Abstract
AIM
To determine the roles of high-mobility group box1 (HMGB1) in pro-inflammation, host immune response and its potential target for treatment in Aspergillus fumigatus (A.fumigatus) keratitis.
METHODS
Expression of HMGB1 was tested in C57BL/6 normal and infected corneas. Dual immunostaining tested co-expression of HMGB1 with TLR4 or LOX-1. C57BL/6 mice were pretreated with Box A or PBS and then infected. Clinical scores, polymerase chain reaction, ELISA, and MPO assay were used to assess the disease response. Flow cytometry were used to test the effect of Box A on reactive oxygen species (ROS) expression after A.fumigatus stimulation in polymorphonuclear neutrophilic leukocytes (PMN). C57BL/6 peritoneal macrophages were pretreated with Box B before A.fumigatus stimulation, and MIP-2, IL-1β, TNF-α, HMGB1 and LOX-1 were measured. Macrophages were pretreated with Box B or Box B combined with Poly(I) (an inhibitor of LOX-1) before stimulating with A.fumigatus, and MIP-2, IL-1β, TNF-α, LOX-1, p38-MAPK, p-p38-MAPK were measured.
RESULTS
HMGB1 levels were elevated in C57BL/6 mice after infection. HMGB1 co-expressed with TLR4, and LOX-1 in infiltrated cells. Box A vs PBS treated C57BL/6 mice had lower clinical scores and down-regulated corneal HMGB1, MIP-2, IL-1β expression and neutrophil influx. Box B treatment amplified expression of MIP-2, IL-1β, TNF-α, HMGB1 and LOX-1 that induced by A.fumigatus in macrophage. Compared to the treatment of Box B only, the protein expression of IL-1β, TNF-α showed inhibition of Box B combined with Poly(I), which also reduced the A.fumigatus-evoked protein level of LOX-1 and phosphorylation level of p38-MAPK. The production of A.fumigatus-stimulated ROS was significantly declined after Box A pretreatment in PMN.
CONCLUSION
Blocking HMGB1 reduces the disease response in C57BL/6 mice. HMGB1 can amplify the host immune response through p38-MAPK, and is a target for treatment of A.fumigatus keratitis.
Keywords: fungal keratitis, HMGB1, mice, macrophage, polymorphonuclear neutrophilic leukocytes
INTRODUCTION
Fungal keratitis (FK) is a serious ocular infectious disease with a high rate of blindness in most developing countries. The infection has become the leading cause of the infectious keratitis in most areas of China due to its high incidence and the unavailability of specific antifungal agents[1]–[2]. The treatment of FK remains a very tricky problem. The innate immune response of the host to infection is considered as the first defense line to identify and combat various microbial infections[3]. Pattern recognition receptors (PRRs), as the major part of innate immunity, could immediately identify the pathogen associated molecular patterns (PAMP), and then mediate the adhesion, absorption and eradication of the pathogen via infiltration of immune cells such as neutrophils and production of cytokines [e.g., interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α)], and chemokines (e.g., CXCL-1, CXCL-2)[3].
In recent years, more and more attention has been attracted by damage associated molecular pattern (DAMP) molecules. As a prototypicalarmin, the high-mobility group box1 (HMGB1) is well-known DAMP[4]. HMGB1 can be secreted by activated immune cells such as monocytes, macrophages, dendritic cells (DCs), natural killer (NK) cells and released by damaged or necrotic cells[5]. Upon cell activation, HMGB1 has cytokine and chemokine activities and involves in inflammasome activation[6]. HMGB1 which can mediate various cellular reactions binds to PRR of cell-surface, such as the receptor for advanced glycation end (RAGE) products, Toll-like receptor2 (TLR2) and TLR4[7]. HMGB1 can affect the functions of immune cells such as T lymphocytes, macrophages, DCs, and NK cells. HMGB1 is responsible for excessive intensification of the inflammatory response[8]. Box A, a conserved DNA-binding domain of HMGB1, plays a role in anti-inflammation and acts as a specific antagonist of HMGB1. For another, Box B, a domain attached to the acidic tail of HMGB1, is correlated with the cytokine activity of full length HMGB1 through stimulating the release of various pro-inflammatory cytokines such as TNF-α[6],[9].
HMGB1 regulates inflammation in various infections and its level is correlated to severity of infections[10]. Recent studies showed that anti-inflammatory action is accompanied by inhibition of HMGB1 during zymosan stimulation of macrophages[8]. HMGB1 also contributes to extensive inflammatory responses[11]. HMGB1 inhibition can alleviate the disease response. HMGB1 inhibitor, thrombomodulin, showed therapeutic effects through increasing synthesis of TGF-β and IL-10 in the lungs in severe acute respiratory distress syndrome [12]. Patel et al[13] showed that the pathology of mice infected with Pseudomonas aeruginosa (P.aeruginosa) can be alleviated by treating with a neutralizing anti-HMGB1 antibody, such as bacterial counts decreased, injury eased, and the number of neutrophils declined in the lungs. Anti-HMGB1 treatment also can protect against sepsis lethality[7]. Recent studies showed that blocking HMGB1 directly promoted better resolution of keratitis by reducing pro-inflammatory mediator expression and HMGB1 knockdown also decreased polymorphonuclear neutrophilic leukocytes (PMN) number in infected cornea[14]–[15].
Mitogen-activated protein kinase (MAPK) signal transduction pathways play a critical role in inflammation[16]–[17]. Recently, many studies proved that the inflammatory amplification of HMGB1 is easier to activate the phosphorylation of p38-MAPK[18]–[20]. However, the relationship between HMGB1 and p38-MAPK pathway is still unclear.
