Abstract
AIM
To analyze the impact of calcitonin gene-related peptide (CGRP) in mouse keratitis after Aspergillus fumigatus (A. fumigatus) infection.
METHODS
C57BL/6 mice were treated subconjunctivally with different concentrations of exogenous CGRP, and BALB/c mice were treated with CGRP8-37 (a CGRP antagonist) before corneas were infected with A. fumigatus. The cornea was assessed under the slit-lamp and the clinical score was recorded. The mRNA levels of IL-1β, TNF-α, IL-6, and MIP-2 were detected by quantitative real-time polymerase chain reaction (PCR), while the protein level of IL-1β was determined by Western blotting. In vitro, RAW264.7 cells were used to investigate NLRP3 and IL-1β expression induced by A. fumigatus after the pretreatment of exogenous CGRP or CGRP8-37. Cytokines expression in RAW264.7 cells was evaluated by real-time PCR and Western blotting.
RESULTS
Using exogenous CGRP resulted in down-regulated synthesis of IL-1β and MIP-2 stimulated by A. fumigatus in C57BL/6 mice keratitis, and the synthesis of IL-1β, MIP-2 and IL-6 was up-regulated in BALB/c mice corneas after the pretreatment with CGRP8-37. Pretreatment with exogenous CGRP and CGRP8-37 did not influence TNF-α mRNA levels either in BALB/c or C57BL/6 mice keratitis. The levels of NLRP3 and IL-1β were both reduced in A. fumigatus stimulated-macrophages after treatment with exogenous CGRP. And CGRP8-37 pretreatment would increase NLRP3 and IL-1β levels.
CONCLUSION
CGRP may alleviate the inflammatory reaction in mice keratitis after infection with A. fumigatus. The anti-inflammatory effect may be related to the inhibition of NLRP3 expression by CGRP.
Keywords: calcitonin gene-related peptide, fungal keratitis, NLRP3, mice
INTRODUCTION
Fungal keratitis (FK) is recognized as a difficult ocular disease which may cause damage to the cornea and visual impairment[1]–[2]. Aspergillus fumigatus (A. fumigatus) is a commom pathogenic fungi, that can induce immune damage[3]–[4] which is involved in corneal injury.
Calcitonin gene-related peptide (CGRP), widely distributed in the nervous systems[5]–[6], plays a role of neuroimmunity in macrophages[7], neutrophils[8] and other cells of inflammation[9]. Many researchers pointed out that CGRP is one of the most abundant neuropeptide in mouse cornea based on the description of nerve architecture and sensory neuropeptides distribution. A recent research has shown that CGRP expression is increased in mouse corneas in response to A. fumigatus infection and it has an anti-inflammatory function by regulating the levels of certain cytokines in mouse macrophages[10]. However, the mechanism of CGRP in keratitis caused by A. fumigatus has never been studied. NLRP3 is one of the pattern recognition receptors (PRRs). It can recognize a variety of pathogens including fungi[11]–[12]. Coupling the Caspase-1, NLRP3 up-regulates the inflammatory factor IL-1β. In the study of lipopolysaccharide (LPS)-induced acute lung injury[7], CGRP expression can significantly down-regulated NLRP3 and its downstream inflammatory factor IL-1β caused by LPS. Study confirmed that the stimulation of A. fumigatus increased the activity of NLRP3, Caspase-1 and IL-1β in human monocytes[11]. And recently some studies have demonstrated that CGRP can attenuate the LPS-induced inflammatory cytokines by inhibiting the inflammasome activation of NLRP3 pathway in stimulated-RAW264.7 macrophages[7],[13]–[14]. Based on previous studies, we considered that CGRP attenuates inflammatory reaction in A. fumigatus keratits through the inhibition of NLRP3 expression. In this study, the mice were pretreated with exogenous CGRP and CGRP8-37 respectively to confirm anti-inflammatory effect of CGRP level in vivo. Then different concentrations of exogenous CGRP and CGRP8-37 were used to pretreat RAW264.7 cells stimulated by A. fumigatus respectively to observe the inhibitory influence of CGRP on inflammation and its mechanism from the NLRP3 expression.
MATERIALS AND METHODS
Ethical Approval
The animals treaty was ratified by the Research Ethics Committee under the Affiliated Hospital of Qingdao University according to the Use of Animals in Ophthalmic and Vision Research of the Association for Research in Vision and Ophthalmology Statement.
