Abstract
We studied 156 individuals with acute, right hemisphere ischemic stroke on a battery of hemispatial neglect tests to distinguish between viewer-centered and stimulus-centered neglect and MRI diffusion weighted imaging. We identified the relative contributions of age, total lesion volume, and damage to subcortical and cortical grey matter regions as well as white matter tracts to both the severity and presence of significant viewer-centered and stimulus-centered neglect, using multivariable regression tests. We found that age, volume of lesion, and percent damage to the regions of interest were each independently associated with the severity of viewer-centered neglect (r2 = .31; p < .001). However, only age (t = 3.20; p = .002) and percent damage to the angular gyrus (t = 2.63, p = .010), a dorsal stream area, predicted severity of viewer-centered neglect independently of the other variables. The same variables predicted the presence of significant viewer-centered neglect. In contrast, these variables did not significantly predict the severity of stimulus-centered neglect. However, we found that percent damage to ventral stream regions of interest (middle temporal gyrus, inferior temporal gyrus, fusiform gyrus, inferior frontal occipital gyrus, sagittal stratum, along with total infarct volume were associated with the presence of significant stimulus-centered neglect (pseudo r2 = .70, p < .0004). Only percent damage to right inferior temporal gyrus predicted stimulus-centered neglect independently of the other variables (p = .018).
Keywords: Hemispatial neglect, Stroke, Inattention, Reference frames, Brain mapping
Hemispatial neglect is a behavioral syndrome, characterized by errors of detection or responsiveness to the contralesional side, most commonly noted after right hemisphere stroke (but see Kleinman, et al., 2007). Mesulam (1981) proposed a neural network of spatial attention, including a parietal spatial map, a frontal area critical for directing exploration and actions to the contralateral side, limbic areas for assigning motivational relevance, and a reticular system involved in vigilance, to account for different types of hemispatial neglect. The assumption was that lesions to different parts of the network would cause distinct spatially-specific deficits. Since then, many investigators have identified distinct types of hemispatial neglect, often associated with different areas of brain damage. Distinct types of neglect have been defined by the sensory modality, the spatial domains, or the space in which it occurs (personal vs extrapersonal) (Rode, Pagliari, Huchon, Rossetti, & Pisella, 2017). The most common sensory modality, visual neglect, is defined as the inability to detect visual stimuli on the contralesional side, while auditory neglect is defined as failure to detect sound or spoken stimuli on the contralesional side of space (Spaccavento, Cellamare, Falcone, Loverre, & Nardulli, 2017). Tactile neglect is defined as the errors in detecting somatosensory stimuli on the contralesional side of the body in the absence of hemianesthesia (Beschin, Cazzani, Cubelli, Della Sala, & Spinazzola, 1996). Types of neglect can be further distinguished by their domains. Spatial neglect refers to failure to detect visual, tactile, or auditory stimuli on the contralesional side of space, whereas exploratory neglect refers to the lack of spontaneous movement towards the contralesional space (Rode et al., 2017). Personal neglect is described as a failure to spontaneously attend to the contralesional side of the body, whereas extrapersonal neglect is a deficit in responding to stimuli in the space beyond the body (Beschin & Robertson, 1997; Bisiach, Perani, Vallar, & Berti, 1986; Caggiano, Beschin, & Cocchini, 2014).
Another distinction is based on the reference frame; that is, neglect on the contralesional side with respect to what? (Hillis & Caramazza, 1995; Ota, Fujii, Suzuki, Fukatsu, & Yamadori, 2001). In viewer-centered neglect (VCN, also called egocentric neglect), the person neglects the contralesional (usually left) side of space with respect to the viewer. In stimulus-centered neglect (SCN, also called allocentric neglect), the person neglects the contralesional side of each stimulus, irrespective of the location with respect to the viewer. Several studies have identified distinct lesion sites associated with these two different types of neglect, using a variety of brain mapping approaches. One study included 171 individuals within 48 h of right supratentorial ischemic stroke using MR perfusion weighted imaging (PWI) along with diffusion weighted imaging (DWI) to identify the entire area of dysfunctional tissue in acute stroke and tests designed to disambiguate VCN and SCN. Results indicated that dysfunction in right supramarginal gyrus was associated with VCN and dysfunction of more ventral areas including the posterior inferior temporal gyrus was associated with SCN (Medina et al., 2009). Another study using both DWI and PWI in acute stroke included 50 patients with pure right subcortical infarcts, and found that left VCN neglect was most strongly associated with significant hypoperfusion of right angular gyrus (Fisher’s exact: p < .0001), while left SCN was most strongly associated with hypoperfusion of more ventral right STG (p < .0001) (Hillis et al., 2005). Those with subcortical stroke without cortical hypoperfusion showed neither type of neglect. Similarly, a study of more chronic stroke patients found distinct areas of lesion overlap in patients with these two types of neglect: dorsal (frontoparietal) lesions associated with VCN and ventral (temporal) lesions associated with SCN (Chen, Caulfield, Hartman, O’Rourke, & Toglia, 2017). Another study of chronic stroke used voxel-based lesion-symptom mapping (VLSM) to identify areas associated with errors on six subtests of the Behavioral Inattention Test (BIT) classified as stimulus-centered tests (line bisection, copying, and drawing tasks) and viewer-centered tests (star, letter, and line cancellation) by 62 right hemisphere stroke. They found errors on viewer-centered (“egocentric”) tests were associated with frontal damage involving precentral gyrus, middle frontal gyrus, insula, and putamen. Errors on the stimulus-centered (“allocentric”) tests were associated damage to superior and middle temporal gyri and superior and inferior parietal cortices (Kenzie et al., 2015).