Reactive oxygen species (ROS), generated during oxidative metabolism, contribute to cellular homeostasis. Excessive ROS contributes to the tissue damage. Active cells, neutrophils, and macrophages produce high levels of ROS[9]. ROS mainly induced by danger signals such as HMGB1. HMGB1 enhances Ca2+ leak through TLR4-ROS signaling pathway[21]. The chemotaxis of inflammatory cells might be interrupted by using ROS scavengers or the neutralizing proteins of HMGB1[22].
The role of HMGB1 in Aspergillus fumigatus (A.fumigatus) keratitis is still not so clear. The studies provided evidence that blocking HMGB1 alleviate disease response of C57BL/6 FK. HMGB1 may amplify immune response and lead to excessive inflammatory response through the pathway of p38-MAPK. The study aimed to determine the effectiveness of HMGB1 as a target for the treatment of patients with A.fumigatus keratitis.
Materials and methods
Ethical Approval
Animals were treated humanely and in strict accordance with the ARVO Statement for the Use of Animals and approved by the Research Ethics Committee of the Affiliated Hospital of Qingdao University (China) and National Institutes of Health Guide (No.8023, NIH Publications, revised 1978) in research.
Culturing and Preparing Aspergillus Fumigatus
Strain of A.fumigatus (No.3.0772), bought from China General Microbiological Culture Collection Center (Beijing, China), was inoculated and then prepared according to the previous method of Li et al[23]. Totally 1×108 colony-forming units (CFU)/mL of A.fumigatus suspension was yielded with activation in the in vivo experiment; for the in vitro experiment, as well as, 5×106 CFU/mL A.fumigatus suspension was inactivated overnight in 75% alcohol.
In Vivo Experiment
Aspergillus fumigatus keratitis animal model establishment
The female C57BL/6 mice (8wk), bought from Jinan Pengyue Experimental Animal Co., Ltd. (Jinan, China), were anesthetized with 10% chloral hydrate. The central corneal epithelium of the C57BL/6 mice's right eyes was removed. Then, 5 µL of A.fumigatus (1×108 CFU/mL) was dropped on the cornea. After that, soft contact lens were covered on the cornea and eyelids were sutured as the way of Li et al[23]. One day after infection, the mice's eyes were opened. Control eyes were treated similarly, but without infection.
Clinical scorning of corneal infection
To investigate clinical score at 1, 3, and 5d post infection (p.i.), the photography was taken by a slit-lamp microscope. Corneal disease was ranked with clinical score ranging from 0 to 12 based on an established classification scale[24].
Box A treatment of C57BL/6 mice
C57BL/6 mice (n=6/group/time) were treated with Box A (LPS free; IBL International GmbH, Hamburg, Germany) subconjunctivally injection (1 µg/5 µL) 1d before infection and topically application (5 µL, 100 µg/mL) 1 time every day through 5d p.i. Control mice were similarly treated with PBS.
In Vitro Experiment
Macrophage isolation and Box B or Box B combined with Poly(I) treatment
C57BL/6 mice were stimulated with 3% thioglycollate medium (1.0 mL; Becton-Dickinson, Sparks, MD, USA) by intraperitoneal (i.p.) injection. Macrophages were collected by peritoneal lavage at 7d p.i. Cells were washed and resuspended in DMEM with 5% fetal calf serum (FCS; Invitrogen, Carlsbad, CA, USA) and plated at the concentrations of 3×106 cells/well. After 3h incubation, cells were treated with Box B (1 µg/mL; IBL International GmbH), A.fumigatus hyphae (5×106 CFU/mL), or Box B and A.fumigatus hyphae together for 18h. For the further experiment, cells were treated with Box B and A.fumigatus hyphae, or Box B combined with Poly(I) (250 µg/mL; Sigma, American) and A.fumigatus hyphae together for 18h.
Polymorphonuclear neutrophilic leukocytes isolation and Box A treatment
Of 1.0 mL 9% casein solution was used to stimulate C57BL/6 mice by i.p. injection. After 24h the same injection was taken again. After 3h, the way of peritoneal lavage was used to collect PMN. After washing and isolating by Percoll gradient, PMN were then resuspended in RPMI 1640 containing 10% FCS (Invitrogen, Carlsbad, CA, USA) and plated. After incubation, cells were treated with Box A (0.1 µg/mL; IBL International GmbH) or control PBS for 2h, and then stimulated A.fumigatus hyphae (5×106 CFU/mL) together for 8h.
Experimental Techniques
Real-time reverse transcriptase polymerase chain reaction
Total RNA isolation, reverse transcription, optimal conditions for polymerase chain reaction (PCR) amplification of cDNA and calculation of relative transcription levels were established using routine methods[23]. Primer sequences used in the research are shown in the Table 1.
Table 1. Nucleotide sequence of mouse primers for real-time PCR.
