at Waves 2–4; IC789 = latent variable for late adolescent inhibitory control at Waves 7–9; ATT789 = latent variable for late adolescent attentional control at Waves 7–9.
p < .05;
p < .01;
p < .001.
Table 3.
Standardized factor loadings and R2 values for combined factor model

| Variable | n | Standardized factor loadings | R2 |
| Alcohol use W2 | 373 | .61 | .38 |
| Alcohol use W3 | 367 | .77 | .59 |
| Alcohol use W4 | 367 | .68 | .46 |
| Marijuana use W2 | 371 | .43 | .18 |
| Marijuana use W3 | 368 | 1.00 | 1.00 |
| Marijuana use W4 | 366 | .61 | .36 |
| Antisocial problems W1 | 387 | .55 | .30 |
| Antisocial problems W2 | 373 | .76 | .58 |
| Antisocial problems W3 | 370 | .98 | .95 |
| Inhibitory control W1 | 352 | .81 | .66 |
| Inhibitory control W2 | 349 | .80 | .64 |
| Inhibitory control W3 | 350 | .84 | .70 |
| Attentional control W1 | 387 | .87 | .76 |
| Attentional control W2 | 373 | .91 | .83 |
| Attentional control W3 | 370 | .88 | .78 |
| Inhibitory control W7 | 352 | .79 | .62 |
| Inhibitory control W8 | 349 | .81 | .65 |
| Inhibitory control W9 | 350 | .77 | .60 |
| Attentional control W7 | 387 | .80 | .64 |
| Attentional control W8 | 373 | .87 | .76 |
| Attentional control W9 | 370 | .75 | .83 |
Notes: W = wave. For standardized factor loadings, all ps < .001.
Path model
Next, prospective structural paths replaced relevant covariances as depicted in Figure 1. In addition, we added gender as a Wave 1 covariate. The model provided an acceptable fit to the data, χ2(190) = 431.86, p < .001; CFI = .92; RMSEA = .057; 90% CI [.050, .065]; SRMR = .061. It explained 28% of the variance in the latent variable for late adolescent inhibitory control (R2 = .28) and 13% of the variance in the latent variable for late adolescent attentional control (R2 = .13).
Auto-regressive paths for attentional (β = .22, p < .001) and inhibitory control (β = .39, p < .001) were statistically significant across early to late adolescence. Regarding cross-sectional associations, early alcohol and marijuana use were positively related, although this association was marginally significant (r = .64, p = .08). In addition, high levels of early antisocial problems were related to high levels of early alcohol use (r = .42, p < .01) and marginally related to high levels of marijuana use (r = .26, p = .06). In early adolescence, attentional and inhibitory control were negatively related to antisocial problems (rs = -.33 and -.44, respectively, p < .001). Early levels of attentional and inhibitory control were associated with neither early alcohol use (rs = -.07 and -.19, ps > .07) nor marijuana use (r = -.01 and -.04, p > .50). Attentional control was significantly related to inhibitory control across early (r = .72, p < .001) and late adolescence (r = .72, p < .001). Gender was significantly related to late adolescent inhibitory control, such that males had higher levels of late adolescent inhibitory control (β = -.14, p < .05).
Prospective effects of substance use on attentional control
High levels of early marijuana use at Waves 2–4 significantly predicted low levels of attentional control at Waves 7–9 (β = -.20, p < .05), above and beyond early attentional control, early alcohol use, and antisocial problems. The relationship between early alcohol use and late adolescent attentional control was also statistically significant (β = .18, p < .05); but, contrary to expectation, high levels of early alcohol use predicted high levels of late adolescent attentional control. This was interpreted as a suppression effect, considering that the measurement model correlation between the latent variable for alcohol use and the latent variable for late adolescent attentional control was nonsignificant with negative sign (r = -.03, p = .60). Other significant relationships between relevant variables helped us to interpret the meaning of this suppression effect, which will be examined further in the discussion.
High levels of early antisocial problems at Waves 1–3 also significantly predicted low levels of late adolescent attentional control at Waves 7–9 (β = -.18, p < .05).
