Abstract
Background
Mitochondrial respiratory chain disorders are the most prevalent group of inherited neurometabolic diseases. They present with central and peripheral neurological features usually in association with other organ involvement including the eye, the heart, the liver, and kidneys, diabetes mellitus and sensorineural deafness. Current treatment is largely supportive and the disorders progress relentlessly causing significant morbidity and premature death. Vitamin supplements, pharmacological agents and exercise therapy have been used in isolated cases and small clinical trials, but the efficacy of these interventions is unclear. The first review was carried out in 2003, and identified six clinical trials. This major update was carried out to identify new studies and grade the original studies for potential bias in accordance with revised Cochrane Collaboration guidelines.
Objectives
To determine whether there is objective evidence to support the use of current treatments for mitochondrial disease.
Search methods
We searched the Cochrane Neuromuscular Disease Group Specialized Register (4 July 2011), CENTRAL (2011, Issue 2, MEDLINE (1966 to July 2011), and EMBASE (January 1980 to July 2011), and contacted experts in the field.
Selection criteria
We included randomised controlled trials (including cross‐over studies). Two of the authors independently selected abstracts for further detailed review. Further review was performed independently by all five authors to decide which trials fit the inclusion criteria and graded risk of bias. Participants included males and females of any age with a confirmed diagnosis of mitochondrial disease based upon muscle histochemistry, respiratory chain complex analysis of tissues or cell lines or DNA studies. Interventions included any pharmacological agent, dietary modification, nutritional supplement, exercise therapy or other treatment. The review authors excluded studies at high risk of bias in any category. The primary outcome measures included an change in muscle strength and/or endurance, or neurological clinical features. Secondary outcome measures included quality of life assessments, biochemical markers of disease and negative outcomes.
Data collection and analysis
Two of the authors (GP and PFC) independently identified studies for further evaluation from all abstracts within the search period. For those studies identified for further review, all five authors then independently assessed which studies met the entry criteria. For the included studies, we extracted details of the number of randomised participants, treatment, study design, study category, allocation concealment and other risk of bias criteria, and participant characteristics. Analysis was based on intention‐to‐treat data. We planned to use meta‐analysis, but this did not prove necessary.
Main results
The authors reviewed 1335 abstracts, and from these identified 21 potentially eligible abstracts. Upon detailed review, 12 studies fulfilled the entry criteria. Of these, eight were new studies that had been published since the previous version of this review. Two studies which were included in the previous version of this review were excluded because of potential for bias. The comparability of the included studies is extremely low because of differences in the specific diseases studied, differences in the therapeutic agents used, dosage, study design, and outcomes. The methodological quality of included studies was generally high, although risk of bias was unclear in random sequence generation and allocation concealment for most studies. Otherwise, the risk of bias was low for most studies in the other categories. Serious adverse events were uncommon, except for peripheral nerve toxicity in a long‐term trial of dichloroacetate (DCA) in adults.
One trial studied high‐dose coenzyme Q10 without clinically meaningful improvement (although there were multiple biochemical, physiologic, and neuroimaging outcomes, in 30 participants). Three trials used creatine monohydrate alone, with one reporting evidence of improved measures of muscle strength and post‐exercise lactate, but the other two reported no benefit (total of 38 participants). One trial studied the effects of a combination of coenzyme Q10, creatine monohydrate, and lipoic acid and reported a statistically significant improvement in biochemical markers and peak ankle dorsiflexion strength, but overall no clinical improvement in 16 participants. Five trials studied the effects of DCA: three trials in children showed a statistically significant improvement in secondary outcome measures of mitochondrial metabolism (venous lactate in three trials, and magnetic resonance spectroscopy (MRS) in one trial; total of 63 participants). One trial of short‐term DCA in adults demonstrated no clinically relevant improvement (improved venous lactate but no change in physiologic, imaging, or questionnaire findings, in eight participants). One longer‐term DCA trial in adults was terminated prematurely due to peripheral nerve toxicity without clinical benefit (assessments included the GATE score, venous lactate and MRS, in 30 participants). One trial using dimethylglycine showed no significant effect (measurements of venous lactate and oxygen consumption (VO2) in five participants). One trial using a whey‐based supplement showed statistically significant improvement in markers of free radical reducing capacity but no clinical benefit (assessments included the Short Form 36 Health Survey (SF‐36) questionnaire and UK Medical Research Council (MRC) muscle strength, in 13 participants).
Authors' conclusions
Despite identifying eight new trials there is currently no clear evidence supporting the use of any intervention in mitochondrial disorders. Further research is needed to establish the role of a wide range of therapeutic approaches. We suggest further research should identify novel agents to be tested in homogeneous study populations with clinically relevant primary endpoints.
Keywords: Humans, Creatine, Creatine/therapeutic use, Dichloroacetic Acid, Dichloroacetic Acid/therapeutic use, Mitochondrial Diseases, Mitochondrial Diseases/drug therapy, Randomized Controlled Trials as Topic, Sarcosine, Sarcosine/analogs & derivatives, Sarcosine/therapeutic use, Thioctic Acid, Thioctic Acid/therapeutic use, Ubiquinone, Ubiquinone/analogs & derivatives, Ubiquinone/therapeutic use
Plain language summary
Treatment for mitochondrial disorders
Mitochondria are found within every human cell and are responsible for the majority of each cell's energy production. When the mitochondria do not function properly, they cause diseases affecting many of the body's organs. Usually, these are the organs with the highest energy needs, such as muscle, brain, the eyes, and heart, although these diseases are highly variable. As a group these conditions are referred to as mitochondrial disorders, and they can cause significant disability or early death. We conducted this review of treatment for mitochondrial disorders to determine whether any available treatments are effective. We identified 12 randomised controlled trials that were of high enough quality to be included in the review. Of these, eight were new studies that had been published since the previous version of this review. Two studies which were included in the previous version of this review were excluded because of a high risk that the study results may be biased.
The included studies are not easily comparable because of differences in the treatment being studied, the dosage of these treatments, the length of study (and other differences in the study methods), and differences in the types of participants included for the research. The studies were generally well designed in order to reduce the possibility of bias, although for most studies the methods describing how participants were randomised were not described in detail. Otherwise, the risk of bias was low for most studies in the other categories. Serious side effects of treatment were uncommon, except for nerve damage in a long‐term trial of dichloroacetate in adults.
One trial studied high‐dose coenzyme Q10 treatment. This treatment had no clinical benefit. Three trials used creatine monohydrate: one trial reported improved muscle strength and biochemical measurements, but the other two trials reported no benefit (total of 38 participants). One trial studied the effects of a combination of coenzyme Q10, creatine monohydrate and lipoic acid, and reported a statistically significant improvement in biochemical measurements and ankle strength, but no clinical improvement (16 participants). Five trials studied the effects of dichloroacetate: three trials in children showed a statistically significant improvement in biochemical measurements but no clinical benefit on physiological measurements and exercise tests (total 63 participants); one trial of short‐term therapy in adults demonstrated no clinical improvement in physiological measurements (total eight participants), and one longer‐term trial in adults was terminated prematurely due to adverse effects without clinical benefit (using a combined scale of treatment effect, in 30 participants). One trial using dimethylglycine showed no significant effect on biochemical markers in five participants. One trial using a whey‐based supplement demonstrated statistically significant improvement in biochemical markers but no clinical benefit in muscle strength or on health questionnaires (13 participants).
Further randomised controlled trials of high quality are needed. They should strictly address outcomes which are relevant to patient care and quality of life, and study these in particular subtypes of mitochondrial disease at a time. The current repertoire of nutritional supplements have been not shown to be effective and future trials should study new treatments.
Background
General introduction
Mitochondria are responsible for converting food into energy within human cells. There are a number of genetically determined abnormalities of mitochondria that cause human diseases. These diseases usually involve organs that are heavily dependent upon the energy produced by mitochondria such as the brain, peripheral nerves, limb muscles, heart and hormone‐producing glands. As a result, mitochondrial disorders can cause muscle weakness on its own, but this is often associated with neurological, heart and hormone problems including diabetes. There is currently no established treatment for mitochondrial disorders, but there have been a number of case reports and small trials describing the positive effects of a number of different drugs, vitamins and food supplements. Exercise therapy has also been shown to help with the muscle symptoms. The purpose of this review was to assess objectively the available evidence for the various treatments that have been tried in mitochondrial myopathy and mitochondrial encephalomyopathy. Leber hereditary optic neuropathy (LHON) is also a primary mitochondrial disorder that usually just affects the eye. This disease has also been included in this study.
Mitochondria and human disease
Mitochondrial disorders are a diverse group of conditions that often involve the nervous system, are usually progressive, and often cause significant disability and premature death (Schapira 2006; DiMauro 2003). Based upon recent epidemiological studies, mitochondrial disorders affect at least 1 in 8000 of the general population (Schaefer 2008).
Mitochondrial function and biogenesis
Mitochondria are complex ubiquitous intracellular organelles that perform an essential role in a number of cellular processes (Wallace 1999). They contain enzymes involved in cellular metabolism, and are involved in many cell death pathways. It is therefore possible that mitochondria play a central role in many disease processes. Mitochondria also play a pivotal role in the final common pathway of aerobic metabolism ‐ oxidative phosphorylation (OXPHOS). Oxidative phosphorylation is carried out by the mitochondrial respiratory chain, which is a group of five multi‐subunit enzyme complexes situated on the inner mitochondrial membrane that generate adenosine triphosphate (ATP) from intermediary metabolites. Adenosine triphosphate is a high‐energy phosphate molecule that provides an energy source for all active cellular processes. The term 'mitochondrial disorders' usually refers to primary disorders of the mitochondrial respiratory chain.
Molecular pathology of mitochondrial disorders
The respiratory chain has a dual genetic basis (DiMauro 1998). The vast majority of the respiratory chain subunits (more than 70) are the products of nuclear genes. These subunits are synthesised within the cytosol and are delivered into mitochondria by a peptide targeting sequence. By contrast, 13 essential respiratory chain subunits are synthesised within the mitochondrial matrix from small 16.5 kb circles of double‐stranded DNA called mitochondrial DNA (mtDNA) (Anderson 1981). MtDNA is different to nuclear DNA in a number of respects. First, there are many thousands of copies of mtDNA within each cell. MtDNA mutations may only affect a proportion of the mtDNA molecules, leading to a mixture of mutant and wild‐type mtDNA within the cell (heteroplasmy) (Holt 1988). Single cell studies have shown that the proportion of mutant mtDNA must exceed a critical threshold level before the cell expresses a biochemical defect of the mitochondrial respiratory chain (Schon 1997). This threshold varies from tissue to tissue, and partly explains the tissue‐selectivity seen in mitochondrial disorders (Wallace 1994). The percentage level of mutant mtDNA can also vary between and within individuals harbouring a pathogenic mtDNA defect, and this partly explains the clinical variability that is a hallmark of mtDNA disorders (Macmillan 1993). In the last decade it has become clear that nuclear gene defects are a major cause of mitochondrial disease, affecting the assembly and structure of the respiratory chain protein complexes or other components of the respiratory chain, such as coenzyme Q10. Nuclear gene defects can also affect the maintenance of mtDNA, leading to secondary mitochondrial DNA mutations which accumulate during life; or they can affect the translation of the mitochondrial genome itself (Spinazzola 2009). Some of these disorders have a distinct clinical phenotype, but others cause an overlapping spectrum of phenotypes similar to primary disorders of mtDNA (Zhu 2009).
Clinical features of mitochondrial diseases
Mitochondrial disorders principally affect tissues that are heavily dependent upon oxidative metabolism. These tissues include the central nervous system, peripheral nerves, eye, skeletal and cardiac muscle, and endocrine organs. Many individuals with mitochondrial diseases have a multi‐system disorder that often involves skeletal muscle and the central nervous system, but some individuals have a disorder that only affects one organ system (DiMauro 2001; Leonard 2000). In general terms, the clinical features of mitochondrial disease can be divided into two groups: central neurological features (including encephalopathy, stroke‐like episodes, seizures, dementia and ataxia), and peripheral neurological features (including myopathy, ophthalmoplegia, and peripheral neuropathy). Some individuals have a mixture of central and peripheral features, whereas others have a pure central or peripheral phenotype.
Many individuals with mitochondrial disease have a clearly defined clinical phenotype (summarised in Table 1: Clinical syndromes associated with mitochondrial disease). Chronic progressive external ophthalmoplegia (CPEO), the Kearns‐Sayre syndrome (KSS) and Pearson syndrome are usually due to a deletion of mtDNA (Moraes 1989; Zeviani 1988). Leber hereditary optic neuropathy (LHON), mitochondrial encephalomyopathy with lactic acidosis and stroke‐like episodes (MELAS), myoclonic epilepsy with ragged‐red fibres (MERRF), maternally inherited diabetes and deafness (MIDD), and neurogenic (or neuropathy) ataxia with retinitis pigmentosa (NARP) are usually due to point mutations of mtDNA (Lamantea 2002). Unlike nuclear DNA, mtDNA is inherited down the maternal line, so these disorders either affect sporadic cases or they are passed from mother to child (Chinnery 1998). Children presenting with a relapsing encephalopathy with prominent brain stem signs and lactic acidosis (Leigh syndrome) may have a mtDNA defect, or an underlying nuclear genetic defect causing a respiratory chain deficiency. These mutations can affect the genes that code for the subunits themselves (complexes I and II), genes important for the assembly of an intact respiratory chain (complexes III and IV), or genes involved in mitochondrial transcription or translation, and they are usually autosomal recessive (Tucker 2010). Mutations in the gene LPPRC cause a specific form of infantile COX deficiency found in the Saguenay‐Lac‐Saint‐Jean region of Canada (Mootha 2003).
