Abstract
AIM
To evaluate and compare the peripapillary and retinal vasculature changes in primary open angle glaucoma (POAG), pseudoexfoliation glaucoma (PXG), ocular hypertension (OHT) and normal eyes using optical coherence tomography angiography (OCTA).
METHODS
A total of 114 POAG, PXG and OHT eyes of 60 patients and 46 eyes of 23 healthy control participants with good quality OCTA images were included. The PXG, POAG, OHT, and control groups (aged 68.17±6.30y, 61.11±10.26y, 58.1±8.9y, and 56.9±4.6y, respectively) contained of 46, 36, 32, and 46 eyes, respectively. Measurements of vessel density (VD) in the peripapillary region and macula, average retinal inner thickness, and retinal nerve fiber layer thickness (RNFLT) were compared among groups. In order to test the accuracy of differentiation between eyes with and without glaucoma, the area was calculated under the receiver operating characteristic (ROC) curves.
RESULTS
The VD in glaucomatous eyes was significantly lower than the control group in all peripapillary sectors (44.35%±6.78% vs 50.47%±1.83%, P<0.001), the superficial (44.08%±5.46% vs 51.28%±2.85%, P<0.001) and the deep (45.13%±8.55% vs 54.20%±5.44%, P<0.001) vascular plexus. There was a significant difference in peripapillary VD between glaucomatous and OHT eyes (44.35%±6.78% vs 49.86%±2.45%, P<0.001). The OHT group featured a lower superficial (48.06%±4.32% vs 51.28%±2.85%, P=0.027) and deep plexus (48.70%±5.99% vs 54.20%±5.44%, P=0.013) whole image vessel density (wiVD) than did the control group. The average macular superficial plexus wiVD was significantly lower in eyes with PXG than in eyes with POAG (42.22%±5.36% vs 46.54%±5.56%, P=0.046).
CONCLUSION
OCTA can measure reduced peripapillary and macular VD in eyes with glaucoma and OHT, and these results are correlated to functional and structural glaucomatous alterations. Peripapillary and macular superficial plexus VD is lower in eyes with PXG than in eyes with POAG. Furthermore, the OHT eyes demonstrate impaired macular vasculature in both superficial and deep plexus.
Keywords: glaucoma, optical coherence tomography angiography, vessel density, ocular hypertension, pseudoexfoliation glaucoma
INTRODUCTION
Glaucoma is a leading cause of irreversible blindness worldwide that is characterized by progressive deterioration and loss of retinal ganglion cells and their axons retinal nerve fiber layer (RNFL)[1]. Although elevated intraocular pressure (IOP) is the most important responsible factor for retinal ganglion cell death and optic nerve damage, reducing IOP does not always prevent the disease progression. It has been shown that ischaemia and vascular dysfunction have been involved in the pathogenesis of glaucoma[2]–[4].
To evaluate and measure the ocular blood flow, various devices and techniques have been developed and used including color Doppler imaging, laser Doppler flowmetry, retinal vessel analyzer, laser speckle flowgraphy, and fluorescein angiography[5]–[6]. However, these techniques have limited ability to provide information on vascular structures because of the high variability of laser Doppler flowmetry and laser speckle flowgraphy measurements, invasive and time consuming nature of fluorescein angiography.
Optical coherence tomography angiography (OCTA) is a novel, non-dye-based imaging technique which enables non-invasive visualization and assessment of the peripapillary retina, macula and optic disc microvasculature[7]–[8]. OCTA applies the principle of split spectrum amplitude-decorrelation angiography (SSADA), to improve signal-to-noise ratio of flow detection and minimize scanning time, thereby enables for more accurate evaluation of microvascular retinal anatomy, and en face visualization[9]. The reproducibility and repeatability of this method have been reported in several studies[10]–[14].
Pseudoexfoliation syndrome is considered to be the most common detectable precursor of open angle glaucoma worldwide and generally develops with age[15]. Pseudoexfoliation glaucoma (PXG) is associated with a faster rate of progression, poorer response to treatment and worse prognosis than primary open angle glaucoma (POAG)[15]–[19]. It is associated with other ocular manifestations such as cataract with zonular instability, lens dislocation, secondary glaucoma and retinal vein occlusion. It is also associated with multiple systemic diseases involving the cardiovascular and central nervous system[15],[20]–[21]. Previous studies showed that the progression rate is more rapid, with higher mean IOP and greater diurnal fluctuation than in eyes with POAG[15]–[19]. Increased IOP, is the only changeable risk factor shown to slow down or stop glaucoma progression, but other than the IOP, possible contribution of non-IOP factors such as ocular ischemia and vascular dysregulation are related to disease progression[15]–[17]. Several papers have reported a decrease in the peripapillary and macular capillary density in eyes with PXG[22]–[25].
Ocular hypertension (OHT) eyes were defined as having an IOP greater than 21 mm Hg with no glaucomatous optic nerve damage or visual field (VF) loss[26]. Previous studies have shown that OCTA can be used to assess the microvascularity changes in the presence of OHT. Reduced OCTA parameters were reported in eyes with OHT, which demonstrates an impairment of the ocular blood flow in these eyes[27]–[29].
The radial peripapillary capillary (RPC) network is a specific plexus of capillary bed within the RNFL around the optic disc which is vulnerable to glaucoma damage. Microvascular changes of optic nerve head (ONH) and peripapillary area measured by OCTA, have been shown in patients with glaucoma previously[8],[12]–[14],[27]–[29]. The mean rate of change in macular vessel density (VD) was found to be significantly higher in glaucomatous eyes than in glaucoma-suspect or normal eyes thereby a change in VD could be determined before a change in inner macular retinal thickness occurs[30]–[31]. Several previous studies have demonstrated the differences in the microvasculature of the optic disc, peripapillary and macular area between glaucoma, glaucoma suspects and normal patients[23]–[25],[27]–[32]. Although previous studies have evaluated the macular and ONH circulation in patients with POAG, PXG and OHT; vascular alterations in different sectors of macular and peripapillary area have not been evaluated among these groups in the same study.
The purpose of this study was to compare the retinal VD measurements of peripapillary and macular regions on OCTA between normal, POAG, PXG and OHT eyes and to analyze correlations between retinal VD measurements to other structural parameters like RNFL and inner macular retinal thickness.
SUBJECTS AND METHODS
Ethical Approval
Ethics approval is obtained from the Institutional Review Board of Ankara University. The study was conducted in accordance with the tenets of the Declaration of Helsinki. Written informed consent was obtained from all study participants.
In this retropective monocentric study data from 114 glaucomatous eyes of 60 patients and 46 eyes of 23 healthy control patients were evaluated. All subjects were Caucasians. Control patients were either hospital workers or patients who consulted for a routine ophthalmological examination. The medical records of patients who visited the glaucoma clinic of the Ankara University, Department of Ophthalmology between March and December 2018 were reviewed retrospectively. POAG, PXG, OHT eyes and normal control eyes were analyzed consecutively. All patients underwent a detailed medical history and a complete ophthalmic examination, including best-corrected visual acuity (BCVA), a refraction test, IOP measurement with Goldmann applanation tonometer, gonioscopy, central corneal thickness measurement, standard automated perimetry with Humphrey field analyzer, dilated fundoscopic examination.