Reagents
Sabouroud culture came from Babio biotech (Jinan, Shandong Province, China). Dulbecco's modified Eagle's medium (DMEM) was derived from Gibco (San Diego, California, USA). RNAiso Plus, TB GreenTM Premix Ex TaqTM II and reverse-transcription polymerase chain reaction (RT-PCR) kit were received from TaKaRa (Dalian, China). BCA Protein Assay Kit, ammonium persulfate, phosphate buffer saline (PBS), phenylmethylsulfonyl fluoride (PMSF), polyvinylidene difluoride (PVDF) membrane and radio immunoprecipitation assay (RIPA) lysis buffer were obtained from Solarbio (Beijing, China). Sample loaded buffer, confining liquid and sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) were obtained from Beyotime Biotechnology (Shanghai, China). Chemiluminescence (ECL) came from Thermo Fisher Scientific (Waltham, MA, USA). Exogenous CGRP neuropeptide was collected from MedChemExpress (New Jersey, USA), and competitive CGRP receptor antagonist (CGRP8-37) was collected from Abcam (Cambridge, UK). NLRP3 and IL-1β antibody were purchased from Abcam (Cambridge, UK).
Mice and Corneal Infection
Eight-week-old female BALB/c and C57BL/6 (specific pathogen-free) mice coming from Jinan Pengyue Experimental Animal Co., Ltd. (Shandong Province, China) were weighed between 20-30 g. Daily care, anesthesia, and corneal treatment of the mice were performed in accordance with laboratory methods[15]. The ocular surfaces were manipulated under a stereoscopic microscope. The left eye of the mouse was set as experimental eye. At the 1, 3, 5d post infection (p.i.), the mice were sacrificed. And corneas were collected for real-time PCR and Western blot.
A. fumigatus Incubation
A. fumigatus were obtained from China General Microbiological Culture Collection Center (No.3.0772) inoculated in Sabouroud medium shaken at 37°C for 2d, and were disrupted into 20-40 µm fractions. Germ-free PBS was used to wash A. fumigatus three times, then the supernatant was removed. The fractions with (for in vitro) or without (for in vivo) inactivation reached the final concentration of 1×108 CFU/mL with DMEM.
RAW264.7 Cells Culture and Incubation with A. fumigatus
RAW264.7 (Shanghai Chinese Academy of Sciences) murine cells plated in 12-well-plate for real-time PCR and 6-well cell culture plate for Western blot were cultured according to the previous methods[15]. When the macrophages reached 80% confluence, CGRP (10 and 30 ng/mL) and CGRP8-37 (100 ng/mL) were used to pretreat the cells for 30min respectively. After pretreatment, the cells of all groups were exposed to A. fumigatus hyphae (final concentration of 5×106 CFU/mL) for 12h for mRNA detection and 24h for protein expression.
CGRP and CGRP8-37 Treatment of C57BL/6 and BALB/c Mice
Subconjunctival injection with CGRP (0.1 and 1 µg/5 µL) or control PBS was performed respectively in the experimental eye (n=6/group) of C57BL/6 mice the day before the experiment. An additional CGRP (0.1 µg/200 µL and 1 µg/200 µL) or control PBS was given to C57BL/6 mice respectively by intraperitoneal injection at 1, 2, 3, 4, and 5d after infection. CGRP8-37 (0.2 µg/5 µL) or control PBS was injected in the experimental eye of BALB/c mice respectively before the infection, then an additional CGRP8-37 (0.2 µg/200 µL) or control PBS was injected respectively at 1, 2, 3, 4, and 5d p.i.
Quantitative Polymerase Chain Reaction
Total RNA extracted from macrophages or corneas was quantified quickly according to the previous methods[10]. Complementary DNA was obtained through reverse transcription of 1 µg total RNA. The 2 µL cDNA diluted in 23 µL diethylpyrocarbonate-treated water was used in the RT-PCR (20 µL reaction volume). β-actin was used as an internal control. The sequences of oligonucleotide primers can be queried in Table 1.
Table 1. Nucleotide sequences of mouse primers for PCR.
| Genes | GenBank No. | Primer sequence (5′-3′) | Size (bp) |
| β-actin | NM_007393.3 | F: GAT TAC TGC TCT GGC TCC TAG C | 147 |
| R: GAC TCA TCG TAC TCC TGC TTG C | |||
| CGRP | NM_007587.2 | F: GGA CTT GGA GAC AAA CCA CCA | 118 |
| R: GAG AGC AAC CAG AGA GGA ACT ACA | |||
| IL-1β | NM_008361.3 | F: CGC AGC AGC ACA TCA ACA AGA GC | 111 |
| R: TGT CCT CAT CCT GGA AGG TCC ACG | |||
| TNF-α | NM_013693.2 | F: ACC CTC ACA CTC AGA TCA TCT T | 148 |
| R: GGT TGT CTT TGA GAT CCA TGC | |||
| MIP-2 | NM_009140.2 | F: AAG TTT GCC TTG ACC CTG AA | 180 |
| R: AGG CAC ATC AGG TAC GAT CC | |||
| IL-6 | NM_001314054.1 | F: CAC AAG TCC GGA GAG GAG AC | 141 |
| R: CAG AAT TGC CAT TGC ACA AC |
F: Forward; R: Reverse.