Several studies have examined white matter tracts where disruption leads to one or more types of neglect. One study involved 45 participants with right hemisphere stroke who underwent neglect testing <3 months after onset and again more than a year post-stroke. The 27 patients who had signs of neglect in the subacute phase had decreased fractional anisotropy in the second and third branches of the right superior longitudinal fasciculus (SLF) and the splenium of the corpus callosum, relative to participants without neglect. Severity of neglect correlated with fractional anisotropy (FA) in SLF branches II and III in the subacute stage of stroke and only the caudal portion of SLF and splenium in the chronic stage. Results were interpreted as evidence of a fronto-parietal disconnection underlying the occurrence and persistence of neglect, and caudal interhemispheric disconnection underlying chronic neglect (Lunven et al., 2015). One large study of 140 stroke patients combined VLSM with a probabilistic cytoarchitectonic atlas. This study also revealed that disruption of SLF, the inferior occipitofrontal fasciculus, and the superior occipitofrontal fasciculus are common in participants with hemispatial neglect. Nevertheless, the largest portion (89.1% – 96.6%) of the damage affected other brain regions, mostly cortical regions like STG, inferior parietal, inferior frontal, and insula, along with caudate and putamen. The authors argued that damage to gray matter structures is a stronger predictor of neglect (Karnath, Rorden, & Ticini, 2009). VCN and SCN were not evaluated separately in these studies. However, another investigation used neuropsychological tests and diffusion tensor imaging (DTI) to identify disruption specific white matter pathways associated with independent components of neglect that reflect perceptual, exploratory, and object-centered deficits. They examined white matter integrity (FA) associated with severity of each type of deficit. They found that FA in posterior regions of the superior longitudinal fasciculus (SLF), and posterior corpus callosum were associated with severity of perceptual spatial deficits, while FA in anterior parts of SLF and inferior fronto-occipital fasciculus (IFOF) were associated with severity of object-centered deficits. (Vaessen, Saj, Lovblad, Gschwind, & Vuilleumier, 2016).
The majority of these studies indicate that more dorsal cortical regions (frontal, parietal, superior temporal) and white matter tracts that connect them (e.g., SLF) may be important for encoding visual stimuli with respect to the viewer, such that lesions in this network cause VSN. In contrast, more ventral cortical regions and deeper areas are important for encoding stimuli irrespective of their location, such that lesions cause SCN. This concept is consistent with a dorsal visual stream (the “where” or “how to” system), important for planning exploratory movements and reaching and a more ventral visual stream (the “what” system) important for recognizing objects. While previous studies have identified cortical regions, subcortical structures, or white matter tracts associated with these different types of neglect, we are not aware of studies that have sought to identify the relative contributions of cortical regions, subcortical structures, white matter tracts, age, and overall lesion volume to the presence and severity of each neglect type. Some studies have shown that premorbid atrophy and lesion volume (Levine, Warach, Benowitz, & Calvanio, 1986), severity of leukoaraiaosis (Bahrainwala, Hillis, Dearborn, & Gottesman, 2014), and age (Gottesman et al., 2008) modify neglect. Therefore, we aimed to identify the relative contributions of cortical regions, subcortical structures, white matter tracts, age, and overall lesion volume to the presence and severity of each neglect type, using multivariable regression. We hypothesized that lesions in more dorsal cortical regions and white matter tracts are associated with both severity of VCN and the presence of significant VCN, while lesions in more ventral cortical regions and white matter tracts are associated with both severity of SCN and the presence of significant SCN. We also hypothesized that age and lesion volume would be associated with severity and presence of both types of neglect, independently of the site of lesion. Finally, we tested the hypothesis that lesions in the basal ganglia or thalamus might be associated with one or more types of neglect, independently of the other variables, as previous studies have documented neglect due to lesions of these regions (e.g., Karnath, Himmelbach, & Rorden, 2002; Sebastian et al., 2014).
1. Methods
We report how we determined our sample size, all data exclusions, all inclusion/exclusion criteria, whether inclusion/exclusion criteria were established prior to data analysis, all manipulations, and all measures in the study. No part of the study procedures or analysis was pre-registered prior to the research being conducted.
1.1. Participants
We enrolled and tested individuals within 48 h of acute ischemic right hemisphere stroke (confirmed by MRI) who provided informed consent for the study. Exclusion criteria included: history of previous neurologic disease affecting the brain, impaired level of consciousness or ongoing sedation, uncorrected visual or hearing impairment, or psychiatric disease other than affective disorder. Of the 156 participants, 76 (48.7%) were female, 140 (89.37%) were right handed (and the 17 left-handed or ambidextrous participants were non-aphasic after right middle cerebral artery stroke), and the mean age was 60.2 (±SD 14.1) years. None of the individuals received thrombolysis, because at the time of the study, patients who were candidates for thrombolysis had acute CT rather than acute MRI. The sample size was not pre-determined; we included all consecutive patients who met the inclusion/exclusion criteria (a convenience sample).
1.2. Assessment of neglect
Participants were administered a battery of neglect tests included the following:
Oral reading: Reading aloud 30 words that were displayed over 2 columns, along with 5 sentences written across the page. All of these words can be made into another word by changing or omitting the first or last letters (e.g., darn could be read as “barn” or “dark” by erring on the first or last letter; rant could be read as “ant” or “ran” by omitting the first or last letter).
Scene copy: Copying of the “Ogden scene,” consisting of a tree, a fence, a house and a second tree presented from left to right (Ogden, 1985). The picture had 36 total components (pen strokes) in total, with 16 components on the left side and 20 on the right side of the page. Within the house and the 2 trees, 14 components were on the left and 14 components on the right side of the stimuli. Each omitted component was scored as an error, and misplaced or distorted components were scored as half an error.
