Abstract
AIM
To introduce a modified technique of internal limiting membrane (ILM) centripetal dragging and peeling to treat idiopathic macular hole (IMH) and to observe the ILM-retina adhesive forces.
METHODS
Twenty-six consecutive patients with stage 3 to 4 IMH and followed up at least six months were enrolled. All patients underwent complete par plana vitrectomy, ILM dragging and peeling, fluid and gas exchange, 15% C3F8 tamponade and 2-week prone position. The best corrected visual acuity, macular hole evaluation by optical coherence tomography, and complications were evaluated.
RESULTS
The mean diameter of IMH was 524±148 µm (range: 201-683 µm), with 21 cases (80.8%) greater than 400 µm. ILM dragging and peeling were successfully performed in all cases. Most of the ILM-retina adhesive forces are severe (42.3%, 11/26), followed by mild (38.5%, 10/26), and moderate (19.2%, 5/26). The mean follow-up duration was 21.2±6.1mo. The IMH was closed in 25 (96.3%) eyes. Visual acuity (logMAR) improved significantly from 1.2±0.6 preoperatively to 0.7±0.5 postoperatively (P<0.001).
CONCLUSION
Preexisting ILM-retina adhesive force is found in IMH patients. With assistance of this force, this modified technique may help to release the IMH edges and improve the closure rate of large IMH.
Keywords: idiopathic macular hole, internal limiting membrane, adhesive force, par plana vitrectomy
INTRODUCTION
In 1991, Kelly and Wendel[1] reported successful surgical treatment for a macular hole (MH) by application of par plana vitrectomy with gas-fluid exchange. Removal of the inner limiting membrane (ILM) was suggested to improve closure rate and vision outcome[2]–[3]. Nowadays, pars plana vitrectomy with posterior hyaloid removal, ILM peeling, and gas tamponade has been the standard treatment for full thickness MH[2]. And idiopathic macular hole (IMH) is treatable with a promising success rate of more than 90%[4]–[7]. Traditional way of ILM peeling was commonly in a circular fashion[8]–[9]. To enhance the MH closure rate, mechanical joining and compression of the retinal edges using 23-gauge GreenTip soft tip cannula/Tano diamond dusted soft silicone tip[10]–[11] and forceps[12]–[13] increase the mobility of the MH edges were performed.
When performing the surgery, we found ILM-retina adhesion exists. Therefore, we come up with a modified technique of ILM centripetal dragging and peeling maneuver in MH surgery. This method uses its own preexisting ILM-retina adhesive force, rather than additional mechanical maneuvers to drag and join the MH edges. This study introduced this technique, evaluated whether ILM dragging and peeling in MH surgery is effective and observe the ILM-retina adhesive forces.
SUBJECTS AND METHODS
Ethical Approval
This is a retrospective and consecutive case series conducted in Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine. The present study adhered to the tenets of the Declaration of Helsinki and was approved by institution review board of Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine. All participants were provided written informed consent of possible benefits and risks.
Subjects
Between July 2015 and February 2017, all patients with stage 3 to 4 IMHs underwent ILM dragging and peeling were enrolled in this study. Patients with previous retinal surgery, trauma, other ocular diseases that could affect the vision, for example choroidal neovascularization, diabetic retinopathy, or opaque corneas were excluded. All surgeries were performed by the same experienced surgeon (Zhao PQ).
In total, 26 consecutive patients (26 eyes) were enrolled. The age, gender, best-corrected visual acuity (BCVA), medical history, lens status, slit-lamp examination, indirect ophthalmoscopy, and size of MH, lamellar hole associated epi-retinal proliferation (LHEP) and epi-retinal membrane (ERM) were recorded before the surgery in all patients. The stages and size of MH were determined by optical coherence tomography (OCT; RTVue-100, Optovue Inc, Fremont, CA, USA). MHs were measured in the middle horizontal diameter across the center by OCT (the shortest distance across the full-thickness defect was defined as the size of the MH). The BCVA, status of the macular assessed by OCT, slit-lamp examination, indirect ophthalmoscopy and complications were recorded after the surgery in all patients by different doctors individually.