Western Blot
Mouse cells or corneas were lysed in RIPA lysis buffer containing PMSF (100:1). The protein separated by SDS-PAGE gels was transferred to PVDF membranes. After incubating the membranes with the target protein by blocking buffer (Beyotime) for 2h, blocked membranes were immersed by the primary antibody of the target protein at 4°C overnight. After washing the membranes with PBS containing 0.05% Tween-20 three times, secondary antibody (Elabscience; Wuhan, China) was used to immerse the membranes at 37°C for 1.5-2h. The primary antibodies against the target protein were chosen: IL-1β (ABcam), NLRP3 (ABcam) and Tubulin (Elbscience). Tubulin was used as control. Those immunoreactive bands on the membranes were developed by ECL.
Statistical Analysis
One-way ANOVA was chosen to analyze statistical difference of PCR and Western blot data with GraphPad 6.0 software. These data are shown as the mean±SD. All the differences were considered significant at P<0.05.
RESULTS
Different Expression of CGRP in the Corneas of BALB/c and C57BL/6 Mice Infected by A. fumigatus
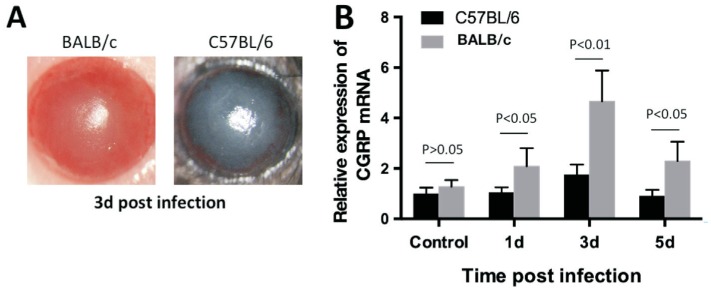
The C57BL/6 and BALB/c mice keratitis models on 1, 3, and 5d were established at the same time by A. fumigatus to compare their CGRP levels (Figure 1A). Relative CGRP mRNA in control and infected mice corneas were tested by RT-PCR. The results showed that both BALB/c and C57BL/6 mouse normal cornea expressed CGRP, and after A. fumigatus infection, the up-regulated expression of CGRP mRNA reached the highest at 3d p.i. BALB/c corneas have higher CGRP expression than C57BL/6 at 1, 3, and 5d p.i. (Figure 1B; P<0.05, P<0.01, P<0.05).
Figure 1. Expression of CGRP in C57BL/6 and BALB/c corneas.
A: Representative eyes of C57BL/6 and BALB/c mice were photographed with a slit lamp after 3d (score 7) infection; B: mRNA levels of CGRP were significantly greater in BALB/c cornea than in C57BL/6 cornea at 1, 3, and 5d p.i.
Inflammatory Response after Exogenous CGRP Pretreatment
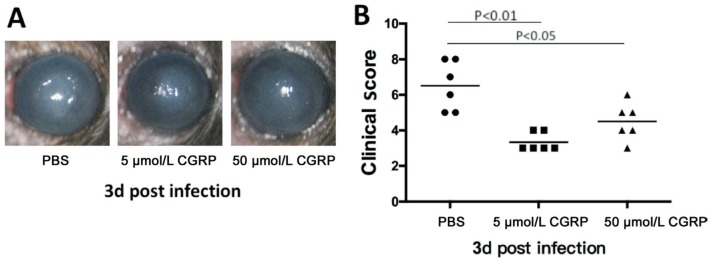
C57BL/6 mice were given exogenous CGRP pretreatment. The role of exogenous CGRP in modulating the inflammatory reaction was evaluated by photographs obtained from silt lamp (Figure 2A), and clinical score[16] was used to quantify the degree of inflammatory response (Figure 2B). The result showed that the pretreatment with exogenous CGRP (a CGRP agonist; 5 and 50 µmol/L) decreased the inflammatory reaction of A. fumigatus keratitis in C57BL/6 mice at 3d p.i. And the levels of clinical scores in exogenous CGRP (5 and 50 µmol/L) treatment groups were less than the scores in the control group (P<0.01 and P<0.05).
Figure 2. Exogenous CGRP treatment alleviates the inflammatory reaction of C57BL/6 mice cornea caused by A. fumigatus-infection.