Inclusion criteria included the following: patient age >18y, open angle on gonioscopic examination, and BCVA of 20/40 or better. Exclusion criteria were the presence of any media opacities that prevented good quality spectral-domain optical coherence tomography (SD-OCT) or OCTA images, refractive error >±6 D sphere and ±3 D cylinder, any retinal or neurologic pathology except for glaucoma, history of intraocular surgery other than uncomplicated cataract extraction, uveitis, ocular trauma, diabetic retinopathy, hypertensive retinopathy, unreliable VF results (≥20% of fixation loss, ≥15% false positive or false negative), retinal vascular occlusive diseases including retinal artery or vein occlusion or any other vascular conditions such as coronary artery disease, peripheral vascular disease or thrombosis. Healthy subjects had IOP<21 mm Hg on the clinical examination day with no history of elevated IOP, glaucoma, OHT, or family history of glaucoma; normal appearing optic disc, intact neuroretinal rim, and RNFL, normal inner macular retina thickness; an open anterior chamber angle on gonioscopic examination, no history of chronic ocular or systemic corticosteroid use, and a minimum of two reliable normal VF tests. Age and gender matched distribution was applied for comparison of control group and the glaucoma subjects.
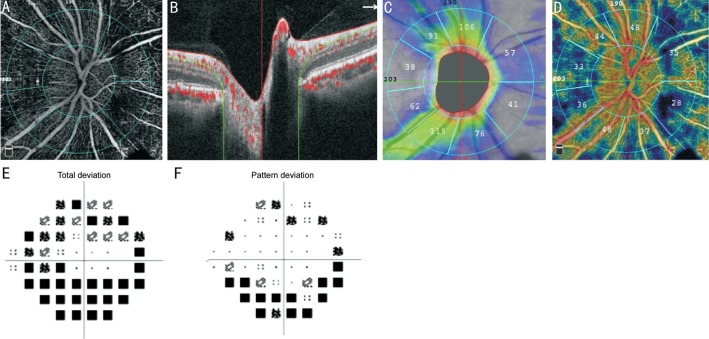
Standard automated perimetry VF tests were completed using Humphrey field analyzer II, model 720i (Zeiss Humphrey Systems, Dublin, CA, USA), with the Swedish interactive threshold algorithm (SITA) standard 24-2 program. Only reliable tests (≤33% fixation losses, ≤15% false-negative errors and ≤15% false-positive errors) with mean deviation (MD), and pattern standard deviation (PSD) within 95% confidence interval were included (Figure 1E, 1F).
Figure 1. OCTA image of the ONH of a representative case with glaucoma.
A: Whole en face image of the ONH; B: Corresponding B-scan segmentation; C: Circumpapillary RNFL thickness map; D: Peripapillary area VD map; E: Visual field mean deviation map; F: Visual field pattern standard deviation map.
All subjects then underwent ONH imaging, peripapillary RNFL (Figure 1C) and inner macular retina thickness (Figure 2F) measurements with a RTVue-XR SD-OCT (Avanti; Optovue, Inc., USA). Average peripapillary RNFL and inner macular retina thickness was obtained automatically from SD-OCT scans. Only good quality scans with a signal strength index (SSI) 37 or more and without segmentation errors or motion artifacts were included.
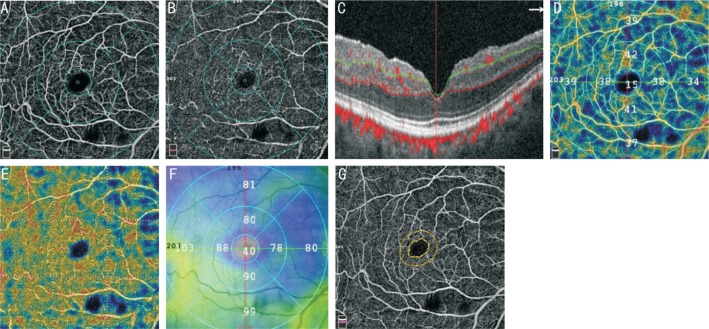
Figure 2. OCTA image of the macula of a representative case with PXG.
A: Whole en face image of macular superficial plexus; B: Whole en face image of macular deep plexus; C: Corresponding B-scan segmentation; D: Superficial plexus VD map; E: Deep plexus VD map; F: Inner macular retina thickness map; G: Foveal avascular zone.
All participants underwent SD-OCT and OCTA (AngioVue; Optovue, Inc., Fremont, CA, USA) imaging of the optic disc region (Figure 1A) and macula (Figure 2A, 2B) were performed on the same day. OCTA uses the SSADA algorithm to minimize scanning time, detect red blood cell movement and provide a high-resolution 3D visualization of retinal circulation. VD is defined as the percentage area occupied by the large vessels and microvasculature in a particular region which is usually represented by clear colours in the greyscale device results, and provided quantitatively. For the ONH scan, several regions of the VD can be assessed, including inside-disc, peripapillary region or whole-image en-face optic disc scan. The peripapillary VD were analyzed in the RPC slab. The RPC slab extends from the internal limiting membrane (ILM) to the posterior boundary of RNFL (Figure 1B). The optic disc scan covers a 4.5×4.5 mm2 area, centered on the ONH. The peripapillary region defined as a 750-µm width elliptical annular area extending from the optic disc margin is divided into 8 sectors. The peripapillary VDs were measured in each sector (nasal-superior, nasal-inferior inferotemporal, inferonasal, superotemporal, superonasal, tempoinferior, temposuperior; Figure 1D) and the average VD measurements of 4 quadrants (nasal, inferior, superior, temporal) were calculated. Whole image vessel density (wiVD) was calculated in the entire 4.5×4.5 mm2 image, and peripapillary VD was calculated in the peripapillary region.
Macular capillary network was visualized in 6.0×6.0 mm2 scans centered on fovea. Each 6.0×6.0 mm2 macular superficial and deep retinal plexus VDs was calculated. The superficial plexus located in a retinal slab extended from 3 µm below the ILM to 15 µm below the inner plexiform layer (IPL), and the deep plexus located in the retinal slab extended from 15 µm to 70 µm below IPL (Figure 2F). The foveal (1 mm diameter central zone), parafoveal (1-3 mm diameter ring) and perifoveal (3-6 mm diameter ring) VDs were measured. The parafoveal and perifoveal regions are divided into 4 sectors of 90 degrees each (nasal, inferior, superior, and temporal sectors; Figure 2D, 2E). In addition, mean VD for the whole image of the macula scan (wiVD macula) was measured. Furthermore, the area of the foveal avascular zone (FAZ) and the area of non-flow regions was also calculated (Figure 2G).
Statistical Analysis
All statistical analysis was performed with SPSS statistics program for Windows Version 21.0 (IBM Corp., Armonk, NY, USA). Different demographic characteristics among the groups, including age, gender and history of systemic disease were compared using the Chi-square test. All analyses were adjusted for age. Significant differences between OCTA VD, inner macular retina thickness, RNFL thickness, VF MD and VF PSD across groups were compared using one-way analysis of variance (ANOVA), and multiple comparisons with Bonferroni adjustments were used for post-hoc analysis. Pearson correlation analysis was used to determine the correlation between OCTA VD and inner macular retina thickness, RNFL thickness, VF MD and VF PSD. The age-adjusted area under the receiver operating characteristic (ROC) curves were calculated for the evaluation of the diagnostic accuracy for differentiating between healthy and glaucoma eyes. A P value of <0.05 was considered statistically significant.
