Abstract
Background
The COVID-19 pandemics required several changes in stroke management and it may have influenced some clinical or functional characteristics. We aimed to evaluate the effects of the COVID-19 pandemics on stroke management during the first month of Italy lockdown. In addition, we described the emergency structured pathway adopted by an Italian University Hub Stroke Unit in the cross-border Italy-Slovenia area.
Methods
We analyzed admitted patients' clinical features and outcomes between 9th March 2020 and 9th April 2020 (first month of lockdown), and compared them with patients admitted during the same period in 2019.
Results
Total admissions experienced a reduction of 45% during the lockdown compared to the same period in 2019 (16 vs 29, respectively), as well as a higher prevalence of severe stroke (NIHSS>10) at admission (n = 8, 50% vs n = 8, 28%). A dramatic prevalence of stroke of unknown symptom onset was observed in 2020 (n = 8, 50% vs n = 3, 10%). During lockdown, worse functional and independence outcomes were found, despite the similar proportion of reperfused patients. Similar ‘symptoms alert-to-admission’ and ‘door-to-treatment’ times were observed. During lockdown hospitalization was shorter and fewer patients completed the stroke work-up.
Conclusion
In conclusion, the adopted strategies for stroke management during the COVID-19 emergency have suggested being effective, while suffering a reduced and delayed reporting of symptoms. Therefore, we recommend raising awareness among the population against possible stroke symptoms onset. Thus, think F.A.S.T. and do not stay-at-home at all costs.
Keywords: Ischemic stroke, COVID, Coronavirus, Stroke unit, Outcome
1. Introduction
As a result of the ongoing pandemic of COVID-19, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), about one-third of the world population is currently living in a lockdown modality [1]. The first case in Italy was diagnosed on February 20th 2020 [2]. The infectious disease spread rapidly throughout northern Italy regions and afterwards the whole country, reaching 143′626 confirmed cases, with 18′279 deaths as of 9th April 2020 (http://www.salute.gov.it/portale/home.html). On the same date, Friuli Venezia Giulia (FVG), a cross-border region between Austria and Slovenia, showed 2′299 confirmed SARS-CoV-2 cases and 171 deaths (Fig. 1 ).
Fig. 1.
Epidemic outbreak in FVG region (data from Italian Ministry of Health daily official report, see http://www.salute.gov.it/portale/home.html). During the study period (9th March - 9th April 2020) a progressive increase of confirmed COVID-19 cases in FVG was observed, up to 2′299 total confirmed cases. Over the same period, there was a modest increase of hospitalized patients that reached a plateau in the first days of April. The same applied to the number of ICU patients. During the study period, 16 patients were admitted to the Stroke Unit, 9 of which as ‘stroke code’.
On 9th March 2020 the Italian government imposed a national quarantine, restricting the movement of the population except for necessity, work, and health circumstances. In many Italian regions, hospitals have been reorganized to properly manage COVID-19 patients, creating new protected wards for SARS-CoV-2 positive patients both for intensive and sub-intensive care, including reorganizing many Stroke Units [3]. The Giuliano-Isontina area of Friuli-Venezia Giulia region represents a peculiar community determined by a high prevalence of elderly with polymorbidities [4], and by an international cooperation program for stroke management between Italy and Slovenia.
2. Aims
To evaluate the effects of the COVID-19 pandemics on stroke management, this report described the emergency structured pathway adopted by an Italian University Hub Stroke Unit in the cross-border Italy-Slovenia area (which serves 373′803 people) (data from Istituto Nazionale di Statistica-ISTAT official report, 30th September 2017, see http://dati.istat.it/), and compared clinical features and outcomes of admitted patients between 9th March 2020 (start of Italy lockdown) and 9th April 2020 with stroke patients admitted during the same period in 2019.