All patients were followed up at Outpatient Clinics for at least 6mo postoperatively. Anatomical surgical success was clinically defined as apposition of the MH edges and absence of subretinal fluid. Anatomical success determined by OCT was restoration of full- or partial-thickness retinal reflection over the retinal pigment epithelium (RPE).
Surgical Procedures
The schematic diagrams of the surgical procedures are shown in Figure 1. A standard 3-port, 23-gauge transconjunctival pars plana vitrectomy was performed on all eyes under retrobulbar anesthesia. Combined phacoemulsification was performed simultaneously for eyes with cataract. After removal of the vitreous (Figure 1A), ILM was then stained by 0.125% indocyanine green (ICG) or brilliant blue G (BBG) followed by its removal within 30s (Figure 1B). Any ERM, which was highlighted after BBG instillation (negative staining), was preserved firstly to avoid ILM laceration. Two horizontal ILM strips was peeled off in the inferior and superior quadrant of macula (Figure 1C). A rectangle ILM flap was peeled from the superior to inferior area in a centripetal way to drag the superior edge of the MH with the assistance of ILM-retina adhesive force (Figure 1D). A rectangle ILM flap in the inferior part was peeled in the same way from the opposite direction (Figure 1E). After centripetally jointing the MH edges with ILM-retina adhesive forces, ILM was peeled in a traditional circular fashion.
Figure 1. The schematic diagrams of the surgical procedures.
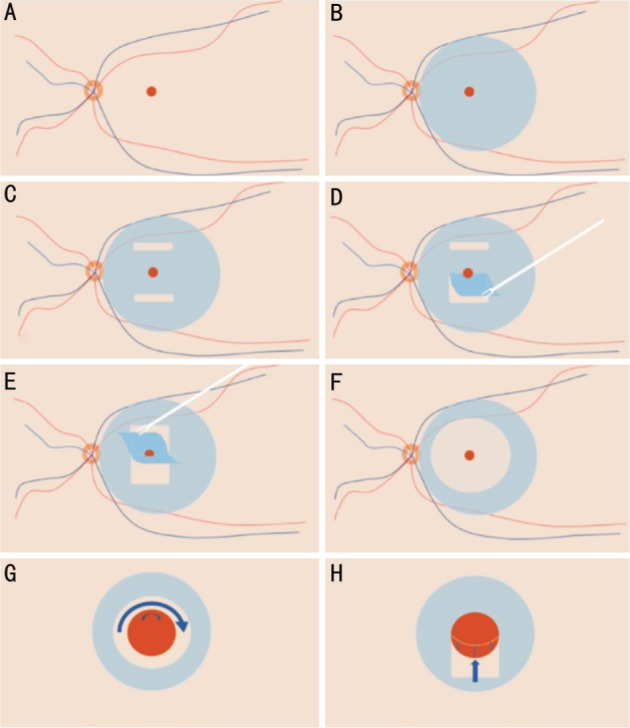
A: The MH; B: ILM was then stained by 0.125% ICG or BBG; C: Two horizontal ILM strips was peeled off in the inferior and superior quadrant of macula hole; D, E: The superior and inferior edges of the MH are jointed with the assistance of ILM-retina adhesive force; F: The ILM was then peeled in a circular fashion; G: In traditional technique with ILM peeled in a circular fashion, the MH edge was not jointed and size of MH remained the same; H: In this modified technique, the MH edges was possibly jointed centripetally to the MH center. The MH edges could be mobilized to different degrees according to the ILM-retina adhesive force.
ILM-retina adhesive forces were graded by the same experienced surgeon and classified into three stages (Figure 2): 1) Mild, only punctate adhesion was noted; 2) Moderate, the force is stronger than the mild ones but weaker than the severe ones; 3) Severe, the adhesive force was strong enough to pull the MH edge to pass the middle line of the MH.
Figure 2. Classifications of ILM-retina adhesive forces.