A: Compared with PBS group, exogenous CGRP (5 and 50 µmol/L) weakened the disease severity of keratitis from photographs obtained from slit lamp at 3d p.i. B: The clinical scores in exogenous CGRP (5 and 50 µmol/L) treated groups were significantly lower than the scores in PBS group.
Effect of Exogenous CGRP on Inflammatory Cytokines in the Cornea of C57BL/6 Mice Infected with A. fumigatus
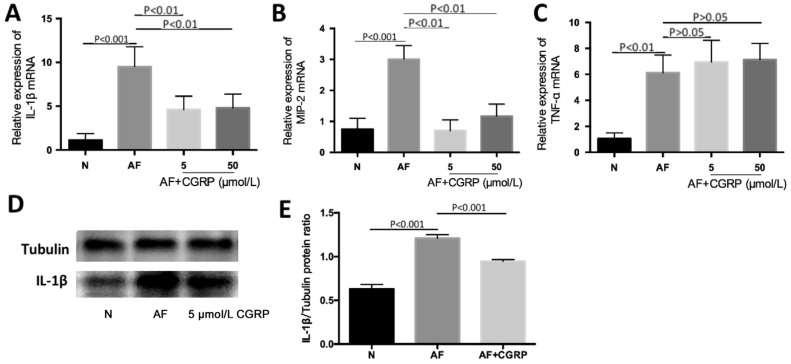
To evaluate the effect of CGRP in inflammation of corneal tissue caused by A. fumigatus, C57BL/6 mice were given exogenous CGRP (a CGRP agonist; 5 and 50 µmol/L) in different concentrations one day before infection. The mRNA expression of IL-1β, TNF-α, and MIP-2 in corneal tissue was measured by RT-PCR, and the protein level of IL-1β was tested by Western blot. Our study observed that the pretreatment of exogenous CGRP (5 and 50 µmol/L) obviously inhibited IL-1β mRNA levels induced by A. fumigatus hyphae (Figure 3A; P<0.01, P<0.01). CGRP (5 and 50 µmol/L) also inhibited mRNA of MIP-2 at 3d p.i. (Figure 3B; both P<0.01). But the exogenous CGRP (5 and 50 µmol/L) did not change TNF-α mRNA levels (Figure 3C; both P>0.05). Through Western blot measurement (Figure 3D-3E; P<0.001), we found that IL-1β protein was reduced after treatment with exogenous CGRP (5 µmol/L).
Figure 3. Exogenous CGRP mitigates cytokines in A. fumigatus-infected C57BL/6 cornea.
A, B: IL-1β and MIP-2 mRNA levels were both significantly mitigated in exogenous CGRP (5 and 50 µmol/L) treatment groups compared with A. fumigatus-infected group at 3d p.i.; C: The change of TNF-α mRNA levels were not statistically significant after various concentrations of exogenous CGRP (5 and 50 µmol/L) treatment at 3d p.i.; D, E: IL-1β protein level in exogenous CGRP (5 µmol/L) treatment group was reduced as compared with A. fumigatus-exposed control group at 3d p.i.
Inhibition of CGRP Magnified Inflammatory Effect of BALB/c Corneas Infected by A. fumigatus
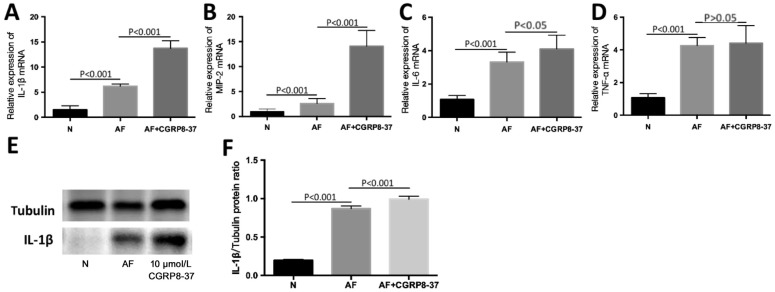
CGRP8-37 pretreatment was applied to BALB/c mice. Pretreatment of CGRP8-37 (a CGRP antagonist; 10 µmol/L) blocked the effect of CGRP in keratitis that supports its role as an anti-inflammatory. The day before corneal infection in BALB/c mice, CGRP8-37 was injected subconjunctivally. In comparison with the infected control group, the mRNA levels of IL-1β (Figure 4A; both P<0.001), MIP-2 (Figure 4B; both P<0.001) and IL-6 (Figure 4C; P<0.001, P<0.05) were significant increased statistically in CGRP8-37 treated group. And Western bands also implicated that the protein levels of IL-1β (Figure 4E-4F; both P<0.001) were basically increased in accordance with mRNA. The RT-PCR results of TNF-α (Figure 4D; P<0.001, P>0.05) showed that that TNF-α was not changed by CGRP8-37.