Prospective effects of substance use on inhibitory control
The relationship between early marijuana use and late adolescent inhibitory control approached significance in the expected direction (β = -.21, p = .07), such that high levels of early marijuana use were trending toward predicting low levels of late adolescent inhibitory control, after controlling for antisocial problems, early inhibitory control, and alcohol use. Early alcohol use significantly predicted late adolescent inhibitory control (β = .19, p < .05); but, again contrary to expectation, high levels of early alcohol use predicted high levels of late adolescent inhibitory control, controlling for antisocial problems, early inhibitory control, and marijuana use. The positive regression coefficient again indicated a possible suppression effect, considering that the measurement model correlation between the latent variable for alcohol use and the latent variable for late adolescent inhibitory control was nonsignificant with negative sign (r = -.10, p = .16).
High levels of early antisocial problems also significantly predicted low levels of late adolescent inhibitory control (β = -.19, p < .01).
Discussion
The current study tested whether marijuana use during a sensitive period of neurobiological development, early adolescence, is related to subsequent deficits in attentional and inhibitory control across adolescence. Strengths of this study included the following: (a) investigating prospective, longitudinal relationships between early adolescent marijuana use and later executive functioning; (b) elucidating the unique effects of early marijuana and alcohol use on executive functioning; and (c) examining these relationships above and beyond the effects of antisocial problems. Results suggested that early adolescent marijuana use (ages 11–14) may derail healthy development of attentional and inhibitory control, above and beyond early alcohol use and antisocial problems. Evidence was stronger for attentional than for inhibitory control.
Our findings are consistent with cross-sectional work suggesting that marijuana users display deficits in attention (Pope & Yurgelun-Todd, 1996) and findings from two longitudinal studies that have reported a significant relationship between adolescent marijuana use and subsequent decreases in attention (Jacobus et al., 2015; Tapert et al., 2002). Findings from the current study extend past work by demonstrating that the adverse effect of early marijuana use on subsequent levels of attentional control persisted above and beyond early alcohol use and antisocial problems.
However, our findings diverge from those reported by Meier and colleagues (2018). The authors reported a significant association between past-year marijuana use at age 18 and poor concurrent executive functioning in their full sample, but twins who used marijuana more frequently than their co-twin did not have lower levels of executive functioning. The authors concluded that family background factors (e.g., shared environment) accounted for the association between marijuana use and performance on executive functioning tests. Although Meier and colleagues did not examine use in early adolescence, it is possible that our effects of marijuana use are attributable to unmeasured shared causes. Our design does not permit an examination of these issues, and this is an important direction for future work considering the potential impact of early marijuana use.
High levels of early marijuana use trended toward significance in predicting low levels of late adolescent inhibitory control, controlling for early levels of inhibitory control, alcohol use, and antisocial problems. Although there is less longitudinal work examining this relationship, findings from prior cross-sectional studies suggest that marijuana use is associated with deficits in inhibition (Gruber &Yurgelun-Todd, 2005; Tapert et al., 2007). Given the limited research in this area and the marginally significant result from the current study, more prospective work is needed to elucidate whether marijuana use during a sensitive period of neurobiological development adversely affects developmental trajectories of inhibitory control.
Evaluation of association of early alcohol use with attentional and inhibitory control in late adolescence was complicated by what appear to be suppression effects1 and findings contrary to expectation. High levels of early alcohol use significantly predicted high levels of late adolescent attentional and inhibitory control. As early alcohol use was strongly related to early antisocial problems and marijuana use, after accounting for these behaviors, remaining overlap between alcohol use and attentional and inhibitory control may represent normative and healthy developmental trajectories. Indeed, alcohol use is normative in American culture (Johnston et al., 2018), and some amount of use may be an indicator of healthy psychosocial adjustment and executive functioning in certain contexts (Christian et al., 1995; Peele & Brodsky, 2000). Social support is viewed as beneficial for cognitive functioning (Seeman et al., 2001). Therefore, it may be that light to moderate drinkers are more socially adjusted, and thereby reap the benefits of social support for the development of attentional and inhibitory control.