1. Clinical syndromes associated with mitochondrial disease.
| Clinical syndrome | Primary features | Additional features |
| Chronic progressive external ophthalmoplegia (CPEO) | External ophthalmoplegia and bilateral ptosis | Mild proximal myopathy |
| Infantile myopathy and lactic acidosis (fatal and non‐fatal forms) | Hypotonia in the first year of life. Feeding and respiratory difficulties | Fatal form may be associated with a cardiomyopathy and/or the Toni‐Fanconi‐Debre syndrome |
| Kearns‐Sayre syndrome (KSS) | CPEO onset before age 20 with pigmentary retinopathy Plus one of the following: CSF protein greater than 1 g/l, cerebellar ataxia, heart block | Bilateral deafness Myopathy Dysphagia Diabetes mellitus and hypoparathyroidism Dementia |
| Leber hereditary optic neuropathy (LHON) | Subacute painless bilateral visual failure Males: females approx. 4:1 Median age of onset 24 years | Dystonia Cardiac pre‐excitation syndromes |
| Leigh syndrome (LS) | Subacute relapsing encephalopathy with cerebellar and brain‐stem signs presenting during infancy | Basal ganglia lucencies |
| Mitochondrial encephalomyopathy with lactic acidosis and stroke‐like episodes (MELAS) | Stroke‐like episodes before age 40 years Seizures and/or dementia Ragged‐red fibres and/or lactic acidosis | Diabetes mellitus Cardiomyopathy (hypertrophic leading to dilated) Bilateral deafness Pigmentary retinopathy Cerebellar ataxia |
| Myoclonic epilepsy with ragged‐red fibres (MERRF) | Myoclonus Seizures Cerebellar ataxia Myopathy | Dementia, optic atrophy Bilateral deafness Peripheral neuropathy Spasticity Multiple lipomata |
| Neurogenic weakness with ataxia and retinitis pigmentosa (NARP) | Late childhood or adult onset peripheral neuropathy with associated ataxia and pigmentary retinopathy | Basal ganglia lucencies Abnormal electroretinogram Sensorimotor neuropathy |
| Pearson Syndome | Sideroblastic anaemia of childhood Pancytopenia Exocrine pancreatic failure | Renal tubular defects |
A further group of mitochondrial disorders have recently been defined at the molecular level. These disorders result from a disorder of mtDNA maintenance. For some of these diseases the primary defect is an abnormality of the intra‐mitochondrial nucleoside pool. Most individuals with autosomal dominant PEO have a mutation in one of three genes: PEO1, ANT1 or POLG, which lead to the formation of multiple mtDNA deletions in muscle (Kaukonen 2000; Spelbrink 2001; Van Goethem 2001). Children presenting with mtDNA depletion syndrome may have mutations in the nuclear genes thymidine kinase 2 (TK2) in the myopathic form, or deoxyguanosine kinase (DGUOK) or POLG in the hepatic form (Mandel 2001; Naviaux 2004; Saada 2001). Secondary mtDNA multiple deletions are also a feature of mitochondrial neurogastrointestinal encephalomyopathy (MNGIE) which is also due to a disturbance of the intra‐mitochondrial nucleoside pool secondary to thymidine phosphorylase (TP) deficiency (Nishino 1999). A final important group are the disorders associated with coenzyme Q10 (ubiquinone) deficiency. This may present with childhood encephalopathy and seizures, recurrent rhabdomyolysis or ataxia with seizures. Case reports suggest that this disorder responds to Q10 replacement therapy (Musumeci 2001).
A large proportion of individuals with mitochondrial disease do not have a clearly defined phenotype. There may be single or multi‐organ involvement including the heart, endocrine organs (particularly the pancreas) and the nervous system. Gastrointestinal complications are an under‐recognised but common feature of mitochondrial disorders. Mitochondrial disease should be considered in any patient presenting with an unexplained progressive multi‐system disorder with prominent neurological features (Chinnery 1997).
Secondary mitochondrial disorders
Secondary mitochondrial disorders will not be covered in this Cochrane review. Many genetic disorders are associated with abnormal mitochondrial function either as a secondary phenomenon, or because mitochondria play a crucial role in the pathophysiology of the disorder. To date these include three X‐linked conditions (Barth syndrome, sideroblastic anaemia with ataxia, deafness and dystonia) and a number of autosomal recessive (Friedreich ataxia, spastic paraparesis SPG4 and SPG13, Wilson disease) and autosomal dominant conditions (hereditary paragangliomas). Increasingly, common neurodegenerative disorders such as Parkinson's disease and Alzheimer's disease are beginning to enter this category as well. Disorders of mitochondrial fusion and fission also result in secondary mitochondrial dysfunction (Westermann 2010). Practically speaking, diseases caused by OPA1 mutation would likely have been covered in this review (autosomal dominant optic atrophy and progressive external ophthalmoplegia), on account of their highly similar clinical presentation with primary mitochondrial disorders. Other fusion/fission defects causing secondary mitochondrial dysfunction (hereditary motor and sensory neuropathy type 2a due to MFN2 mutation, and various other neurological diseases which involve fusion/fission defects in their pathogenesis) have not been included.
The clinical management of mitochondrial disease
There is currently no established treatment for mitochondrial disorders, and the clinical management of individuals is largely supportive. The aims are to provide prognostic information and genetic counselling.
Treatments used to modify the underlying disease process fall into three groups: pharmacological and nutritional agents, modification of macronutrient composition in the diet (dietary supplementation with vitamins and co‐factors), and exercise therapy. A number of different pharmacological treatments and nutritional supplements have been used in individuals with mitochondrial disease, with varying reports of success. These include antioxidants (coenzyme Q10, idebenone, vitamin C, vitamin E and menadione), agents that specifically improve lactic acidosis (dichloroacetate and dimethylglycine, which is a component of pangamic acid (vitamin B15), agents that correct secondary biochemical deficiencies (carnitine, creatine), respiratory chain co‐factors (nicotinamide, thiamine, riboflavin, succinate, and coenzyme Q10), and hormones (growth hormone and corticosteroids) (reviewed in Chinnery 2001). Much of the evidence used to support specific treatments comes from single case reports, but there have been a number of small quasi‐randomised trials and open‐labelled case series. Improvements following dietary modification (for example, a ketogenic diet) and exercise therapy (for example, endurance training) have also been documented in individual cases, and open‐labelled trials (Taivassalo 2001). These reports suggest that there might be benefits from these treatments.
To our knowledge this is the only systematic review of clinical trials in mitochondrial disease.
Novel treatment strategies
A number of groups are developing treatments that act on the genetic or cellular level, but it is unlikely that these will be available for individuals in the near future (for review see Hassani 2010).
The aim of this review is to critically appraise the available evidence from RCTs for currently available treatments for individuals with mitochondrial disorders with peripheral neurological features. Our initial protocol for the first review subdivided mitochondrial disorders into encephalopathies and myopathies. After completing the first review, we amalgamated our results into a single report because (a) only a small number of studies were identified, (b) the majority of participants in these studies had both central neurological and neuromuscular features which were both assessed in the same trial. We used the same approach in this revision (completed in 2012).
Objectives
This review will focus on the treatment of all primary mitochondrial disorders, including specific syndromes, complex multi‐system disorders and specific phenotypes such as LHON.
The objective of this review is to examine the effects of pharmacological treatments, and non‐pharmacological treatments (vitamins and food supplements), and physical training in improving the symptoms, signs, disability and quality of life in individuals with mitochondrial disorders.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs (including cross‐over studies). We decided to change our protocol when we updated the review in 2012, and excluded quasi‐RCTs (trials in which randomisation is intended but which might be flawed, such as alternate allocation) and RCTs at high risk of bias in any category, since such trials might have results that are not valid.
Types of participants
We included participants of any age with a confirmed diagnosis of mitochondrial disease based upon muscle histochemistry and/or respiratory chain complex analysis of tissues or cell lines and/or DNA studies in mitochondrial DNA or relevant nuclear genes.
Types of interventions
We included any pharmacological agent, dietary modification, nutritional supplement, exercise therapy or other treatment. We did not study the effects of treatments for the complications of mitochondrial disorders (such as ptosis surgery, or cardiac pacing).
Types of outcome measures
Primary outcomes
The primary outcome measures included an change in muscle strength and/or endurance (including the UK Medical Research Council (MRC) muscle strength scale, isometric dynamometer, custom made strain device, vital capacity or maximal voluntary inspiratory or expiratory capacity, or walking speed). For central neurological features, our primary end points were an alteration in a system‐specific neurological function score, a reduction of paroxysmal events (such as seizures or stroke‐like episodes), visual acuity, pure tone audiometry or cognitive performance. We chose a number of outcome measures because a preliminary literature search identified only a few studies, and each one incorporated different outcome measures. Focussing on one outcome measure would severely limit the scope of this review for a group of disorders with such a complex clinical phenotype. We intended to identify treatment effect at three months after initiation of treatment. This did not prove to be possible, and ultimately we did not choose a specific time point for the primary outcomes. This is because a specific time point would exclude most of the identified studies, as no universal time point has been agreed upon for the assessment of treatments in these disorders.
Secondary outcomes
Secondary outcome measures were as follows.
An alteration in quality of life as measured by a recognised scale.
Biochemical markers of disease (normalisation of plasma lactate/pyruvate ratio, lowered Vmax as measured by magnetic resonance spectroscopy).
Negative outcomes. These included all adverse events attributable to the treatment. Serious adverse events, namely disabling or life‐threatening complications, complications which require hospitalisation, and death were recorded separately. A record was made if it was not possible to determine whether the negative outcome was a consequence of the treatment or part of the natural history of the disease.
If it had been possible to produce a 'Summary of findings' table, we would have selected outcomes that included change in MRC muscle strength, change in quality of life scores, and change in activity of daily living scores. We would also have considered adverse events among the outcomes, including peripheral neuropathy, somnolence, nausea, and hospitalisation. Again, the heterogeneity of these conditions makes the selection of these outcomes extremely difficult, and potentially with limited comparability between studies.
Search methods for identification of studies
We searched the Cochrane Neuromuscular Disease Group Specialized Register (4 July 2011) using the terms 'mitochondrial disease' or 'mitochondrial myopathy' or 'mitochondrial disorder' or 'Disorders of mitochondrial function', or 'chronic progressive external ophthalmoplegia' or 'CPEO' or 'Kearns syndrome' or 'KSS' or 'Kearns Sayre syndrome' or 'Pearson syndrome' or 'Leber hereditary optic neuropathy' or 'LHON' or 'MELAS syndrome' or 'MERRF syndrome' or 'MIDD' or 'NARP' or 'Leigh syndrome' or 'MNGIE'. We adapted this strategy to search the Cochrane Central Register of Controlled Trials (CENTRAL) (2011, Issue 2), MEDLINE (January 1966 to July 2011), EMBASE (January 1980 to July 2011). We searched for RCTs and quasi‐RCTs for possible inclusion in the analysis. We also searched for informative single case reports and observational studies, and incorporated these in the discussion. Wherever possible we contacted the authors of these studies for long‐term follow up on the individual cases. We also planned to include any unpublished studies conducted by experts in the field by contacting the authors of all published studies and other experts in the field. The search strategies for MEDLINE, EMBASE and The Cochrane Central Register of Controlled Trials (CENTRAL) are in Appendix 1, Appendix 2 and Appendix 3 respectively.
Data collection and analysis
GP and PFC independently checked titles and abstracts identified using the search strategy, and identified 21 studies for further review. All five authors then independently decided which of these 21 trials fit the inclusion criteria and graded risk of bias according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Specifically, the bias assessment addressed the following domains: (1) Random sequence generation (method used to randomise participants), (2) Allocation concealment (how randomisation was performed), (3) Blinding of participants and personnel, (4) Blinding of outcome data, (5) Incomplete outcome data, (6) Selective outcome reporting, and (7) Other. For each category, the risk of bias was graded as "Low risk", "High risk", or "Unclear risk" based upon the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
In the original review (2003), four authors (PFC, KM, DMT, DT) performed data extraction and it was concordant in each case. GP and PFC performed data extraction for the 2011 update. We obtained missing data from the trial authors wherever possible. If there had been adequate data we planned to carry out a meta‐analysis using mean differences to analyse continuous data and risk ratios to analyse dichotomous data. If data were available for more than one trial with a specific intervention, we planned to use the Cochrane statistical software Review Manager 5 (RevMan) software using a fixed‐effect model. If there was heterogeneity we planned to explore possible reasons for differences between studies such as type of participants, intervention or quality, and we planned to perform sensitivity analyses by omitting trials which lacked one or more of the methodological attributes. We planned to express uncertainty as 95% confidence intervals (CI).
Results
Description of studies
The review authors scanned 1335 abstracts and identified 21 studies for further review, from which the authors selected 12 for inclusion in the review. These studies evaluated the following treatments: coenzyme Q10, creatine, a combination (including creatine, coenzyme Q10 and lipoic acid), dichloroacetate (DCA), dimethylglycine (DMG), or a whey‐based cysteine supplement. Nine studies were excluded, and the reasons for their exclusion have been summarised in Characteristics of excluded studies. The most common reasons for exclusion of these studies was the lack of randomisation (Bresolin 1990; Muller 1990; DeStefano 1995) and/or blinding (Suzuki 1998; Cejudo 2005; Koga 2005; Taivassalo 2006; Stacpoole 2008) in most of these studies, which would have a high risk of biasing the results. Other methodological issues which produced a high risk of bias were present in two studies (Bresolin 1990; Chen 1997). Two of these studies were in abstract format only and could not be adequately assessed (Muller 1990; Gimenes 2007).
Risk of bias in included studies
See Characteristics of included studies for 'Risk of bias' ratings for the included studies and Figure 1 for a summary of 'Risk of bias' assessments. The majority of studies included only a small number of participants. Given the various treatments studied, limited size of these studies, the heterogeneous patient groups, and the different end points, we elected not to perform meta‐analysis.
1.
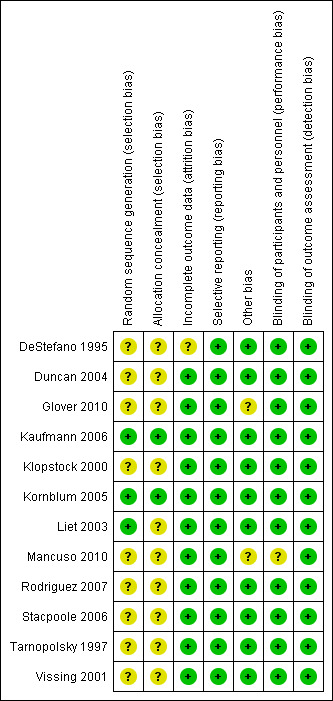
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
All 12 studies involved an oral agent (either a pharmacological agent, or a food supplement). The studies are reported in alphabetical order according to the treatment used.