Figure 4. CGRP8-37 pretreatment regulated inflammatory cytokines in BALB/c cornea infected by A. fumigatus.
A-C: In CGRP8-37 (10 µmol/L) treatment group, mRNA levels of IL-1β, MIP-2, and IL-6 were elevated compared with A. fumigatus-exposed control group at 3d p.i.; D: The change of TNF-α mRNA was not statistically significant after the injection of CGRP8-37 (10 µmol/L); E, F: CGRP8-37 (10 µmol/L) treatment increased the level of IL-1β protein induced by A. fumigatus at 3d p.i.
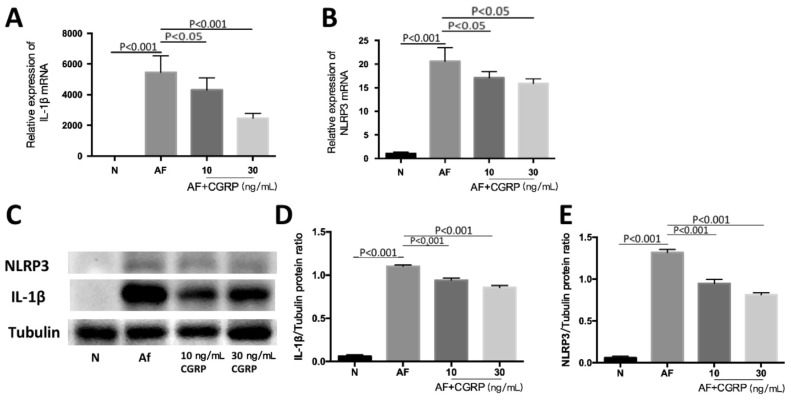
CGRP Regulates NLPR3 and IL-1β mRNA and Protein Levels in Macrophages Interacted with A. fumigatus
We further investigated the mechanism of CGRP regulating inflammatory cytokines by testing NLRP3 and IL-1β levels in A. fumigatus stimulated RAW264.7 cells with and without CGRP pretreatment by RT-PCR and Western blot. After stimulation, the mRNA and protein levels of NLRP3 increased (Figure 5B-5C, 5E; both P<0.001). The results also showed that the decrease of NLRP3 expression levels in A. fumigatus stimulated RAW264.7 cells after exogenous CGRP pretreatment. The exogenous CGRP (10 ng/mL) had an influence on mRNA levels of NLRP3 (Figure 5B; P<0.05). When the cells were treated with exogenous CGRP (30 ng/mL) before infection, the change of NLRP3 mRNA was apparent (Figure 5B; P<0.05). And the protein levels of NLRP3 (Figure 5C, 5E; both P<0.001) were also suppressed under the pretreatment of exogenous CGRP (10 and 30 ng/mL). We also detected changes in downstream inflammatory molecule IL-1β. The results showed that the level of IL-1β mRNA decreased after the treatment of exogenous CGRP (10 ng/mL), and IL-1β mRNA decreased when the concentration of exogenous CGRP reached 30 ng/mL (Figure 5A; P<0.05, P<0.001). Protein levels of IL-1β were decreased with the pretreatment of exogenous CGRP (10 and 30ng/mL) after A. fumigatus stimulation (Figure 5C, 5D; both P<0.001).
Figure 5. CGRP regulates NLRP3 levels induced by A. fumigatus in RAW264.7 cells.
A, B: The levels of NLRP3 and IL-1β mRNA were decreased in certain concentrations exogenous CGRP (10 and 30 ng/mL) after 12h stimulation with A. fumigatus; C-E: The levels of NLRP3 and IL-1β protein induced by A. fumigatus were decreased with exogenous CGRP (10 and 30 ng/mL) treatment at 24h.