RESULTS
A total of 114 eyes from 60 patients and 46 eyes from 23 healthy controls underwent VD and structural imaging with OCTA. The PXG group contained of 46 eyes (24 patients) with an average age of 68.17±6.30y, the POAG group contained of 36 eyes (20 patients) with an average age of 61.11±10.26y, the OHT group contained of 32 eyes (16 patients) with an average age of 58.1±8.9y and the control group contained of 46 eyes (23 patients) with an average age of 56.9±4.6y. Table 1 summarizes the demographic, clinical and ophthalmic characteristics of each group. Mean age in the OHT and healthy control groups was significantly lower than both glaucoma groups (P<0.001). Therefore, age-adjustment was applied for all comparisons and area under ROC curves. There were no significant differences in sex, IOP and proportion of hypertension and diabetes mellitus between the studied groups.
Table 1. Demographic and ophthalmic characteristics of the study groups.
| Parameters | PXG | POAG | OHT | Control | P |
| No. of eyes | 46 | 36 | 32 | 46 | |
| Age (y) | 68.17±6.30 | 61.11±10.26 | 58.1±8.9 | 56.9±4.6 | <0.001 |
| Sex (M/F) | 14/10 | 12/8 | 10/6 | 13/10 | 0.24 |
| IOP (mm Hg) | 15.6±2 | 14.9±3.6 | 17.5±3.1 | 13.4±2 | 0.12 |
| CCT (µm) | 536.4±23.4 | 534.2±21 | 540.7±31.6 | 532.5±16.7 | 0.56 |
| Diabetes mellitus | 2/24 | 2/20 | 1/16 | 2/23 | 0.21 |
| Hypertension | 6/24 | 4/20 | 3/16 | 3/23 | 0.15 |
| Antiglaucomatous eye drops | 2.7±0.3 | 2.2±0.4 | 1.1±0 | 0 |
OHT: Ocular hypertension; PXG: Pseudoexfoliation glaucoma; POAG: Primary open angle glaucoma; IOP: Intraocular pressure; CCT: Central corneal thickness.
Peripapillary and macular OCTA VDs, inner macular retina thickness, RNFL thickness, VF MD and VF PSD's in three groups are shown in Table 2. As expected, all diagnostic measurements were significantly decreased in the glaucoma group compared to the control and OHT groups (ANOVA, all P<0.001). There were no statistically significant differences in peripapillary VD and RNFLT between OHT and control group; however, the OHT group featured a lower superficial (P=0.027) and deep plexus (P=0.013) wiVD than did the control group. The average peripapillary wiVD was significantly lower in eyes with PXG than in eyes with POAG (P=0.023). There was a significant reduction in peripapillary VD in the inferonasal sector, but not in the other sectors of eyes with PXG. The average macular superficial plexus wiVD was significantly lower in eyes with PXG than in eyes with POAG (P=0.046). There were no statistically significant differences in deep plexus wiVD. PXG group featured a lower foveal VD in both superficial (P=0.005) and deep plexus (P=0.031) than POAG group. Additionally, PXG group had lower average values for inner macular retina thickness than the POAG group in all quadrants (Table 2).
Table 2. Diagnostic measurements analysis among the study groups after adjusting for age.
| Vessel density | PXG group | POAG group | OHT group | Control group | P (total) | P1 | P2 | P3 |
| Peripapillary | ||||||||
| wiVD | 42.15±8.59 | 47.21±5.87 | 49.86±2.45 | 50.47±1.83 | <0.001 | >0.05 | 0.002 | 0.023 |
| Inside disc | 44.22±11.43 | 47.55±8.39 | 50.61±5 | 50.65±2.99 | 0.002 | >0.05 | 0.015 | >0.05 |
| Nasal | 42.62±7.71 | 47.15±7.25 | 48.79±4.32 | 50.49±3.65 | <0.001 | >0.05 | 0.073 | 0.048 |
| Inferior | 46.79±9.87 | 52.41±10.83 | 55.92±7.28 | 54.93±4.56 | <0.001 | >0.05 | 0.023 | 0.037 |
| Superior | 44.32±12.65 | 49.86±11.25 | 52.79±4.9 | 53.78±4.67 | <0.001 | >0.05 | 0.064 | >0.05 |
| Temporal | 46.47±10.61 | 51.57±8.02 | 54.52±3.92 | 53.83±3.41 | <0.001 | >0.05 | 0.034 | >0.05 |
| Macula (superficial plexus) | ||||||||
| wiVD | 42.22±5.36 | 46.54±5.56 | 48.06±4.32 | 51.28±2.85 | <0.001 | 0.027 | 0.034 | 0.046 |
| Foveal VD | 15.17±7.64 | 19.71±8.15 | 19.22±7.9 | 20.22±5.34 | 0.001 | >0.05 | 0.075 | 0.005 |
| pfVD nasal | 42.59±8.84 | 48.09±6.59 | 50.39±4.80 | 52.96±4 | <0.001 | >0.05 | 0.024 | >0.05 |
| pfVD inferior | 44.87±6.83 | 49.18±6.35 | 50.24±5.62 | 53.68±4.86 | <0.001 | >0.05 | >0.05 | >0.05 |
| PfVD superior | 44.85±9.05 | 49.12±6.88 | 50.91±5.30 | 54.61±4.23 | <0.001 | >0.05 | >0.05 | >0.05 |
| pfVD temporal | 43.61±6.35 | 48.74±6.22 | 50.88±5.39 | 52.72±4.14 | <0.001 | >0.05 | 0.012 | 0.032 |
| Macula (deep plexus) | ||||||||
| wiVD | 43.05±9.45 | 47.81±7.69 | 48.70±5.99 | 54.20±5.44 | <0.001 | 0.013 | >0.05 | >0.05 |
| Foveal VD | 32.04±8.57 | 36.22±10.23 | 34.39±8.65 | 36.47±6.60 | 0.002 | >0.05 | >0.05 | 0.031 |
| pfVD nasal | 50.56±5.76 | 53.98±7.59 | 54.42±4.41 | 52.96±4 | 0.01 | >0.05 | >0.05 | >0.05 |
| pfVD inferior | 47.31±9.92 | 51.19±8.04 | 52.10±5.45 | 53.68±4.86 | 0.026 | >0.05 | >0.05 | >0.05 |
| PfVD superior | 48.23±10.43 | 52.81±6.74 | 53.39±5.19 | 56.32±4.62 | <0.001 | >0.05 | >0.05 | >0.05 |
| pfVD temporal | 50.73±6.42 | 54.24±6.11 | 54.84±3.98 | 57.31±4.11 | 0.007 | >0.05 | >0.05 | >0.05 |
| FAZ size (mm2) | 0.263±0.152 | 0.251±0.129 | 0.298±0.09 | 0.0305±0.08 | 0.017 | >0.05 | 0.047 | 0.037 |
| Parafovea inner macular retina thickness (µm) | ||||||||
| Fovea | 45.05±9.63 | 50.06±10.96 | 50.97±6.61 | 51.78±7.89 | 0.013 | >0.05 | >0.05 | >0.05 |
| Nasal | 87.84±16.29 | 100.29±16.31 | 104.29±7.29 | 111.0±6.05 | <0.001 | >0.05 | 0.057 | 0.011 |
| Inferior | 85.98±22.67 | 103.23±15.53 | 107.19±7.31 | 114.5±7.37 | <0.001 | >0.05 | 0.012 | 0.002 |
| Superior | 86.70±21.23 | 100.29±17.06 | 107.06±7.35 | 114.15±6.79 | <0.001 | >0.05 | 0.026 | 0.048 |
| Temporal | 81.02±15.37 | 92.00±14.49 | 96.71±6.14 | 103.57±6.97 | <0.001 | >0.05 | 0.042 | 0.007 |
| RNFLT (µm) | ||||||||
| Nasal | 75.4±18.65 | 89.73±21.05 | 92.5±13.7 | 98.73±12.5 | <0.001 | >0.05 | 0.037 | 0.083 |
| Inferior | 100.53±30.11 | 115.86±28.27 | 126.56±15.39 | 139.72±24.03 | <0.001 | >0.05 | 0.048 | >0.05 |
| Superior | 83.45±22.67 | 102.67±15.85 | 112.4±15.14 | 124.43±18.8 | <0.001 | >0.05 | 0.020 | >0.05 |
| Temporal | 59.57±13.59 | 67,07±14.55 | 71.41±10.34 | 72.31±8.4 | 0.002 | >0.05 | 0.025 | >0.05 |
| VF MD | -5.63±6.05 | -2.93±3.88 | -1.53±2.23 | -1.64±1.25 | 0.001 | >0.05 | 0.041 | >0.05 |
| VF PSD | 3.85±2.49 | 3.00±2.71 | 2.19±1.02 | 1.92±0.69 | 0.001 | >0.05 | 0.077 | >0.05 |
P (total): The P-value obtained from one-way analysis of variance (ANOVA); P1: The P-value obtained from post-hoc test (Bonferroni) between the OHT and control groups; P2: The P-value obtained from post-hoc test (Bonferroni) between the glaucoma (PXG+POAG) and OHT groups; P3: The P-value obtained from post-hoc test (Bonferroni) between the POAG and PXG comparison groups. wiVD: Whole image vessel density; pfVD: Parafoveal vessel density; RNFLT: Retinal nerve fiber layer thickness; VF MD: Visual field mean deviation; VF PSD: Visual field pattern standard deviation.