| Gene | GenBank No. | Primer sequence (5′-3′) |
| mβ-actin | NM_007393.3 | Forward: GAT TAC TGC TCT GGC TCC TAG C |
| Reverse: GAC TCA TCG TAC TCC TGC TTG C | ||
| HMGB1 | NM_010439.3 | Forward: TGG CAA AGG CTG ACA AGG CTC |
| Reverse: GGA TGC TCG CCT TTG ATT TTG G | ||
| IL-1β | NM_008361.3 | Forward: CGC AGC AGC ACA TCA ACA AGA GC |
| Reverse: TGT CCT CAT CCT GGA AGG TCC ACG | ||
| TNF-α | NM_013693.2 | Forward: ACC CTC ACA CTC AGA TCA TCT T |
| Reverse: GGT TGT CTT TGA GAT CCA TGC | ||
| MIP-2 | NM_009140.2 | Forward: TGT CAA TGC CTG AAG ACC CTG CC |
| Reverse: AAC TTT TTG ACC GCC CTT GAG AGT GG |
Immunofluorescent staining
Eyeballs were removed from the C57BL/6 mice (n=6/group/time) after 3d p.i. The sections preparation was according to the methods used by Li et al[23]. After fixation and blocking, the sections were incubated at a 1:100 dilution of rabbit anti-mouse HMGB1 antibody (Abcam, Cambridge, MA, USA) for 1h, and then incubated at Alexa Fluor 546-conjugated donkey anti-rabbit antibody (1:1000, Invitrogen) for 1h. After that, sections were incubated with SYTOX Green nuclear acid stain (1:30000, Lonza, Walkersville, MD, USA). For the co-expression experiments, sections were incubated in rabbit anti-mouse TLR4 (1:100, Santa Cruz Biotechnology, San Jose, CA, USA) antibody, or anti-mouse LOX-1 (Abcam), then incubated in Alexa Fluor 546-conjugated donkey anti-rabbit antibody (1:1000, Invitrogen) for 1h. Then incubated at rabbit anti-mouse HMGB1 (1:100, Abcam, Cambridge, MA, USA) for 1h, then Alexa Fluor 647-conjugated donkey anti-rabbit antibody at 1:1000. Sections were incubated with SYTOX Green nuclear acid stain. Controls were disposed by the similar way, however, the primary antibodies were in the place of the same host IgG. Digital images were captured.
Enzyme-linked immunosorbent assay
Complete corneas were collected after 3d p.i. (n=6/group/time). Corneas were homogenized according to the routine method[25]. The 25 µL sample was used for the assay of IL-1β and MIP-2 protein (R&D Systems, Minneapolis, MN, USA). For the cell experiment, 50 µL of supernatant was used for testing the protein level of IL-1β, TNF-α and MIP-2 according to the R&D Systems' instructions.
Western blotting analysis
Cells and corneas were harvested and lysed in RIPA buffer. Total protein was separated by running acrylamide sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), after that the separated protein on SDS-PAGE was transferred to the polyvinylidene difluoride (PVDF) membranes. After blocking, membranes were incubated with primary antibodies overnight at 4°C, which used in this research are as follows: β-actin (1:2000, Bioss, Beijing, China) monoclonal antibody, or IL-1β (1:500, Bioss, Beijing, China) primary antibody, or TNF-α (1:500, CST, American) primary antibody, or LOX-1 (1:500, Abcam, Cambridge, MA, USA) primary antibody, or HMGB1 (1:10000, Abcam, Cambridge, MA, USA) primary antibody, or p38-MAPK (1:500, Elabscience, Wuhan, China) primary antibody, p-p38-MAPK (1:500, Elabscience, Wuhan, China) primary antibody. After washing for 3 times with PBST, the membranes were incubated with corresponding peroxidase-conjugated secondary antibodies (1:5000, Santa Cruz Biotechnology, San Jose, CA, USA) at 37°C for 1h. The blots were showed by usage of chemiluminescence (ECL; Thermo Scientific).
Quantitation of Polymorphonuclear Neutrophilic Leukocytes in Cornea
Corneas were havest at 3 and 5d p.i. (n=6/group/time). And homogenized in 1.0 mL of the No.2 reagent of the MPO test kit (Njjcbio, Nanjing, China) according to the operating table. Samples were made to tissue homogenate, mixing with No.3 reagent in a water bath, next adding No.4 agent and color agent with water bath. After that No.7 agent was added into, and then water bath. The change in absorbance value was measured at 460 nm at once. The slope of the line was depended on each sample and used to calculate units of MPO in each cornea.
Measurement of ROS in Polymorphonuclear Neutrophilic Leukocytes
PMN were pretreated with Box A (0.1 µg/mL, IBL International GmbH) or control PBS for 2h, and then incubated with A.fumigatus hyphae (5×106 CFU/mL) for 8h. The generation of ROS was detected with the 2′, 7′-dichlorofluorescin diacetate (DCFH-DA; Sigma, St. Louis, MO, USA) through flow cytometry according to the routine methods[26]. Data was recorded as percentage of ROS productive cells.
Statistical Analysis
To identify the difference of clinical score in two groups, the Mann-Whitney U test was used. As to the evaluation of statistical significance about real-time reverse transcriptase polymerase chain reaction (RT-PCR), enzyme-linked immunosorbent assay (ELISA), Western blot, MPO and flow cytometry data for animal experiment, an unpaired, two-tailed Student's t-test was adopted, while in vitro experiments, a one-way ANOVA was taken to analyze the statistical significance of relative experiments including RT-PCR, Western blot, and the ELISA data. The P-value less than 0.05 was considered to be statistically significant. To guarantee the reproducibility of all experiments, each one was repeated, and datum from representative experiments are presented as mean±standard deviation (SD).