Coenzyme Q10
Glover et al (Glover 2010) studied the effects of high‐dose coenzyme Q10 on 30 participants with mitochondrial disease, including 15 with mitochondrial encephalomyopathy with lactic acidosis and stroke‐like episodes (MELAS), 11 with progressive external ophthalmoplegia (PEO), two with ataxia‐neuropathy syndromes, one with complex I deficiency, and one with Leber hereditary optic neuropathy (LHON). Participants received coenzyme Q10 600 mg orally twice daily for 60 days and placebo for 60 days, with an intervening washout period of 67 +/‐ 8.3 days. This was a randomised, placebo‐controlled, cross‐over trial. Outcome assessments were performed at the end of each trial period. These included urine analysis of 8‐isoprostane and 8‐hydroxy‐2‐deoxyguanosine. Blood analysis was performed for lactate, glucose, PCO2, coenzyme Q10, bilirubin, creatine kinase and gamma‐glutamyltransferase. Questionnaires were given for quality of life and activities of daily living. Maximal isometric force and near‐infrared spectroscopy was measured during a 90 s forearm ischaemic exercise test, with venous lactate, PCO2, PO2 at 1 min and 3 min post‐test. Dual‐energy X‐ray absorptometry was performed for body composition. Testing during 15 min cycle ergometry (0 W for 5 min and 30 W for 10 min) monitored heart rate, inspired O2, expired CO2, VO2 and VCO2 measured at several time points. Venous lactate was measured prior, and at various points during and after testing. Magnetic resonance spectroscopy (MRS) of cerebrospinal fluid (CSF) lactate, N‐acetylaspartate and choline was completed. Statistical analysis included paired t‐tests for all measurements. The exception was handgrip PO2 and PCO2, which was analysed using two‐way repeated measures analysis of variance with Tukey's post hoc test. P values of less than 0.05 were considered to be statistically significant. Results included a statistically significantly increased coenzyme Q10 level. Lactate was statistically significantly decreased at 1 min of cycle ergometry, but there was no difference at other time points. All other endpoints did not differ significantly.
Creatine
Tarnopolsky et al (Tarnopolsky 1997) studied the effects of creatine monohydrate on seven patients with mitochondrial disease, including six with MELAS and one with a mitochondrial myopathy. In this randomised cross‐over study, participants received placebo in one period, and in the treatment period received 5 g creatine twice daily for two weeks followed by 2 g twice daily for one week (although measurements were performed at the end of the treatment period, i.e after three weeks). Measurements included activities of daily living on a visual analogue scale, ischaemic isometric handgrip strength for 1 min, evoked and voluntary contraction strength of dorsiflexor muscles using a dynamometer, nonischaemic isometric dorsiflexion torque for 2 min (NIDFT), and aerobic cycle ergometry (15 to 30 W for 5 to 10 min) with basal and post‐ischaemic lactate measurements. Statistical analysis included two‐way repeated measures analysis of variance with Tukey's post hoc test for all outcomes (except for body composition variables which used paired t‐tests). P values of less than 0.05 were considered to be statistically significant. Creatine resulted in statistically significantly increased handgrip strength (19% increase in torque in N m, P < 0.01), NIDFT (11%, P < 0.01) and post‐exercise lactate (P < 0.05) compared with values after treatment with placebo (although we note that these data are represented graphically, with no reported SD, and it is unclear whether mean percentage difference or percentage difference of the means are reported). There was no change in the other variables. No side‐effects were noted.
Klopstock et al (Klopstock 2000) studied the effects of creatine monohydrate in 13 participants with PEO and three with mitochondrial myopathy in a randomised placebo controlled cross‐over trial. Participants received either 20 g of creatine/day or placebo for four weeks. Measurements included visual analogue scales of subjective weakness and general activity, testing muscle strength in 32 muscles according to the Medical Research Council (MRC) scale, the Hammersmith motor ability score, a neuromuscular symptom score, a function time test, a function ranking test and an ataxia score. Resting and post‐exercise lactate was determined following cycle ergometry, and maximal voluntary muscle torque was measured for elbow flexion (biceps at 90º) and knee extension (quadriceps at 110º) using the multifunctional training machine. Aerobic exercise was tested by nonischaemic isokinetic biceps flexion and knee extension (at a speed of 80 º/s with 15% of maximal muscle force until muscular exhaustion). Eye motility and eyelid drooping, and the velocity, gain and latency of visually guided horizontal saccades were also measured. Data were reported according to an "estimated treatment effect" which was generated by an ordinary least squares model using patients, treatment, period and pretreatment measurement as factors and 95% confidence intervals were reported. No significant effects of treatment were noted. Two participants experienced muscle cramps whilst being treated with creatine.
Kornblum et al (Kornblum 2005) studied 15 participants with CPEO or Kearns‐Sayre syndrome (KSS) due to single deletions of mitochondrial DNA (mtDNA) in a double‐blind, placebo‐controlled cross‐over study. Participants were treated with 150 mg/kg of creatine monohydrate or lactulose for six weeks. Assessments included phosphorus‐31 magnetic resonance spectroscopy (31P‐MRS: parameters included the phosphocreatine/adenosine triphosphate (PCr/ATP) ratio at rest and the time constant for oxidative PCr recovery following aerobic and ischaemic exercise), a modified Boston score of skeletal muscle function, maximum voluntary contraction tensiometric hand grip, and serum creatine kinase levels. The primary outcomes were analysed statistically using two‐sided t‐tests, with significance for P values less than 0.05. The secondary outcomes were not analysed statistically. There were no statistically significant differences between the creatine and lactulose treatments. No adverse effects were noted.
Given that these three trials used different doses of creatine, different treatment period, and participants with different genetic, biochemical, and clinical subtypes of mitochondrial disease, we did not attempt to calculate a summary effect for any of the outcomes.
Creatine, coenzyme Q10 and lipoic acid combination
Rodriguez et al (Rodriguez 2007) studied 16 participants with different mitochondrial disorders, including three with CPEO and one with KSS due to a single mtDNA deletion, three with MELAS due to m.3271T>C, two with LHON due to m.3460G>A, one with mitochondrial neurogastrointestinal encephalomyopathy (MNGIE), three with mitochondrial disorders due to rare point mutations (m.9053T>C, m.4452T>C and m.9035T>C), and three with a biochemically defined mitochondrial disorder. The 16 participants enrolled in a randomised, double‐blind, placebo‐controlled cross‐over study receiving the active cocktail or placebo for two months, with a five‐week washout period in between the treatment arms. The active cocktail comprised: 3 g creatine monohydrate with 2 g of dextrose, 300 mg alpha‐lipoic acid, and 120 mg coenzyme Q10. Participants were evaluated after a four hour fast before and after each intervention at the same time of day. Clinical assessments included: handgrip, ankle dorsiflexion (at 90°) and knee extension of the right limbs using a custom‐made force transducer. The best measurement from three 5 s trials was recorded. Additional measures included forced vital capacity and forced expiratory volume, free fat mass, percentage body fat and total body water. Plasma lactate, urinary 8‐isoprostanes and 8‐hydroxy‐2‐deoxyguanosine were also measured. Statistical analysis included two‐ or three‐way repeated measures analysis of variance, with Tukey's post hoc test for significant values. P values of less than 0.05 were considered significant. One‐tailed P values were used for antioxidant levels although two‐tailed values for all others. Across the whole study group, the treatment statistically significantly lowered plasma lactate (one‐tailed P < 0.05, mean + SD shown in graphical form) and urinary 8‐isoprostanes, and there was evidence of attenuated the decline in peak angle dorsiflexion strength. There was evidence of higher free‐fat mass with the treatment in participants with the MELAS phenotype (P < 0.05, mean + SD shown in graphical form). All other outcomes demonstrated non‐significant differences.
Dichloroacetate
De Stefano et al (DeStefano 1995) studied 11 participants with mitochondrial disease, including four with myopathy, one with chronic CPEO and myopathy, two with KSS, two with Leigh syndrome, one with mitochondrial MELAS, and one with the mitochondrial DNA depletion syndrome. The study was a double‐blind, placebo‐controlled cross‐over trial using 25 mg/kg twice daily dichloroacetate (DCA) or placebo for one week, followed by a three month washout period before the second period. Assessments were performed prior to each treatment period and on termination of the treatment, and included: a complete neurological examination, isometric force generation on dynamometry in proximal muscles (deltoid and iliopsoas), gait performance evaluation, resting venous blood lactate, alanine and pyruvate; incremental exercise in four participants with measurements of venous blood lactate, alanine and pyruvate; 31P‐MRS of muscle and proton MRS (1H‐MRS) of the brain. Statistical analysis of participants was done using aggregate scores of all participants while on placebo compared with DCA. Two‐tailed P values of less than 0.05 were considered statistically significant. For the MRS component of the study, the above statistical analysis was performed, as well as comparison with normal controls using one‐tailed t‐test. Variance of the results was reported as standard deviation. DCA produced statistically significant decreases in blood lactate, pyruvate and alanine at rest and after exercise (P < 0.05, values for each individual shown graphically), and statistically significant improvements on brain MRS were also noted in seven participants, including a statistically significant reduction of the brain lactate/creatine ratio (42%, P < 0.05, relative values shown graphically, no SD given), a statistically significant increase in brain choline/creatine ratio (18%, P < 0.01), and statistically significant increase in the acetylaspartate/creatine ratio (8%, P < 0.05) (for all of the statistics relating to MRS, only relative values were given, represented graphically, and SD was not provided). In two participants similar results were seen in a different volume of interest including the basal ganglia. Muscle MRS and self assessed clinical disability were unchanged. No adverse effects were reported.
Vissing et al (Vissing 2001) studied eight adults with various molecularly proven mitochondrial myopathies, having exercise intolerance. This was a randomised, double‐blind, placebo‐controlled cross‐over study of DCA. Treatment was for either 15 days of DCA at 25 mg/kg twice daily or placebo, with a six‐week washout period before entering the opposite arm of the study. Outcome measures included oxygen consumption (VO2), workload and blood pressure during cycle ergometry, with measurements of venous lactate and pyruvate. 31P‐MRS of forearm muscles before and after forearm exercise was measured. All participants filled a 20‐item questionnaire commenting on activity levels and symptoms. Outcome measurements were performed before and at the end of each treatment phase. The participants' results at the end of each treatment period were compared in aggregate. P‐values of less than 0.05 using a two‐tailed t‐test was considered to be statistically significant. One participant withdrew before data were collected because of sedation as a side‐effect of DCA. Three other participants also experienced sedation on DCA but completed the study. Results demonstrated evidence of normalisation of the resting and exercise lactate and pyruvate levels. However, there was no difference in any of the other outcomes on cycle ergometry, 31P‐MRS, or the questionnaire.
Duncan et al (Duncan 2004) published a randomised, placebo‐controlled, double‐blind cross‐over study of DCA versus placebo in nine children and adults with varied respiratory chain disorders. Treatment with DCA (25 mg/kg) or placebo was for three months, with two‐week washout in between. Measurements included physiological values and blood lactate at maximal and submaximal effort during treadmill exercise testing. Statistical analysis included analysis of variance for each of the variables, with two‐sided tests at an alpha of 0.05. Mean blood lactate during submaximal exercise was statistically significantly lower during DCA therapy (1.71 (SD +/‐ 1.37) versus 2.39 (SD +/‐ 1.32)), although a variety of other measurements (VO2 max, work, lactate, heart rate and blood pressure) were unchanged.
Kaufmann et al (Kaufmann 2006) reported a double‐blind, randomised, placebo‐controlled, three‐year cross‐over trial using 25 mg/kg/day of DCA for 12 months in 30 participants harbouring the m.3243A>G mtDNA mutation. Primary outcome measures included a purpose‐designed integrated clinical assessment score (GATE, or Global Assessment of Treatment Efficacy, which was based on a health‐related inventory, neurological and psychological evaluation, and activities of daily living). Additional endpoints included lactate measurements in venous blood and CSF, measured by 1H‐MRS. Nerve conduction tests were used to monitor safety. For the primary outcome, the Fisher exact test was used to determine whether significant differences existed in the aggregate GATE scores for participants while on DCA versus placebo therapy. Analysis of the secondary outcomes was performed using type 3 analysis of variance. The DCA was withdrawn from all 15 of the group receiving DCA in the first 24 month study period, compared to 4 of 15 participants on placebo. The trial was discontinued due to peripheral nerve toxicity. No significant differences were noted for the GATE score, or any of the secondary outcome measures.
Stacpoole et al (Stacpoole 2006) carried out a randomised, double‐blind, controlled trial on 43 children with congenital lactic acidosis. The study group largely comprised children with mitochondrial respiratory chain disease (25 had one or more defects of the respiratory chain and 7 had a mutation of mtDNA), but also included 11 children with pyruvate dehydrogenase deficiency. Following six months' preconditioning on placebo, the children were randomised to receive 12.5 mg/kg of DCA or placebo every 12 hours for six months. The primary outcome measures were: a purpose designed integrated clinical assessment score (GATE, which was based on a health‐related inventory, neurological and psychological evaluation, and activities of daily living); a measurement of linear growth; fasting blood lactate, and lactate after a carbohydrate meal; the frequency of severe or recurrent illnesses or hospital admissions; and safety tests of liver and peripheral nerve function. For the primary outcome, a generalised odds ratio (with 95% CI) was calculated for the effect of DCA on the GATE score. The secondary outcomes were analysed using two‐sided t‐tests, with P values less than 0.05 considered as statistically significant. There was evidence that treatment attenuated the rise in blood lactate following a carbohydrate meal, but had no effect on the other primary outcome measures. There were no significant side‐effects, and specifically no objective evidence of peripheral nerve dysfunction as a consequence of the treatment.
Dimethylglycine
Based on anecdotal reports of an improvement in patients with congenital lactic acidosis, Liet et al (Liet 2003) studied five children with Saguenay‐Lac‐Saint‐Jean cytochrome c oxidase (SLSJ‐COX) deficiency in a randomised, double‐blind study comparing dimethylglycine (DMG) to placebo. Children weighing < 33 kg were given 50 mg/kg/day in three divided doses, and those weighing > 33 kg were given 5 g per day in three doses over three days. The washout period was at least two weeks. Four measurements of VO2 were performed using indirect calorimetry before and after treatment, and blood lactate, pyruvate, bicarbonate and pH. Dietary caloric intake was calculated for three days prior to each measurement. The primary outcome of change in VO2 after placebo or DMG was analysed statistically using the Wilcoxon signed rank test for related samples for each participant. P values of less than 0.05 were considered to be statistically significant (two‐tailed). The mean VO2 was lower after administration of both DMG and placebo, but neither value reached statistical significance. There was no detectable effect on blood lactate, pyruvate, bicarbonate or pH. No significant side‐effects were noted.