Line cancellation (Albert, 1973): Crossing out all of the 28 vertical lines presented at the midsagittal plane and at 45° both right and left of the viewer’s midsagittal plane.
Gap detection (Ota et al., 2001): Circling all complete circles, and crossing out (with an X) all of the circles with gaps. There were 30 circles total, with 15 circles on each side of the page. Ten circles had a gap on the left side, and 10 had a gap on the right side. The remaining stimuli were full circles. Two versions of this task were administered: one with large circles and one with small circles. Errors included stimuli either omitted or marked incorrectly.
This battery may be obtained from our laboratory website: http://www.score-lab.net/test-materials-for-public-use.html.
In addition, all patients were tested for visual field deficits using confrontation testing at bedside (see Table 1 for numbers in each group with visual field cuts). We did not exclude patients with homonymous hemianopia as severe neglect can masquerade as homonymous hemianopia, and many patients with pure hemianopia perform normally on our neglect battery (as shown in Table 1). Patients were not tested for personal neglect.
Table 1 –
Demographics of participants with VCN, SCN, both, and neither.
| Group | Mean Age in Years (SD) | Mean Education in Years (SD | Sex # (%) Female | Handedness # (%) Left Handed or Ambidextrous | Visual Field Cut # (%) with HHa # (%) with Quada |
|---|---|---|---|---|---|
| VCN only | 60.2 (14.1) | 12.7 (14.1) | 16 (19.3) | 1 (20) | 9 (29.0) HH 2 (6.5) Quad |
| SCN only | 60.0 (15.0) | 12.3 (2.5) | 1 (16.7) | 1 (16.7) | 2 (33.3) HH 0 Quad |
| VCN + SCN | 64.4 (14.0) | 14.0 (4.1) | 4 (80) | 3 (9.6) | 3 (75) HH 0 Quad |
| No Neglect | 58.6 (13.3) | 12.8 (3.1) | 58 (48.3) | 12 (10) | 10 (8.3) HH 5 (4.2) Quad |
Quad = Quadrantanopia.
HH = Homonymous hemianopia or dense neglect masquerading as homonymous hemianopia.
We dichotomously defined VCN and SCN. We defined VCN as significantly more errors (by Fisher’s Exact test; p < .05) on the contralesional (left) side than the ipsilesional (right) side of the page on the gap detection tests (failure to mark the stimuli at all), significantly more errors (by Fisher’s Exact test, p < .05) on the left than the right side of the page in the copy scene task, significantly more errors (by Fisher’s Exact test, p < .05) on the left position than the right position with respect to the viewer on the line cancellation task, and/or or significantly more errors (by Fisher’s Exact test, p < .05) in reading words on the left than the right column in oral reading.
We defined SCN as significantly more errors (by Fisher’s Exact test; p < .05) on the contralesional (left) side than the ipsilesional (right) side of stimuli in the gap detection test (failure to detect gaps in circles with gaps on left or right) or the copy scene task (omitting components on the contralesional side of the house and trees), or oral reading (errors on the left vs the right sides of words).
To determine VCN severity, we calculated the average number of errors (failure to mark the stimuli) on the contralesional side of the page in the gap detection test. We determined SCN severity by calculating the average number of failures to detect the contralesional (left) gap on circles, irrespective of the side of the page, in gap detection. We used only this test because it was the test most frequently completed by participants, and we wanted to define severity the same way for all participants.
Not all participants were administered every test. However, we only evaluated severity in those who completed the gap detection test (n = 155/156).
1.3. Image processing
The stroke area was defined on diffusion weighted images (DWI) b1000 and trace images, which are highly sensitive to acute infarct. DWIs were acquired using single-shot spin-echo echo-planar sequence in the axial plane covering the entire brain with a b-value of 1000 sec/mm2 and with a least diffusion-weighted (b0) image. A neurologist (K.O.), who was blinded to clinical information, delineated boundaries to create maps of acute lesions (stroke lesion map) in semi-automated way as described in previous studies (Agis et al., 2016; Davis et al., 2016; Leigh et al., 2013). The b0 image was transformed to the JHU-MNI-b0 atlas (www.MRIStudio.org), using linear and diffeomorphic image transformation (Oishi et al., 2009) to normalize the stroke lesion map. Regions-of-interest (ROIs) of 23 anatomical structures, determined by the JHU-MNI Brain Parcellation Map (cmrm.med.jhmi.edu) were overlaid on the normalized stroke lesion map to investigate the percent volume of each of the selected anatomical structures that were affected by acute stroke. The percent damage to each of the following ROIs was identified: right inferior frontal gyrus pars opercularis (IFG-op), pars orbitalis (IFG-orb), and pars triangularis (IFG-tri); supramarginal gyrus (SMG), angular gyrus (AG), superior temporal gyrus (STG), fusiform gyrus (FG), middle temporal gyrus (MTG), inferior temporal gyrus (ITG), caudate, putamen, globus pallidus, thalamus, anterior limb of internal capsule (alic), posterior limb of internal capsule (plic), retro-lenticular part of internal capsule (rlic), inferior frontal occipital tract (ifo), superior frontal occipital tract (sfo), sagittal stratum (ss), superior longitudinal fasciculus (slf), ansa lenticularis (al), posterior thalamic radiation (ptr), and uncinate fasciculus (unc) (Fig. 1). In secondary analyses, we evaluated the independent contributions of ventral stream ROIs (MTG, ITG, FUG, ifo, and ss) and dorsal stream ROIs (IFG-op, IFG-orb, IFG-tri, SMG, AG, sfo, and slf). These ROIs were included based on previous studies that have implicated damage to these regions in hemispatial neglect. We included ptr (also known as optic radiation) because surgical resection of the ptr combined with forebrain commissurotomy caused hemispatial neglect in six Macaque monkeys who were trained preoperatively in a visual search task (Gaffan & Hornak, 1997). Cerebral edema soon after stroke compromises the function of cerebral tissue in the perilesional areas and its corresponding cognitive functions. However, we did not include patients with observable cerebral edema, as those with observable cerebral edema in the first 48 h typically have compromised level of consciousness, which was an exclusion criteria.