RESULTS
HMGB1 Expression in Cornea of C57BL/6 Mice
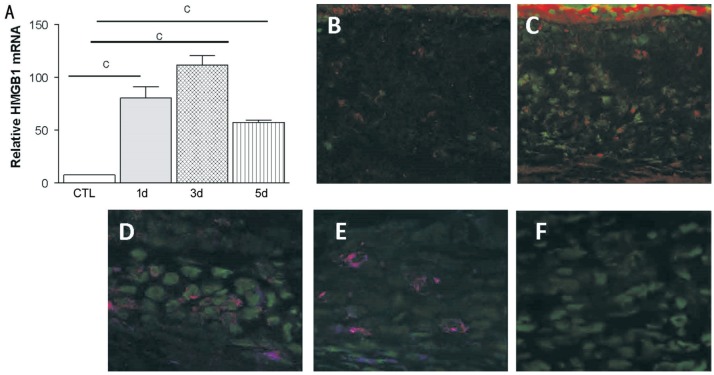
To identify the cornea expression of HMGB1, the mRNA and protein levels of HMGB1 are measured in control and infected C57BL/6 corneas by the RT-PCR and immunostaining. Our data showed that the level of relative HMGB1 mRNA was significantly up-regulated at 1, 3, and 5d p.i. (Figure 1A, all P<0.001) and peaked at the third day p.i. Immunostaining showed that HMGB1 protein expressed on control uninfected corneas (Figure 1B). HMGB1 protein was elevated at the third day p.i with A.fumigatus (Figure 1C) compared with the control group (Figure 1B). Also, dual immunostaining was used to detect whether HMGB1 co-expressed with TLR4 or LOX-1. Positive dual staining (magenta) for TLR4 and HMGB1 (Figure 1D), LOX-1 and HMGB1 (Figure 1E) was seen in C57BL/6 corneal stroma at the third day p.i with A.fumigatus. While the negative staining was showed in the control (Figure 1F).
Figure 1. HMGB1 expression in cornea of C57BL/6 mice.
Relative HMGB1 mRNA levels (A) were significantly higher at 1, 3, and 5d p.i. and peaked at 3d p.i. HMGB1 protein expressed (red) on control uninfected corneas (B). Protein level of HMGB1 (C) were significantly increased in the infected cornea of C57BL/6 mice at 3d p.i. Nuclei are labeled with SYTOX Green (green). Dual-immunostaining (magenta) for TLR4 (red) and HMGB1 (blue) was seen in C57BL/6 corneal stroma at 3d p.i. (D). Positive dual staining (magenta) for LOX-1 (red) and HMGB1 (blue) was seen in C57BL/6 corneal stroma at 3d p.i. (E). Primary antibody was replaced with species-specific IgG in control sections, which was positive for SYTOX green nuclear stain only (F). B-C: 250×; D-F: 400×. cP<0.001.
Box A Treatment of C57BL/6 Mice
To test whether blocking HMGB1 resulted in better disease, Box A was used to treat C57BL/6 mice. Box A treatment significantly down-regulated the clinical scores of C57BL/6 mice at 3 and 5d p.i. compared to the control treated with PBS (Figure 2A, both P<0.01). Photographs of corneas taken by a slit-lamp at 3d p.i. dedicated a worsened infection after PBS treatment (Figure 2B) compared to Box A treatment (Figure 2C). And then, we explored the effect of Box A treatment on the expression of MIP-2 and IL-1β. The protein level of MIP-2 (Figure 2D, P<0.05, P<0.001) and IL-1β (Figure 2E, both P<0.05) were decreased in Box A treated cornea at 3 and 5d p.i. compared to control-treated mice. Also, MPO level (Figure 2F, P<0.05, P<0.01) meaningfully down-regulated in Box A treated cornea vs the control treated with PBS at 3 and 5d p.i.
Figure 2. Box A treatment of C57BL/6 mice.
Box A treatment significantly down-regulated the clinical scores of C57BL/6 mice at 3 and 5d p.i. compared with PBS-treated mice (A). Photographs showed worsened disease after PBS treatment (B) compared with Box A treatment (C) at 3d p.i. Effect of Box A on MIP-2 and IL-1β expression and MPO levels. Corneal protein level of MIP-2 (D) and IL-1β (E) were decreased in Box A treated cornea of C57BL/6 mice at 3 and 5d p.i. compared to control-treated mice. MPO level (F) significantly down-regulated in Box A treated cornea vs PBS treated cornea of C57BL/6 mice at 3 and 5d p.i. aP<0.05; bP<0.01; cP<0.001.
Box B Treatment of Macrophages from C57BL/6 Mice
Pretreated macrophages with Box B to test whether stimulating HMGB1 resulted in up-regulation of pro-inflammatory cytokines and whether HMGB1 amplified inflammation responses. Data showed that Box B treatment significantly up-regulated expression of MIP-2 (Figure 3A, P<0.05), IL-1β (Figure 3B, P<0.01), TNF-α (Figure 3C, P<0.05) in mRNA levels that induced by A.fumigatus compared with A.fumigatus treatment only. Box B treatment significantly up-regulated expression of IL-1β (Figure 3E, P<0.05), TNF-α (Figure 3F, P<0.05), LOX-1 (Figure 3G, P<0.0001), HMGB1 (Figure 3F, P<0.001) in protein level, that induced by A.fumigatus while MIP-2 level had no change after Box B and A.fumigatus treatment compared with A.fumigatus treatment only (Figure 3D). Only Box B treatment did not effect on MIP-2, IL-1β, TNF-α, LOX-1 and HMGB1 expression.
Figure 3. Effect of Box B on macrophages from C57BL/6 mice.
mRNA levels of MIP-2 (A), IL-1β (B), TNF-α (C) that induced by A.fumigatus were upregulated after Box B treatment. Box B treatment also up-regulated protein levels of IL-1β (E), TNF-α (F), LOX-1 (G) and HMGB1 (G) that induced by A.fumigatus while MIP-2 level had no change after Box B and A.fumigatus treatment compared with A.fumigatus treatment only (D). Only Box B treatment did not effect on MIP-2, IL-1β, TNF-α, LOX-1 (G) and HMGB1 (G) expression. aP<0.05; bP<0.01; cP<0.001; dP<0.0001.