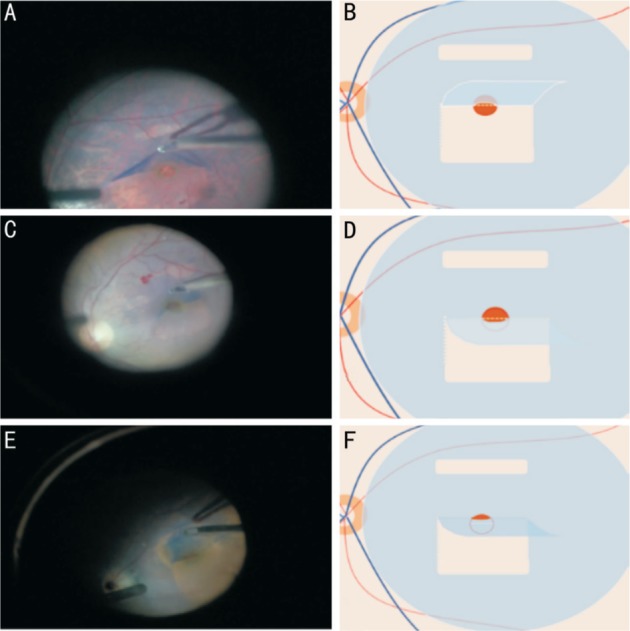
A, B: Grade mild, only punctate adhesion was noted; C, D: Grade moderate, the force is stronger than the mild ones but weaker than the severe ones; E, F: Grade severe, the adhesive force was strong enough to pull the MH edge to pass the middle line of the MH. In this case, the MH was almost closed during the surgery.
All patients underwent complete vitrectomy, ILM dragging and peeling, fluid and gas exchange, 15% C3F8 tamponade and 2-week prone position. No intraoperative or postoperative laser photocoagulation was applied to the hole margin or to the central retina.
Statistical Analysis
BCVA recorded as decimal visual acuity was converted to logarithm of minimal angle of resolution (logMAR) value for statistics. The preoperative and postoperative BCVA was compared with paired t-test. All statistics were processed using SPSS 24.0 software (SPSS, Inc, Chicago, IL, USA). A P value of less than 0.05 was considered statistically significant.
RESULTS
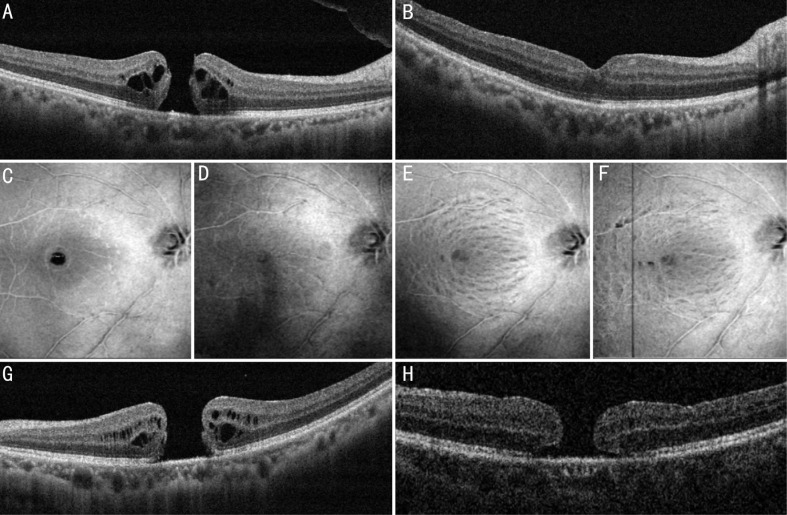
A total of 26 cases (26 eyes in 26 patients; 17 females and 9 males) were enrolled in this study. The mean age was 61.2±7.0y (range: 45-75y), and the mean size of the MH before the surgery was 524.2±147.9 µm (range: 201-683 µm). Ten (38.5%) eyes had MH with a diameter no less than 400 µm but smaller than 600 µm, and 11 (42.3%) eyes had MH with a diameter no less than 600 µm. No patients had pathological myopia. The median duration of visual symptoms, such as blurring, metamorphopsia, or scotoma, was 3mo (range: 10d to 2y). Twenty phakic eyes with cataract underwent simultaneous phacoemulsification and intraocular lens (IOL) implantation, 3 eyes were pseudophakic, and the remaining 3 eyes had transparent lens and underwent merely vitrectomy. The mean follow-up duration was 21.2±6.1mo (range:13.1-36mo). MH was closed in 25 (96.2%) eyes (Figure 3).