Fig. 1 –
Regions of Interest Included in the Analyses. IFG-op = inferior frontal gyrus pars opercularis; IFG-orb = inferior frontal gyrus pars orbitalis; IFG-tri = inferior frontal gyrus pars triangularis; SMG = supramarginal gyrus; AG = angular gyrus; STG = superior temporal gyrus; FG = fusiform gyrus; MTG = middle temporal gyrus; ITG = inferior temporal gyrus, ALIC = anterior limb of internal capsule; PLIC = posterior limb of internal capsule; RLIC = retro-lenticular part of internal capsule; ifo = inferior frontal occipital tract; sfo = superior frontal occipital tract; ss = sagittal stratum; slf = superior longitudinal fasciculus; al = ansa lenticularis; unc = uncinate fasciculus; ptr = posterior thalamic radiation.
1.4. Statistical analyses
All statistical analyses were carried out in STATA (Version 14). We compared means between groups of participants with different forms of neglect on continuous variables using two-tailed, two sample t-tests, and compared frequency of dichotomous variables across groups using Pearson chi squared tests. We carried out multivariable linear regressions to evaluate contributions of linear variables (age, percent damage to each region of interest, infarct volume) to severity of each type of neglect. We carried out multivariable logistic regressions to evaluate contributions of linear variables (age, total infarct volume) and dichotomous variables (e.g., damage to >1% of each region of interest) to the presence of each type of neglect. Regions of interest (ROIs) included cortical regions, subcortical structures, and white matter tracts that have been associated with each type of left neglect (see Image Processing and Fig. 1) in previous studies.
The conditions of our ethics approval do not permit public archiving of any anonymised individual data associated with this study. Readers seeking access to the data should contact the corresponding author, Argye E. Hillis (argye@jhmi.edu). Access will be granted to named individuals in accordance with ethical procedures governing the reuse of sensitive data. Specifically, requestors must meet the following conditions to obtain the data: completion of a formal data sharing agreement.
2. Results
Among the 156 participants with right hemisphere stroke, 36 had statistically significant left VCN on at least one test; 11 had statistically significant left SCN on at least one test, and 9; 5 had both. The mean age of participants with VCN was significantly higher than the mean age of those without VCN (65.5± SD 15.3 years vs 58.7± 13.3; t = 2.64; df 157; p = .0091). There was no significant difference in age for those with and without SCN (mean 60.1 ± 14.1 vs 62.4 ± 14.0). There was no difference in sex distribution for VCN (20 women, 16 men) or SCN (5 women, 6 men). Demographics for the four groups (VCN only, SCN only, both, neither) are shown in Table 1. Their mean scores on the various neglect tests are shown in Table 2. Lesion overlap maps for each group are shown in Fig. 2. Although most of our participants were right-handed (by self report), we did include 17 non-aphasic left handed participants. There was no significant difference by t-tests in severity of neglect between left- and right-handed participants (Table 3).
Table 2 –
Scores on neglect tests by each group.
| Test | VCN |
SCN |
Both |
Neither |
||||
|---|---|---|---|---|---|---|---|---|
| mean | SD | mean | SD | mean | SD | mean | SD | |
| Gap Detection: % of stimuli omitted on left side of page | 21.8 | 20.0 | 16.7 | 20.5 | 26.7 | 25.7 | 1.1 | 3.0 |
| Gap Detection: % of left gaps undetected | 7.6 | 10.7 | 13.0 | 27.7 | 7.5 | 8.7 | 1.1 | 3.4 |
| Copy: % of marks omitted on left side of paper | 37.0 | 31.6 | 9.5 | 14.9 | 17.1 | 24.4 | 3.2 | 6.9 |
| Copy: % of marks omitted on left side of each figure | 7.7 | 14.1 | 24.6 | 28.0 | 22.6 | 39.2 | 2.5 | 6.9 |
| Line cancellation: % of lines omitted | 13.3 | 12.8 | 19.0 | 15.5 | 25 | 12.5 | .7 | 1.4 |
| Line Bisection (difference between left and right % deviation from center of the line) | 11.7 | 13.0 | 10.3 | 12.6 | 6.9 | 7.5 | 3.3 | 4.6 |
