Abstract
Objective
To investigate the clinical features of COVID-19 cases in Suzhou China. Biomarkers were screened out of hematological parameters for risk stratification.
Method
Confirmed COVID-19 adult patients in Suzhou were included. The patient data was collected, and the results of laboratory examinations were compared between the mild/moderate and severe COVID-19 groups. A ROC was calculated to compare the diagnostic performance of candidate indexes, and dynamic levels of hematological indexes were compared between the two groups.
Result
75 patients were enrolled, with a mean age of 46.6 ± 14 years, and 45 patients were male. All patients were classified into two groups: the mild/moderate group and the severe group. WBC, neutrophil to lymphocyte ratio (NLR), D-dimer, and fibrinogen levels of the severe group were significantly higher (P < 0.05) than the mild/moderate, and the lymphocyte was lower. The ROC test showed that the hematological parameters had a larger AUC than that of inflammatory factors. There was a significant difference in lymphocyte and fibrinogen levels between the two groups on day 1 (P < 0.05). However, NLR of the severe group was higher than the mild/moderate on days 1, 4 and 14 (P < 0.01), and so was D-dimer on days 1, 7 and 14 (P < 0.05).
Conclusion
The common COVID-19 abnormal hematological indexes on admission included hyperfibrinogenemia, lymphopenia, the elevation of D-dimer, and leukopenia, which were significantly different between the mild/moderate and severe COVID-19 groups. Furthermore, the dynamic change of NLR and D-dimer level can distinguish severe COVID-19 cases from the mild/moderate.
Keywords: Coronavirus disease 2019, Neutrophil to lymphocyte ratio, D-dimer
Highlights
-
•
Most common symptoms of Suzhou COVID-19 are fever, cough and sore throat.
-
•
COVID-19 has characteristics of abnormal hematological indexes.
-
•
NLR and D-dimer can be used as valuable biomarkers for risk stratification of COVID-19.
1. Introduction
Since December 2019, an outbreak of cluster pneumonia of unknown cause happened in Wuhan, the capital city of Central China province, Hubei [1,2]. Subsequently, a novel coronavirus was isolated from patient groups in Wuhan and soon identified to be the causative pathogen of this highly contagious pneumonia [3,4]. In February 2020, the World Health Organization formally designated the disease COVID-19 (coronavirus disease 2019), and the novel coronavirus was designated severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). In two months, the outbreak of COVID-19 spread from Wuhan to all other districts of China. SARS-CoV-2 also caused many hospital transmissions and more than three thousand Chinese health workers were infected until March 15, 2020 [5]. On January 26, Wuhan city was locked down in an unprecedented manner with the majority of public transport cancelled. The WHO recently announced the current situation of COVID-19 as a global pandemic. Up to now there are no available specific curative medicines and vaccines, and the treatment methods for COVID-19 are largely supportive.
Several abnormal hematological parameters were reported in COVID-19 patients [[6], [7], [8], [9]], including lymphopenia, neutrophilia, elevated levels of D-dimer and fibrinogen, but the clinical implication of these indexes remains elusive. The neutrophil to lymphocyte ratio (NLR) is a convenient index that can be calculated from a complete blood count, and many studies showed that NLR had a prognostic value in various conditions, including sepsis, cardiovascular diseases, and malignant tumors, etc. [[10], [11], [12], [13]]. Increased thrombogenicity and higher platelet aggregation had been proven in community acquired pneumonia, and a recent study reported that COVID-19 can induce a massive prothrombotic status [14,15]. D-dimer was also found to correlate with the prognosis of 2009 novel influenza A (H1N1) pneumonia [16]. Herein we performed a retrospective study of COVID-19 patients in the designated hospital in Suzhou China, and the correlation between hematological parameters and different severity groups of COVID-19 was analyzed.
2. Methods
Consecutive adult patients (≥18 years old) with a confirmed diagnosis of COVID-19 admitted to the Affiliated Infectious Diseases Hospital of Soochow University from January 20 to February 20, 2020, were enrolled in this retrospective study. Many senior doctors from other medical centers in Suzhou were sent to this hospital to care COVID-19 patients. The diagnosis and classification of COVID-19 were based on the trial version 1–5 guidelines on the novel coronavirus-infected pneumonia diagnosis and treatment (issued by the National Health Commission of China). All patients were followed up till March 10. The study was approved by the hospital's Ethics Committee and the written informed consent was obtained from patients enrolled.