Limitations and implications
One limitation of the current study involves measurement of marijuana use that did not provide information on potency. Composition of illicit marijuana has changed in recent decades, with the potency of cannabis plant material increasing from 4% in 1995 to 12% in 2014 (ElSohly et al., 2016), and more potent marijuana may be more harmful (Bolla et al., 2002; ElSohly et al., 2016). However, measurement of quantity of marijuana use is difficult, given that no standard dose exists and that there are many means by which marijuana is ingested (e.g., joints, bowls, bongs, oils, food, etc.) (Cuttler & Spradlin, 2017). Therefore, it remains unclear in what ways dose and composition of marijuana may be important when examining the long-term effects of use of this drug on the developing adolescent brain.
Another limitation is reliance on self-report questionnaires of substance use, and of attentional and inhibitory control and concerns about social desirability and poor recall. With respect to attention and inhibitory control, the executive functioning literature faces many definitional and measurement issues (Nigg, 2017); given this lack of consensus, no “gold standard” of measurement exists (Campbell et al., 2016). Self-reports provide an opportunity to access the totality of a person’s experience, including their regulation of attention and behavior in real situations, thus providing ecological validity across a variety of contexts (Cyders & Coskunpinar, 2011). Laboratory assessments of executive functioning eliminate potential reporter bias and provide greater objectivity, but they characterize executive functioning in a limited context that may not reflect how people behave in daily life. Specific to our measure, the EATQ-R has been linked to development and activity in brain regions that underlie executive functioning (Vijayakumar et al., 2014).
Still, findings from the current study demonstrate that marijuana use may adversely affect cognitive development. Moreover, they provide important information for policy makers to consider as they grapple with legalization. Given recent decreases in the perceived harmful effects of marijuana among youth, it is important to disseminate educational resources and scientific findings regarding adverse outcomes associated with marijuana use.
Acknowledgment
The authors thank the families and children for their gracious participation in this work.
Footnotes
The model was estimated without marijuana use and antisocial problems. The regression paths between early alcohol use and late adolescent attentional (β = -.009, p = .87) and inhibitory control (β = -.008, p = .88) were nonsignificant, small, and negative.
This research was supported by National Institute on Drug Abuse Grant R01 DA020171 awarded to Craig R. Colder. The authors have no conflicts of interest to declare.
References
- Achenbach T. M., Dumenci L., Rescorla L. A. Ratings of relations between DSM-IV diagnostic categories and items of the Adult Self-Report (ASR) and Adult Behavior Checklist (ABCL) Burlington, VT: University of Vermont, Research Center for Children, Youth, and Families; 2003. [Google Scholar]
- Achenbach T. M., Rescorla L. A. Manual for the ASEBA school-age forms & profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, and Families; 2001. [Google Scholar]
- Aytaclar S., Tarter R. E., Kirisci L., Lu S. Association between hyperactivity and executive cognitive functioning in childhood and substance use in early adolescence. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38:172–178. doi: 10.1097/00004583-199902000-00016. doi:10.1097/00004583-199902000-00016. [DOI] [PubMed] [Google Scholar]
- Behrendt S., Beesdo-Baum K., Höfler M., Perkonigg A., Bühringer G., Lieb R., Wittchen H.-U. The relevance of age at first alcohol and nicotine use for initiation of cannabis use and progression to cannabis use disorders. Drug and Alcohol Dependence. 2012;123:48–56. doi: 10.1016/j.drugalcdep.2011.10.013. doi:10.1016/j.drugalcdep.2011.10.013. [DOI] [PubMed] [Google Scholar]