| Oral reading: % words omitted on left side of page/sentence | 27.2 | 43.2 | 35.2 | 45.5 | 56.7 | 51.2 | 1.6 | 4.1 |
| Oral reading: % errors on left side of each word | 11.0 | 17.5 | 16.7 | 19.8 | 21.7 | 25.6 | 1.3 | 2.9 |
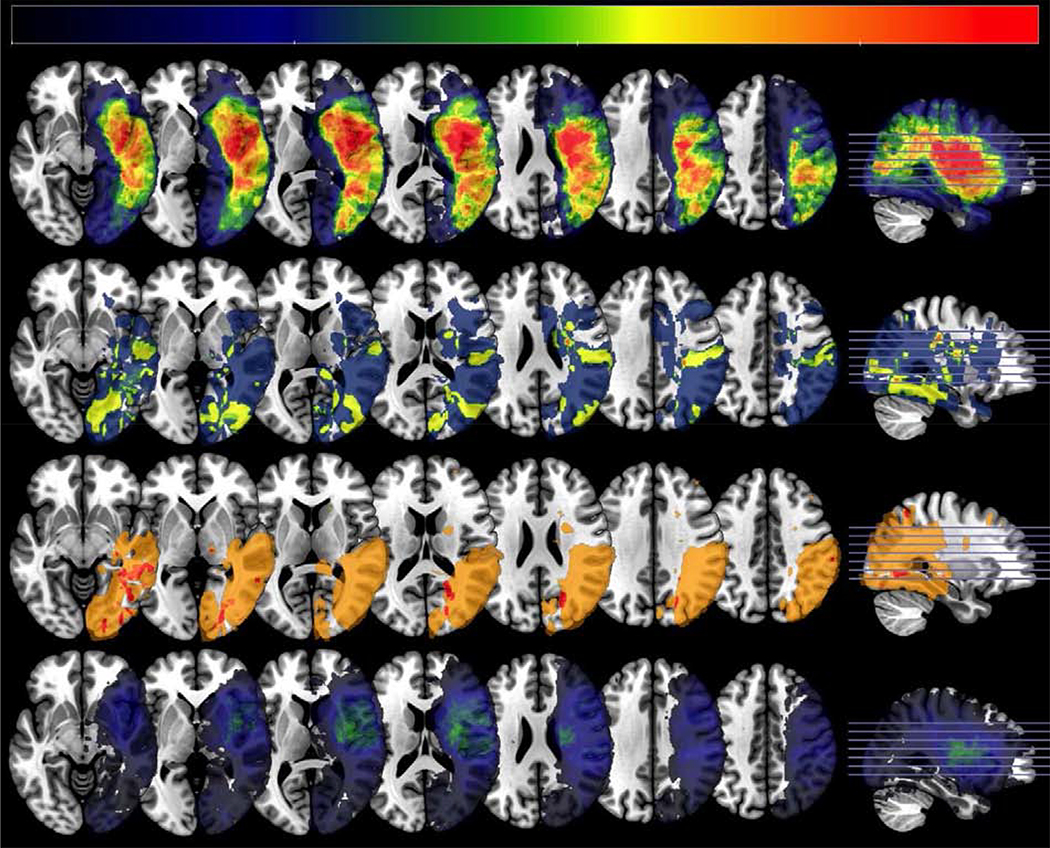
Fig. 2 –
Lesion overlap map for patient groups. Lesion density plots showing brain regions typically injured in each patient group. The top panel shows the VCN only group, the second panel shows the SCN only group, the third panel shows the group with both impairments (VCN + SCN) and the bottom panel shows individuals with no neglect. The axial slices correspond to z = −8,0, 8, 16, 24, 32, and 40mm in Montreal Neurological Institute coordinates. The sagittal slice is at x = 30mm. Images are shown in neurological convention (right hemisphere injury). The color bar shows lesion incidence, to account for different group sizes the scale is from 0 to 33%.
Table 3 –
Mean errors for dextral and sinistral participants.
| Mean (SD) viewer-centered errors | Mean (SD) stimulus-centered errors | |
|---|---|---|
| Left-handed | .63 (2.2) | .13 (.34) |
| Right-handed | 1.03 (2.8) | .51 (.12) |
| t-test | t = −.55; p = .58 | t = 1.07; p = .29 |
2.1. Analysis of continuous outcomes
When we included percent damage to all ROIs, age, and infarct volume as independent variables, severity of VCN as the dependent variable, and excluded participants with co-occurring significant SCN, the model explained about 30% of the variance (r2 = .31) in severity of VCN [F (25, 122) = 3.10; p < .0001]. However, only age (t = 3.20; p = .002) and percent damage to the angular gyrus (t = 2.63, p = .010) predicted severity of VCN independently of the other variables. All the variables together did not significantly account for severity of SCN (excluding participants who also had significant VCN), although percent damage to inferior frontal occipital tract was independently associated with severity of SCN (t = 2.05, p = .043).
2.2. Analyses of dichotomous outcomes
Given the important effect of age, we examined the data to determine if the frequency of VCN increased after a specific age. We found that age >60 years was strongly associated with VCN; 51.0% of participants >60 had VCN, while 16.7% 60 years and younger had VCN (X2 df1 = 10.55; p = .001). Therefore, we carried out logistic regressions separately for participants age >60 and age ≤60 years. Together, infarct volume and percent damage to each of the ROIs together predicted the presence of significant VCN for participants <60 (X2 = 51.05; df21; p = .0003) and accounted for about 84% of the variance (pseudo r2 = .84), but none of the variables independently predicted the presence of significant VCN. Likewise, for participants >60, the model predicted the presence of VCN (X2 = 56.80; df21; p < .00001; pseudo r2 = 0.58), but there were no independent predictors.
The same variables, infarct volume and percent damage to each ROI did not predict SCN. However, when we included only ventral stream ROIs (middle temporal gyrus, inferior temporal gyrus, fusiform gyrus, inferior frontal occipital gyrus, sagittal stratum, along with total infarct volume, for participants ≤60 years old, these variables predicted the presence of significant SCN (X2 = .0002; pseudo r2 = .70). Percent damage to right inferior temporal gyrus predicted SCN independently of the other variables (p = .018). Likewise, for those >60 the ventral stream ROIs and infarct volume predicted SCN (X2= 26.41; p = .0004; pseudo r2 = .70), and percent damage to right inferior temporal gyrus predicted SCN independently of the other variables (p = .015). When we carried out multivariable logistic regression, including SCN as the dependent variable, and percent damage to dorsal stream ROIs (IFGop, IFGorb, IFGtri, SMG, AG, SFO, and SLF) and infarct volume, the only independent predictor, for age >60 only, was total infarct volume (OR 1.000464; 95%CI: 1.000058 –1.0008795; p = .025). There were no independent predictors for patients ≤60 years.