Figure 3. Results of the surgery.
A, B: A 64-year old male presented with an MH of 365 µm. A: The intraoperative ILM-retina adhesive force was graded mild; B: Three months later, the MH showed a good configuration and the BCVA improved from 20/100 preoperatively to 20/32 postoperatively. C-F: 3D wide-field en-face scans of SD-OCT showing the ILM layer. C: Before the surgery; D: One month after the surgery, the MH was closed and no severe iatrogenic damage caused by the modified technique was detected; E: Three months after the surgery; F: One year after the surgery. Inner retinal dimplings were observed in the ILM-peeled area, which is similar to other patients who underwent conventional ILM peeling. G, H: A 47-year old female with an MH of 659 µm. G: The intraoperative ILM-retina bonding force was graded mild; H: After one-month follow-up, the MH remained unclosed with a smaller diameter of 540 µm. She received another lens capsular flap transplantation and the MH was closed.
The ILM-retina adhesive force was found in all patients. Most of the ILM-retina adhesive forces were severe (42.3%, 11/26), followed by mild (38.5%, 10/26), and moderate (19.2%, 5/26). BBG was preferred during the surgeries, while ICG was alternative when BBG shortage occurred. ICG was used in only one case with moderate ILM-retina adhesive force, while BBG was used in the other 25 cases.
The surgical complications included transient intraocular pressure elevations in 1 eye (3.8%), which were controlled by glaucoma drugs and postoperative corneal epithelium lesions in 1 eye (3.8%). No retinal hemorrhages or retinal break due to ILM dragging technique was noted.
With respect to the functional outcomes, the BCVA was improved in 23 (88.5%) eyes and remained stable in 3 eyes (11.5%; one cataractous, one pseudophakic eye and one eye with clean crystalline) at the final follow-up examination. Visual acuity (logMAR) improved from 1.2±0.6 preoperatively to 0.7±0.5 postoperatively (P<0.001).
LHEP was found in 19.2% (5/26) cases. ERM was found in 57.7% (15/26) cases. Cystoid macular edema was found in all cases. The demographic data were listed in Table 1.
Table 1. The demographic data of the patients.
| Parameters | ILM-retina adhesive force |
Total | ||
| Mild | Moderate | Severe | ||
| Total cases | 10 | 5 | 11 | 26 |
| Sex | ||||
| Male | 4 | 2 | 3 | 9 |
| Female | 6 | 3 | 8 | 17 |
| Age (y) | 60.0±6.6 | 60.8±10.6 | 62.5±5.8 | 61.2±7.0 |
| MH diameter (range), µm | 582 (498-647) | 588 (326-656) | 571 (402-624) | 524±148 (201-683) |
| MH duration | ||||
| <6mo | 6 | 3 | 7 | 16 |
| ≥6mo | 4 | 2 | 4 | 10 |
| LHEP | ||||
| Yes | 2 | 0 | 3 | 5 |
| No | 8 | 5 | 8 | 21 |
| ERM | ||||
| Yes | 4 | 3 | 8 | 15 |
| No | 6 | 2 | 3 | 11 |
| ME | ||||
| Yes | 10 | 5 | 11 | 26 |
| No | 0 | 0 | 0 | 0 |
ME: Macular edema; MH: Macular hole; LHEP: Lamellar hole associated epi-retinal proliferation; ERM: Epi-retinal membrane.
DISCUSSION
Standard vitrectomy with or without ILM peeling is commonly used to treat MH with a high success rate of more than 90%[3],[5]. To improve closure rates in MH surgery, intraoperative application of various methods and adjuncts have been used. These adjuncts include intraoperative applications of transforming growth factor-beta2, autologous serum and autologous platelet concentrates[14], laser photocoagulation to the RPE in the bed of the MH, ILM free flap insertion[15] and free lens capsular flap transplantation[2],[5].