According to the severity of COVID-19, all patients were classified into two groups: the mild/moderate group, and the severe group. The mild/moderate group included mild and moderate cases. The severe group consisted of severe and critical cases which fulfilled one of these criteria as follows: 1) respiratory distress (RR ≥ 30 bpm); 2) oxygen saturation ≤ 93%; 3) arterial partial pressure of oxygen (PaO2)/fraction of inspired oxygen (FiO2) < 300 mm Hg; 4) patients with chest imaging that indicates an obvious progression of infiltrations within 24–48 h; 5) respiratory failure and requiring mechanical ventilation, shock or other organ failure need ICU support.
Every patient received a detailed investigation of clinical and epidemiological history. The laboratory tests on admission included complete blood count (CBC), coagulation profile, arterial blood gas analysis, blood biochemistry, myocardial biomarker and inflammation biomarker (C-reactive protein and procalcitonin). Serial peripheral hematological analyses and other blood test items were ordered based on the clinical condition of each patient. Patients who had full CBC results on days 1, 4, 7, 14 or full coagulation profile result on days 1, 7, 14 were selected for a further analysis.
The treatment protocol included oxygenation therapy for hypoxemia, antivirus medications, corticosteroids for patients with diffuse infiltration or rapid progression (1–2 mg/kg for 3–5 days) and antibiotics for secondary bacterial infection. The patient with signs of deterioration in the normal unit was considered to be transferred to ICU. The discharge criteria are as follows: body temperature back to normal at least for three days, and two consecutive negative results of SARS-CoV-2 RT-PCR test (sampling at least 24 hour interval), and an obvious absorption of lung infiltration proved by chest CT.
The statistical software SPSS version 21.0 for Windows (IBM, Armonk, NY, USA) was used for data analysis. All data are presented as means ± SD or median (range). Independent sample t-tests and chi-square tests were used to compare the differences between the two groups. The receiver operating curve (ROC) and the area under the curve (AUC) were calculated to compare the diagnostic performance of each parameter. A value of P < 0.05 was considered statistically significant.
3. Results
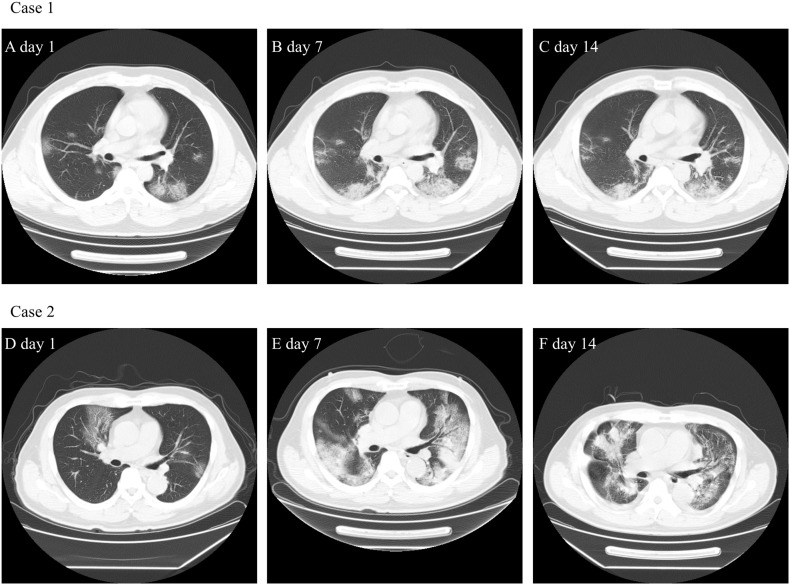
In this retrospective study, 75 confirmed COVID-19 patients were included until February 20, 2020. The mean age of patients was 46.6 ± 14 (22–77) years, and 45 patients (60%) were male (Table 1 ). The epidemiological survey found that 41 patients (54.7%) had a history of Hubei travel or residency. The common clinical manifestations of COVID-19 at admission were fever (62, 86.7%), cough (54, 72%) and sore throat (12, 16%), and other less common symptoms included myalgia, diarrhea, shortness of breath and rhinorrhea. Thirteen patients had underlying chronic diseases, including seven with hypertension, four with chronic respiratory disease, four with diabetes mellitus, and one with malignant tumor history. The median period from onset to admission was 5.6 ± 3.5 days (1–14). Sixteen patients were classified into the severe group and admitted to the special ICU ward, and 59 patients were classified into the mild/moderate group and admitted to the normal isolation ward. Most patients were reported with bilateral pneumonia proved by chest CT, and 84% positive findings of CT were characterized as multiple mottling and ground-grass opacity. Fig. 1 showed the representative pulmonary CT of two COVID-19 patients.