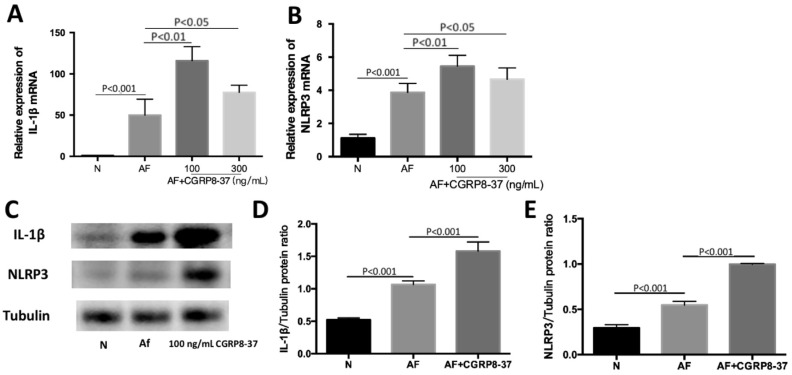
Effect of CGRP8-37 on NLRP3 Level and its Downstream Molecule IL-1β in RAW264.7 Cells after Stimulation with A. fumigatus
To further confirm the effect of CGRP in NLRP3 pathway, cells were incubated with pre-tested concentration of CGRP8-37 (a CGRP antagonist, 100 ng/mL) 30min, then followed by exposure to A. fumigatus. RT-PCR was used to test the levels of cytokines mRNA in macrophages. Data showed that antagonist promoted the levels of NLRP3 and IL-1β mRNA after A. fumigatus infection (Figure 6A, 6B; P<0.05). The results from Western blot showed that the production of NLRP3 and IL-1β protein was increased at 24h p.i. after the pretreatment of CGRP8-37 (100 ng/mL) for 30min (Figure 6C-6E; all P<0.001).
Figure 6. Inhibiting CGRP role was to prove the effect of CGRP on NLRP3 expression in RAW264.7 cells stimulated by A. fumigatus.
A, B: CGRP8-37 (100 and 300 ng/mL) increased mRNA levels of NLRP3 and IL-1β compared with A. fumigatus hyphae-exposed control group at 12h; C-E: After CGRP8-37 (100 ng/mL) pretreatment, the protein levels of NLRP3 and IL-1β were up-regulated at 24h in comparison with infected control group.
DISCUSSION
Neuropeptides are involved in many fungal diseases[17]–[18]. CGRP, as an important neuropeptide, can regulate the expression of inflammatory cytokines and inhibit excessive inflammation[19]–[22].
Results in this research revealed that C57BL/6 and BALB/c mice implicated constitutive expression of corneal CGRP. Using the mouse model of keratitis caused by A. fumigatus, the CGRP level of mice cornea was increased and reached the highest at 3d p.i. And BALB/c corneas expressed more CGRP than C57BL/6 corneas at 1, 3, and 5d p.i. These implied that CGRP may have a protective role in A. fumigatus keratitis as evidenced by previous studies[10],[23]. Analysing the clinical scores of A. fumgiatus keratitis after 3d infection of C57BL/6 and BALB/c mouse pretreated by exogenous CGRP and CGRP8-37 respectively, we confirmed that exogenous CGRP at the certain concentration can shorten the course of disease and protect cornea in mice with A. fumigatus keratitis. These results support the previous study[10] revealing that the neuropeptide CGRP plays a protective role through the experiments of mouse macrophages. Since treatment with CGRP or CGRP8-37 can result in decreased or increased inflammatory response in mice cornea, inflammatory cytokines were used to test if changing CGRP level in mice had a corresponding inflammatory regulation. Exogenous CGRP at different concentrations can down-regulate the production of IL-1β and MIP-2 caused by A. fumigatus in mouse corneas, while CGRP8-37 (a CGRP inhibitor) can increase the secretion of IL-1β, MIP-2 and IL-6 in response to A. fumigatus in mice corneas. However, we did not find any statistical differences in the level of TNF-α synthesis induced by A. fumigatus in mice corneas which were pretreated with exogenous CGRP or CGRP8-37. This is in line with the previous study indicating that different concentrations of exogenous CGRP treatment did not change TNF-α level in LPS-stimulated RAW264.7 cells[7], while IL-1β and MIP-2 levels were down-regulated after CGRP treatment and IL-1β, MIP-2, and IL-6 levels were up-regulated after CGRP8-37 treatment, which are in keeping with Yin et al's study[10], supporting the anti-inflammatory action of CGRP in A. fumigatus keratitis.
As a member of the NLR family, NLRP3 is released by neutrophils and macrophages[13],[24]. A previous study[25] showed that the NLRP3/Caspase-1 pathway played a pro-inflammatory role in A. fumigatus keratitis. In our study, mouse macrophages were pretreated with exogenous CGRP to enhance the effect of CGRP, while CGRP8-37 was used to weaken the effect of CGRP. Data showed that CGRP down-regulated the NLRP3 expression induced by A. fumigatus stimulation, while it was observed that the processing of downstream inflammatory cytokines IL-1β was reduced in cells. Blocking CGRP effect can up-regulate NLRP3 expression in A. fumigatus stimulated cells, and inflammatory cytokine IL-1β level was tested to be up-regulated after pretreatment of CGRP8-37. This finding is consistent with previous studies[7],[13]–[14] showing that CGRP has anti-inflammatory effect through inhibiting NLRP3 pathway stimulated by LPS. Our study provides support for the inhibition of the NLRP3 pathway by CGRP and played anti-inflammatory role in A. fumigatus infection.