Because it is unclear whether the severity of damage to a ROI versus any damage to a ROI should make a more important contribution to neglect, we then carried out logistic regressions with significant VCN or SCN as the dependent variable, and damage to >1% of each of the ROIs that were found to be significantly associated with VCN or SCN, along with infarct volume and age, as the independent predictors. These dichotomous lesion variables, infarct volume, and age predicted the presence of significant VCN only: [X2 (df6) = 42.42; p < .00001; r2= .28]. Age, infarct volume, and damage to the angular gyrus were the only variables that independently predicted VCN (Table 4). Participants with angular gyrus lesions were four times more likely to have VCN than those without VCN.
Table 4 –
Multivariable Logistic Regression to Identify Contributions of independent variables to the likelihood of significant viewer-centered neglect.
| Independent Variable | Odds Ratio | Standard Error | z | p | [95% Conf. Interval] |
|---|---|---|---|---|---|
| Age | 1.08 | .024 | 3.33 | .001 | 1.03–1.13 |
| Infarct Volume | 1.00000 | .000012 | 2.22 | .026 | 1.000003–1.000051 |
| IFG-op Lesion | 1.12 | .73 | .17 | .86 | .31–4.03 |
| ITG Lesion | .65 | .69 | −.41 | .69 | .08–5.22 |
| AG Lesion | 4.06 | 2.48 | 2.30 | .022 | 1.23–13.44 |
| SFO Lesion | 1.23 | .82 | .31 | .76 | .33–4.52 |
Bolded variables are significant.
These dichotomous lesion variables, infarct volume, and age did not predict the presence of significant SCN only: [X2 (df6) = 7.9; p = .26; pseudo r2 = .16]. However, damage to inferior temporal gyrus predicted SCN independently of the other variables (Table 5).
Table 5 –
Multivariable logistic regression to identify contributions of independent variables to the likelihood of significant stimulus-centered neglect.
| Independent Variable | Odds Ratio | Standard Error | z | p | [95% Conf. Interval] |
|---|---|---|---|---|---|
| Age | .99 | .036 | −.15 | .88 | .93–1.07 |
| Infarct Volume | 1.00 | −.07 | .94 | 1.00 | 1.00–1.00 |
| IFG-op Lesion | 2.47 | 3.24 | .69 | .49 | .19–32.43 |
| ITG Lesion | 42.75 | 69.08 | 2.32 | .02 | 1.80–1014.75 |
| AG Lesion | 1.35 | 2.09 | .20 | .84 | .07–27.96 |
| Sfo Lesion | .13 | .24 | −1.10 | .27 | .00–5.01 |
| Constant | .05 | .10 | −1.44 | .0 | .00–3.03 |
We confirmed these associations between damage to specific sites and each type of neglect with Pearson chi squared tests. There was a strong association between damage to angular gyrus and the presence of significant VCN (X2 df1 = 16.1; p < .0001). Also, there was a strong association between damage to right inferior temporal gyrus and the presence of significant SCN (X2 df1= 11.2; p = .001).
3. Discussion
Our results are consistent with previous studies reporting that regions of the right dorsal stream of visual processing (the “where” or “how to” stream, critical for directing gaze and exploratory movements) underlie viewer-center or egocentric representations, such that damage can cause contralesional VCN (Hillis et al., 2005; Medina et al., 2009; Shirani et al., 2009; Verdon, Schwartz, Lovblad, Hauert, & Vuilleumier, 2010). Damage to right angular gyrus in the inferior parietal lobule predicted both severity and presence of VCN, independently of the other lesion sites, age, and lesion volume. Age and lesion volume were also independent predictors, consistent with previous studies showing a strong effect of age (Gottesman et al., 2009) and lesion volume (Agis et al., 2016; Gottesman et al., 2008).
Our findings also provide additional evidence for the importance of right ventral stream of visual processing for stimulus-centered processing, such that damage can cause contralesional SCN (Hillis et al., 2005; Medina et al., 2009; Shirani et al., 2009; Verdon et al., 2010). Together, damage to ventral stream regions, particularly inferior temporal gyrus, was associated with both severity and presence of SCN. Inferior temporal gyrus is supplied by the posterior cerebral artery, whereas as the areas associated with VCN are supplied by the middle cerebral artery. Therefore, it seems likely that posterior cerebral artery strokes or posterior cerebral/middle cerebral artery “watershed” strokes are more likely than middle cerebral artery strokes to cause SCN. This fact may account for the lower frequency of SCN than VCN in this and other studies, as middle cerebral artery strokes are more common than posterior cerebral artery strokes.
Note that we use the term SCN rather than the commonly used term object-centered neglect, to differentiate two dissociable types of allocentric neglect. We reserve the term object-centered neglect for the neglect of the left side of the canonical representation of an object, irrespective of its orientation. For example, patients have been described who make errors selectively on the contralesional side of words, irrespective of the orientation of the word (vertical, mirror-reversed, or spelled aloud; Barbut & Gazzaniga, 1987; Baxter & Warrington, 1983; Caramazza & Hillis, 1990; Hillis & Caramazza, 1995). In contrast, patients with SCN make errors on the left side of the stimulus (e.g., the initial letters in normal print and the final letters in mirror-reversed words) (Hillis & Caramazza, 1991; Subbiah & Caramazza, 2000). In this study we did not test for object-centered neglect (by assessing the effect of orientation of the stimulus), so what we have referred to as stimulus-centered neglect (left side of the stimulus, irrespective of the location) might have included those with object-centered neglect. Both are types of allocentric neglect.