Whey‐based cysteine supplement
Mancuso et al (Mancuso 2010) studied the effect of a whey‐based cysteine supplement in 13 PEO patients in a randomised, double‐blind, placebo‐controlled cross‐over study. Participants received 10 g/day of the supplement or placebo for 30 days, with no washout period. Measurements included markers of free radical reducing capacity (advanced oxidation protein products (AOPP); ferric reducing antioxidant power (FRAP); glutathione (GSH) level). Serum lactate was measured at various time points during cycle ergometry. Quality of life was measured using the Short Form 36 Health Survey (SF‐36) questionnaire. Muscle power was assessed using the UK Medical Research Council (MRC) scale in various muscle groups. The baseline data were analysed using unpaired two‐tailed t‐tests, and Pearson correlation tests (for oxidative markers to the clinical measurements). Changes after therapy or placebo were analysed with paired t‐tests. P values of less than 0.05 were considered significant. Results demonstrated that AOPP, FRAP and GSH were statistically significantly increased in the treatment period. Lactate, quality of life, and muscle power were unchanged.
Discussion
Included studies
Assessing the efficacy of treatment for mitochondrial disorders is difficult for a number of reasons. First, the complex and variable phenotypes make it difficult to compare two or more individuals. Second, many mitochondrial disorders affect multiple organ systems which are difficult to compare (for example, it is difficult to compare an improvement in diabetic control with a reduction in seizure frequency). Thirdly, there is a lack of natural history data on individuals with mitochondrial disease, and related to this, many mitochondrial disorders involve infrequent paroxysmal events (such as stroke‐like episodes, encephalopathy or seizures), making short studies unhelpful. These difficulties explain why we were only able to find 12 studies fulfilling our entry criteria, and most of these focussed on peripheral neuromuscular features, which are easier to assess.
The 12 studies assessed the effect of oral agents: coenzyme Q10, creatine monohydrate; a combination of coenzyme Q10 with creatine and lipoic acid; dichloroacetate (DCA); dimethylglycine (DMG); and whey‐based cysteine supplement. There was objective evidence of locomotor functional improvement with creatine, but there were conflicting data. Creatine statistically significantly improved handgrip strength and nonischaemic isometric dorsiflexion torque (NIDFT) but did not improve muscle strength in two other studies. Paradoxically, the trials showing no effect used higher doses of the oral agent, ruling out inadequate dosage as an explanation for the lack of response. Although differences in study design may explain the discrepancies, it is intriguing, that the two longer trials showed no effect, possibly indicating that if there is a treatment response to creatine, it is not sustained. Although secondary measures were shown to statistically significantly improve with DCA in three studies in children (DeStefano 1995; Duncan 2004; Stacpoole 2006) and one short‐term trial in adults (Vissing 2001), this was not associated with any improvement in physical function. The longer‐term study carried out in adults used the same dose, but had to be terminated prematurely due to unacceptable side‐effects without any evidence of clinical benefit (Kaufmann 2006). There was no evidence of a positive clinical response to DMG (Liet 2003) or whey‐based cysteine supplement (Mancuso 2010).
It is important to mention some of the methodological similarities and differences between the studies. Of the 12 included studies, all but one (Stacpoole 2006) were cross‐over studies, enabling each participant to act as his/her own control. In most of the studies, this was appropriate because the participants had a stable and chronic clinical course. However, this may not be the case for mitochondrial diseases with a fluctuating clinical course, such as Leigh syndrome, and patients with other fluctuating encephalopathies. All of the cross‐over studies except one (Mancuso 2010) included a washout period, which is an essential component of a cross‐over study design. The results of the statistical analyses were appropriately reported in each study. The variance of recorded measurements was reported as standard deviation in eight of the studies, standard error in two studies, and 95% CI in two studies. However, in order to maximise the power of a cross‐over study, the usual approach involves a comparison of the difference between pre‐ and post‐treatment measurements for each participant receiving either the active drug or placebo. This minimises the effect of inter‐individual variability which is a major concern for small studies of a heterogeneous disorder such as mitochondrial disease. In simple terms, the analysis uses each participant as his/her own control. Studies which used statistical methods that would compare within‐subject treatment differences used appropriate statistical methodology for the study design, and this included four studies using analysis of variance or (in the case of one study) the Wilcoxon signed‐rank test. One study used analysis of variance for the secondary outcomes but did not include this for the primary outcome. Five studies used statistical methodology that did not include within‐subject differences, potentially limiting the power of the studies to detect a treatment effect. One conclusion of this review is, therefore, to recommend an analysis of within‐subject treatment effect in all future treatment studies. Another comment is that where statistically significant differences exist between placebo and treatment for a cross‐over study, ideally the treatment difference with its 95% CI should be reported. This treatment difference was not reported in the studies which demonstrated statistically significant differences for any of their endpoints, and we suggest that future studies include this statistic. Finally, the small number of participants per study may also have made the detection of treatment effect difficult, although the fact that all 12 studies did not detect a difference suggests that if any treatment effect exists, it is likely too small to be of any clinical significance.
With regard to the trial endpoints, numerous measures were employed in most trials, usually a combination of biochemical, physiologic, imaging, and questionnaire data. In most cases, the endpoints chosen were not directly clinically relevant, and the inclusion of so many endpoints increases the chances of a significant result arising due to chance. Ideally, further studies should focus on a small number of endpoints which are likely to reflect actual clinical benefit. In these regards, the GATE score (an aggregate measurement of neurological/psychological exam results, with questionnaire data on health and function), as described in the trials by Stacpoole (Stacpoole 2006) and Kaufmann et al (Kaufmann 2006), is one means of attempting to provide a clinically meaningful measurement of improvement.
Adverse events were considered for all of the above studies. With the exception of peripheral neuropathy in one of the DCA trials (Kaufmann 2006), there were no significant side‐effects in the other trials. However the full evaluation of adverse reactions was difficult to assess because of the small number of participants per study, heterogeneous study populations, and lack of comparison of within‐subject differences.
Excluded studies
Upon reassessment of the studies included in the previous version of this review, it was decided that two studies should be excluded. Both are negative studies of coenzyme Q10 in mitochondrial myopathy. One study (Muller 1990) found no clinical benefit of nine months of coenzyme Q10 versus placebo in a non‐randomised, double‐blind, cross‐over design. Full assessment of this study is not possible because it exists only in abstract format. Data were not reported, except to state that there was no benefit from coenzyme Q10 therapy. There is a high risk of bias in this study because of the non‐randomised design, and possibility of selective outcome reporting given that no data were reported. The other study of coenzyme Q10 (Chen 1997) was a non‐randomised, double‐blind, placebo‐controlled cross‐over study. Participants either had one month of placebo followed by three months of coenzyme Q10, or three months of coenzyme Q10 followed by a one‐month washout and one month of placebo. Outcome assessments (subjective fatiguability in activities of daily living score, global muscle strength based on MRC scale, bedside endurance tests, exercise lactate test, serum Q10 level) occurred at monthly intervals. Results of the study reported that global MRC scale was significantly improved after three months coenzyme Q10, although the standard deviation (SD) was not reported for this outcome. The other outcomes were nonsignificant. This study has a high risk of bias because it was nonrandomised, and the design (in which participants starting with coenzyme Q10 spend more time in the study than participants starting on placebo) could have allowed the blinded assessors to determine whether they were in the placebo or study group based upon their time in the study. Furthermore, there is the possibility of selective outcome reporting because results for the coenzyme Q10 group were only reported at the end of three months, although data measurements occurred monthly.
On a practical level, the high risk of bias in these studies would presumably have altered the outcome in favour of treatment, and despite this both studies were negative. Several small case series have evaluated the effect of coenzyme Q10 in non‐controlled or non‐blinded formats (Ogasahara 1986; Ihara 1989; Bendahan 1992; Gold 1996; Papadimitriou 1996; Abe 1999) with results suggesting a possible beneficial effect of treatment. Recently a trial studying coenzyme Q10 with creatine and lipoic acid (Rodriguez 2007), which is summarized above, was positive for some surrogate endpoints although it is unclear if these are clinically relevant. Treatment with high‐dose coenzyme Q10 (Glover 2010) was also not demonstrated to have any clinical effect in a RCT.
The literature contains hundreds of case studies describing benefits and harmful effects of a wide range of different therapies in the treatment of mitochondrial disease (examples are summarised in Table 2). There have also been a number of open studies showing improvement with pharmacological and non‐pharmacological treatments, including a recent study of L‐arginine for the acute and long‐term treatment of stroke‐like episodes in patients with mitochondrial encephalomyopathy with lactic acidosis and stroke‐like episodes (MELAS) (Koga 2005). Given the inherent bias in studies of this type, we have deliberately not used these reports when reaching a view about treatment. These interventions should be studied rigorously in RCTs, particularly to evaluate the longer term effects.
2. Oral agents used in single case studies and open trials.
| Class | Agent (route) | Indication | Proposed mechanism | Dose | Effects | |
| Quinone derivatives | Ubiquinone (coenzyme Q10)(oral) | Isolated ubiquinone deficiency | Redox bypass corrects the deficiency | 60 ‐ 250 mg/day | Significant clinical improvement (Ogasahara 1989; Sobreira 1997; Rotig 2000; Montini 2008) |
|
| All mitochondrial disorders | Redox bypass, free radical scavenger | 30 ‐ 260 mg/day | Subjective improvement, particularly reduced fatigue and reduced muscle cramps. Isolated reports of clinical and metabolic improvement (Ogasahara 1986; Ihara 1989; Nishikawa 1989; Abe 1991; Bendahan 1992; Gold 1996; Papadimitriou 1996) | |||
| Idebenone (oral) | All mitochondrial disorders, especially LHON | Free radical scavenger, redox bypass of complex I |
90 ‐ 270 mg/day | Improved brain and skeletal muscle metabolism in isolated cases (Ihara 1989; Cortelli 1997). May enhance the rate and degree of visual recovery in LHON (Mashima 1992; Mashima 2000) | ||
| Vitamin supplements | Thiamine (B1)(oral) | KSS and other mitochondrial disorders | Coenzyme for pyruvate dehydrogenase complex (PDHC) | Up to 900 mg/day | Isolated reports of improvement (Lou 1981). No significant effect in a larger study (Mathews 1993) | |
| Riboflavin (B2)(oral) | Complex I and complex II deficiency | Acts as flavin precursor for complex I and II | 100 mg/day | Clinical and biochemical improvements in small groups of patients (Penn 1992; Bernsen 1993). A larger study of 16 different patients failed to show a benefit (Mathews 1993) | ||
| Ascorbate (C) and menadione (K3)(oral) | Complex III deficiency. Other mitochondrial disorders | Antioxidant Bypass of complex III (with vitamin C) | 10 mg qds (four times daily) | Symptomatic and bioenergetic improvements in isolated cases (Eleff 1984; Mowat 1999) | ||
| Nicotinamide (B3) (oral) | MELAS and complex I deficiency | Increase of NAD + NADH pool | Clinical and biochemical improvements in isolated cases treated with nicotinamide alone (Majamaa 1997) or in combination with ubiquinone (Remes 2002) or riboflavin (Penn 1992) | |||
| Metabolic supplements | Succinate (oral) | Complex I deficiency, KSS and MELAS | Donates electrons directly to complex II | 6 g/day | Improvements reported in isolated cases (Shoffner 1989; Oguro 2004) | |
| Creatine | Mitochondrial myopathy | Enhances muscle phosphocreatine | Up to 10 g/day | Reduced fatigue and enhanced muscle strength and aerobic exercise capacity (Tarnopolsky 1999) | ||
| Carnitine | Secondary carnitine deficiency | Replacement | Up to 3 g/day | Improvements in isolated cases (Hsu 1995) | ||
| Lipoic acid (oral) | CPEO | Enhancing PDHC activity | 600 mg/day | Clinical and biochemical improvement in an isolated case (Barbiroli 1995) | ||
| High fat diet | Mitochondrial disorders, especially complex I deficiency | Increased proportion of electrons donated after (ie bypass) Complexes I and II | 50 ‐ 60% of caloric intake | Short‐term clinical improvement in small series treated with high‐fat diet plus ubiquinone and vitamins B1, B1 and C (Panetta 2004) | ||
| Dimethylglycine | Congenital lactic acidosis | Minor contributor to respiratory chain and possible free radical scavenger. | Not specified | Unspecified improvement in unpublished observations (Liet 2003) | ||
| Dichloroacetate | Mitochondrial disorders especially with lactic acidosis | Reduces lactic acidosis by enhancing PDHC activity | 25 mg/kg/day | Short‐term improvements in muscle and brain oxidative metabolism (DeStefano 1995). Potential complications include a painful peripheral neuropathy (Kurlemann 1995). Temporary benefits noted in two recent open trials (Barshop 2004; Mori 2004) | ||
| Corticosteroids | No clear indication | Unclear, if any | Up to 100 mg/day Prednisone | Improvements have been reported in isolated cases (Gubbay 1989), but steroids may exacerbate the metabolic encephalopathy (Curless 1986) and have long‐term consequences (Mitsui 2002) | ||
| Vasodilators | L‐arginine | 3243A>G MELAS stroke‐like episodes | Endothelial relaxation through enhanced nitric oxide production | 0.15 ‐ 0.3 g/kg/d IV in the acute phase, oral between episodes | Improved stroke‐like symptoms (Koga 2005) |
CPEO: chronic progressive external ophthalmoplegia
IV: intravenous
KSS: Kearns‐Sayre syndrome
LHON: Leber hereditary optic neuropathy
MELAS; mitochondrial encephalomyopathy with lactic acidosis and stroke‐like episodes
NAD: nicotinamide adenine dinucleotide
PDHC: pyruvate dehydrogenase complex
In recent years there has been growing interest in the potential benefits of exercise for mitochondrial myopathy. A number of open‐labelled studies have reported evidence of improved exercise tolerance and well‐being following aerobic exercise training (Taivassalo 1998; Taivassalo 2006; Jeppesen 2006), and resistance training is also being explored as a potential means of correcting the underlying biochemical defect in muscle (Murphy 2008). However, only one RCT has been carried out to date (Cejudo 2005). Although this study had a positive outcome, it was not included in this review because of the high risk of bias in an unblinded study, where both participant and physician have a vested interest in showing a positive treatment effect. Whilst we recognise the difficulty of designing an exercise trial that is double blind, blinding is of particular importance for this intervention because the measured outcomes are known to depend on the effort of the study participant, and the approach taken by the investigator making the measurement.
Future directions
Over the last five years a number of multicentre research collaborations have been forged throughout Europe and North America (United Mitochondrial Disease Foundation: www.umdf.org), and clinical rating scales have been produced through an EU consortium (EUmitocombat www.eumitocombat.org/ and MITOCIRCLE http://mitocircle.unimaas.nl/). These developments have enabled a number of multicentre trials on large cohorts of phenotypically similar patients. Several trials are currently ongoing. Finally, the pharmaceutical industry has become interested in mitochondrial disorders in recent years, opening up the opportunity for commercially sponsored trials. These developments provide hope for the immediate future.