One of the important conditions for MH closure is the mobility of the MH edges. For some instances, ILM peeling may not provide sufficient mobility, especially in chronic or large MH[12]. As a result, additional mechanical joining and compression of the retinal edges were applied, using 23-gauge GreenTip soft tip cannula/Tano diamond dusted soft silicone tip[10]–[11] and forceps[12]–[13]. Traditional way of ILM peeling was commonly in a circular fashion[8]. In this study, we took advantage of the preexisting ILM-retina adhesive forces to increase the mobility of the MH edges. No additional destructive changes in this group were noted.
The MH closure rate of the whole group was 96.2%. In subgroups with a diameter no more than 400 µm, 400 µm to 600 µm, and more than 600 µm was 100.0%, 100.0% and 90.1%, respectively, which is higher than the the Manchester Large Macular Hole Study (the closure rate of FTMH was 98% in the 400-477 µm quartile, 91% in the 478-558 µm quartile, 94% in the 559-649 µm quartile, and 76% in the 650-1416 µm quartile)[16]. No severe perioperative complications were found. This method turned out to be effective and safe. In the unclosed case, the MH was 659 µm while the ILM-retina adhesive was mild, which may not be enough to provide sufficient mobility to close the MH.
In this study, most of the ILM-retina adhesive forces are severe (42.3%, 11/26), followed by mild (38.5%, 10/26), and moderate (19.2%, 5/26). In group with mild adhesive force, 90.0% (9/10) of the MH were closed. We believe the ILM-retina adhesive forces are not the dominant but an assistant factor for MH closure. The ILM-retina adhesive forces are preexisting; no additional instrument is needed and no possible additional surgical damage could be incurred. Additional application of the adhesive forces theoretically improves the MH closure rate. These facts make this modified technique useful and of interest. Except anatomical improvement, we also observed improved visual acuity in the majority of cases (88.5%). Although a combined cataract surgery in the same eye group may make the postoperative visual improvement more difficult to interpret. Visual improvement was also observed in 2 pseudophakic eyes and 2 eyes without cataract surgeries. Therefore, this clinical finding may indicate the high anatomic success rate of this technique also resulted in postoperative functional improvement.
The ILM is the boundary between the retina and the vitreous body, formed by astrocytes and the end feet of Müller cells. It is separated from the vitreous humor by a basal lamina[16]. ILM, part of retina, has its natural adhesive force with underneath retinal layers. The dye used to stain the ILM stays on the surface of the ILM rather than ILM-retina interface, which is applied in every case in less than 30s. Variable degrees of ILM-retina forces show the fact that dye is not one of the major factors causing different degrees of ILM-retina forces. In our study, ICG was applied in only one case with moderate ILM-retina adhesive force with BBG in the other cases, which makes it hard to analyze the impact of the dye on the adhesive force. In our opinion, preexisting ILM-retina adhesive forces may possibly associate with the following factors: 1) age, human ILM undergoes age-dependent alterations including a dramatic increase in thickness, a loss of the typical basement membrane ultrastructure, an increase in stiffness and age-related changes in its biochemical composition[16]. The adult human ILM is thicker and irregular with long indentations into the retinal tissue, which may increase ILM-retina adhesive forces theoretically. 2) LHEP was firstly found in 2006[17] and named in 2014[18]. LHEP is contiguous with the middle retinal layers, which is thicker than ERM and causes no contraction[19]–[20]. The LHEP overlies the ILM and makes the ILM or ERM harder to be removed. This may be a promising factor to increase ILM-retina adhesive forces. 3) elevation of the MH edges, this may provide additional mobility of the MH edges, which indicates less sensory retina-RPE adhesive force. This may result in a relatively stronger ILM-retina adhesive force. The exact mechanism of the ILM-retina adhesive forces remains unclear and to be explored.