The glaucoma group had a larger FAZ than the control and OHT groups. Similarly, the superficial non-flow area of the macula in the glaucoma group was larger than that of the OHT and the control groups (all P<0.05). Additionally, PXG group had a larger FAZ area than POAG group (P=0.037). There were no statistically significant differences between the OHT and control groups in these parameters.
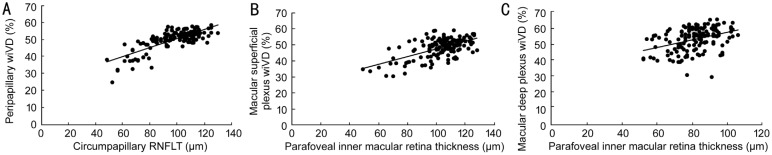
Pearson correlation indexes of macular and peripapillary VDs with RNFL thickness, inner macular retina thickness, VF MD and VF PSD are shown in the Table 3. Figure 3 shows the correlation between OCTA VDs and structural parameters. In the glaucoma group, the linear regression analysis showed that whole image peripapillary VD was most significantly correlated with RNFL thickness (r=0.808, P<0.001); more strongly in inferior quadrant (r=0.723, P<0.001; Figure 3A). In addition, macular wiVD were significantly correlated (more strongly in superior and inferior quadrant) with inner macular retina thickness in both superficial (r=0.667, P<0.001) and deep plexus (r=0.546, P<0.001), except foveal region (Figure 3B, 3C). The correlation between macular and peripapillary VD with structural parameters (RNFL or inner macular retina thickness) and functional parameters (MD and PSD on VF) were statistically significant within the glaucoma group, while there was no correlation within normal subjects. The VF MD was significantly correlated with inner macular retina thickness (r=0.53, P<0.001), RNFL thickness (r=0.59, P<0.001), superficial plexus VD (r=0.48, P<0.001), deep plexus VD (r=0.25, P=0.002) and peripapillary VD (r=0.59, P<0.001) in the glaucoma group. The VF PSD was significantly correlated with inner macular retina thickness (r=-0.52, P<0.001), RNFL thickness (r=-0.77, P<0.001), superficial plexus VD (r=-0.52, P<0.001), deep plexus VD (r=-0.24, P=0.003) and peripapillary VD (r=-0.56, P<0.001) in the glaucoma group.
Table 3. Pearson correlation indexes of macular and peripapillary VDs with RNFL, inner macular retina thickness, VF MD and VF PSD.
| Parameters | P (IMRT) | r (IMRT) | P (RNFLT) | r (RNFLT) | P (MD) | r (MD) | P (PSD) | r (PSD) |
| pp wiVD | <0.001 | 0.603 | <0.001 | 0.808 | <0.001 | 0.590 | <0.001 | -0.561 |
| pp nas VD | <0.001 | 0.417 | <0.001 | 0.604 | <0.001 | 0.384 | <0.001 | -0.416 |
| pp inf VD | <0.001 | 0.443 | <0.001 | 0.723 | <0.001 | 0.578 | <0.001 | -0.475 |
| pp sup VD | <0.001 | 0.428 | <0.001 | 0.660 | <0.001 | 0.544 | <0.001 | -0.450 |
| pp tem VD | <0.001 | 0.382 | <0.001 | 0.645 | <0.001 | 0.311 | <0.001 | -0.396 |
| SP wiVD | <0.001 | 0.667 | <0.001 | 0.644 | <0.001 | 0.482 | <0.001 | -0.519 |
| SP fov VD | 0.150 | 0.117 | 0.008 | 0.215 | 0.031 | 0.177 | 0.004 | -0.235 |
| SP nas VD | <0.001 | 0.554 | <0.001 | 0.445 | <0.001 | 0.422 | <0.001 | -0.408 |
| SP inf VD | <0.001 | 0.558 | <0.001 | 0.472 | 0.001 | 0.269 | <0.001 | -0.352 |
| SP sup VD | <0.001 | 0.628 | <0.001 | 0.474 | <0.001 | 0.316 | <0.001 | -0.388 |
| SP tem VD | <0.001 | 0.566 | <0.001 | 0.412 | <0.001 | 0.308 | <0.001 | -0.402 |
| DP wiVD | <0.001 | 0.546 | <0.001 | 0.492 | 0.002 | 0.245 | 0.003 | -0.240 |
| DP fov VD | 0.202 | 0.103 | 0.03 | 0.179 | 0.039 | 0.168 | 0.133 | -0.123 |
| DP nas VD | <0.001 | 0.357 | <0.001 | 0.417 | <0.001 | 0.343 | <0.001 | -0.316 |
| DP inf VD | <0.001 | 0.441 | <0.001 | 0.333 | 0.126 | 0.125 | 0.083 | -0.142 |
| DP sup VD | <0.001 | 0.438 | <0.001 | 0.374 | 0.056 | 0.156 | 0.022 | -0.187 |
| DP tem VD | <0.001 | 0.324 | <0.001 | 0.340 | 0.008 | 0.256 | 0.002 | -0.252 |
pp: Peripapillary; SP: Superficial plexus; DP: Deep plexus; nas: Nasal; inf: Inferior; sup: Superior; tem: Temporal; fov: fovea; wiVD: Whole image vessel density; IMRT: Inner macular retina thickness; RNFLT: Retinal nerve fiber layer thickness; VF MD: Visual field mean deviation; VF PSD: Visual field pattern standard deviation.