Box B or Box B combined with Poly(I) Treatment of Macrophage from C57BL/6 Mice
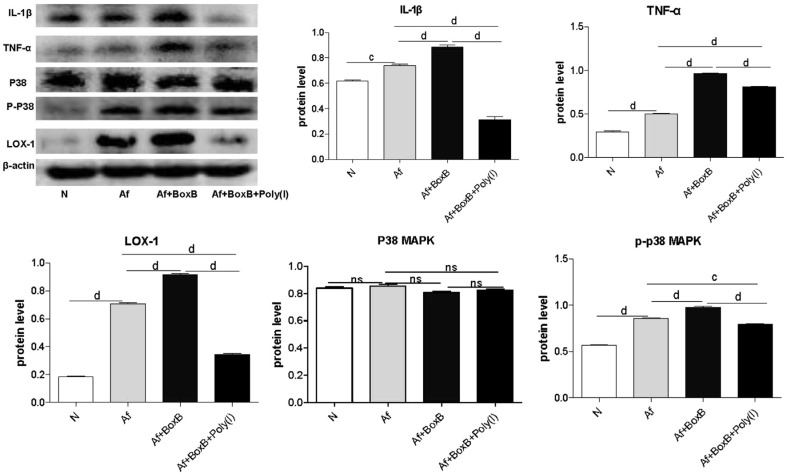
Pretreated macrophage with Box B or Box B combined with Poly(I). Data showed that Box B combined with Poly(I) and A.fumigatus treatment significantly reduced the protein levels of IL-1β (Figure 4, P<0.0001), TNF-α (Figure 4, P<0.0001), LOX-1 (Figure 4, P<0.0001), and also down-regulated the phosphorylation level of p-p38-MAPK (Figure 4, P<0.001) that induced by A.fumigatus compared to Box B and A.fumigatus treatment.
Figure 4. Effect of Box B or Box B combined with Poly(I) from C57BL/6 mice.
Protein levels of IL-1β, TNF-α, LOX-1 and p-p38MAPK induced by A.fumigatus were significantly down-regulated after Box B combined with Poly(I) treatment. cP<0.001; dP<0.0001.
Effect of Box A on ROS generation of PMN from C57BL/6 Mice
Flow cytometry data showed that A.fumigatus stimulation up-regulated ROS generation significantly (62.5%; Figure 5B, 5E) compared with normal cells (19.5%; Figure 5A, 5D). Box A treatment down-regulated the ROS generation (20.3%; Figure 5C, 5F) that induced by A.fumigatus stimulation compared with A.fumigatus control (62.5%; Figure 5B, 5E), and the difference is significant (P<0.01) shown as Figure 5G, 5H.
Figure 5. Effect of Box A on ROS generation of PMN from C57BL/6 mice.
ROS generation was up-regulated after A.fumigatus stimulation (B, E) compared with normal cells (A, D). ROS expression induced by A.fumigatus stimulation was down-regulated after Box A treatment (C, F). The difference was significant (G, H). bP<0.01.
DISCUSSION
There are more and more studies aimed at the role of HMGB1 in inflammatory diseases. Until recently, little was known about whether HMGB1 signaling contributes to the disease process of A.fumigatus keratitis.
It has been reported that HMGB1 up-regulates and plays pro-inflammatory role in P.aeruginosa keratitis[14]–[15]. Results in this research showed that after A.fumigatus infection, HMGB1 expression up-regulated in C57BL/6 corneas which are consistent with results showing that HMGB1 expression increased and played important role in sepsis[27], haemorrhagic shock, rheumatoid arthritis[28], systemic lupus erythematosus[29], lung injury[30] and cystic fibrosis lung[31]. It has been proved that HMGB1 has the ability to amplify inflammation and plays a pro-inflammatory role in various disease[32].
Using dual immunostaining, HMGB1 was seen co-expressed with TLR4 and LOX-1. Previous studies showed that HMGB1 binds TLRs and the RAGE[7],[33]. TLRs can recognize fungi and trigger a complex signal transduction cascade that induces the production of inflammatory cytokines, thus initiating innate and adaptive immunity[34]. During the C.albicans keratitis, fungal growth could be controlled by pro-inflammatory cytokines which are produced by the activated TLR2 and TLR4 signals pathway[35]. The neutrophil-mediated bactericidal action and the activation of NADPH oxidase can be declined by HMGB1 through a RAGE-dependent mechanism[36]. As to the function of LOX-1 in FK, our previous studies and data from other labs indicated LOX-1 played a pro-inflammatory role during FK[23],[37]–[38]. The present study showed that HMGB1 co-expressed with TLR4 and LOX-1 in the corneal stroma. But further work will be required to test the function of TLR4 and LOX-1 in HMGB1 induced inflammation.
Previous studies have proved that when HMGB1 was neutralized at the period of corneal infection with P.aeruginosa, the PMN influx would be reduced and the pathology would be alleviated in C57BL/6 mice at p.i. 48h[12]. For purpose of fully identify whether HMGB1 play pro-inflammatory role in fungal infection, we treated C57BL/6 mice with HMGB1 antagonist Box A, and result indicated that the disease outcome of C57BL/6 mice could be alleviated with injection of Box A. Treatment with Box A compared with PBS significantly decreased expression of MIP-2 and IL-1β. All findings are in alignment with a previous study indicating that HMGB1 inhibition significantly enhanced the survival rate of rodents[39]. Our in vivo data also showed that HMGB1 inhibition can reduce PMN influx, which is consistent with study, indicating that HMGB1 reduces the inflammation by RAGE-dependent mechanisms[36]. Also, it has been reported that the appropriate inflammatory responses could be necessary for the cornea healing, excessive inflammatory response led to a worsen corneal damage[40]–[41]. Thus, the control of inflammatory response by target of HMGB1 may contribute a better outcome.