Authors' conclusions
Implications for practice.
There have been very few RCTs for the treatment of mitochondrial disease. We conclude that there is currently no clear evidence supporting the use of any of these agents in mitochondrial disorders. Dichloracetate should not be used in adults because of the high incidence of potentially irreversible side‐effects.
Implications for research.
Further research is needed to establish the role of a wide range of therapeutic approaches in the treatment of mitochondrial disorders. Future studies should concentrate on designs featuring homogeneous study populations and clinically relevant outcomes. Trials should designate a much more limited number of primary and secondary outcomes to study, and focus strictly on those which are clinically relevant.
What's new
| Date | Event | Description |
|---|---|---|
| 29 March 2011 | New citation required but conclusions have not changed | G Pfeffer included among authors |
| 29 March 2011 | New search has been performed | New searches to July 2011. Six new studies included and two previously included studies now excluded, resulting in 10 included studies. Incorporated revised 'Risk of bias' methods |
History
Protocol first published: Issue 4, 2003 Review first published: Issue 1, 2006
| Date | Event | Description |
|---|---|---|
| 27 April 2008 | Amended | Converted to new review format |
| 12 September 2005 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
With thanks to the staff of University of British Columbia libraries. PFC is a Wellcome Trust Senior Fellow in Clinical Science and a UK NIHR Senior Investigator who also receives funding from the Medical Research Council (UK), the Association Française contre les Myopathies, and the UK NIHR Biomedical Research Centre for Ageing and Age‐related disease award to the Newcastle upon Tyne Foundation Hospitals NHS Trust.
The editorial base of the Cochrane Neuromuscular Disease Group is supported by the MRC Centre for Neuromuscular Diseases and the Muscular Dystrophy Campaign.
Appendices
Appendix 1. OVID MEDLINE strategy
1 randomized controlled trial.pt. 2 controlled clinical trial.pt. 3 randomized.ab. 4 placebo.ab. 5 drug therapy.fs. 6 randomly.ab. 7 trial.ab. 8 groups.ab. 9 or/1‐8 10 animals not (animals and humans)).sh. 11 9 not 10 12 exp Mitochondrial Diseases/ 13 mitochondrial myopath$.mp. 14 (mitochondrial encephalomyopath$ or mitochondrial encephalopath$).mp. 15 (mitochondrial disease$ or mitochondrial disorder$).mp. 16 or/12‐15 17 ((CPEO or ophthalmoplegia) adj5 progressive).mp. 18 cytochrome‐c oxidase deficiency.mp. or Ubiquinol‐Cytochrome‐c reductase/df 19 (Kearns adj3 syndrome$).mp. 20 KSS.mp. 21 or/17‐20 22 ((leber adj5 neuropath$) or (Pearson adj5 syndrome$) or (pearson adj5 disease$)).mp. 23 optic nerve/ and (atrophy/ or optic atrophy/) 24 LHON.mp. 25 (MELAS adj6 syndrome$).mp. 26 lactic acidosis.mp. or Lactic Acidosis/ 27 (stroke adj episode$).mp. 28 26 and 27 29 22 or 23 or 24 or 25 or 28 30 ((MERFF adj5 syndrome$) or MERFF).mp. 31 MIDD.mp. 32 (maternal$ adj5 diabetes adj5 deafness).mp. 33 or/30‐32 34 ((leigh adj5 syndrome$) or (leigh adj5 disease$)).mp. 35 MNGIE.mp. 36 (mitochondrial adj5 neurogastrointestinal adj5 encephalomyopath$).mp. 37 or/34‐36 38 retinitis pigmentosa.mp. or Retinitis Pigmentosa/ 39 ATAXIA/ 40 ataxia.mp. 41 38 and (39 or 40) 42 NARP.mp. 43 (pyruvate adj5 carboxylase adj5 deficiency adj5 disease).mp. 44 pyruvate dehydrogenase complex deficiency disease.mp. 45 or/41‐44 46 16 or 21 or 29 or 33 or 37 or 45 47 drug$.mp. or Drug Therapy/ 48 treatment.mp. or Therapeutics/ 49 (coenzyme Q10 or co enzyme Q10 or carnitine$ or creatine$ or idebenone$ or ubidecarenone$ or vitamin C or vitamin E or menadione$ or nicotinamide$ or dichloracetate$ or thiamine$ or riboflavin$ or succinate$ or corticosteroid$).mp. 50 (growth adj5 hormone$).mp. 51 exercise.mp. or Exercise Therapy/ 52 diet$.mp. or Diet Therapy/ 53 or/47‐52 54 11 and 46 and 53
Appendix 2. EMBASE search strategy
1 crossover‐procedure/ 2 double‐blind procedure/ 3 randomized controlled trial/ 4 single‐blind procedure/ 5 (random$ or factorial$ or crossover$ or cross over$ or cross‐over$ or placebo$ or (doubl$ adj blind$) or (singl$ adj blind$) or assign$ or allocat$ or volunteer$).tw. 6 or/1‐5 7 human/ 8 6 and 7 9 nonhuman/ or human/ 10 6 not 9 11 8 or 10 12 exp Disorders of Mitochondrial Functions/ 13 mitochondrial myopath$.mp. 14 12 or 13 15 Encephalomyopathy/ 16 (mitochondrial encephalomyopath$ or mitochondrial encephalopath$).mp. 17 15 or 16 18 External Ophthalmoplegia/ or CPEO.mp. 19 chronic progressive external ophthalmoplegia.mp. 20 or/18‐19 21 (Kearns adj5 syndrome$).mp. 22 Kearns Sayre Syndrome/ 23 KSS.mp. 24 or/21‐23 25 ((Pearson adj5 syndrome$) or (pearson adj5 disease$)).mp. 26 ((Leber adj5 neuropath$).mp. or optic nerve atrophy/) and leber.mp. 27 LHON.mp. 28 25 or 26 or 27 29 (MELAS adj5 syndrome$).mp. or Melas Syndrome/ 30 lactic acidosis.mp. or Lactic Acidosis/ 31 (stroke adj episode$).mp. 32 30 and 31 33 29 or 32 34 ((Merff adj5 syndrome$) or MERFF).mp. 35 Merrf Syndrome/ 36 34 or 35 37 MIDD.mp. 38 (maternal$ adj5 diabetes adj5 deafness).mp. 39 37 or 38 40 ((leigh adj5 syndrome$) or (leigh adj5 disease)).mp. or Leigh Disease/ 41 NARP.mp. or narp syndrome/ 42 ataxia.mp. 43 (retinitis adj3 pigmentosa).mp. or retinitis pigmentosa/ 44 42 and 43 45 41 or 44 46 MNGIE.mp. 47 mitochondrial neurogastrointestinal encephalomyopathy/ or (mitochondrial adj5 neurogastrointestinal adj5 encephalomyopath$).mp. 48 ubiquinol‐cytochrome‐c reductase.mp. or Ubiquinol Cytochrome C Reductase/ 49 enzyme deficiency.mp. or Enzyme Deficiency/ 50 48 and 49 51 46 or 47 52 12 or 14 or 17 or 20 or 24 or 28 or 33 or 36 or 39 or 40 or 45 or 50 or 51 53 diet.mp. or exp Diet Therapy/ 54 drug.mp. or Drug Therapy/ 55 (coenzyme Q10 or co enzyme Q10 or carnitine$ or creatine$ or idebenone$ or ubidecarenone$ or vitamin C or vitamin E or menadione$ or nicotinamide$ or thiamine$ or riboflavin$ or succinate$ or corticosteroid$ or dichloracetate$).mp. 56 (growth adj5 hormone$).mp. 57 exp Kinesiotherapy/ or physiotherapy/ 58 exercise.mp. 59 exp Therapy/ 60 or/53‐59 61 52 and 60 62 11 and 61
Appendix 3. The Cochrane Central Register of Controlled Trials (CENTRAL)
#1MeSH descriptor Mitochondrial Diseases explode all trees #2mitochondrial myopath* #3(mitochondrial encephalomyopath* or mitochondrial encephalopath*) #4(mitochondrial disease* or mitochondrial disorder*) #5(#1 OR #2 OR #3 OR #4) #6((CPEO or ophthalmoplegia) near5 progressive) #7cytochrome‐c oxidase deficiency#8MeSH descriptor Electron Transport Complex III, this term only #9(Kearns near3 syndrome*) #10KSS #11(#6 OR #7 OR #8 OR #9 OR #10) #12((leber near5 neuropath*) or (Pearson near5 syndrome*) or (pearson near5 disease*)) #13MeSH descriptor Optic Nerve, this term only #14MeSH descriptor Atrophy, this term only #15MeSH descriptor Optic Atrophy, this term only #16(#13 AND ( #14 OR #15 )) #17LHON #18(MELAS near6 syndrome*) #19"lactic acidosis" #20MeSH descriptor Acidosis, Lactic, this term only #21(stroke near episode*) #22(( #19 OR #20 ) AND #21) #23(#12 OR #16 OR #17 OR #18 OR #22) #24MERFF #25MIDD #26(maternal* near5 diabetes near5 deafness) #27(#24 OR #25 OR #26) #28((leigh syndrome*) or (leigh disease*)) #29MNGIE #30 (mitochondrial neurogastrointestinal encephalomyopath*) #31(#28 OR #29 OR #30) #32retinitis pigmentosa #33MeSH descriptor Retinitis Pigmentosa, this term only #34MeSH descriptor Ataxia, this term only #35ATAXIA #36(#32 OR #33 OR #34 OR #35) #37NARP #38(pyruvate near5 carboxylase near5 deficiency near5 disease) #39pyruvate dehydrogenase complex deficiency disease #40(#36 OR #37 OR #38 OR #39) #41(#5 OR #11 OR #23 OR #27 OR #31 OR #36 OR #40) #42drug* #43MeSH descriptor Drug Therapy, this term only #44treatment #45MeSH descriptor Therapeutics, this term only #46 ("coenzyme Q10" or "co enzyme Q10" or carnitine* or creatine* or idebenone* or ubidecarenone* or "vitamin C" or "vitamin E" or menadione* or nicotinamide* or dichloracetate* or thiamine* or riboflavin* or succinate* or corticosteroid*) #47(growth near5 hormone*) #48exercise #49MeSH descriptor Exercise Therapy, this term only #50diet* #51MeSH descriptor Diet Therapy, this term only #52(#42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50) #53(#41 AND #52)
Characteristics of studies
Characteristics of included studies [ordered by study ID]
DeStefano 1995.
| Methods | Randomised, placebo‐controlled, double‐blind cross‐over | |
| Participants | 11 participants (4 myopathy, 1 CPEO, 2 KSS, 2 Leigh syndrome, one MELAS) | |
| Interventions | Dichloroacetate 25 mg/kg twice daily or placebo for one week. 3 month washout | |
| Outcomes | Neurological exam, dynamometry, gait evaluation, venous blood lactate, alanine and pyruvate at rest and on incremental exercise; 31P‐MRS of muscle and 1H‐MRS of the brain | |
| Notes | Statistically significantly decreased blood lactate, pyruvate and alanine at rest and after exercise (P < 0.05), and statistically significant improvements on brain MRS (brain lactate/creatine ratio fell by 42%, P < 0.05; brain choline/creatine ratio increased by 18%, P < 0.01; brain acetylaspartate/creatine ratio increased by 18%, P < 0.05) (SD not provided for these values) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not specified |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation not specified |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not all participants underwent all outcome assessments |
| Selective reporting (reporting bias) | Low risk | All outcome measures in methods section were reported in results section |
| Other bias | Low risk | None identified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Groups were "randomized and unknown to the patient, physicians, or other evaluators." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Each patient underwent two treatment courses, one with DCA and one with placebo. The order of these was randomized..." |
Duncan 2004.
| Methods | Randomised, placebo‐controlled, double‐blind cross‐over study | |
| Participants | 9 children and adults with various mitochondrial respiratory chain disorders | |
| Interventions | Dichloroacetate (25 mg/kg daily) or placebo, 2 week washout | |
| Outcomes | Physiologic exercise measurements (VO2 max, work output, lactate, heart rate, blood pressure), and rating of perceived exertion scale | |
| Notes | Blood lactate during submaximal exercise statistically significantly lower in DCA group (1.71 (CI 0.81 to 2.61) versus 2.39 (CI 1.53 to 3.25)). All other outcomes not significant | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not specified |
| Allocation concealment (selection bias) | Unclear risk | Method of sequence allocation not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed all outcome assessments |
| Selective reporting (reporting bias) | Low risk | All outcome measures in methods section were reported in results section |
| Other bias | Low risk | None identified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "subjects received either oral DCA or PL (25 mg/kg)..., administered randomly in a double‐blind fashion" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "subjects received either oral DCA or PL (25 mg/kg)..., administered randomly in a double‐blind fashion" |
Glover 2010.
| Methods | Randomised, double‐blind, placebo‐controlled cross‐over study | |
| Participants | 30 adult participants with various mitochondrial disorders: 15 MELAS, 11 PEO, 1 complex I deficiency, 1 NARP, 1 ataxia‐neuropathy, 1 LHON | |
| Interventions | Coenzyme Q10 600 mg orally twice daily for 60 days and placebo for 60 days. Washout period of 67 +/‐ 8.3 days | |
| Outcomes | Outcome assessment at end of each trial period. Urine analysis of 8‐isoprostane and 8‐hydroxy‐2‐deoxyguanosine. Blood analysis of lactate, glucose, PCO2, CoQ10, bilirubin, creatine kinase and gamma‐glutamyltransferase. Questionnaires for quality of life and ADL. Maximal isometric force and near‐infrared spectroscopy on 90 s forearm ischaemic exercise test, with venous lactate, PCO2, PO2 at 1 min and 3 min post‐test. Dual‐energy X‐ray absorptometry for body composition. 15 min cycle ergometry with monitoring heart rate, inspired O2, expired CO2. VO2 and VCO2 measured at several time points. Venous lactate measured prior, and at various points during and after testing | |
| Notes | MRS of CSF lactate, N‐acetyl aspartate and choline. Serum CoQ10 was statistically significantly increased. Lactate was statistically significantly decreased at 1 min of cycle ergometry, but there was no difference at other time points. All other endpoints did not differ significantly | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not specified |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete outcome data adequately addressed |
| Selective reporting (reporting bias) | Low risk | All outcome measures in methods section were reported in results section |
| Other bias | Unclear risk | The inclusion of participants with mitochondrial diseases that do not have myopathy may have biased the study against a treatment effect |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | This was a double‐blind trial using "identical‐appearing placebo capsules" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants were randomly assigned to placebo or CoQ10 treatment |
Kaufmann 2006.