Figure 3. Correlations between peripapillary and macular VDs and structural parameters.
A: Correlation between peripapillary wiVD and circumpapillary RNFLT; B: Correlation between macular superficial plexus wiVD and parafoveal inner macular retina thickness; C: Correlation between macular deep plexus wiVD and parafoveal inner macular retina thickness.
To differentiate between healthy and glaucoma eyes, we used area under the ROC curves as quality measures. The age-adjusted area under ROC for differentiating between glaucoma and healthy eyes was highest for inner macular retina thickness (0.897±0.025, 0.847-0.947), followed by RNFL thickness (0.849±0.030, 0.791-0.908), superficial plexus VD (0.820±0.033, 0.755-0.884), deep plexus VD (0.793±0.037, 0.721-0.866), and peripapillary VD (0.645±0.044, 0.560-0.731; Figure 4).
Figure 4. Comparison between peripapillary wiVD, macular superficial plexus wiVD, macular deep plexus wiVD, peripapillary RNFL thickness, parafoveal inner macular retina thickness in terms of area under ROC curves.
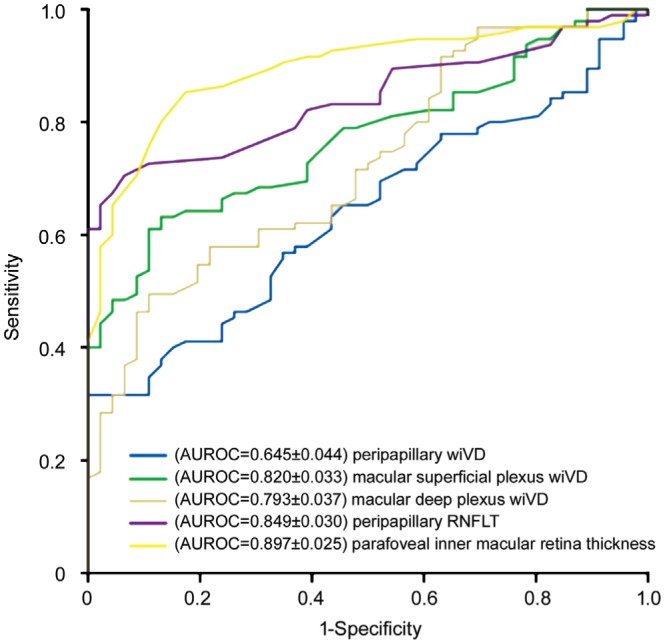
AUROC: Area under the receiver operating characteristic.
DISCUSSION
In this study we evaluated and compared the peripapillary and macular microvascular parameters between POAG, PXG, OHT patients and age-matched healthy controls using OCTA. We have demonstrated that peripapillary and macular VDs are reduced in glaucomatous eyes. In addition, in our study we were able to demonstrate lower superficial and deep perifoveal VDs in OHT group compared with normal eyes. However, there was no difference in peripapillary VDs or other optic disc parameters between OHT and normal eyes. To the best of our knowledge, this is the first study to compare peripapillary and macular VDs between patients with OHT and those with POAG, PXG and healthy controls using OCTA.
Our study showed a difference in peripapillary and macular superficial wiVD between eyes with PXG and POAG. In addition, we found a significant reduction in peripapillary VD in the inferonasal sector, but not in the other sectors of eyes with PXG. Park et al[25] showed that peripapillary VD was lower in eyes with PXG than in eyes with POAG primarily in the nasal and inferonasal sectors in their comparative study. They suggested that eyes with PXG could be more vulnerable to glaucomatous damage because of the decreased peripapillary VD and the resulting ischemia. Additionally, Suwan et al[22] reported lower peripapillary VD in exfoliation glaucoma compared to POAG. Philip et al[24] reported that superficial capillary plexus VD was significantly lower in the superior and nasal quadrants in exfoliation glaucoma versus POAG in their study.
Exfoliation syndrome is characterized with intraocular and extraocular vascular manifestations, reduced ocular and retrobulbar blood flow and thin RNFL thickness[15]–[17]. Previous studies have shown that, eyes with PXG have significantly more severe glaucomatous optic nerve damage at the time of diagnosis, faster VF loss, more serious clinical course, worse response to anti-glaucomatous agents and more often necessity for surgical procedures than eyes with POAG at similar levels of IOP[15]–[19]. This ocular perfusion impairment in optic disc and macula measured by OCTA may show an contributing risk factor for glaucoma progression, making eyes with PXG more sensitive to a faster progression and supporting the serious prognosis of PXG glaucoma. Furthermore, it has been shown that the nasal part of the lamina cribrosa in PXG eyes could be more vulnerable to glaucomatous damage[33]. In addition, previous studies have demontstrated that the glaucomatous damage tends to be most commonly inferior locations[34]–[35]. These prior findings may be considered for a possible explanation of the regional decrease in inferonasal sector in eyes with PXG in our study.
According to our results, PXG and POAG eyes had was significantly higher FAZ area than OHT and normal control eyes. Previous studies have demonstrated the effect of glaucoma on FAZ parameters and the diagnostic power of these parameters to evaluate the glaucomatous changes[24]. These findings suggest that, FAZ size may be a potential considerable diagnostic parameter for discriminating glaucoma from healthy controls.
We have shown the positive correlation of peripapillary and macular VDs with inner retinal thickness, RNFL thickness, VF loss measured as MD and PSD mean sensitivity. Additionally, we have shown that peripapillary and macular VDs were highly correlated to the structural damage and VF damage in glaucoma eyes. Previous studies have reported a correlation between OCTA-derived measurements and VF damage in ONH in patients with glaucoma[14],[36]–[39]. Our results have shown better correlations of peripapillary and macular VD with structural parameters than with functional parameters. According to our results, the strength of the structural and functional assocation was stronger for mean macular superficial plexus VD than mean macular deep plexus VD. Previous studies have reported reduced VD in the superficial plexus in glaucomatous eyes compared with normal eyes[36],[40]–[42]. Macular damage has been shown in early stages of glaucoma and glaucoma is associated with reduced VD in the macula region[40],[42]. Chen et al[43] showed that macular superficial VD had similar diagnostic accuracy as peripapillary RNFL and inner macular retina thickness for distinguishing between glaucoma affected and normal eyes. Additionally, Takusagawa et al[40] also reported the early decrease of macular superficial VD using the Project Resolved OCTA. The blood supply to the RNFL and ganglion cell layer is superficial retinal vascular plexus, thereby this difference between the VD of the superficial and deep plexus could demonstrate a different involvement of each layer in the pathophysiology of glaucoma.
According to our results, OCTA VDs in inferior and superior quadrants showed a stronger correlation with structural and functional parameters. Hood et al[34] demonstrated that, superior and inferior quadrants are vulnerable to glaucomatous damage from OCT RNFL and HRT neural rim measurements, and analysis of fundus photographs in their study. They suggested that, the collection of RNFL bundles is thicker in the superior and inferior quadrants than the other quadrants of the optic disc. Additionally, previous studies have shown that, RNFL thinning in the superior and inferior quadrants of the disc is a more sensitive indicator of glaucomatous damage than are changes in the temporal or nasal quadrants[44]–[45].