HMGB1 is released by two ways, one is an active secretion by monocytes and macrophages, while another is a passive secretion by necrotic or damaged cells[42]. HMGB1 could be considered as a secreted cytokine in bacterial infection and amplify innate defense by activating macrophages[39]. So, we next extracted macrophages from C57BL/6 mice and pretreated with Box B to see whether HMGB1 can amplify the inflammation. Data showed that Box B treatment significantly up-regulated levels of MIP-2, IL-1β, TNF-α that stimulated by A.fumigatus. This is consistent with a research showing that upon co-activation with HMGB1, cytokines with pro-inflammatory activity, such as TNF-α and IL-1β, can be generated by macrophages[32]. Our data is also consistent with a study showed that a mixture of human peripheral blood monocytes which are stimulated with HMGB1 and LPS results in a higher increase in TNF-α production than LPS or HMGB1 alone[43].
HMGB1 has been reported as a protein which had an important role in some nuclear transaction and transcription[44]–[45]. To confirm the mechanisms of HMGB1 up-regulation of pro-inflammatory cytokines expression, we pretreated the macrophage from C57BL/6 mice with Box B combined with Poly(I). Our data indicated that the expression level of LOX-1 and p-p38-MAPK decreased when treated by the Box B combined with Poly(I) and A.fumigatus together, which also down-regulated the protein level of TNF-α and IL-1β. These findings explained that HMGB1 could through the p38-MAPK pathway amplify inflammatory response in A.fumigatus infection. This is in accordance with the study, showed that HMGB1-LPS complex can induce the macrophages to release inflammatory cytokines through RAGE, and the signal pathway of p38-MAPK[46].
Excessive ROS contribute to tissue damage. High-level of ROS can be produced by the PMN and macrophages from the immune system[9]. ROS mainly induced by danger signals such as HMGB1. Our data indicated that A.fumigatus stimulation up-regulated the ROS expression. Box A treatment down-regulated the ROS expression induced by A.fumigatus stimulation. This is consistent with study showed that HMGB1 enhances TLR4-ROS signaling pathway[21]. Results suggested that HMGB1 may play pro-inflammatory role through the way of ROS.
In summary, our study indicates that blocking HMGB1 alleviate disease response of C57BL/6 mice. Our data also shows that HMGB1 can amplify immune response through p38-MAPK pathway. Further studies of HMGB1 receptors and signaling pathway may provide novel ways for the treatment of FK.
Acknowledgments
Foundation: Supported by Natural Science Foundation of Shandong Province (No.ZR2017BH025).
Conflicts of Interest: Wu MQ, None; Li C, None; Zhang LN, None; Lin J, None; He K, None; Niu YW, None; Che CY, None; Jiang N, None; Jiang JQ, None; Zhao GQ, None.
REFERENCES
- 1.Niu L, Liu X, Ma Z, Yin Y, Sun L, Yang L, Zheng Y. Fungal keratitis: Pathogenesis, diagnosis and prevention. Microb Pathog. 2020;138:103802. doi: 10.1016/j.micpath.2019.103802. [DOI] [PubMed] [Google Scholar]
- 2.Xu Y, He Y, Li X, Gao C, Zhou L, Sun S, Pang G. Antifungal effect of ophthalmic preservatives phenylmercuric nitrate and benzalkonium chloride on ocular pathogenic filamentous fungi. Diagn Microbiol Infect Dis. 2013;75(1):64–67. doi: 10.1016/j.diagmicrobio.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 3.Li N, Zhao GQ. Mechanism of the immune response to keratomycosis. Zhonghua Yan ke Za Zhi. 2011;47(4):378–381. [PubMed] [Google Scholar]
- 4.Pandolfi F, Altamura S, Frosali S, Conti P. Key role of DAMP in inflammation, cancer, and tissue repair. Clin Ther. 2016;38(5):1017–1028. doi: 10.1016/j.clinthera.2016.02.028. [DOI] [PubMed] [Google Scholar]
- 5.Li G, Liang X, Lotze MT. HMGB1: the central cytokine for all lymphoid cells. Front Immunol. 2013;4:68. doi: 10.3389/fimmu.2013.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee SA, Kwak MS, Kim S, Shin JS. The role of high mobility group box 1 in innate immunity. Yonsei Med J. 2014;55(5):1165–1176. doi: 10.3349/ymj.2014.55.5.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang LF, Yao YM, Sheng ZY. Novel insights for high mobility group box 1 protein-mediated cellular immune response in sepsis: a systemic review. World J Emerg Med. 2012;3(3):165–171. doi: 10.5847/wjem.j.issn.1920-8642.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mazur-Bialy AI, Pocheć E. HMGB1 inhibition during zymosan-induced inflammation: the potential therapeutic action of riboflavin. Arch Immunol Ther Exp (Warsz) 2016;64(2):171–176. doi: 10.1007/s00005-015-0366-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barreiro-Alonso A, Lamas-Maceiras M, Rodríguez-Belmonte E, Vizoso-Vázquez Á, Quindós M, Cerdán ME. High mobility group B proteins, their partners, and other redox sensors in ovarian and prostate cancer. Oxid Med Cell Longev. 2016;2016:5845061. doi: 10.1155/2016/5845061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johansson L, Snäll J, Sendi P, Linnér A, Thulin P, Linder A, Treutiger CJ, Norrby-Teglund A. HMGB1 in severe soft tissue infections caused by Streptococcus pyogenes. Front Cell Infect Microbiol. 2014;4:4. doi: 10.3389/fcimb.2014.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hou XQ, Qin JL, Zheng XX, Wang L, Yang ST, Gao YW, Xia XZ. Potential role of high-mobility group box 1 protein in the pathogenesis of influenza H5N1 virus infection. Acta Virol. 2014;58(1):69–75. doi: 10.4149/av_2014_01_69. [DOI] [PubMed] [Google Scholar]