Table 1.
Demographic and clinical characteristic of COVID-19 patients in Suzhou.
| Patients (n = 75) | |
|---|---|
| Age (years) | 46.6 ± 14 (22–77) |
| Sex (male/female) | 45 (60%) |
| Exposure to Wuhan/Hubei | 41 (54.7%) |
| Medical chronic illness | 13 (17.3%) |
| Hypertension | 7 (9.3%) |
| Respiratory disease | 4 (5.3%) |
| Diabetes | 4 (5.3%) |
| Malignant tumor | 1 (1.3%) |
| Digestive system disease | 2 (2.7%) |
| Nervous system disease | 2 (2.7%) |
| Symptoms at admission | |
| Fever | 62 (82.7%) |
| Cough | 54 (72%) |
| Sore throat | 12 (16%) |
| Muscle ache | 6 (8%) |
| Diarrhea | 6 (8%) |
| Short of breath | 4 (5.3%) |
| Rhinorrhea | 4 (5.3%) |
| Days from onset to admission | 5.6 ± 3.5 (1–14) |
| Chest CT positive findings | 73 (4%) |
| Unilateral pneumonia | 16 (21.3%) |
| Bilateral pneumonia | 57 (76%) |
| Multiple mottling and ground-grass opacity | 63 (84%) |
| ICU admission | 16 (21.3%) |
| Severity classification | |
| Mild/moderate | 59 (78.7%) |
| Severe | 16 (21.3%) |
CT computed tomography, ICU intensive care unit.
Fig. 1.
The computed tomography of two COVID-19 patients.
Case 1. A 41-year-old man who had traveled to Wuhan. On January 29 he visited a fever clinic in Suzhou because of mild fever and dry cough. The RT-PCR nucleic acid for SARS-CoV-2 turned out positive and he was admitted to our center. Chest CT 1A, 1B, 1C showed multiple ground-glass opacity with mild progression of infiltrations on day 1, day 7 and day14.
Case 2. A 70-year-old man who was admitted to our ICU and received HFNC and NIV support. Chest CT showed lung lesions were multiple patchy ground glass shadows (2D), but on day 7 the lung infiltrations became diffuse and focal consolidation emerged (2E) and on day 14 mild absorption of lung lesions on CT can be seen (2F). His symptoms improved obviously and discharge on March 10.
On admission, obvious abnormalities of laboratory blood examinations were found, especially for peripheral blood cell analysis and coagulation profile (Table 2 ). Hemocytopenia was common, including lymphopenia, leukopenia, and thrombocytopenia (45.3%, 21.3%, and 12%, respectively). The rate of anemia was 8%. The neutrophil-to-lymphocyte ratio was 2.3 (0.6–16.0). The coagulation profile revealed an obvious elevation of fibrinogen (60%) and D-dimer (12%) despite a normal level of prothrombin time (PT) and partial thromboplastin time (APTT). Regarding the tests of the liver and renal function, PCT and NT-proBNP, most results were within the normal range. The elevation of the C-reactive protein level was seen in 58.7% of COVID-19 patients.
Table 2.