The age-adjusted area under ROC value for differentiating between glaucoma and healthy eyes was found to be higher for inner macular retina thickness and circumpapillary RNFL thickness than macular and peripapillary VDs in our study. Previous studies have reported macular OCTA parameters' diagnostic accuracies more inferior compared to that of macular OCT parameters[46]–[49]. Similarly to our results, former studies demonstrated that the area under ROC of inner macular retina thickness was higher than the area under ROC of inner macula VD for distinguishing between glaucoma affected and healthy eyes[37],[50]–[51].
In this study, patients in the OHT group had lower wiVD in both superficial and deep macular plexus than those in the control group with the exception of foveal region. The OHT group showed decreased macular circulation than did the control group prior to significant VF, RNFL, and inner macular retinal defects. To the best of our knowledge, this is a novel and critical finding that requires further investigation. Vascular factors may play a role in the pathogenesis of glaucoma therefore, differences in vasculature between glaucoma, OHT and normal subjects may exist. Chihara et al[52] reported decreased VD in OHT eyes in the superficial peripapillary retina than those in normal eyes. They suggested that, decreases in the VD in OHT eyes might reflect dysregulation of the blood flow in the superficial optic disc. According to OHT treatment study, the high IOP and splinter hemorrhage of the optic disc were risk factors for development of POAG[53]–[54]. These findings and our study's results suggest that, the reduced VD before changes in the other structural and functional parameters in eyes with OHT might reflect disrupted autoregulation that was found in experimental nonhuman glaucoma eyes. Holló[28] reported that large IOP reduction may develop increased peripapillary capillary perfusion in both glaucoma and OHT eyes. According to this study, reduced OCTA parameters in eyes with OHT caused by a high IOP. All eyes with OHT included in our study, were under medical treatment and the mean IOP was 17.5±3.1 mm Hg on the same day with OCTA imaging.
There are several limitations to our study. First, relatively small sample size of groups with different amount of subjects. Second, the subjects of the OHT and control group were significantly younger than the glaucoma patients. For this reason, all analyses were adjusted for age and after age-adjustment, our results remained significant. In addition, most patients in our glaucoma group were on multiple antiglaucoma eye drops and systemic blood pressure medications. Due to the cross-sectional, observational design of our study, we can not eliminate the possibility that the antiglaucoma drops, antihypertensive medications and diabetes melltius could somehow be responsible for the reduced vascular measurements. Even though we excluded eyes with manifest diabetic retinopathy and hypertensive retinopathy, the effect of subclinical vascular change on retinal vascular measurements cannot be dismissed.
To the best of our knowledge, our study is the first study to compare peripapillary and macular VDs between patients with OHT and those with POAG, PXG and healthy controls using OCTA. This is the first report that OCTA-derived macular VD measures showed a decrease from normal eyes to OHT eyes and glaucomatous eyes in both superficial and deep plexus. We can conclude that, OCTA can measure reduced peripapillary and macular VD in eyes with glaucoma and OHT, and these results are correlated to functional and structural glaucomatous changes. Our findings would appear to support the early macular perfusion impairment for OHT, though further large-scale, longitudinal studies are required to better define the predictive and diagnostic value of these vascular parameters. Furthermore, our data suggest that peripapillary and macular superficial wiVD was lower in eyes with PXG than in eyes with POAG. However, it is necessary to investigate the roles of OCTA as a diagnostic parameter to predict the risk of glaucoma development in patients with exfoliation syndrome. Although the use of OCTA has good diagnostic accuracy for distinguishing healthy subjects from patients with OHT and types of glaucoma, there are more questions to be answered: Is the clinical use of OCTA the same in different subtypes of glaucoma? Do physiologic parameters, systemic and topical medications affect retinal perfusion? Is OCTA useful for prediction of glaucoma progression? Further studies are needed to confirm our results and try to find an answer to these questions.
Acknowledgments
Conflicts of Interest: Köse HC, None; Tekeli O, None.
REFERENCES
- 1.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sommer A, Tielsch JM, Katz J, Quigley HA, Gottsch JD, Javitt J, Singh K. Relationship between intraocular pressure and primary open angle glaucoma among white and black Americans. The Baltimore Eye Survey. Arch Ophthalmol. 1991;109(8):1090–1095. doi: 10.1001/archopht.1991.01080080050026. [DOI] [PubMed] [Google Scholar]
- 3.Flammer J, Orgül S. Optic nerve blood-flow abnormalities in glaucoma. Prog Retin Eye Res. 1998;17(2):267–289. doi: 10.1016/s1350-9462(97)00006-2. [DOI] [PubMed] [Google Scholar]
- 4.Flammer J, Orgül S, Costa VP, Orzalesi N, Krieglstein GK, Serra LM, Renard JP, Stefánsson E. The impact of ocular blood flow in glaucoma. Prog Retin Eye Res. 2002;21(4):359–393. doi: 10.1016/s1350-9462(02)00008-3. [DOI] [PubMed] [Google Scholar]
- 5.Rechtman E, Harris A, Kumar R, Cantor LB, Ventrapragada S, Desai M, Friedman S, Kagemann L, Garzozi HJ. An update on retinal circulation assessment technologies. Curr Eye Res. 2003;27(6):329–343. doi: 10.1076/ceyr.27.6.329.18193. [DOI] [PubMed] [Google Scholar]
- 6.Burgansky-Eliash Z, Bartov E, Barak A, Grinvald A, Gaton D. Blood-flow velocity in glaucoma patients measured with the retinal function imager. Curr Eye Res. 2016;41(7):965–970. doi: 10.3109/02713683.2015.1080278. [DOI] [PubMed] [Google Scholar]