- 12.Kudo D, Toyama M, Aoyagi T, Akahori Y, Yamamoto H, Ishii K, Kanno E, Maruyama R, Kaku M, Kushimoto S, Kawakami K. Involvement of high mobility group box 1 and the therapeutic effect of recombinant thrombomodulin in a mouse model of severe acute respiratory distress syndrome. Clin Exp Immunol. 2013;173(2):276–287. doi: 10.1111/cei.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel VS, Sitapara RA, Gore A, Phan B, Sharma L, Sampat V, Li JH, Yang H, Chavan SS, Wang H, Tracey KJ, Mantell LL. High mobility group box-1 mediates hyperoxia-induced impairment of Pseudomonas aeruginosa clearance and inflammatory lung injury in mice. Am J Respir Cell Mol Biol. 2013;48(3):280–287. doi: 10.1165/rcmb.2012-0279OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McClellan SA, Ekanayaka SA, Li C, Jiang X, Barrett RP, Hazlett LD. Thrombomodulin protects against bacterial keratitis, is anti-inflammatory, but not angiogenic. Invest Ophthalmol Vis Sci. 2015;56(13):8091–8100. doi: 10.1167/iovs.15-18393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McClellan S, Jiang X, Barrett R, Hazlett LD. High-mobility group box 1: a novel target for treatment of Pseudomonas aeruginosa keratitis. J Immunol. 2015;194(4):1776–1787. doi: 10.4049/jimmunol.1401684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meili N, Christen V, Fent K. Nodularin induces tumor necrosis factor-alpha and mitogen-activated protein kinases (MAPK) and leads to induction of endoplasmic reticulum stress. Toxicol Appl Pharmacol. 2016;300:25–33. doi: 10.1016/j.taap.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 17.Shi JH, Sun SC. Tumor necrosis factor receptor-associated factor regulation of nuclear factor κB and mitogen-activated protein kinase pathways. Front Immunol. 2018;9:1849. doi: 10.3389/fimmu.2018.01849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He Q, You H, Li XM, Liu TH, Wang P, Wang BE. HMGB1 promotes the synthesis of pro-IL-1β and pro-IL-18 by activation of p38 MAPK and NF-κB through receptors for advanced glycation end-products in macrophages. Asian Pac J Cancer Prev. 2012;13(4):1365–1370. doi: 10.7314/apjcp.2012.13.4.1365. [DOI] [PubMed] [Google Scholar]
- 19.He ZW, Qin YH, Wang ZW, Chen Y, Shen Q, Dai SM. HMGB1 acts in synergy with lipopolysaccharide in activating rheumatoid synovial fibroblasts via p38 MAPK and NF-κB signaling pathways. Mediators Inflamm. 2013;2013:596716. doi: 10.1155/2013/596716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou YH, Han QF, Wang LH, et al. High mobility group box 1 protein attenuates myocardial ischemia reperfusion injury via inhibition of the p38 mitogen-activated protein kinase signaling pathway. Exp Ther Med. 2017;14(2):1582–1588. doi: 10.3892/etm.2017.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang C, Mo M, Ding W, Liu W, Yan D, Deng J, Luo X, Liu J. High-mobility group box 1 (HMGB1) impaired cardiac excitation-contraction coupling by enhancing the sarcoplasmic reticulum (SR) Ca(2+) leak through TLR4-ROS signaling in cardiomyocytes. J Mol Cell Cardiol. 2014;74:260–273. doi: 10.1016/j.yjmcc.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Han SJ, Min HJ, Yoon SC, Ko EA, Park SJ, Yoon JH, Shin JS, Seo KY. HMGB1 in the pathogenesis of ultraviolet-induced ocular surface inflammation. Cell Death Dis. 2015;6:e1863. doi: 10.1038/cddis.2015.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li C, Zhao G, Che C, Lin J, Li N, Hu L, Jiang N, Liu Y. The role of LOX-1 in innate immunity to aspergillus fumigatus in corneal epithelial cells. Invest Ophthalmol Vis Sci. 2015;56(6):3593–3603. doi: 10.1167/iovs.14-15989. [DOI] [PubMed] [Google Scholar]
- 24.Wu TG, Wilhelmus KR, Mitchell BM. Experimental keratomycosis in a mouse model. Invest Ophthalmol Vis Sci. 2003;44(1):210–216. doi: 10.1167/iovs.02-0446. [DOI] [PubMed] [Google Scholar]
- 25.Li C, McClellan SA, Barrett R, Hazlett LD. Interleukin 17 regulates mer tyrosine kinase-positive cells in pseudomonas aeruginosa keratitis. Investig Ophthalmol Vis Sci. 2014;55(10):6886–6900. doi: 10.1167/iovs.14-14522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao XR, Zhao GQ, Li C, Lin J, Jiang N, Wang Q, Hu L, Xu Q, Peng X, He K, Zhu G. LOX-1 and TLR4 affect ech other and regulate the generation of ROS in A.fumigatus keratitis. Int Immunopharmacol. 2016;40:392–399. doi: 10.1016/j.intimp.2016.09.027. [DOI] [PubMed] [Google Scholar]