We also did not evaluate neglect after left hemisphere stroke. Previous studies have shown similar rates of right neglect after left hemisphere stroke as left neglect after right hemisphere stroke, although the type and severity differed (Kleinman et al., 2007). SCN was found to be more common after left hemisphere stroke, while VCN was more common after right hemisphere stroke.
One novel finding is the effect of age, independently of size and site of stroke, on VCN only. Likewise, a new finding is that both the extent and presence of damage to right angular gyrus predicts VCN, independently of both age and lesion volume. Although previous studies have shown the association between angular gyrus lesions and neglect, they have not shown that the association is independent of age and lesion volume. Similarly, we showed the importance of damage to right inferior temporal gyrus, independently of the effects of age and lesion volume. Interestingly, neither of the latter variables was associated with either the severity or the presence of SCN, despite their important influence on VCN.
Like Karnath et al. (2009), we found no significant contribution of disruption of specific white matter tracts, independently of age, lesion volume, and damage to cortical lesions. However, previous studies have shown that the severity of diffuse white matter disease (leukoaraiosis) is strongly associated with the severity of neglect, independently of age and lesion volume (Bahrainwala et al., 2014). It is likely that the absence of an association between disruption of any white matter tract and neglect in this study reflects the fact that damage to any one of several white matter tracts could result in neglect (e.g., SLF and splenium, as reported by Lunven, et al., 2015), but disruption of these tracts is not independent of lesion volume. Unlike Karnath et al. (2009), we found no independent contribution of damage to caudate or putamen to neglect. It is possible that we had insufficient numbers of patients with damage to these subcortical gray matter structures to identify their contributions.
Despite the limitations that we did not assess left hemisphere stroke or assess the effects of stimulus orientation (to identify object-centered neglect), this study provides some new insights regarding the variables (age, volume of lesion, and site of lesion) that influence VCN and SCN in a relatively large study of patients with acute right hemisphere stroke, before the opportunity for reorganization or recovery.
Acknowledgements
This study was made possible by support from NIH (NIDCD) through R01 DC015466 and P50014664. We are grateful to the stroke patients who participated in the study and to previous members of the SCORE lab who collected the behavioral data (Melissa Newhart Gross, Cameron Davis, Amy Wright, Emily Sherry, Joey Posner, Yessenia Gomez, Kevin Kim, Arianna Wright, and others).
Footnotes
Open practices
The study in this article earned an Open Materials badge for transparent practices.
REFERENCES
- Agis D, Goggins MB, Oishi K, Oishi K, Davis C, Wright A, … Hillis AE (2016). Picturing the size and site of stroke with an expanded national institutes of health stroke scale. Stroke, 47(6), 1459–1465. 10.1161/STROKEAHA.115.012324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert ML (1973). A simple test of visual neglect. Neurology, 23, 658–64 [DOI] [PubMed] [Google Scholar]
- Bahrainwala ZS, Hillis AE, Dearborn J, & Gottesman RF (2014). Neglect performance in acute stroke is related to severity of white matter hyperintensities. Cerebrovascular Diseases (Basel, Switzerland), 37(3), 223–230. 10.1159/000357661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbut D, & Gazzaniga MS (1987). Disturbances in conceptual space involving language and speech. Brain, 110(6), 1487–1496. [DOI] [PubMed] [Google Scholar]
- Baxter DM, & Warrington EK (1983). Neglect dysgraphia. Journal of Neurology, Neurosurgery & Psychiatry, 46(12), 1073–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beschin N, Cazzani M, Cubelli R, Della Sala S, & Spinazzola L (1996). Ignoring left and far: An investigation of tactile neglect. Neuropsychologia, 34(1), 41–49. [DOI] [PubMed] [Google Scholar]
- Beschin N, & Robertson IH (1997). Personal versus extrapersonal neglect: A group study of their dissociation using a reliable clinical test. Cortex, 33(2), 379–384. [DOI] [PubMed] [Google Scholar]
- Bisiach E, Perani D, Vallar G, & Berti A (1986). Unilateral neglect: Personal and extra-personal. Neuropsychologia, 24(6), 759–767. [DOI] [PubMed] [Google Scholar]
- Caggiano P, Beschin N, & Cocchini G (2014). Personal neglect following unilateral right and left brain damage. Procedia-Social and Behavioral Sciences, 140, 164–167. [Google Scholar]
- Caramazza A, & Hillis AE (1990). Spatial representation of words in the brain implied by studies of a unilateral neglect patient. Nature, 346(6281), 267. [DOI] [PubMed] [Google Scholar]
- Chen P, Caulfield MD, Hartman AJ, O’Rourke J, & Toglia J (2017). Assessing viewer-centered and stimulus-centered spatial bias: The 3s spreadsheet test version 1. Applied Neuropsychology: Adult, 24(6), 532–539. [DOI] [PubMed] [Google Scholar]
- Davis CL, Oishi K, Faria AV, Hsu J, Gomez Y, Mori S, et al. (2016). White matter tracts critical for recognition of sarcasm. Neurocase, 22(1), 22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffan D, & Hornak J (1997). Visual neglect in the monkey. Representation and disconnection. Brain, 120(9), 1647–1657. [DOI] [PubMed] [Google Scholar]