- 7.Spaide RF, Fujimoto JG, Waheed NK, Sadda SR, Staurenghi G. Optical coherence tomography angiography. Prog Retin Eye Res. 2018;64:1–55. doi: 10.1016/j.preteyeres.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jia YL, Morrison JC, Tokayer J, Tan O, Lombardi L, Baumann B, Lu CD, Choi W, Fujimoto JG, Huang D. Quantitative OCT angiography of optic nerve head blood flow. Biomed Opt Express. 2012;3(12):3127–3137. doi: 10.1364/BOE.3.003127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jia YL, Tan O, Tokayer J, Potsaid B, Wang YM, Liu JJ, Kraus MF, Subhash H, Fujimoto JG, Hornegger J, Huang D. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt Express. 2012;20(4):4710. doi: 10.1364/OE.20.004710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lei JQ, Durbin MK, Shi Y, Uji A, Balasubramanian S, Baghdasaryan E, Al-Sheikh M, Sadda SR. Repeatability and reproducibility of superficial macular retinal vessel density measurements using optical coherence tomography angiography en face images. JAMA Ophthalmol. 2017;135(10):1092–1098. doi: 10.1001/jamaophthalmol.2017.3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao Q, Yang WL, Wang XN, Wang RK, You QS, Chu ZD, Xin C, Zhang MY, Li DJ, Wang ZY, Chen W, Li YF, Cui R, Shen L, Wei WB. Repeatability and reproducibility of quantitative assessment of the retinal microvasculature using optical coherence tomography angiography based on optical microangiography. Biomed Environ Sci. 2018;31(6):407–412. doi: 10.3967/bes2018.054. [DOI] [PubMed] [Google Scholar]
- 12.Liu L, Jia YL, Takusagawa HL, Pechauer AD, Edmunds B, Lombardi L, Davis E, Morrison JC, Huang D. Optical coherence tomography angiography of the peripapillary retina in glaucoma. JAMA Ophthalmol. 2015;133(9):1045–1052. doi: 10.1001/jamaophthalmol.2015.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee EJ, Lee KM, Lee SH, Kim TW. OCT angiography of the peripapillary retina in primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2016;57(14):6265–6270. doi: 10.1167/iovs.16-20287. [DOI] [PubMed] [Google Scholar]
- 14.Yarmohammadi A, Zangwill LM, Diniz-Filho A, Suh MH, Manalastas PI, Fatehee N, Yousefi S, Belghith A, Saunders LJ, Medeiros FA, Huang D, Weinreb RN. Optical coherence tomography angiography vessel density in healthy, glaucoma suspect, and glaucoma eyes. Invest Ophthalmol Vis Sci. 2016;57(9):OCT451–OCT459. doi: 10.1167/iovs.15-18944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ritch R, Schlötzer-Schrehardt U. Exfoliation syndrome. Surv Ophthalmol. 2001;45(4):265–315. doi: 10.1016/s0039-6257(00)00196-x. [DOI] [PubMed] [Google Scholar]
- 16.Grødum K, Heijl A, Bengtsson B. Risk of glaucoma in ocular hypertension with and without pseudoexfoliation. Ophthalmology. 2005;112(3):386–390. doi: 10.1016/j.ophtha.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 17.De Moraes CG, Liebmann JM, Liebmann CA, Susanna R, Jr, Tello C, Ritch R. Visual field progression outcomes in glaucoma subtypes. Acta Ophthalmol. 2013;91(3):288–293. doi: 10.1111/j.1755-3768.2011.02260.x. [DOI] [PubMed] [Google Scholar]
- 18.Leske MC, Heijl A, Hyman L, Bengtsson B, Dong LM, Yang ZM, EMGT Group Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology. 2007;114(11):1965–1972. doi: 10.1016/j.ophtha.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 19.Koz OG, Turkcu MF, Yarangumeli A, Koz C, Kural G. Normotensive glaucoma and risk factors in normotensive eyes with pseudoexfoliation syndrome. J Glaucoma. 2009;18(9):684–688. doi: 10.1097/IJG.0b013e31819c4311. [DOI] [PubMed] [Google Scholar]
- 20.Aboobakar IF, Johnson WM, Stamer WD, Hauser MA, Allingham RR. Major review: Exfoliation syndrome; advances in disease genetics, molecular biology, and epidemiology. Exp Eye Res. 2017;154:88–103. doi: 10.1016/j.exer.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 21.Helbig H, Schlötzer-Schrehardt U, Noske W, Kellner U, Foerster MH, Naumann GO. Anterior-chamber hypoxia and iris vasculopathy in pseudoexfoliation syndrome. Ger J Ophthalmol. 1994;3(3):148–153. [PubMed] [Google Scholar]
- 22.Suwan Y, Geyman LS, Fard MA, Tantraworasin A, Chui TY, Rosen RB, Ritch R. Peripapillary perfused capillary density in exfoliation syndrome and exfoliation glaucoma versus POAG and healthy controls: an OCTA study. Asia Pac J Ophthalmol (Phila) 2018;7(2):84–89. doi: 10.22608/APO.2017318. [DOI] [PubMed] [Google Scholar]
- 23.Rebolleda G, Pérez-Sarriegui A, De Juan V, Ortiz-Toquero S, Muñoz-Negrete FJ. A comparison of two optical coherence tomography-angiography devices in pseudoexfoliation glaucoma versus primary open-angle glaucoma and healthy subjects. Eur J Ophthalmol. 2019;29(6):636–644. doi: 10.1177/1120672118805882. [DOI] [PubMed] [Google Scholar]
- 24.Philip S, Najafi A, Tantraworasin A, Chui TYP, Rosen RB, Ritch R. Macula vessel density and foveal avascular zone parameters in exfoliation glaucoma compared to primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2019;60(4):1244–1253. doi: 10.1167/iovs.18-25986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park JH, Yoo C, Girard MJA, Mari JM, Kim YY. Peripapillary vessel density in glaucomatous eyes: comparison between pseudoexfoliation glaucoma and primary open-angle glaucoma. J Glaucoma. 2018;27(11):1009–1016. doi: 10.1097/IJG.0000000000001062. [DOI] [PubMed] [Google Scholar]
- 26.Quigley HA, Enger C, Katz J, Sommer A, Scott R, Gilbert D. Risk factors for the development of glaucomatous visual field loss in ocular hypertension. Arch Ophthalmol. 1994;112(5):644–649. doi: 10.1001/archopht.1994.01090170088028. [DOI] [PubMed] [Google Scholar]
- 27.Holló G. Vessel density calculated from OCT angiography in 3 peripapillary sectors in normal, ocular hypertensive, and glaucoma eyes. Eur J Ophthalmol. 2016;26(3):e42–e45. doi: 10.5301/ejo.5000717. [DOI] [PubMed] [Google Scholar]
- 28.Holló G. Influence of large intraocular pressure reduction on peripapillary OCT vessel density in ocular hypertensive and glaucoma eyes. J Glaucoma. 2017;26(1):e7–e10. doi: 10.1097/IJG.0000000000000527. [DOI] [PubMed] [Google Scholar]
- 29.Chao SC, Yang SJ, Chen HC, Sun CC, Liu CH, Lee CY. Early macular angiography among patients with glaucoma, ocular hypertension, and normal subjects. J Ophthalmol. 2019;2019:7419470. doi: 10.1155/2019/7419470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lommatzsch C, Rothaus K, Koch JM, Heinz C, Grisanti S. OCTA vessel density changes in the macular zone in glaucomatous eyes. Graefes Arch Clin Exp Ophthalmol. 2018;256(8):1499–1508. doi: 10.1007/s00417-018-3965-1. [DOI] [PubMed] [Google Scholar]
- 31.Shoji T, Zangwill LM, Akagi T, Saunders LJ, Yarmohammadi A, Manalastas PIC, Penteado RC, Weinreb RN. Progressive macula vessel density loss in primary open-angle glaucoma: a longitudinal study. Am J Ophthalmol. 2017;182:107–117. doi: 10.1016/j.ajo.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mansoori T, Sivaswamy J, Gamalapati JS, Agraharam SG, Balakrishna N. Measurement of radial peripapillary capillary density in the normal human retina using optical coherence tomography angiography. J Glaucoma. 2017;26(3):241–246. doi: 10.1097/IJG.0000000000000594. [DOI] [PubMed] [Google Scholar]
- 33.Yüksel N, Karabaş VL, Arslan A, Demirci A, Cağlar Y. Ocular hemodynamics in pseudoexfoliation syndrome and pseudoexfoliation glaucoma. Ophthalmology. 2001;108(6):1043–1049. doi: 10.1016/s0161-6420(01)00572-3. [DOI] [PubMed] [Google Scholar]