Laboratory results on admission and treatments of COVID-19 in Suzhou.
| Normal range | Patients (n = 75) | |
|---|---|---|
| White blood cell (109/L) | 3.5–9.5 | 5.12 (1.68–17.60) |
| Increased | 4 (5.3%) | |
| Decreased | 16 (21.3%) | |
| Lymphocyte (109/L) | 1.10–3.20 | 1.33 ± 0.63 |
| Increased | 0 | |
| Decreased | 34 (45.3%) | |
| Neutrophil (109/L) | 1.80–6.30 | 3.50 ± 2.21 |
| Increased | 4 (5.3%) | |
| Decreased | 6 (8%) | |
| NLR | 2.3 (0.6–16.0) | |
| Hemoglobin (g/L) | 130–175 | 138 ± 16 |
| Anemia (<120 g/L) | 6 (8%) | |
| Platelet (109/L) | 125–350 | 178.7 ± 66.0 |
| Increased | 1 (1.3%) | |
| Decreased | 9 (12%) | |
| Prothrombin time (s) | 10–14 | 12.2 ± 0.8 |
| Partial thromboplastin time (s) | 20–40 | 24.5 ± 3.3 |
| Fibrinogen (g/L) | 2.0–4.0 | 4.30 ± 1.19 |
| Increased | 45 (60%) | |
| Decreased | 0 | |
| D-dimer (media) | 0–550 | 210 (70–1220) |
| Increased | 9 (12%) | |
| Decreased | 0 | |
| Total bilirubin (μmol/L) | 4.0–17.1 | 9.6 (3.5–120.3) |
| Albumin (g/L) | 38–55 | 37.1 ± 4.3 |
| AST (U/L) | 8–40 | 27 (15–158) |
| ALT (U/L) | 5–40 | 40 (16–181) |
| Lactate dehydrogenase (U/L) | 135–225 | 464.5 ± 234.1 |
| Creatinine (μmol/L) | 44–106 | 64.5 ± 22.2 |
| NT-proBNP positivity (%) | <125 pg/mL | 8 (10.7%) |
| CRP positivity (%) | 0–10 mg/L | 44 (58.7%) |
| PCT positivity (%) | <0.046 ng/mL | 3 (4%) |
| Antivirus treatment | ||
| Arbidol | 42 (56%) | |
| Lopinavir/ritonavir | 46 (61.3%) | |
| Hydroxychloroquine | 6 (8%) | |
| Ribavirin | 66 (8%) | |
| Oseltamivir | 14 (18.7%) | |
| Antibiotics | 65 (86.7%) | |
| Corticosteroids | 25 (33.3%) | |
| Low molecular heparin | 16 (21.3%) | |
| Respiratory therapy | ||
| Nasal cava only | 68 (90.7%) | |
| High flow nasal cannula | 7 (9.3%) | |
| Noninvasive mechanical ventilation | 4 (5.3%) |
NLR neutrophil-to-lymphocyte ratio, AST aspartate aminotransferase, ALT alanine aminotransferase, NT-proBNP N-terminal proBNP, PCT procalcitonin.
The treatment protocol of COVID-19 in our center consisted of pharmacotherapy and respiratory support modalities. The pharmacotherapy mainly included antivirus medications, antibiotics, corticosteroids, and anticoagulation. Antivirus medications included lopinavir/ritonavir (46, 61.3%), arbidol (42, 56%), and other agents such as ribavirin, hydroxychloroquine and oseltamivir were less prescribed. Most patients (86.7%) were treated with antibiotics, and 25 patients received moderate-dose Methylprednisolone for a short period. For 16 hyperfibrinogenemia patients combined with an elevated risk of thrombosis, low molecular heparin was used to prevent potential venous deep thrombosis (DVT). The respiratory symptoms of most COVID-19 patients were mild, and 68 patients (90.7%) were given oxygen therapy by nasal cava only. Seven patients received HFNC (high flow nasal cannula) and four patients underwent NIV (noninvasive mechanical ventilation), and all 75 patients recovered and were discharged from the hospital.