- 27.Wang H, Bloom O, Zhang M, et al. HMGB-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285(5425):248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 28.Kokkola R, Sundberg E, Ulfgren AK, et al. High mobility group box chromosomal protein 1: a novel proinflammatory mediator in synovitis. Arthritis Rheum. 2002;46(10):2598–2603. doi: 10.1002/art.10540. [DOI] [PubMed] [Google Scholar]
- 29.Jiang W, Pisetsky DS. Expression of high mobility group protein 1 in the sera of patients and mice with systemic lupus erythematosus. Ann Rheum Dis. 2008;67(5):727–728. doi: 10.1136/ard.2007.074484. [DOI] [PubMed] [Google Scholar]
- 30.Ogawa EN, Ishizaka A, Tasaka S, et al. Contribution of high-mobility group box-1 to the development of ventilator-induced lung injury. Am J Respir Crit Care Med. 2006;174(4):400–407. doi: 10.1164/rccm.200605-699OC. [DOI] [PubMed] [Google Scholar]
- 31.Gaggar A, Rowe SM, Hardision M, Blalock JE. Proline-glycine-proline (PGP) and high mobility group box protein-1 (HMGB1): potential mediators of cystic fibrosis airway inflammation. Open Respir Med J. 2010;4(2):32–38. doi: 10.2174/1874306401004010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Passali D, Kern E, Lei Chen R, Bellussi L. High mobility group box 1 (HMGB 1): a new protein in the pathogenesis of ENT inflammatory and infectious diseases. Acta Otorhinolaryngol Ital. 2012;32(1):46–47. [PMC free article] [PubMed] [Google Scholar]
- 33.Ojo OO, Ryu MH, Jha A, Unruh H, Halayko AJ. High-mobility group box 1 promotes extracellular matrix synthesis and wound repair in human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2015;309(11):L1354–L1366. doi: 10.1152/ajplung.00054.2015. [DOI] [PubMed] [Google Scholar]
- 34.Chen P, Xie LX. Signal transduction pathways mediated by Toll-like receptors and their relations with fungal keratitis. Zhonghua Yan Ke Za Zhi. 2012;48(1):80–84. [PubMed] [Google Scholar]
- 35.Yuan X, Wilhelmus KR. Toll-like receptors involved in the pathogenesis of experimental Candida albicans keratitis. Invest Ophthalmol Vis Sci. 2010;51(4):2094–2100. doi: 10.1167/iovs.09-4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tadié JM, Bae HB, Banerjee S, Zmijewski JW, Abraham E. Differential activation of RAGE by HMGB1 modulates neutrophil-associated NADPH oxidase activity and bacterial killing. Am J Physiol Cell Physiol. 2012;302(1):C249–C256. doi: 10.1152/ajpcell.00302.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.He K, Yue LH, Zhao GQ, et al. The role of LOX-1 on innate immunity against Aspergillus keratitis in mice. Int J Ophthalmol. 2016;9(9):1245–1250. doi: 10.18240/ijo.2016.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao X, Zhao G, Li C, et al. LOX-1 and TLR4 affect each other and regulate the generation of ROS in A.fumigatus keratitis. International immunopharmacology. 2016;40:392–399. doi: 10.1016/j.intimp.2016.09.027. [DOI] [PubMed] [Google Scholar]
- 39.Zhao L, Hu YH, Sun JS, Sun L. The high mobility group box 1 protein of Sciaenops ocellatus is a secreted cytokine that stimulates macrophage activation. Dev Comp Immunol. 2011;35(10):1052–1058. doi: 10.1016/j.dci.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Y, Kobayashi T, Hayashi Y, Yoshioka R, Shiraishi A, Shirasawa S, Higashiyama S, Ohashi Y. Important role of epiregulin in inflammatory responses during corneal epithelial wound healing. Invest Ophthalmol Vis Sci. 2012;53(4):2414–2423. doi: 10.1167/iovs.11-8869. [DOI] [PubMed] [Google Scholar]
- 41.Zhou R, Zhang R, Sun Y, Platt S, Szczotka-Flynn L, Pearlman E. Innate immune regulation of Serratia marcescens-induced corneal inflammation and infection. Invest Ophthalmol Vis Sci. 2012;53(11):7382–7388. doi: 10.1167/iovs.12-10238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Apetrei NS, Călugăru A, Kerek F, Panteli M, Rasit I, Cremer L, Szegli G, Lupu AR. A highly purified vegetal fraction able to modulate HMGB1 and to attenuate septic shock in mice. Roum Arch Microbiol Immunol. 2011;70(3):114–123. [PubMed] [Google Scholar]
- 43.Youn JH, Oh YJ, Kim ES, Choi JE, Shin JS. High mobility group box 1 protein binding to lipopolysaccharide facilitates transfer of lipopolysaccharide to CD14 and enhances lipopolysaccharide-mediated TNF-alpha production in human monocytes. J Immunol. 2008;180(7):5067–5074. doi: 10.4049/jimmunol.180.7.5067. [DOI] [PubMed] [Google Scholar]
- 44.Mandke P, Vasquez KM. Interactions of high mobility group box protein 1 (HMGB1) with nucleic acids: Implications in DNA repair and immune responses. DNA Repair (Amst) 2019;83:102701. doi: 10.1016/j.dnarep.2019.102701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xie Y, Zhang K, Zhang K, Zhang J, Wang L, Wang X, Hu X, Liang Z, Li J. Toll-like receptors and high mobility group box 1 in granulosa cells during bovine follicle maturation. J Cell Physiol. 2020;235(4):3447–3462. doi: 10.1002/jcp.29234. [DOI] [PubMed] [Google Scholar]
- 46.Qin YH, Dai SM, Tang GS, Zhang J, Ren D, Wang ZW, Shen Q. HMGB1 enhances the proinflammatory activity of lipopolysaccharide by promoting the phosphorylation of MAPK p38 through receptor for advanced glycation end products. J Immunol. 2009;183(10):6244–6250. doi: 10.4049/jimmunol.0900390. [DOI] [PubMed] [Google Scholar]