3. Materials and methods
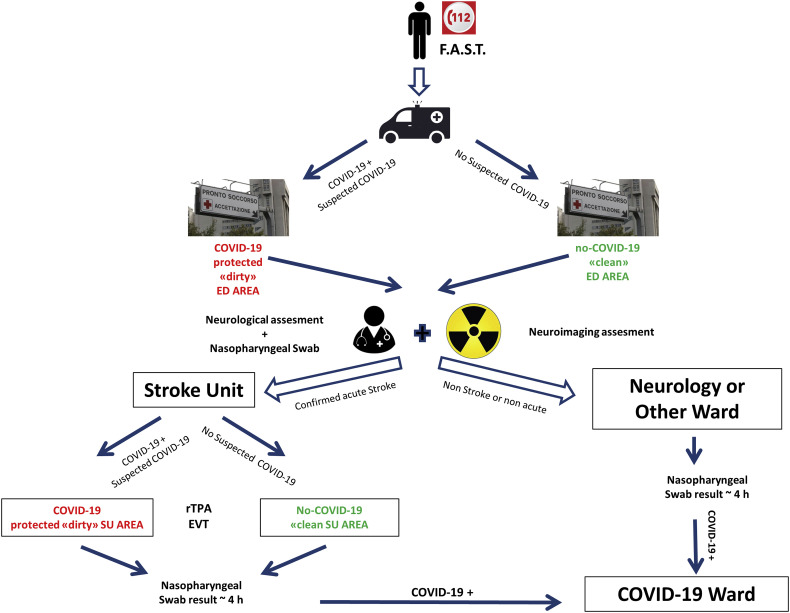
This retrospective study was conducted on patients admitted to the Hub Stroke Unit (8 monitored beds dedicated to acute stroke patients and 4 beds for sub-acute stroke phase management) of the University Medical Hospital of Trieste between 9th March 2020 (start of Italy lockdown) and 9th April 2020 (COVID-19 period) and the same period in 2019 (no-COVID-19 period). A standardized protocol for diagnosis and treatment of acute stroke during the COVID-19 pandemics was established between the Clinical Unit of Neurology, Neuroradiology, and EDs of the University Hospital of Trieste. The protocol is summarized in Fig. 2 .
Fig. 2.
Block diagram of protocol for acute stroke management during the COVID-19 pandemics in our hub university hospital. Following national and institutional regulations, all the patients and healthcare personnel were provided with personal protection equipment (PPE). All patients from the Giuliano-Isontina area with acute onset of neurological symptoms compatible with suspected cerebrovascular disease were transported to the Trieste University Hospital Emergency Department (ED) and the neurologist advice was immediately requested. The pre-hospital healthcare personnel identified possible COVID-19 positive cases if symptoms such as cough, fever, flu-like syndrome or dyspnea were present or reported. If the patient was suspected of being positive to SARS-CoV-2 infection, they were admitted to a specific protected ‘dirty ED area’ (separated from the ‘clean ED area’, for non-suspected COVID-19 patients) where neurological examination and urgent hematological tests were performed. In “code stroke” patients, Multimodal CT (including Non-enhanced CT, CT angiography of the supra-aortic and intracranial arteries, and - in the cases of ischemic strokes - whole brain volume CT Perfusion) was performed as usual. After neuroradiological examination in suspected COVID-19 positive patients, the CT-room and equipment were properly sanitized. Patients with diagnosis of definite or probable acute cerebrovascular disease were hospitalized in Stroke Unit where, similarly to the ED, ‘dirty’ and ‘clean’ areas were arranged.
In both areas, patients were treated with the usual standardized protocols. All patients admitted to ED with stroke symptoms performed nasopharyngeal swab during the assessment process. The median time from swab collection to examination results was 4 h. If COVID-19 diagnosis was confirmed, the patient was transferred to a protected intensive care unit (ICU) or other wards dedicated to COVID-19 for sub-acute care.
The study population was composed of consecutive patients of both sexes, above 18 years of age, with acute focal neurological symptoms compatible with acute stroke. We excluded patients with acute and sub-acute stroke admitted to other departments. Intravenous thrombolytic therapy (rtPA) (0.9 mg/kg) and endovascular thrombectomy (EVT) were administered following the international guidelines [5], with the support of computed tomography perfusion (CTP) for tissue-based selection. NIHSS evaluation was carried out at the time of presentation at the Stroke Unit by a vascular neurologist trained in performing NIHSS examination. For this report, demographic characteristics, clinical and functional features, pre-hospital and intrahospital management characteristics were included in the analysis. For a complete description, see Table 1 .
Table 1.
Participants' demographics, clinical, neuroimaging data and pre-hospital and intrahospital management characteristics of patients admitted in COVID-19 period versus no-COVID-19 period. Data are presented as medians (IQR) and frequencies.