- 34.Hood DC, Raza AS, de Moraes CG, Liebmann JM, Ritch R. Glaucomatous damage of the macula. Prog Retin Eye Res. 2013;32:1–21. doi: 10.1016/j.preteyeres.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi JA, Park HY, Shin HY, Park CK. Optic disc characteristics in patients with glaucoma and combined superior and inferior retinal nerve fiber layer defects. JAMA Ophthalmol. 2014;132(9):1068–1075. doi: 10.1001/jamaophthalmol.2014.1056. [DOI] [PubMed] [Google Scholar]
- 36.Rao HL, Pradhan ZS, Weinreb RN, Riyazuddin M, Dasari S, Venugopal JP, Puttaiah NK, Rao DAS, Devi S, Mansouri K, Webers CAB. Vessel density and structural measurements of optical coherence tomography in primary angle closure and primary angle closure glaucoma. Am J Ophthalmol. 2017;177:106–115. doi: 10.1016/j.ajo.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 37.Rao HL, Pradhan ZS, Weinreb RN, Dasari S, Riyazuddin M, Raveendran S, Puttaiah NK, Venugopal JP, Rao DAS, Devi S, Mansouri K, Webers CAB. Relationship of optic nerve structure and function to peripapillary vessel density measurements of optical coherence tomography angiography in glaucoma. J Glaucoma. 2017;26(6):548–554. doi: 10.1097/IJG.0000000000000670. [DOI] [PubMed] [Google Scholar]
- 38.Sakaguchi K, Higashide T, Udagawa S, Ohkubo S, Sugiyama K. Comparison of sectoral structure-function relationships in glaucoma: vessel density versus thickness in the peripapillary retinal nerve fiber layer. Invest Ophthalmol Vis Sci. 2017;58(12):5251–5262. doi: 10.1167/iovs.17-21955. [DOI] [PubMed] [Google Scholar]
- 39.Yarmohammadi A, Zangwill LM, Diniz-Filho A, Suh MH, Yousefi S, Saunders LJ, Belghith A, Manalastas PI, Medeiros FA, Weinreb RN. Relationship between optical coherence tomography angiography vessel density and severity of visual field loss in glaucoma. Ophthalmology. 2016;123(12):2498–2508. doi: 10.1016/j.ophtha.2016.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takusagawa HL, Liu L, Ma KN, Jia YL, Gao SS, Zhang M, Edmunds B, Parikh M, Tehrani S, Morrison JC, Huang D. Projection-resolved optical coherence tomography angiography of macular retinal circulation in glaucoma. Ophthalmology. 2017;124(11):1589–1599. doi: 10.1016/j.ophtha.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu H, Kong XM. Study of retinal microvascular perfusion alteration and structural damage at macular region in primary open-angle glaucoma patients. Zhonghua Yan Ke Za Zhi. 2017;53(2):98–103. doi: 10.3760/cma.j.issn.0412-4081.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 42.Kurysheva NI, Maslova EV, Trubilina AV, Ardzhevnishvili TD, Fomin AV. Macular blood flow in glaucoma. Vestn Oftalmol. 2017;133(2):29–38. doi: 10.17116/oftalma2017133229-37. [DOI] [PubMed] [Google Scholar]
- 43.Chen HS, Liu CH, Wu WC, Tseng HJ, Lee YS. Optical coherence tomography angiography of the superficial microvasculature in the macular and peripapillary areas in glaucomatous and healthy eyes. Invest Ophthalmol Vis Sci. 2017;58(9):3637–3645. doi: 10.1167/iovs.17-21846. [DOI] [PubMed] [Google Scholar]
- 44.Leite MT, Zangwill LM, Weinreb RN, Rao HL, Alencar LM, Medeiros FA. Structure-function relationships using the Cirrus spectral domain optical coherence tomograph and standard automated perimetry. J Glaucoma. 2012;21(1):49–54. doi: 10.1097/IJG.0b013e31822af27a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rao HL, Babu JG, Addepalli UK, Senthil S, Garudadri CS. Retinal nerve fiber layer and macular inner retina measurements by spectral domain optical coherence tomograph in Indian eyes with early glaucoma. Eye (Lond) 2012;26(1):133–139. doi: 10.1038/eye.2011.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Richter GM, Madi I, Chu ZD, Burkemper B, Chang R, Zaman A, Sylvester B, Reznik A, Kashani A, Wang RK, Varma R. Structural and functional associations of macular microcirculation in the ganglion cell-inner plexiform layer in glaucoma using optical coherence tomography angiography. J Glaucoma. 2018;27(3):281–290. doi: 10.1097/IJG.0000000000000888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rao HL, Pradhan ZS, Weinreb RN, Reddy HB, Riyazuddin M, Dasari S, Palakurthy M, Puttaiah NK, Rao DA, Webers CA. Regional comparisons of optical coherence tomography angiography vessel density in primary open-angle glaucoma. Am J Ophthalmol. 2016;171:75–83. doi: 10.1016/j.ajo.2016.08.030. [DOI] [PubMed] [Google Scholar]
- 48.Triolo G, Rabiolo A, Shemonski ND, Fard A, Di Matteo F, Sacconi R, Bettin P, Magazzeni S, Querques G, Vazquez LE, Barboni P, Bandello F. Optical coherence tomography angiography macular and peripapillary vessel perfusion density in healthy subjects, glaucoma suspects, and glaucoma patients. Invest Ophthalmol Vis Sci. 2017;58(13):5713–5722. doi: 10.1167/iovs.17-22865. [DOI] [PubMed] [Google Scholar]
- 49.Kim JS, Kim YK, Baek SU, Ha A, Kim YW, Jeoung JW, Park KH. Topographic correlation between macular superficial microvessel density and ganglion cell-inner plexiform layer thickness in glaucoma-suspect and early normal-tension glaucoma. Br J Ophthalmol. 2020;104(1):104–109. doi: 10.1136/bjophthalmol-2018-313732. [DOI] [PubMed] [Google Scholar]
- 50.Penteado RC, Zangwill LM, Daga FB, Saunders LJ, Manalastas PIC, Shoji T, Akagi T, Christopher M, Yarmohammadi A, Moghimi S, Weinreb RN. Optical coherence tomography angiography macular vascular density measurements and the central 10-2 visual field in glaucoma. J Glaucoma. 2018;27(6):481–489. doi: 10.1097/IJG.0000000000000964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wan KH, Lam AKN, Leung CK. Optical coherence tomography angiography compared with optical coherence tomography macular measurements for detection of glaucoma. JAMA Ophthalmol. 2018;136(8):866–874. doi: 10.1001/jamaophthalmol.2018.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chihara E, Dimitrova G, Amano H, Chihara T. Discriminatory power of superficial vessel density and prelaminar vascular flow index in eyes with glaucoma and ocular hypertension and normal eyes. Invest Ophthalmol Vis Sci. 2017;58(1):690–697. doi: 10.1167/iovs.16-20709. [DOI] [PubMed] [Google Scholar]
- 53.Ocular Hypertension Treatment Study Group, European Glaucoma Prevention Study Group. Gordon MO, Torri V, Miglior S, Beiser JA, Floriani I, Miller JP, Gao F, Adamsons I, Poli D, D'Agostino RB, Kass MA. Validated prediction model for the development of primary open-angle glaucoma in individuals with ocular hypertension. Ophthalmology. 2007;114(1):10–19. doi: 10.1016/j.ophtha.2006.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Budenz DL, Anderson DR, Feuer WJ, Beiser JA, Schiffman J, Parrish RK, 2nd, Piltz-Seymour JR, Gordon MO, Kass MA, Ocular Hypertension Treatment Study Group Detection and prognostic significance of optic disc hemorrhages during the Ocular Hypertension Treatment Study. Ophthalmology. 2006;113(12):2137–2143. doi: 10.1016/j.ophtha.2006.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]