In conclusion, we first discovered that CGRP can down-regulate the activation of NLRP3 pathway caused by A. fumigatus and prevent excessive inflammatory reaction in A. fumigatus keratitis. Previous studies and our study further indicate that CGRP might be an important target for the treatment of A. fumigatus keratitis. However, the mechanism by which CGRP plays anti-inflammatory role in vivo is needed. This basic research also confirmed the role of neuro-immune regulation in FK.
Acknowledgments
Foundations: Supported by National Natural Science Foundation of China (No.81870632); the Youth National Natural Science Foundation of China (No.81800800; No.81700800); the Natural Science Foundation of Shandong Province (No.ZR2017BH025).
Conflicts of Interest: Xu M, None; Li C, None; Zhao GQ, None; Lin J, None; Yin M, None; Zheng HR, None; Zhang L, None; Wu MQ, None.
REFERENCES
- 1.Bourcier T, Sauer A, Dory A, Denis J, Sabou M. Fungal keratitis. J Français D'ophtalmologie. 2017;40(9):e307–e313. doi: 10.1016/j.jfo.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Li C, Zhao GQ, Che CY, Lin J, Li N, Jia WY, Zhang QQ, Jiang N, Hu LT. Effect of corneal graft diameter on therapeutic penetrating keratoplasty for fungal keratitis. Int J Ophthalmol. 2012;5(6):698–703. doi: 10.3980/j.issn.2222-3959.2012.06.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heinekamp T, Schmidt H, Lapp K, Pähtz V, Shopova I, Köster-Eiserfunke N, Krüger T, Kniemeyer O, Brakhage AA. Interference of Aspergillus fumigatus with the immune response. Semin Immunopathol. 2015;37(2):141–152. doi: 10.1007/s00281-014-0465-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garg P, Roy A, Roy S. Update on fungal keratitis. Curr Opin Ophthalmol. 2016;27(4):333–339. doi: 10.1097/ICU.0000000000000272. [DOI] [PubMed] [Google Scholar]
- 5.Ma W, Chabot JG, Powell KJ, Jhamandas K, Dickerson IM, Quirion R. Localization and modulation of calcitonin gene-related peptide-receptor component protein-immunoreactive cells in the rat central and peripheral nervous systems. Neuroscience. 2003;120(3):677–694. doi: 10.1016/s0306-4522(03)00159-3. [DOI] [PubMed] [Google Scholar]
- 6.Rosenfeld MG, Mermod JJ, Amara SG, Swanson LW, Sawchenko PE, Rivier J, Vale WW, Evans RM. Production of a novel neuropeptide encoded by the calcitonin gene via tissue-specific RNA processing. Nature. 1983;304(5922):129–135. doi: 10.1038/304129a0. [DOI] [PubMed] [Google Scholar]
- 7.Duan JX, Zhou Y, Zhou AY, Guan XX, Liu T, Yang HH, Xie H, Chen P. Calcitonin gene-related peptide exerts anti-inflammatory property through regulating murine macrophages polarization in vitro. Mol Immunol. 2017;91:105–113. doi: 10.1016/j.molimm.2017.08.020. [DOI] [PubMed] [Google Scholar]
- 8.Pinho-Ribeiro FA, Baddal B, Haarsma R, O'Seaghdha M, Yang NJ, Blake KJ, Portley M, Verri WA, Dale JB, Wessels MR, Chiu IM. Blocking neuronal signaling to immune cells treats streptococcal invasive infection. Cell. 2018;173(5):1083–1097.e22. doi: 10.1016/j.cell.2018.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asahina A, Moro O, Hosoi J, Lerner EA, Xu S, Takashima A, Granstein RD. Specific induction of cAMP in Langerhans cells by calcitonin gene-related peptide: relevance to functional effects. Proc Natl Acad Sci USA. 1995;92(18):8323–8327. doi: 10.1073/pnas.92.18.8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yin M, Li C, Peng XD, Zhao GQ, Wu Y, Zheng HR, Wang Q, Xu Q, Jiang N. Expression and role of calcitonin gene-related peptide in mouse Aspergillus fumigatus keratitis. Int J Ophthalmol. 2019;12(5):697–704. doi: 10.18240/ijo.2019.05.