| Personal characteristics | COVID-19 (2020) |
no-COVID-19 (2019) |
|---|---|---|
| (n = 16) | (n = 29) | |
| Age [y] | 77 (67–81) | 78 (70–85) |
| Female:Male | 10:6 | 17:12 |
| Final diagnosis | ||
| Ischemic stroke (%) | 14 (88%) | 23 (79%) |
| Haemorrhagic stroke (%) | 1 (6%) | 2 (7%) |
| TIA (%) | 1 (6%) | 4 (14%) |
| SUSO (%) | 8 (50%) | 3 (10%) |
| Code stroke (%) | 9 (56%) | 17 (59%) |
| rTPA alone (%) | 4 (25%) | 10 (35%) |
| rTPA + EVT (%) | 2 (13%) | 2 (7%) |
| EVT alone (%) | 0 (0%) | 1 (3%) |
| Timespan in treated patients | ||
| Alert – admission [min] | 128 (56–146) | 91 (46–165) |
| Door to needle [min] | 51 (41–58) | 57 (40–109) |
| Door to groin [min] | 81 (74–87) | 83 (70–99) |
| Neurorad. assessment | ||
| ASPECTS | 10 (8–10) | 10 (9–10) |
| Large vessel occlusion (%) | 4 (25%) | 7 (24%) |
| NIHSS at baseline | 10 (3–18) | 6 (3–11) |
| NIHSS > 10 (%) | 8 (50%) | 8 (28%) |
| NIHSS at discharge | 5 (1–17) | 1 (0–6) |
| Barthel index at baseline | 10 (0–100) | 90 (18–100) |
| Barthel index at discharge | 37 (7–100) | 95 (30–100) |
| mRS 0–2 anamnestic (%) | 16 (100%) | 29 (100%) |
| mRS 0–2 at discharge (%) | 4 (25%) | 14 (48%) |
| Intrahospital mortality (%) | 2 (12%) | 3 (10%) |
| Bamford classification (%) | ||
| TACI | 4 (27%) | 5 (19%) |
| PACI | 9 (60%) | 13 (48%) |
| LACI | 0 | 4 (15%) |
| POCI | 2 (13%) | 5 (18%) |
| TOAST (%) | ||
| Atherotrombotic | 1 (6%) | 3 (11%) |
| Small vessel | 0 | 4 (15%) |
| Cardioembolic | 7 (47%) | 9 (33%) |
| Cryptogenic | 7 (47%) | 10 (37%) |
| Other | 0 | 1 (4%) |
| Risk factors (%) | ||
| Hypertension | 15 (94%) | 22 (76%) |
| Diabetes | 6 (37%) | 8 (28%) |
| Dyslipidemia | 10 (62%) | 18 (62%) |
| Atrial Fibrillation | 7 (44%) | 10 (34%) |
| Ischemic cardiomyopathy | 2 (12%) | 6 (21%) |
| Infective complication (%) | 5 (33%) | 3 (10%) |
| Pneumonia | 3 (19%) | 2 (7%) |
| Antibiotic treatment | 5 (31%) | 4 (14%) |
| Lenght of hospitalization (%) | 13 (12–16) | 18 (11–24) |
| Rehab. treatment (%) | 7 (44%) | 15 (52%) |
| Admission – Rehab (day) | 3 (2–5) | 4 (2–4) |
| Number of advice | 1 (1–2) | 3 (2–3) |
| Complete stroke work–up (%) | 5 (31%) | 20 (69%) |
| Destination at discharge (%) | ||
| Home | 6 (43%) | 12 (46%) |
| Rehabilitation | 2 (15%) | 4 (15%) |
| Neuro spoke | 5 (35%) | 2 (8%) |
| Other | 1 (7%) | 8 (31%)) |
Notes: Participants' reported characteristics. Stroke of Unknown Symptoms Onset (SUSO), thrombolysis (rTPA) and thrombectomy (EVT), modified Rankin Scale (mRS). Results are summarized for patients admitted in our Stroke Unit in COVID-19 period (9 March - 9 April 2020) and in no-COVID-19 period (9 March - 9 April 2019). Bold values for significance value for intergroup comparison. (p < .05).
The study was conducted according to the principles of the Declaration of Helsinki. Approval for the study was obtained from the local ethics committee (CEUR FVG).
3.1. Statistical analysis
Subgroup analysis and data presentation was proposed for 2019 and 2020 patients, continuous variables were presented as medians (25th–75th percentile) and non-continuous variables as percentages. Differences between the two groups were tested with the appropriate nonparametric tests (namely, Mann-Whitney U test) and chi-square. A level of p < .05 was regarded as statistically significant.
4. Results
During the study period, 16 patients were admitted to the Stroke Unit compared to 29 who were admitted in the same period of 2019 (−45%). All patients admitted to our Stroke Unit performed nasopharyngeal swab. None of the patients was positive to SARS-CoV-2. Among these, no differences were present in terms of demographic characteristics and stroke subtypes. In general, a lower absolute number of ‘code stroke’ activations (9 vs 17) and rtPA treatments (6 vs 12) was found in 2020 compared to 2019. Despite similar alert-to-admission and door-to-treatment times, a higher prevalence of severe stroke (NIHSS>10) was found in 2020 (n = 8, 50%) compared to 2019 (n = 8, 28%), thus leading to worse functional outcomes. Intrahospital management and complications highlighted a shorter hospitalization with a faster commencement of physiatric consultancy and a higher absolute number of respiratory infections in 2020. A dramatic prevalence of stroke of unknown symptom onset (SUSO) was found in 2020 (n = 8, 50%) compared to 2019 (n = 3, 10%). A complete summary of these findings is reported in Table 1.