- Blakemore S.-J., Choudhury S. Development of the adolescent brain: Implications for executive function and social cognition. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2006;47:296–312. doi: 10.1111/j.1469-7610.2006.01611.x. doi:10.1111/j.1469-7610.2006.01611.x. [DOI] [PubMed] [Google Scholar]
- Bolla K. I., Brown K., Eldreth D., Tate K., Cadet J. L. Dose-related neurocognitive effects of marijuana use. Neurology. 2002;59:1337–1343. doi: 10.1212/01.wnl.0000031422.66442.49. doi:10.1212/01.WNL.0000031422.66442.49. [DOI] [PubMed] [Google Scholar]
- Brown S. A., Tapert S. F., Granholm E., Delis D. C. Neurocognitive functioning of adolescents: Effects of protracted alcohol use. Alcoholism: Clinical and Experimental Research. 2000;24:164–171. doi:10.1111/j.1530-0277.2000.tb04586.x. [PubMed] [Google Scholar]
- Campbell S. B., Denham S. A., Howarth G. Z., Jones S. M., Whittaker J. V., Williford A. P., Darling-Churchill K. Commentary on the review of measures of early childhood social and emotional development: Conceptualization, critique, and recommendations. Journal of Applied Developmental Psychology. 2016;45:19–41. doi:10.1016/j.appdev.2016.01.008. [Google Scholar]
- Castellanos-Ryan N., Pingault J.-B., Parent S., Vitaro F., Tremblay R. E., Séguin J. R. Adolescent cannabis use, change in neurocognitive function, and high-school graduation: A longitudinal study from early adolescence to young adulthood. Development and Psychopathology. 2017;29:1253–1266. doi: 10.1017/S0954579416001280. doi:10.1017/S0954579416001280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerdá M., Wall M., Feng T., Keyes K. M., Sarvet A., Schulenberg J., Hasin D. S. Association of state recreational marijuana laws with adolescent marijuana use. JAMA Pediatrics. 2017;171:142–149. doi: 10.1001/jamapediatrics.2016.3624. doi:10.1001/jamapediatrics.2016.3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian J. C., Reed T., Carmelli D., Page W. F., Norton J. A., Jr., Breitner J. C. Self-reported alcohol intake and cognition in aging twins. Journal of Studies on Alcohol. 1995;56:414–416. doi: 10.15288/jsa.1995.56.414. doi:10.15288/jsa.1995.56.414. [DOI] [PubMed] [Google Scholar]
- Crone E. A. Executive functions in adolescence: Inferences from brain and behavior. Developmental Science. 2009;12:825–830. doi: 10.1111/j.1467-7687.2009.00918.x. doi:10.1111/j.1467-7687.2009.00918.x. [DOI] [PubMed] [Google Scholar]
- Cuttler C., Spradlin A. Measuring cannabis consumption: Psychometric properties of the Daily Sessions, Frequency, Age of Onset, and Quantity of Cannabis Use Inventory (DFAQ-CU) PLoS One. 2017;12:e0178194. doi: 10.1371/journal.pone.0178194. doi:10.1371/journal.pone.0178194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyders M. A., Coskunpinar A. Measurement of constructs using self-report and behavioral lab tasks: Is there overlap in nomothetic span and construct representation for impulsivity? Clinical Psychology Review. 2011;31:965–982. doi: 10.1016/j.cpr.2011.06.001. doi:10.1016/j.cpr.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Del Boca F. K., Darkes J. The validity of self-reports of alcohol consumption: State of the science and challenges for research. Addiction. 2003;98(Supplement 2):1–12. doi: 10.1046/j.1359-6357.2003.00586.x. doi:10.1046/j.1359-6357.2003.00586.x. [DOI] [PubMed] [Google Scholar]
- Eisenberg N., Smith C. L., Spinrad T. L. Effortful control: Relations with emotion regulation, adjustment, and socialization in childhood. In: Vohs K. D., Baumeister R. F., editors. Handbook of self-regulation: Research, theory, and applications. 2nd ed. New York, NY: Guilford Press; 2011. pp. 263–283. [Google Scholar]