- Gottesman RF, Kleinman JT, Davis C, Heidler-Gary J, Newhart M, & Hillis AE (2009). The NIHSS-plus: Improving cognitive assessment with the NIHSS. Behavioural Neurology, 22(1), 11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman RF, Kleinman JT, Davis C, Heidler-Gary J, Newhart M, Kannan V, et al. (2008). Unilateral neglect is more severe and common in older patients with right hemispheric stroke. Neurology, 71(18), 1439–1444. 10.1212/01.wnl.0000327888.48230.d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillis AE, & Caramazza A (1991). Deficit to stimulus-centered, letter shape representations in a case of “unilateral neglect”. Neuropsychologia, 29(12), 1223–1240. [DOI] [PubMed] [Google Scholar]
- Hillis AE, & Caramazza A (1995). A framework for interpreting distinct patterns of hemispatial neglect. Neurocase, 1(3), 189–207. [Google Scholar]
- Hillis AE, Newhart M, Heidler J, Barker PB, Herskovits EH, & Degaonkar M (2005). Anatomy of spatial attention: Insights from perfusion imaging and hemispatial neglect in acute stroke. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 25(12), 3161–3167. doi:25/12/3161 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnath H, Himmelbach M, & Rorden C (2002). The subcortical anatomy of human spatial neglect: Putamen, caudate nucleus and pulvinar. Brain, 125(2), 350–360. [DOI] [PubMed] [Google Scholar]
- Karnath H, Rorden C, & Ticini LF (2009). Damage to white matter fiber tracts in acute spatial neglect. Cerebral Cortex, 19(10), 2331–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenzie JM, Girgulis KA, Semrau JA, Findlater SE, Desai JA, & Dukelow SP (2015). Lesion sites associated with allocentric and egocentric visuospatial neglect in acute stroke. Brain Connectivity, 5(7), 413–422. [DOI] [PubMed] [Google Scholar]
- Kleinman JT, Newhart M, Davis C, Heidler-Gary J, Gottesman RF, & Hillis AE (2007). Right hemispatial neglect: Frequency and characterization following acute left hemisphere stroke. Brain and Cognition, 64(1), 50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh R, Oishi K, Hsu J, Lindquist M, Gottesman RF, Jarso S, … Hillis, A. E. (2013). Acute lesions that impair affective empathy. Brain : A Journal of Neurology, 136(Pt 8), 2539–2549. 10.1093/brain/awt177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine DN, Warach JD, Benowitz L, & Calvanio R (1986). Left spatial neglect: Effects of lesion size and premorbid brain atrophy on severity and recovery following right cerebral infarction. Neurology, 36(3), 362. [DOI] [PubMed] [Google Scholar]
- Lunven M, Thiebaut de Schotten M, Bourlon C, Duret C, Migliaccio R, Rode G, et al. (2015). White matter lesional predictors of chronic visual neglect: A longitudinal study. Brain, 138(3), 746–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina J, Kannan V, Pawlak MA, Kleinman JT, Newhart M, Davis C, … Hillis AE (2009). Neural substrates of visuospatial processing in distinct reference frames: Evidence from unilateral spatial neglect. Journal of Cognitive Neuroscience, 21(11), 2073–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M (1981). A cortical network for directed attention and unilateral neglect. Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society, 10(4), 309–325. [DOI] [PubMed] [Google Scholar]
- Ogden J Contralesional neglect of constructed visual images in right and left brain-damaged patients. Neuropsychologia 1985;23:273–277. [DOI] [PubMed] [Google Scholar]
- Oishi K, Faria A, Jiang H, Li X, Akhter K, Zhang J, et al. (2009). Atlas-based whole brain white matter analysis using large deformation diffeomorphic metric mapping: application to normal elderly and Alzheimer’s disease participants. Neuroimage, 46(2), 486–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota H, Fujii T, Suzuki K, Fukatsu R, & Yamadori A (2001). Dissociation of body-centered and stimulus-centered representations in unilateral neglect. Neurology, 57(11), 2064–2069. [DOI] [PubMed] [Google Scholar]
- Rode G, Pagliari C, Huchon L, Rossetti Y, & Pisella L (2017). Semiology of neglect: An update. Annals of Physical and Rehabilitation Medicine, 60(3), 177–185. [DOI] [PubMed] [Google Scholar]
- Sebastian R, Schein MG, Davis C, Gomez Y, Newhart M, Oishi K, et al. (2014). Aphasia or neglect after thalamic stroke: The various ways they may be related to cortical hypoperfusion. Frontiers in Neurology, 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirani P, Thorn J, Davis C, Heidler-Gary J, Newhart M, Gottesman RF, et al. (2009). Severity of hypoperfusion in distinct brain regions predicts severity of hemispatial neglect in different reference frames. Stroke; a Journal of Cerebral Circulation, 40(11), 3563–3566. 10.1161/STROKEAHA.109.561969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaccavento S, Cellamare F, Falcone R, Loverre A, & Nardulli R (2017). Effect of subtypes of neglect on functional outcome in stroke patients. Annals of Physical and Rehabilitation Medicine, 60(6), 376–381. [DOI] [PubMed] [Google Scholar]
- Subbiah I, & Caramazza A (2000). Stimulus-centered neglect in reading and object recognition. Neurocase, 6(1), 13–31. [Google Scholar]
- Vaessen MJ, Saj A, Lovblad K, Gschwind M, & Vuilleumier P (2016). Structural white-matter connections mediating distinct behavioral components of spatial neglect in right brain-damaged patients. Cortex, 77, 54–68. [DOI] [PubMed] [Google Scholar]
- Verdon V, Schwartz S, Lovblad KO, Hauert CA, & Vuilleumier P (2010). Neuroanatomy of hemispatial neglect and its functional components: A study using voxel-based lesion-symptom mapping. Brain : A Journal of Neurology, 133(Pt 3), 880–894. 10.1093/brain/awp305. [DOI] [PubMed] [Google Scholar]