5. Discussion and conclusions
During the state of emergency, the attention of healthcare providers and health authorities is primarily focused on infected patients and the frontline responders. This had an impact also on other units dedicated to highly invalidant pathologies such as a stroke, which still is one of the leading causes of death and disability worldwide. The main finding is a reduction of 45% of total admissions in our Hub Stroke Unit compared to the same period in 2019 and a higher prevalence of severe stroke (NIHSS >10) at admission during the COVID-19 pandemics.
This may be explained with the lower number of ED admissions of transient ischemic attack (TIA) and minor strokes probably related to the diffused fear of going to the hospital during the pandemia when showing mild or transient stroke symptoms. A similar hypothesis was stated by Baracchini et al. [6]. In addition, in our study we also observed a higher prevalence of SUSO patients with high NIHSS, who may have underestimated their own symptoms and thus delayed the alert. The increased number of SUSOs supports the adoption of a multimodal neuroimaging tissue-based approach (instead of time-based approach) to better define patients eligible to reperfusion therapy [7]. Considering ‘code stroke’ patients, the comparison of prehospital time response (from alert-to-admission) and intrahospital time response (door-to-treatment) showed no significant differences between 2020 and 2019 periods, suggesting the efficacy of the structured pathways adopted by our local health system to treat stroke patients during the COVID-19 emergency.
The acute stroke emergency structured pathway for acute stroke, characterized by the suspicious case pre-alert, the presence of two well separated ED and Stroke Unit areas and the extensive and early use of the swabs, made it possible to offer reperfusion treatments in suitable times and protect staff and other inpatients from infection.
The worse functional and independence outcomes, despite the similar proportion of treated patients, may be related to the more severe clinical presentation and to a slightly faster time course to discharge. However, the proportion of patients discharged at home and intra-hospital mortality were similar between the two periods. The shorter hospitalization and the associated difficulties in performing all the diagnostic etiology exams (that may involve other specialists) resulted in a lower number of patients that were discharged with a complete stroke work-up (31% vs 69%). Tele-monitoring and tele-assistance solutions, using also smart wearable technologies, in this scenario could reduce this gap and assure patients security in the post-acute phase outside the hospital.
Particular attention during pandemics-related countries lockdown should be given to cross-border areas. Indeed, among 16 patients admitted in 2020, 2 of them were part of an international management protocol between Italy and Slovenia, having been cared both in a Slovenian spoke cross-border hospital and in our Hub Stroke Unit despite the strict mobility restrictions.
In conclusion, the adopted strategies for stroke management during the COVID-19 emergency have suggested being effective, while suffering a reduced and delayed reporting of symptoms. Therefore, we recommend raising awareness among the population against possible stroke symptoms onset.
Declaration of Competing Interest
The Authors declare that there is no conflict of interest.
Acknowledgements
The authors would like to thank Matteo di Franza for editorial assistance and english proof-reading. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Caso V., Federico A. No lockdown for neurological diseases during COVID19 pandemic infection. Neurol. Sci. 2020:1–3. doi: 10.1007/s10072-020-04389-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grasselli G., Pesenti A., Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA. 2020 doi: 10.1001/jama.2020.4031. [DOI] [PubMed] [Google Scholar]
- 3.Bersano A., Pantoni L. On being a neurologist in Italy at the time of the COVID-19 outbreak. Neurology. 2020 doi: 10.1212/WNL.0000000000009508. [DOI] [PubMed] [Google Scholar]
- 4.Marcon G., Manganotti P., Tettamanti M. Is Parkinson’s disease a very rare pathology in centenarians? A clinical study in a cohort of subjects. J. Alzheimers Dis. 2020;73:73–76. doi: 10.3233/JAD-190717. [DOI] [PubMed] [Google Scholar]
- 5.Powers W.J., Rabinstein A.A., Ackerson T. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49:e46–e110. doi: 10.1161/STR.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 6.Baracchini C., Pieroni A., Viaro F. Acute stroke management pathway during Coronavirus-19 pandemic. Neurol Sci. 2020:1–3. doi: 10.1007/s10072-020-04375-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Furlanis G., Ajčević M., Buoite Stella A. Wake-up stroke: thrombolysis reduces ischemic lesion volume and neurological deficit. J. Neurol. 2020;267:666–673. doi: 10.1007/s00415-019-09603-7. [DOI] [PubMed] [Google Scholar]