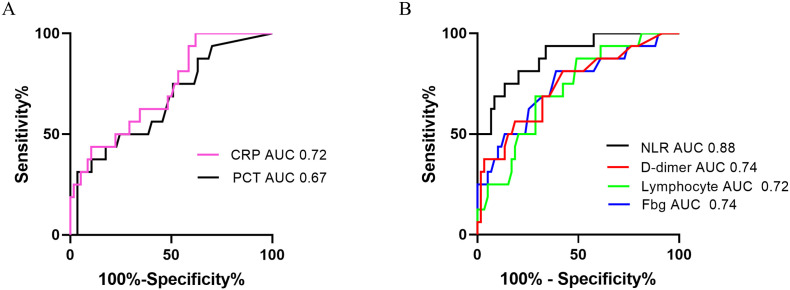
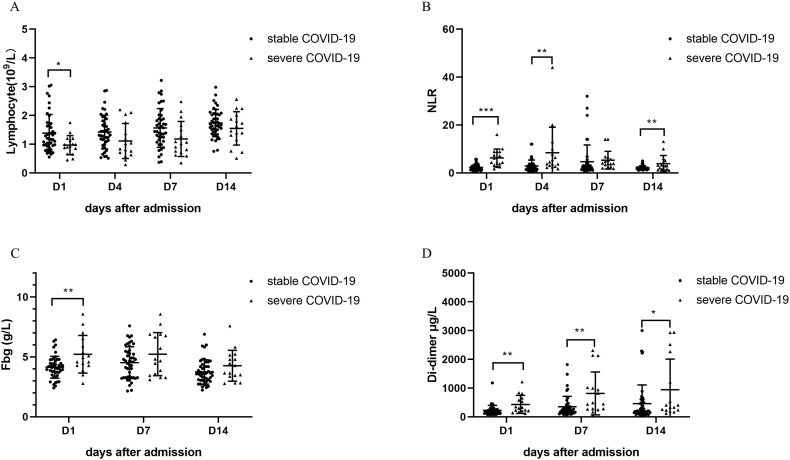
The demographic and hematological parameters on admission were compared between patients in the mild/moderate and severe groups (Table 3 ). There was no statistical difference in age, gender, days from onset to admission and hemoglobin level between the two groups. However, compared to the mild/moderate COVID-19 group, WBC, NLR, D-dimer, and fibrinogen levels of severe group were significantly higher (P < 0.05), and the lymphocyte level was lower (P < 0.01). The ROC and AUC of hematological and inflammatory indexes were calculated between the two groups (Fig. 2 ). Of the six indexes, the highest AUC is NLR (0.88), and the AUC of D-dimer and fibrinogen was 0.74, and those of lymphocyte and PCT were 0.72 and 0.67 respectively. Four hematological indexes (lymphocyte, NLR, fibrinogen, and D-dimer) were compared between the two groups on different hospitalization days. As seen in Fig. 3 , lymphocyte and fibrinogen levels between the two groups had a significant difference on day 1 (P < 0.05), but the difference disappeared over time. However, NLR distinguished the severe COVID-19 cases from the mild/moderate on days 1, 4 and 14 (P < 0.01), and so did D-dimer on days 1, 7 and 14 (P < 0.05).
Table 3.
The correlation of hematological parameters and inflammatory factors on admission with the severity of COVID-19 in Suzhou.
| Severe group (n = 16) | Mild/moderate group (n = 59) | P value | |
|---|---|---|---|
| Age (years) | 51.8 ± 12.8 | 45.1 ± 14.0 | 0.095 |
| Gender (male/female) | 10/6 | 35/24 | 0.818 |
| Days from onset to admission | 6.1 ± 3.2 | 5.5 ± 3.5 | 0.531 |
| Chronic illness (%) | 4 | 10 | 0.481 |
| WBC count (109/L) | 7.14 ± 3.61 | 4.87 ± 1.70 | 0.026⁎ |
| Neutrophil count (109/L) | 5.63 ± 3.50 | 2.92 ± 1.21 | 0.008⁎⁎ |
| Increased | 4 (25%) | 0 | |
| Decreased | 0 | 6 (10.1%) | |
| Lymphocyte level (109/L) | 0.97 ± 0.33 | 1.42 ± 0.66 | 0.009⁎⁎ |
| Decreased (0.6–1.1) | 11 (68.8%) | 24 (40.7%) | 0.046⁎ |
| NLR | 6.29 ± 3.72 | 2.33 ± 1.22 | 0.001⁎⁎ |
| Hemoglobulin (%) | 135.9 ± 16.5 | 138.6 ± 16.0 | 0.548 |
| Fibrinogen level (g/L) | 1.57 ± 0.39 | 0.94 ± 0.12 | 0.010⁎ |
| D-dimer level (μg/L) | 315 (70–1220) | 190 (70–1180) | 0.001⁎⁎ |
| CRP (mg/L) | 39.0 (2.6–200) | 11.3 (0–59.4) | 0.001⁎⁎ |
| PCT (ng/ml) | 0.032 (0–4.77) | 0.046 (0–0.89) | 0.843 |
WBC white blood cell, CRP C-reactive protein, PCT procalcitonin.