Another concern about the technique is the 2 to 4 incisions on ILM needed to make a rectangle ILM flap in both superior and inferior area. The en-face OCT images showed no severe pit or severe inner retinal surface damage in the corresponding areas (Figure 3). Besides, the incisions on ILM is near the arcade and far from the fovea, which may have little impact on vision function.
However, there are several limitations to this study, which include its retrospective nature, small case number, short-term follow-up, and lack of a control group. However, this is the first case series using preexisting ILM-retina adhesive force as assistance to increase mobility of the MH edges. Another limitation is lack of objective technique to test the ILM-retina adhesive forces. Combined phacoemulsification surgery interferes with visual acuity analysis making the functional evaluation hard to interpret. Due to the small sample, it is very hard to find significant association between adhesive force and closure rate or postoperative visual acuity in this pilot study. A prospective and randomized controlled trial with a larger scale of patients and longer follow-up time is necessary to assess the efficacy and complications of the method in the long run and to unveil the mechanism and possible predicting factors of the ILM-retina adhesive forces. Pathological studies of the ILM and tissues covering and beneath it are planned in the future.
In conclusion, preexisting ILM-retina adhesive force may help to increase MH edges mobility without additional mechanical damage. Standard vitrectomy with ILM dragging and jointing MH edges centripetally by ILM-retina adhesive forces could be an effective treatment for IMH and showed a high success rate of anatomic restoration and visual recovery, even in large MHs. The preexisting ILM-retina adhesive force may enhance the closure rate without additional mechanical damage to the retina. It is a simple, safe and effective method to treat IMH.
Acknowledgments
Foundations: Supported by the National Natural Science Foundation of China (No.81470642; No.81770964); the Science and Technology Commission of Shanghai Municipality (No.17411952900).
Conflicts of Interest: Peng J, None; Zhang LH, None; Chen CL, None; Liu JJ, None; Zhu XY, None; Zhao PQ, None.
REFERENCES
- 1.Kelly NE, Wendel RT. Vitreous surgery for idiopathic macular holes. Results of a pilot study. Arch Ophthalmol. 1991;109(5):654–659. doi: 10.1001/archopht.1991.01080050068031. [DOI] [PubMed] [Google Scholar]
- 2.Chen SN, Yang CM. Lens capsular flap transplantation in the management of refractory macular hole from multiple etiologies. Retina. 2016;36(1):163–170. doi: 10.1097/IAE.0000000000000674. [DOI] [PubMed] [Google Scholar]
- 3.Bikbova G, Oshitari T, Baba T, Yamamoto S, Mori K. Pathogenesis and management of macular hole: review of current advances. J Ophthalmol. 2019;2019:3467381. doi: 10.1155/2019/3467381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ip MS, Baker BJ, Duker JS, Reichel E, Baumal CR, Gangnon R, Puliafito CA. Anatomical outcomes of surgery for idiopathic macular hole as determined by optical coherence tomography. Arch Ophthalmol. 2002;120(1):29–35. doi: 10.1001/archopht.120.1.29. [DOI] [PubMed] [Google Scholar]
- 5.Peng J, Chen CL, Jin HY, Zhang HT, Zhao PQ. Autologous lens capsular flap transplantation combined with autologous blood application in the management of refractory macular hole. Retina. 2018;38(11):2177–2183. doi: 10.1097/IAE.0000000000001830. [DOI] [PubMed] [Google Scholar]
- 6.Ch'ng SW, Patton N, Ahmed M, Ivanova T, Baumann C, Charles S, Jalil A. The Manchester large macular hole study: is it time to reclassify large macular holes? Am J Ophthalmol. 2018;195:36–42. doi: 10.1016/j.ajo.2018.07.027. [DOI] [PubMed] [Google Scholar]