- Eisenberg N., Taylor Z. E., Widaman K. F., Spinrad T. L. Externalizing symptoms, effortful control, and intrusive parenting: A test of bidirectional longitudinal relations during early childhood. Development and Psychopathology. 2015;27:953–968. doi: 10.1017/S0954579415000620. doi:10.1017/S0954579415000620. [DOI] [PubMed] [Google Scholar]
- Elliott D. S., Huizinga D. Social class and delinquent behavior in a national youth panel. Criminology. 1983;21:149–177. doi:10.1111/j.1745-9125.1983.tb00256.x. [Google Scholar]
- Ellis L., Rothbart M. Early adolescent temperament questionnaire-revised (EATQ-R) 1999. Retrieved from https://research.bowdoin.edu/rothbart-temperament-questionnaires/instrument-descriptions/the-early-adolescent-temperament-questionnaire. [Google Scholar]
- ElSohly M. A., Mehmedic Z., Foster S., Gon C., Chandra S., Church J. C. Changes in cannabis potency over the last 2 decades (1995–2014): Analysis of current data in the United States. Biological Psychiatry. 2016;79:613–619. doi: 10.1016/j.biopsych.2016.01.004. doi:10.1016/j.biopsych.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engle R. W. Working memory capacity as executive attention. Current Directions in Psychological Science. 2002;11:19–23. doi:10.1111/1467-8721.00160. [Google Scholar]
- Evans D. E., Rothbart M. K. Developing a model for adult temperament. Journal of Research in Personality. 2007;41:868–888. doi:10.1016/j.jrp.2006.11.002. [Google Scholar]
- Fontes M. A., Bolla K. I., Cunha P. J., Almeida P. P., Jungerman F., Laranjeira R. R. Lacerda A. L. T. Cannabis use before age 15 and subsequent executive functioning. British Journal of Psychiatry. 2011;198:442–447. doi: 10.1192/bjp.bp.110.077479. doi:10.1192/bjp.bp.110.077479. [DOI] [PubMed] [Google Scholar]
- Giancola P. R., Mezzich A. C., Tarter R. E. Disruptive, delinquent and aggressive behavior in female adolescents with a psychoactive substance use disorder: Relation to executive cognitive functioning. Journal of Studies on Alcohol. 1998;59:560–567. doi: 10.15288/jsa.1998.59.560. doi:10.15288/jsa.1998.59.560. [DOI] [PubMed] [Google Scholar]
- Gruber S. A., Yurgelun-Todd D. A. Neuroimaging of marijuana smokers during inhibitory processing: A pilot investigation. Cognitive Brain Research. 2005;23:107–118. doi: 10.1016/j.cogbrainres.2005.02.016. doi:10.1016/j.cogbrainres.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Guttmannova K., Bailey J. A., Hill K. G., Lee J. O., Hawkins J. D., Woods M. L., Catalano R. F. Sensitive periods for adolescent alcohol use initiation: Predicting the lifetime occurrence and chronicity of alcohol problems in adulthood. Journal of Studies on Alcohol and Drugs. 2011;72:221–231. doi: 10.15288/jsad.2011.72.221. doi:10.15288/jsad.2011.72.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall W., Degenhardt L. Adverse health effects of non-medical cannabis use. The Lancet. 2009;374:1383–1391. doi: 10.1016/S0140-6736(09)61037-0. doi:10.1016/S0140-6736(09)61037-0. [DOI] [PubMed] [Google Scholar]
- Hofmann W., Gschwendner T., Friese M., Wiers R. W., Schmitt M. Working memory capacity and self-regulatory behavior: Toward an individual differences perspective on behavior determination by automatic versus controlled processes. Journal of Personality and Social Psychology. 2008;95:962–977. doi: 10.1037/a0012705. doi:10.1037/a0012705. [DOI] [PubMed] [Google Scholar]
- Hofmann W., Schmeichel B. J., Baddeley A. D. Executive functions and self-regulation. Trends in Cognitive Sciences. 2012;16:174–180. doi: 10.1016/j.tics.2012.01.006. doi:10.1016/j.tics.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Houben K., Wiers R. W. Response inhibition moderates the relationship between implicit associations and drinking behavior. Alcoholism: Clinical and Experimental Research. 2009;33:626–633. doi: 10.1111/j.1530-0277.2008.00877.x. doi:10.1111/j.1530-0277.2008.00877.x. [DOI] [PubMed] [Google Scholar]
- Hussong A. M., Curran P. J., Moffitt T. E., Caspi A., Carrig M. M. Substance abuse hinders desistance in young adults’ antisocial behavior. Development and Psychopathology. 2004;16:1029–1046. doi: 10.1017/s095457940404012x. doi:10.1017/S095457940404012X. [DOI] [PubMed] [Google Scholar]