| Methods | Randomised, placebo controlled cross‐over study | |
| Participants | 30 participants with m.3243A>G | |
| Interventions | 25mg/kg/day of dichloracetate for 12 months | |
| Outcomes | GATE, lactate measurements in venous blood, CSF and measured by 1H‐MRS. Nerve conduction tests were used to monitor safety | |
| Notes | No benefit The trial was discontinued due to peripheral nerve toxicity |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Based on a computer‐generated randomization list" |
| Allocation concealment (selection bias) | Low risk | "the research pharmacy at the University of Florida mailed study medication for each participant to the clinical investigators" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete outcome data for some participants, although this was due to withdrawal of participants due to side‐effects in the DCA group, and early termination of the study |
| Selective reporting (reporting bias) | Low risk | All outcome measures were reported for all participants up to the termination of the trial |
| Other bias | Low risk | None identified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "clinical researchers remained blinded to treatment assignment throughout the trial" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants were randomly assigned to start in either the DCA or placebo arm |
Klopstock 2000.
| Methods | Randomised, placebo‐controlled, double‐blind cross‐over | |
| Participants | 16 participants (13 CPEO, 3 myopathy) | |
| Interventions | Creatine monohydrate 20 g /day or placebo for 4 weeks. Washout ≥ 24 days in all participants | |
| Outcomes | Visual analogue scales of subjective weakness and general activity, neuromuscular symptom score, function time test, function ranking test, ataxia score, muscle strength, motor ability score. Resting and post‐exercise lactate, maximal voluntary muscle torque, aerobic exercise performance, eye motility and eyelid drooping | |
| Notes | No effect | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not specified |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed all outcome assessments |
| Selective reporting (reporting bias) | Low risk | All outcome measures in methods section were reported in results section |
| Other bias | Low risk | None identified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "double‐blind, placebo‐controlled 2x2 crossover trial" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants were randomly assigned to start in either the creatine or placebo arm |
Kornblum 2005.
| Methods | Randomised, placebo controlled cross‐over study | |
| Participants | 15 participants with CPEO or KSS due to single deletions of mtDNA | |
| Interventions | 150 mg/kg of creatine monohydrate or placebo containing lactose for 6 weeks. 4‐week washout | |
| Outcomes | 31P‐MRS (parameters included the PCr/ATP ratio at rest and the time constant for oxidative PCr | |
| Notes | No significant effect | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were individually randomized... using standard PC software." |
| Allocation concealment (selection bias) | Low risk | Allocation conducted by the central pharmacy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete outcome data adequately addressed |
| Selective reporting (reporting bias) | Low risk | All outcome measures in methods section were reported in results section |
| Other bias | Low risk | None identified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | In this double‐blind trial, creatine and placebo provided in "identical‐looking capsules." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants were randomly assigned to start in either the creatine or placebo arm. "Evaluations and calculations on efficacy measures were performed blinded." |
Liet 2003.
| Methods | Randomised, placebo‐controlled cross‐over study | |
| Participants | 5 children with SLSJ‐COX deficiency | |
| Interventions | Dimethylglycine or placebo (50 mg/kg/day if < 33 kg, 5 g/day if > 33 kg). 2‐week washout | |
| Outcomes | Oxygen consumption (VO2) by indirect calorimetry, venous lactate, pyruvate, bicarbonate and pH | |
| Notes | No significant effect | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The order of administration of placebo and DMG was randomized by using a one‐block sequence generated from a random number table." |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed all outcome assessments |
| Selective reporting (reporting bias) | Low risk | All outcome measures in methods section were reported in results section |
| Other bias | Low risk | None identified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Capsules containing placebo or DMG were indistinguishable." This was a double‐blind cross‐over trial, involved dieticians were also blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants were randomly assigned to start in either the DMG or placebo arm. |
Mancuso 2010.
| Methods | Randomised, double‐blind, placebo‐controlled cross‐over study | |
| Participants | Thirteen participants with progressive external ophthalmoplegia | |
| Interventions | 10 g/day of whey‐based oral supplement or placebo for 30 days, then immediately proceed to next group | |
| Outcomes | Measurements of markers of free radical reducing capacity (advanced oxidation protein products (AOPP); ferric reducing antioxidant power (FRAP); glutathione (GSH) level). Serum lactate measured at various time points during cycle ergometry. Quality of life measured using SF‐36 questionnaire. Muscle power measured with MRC scale in various muscle groups | |
| Notes | AOPP, FRAP and GSH were statistically significantly increased in the treatment period. Lactate, quality of life, and muscle power were unchanged | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not specified |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete outcome data adequately addressed |
| Selective reporting (reporting bias) | Low risk | All outcome measures in methods section were reported in results section |
| Other bias | Unclear risk | There was no washout period between trials which could potentially decrease the treatment effect |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It is not specified whether the placebo had identical characteristics (appearance/taste) to the treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants were randomly assigned to placebo or CoQ10 treatment |
Rodriguez 2007.
| Methods | Randomised, double‐blind, placebo‐controlled cross‐over study | |
| Participants | 16 participants with different mitochondrial disorders (3 CPEO, 1 KSS, 3 MELAS, 1 MNGIE, 3 with mitochondrial disorders due to rare point mutations (m.9053T>C, m.4452T>C and m.9035T>C)) | |
| Interventions | Combination of 3 g creatine monohydrate with 2 g of dextrose, 300 mg alpha‐lipoic acid, and 120 mg coenzyme Q10 | |
| Outcomes | Handgrip, ankle dorsiflexion (at 90°) and knee extension, forced vital capacity and forced expiratory volume, free fat mass, percentage body fat and total body water, plasma lactate, urinary 8‐isoprostanes and 8‐hydroxy‐2‐deoxyguanosine | |
| Notes | Across the whole study group, the treatment statistically significantly lowered plasma lactate and urinary 8‐isoprostanes, and there was evidence it attenuated the decline in peak angle dorsiflexion strength | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not specified |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete outcome data adequately addressed |
| Selective reporting (reporting bias) | Low risk | All outcome measures in methods section were reported in results section |
| Other bias | Low risk | None identified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | In this double‐blind study, placebo provided as "identical‐appearing and tasting powder... and gel capsules." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants were randomly assigned to start in either the treatment or placebo arm |
Stacpoole 2006.
| Methods | Randomised, double‐blind, placebo‐controlled parallel‐group study | |
| Participants | 43 participants with congenital lactic acidosis, including 25 with one or more defects of the respiratory chain and 7 with a mutation of mtDNA | |
| Interventions | 12.5 mg/kg of dichloracetate every 12 hours for 6 months | |
| Outcomes | GATE; linear growth; fasting blood lactate, and lactate after a carbohydrate meal; the frequency of severe or recurrent illnesses or hospital admissions; and safety tests of liver and peripheral nerve function | |
| Notes | Statistically significant attenuation of the rise in blood lactate following a carbohydrate meal. No clinical benefit | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not specified |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete outcome data adequately addressed |
| Selective reporting (reporting bias) | Low risk | All outcome measures in methods section were reported in results section |
| Other bias | Low risk | None identified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | This was a double‐blind trial. "[P]lacebo solution was formulated in an identical manner [to the DCA solution], except for the absence of DCA." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants were randomly assigned to placebo or DCA treatment |
Tarnopolsky 1997.
| Methods | Randomised, placebo‐controlled, double‐blind cross‐over | |
| Participants | 7 participants (6 MELAS, 1 myopathy) | |
| Interventions | Creatine monohydrate 5 g twice daily for two weeks followed by 2 g twice daily for 1 week | |
| Outcomes | Visual analogues ADL scale, ischaemic isometric handgrip strength, evoked and voluntary muscle contraction strength, NIDFT, aerobic cycle ergometry with basal and post‐ischaemic lactate measurements | |
| Notes | Statistically significantly increased handgrip strength (19% increase in torque in N m, P < 0.01), NIDFT (11%, P < 0.01) and post‐exercise lactate (P < 0.05) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not specified |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed all outcome assessment. |
| Selective reporting (reporting bias) | Low risk | All outcome measures in methods section were reported in results section |
| Other bias | Low risk | None identified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | This was a double‐blind study. Treatment and placebo provided as "identical and indistinguishable powders." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants were randomly assigned to start in either the treatment or placebo arm |
Vissing 2001.
| Methods | Randomised, placebo‐controlled, double‐blind cross‐over | |
| Participants | 8 participants (3 CPEO, 1 MERRF, 5 mitochondrial myopathy) | |
| Interventions | Dichloroacetate 25 mg/kg twice daily or placebo for 15 days. Washout of 6 weeks in all participants | |
| Outcomes | VO2, workload and blood pressure during cycle ergometry, with measurements of venous lactate and pyruvate. 31P‐MRS of forearm muscles before and after forearm exercise was measured. All participants filled a 20‐item questionnaire commenting on activity levels and symptoms. Outcome measurements were performed before and at the end of each treatment phase | |
| Notes | Normalisation of resting and exercise lactate and pyruvate levels. All other outcomes were non‐significant or did not favour treatment. One participant withdrew due to sedation on treatment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not specified |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed all outcome assessments |
| Selective reporting (reporting bias) | Low risk | All outcome measures in methods section were reported in results section |
| Other bias | Low risk | None identified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | This was a double‐blind study. Placebo was "the same volume, color and flavor as the DCA mixture." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants were randomly assigned to start in either the treatment or placebo arm |
ADL: activities of daily living
ATP: adenosine triphosphate
CPEO: chronic progressive external ophthalmoplegia
DCA: dichloroacetate
DMG: dimethylglycine
GATE: Global Assessment of Treatment Efficacy score, based on a health‐related inventory, neurological and psychological evaluation, and activities of daily living
1H‐MRS: proton magnetic resonance spectroscopy
KSS: Kearns‐Sayre syndrome
MELAS: mitochondrial encephalomyopathy with lactic acidosis and stroke‐like episodes
MERRF: myoclonic epilepsy with ragged‐red fibres
MRC: UK Medical Research Council
mtDNA: mitochondrial DNA
NIDFT: nonischaemic isometric dorsiflexion torque 31P‐MRS: phosphorus magnetic resonance spectroscopy
PCr: phosphocreatine
SD: standard deviation
SF‐36 Short Form 36 Health Survey
SLSJ‐COX: Saguenay‐Lac‐Saint‐Jean cytochrome c oxidase deficiency
VO2: oxygen consumption
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bresolin 1990 | High risk of bias because entry criterion for the double‐blind portion of the study was decided retrospectively based upon the results of the open‐label phase. Furthermore, the double‐blind portion of the study was not randomised. |
| Cejudo 2005 | High risk of bias due to lack of blinding. |
| Chen 1997 | High risk of bias. Participants were not randomised. The study design (3 months of coenzyme Q10 or 1 month placebo, with one month washout if the participant started on coenzyme Q10) would allow a blinded assessor to know whether participant was on treatment or placebo depending on the duration of time in the study. There is a possibility of selective outcome reporting since participants were assessed monthly although results were only reported for end of three‐month treatment period |
| Gimenes 2007 | High risk of bias due to possibility of selective outcome reporting, and inability to fully assess risk of bias in other domains, since this publication consists of an abstract only, and the study has not been published in a peer‐reviewed format |
| Koga 2005 | High risk of bias due to the non‐randomised, open‐label design |
| Muller 1990 | High risk of bias. Participants were not randomised. Data and statistical analysis were not reported. There is a possibility of selective outcome reporting given that study endpoints were not clearly stated, and only 7 participants were reported. This evidence is in abstract format and therefore it is not possible to fully assess the risk of bias |
| Stacpoole 2008 | High risk of bias due to the non‐randomised, open‐label, non‐controlled study design |
| Suzuki 1998 | High risk of bias due to the non‐randomised, open‐label, non‐placebo controlled study design |
| Taivassalo 2006 | High risk of bias due to the non‐randomised, non‐controlled, non‐blinded study design |
Differences between protocol and review
We removed the European Neuromuscular Centre (ENMC) clinical trials register from the resources to be searched.
For the 2012 update we incorporated 'Risk of bias' tables.
In the protocol we stated that all authors would review search results and select studies. For the update, two authors screened search results and selected studies for further review which were then assessed by all five authors.
For the update the study inclusion criteria were changed and the authors excluded quasi‐randomised studies and studies at high risk of bias for any domain.
Contributions of authors
For the first version of the review, all four authors agreed the criteria for inclusion of studies and their methodological quality. All four authors independently assessed all of the studies reviewed. PFC completed the first draft which was modified with comments from the other authors. GP and PFC updated the review. GP and PFC independently assessed all of the abstracts, including the original studies, to determine which required further review. All five reviewers agreed the criteria for inclusion of studies and their methodological quality. PFC modified the original review with GP, which was further modified following comments from the other reviewers.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Wellcome Trust, UK.
- Funding for PFC and DMT
-
Muscular Dystrophy Campaign, UK.
- Funding for DRT
-
Medical Research Council, UK.
- Funding for PFC and DMT
Ataxia, UK.
-
Association Francaise contre les myopathies, France.
- Funding for PFC and DMT
Council for Health Research, Finland.
Sigrid Juselius Foundation, Finland.
-
Australian National Health and Medical Research Council, Australia.
- Funding for DRT
-
Muscular Dystrophy Association, USA.
- Funding for DRT
-
Parkinson's UK, UK.
- Funding for PFC and DMT
-
National Institute for Health Research (NIHR), UK.
- Funding for PFC and DMT through the Newcastle NIHR Biomedical Research Centre in ageing and age‐related disease
-
Clinician Investigator Program, University of British Columbia, Canada.
- Funding for GP
-
Canadian Institutes of Health Research, Canada.
- GP is in receipt of a Bisby Fellowship
-
Juvenile Diabetes Research Foundation, Not specified.
- Funding for DRT
-
Foundation for Children, Not specified.
- Funding for DRT
Declarations of interest
Patrick Chinnery: none known.
Kari Majamaa: has received funding for travel from Octapharma and Lundbeck and speaker honoraria from OrionPharma.
Gerald Pfeffer: is the recipient of funding from the Clinician Investigator Program from the University of British Columbia in Vancouver, Canada, and a Bisby fellowship from the Canadian Institutes of Health Research. He reports no competing interests.