P < 0.05.
P < 0.01.
Fig. 2.
The receiver operation curve of inflammatory indexes and hematological parameters for disease severity classification of COVID-19.
A. ROC analysis of C-reactive protein and procalcitonin for discriminating 16 severe COVID-19 cases from 59 mild/moderate cases. B. ROC analysis of peripheral hematological parameters (lymphocyte, NLR, fibrinogen and D-dimer) analysis for discriminating 16 severe cases from 59 mild/moderate cases.
Fig. 3.
Dynamic change of peripheral blood cell indexes and coagulation factors between severe and mild/moderate patient group.
A. Lymphocyte level on four different days (1, 4, 7, 14) between the mild/moderate and severe patient group. B. Neutrophil-to-lymphocyte ratio (NLR) on four different days (1, 4, 7, 14) between the mild/moderate and severe patient group. C. Fibrinogen level on three different days (1, 7, 14) between the mild/moderate and severe patient group. D. D-dimer level on three different days (1, 7, 14) between the mild/moderate and severe patient group.
4. Discussion
From the beginning of COVID-19 outbreak, Wuhan and surrounding districts of Hubei became the center of the epidemic, and the number of confirmed cases increased from 41 to >80,000 and caused about 3200 deaths in China as of March 15. The early clinical studies mainly focused on COVID-19 cases in Wuhan, and part of patients included in studies were during their hospitalization [6,7,17]. It is essential to conclude the feature of COVID-19 early to fight against it. However, some limitations of these studies also exist. To explore features of COVID-19 patients with complete hospitalization and outside Hubei province, we enrolled adult COVID-19 patients discharged in Suzhou, one city in East China with an 11-million population.
During the past twenty years, there had been two coronavirus-caused serious epidemics, Severe Acute Respiratory Syndrome (SARS, 2003) and Middle East Respiratory Syndrome (MERS, 2014) [18]. The newly identified SARS-CoV-2 belongs to the betacoronavirus genus and shares >85% homology with bat SARS-like coronavirus. Furthermore, the receptor-binding gene region structure of SARS-CoV-2 resembles to that of SARS-CoV. After isolated and cultured in vitro, SARS-CoV-2 can be detected in human respiratory epithelial cells in 96 h. SARS-CoV-2 can bind the angiotensin-converting enzyme 2 (ACE2) for cell invasion as SARS-CoV [3].
The common symptoms of Suzhou COVID-19 cohort were fever (82.7%), cough (73.1%) and sore throat (26%), 13% of patients had an underlying medical chronic illness. 76% of patients with a bilateral ground-glass opacity on CT. In our study, 16 patients (21.3%) were classified as severe cases and admitted to ICU. Of them, seven received HFNC while four received NIV therapy and no patient died. This is in agreement with early studies that most COVID-19 cases had a mild clinical course and recovered in two weeks with supportive therapy, but the rest 15%–20% cases will develop severe symptoms of pneumonia and even progress to multiple organ failure which will warrant mechanical ventilation or intensive care [19]. It is crucial to discriminate severe COVID-19 cases because early intervention can make a different outcome.