- Jacobus J., Squeglia L. M., Infante M. A., Castro N., Brumback T., Meruelo A. D., Tapert S. F. Neuropsychological performance in adolescent marijuana users with co-occurring alcohol use: A three-year longitudinal study. Neuropsychology. 2015;29:829–843. doi: 10.1037/neu0000203. doi:10.1037/neu0000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston L. D., Miech R. A., O’Malley P. M., Bachman J. G., Schulenberg J. E., Patrick M. E. Monitoring the Future national survey results on drug use: 1975-2017: Overview, key findings on adolescent drug use. Ann Arbor, MI: Institute for Social Research, The University of Michigan; 2018. [Google Scholar]
- Karreman A., van Tuijl C., van Aken M. A. G., Dekovi M. Predicting young children’s externalizing problems: Interactions among effortful control, parenting, and child gender. Merrill-Palmer Quarterly. 2009;55:111–134. doi:10.1353/mpq.0.0020. [Google Scholar]
- Meier M. H., Caspi A., Danese A., Fisher H. L., Houts R., Arseneault L., Moffitt T. E. Associations between adolescent cannabis use and neuropsychological decline: A longitudinal co-twin control study. Addiction. 2018;113:257–265. doi: 10.1111/add.13946. doi:10.1111/add.13946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A., Friedman N. P., Emerson M. J., Witzki A. H., Howerter A., Wager T. D. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: A latent variable analysis. Cognitive Psychology. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. doi:10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Muris P., Meesters C. Reactive and regulative temperament in youths: Psychometric evaluation of the Early Adolescent Temperament Questionnaire-Revised. Journal of Psychopathology and Behavioral Assessment. 2009;31:7–19. doi:10.1007/s10862-008-9089-x. [Google Scholar]
- Muthén L. K., Muthén B. O. Mplus user’s guide. eighth edition. Los Angeles, CA: Authors; 1998–2017. [Google Scholar]
- Nigg J. T. Annual research review: On the relations among self-regulation, self-control, executive functioning, effortful control, cognitive control, impulsivity, risk-taking, and inhibition for developmental psychopathology. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2017;58:361–383. doi: 10.1111/jcpp.12675. doi:10.1111/jcpp.12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peele S., Brodsky A. Exploring psychological benefits associated with moderate alcohol use: A necessary corrective to assessments of drinking outcomes? Drug and Alcohol Dependence. 2000;60:221–247. doi: 10.1016/s0376-8716(00)00112-5. doi:10.1016/S0376-8716(00)00112-5. [DOI] [PubMed] [Google Scholar]
- Pope H. G., Jr., Gruber A. J., Hudson J. I., Cohane G., Huestis M. A., Yurgelun-Todd D. Early-onset cannabis use and cognitive deficits: What is the nature of the association? Drug and Alcohol Dependence. 2003;69:303–310. doi: 10.1016/s0376-8716(02)00334-4. doi:10.1016/S0376-8716(02)00334-4. [DOI] [PubMed] [Google Scholar]
- Pope H. G., Jr., Yurgelun-Todd D. The residual cognitive effects of heavy marijuana use in college students. JAMA. 1996;275:521–527. doi:10.1001/jama.1996.03530310027028. [PubMed] [Google Scholar]
- Rothbart M. K., Ahadi S. A., Evans D. E. Temperament and personality: Origins and outcomes. Journal of Personality and Social Psychology. 2000;78:122–135. doi: 10.1037//0022-3514.78.1.122. doi:10.1037/0022-3514.78.1.122. [DOI] [PubMed] [Google Scholar]
- Schneider M., Koch M. Chronic pubertal, but not adult chronic cannabinoid treatment impairs sensorimotor gating, recognition memory, and the performance in a progressive ratio task in adult rats. Neuropsychopharmacology. 2003;28:1760–1769. doi: 10.1038/sj.npp.1300225. doi:10.1038/sj.npp.1300225. [DOI] [PubMed] [Google Scholar]
- Schwartz R. H., Gruenewald P. J., Klitzner M., Fedio P. Short-term memory impairment in cannabis-dependent adolescents. American Journal of Diseases of Children. 1989;143:1214–1219. doi: 10.1001/archpedi.1989.02150220110030. [DOI] [PubMed] [Google Scholar]