- 7.Liu L, Enkh-Amgalan I, Wang NK, Chuang LH, Chen YP, Hwang YS, Chang CJ, Chen KJ, Wu WC, Chen TL, Lai CC. Results of macular hole surgery: evaluation based on the international vitreomacular traction study classification. Retina. 2018;38(5):900–906. doi: 10.1097/IAE.0000000000001647. [DOI] [PubMed] [Google Scholar]
- 8.Lai CC, Hwang YS, Liu L, Chen KJ, Wu WC, Chuang LH, Kuo JZ, Chen TL. Blood-assisted internal limiting membrane peeling for macular hole repair. Ophthalmology. 2009;116(8):1525–1530. doi: 10.1016/j.ophtha.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 9.Chatziralli IP, Theodossiadis PG, Steel DHW. Internal limiting membrane peeling in macular hole surgery; why, when, and how? Retina. 2018;38(5):870–882. doi: 10.1097/IAE.0000000000001959. [DOI] [PubMed] [Google Scholar]
- 10.Kumar A, Tinwala SI, Gogia V, Sehra SV. Tapping of macular hole edges: the outcomes of a novel technique for large macular holes. Asia Pac J Ophthalmol (Phila) 2013;2(5):305–309. doi: 10.1097/APO.0b013e31829a1919. [DOI] [PubMed] [Google Scholar]
- 11.Manasa S, Kakkar P, Kumar A, Chandra P, Kumar V, Ravani R. Comparative evaluation of standard ILM peel with inverted ILM flap technique in large macular holes: a prospective, randomized study. Ophthalmic Surg Lasers Imaging Retina. 2018;49(4):236–240. doi: 10.3928/23258160-20180329-04. [DOI] [PubMed] [Google Scholar]
- 12.Alpatov S, Shchuko A, Malyshev V. A new method of treating macular holes. Eur J Ophthalmol. 2007;17(2):246–252. doi: 10.1177/112067210701700215. [DOI] [PubMed] [Google Scholar]
- 13.Ohana O, Barak A, Schwartz S. Internal aspiration under perfluorocarbon liquid for the management of large macular holes. Retina. 2017;37(11):2145–2150. doi: 10.1097/IAE.0000000000001449. [DOI] [PubMed] [Google Scholar]
- 14.Coca M, Makkouk F, Picciani R, Godley B, Elkeeb A. Chronic traumatic giant macular hole repair with autologous platelets. Cureus. 2017;9(1):e955. doi: 10.7759/cureus.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong D, Steel DH. Free ILM patch transplantation for recalcitrant macular holes; should we save some internal limiting membrane for later? Graefes Arch Clin Exp Ophthalmol. 2016;254(11):2093–2094. doi: 10.1007/s00417-016-3462-3. [DOI] [PubMed] [Google Scholar]
- 16.Candiello J, Cole GJ, Halfter W. Age-dependent changes in the structure, composition and biophysical properties of a human basement membrane. Matrix Biol. 2010;29(5):402–410. doi: 10.1016/j.matbio.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Witkin AJ, Ko TH, Fujimoto JG, Schuman JS, Baumal CR, Rogers AH, Reichel E, Duker JS. Redefining lamellar holes and the vitreomacular interface: an ultrahigh-resolution optical coherence tomography study. Ophthalmology. 2006;113(3):388–397. doi: 10.1016/j.ophtha.2005.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pang CE, Spaide RF, Freund KB. Epiretinal proliferation seen in association with lamellar macular holes: a distinct clinical entity. Retina. 2014;34(8):1513–1523. doi: 10.1097/IAE.0000000000000163. [DOI] [PubMed] [Google Scholar]
- 19.Pang CE, Maberley DA, Freund KB, White VA, Rasmussen S, To E, Matsubara JA. Lamellar hole-associated epiretinal proliferation: a clinicopathologic correlation. Retina. 2016;36(7):1408–1412. doi: 10.1097/IAE.0000000000001069. [DOI] [PubMed] [Google Scholar]
- 20.dell'Omo R, Virgili G, Rizzo S, De Turris S, Coclite G, Giorgio D, dell'Omo E, Costagliola C. Role of lamellar hole-associated epiretinal proliferation in lamellar macular holes. Am J Ophthalmol. 2017;175:16–29. doi: 10.1016/j.ajo.2016.11.007. [DOI] [PubMed] [Google Scholar]