A recent finding from three COVID-19 patients by minimally invasive autopsies revealed valuable pathological characteristics [20]. Besides severe lung lesions, the prominent derangement of the lymphohematopoietic system was noted. Decreased lymphocytes, cell degeneration, and necrosis were observed in spleen, and scarcity of lymphocytes and focal necrosis were observed in lymph nodes. CD4+ and CD8+ immunohistochemistry confirmed decreased T cells in the spleen and lymph nodes. Impaired myelopoiesis was also proved in the bone marrow. Another important study reported that SARS-CoV-2 nucleic acid can be readily detected in blood stream of 10.5% COVID-19 patients, and there was a strong correlation of serum viral RNA load in blood with the disease severity [21]. The systematic inflammatory response, especially cytokine release syndrome which happened in some COVID-19 patients, usually involved various degrees of blood cell component abnormalities and coagulation factor activation. Huang et al. found an elevated level of NLR in community-acquired pneumonia which correlated with disease severity [22]. Serum D-dimer level was found to have a prognostic value in community-acquired pneumonia and 2009 novel influenza A(H1N1) [16,23].
Our study aimed to find meaningful COVID-19 biomarkers out of conventional hematological examinations for disease severity classification and early warning of progression. We compared the level of inflammatory factors and hematological parameters between two different groups of disease severity (Table 3), and six candidate indexes (CRP, PCT, lymphocyte, NLR, fibrinogen, and D-dimer) were chosen for ROC test. As seen in Fig. 2, the hematological parameters had a higher AUC than inflammatory factors (NLR 0.88, D-dimer 0.74, fibrinogen 0.74, lymphocyte 0.72 vs C-reactive protein 0.72, PCT 0.67). We then evaluated the diagnostic performance of four hematological parameters on different days after admission. The lymphocyte and fibrinogen levels can discriminate the two COVID-19 groups of different severity only on admission. In contrast, NLR and D-dimer levels showed superior performance not only on admission but also on subsequent different days after admission.
Two main limitations must be acknowledged. First, as a retrospective cohort study in a single center, there were only 75 COVID-19 patients included and most of them were exported cases. A multiple-center, prospective research is needed to further clarify the value of D-dimer and NLR in COVID-19. Second, we did not test cytokines like interleukin-2 receptor (IL-2R) and interleukin-6 (IL-6) because of a shortage of biosafety labs. However, the benefit of routine cytokines monitor is still of controversy. We believed that ideal biomarkers for COVID-19 should be easily accessible, inexpensive and correct. Tests or techniques that incur unnecessary exposure need careful consideration of risk and benefit.
In summary, our results indicated that the epidemiological and clinical feature of COVID-19 cases in Suzhou is similar to previous reports. The common abnormalities in hematological parameters in COVID-19 patients included hyperfibrinogenemia (60%), lymphocytopenia (45.3%), leukopenia (21.3%), while thrombocytopenia (12%) and anemia (8%) was less common. There was a significant difference concerning four hematological parameters and CRP between the mild/moderate and severe COVID-19 groups. The dynamic change of neutrophil to lymphocyte ratio (NLR) and D-dimer level can discriminate severe COVID-19 cases from mild/moderate ones on consequent days after admission.
Declaration of competing interest
The authors have no conflict of interest to disclose.
Acknowledgements
This study is supported by National Natural Science Foundation of China (81730003) and National Science and Technology Major Project (2017ZX09304021).
Contributor Information
Depei Wu, Email: wudepei@suda.edu.cn.
Xin Yu, Email: yuxinsuzhou@163.com.