David Thorburn: none known.
Douglass Turnbull: our work was supported by The Wellcome Trust Centre for Mitochondrial Research (906919), the Newcastle University Centre for Brain Ageing and Vitality supported by BBSRC, EPSRC, ESRC and MRC as part of the cross‐council Lifelong Health and Wellbeing Initiative (G0700718)
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
DeStefano 1995 {published data only}
- DeStefano N, Matthews PM, Ford B, Genge A, Karpati G, Arnold DL. Short‐term dichloracetate treatment improves indices of cerebral metabolism in patients with mitochondrial disorders. Neurology 1995;45(6):1193‐8. [PUBMED: 7783888] [DOI] [PubMed] [Google Scholar]
Duncan 2004 {published data only}
- Duncan GE, Perkins LA, Theriaque DW, Neiberger RE, Stacpoole PW. Dichloroacetate therapy attenuates the blood lactate response to submaximal exercise in patients with defects in mitochondrial energy metabolism. Journal of Clinical Endocrinology and Metabolism 2004;89(4):1733‐8. [PUBMED: 15070938] [DOI] [PubMed] [Google Scholar]
Glover 2010 {published data only}
- Glover EI, Martin J, Maher A, Thornhill RE, Moran GR, Tarnopolsky MA. A randomized trial of coenzyme Q10 in mitochondrial disorders. Muscle & Nerve 2010;42(5):739‐48. [PUBMED: 20886510] [DOI] [PubMed] [Google Scholar]
Kaufmann 2006 {published data only}
- Kaufmann P, Engelstad K, Wei Y, Jhung S, Sano MC, Shungu DC, et al. Dichloroacetate causes toxic neuropathy in MELAS. Neurology 2006;66(3):324‐30. [PUBMED: 16476929] [DOI] [PubMed] [Google Scholar]
Klopstock 2000 {published data only}
- Klopstock T, Querner V, Schmidt F, Gekeler F, Walter M, Hartard M, et al. A placebo‐controlled crossover trial of creatine in mitochondrial diseases. Neurology 2000;55(11):1748‐51. [PUBMED: 11113239] [DOI] [PubMed] [Google Scholar]
Kornblum 2005 {published data only}
- Kornblum C, Schröder R, Müller K, Vorgerd M, Eggers J, Bogdanow M, et al. Creatine has no beneficial effect on skeletal muscle energy metabolism in patients with single mitochondrial deletions: a placebo‐controlled, double‐blind 31P‐MRS crossover study. European Journal of Neurology 2005;12(4):300‐9. [PUBMED: 15804248] [DOI] [PubMed] [Google Scholar]
Liet 2003 {published data only}
- Liet JM, Pelletier V, Robinson BH, Laryea MD, Wendel U, Morneau S, et al. The effect of short‐term dimethylglycine treatment on oxygen consumption in cytochrome oxidase deficiency: a double‐blind randomized crossover clinical trial. Journal of Pediatrics 2003;142(1):62‐6. [PUBMED: 12520257] [DOI] [PubMed] [Google Scholar]
Mancuso 2010 {published data only}
- Mancuso M, Orsucci D, LoGerfo A, Rocchi A, Petrozzi L, Nesti C, et al. Oxidative stress biomarkers in mitochondrial myopathies, basally and after cysteine donor supplementation. Journal of Neurology 2010;257(5):774‐781. [DOI] [PubMed] [Google Scholar]
Rodriguez 2007 {published data only}
- Rodriguez MC, MacDonald JR, Mahoney DJ, Parise G, Beal MF, Tarnopolsky MA. Beneficial effects of creatine, CoQ10, and lipoic acid in mitochondrial disorders. Muscle & Nerve 2007;35(2):235‐42. [PUBMED: 17080429] [DOI] [PubMed] [Google Scholar]
Stacpoole 2006 {published data only}
- Stacpoole PW, Kerr DS, Barnes C, Bunch ST, Carney PR, Fennell EM, et al. Controlled clinical trial of dichloroacetate for treatment of congenital lactic acidosis in children. Pediatrics 2006;117(5):1519‐31. [PUBMED: 16651305] [DOI] [PubMed] [Google Scholar]
Tarnopolsky 1997 {published data only}
- Tarnopolsky MA, Roy BD, MacDonald JR. A randomized controlled trial of creatine monohydrate in patients with mitochondrial cytopathies. Muscle & Nerve 1997;20(12):1502‐9. [PUBMED: 9390662] [DOI] [PubMed] [Google Scholar]
Vissing 2001 {published data only}
- Vissing J, Gansted U, Quistorff B. Exercise intolerance in mitochondrial myopathy is not related to lactic acidosis. Annals of Neurology 2001;49(5):672‐6. [PUBMED: 11357960] [PubMed] [Google Scholar]
References to studies excluded from this review
Bresolin 1990 {published data only}
- Bresolin N, Doriguzzi C, Ponzetto C, Angelini C, Moroni I, Castelli E, et al. Ubidecarenone in the treatment of mitochondrial myopathies: a multi‐center double‐blind trial. Journal of the Neurological Sciences 1990;100(1‐2):70‐8. [PUBMED: 2089142] [DOI] [PubMed] [Google Scholar]
Cejudo 2005 {published data only}
- Cejudo P, Bautista J, Montemayor T, Villagómez R, Jiménez L, Ortega F, et al. Exercise training in mitochondrial myopathy: a randomized controlled trial. Muscle & Nerve 2005;32(3):342‐50. [PUBMED: 15962332] [DOI] [PubMed] [Google Scholar]
Chen 1997 {published data only}
- Chen RS, Huang CC, Chu NS. Coenzyme Q10 treatment in mitochondrial encephalomyopathies. Short‐term double‐blind, crossover study. European Neurology 1997;37(4):212‐8. [PUBMED: 9208260] [DOI] [PubMed] [Google Scholar]
Gimenes 2007 {published data only}
- AC Gimenes, LM Nápolis, NL Silva, GO Siqueira, CR Nogueira, AS Bulle, et al. The effects of L‐carnitine supplementation on respiratory muscle strength and exercise tolerance in patients with mitochondrial myopathies [Abstract]. European Respiratory Journal 2007;Suppl 51:21S[E297]. [Google Scholar]
Koga 2005 {published data only}
- Koga Y, Akita Y, Nishioka J, Yatsuga S, Povalko N, Tanabe Y, et al. L‐arginine improves the symptoms of strokelike episodes in MELAS. Neurology 2005;64(4):710‐2. [PUBMED: 15728297] [DOI] [PubMed] [Google Scholar]
Muller 1990 {published data only}
- Muller W, Reimers CD, Berninger T, Boergen K‐P, Frey A, Zrenner E, et al. Coenzyme Q10 in ophthalmoplegia plus ‐ a double blind, cross over therapeutic trial. Journal of the Neurological Sciences 1990;98 Supplement:442. [Google Scholar]
Stacpoole 2008 {published data only}
- Stacpoole PW, Gilbert LR, Neiberger RE, Carney PR, Valenstein E, Theriaque DW, et al. Evaluation of long‐term treatment of children with congenital lactic acidosis with dichloroacetate. Pediatrics 2008;121(5):e1223‐8. [PUBMED: 18411236] [DOI] [PMC free article] [PubMed] [Google Scholar]
Suzuki 1998 {published data only}
- Suzuki S, Hinokio Y, Ohtomo M, Hirai M, Hirai A, Chiba M, et al. The effects of coenzyme Q10 treatment on maternally inherited diabetes mellitus and deafness, and mitochondrial DNA 3243 (A to G) mutation. Diabetologia 1998;41(5):584‐8. [PUBMED: 9628277] [DOI] [PubMed] [Google Scholar]
Taivassalo 2006 {published data only}
- Taivassalo T, Gardner JL, Taylor RW, Schaefer AM, Newman J, Barron MJ, et al. Endurance training and detraining in mitochondrial myopathies due to single large‐scale mtDNA deletions. Brain 2006;129(Pt 12):3391‐401. [PUBMED: 17085458] [DOI] [PubMed] [Google Scholar]
Additional references
Abe 1991
- Abe K, Fujimura H, Nishikawa Y, Yorifuji S, Mezaki T, Hirono N, et al. Marked reduction in CSF lactate and pyruvate levels after CoQ therapy in a patient with mitochondrial myopathy, encephalopathy, lactic acidosis and stroke‐like episodes (MELAS). Acta Neurologica Scandinavica 1991;83(6):356‐9. [DOI] [PubMed] [Google Scholar]
Abe 1999
- Abe K, Matsuo Y, Kadekawa J, Inoue S, Yanagihara T. Effect of coenzyme Q10 in patients with mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke‐like episodes (MELAS): evaluation by noninvasive tissue oximetry. Journal of the Neurological Sciences 1999;162(1):65‐8. [DOI] [PubMed] [Google Scholar]
Anderson 1981
- Anderson S, Bankier AT, Barrell BG, Bruijn MH, Coulson AR, Drouin J, et al. Sequence and organization of the human mitochondrial genome. Nature 1981;290(5806):457‐65. [DOI] [PubMed] [Google Scholar]
Barbiroli 1995
- Barbiroli B, Montagna P, Cortelli P, Iotti S, Lodi R, Barboni P, et al. Lipoic (thioctic) acid increases brain energy availability and skeletal muscle performance as shown by in vivo 31P‐MRS in a patient with mitochondrial cytopathy. Journal of Neurology 1995;242(7):472‐7. [DOI] [PubMed] [Google Scholar]
Barshop 2004
- Barshop BA, Naviaux RK, McGowan KA, Levine F, Nyhan WL, Loupis‐Geller A, et al. Chronic treatment of mitochondrial disease patients with dichloroacetate. Molecular Genetics and Metabolism 2004;83(1‐2):138‐49. [DOI] [PubMed] [Google Scholar]
Bendahan 1992
- Bendahan D, Desnuelle C, Vanuxem D, Confort‐Gouny S, Figarella‐Branger D, Pellissier JF, et al. 31P NMR spectroscopy and ergometer exercise test as evidence for muscle oxidative performance improvement with coenzyme Q in mitochondrial myopathies. Neurology 1992;42(6):1203‐8. [DOI] [PubMed] [Google Scholar]
Bernsen 1993
- Bernsen PL, Gabreels FJ, Ruitenbeek W, Hamburger HL. Treatment of complex I deficiency with riboflavin. Journal of the Neurological Sciences 1993;118(2):181‐7. [DOI] [PubMed] [Google Scholar]
Chinnery 1997
- Chinnery PF, Turnbull DM. Clinical features, investigation and management of patients with defects of mitochondrial DNA. Journal of Neurology, Neurosurgery and Psychiatry 1997;63(5):559‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chinnery 1998
- Chinnery PF, Howell N, Lightowlers RN, Turnbull DM. Genetic counseling and prenatal diagnosis for mtDNA disease. American Journal of Human Genetics 1998;63(6):1908‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chinnery 2001
- Chinnery PF, Turnbull DM. Epidemiology and treatment of mitochondrial disease. American Journal of Medical Genetics 2001;106(1):94‐101. [DOI] [PubMed] [Google Scholar]
Cortelli 1997
- Cortelli P, Montagna P, Pierangeli G, Lodi R, Barboni P, Liguori R, et al. Clinical and brain bioenergetics improvement with idebenone in a patient with Leber's hereditary optic neuropathy: a clinical and 31P‐MRS study. Journal of the Neurological Sciences 1997;148(1):25‐31. [DOI] [PubMed] [Google Scholar]
Curless 1986
- Curless RG, Flynn J, Bachynski B, Gregorios JB, Benke P, Cullen R. Fatal metabolic acidosis, hyperglycemia, and coma after steroid therapy for Kearns‐Sayre syndrome. Neurology 1986;36(6):872‐3. [DOI] [PubMed] [Google Scholar]
DiMauro 1998
- DiMauro S, Schon EA. Nuclear power and mitochondrial disease. Nature Genetics 1998;19(3):214‐5. [DOI] [PubMed] [Google Scholar]
DiMauro 2001
- DiMauro S, Schon EA. Mitochondrial DNA mutations in human disease. American Journal of Medical Genetics 2001;106(1):18‐26. [DOI] [PubMed] [Google Scholar]
DiMauro 2003
- DiMauro S, Schon EA. Mitochondrial respiratory‐chain diseases. New England Journal of Medicine 2003;348(26):2656‐68. [DOI] [PubMed] [Google Scholar]
Eleff 1984
- Eleff S, Kennaway NG, Buist NR, Darley‐Usmar VM, Capaldi RA, Bank WJ, et al. 31P NMR study of improvement in oxidative phosphorylation by vitamins K3 and C in a patient with a defect in electron transport at complex III in skeletal muscle. Proceedings of the National Academy of Sciences of the United States of America 1984;81(11):3529‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gold 1996
- Gold R, Seibel P, Reinelt G, Schinder R, Landwehr P, Beck A, et al. Phosphorus magnetic resonance spectroscopy in the evaluation of mitochondrial myopathies: results of a 6‐month therapy study with coenzyme Q. European Neurology 1996;36(4):191‐6. [DOI] [PubMed] [Google Scholar]
Gubbay 1989
- Gubbay SS, Hankey GJ, Tan NT, Fry JM. Mitochondrial encephalomyopathy with corticosteroid dependence. The Medical Journal of Australia 1989;151(2):100‐8, 10. [DOI] [PubMed] [Google Scholar]
Hassani 2010
- Hassani A, Horvath R, Chinnery PF. Mitochondrial myopathies: developments in treatment. Current Opinion in Neurology 2010;23(5):459‐65. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Holt 1988
- Holt IJ, Harding AE, Morgan‐Hughes JA. Deletion of muscle mitochondrial DNA in patients with mitochondrial myopathies. Nature 1988;331(6158):717‐9. [DOI] [PubMed] [Google Scholar]
Hsu 1995
- Hsu CC, Chuang YH, Tsai JL, Jang HJ, Shen YY, Huang HL, et al. CPEO and carnitine deficiency overlapping in MELAS syndrome. Acta Neurologica Scandinavica 1995;92(3):252‐5. [DOI] [PubMed] [Google Scholar]
Ihara 1989