- Schweinsburg A. D., Brown S. A., Tapert S. F. The influence of marijuana use on neurocognitive functioning in adolescents. Current Drug Abuse Reviews. 2008;1:99–111. doi: 10.2174/1874473710801010099. doi:10.2174/1874473710801010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman T. E., Lusignolo T. M., Albert M., Berkman L. Social relationships, social support, and patterns of cognitive aging in healthy, high-functioning older adults: MacArthur studies of successful aging. Health Psychology. 2001;20:243–255. doi: 10.1037//0278-6133.20.4.243. doi:10.1037/0278-6133.20.4.243. [DOI] [PubMed] [Google Scholar]
- Solowij N., Stephens R. S., Roffman R. A., Babor T., Kadden R., Miller M., Vendetti J. & the Marijuana Treatment Project Research Group. Cognitive functioning of long-term heavy cannabis users seeking treatment. JAMA. 2002;287:1123–1131. doi: 10.1001/jama.287.9.1123. doi:10.1001/jama.287.9.1123. [DOI] [PubMed] [Google Scholar]
- Squeglia L. M., Jacobus J., Tapert S. F. The influence of substance use on adolescent brain development. Clinical EEG and Neuroscience. 2009;40:31–38. doi: 10.1177/155005940904000110. doi:10.1177/155005940904000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swendsen J., Burstein M., Case B., Conway K. P., Dierker L., He J., Merikangas K. R. Use and abuse of alcohol and illicit drugs in US adolescents: Results of the National Comorbidity Survey— Adolescent Supplement. Archives of General Psychiatry. 2012;69:390–398. doi: 10.1001/archgenpsychiatry.2011.1503. doi:10.1001/archgenpsychiatry.2011.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapert S. F., Granholm E., Leedy N. G., Brown S. A. Substance use and withdrawal: Neuropsychological functioning over 8 years in youth. Journal of the International Neuropsychological Society. 2002;8:873–883. doi: 10.1017/s1355617702870011. doi:10.1017/S1355617702870011. [DOI] [PubMed] [Google Scholar]
- Tapert S. F., Schweinsburg A. D., Drummond S. P. A., Paulus M. P., Brown S. A., Yang T. T., Frank L. R. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology. 2007;194:173–183. doi: 10.1007/s00213-007-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trucco E. M., Colder C. R., Wieczorek W. F., Lengua L. J., Hawk L. W., Jr. Early adolescent alcohol use in context: How neighborhoods, parents, and peers impact youth. Development and Psychopathology. 2014;26:425–436. doi: 10.1017/S0954579414000042. doi:10.1017/S0954579414000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayakumar N., Whittle S., Dennison M., Yücel M., Simmons J., Allen N. B. Development of temperamental effortful control mediates the relationship between maturation of the prefrontal cortex and psychopathology during adolescence: A 4-year longitudinal study. Developmental Cognitive Neuroscience. 2014;9:30–43. doi: 10.1016/j.dcn.2013.12.002. doi:10.1016/j.dcn.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L., Guo N., Baeken C., Bi M., Wang X., Qiu J., Wu G.-R. Grey matter volumes in the executive attention system predict individual differences in effortful control in young adults. Brain Topography. 2019;32:111–117. doi: 10.1007/s10548-018-0676-1. doi:10.1007/s10548-018-0676-1. [DOI] [PubMed] [Google Scholar]
- Windle M. A longitudinal study of antisocial behaviors in early adolescence as predictors of late adolescent substance use: Gender and ethnic group differences. Journal of Abnormal Psychology. 1990;99:86–91. doi: 10.1037//0021-843x.99.1.86. doi:10.1037/0021-843X.99.1.86. [DOI] [PubMed] [Google Scholar]
- Winters K. C., Stinchfield R. D., Henly G. A., Schwartz R. H. Validity of adolescent self-report of alcohol and other drug involvement. International Journal of the Addictions. 1990;25(Supplement 11):1379–1395. doi: 10.3109/10826089009068469. doi:10.3109/10826089009068469. [DOI] [PubMed] [Google Scholar]