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson R. Pandemic potential of 2019-nCoV. Lancet Infect Dis. 2020;20(3):280. doi: 10.1016/S1473-3099(20)30068-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., Ren R., Leung K.S.M., Lau E.H.Y., Wong J.Y. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. New Engl J Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in ChinaZhonghua Liu Xing Bing Xue Za Zhi. 2020;41(2):145–151. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Guan W., Ni Z., Hu Y., Liang W., Ou C., He J., Liu L., Shan H., Lei C., Hui D.S.C. Clinical characteristics of coronavirus disease 2019 in China. New Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fan B.E., Chong V., Chan S., Lim G.H., Lim K., Tan G.B., Mucheli S.S., Kuperan P., Ong K.H. Hematologic parameters in patients with COVID-19 infection. Am J Hematol. 2020 doi: 10.1002/ajh.25774. [DOI] [PubMed] [Google Scholar]
- 10.Russell C.D., Parajuli A., Gale H.J., Bulteel N.S., Schuetz P., de Jager C., Loonen A., Merekoulias G.I., Baillie J.K. The utility of peripheral blood leucocyte ratios as biomarkers in infectious diseases: a systematic review and meta-analysis. J Infect. 2019;78(5):339–348. doi: 10.1016/j.jinf.2019.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huguet E., Maccallini G., Pardini P., Hidalgo M., Obregon S., Botto F., Koretzky M., Nilsson P.M., Ferdinand K., Kotliar C. Reference values for neutrophil to lymphocyte ratio (NLR), a biomarker of cardiovascular risk, according to age and sex in a latin american population. Curr Probl Cardiol. 2019;100422 doi: 10.1016/j.cpcardiol.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Park J.S., Seo K.W., Choi B.J., Choi S.Y., Yoon M.H., Hwang G.S., Tahk S.J., Shin J.H. Importance of prognostic value of neutrophil to lymphocyte ratio in patients with ST-elevation myocardial infarction. Medicine (Baltimore) 2018;97(48) doi: 10.1097/MD.0000000000013471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mei Z., Shi L., Wang B., Yang J., Xiao Z., Du P., Wang Q., Yang W. Prognostic role of pretreatment blood neutrophil-to-lymphocyte ratio in advanced cancer survivors: a systematic review and meta-analysis of 66 cohort studies. Cancer Treat Rev. 2017;58:1–13. doi: 10.1016/j.ctrv.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Santos-Gallego C.G., Badimon J.J. The sum of two evils: pneumonia and myocardial infarction: is platelet activation the missing link? J Am Coll Cardiol. 2014;64(18):1926–1928. doi: 10.1016/j.jacc.2014.08.023. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y., Xiao M., Zhang S., Xia P., Cao W., Jiang W., Chen H., Ding X., Zhao H., Zhang H. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. New Engl J Med. 2020;382(17) doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Z.F., Su F., Lin X.J., Dai B., Kong L.F., Zhao H.W., Kang J. Serum D-dimer changes and prognostic implication in 2009 novel influenza A(H1N1) Thromb Res. 2011;127(3):198–201. doi: 10.1016/j.thromres.2010.11.032. [DOI] [PubMed] [Google Scholar]
- 17.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hui D., Zumla A. Severe acute respiratory syndrome: historical, epidemiologic, and clinical features. Infect Dis Clin N Am. 2019;33:869–889. doi: 10.1016/j.idc.2019.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., Huang H., Zhang L., Zhou X., Du C. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. Jama Intern Med. 2020 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yao X.H., Li T.Y., He Z.C., Ping Y.F., Liu H.W., Yu S.C., Mou H.M., Wang L.H., Zhang H.R., Fu W.J. A pathological report of three COVID-19 cases by minimally invasive autopsies. Zhonghua Bing Li Xue Za Zhi. 2020;49(0) doi: 10.3760/cma.j.cn112151-20200312-00193. [DOI] [PubMed] [Google Scholar]
- 21.Chen W., Lan Y., Yuan X., Deng X., Li Y., Cai X., Li L., He R., Tan Y., Deng X. Detectable 2019-nCoV viral RNA in blood is a strong indicator for the further clinical severity. Emerg Microbes Infect. 2020;9(1):469–473. doi: 10.1080/22221751.2020.1732837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang Y., Liu A., Liang L., Jiang J., Luo H., Deng W., Lin G., Wu M., Li T., Jiang Y. Diagnostic value of blood parameters for community-acquired pneumonia. Int Immunopharmacol. 2018;64:10–15. doi: 10.1016/j.intimp.2018.08.022. [DOI] [PubMed] [Google Scholar]
- 23.Karakioulaki M., Stolz D. Biomarkers in pneumonia-beyond procalcitonin. Int J Mol Sci. 2019;20(8) doi: 10.3390/ijms20082004. [DOI] [PMC free article] [PubMed] [Google Scholar]