- Ihara Y, Namba R, Kuroda S, Sato T, Shirabe T. Mitochondrial encephalomyopathy (MELAS): pathological study and successful therapy with coenzyme Q10 and idebenone. Journal of the Neurological Sciences 1989;90(3):263‐71. [DOI] [PubMed] [Google Scholar]
Jeppesen 2006
- Jeppesen TD, Schwartz M, Olsen DB, Wibrand F, Krag T, Dunø M, et al. Aerobic training is safe and improves exercise capacity in patients with mitochondrial myopathy. Brain 2006;129(Pt 12):3402‐12. [DOI] [PubMed] [Google Scholar]
Kaukonen 2000
- Kaukonen J, Juselius JK, Tiranti V, Kyttala A, Zeviani M, Comi GP, et al. Role of adenine nucleotide translocator 1 in mtDNA maintenance. Science 2000;289(5480):782‐5. [DOI] [PubMed] [Google Scholar]
Kurlemann 1995
- Kurlemann G, Paetzke I, Maler H, Masur H, Schuierer G, Weglage J, et al. Therapy of complex I deficiency: peripheral neuropathy during dichloracetate therapy. European Journal of Pediatrics 1995;154(11):928‐32. [DOI] [PubMed] [Google Scholar]
Lamantea 2002
- Lamantea E, Tiranti V, Bordoni A, Toscano A, Bono F, Servidei S, et al. Mutations of mitochondrial DNA polymerase gammaA are a frequent cause of autosomal dominant or recessive progressive external ophthalmoplegia. Annals of Neurology 2002;52(2):211‐9. [DOI] [PubMed] [Google Scholar]
Leonard 2000
- Leonard JV, Schapira AVH. Mitochondrial respiratory chain disorders I: mitochondrial DNA defects. Lancet 2000;355(9200):299‐304. [DOI] [PubMed] [Google Scholar]
Lou 1981
- Lou HC. Correction of increased plasma pyruvate and plasma lactate levels using large doses of thiamine in a patient with Kearns‐Sayre syndrome. Archives of Neurology 1981;38(7):469. [DOI] [PubMed] [Google Scholar]
Macmillan 1993
- Macmillan C, Lach B, Shoubridge EA. Variable distribution of mutant mitochondrial DNAs (tRNA(Leu[3243])) in tissues of symptomatic relatives with MELAS: the role of mitotic segregation. Neurology 1993;43(8):1586‐90. [DOI] [PubMed] [Google Scholar]
Majamaa 1997
- Majamaa K, Rusanen H, Remes A, Hassinen IE. Metabolic interventions against complex I deficiency in MELAS syndrome. Molecular and Cellular Biochemistry 1997;174(1‐2):291‐6. [PubMed] [Google Scholar]
Mandel 2001
- Mandel H, Szargel R, Labay V, Elpeleg O, Saada A, Shalata A. The deoxyguanosine kinase gene is mutated in individuals with depleted hepatocerebral mitochondrial DNA. Nature Genetics 2001;29(3):337‐41. [DOI] [PubMed] [Google Scholar]
Mashima 1992
- Mashima Y, Hiida Y, Oguchi Y. Remission of Leber's hereditary optic neuropathy with idebenone [letter]. Lancet 1992;340(8815):368‐9. [PUBMED: 1353825] [DOI] [PubMed] [Google Scholar]
Mashima 2000
- Mashima Y, Kigasawa K, Wakakura M, Oguchi Y. Do idebenone and vitamin therapy shorten the time to achieve visual recovery in Leber hereditary optic neuropathy?. Journal of Neuro‐Ophthalmology 2000;20(3):166‐70. [DOI] [PubMed] [Google Scholar]
Mathews 1993
- Mathews PM, Andermann F, Silver K, Karpati G, Arnold DL. Proton MR spectroscopic characterization of differences in regional brain metabolic abnormalities in mitochondrial encephalomyopathies. Neurology 1993;43(12):2484‐90. [DOI] [PubMed] [Google Scholar]
Mitsui 2002
- Mitsui T, Umaki Y, Nagaswa M, Akaike M, Aki K, Azuma H, et al. Mitochondrial damage in patients with long‐term corticosteroid therapy: development of oculoskeletal symptoms similar to mitochondrial disease. Acta Neuropathologica 2002;104(3):260‐6. [DOI] [PubMed] [Google Scholar]
Montini 2008
- Montini G, Malaventura C, Salviati L. Early coenzyme Q10 supplementation in primary coenzyme Q10 deficiency. New England Journal of Medicine 2008;358(26):2849‐50. [DOI] [PubMed] [Google Scholar]
Mootha 2003
- Mootha VK, Lepage P, Miller K, Bunkenborg J, Reich M, Hjerrild M, et al. Identification of a gene causing human cytochrome c oxidase deficiency by integrative genomics. Proceedings of the National Academy of Sciences of the United States of America 2003;100(2):605‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moraes 1989
- Moraes CT, DiMauro S, Zeviani M, Lombes A, Shanske S, Miranda AF, et al. Mitochondrial DNA deletions in progressive external ophthalmoplegia and Kearns‐Sayre syndrome. New England Journal of Medicine 1989;320(20):1293‐9. [DOI] [PubMed] [Google Scholar]
Mori 2004
- Mori M, Yamagata T, Goto T, Saito S, Momoi MY. Dichloroacetate treatment for mitochondrial cytopathy: long‐term effects in MELAS. Brain & Development 2004;26(7):453‐8. [DOI] [PubMed] [Google Scholar]
Mowat 1999
- Mowat D, Kirby DM, Kamatu KR, Kan A, Thorburn DR, Christodoulou J. Respiratory chain complex III deficiency with pruritis: a novel vitamin responsive clinical feature. Journal of Pediatrics 1999;134(3):352‐4. [DOI] [PubMed] [Google Scholar]
Murphy 2008
- Murphy JL, Blakely EL, Schaefer AM, He L, Wyrick P, Haller RG. Resistance training in patients with single, large‐scale deletions of mitochondrial DNA. Brain 2008;131(Pt 11):2832‐40. [DOI] [PubMed] [Google Scholar]
Musumeci 2001
- Musumeci O, Naini A, Slonim AE, Skavin N, Hadjigeorgiou GL, Krawiecki N, et al. Familial cerebellar ataxia with muscle coenzyme Q10 deficiency. Neurology 2001;56(7):849‐55. [DOI] [PubMed] [Google Scholar]
Naviaux 2004
- Naviaux RK, Nguyenk V. POLG mutations associated with Alpers' syndrome and mitochondrial DNA depletion. Annals of Neurology 2004;55(5):706‐12. [DOI] [PubMed] [Google Scholar]
Nishikawa 1989
- Nishikawa K, Takahashi M, Yorifuji S, Nakamura Y, Veno S, Tarui S, et al. Long‐term coenzyme Q10 therapy for a mitochondrial encephalomyopathy with cytochrome c oxidase deficiency: a 31P NMR study. Neurology 1989;39(3):399‐403. [DOI] [PubMed] [Google Scholar]
Nishino 1999
- Nishino I, Spinazzola A, Hirano M. Thymidine phosphorylase gene mutations in MNGIE, a human mitochondrial disorder. Science 1999;283(5402):689‐92. [DOI] [PubMed] [Google Scholar]
Ogasahara 1986
- Ogasahara S, Nishikawa Y, Yorifuji S, Soga F Nakamura Y, Takahashi M, et al. Treatment of Kearns‐Sayre syndrome with coenzyme Q10. Neurology 1986;36(1):45‐53. [DOI] [PubMed] [Google Scholar]
Ogasahara 1989
- Ogasahara S, Engel AG, Frens D, Mack D. Muscle coenzyme Q deficiency in familial mitochondrial encephalomyopathy. Proceedings of the National Academy of Sciences of the United States of America 1989;86(7):2379‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Oguro 2004
- Oguro H, Iijima K, Takahasi K, Nagai A, Bokwa H, Yamaguchi S, et al. Successful treatment with succinate in a patient with MELAS. Internal Medicine 2004;43(5):427‐31. [DOI] [PubMed] [Google Scholar]
Panetta 2004
- Panetta J, Smith LJ, Boneh A. Effect of high‐dose vitamins, coenzyme Q and high‐fat diet in paediatric patients with mitochondrial diseases. Journal of Inherited Metabolic Disease 2004;27(4):487‐98. [DOI] [PubMed] [Google Scholar]
Papadimitriou 1996
- Papadimitriou A, Hadjigeorgiou GM, Divavi R, Pagagalanis N, Comi G, Bresolin N. The influence of coenzyme Q10 on total serum calcium concentration in two patients with Kearns‐Sayre syndrome and hypoparathyroidism. Neuromuscular Disorders 1996;6(1):49‐53. [DOI] [PubMed] [Google Scholar]
Penn 1992
- Penn AM, Lee JW, Thuillier P, Wagner M, Machire KM Menard MR, et al. MELAS syndrome with mitochondrial tRNA(Leu)(UUR) mutation: correlation of clinical state, nerve conduction, and muscle 31P magnetic resonance spectroscopy during treatment with nicotinamide and riboflavin. Neurology 1992;42(11):2147‐52. [DOI] [PubMed] [Google Scholar]
Remes 2002
- Remes AM, Liimatta EV, Winqvist S, Tolonen U, Ranua JA, Reinikaineuk, et al. Ubiquinone and nicotinamide treatment of patients with the 3243A‐‐>G mtDNA mutation. Neurology 2002;59(8):1275‐7. [DOI] [PubMed] [Google Scholar]
Rotig 2000
- Rotig A, Appelkvist EL, Geromel V, Chretien D, Kadahan N, Edery P, et al. Quinone‐responsive multiple respiratory chain deficiency due to widespread coenzyme Q10 deficiency. Lancet 2000;356(9227):391‐5. [DOI] [PubMed] [Google Scholar]
Saada 2001
- Saada A, Shaag A, Mandel H, Nevo Y, Eriksson S, Elpeleg O. Mutant mitochondrial thymidine kinase in mitochondrial DNA depletion myopathy. Nature Genetics 2001;29(3):342‐4. [DOI] [PubMed] [Google Scholar]
Schaefer 2008
- Schaefer AM, McFarland R, Blakely EL, He L, Whittaker RG. Prevalence of mitochondrial DNA disease in adults. Annals of Neurology 2008;63(1):35‐9. [DOI] [PubMed] [Google Scholar]
Schapira 2006
- Schapira AH. Mitochondrial disease. Lancet 2006;368(9529):70‐82. [DOI] [PubMed] [Google Scholar]
Schon 1997
- Schon EA, Bonilla E, DiMauro S. Mitochondrial DNA mutations and pathogenesis. Journal of Bioenergetics and Biomembranes 1997;29(2):131‐49. [DOI] [PubMed] [Google Scholar]
Shoffner 1989
- Shoffner JM, Lott MT, Voljavec AS, Soueidan SA, Costigan DA, Wallace DC. Spontaneous Kearn‐Sayre/chronic external ophthalmoplegia plus syndromes associated with a mitochondrial DNA deletion: a slip‐replication model and metabolic therapy. Proceedings of the National Academy of Sciences of the United States of America 1989;86(20):7952‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sobreira 1997
- Sobreira C, Hirano M, Shanske S, Keller RK, Haller RG, Davidson E, et al. Mitochondrial encephalomyopathy with coenzyme Q10 deficiency. Neurology 1997;48(5):1238‐43. [DOI] [PubMed] [Google Scholar]
Spelbrink 2001
- Spelbrink JN, Li FY, Tiranti V, Nikali K, Yuan QP, Tariq M, et al. Human mitochondrial DNA deletions associated with mutations in the gene encoding Twinkle, a phage T7 gene 4‐like protein localised in mitochondria. Nature Genetics 2001;28(3):223‐31. [DOI] [PubMed] [Google Scholar]
Spinazzola 2009
- Spinazzola A, Zeviani M. Mitochondrial diseases: a cross‐talk between mitochondrial and nuclear genomes. Advances in Experimental Medicine and Biology 2009;652:69‐84. [DOI] [PubMed] [Google Scholar]
Taivassalo 1998
- Taivassalo T, Stefano N, Argov Z, Matthews PM, Chen J, Genge A, et al. Effects of aerobic training in patients with mitochondrial myopathies. Neurology 1998;50(4):1055‐60. [DOI] [PubMed] [Google Scholar]
Taivassalo 2001
- Taivassalo T, Shoubridge EA, Chen J, Kennaway NG, Dimauro S, Arnold DL, et al. Aerobic conditioning in patients with mitochondrial myopathies: physiological, biochemical, and genetic effects. Annals of Neurology 2001;50(2):133‐41. [DOI] [PubMed] [Google Scholar]
Tarnopolsky 1999
- Tarnopolsky M, Martin J. Creatine monohydrate increases strength in patients with neuromuscular disease. Neurology 1999;52(4):854‐7. [DOI] [PubMed] [Google Scholar]
Tucker 2010
- Tucker EJ, Compton AG, Thorburn DR. Recent advances in the genetics of mitochondrial encephalomyopathies. Current Neurology and Neuroscience Reports 2010;10(4):277–85. [DOI] [PubMed] [Google Scholar]
Van Goethem 2001
- Goethem G, Dermaut B, Lofgren A, Martin JJ, Broeckhoven C. Mutation of POLG is associated with progressive external ophthalmoplegia characterized by mtDNA deletions. Nature Genetics 2001;28(3):211‐2. [DOI] [PubMed] [Google Scholar]
Wallace 1994
- Wallace DC. Mitochondrial DNA mutations in diseases of energy metabolism. Journal of Bioenergetics & Biomembranes 1994;26(3):241‐50. [DOI] [PubMed] [Google Scholar]
Wallace 1999
- Wallace DC. Mitochondrial diseases in mouse and man. Science 1999;283(5407):1482‐8. [DOI] [PubMed] [Google Scholar]
Westermann 2010
- Westermann B. Mitochondrial fusion and fission in cell life and death. Nature Reviews Molecular Cell Biology 2010;11(12):872‐4. [DOI] [PubMed] [Google Scholar]
Zeviani 1988
- Zeviani M, Moraes CT, DiMauro S, Nakase H, Bonilla E, Schon EA, et al. Deletions of mitochondrial DNA in Kearns‐Sayre syndrome. Neurology 1988;38(9):1339‐46. [DOI] [PubMed] [Google Scholar]
Zhu 2009
- Zhu X, Peng X, Guan MX, Yan Q. Pathogenic mutations of nuclear genes associated with mitochondrial disorders. Acta Biochimica et Biophysica Sinica 2009;41(3):179‐87. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Chinnery 2006
- Chinnery P, Majamaa K, Turnbull D, Thorburn D. Treatment for mitochondrial disorders. Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI: 10.1002/14651858.CD004426.pub2] [DOI] [PubMed] [Google Scholar]