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saïd-Sadier N, Padilla E, Langsley G, Ojcius DM. Aspergillus fumigatus stimulates the NLRP3 inflammasome through a pathway requiring ROS production and the Syk tyrosine kinase. PLoS One. 2010;5(4):e10008. doi: 10.1371/journal.pone.0010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lei GW, Chen MK, Li H, et al. Biofilm from a clinical strain of Cryptococcus neoformans activates the NLRP3 inflammasome. Cell Res. 2013;23(7):965–968. doi: 10.1038/cr.2013.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Y, Guo JF, Cai J, Yang Y, Li X, Zhang HY, Zhang G. Calcitonin gene-related peptide inhibits the function of macrophage through inhibiting inflammasome activation. Immunological Journal. 2017;33(5):400–404. [Google Scholar]
- 14.Xiao MF, Lin ZP, Lu Z, Liu QK. The role of CGRP in inhibiting inflammatory regulation of murine macrophages. China Journal of Modern Medicine. 2019:1–10. [Google Scholar]
- 15.Jiang JQ, Li C, Cui CX, Ma YN, Zhao GQ, Peng XD, Xu Q, Wang Q, Zhu GQ, Li CY. Inhibition of LOX-1 alleviates the proinflammatory effects of high-mobility group box 1 in Aspergillus fumigatus keratitis. Int J Ophthalmol. 2019;12(6):898–903. doi: 10.18240/ijo.2019.06.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu TG, Wilhelmus KR, Mitchell BM. Experimental keratomycosis in a mouse model. Invest Ophthalmol Vis Sci. 2003;44(1):210. doi: 10.1167/iovs.02-0446. [DOI] [PubMed] [Google Scholar]
- 17.Maruyama K, Takayama Y, Kondo T, et al. Nociceptors boost the resolution of fungal osteoinflammation via the TRP channel-CGRP-Jdp2 axis. Cell Rep. 2017;19(13):2730–2742. doi: 10.1016/j.celrep.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Li C, Liu YY, Zhao GQ, Lin J, Che CY, Jiang N, Li N, Zhang J, He K, Peng XD. Role of vasoactive intestinal peptide in Aspergillus fumigatus-infected cornea. Int J Ophthalmol. 2018;11(2):183–188. doi: 10.18240/ijo.2018.02.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li WJ, Wang TK, Ma CM, Xiong TT, Zhu Y, Wang X. Calcitonin gene-related peptide inhibits interleukin-1β-induced endogenous monocyte chemoattractant protein-1 secretion in type II alveolar epithelial cells. Am J Physiol Cell Physiol. 2006;291(3):C456–C465. doi: 10.1152/ajpcell.00538.2005. [DOI] [PubMed] [Google Scholar]
- 20.Yang W, Xv M, Yang WC, Wang N, Zhang XZ, Li WZ. Exogenous α-calcitonin gene-related peptide attenuates lipopolysaccharide-induced acute lung injury in rats. Mol Med Rep. 2015;12(2):2181–2188. doi: 10.3892/mmr.2015.3620. [DOI] [PubMed] [Google Scholar]
- 21.Reinshagen M, Flämig G, Ernst S, Geerling I, Wong H, Walsh JH, Eysselein VE, Adler G. Calcitonin gene-related peptide mediates the protective effect of sensory nerves in a model of colonic injury. J Pharmacol Exp Ther. 1998;286(2):657–661. [PubMed] [Google Scholar]
- 22.Rochlitzer S, Veres TZ, Kühne K, et al. The neuropeptide calcitonin gene-related peptide affects allergic airway inflammation by modulating dendritic cell function. Clin Exp Allergy. 2011;41(11):1609–1621. doi: 10.1111/j.1365-2222.2011.03822.x. [DOI] [PubMed] [Google Scholar]
- 23.Gomes RN, Castro-Faria-Neto HC, Bozza PT, Soares MB, Shoemaker CB, David JR, Bozza MT. Calcitonin gene-related peptide inhibits local acute inflammation and protects mice against lethal endotoxemia. Shock. 2005;24(6):590–594. doi: 10.1097/01.shk.0000183395.29014.7c. [DOI] [PubMed] [Google Scholar]
- 24.Gurung P, Lukens JR, Kanneganti TD. Mitochondria: diversity in the regulation of the NLRP3 inflammasome. Trends Mol Med. 2015;21(3):193–201. doi: 10.1016/j.molmed.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang XJ, Zhao GQ, Yan JW, Xu R, Che CY, Zheng HR, Zhu GQ, Zhang J. Pannexin 1 channels contribute to IL-1β expression via NLRP3/caspase-1 inflammasome in Aspergillus fumigatus keratitis. Curr Eye Res. 2019;44(7):716–725. doi: 10.1080/02713683.2019.1584321. [DOI] [PubMed] [Google Scholar]