Abstract
Pomegranate (Punica granatum L.) fruit and flower extracts, which are rich sources of bioactive phenolics, are widely utilized as ingredients in botanical dietary supplements. While the phenolic characterization of extracts of pomegranate fruit have been previously studied by LC-MS/MS, there is lack of similar data for pomegranate flowers. Herein, LC-TOF-MS/MS was utilized to comprehensively characterize the phenolics present in two pomegranate extracts, previously studied for their in vitro and in vivo biological effects, namely, a patented commercial pomegranate fruit extract (Pomella®), and a pomegranate flower extract. Seventy-one phenolics were characterized in the pomegranate fruit extract with the vast majority identified in the flower extract. However, only the pomegranate fruit extract contained tannin-glucuronides and two punicalagin isomers (a characteristic pomegranate phenolic) were identified in this extract while four were identified in the flower extract. The previously reported compounds, pomellatannin and punicatannins A/B, were identified as unique chemical markers in the pomegranate fruit and flower extracts, respectively. This study will aid in the quality control, authentication, and standardization of these botanical ingredients to evaluate their potential health benefits in future planned pre-clinical and clinical studies. Also, this is the first phenolic characterization of a pomegranate flower extract using LC-MS/MS.
Keywords: Phenolics, Pomegranate, Punica granatum, Tandem mass spectrometry
1. INTRODUCTION
Botanical dietary supplements are widely consumed worldwide for a variety of potential human health benefits and biological effects. However, the industry is commonly plagued by lack of comprehensive characterization of botanical extract ingredients which is critical to ensure authentication of study materials used in pre-clinical and clinical studies designed to evaluate their potential health benefits [1]. In addition, the identification of potential unique chemical markers present in various botanical extracts would aid in ensuring product integrity and prevent economic adulteration and counterfeiting of study materials. This has led to the utilization of analytical techniques, including, HPLC and LC-MS/MS analyses, for the characterization of botanical ingredients [1–5].
The pomegranate (Punica granatum L.) fruit, a rich source of bioactive phenolics, especially ellagitannins, is highly regarded for its cardiovascular, antidiabetic, antimicrobial, antiviral, anticancer, anti-inflammatory, and neuroprotective properties [6–13]. Pomegranate fruits are not only widely consumed as a food, as its edible arils and squeezed fruit juice, but also as extracts which are widely used as ingredients in oral botanical dietary supplements [7]. However, apart from its fruits, other parts of the pomegranate plant, including its flowers, which have been used as a traditional medicine for treating various ailments such as diabetes [14], are also being investigated for botanical ingredient applications. Whilst the phenolic constituents of various parts of the pomegranate fruit have been previously characterized by LC-MS/MS [15–21], there is lack of similar data on pomegranate flower extracts. Moreover, there is limited data available on the utilization of LC-MS/MS methods for the comprehensive phenolic characterization of commercial pomegranate fruit extracts used as ingredients in botanical dietary supplements. This is unfortunate considering that the economic adulteration of commercial pomegranate extracts with lower-cost exogenous ingredients has been noted as a significant problem in the botanical dietary supplement industry [7]. This has led to the recent issuance of a pomegranate laboratory guidance document for the Botanical Adulterants Program by the joint non-profit organizations, the American Botanical Council, the American Herbal Pharmacopoeia, and the University of Mississippi’s National Center for Natural Product Research [22].
A variety of pomegranate extracts are available in the botanical dietary supplement markets, some containing high concentrations (> 90%) of ellagic acid, indicating potential adulteration to enhance the perceived value of the product [22]. This is because ellagic acid, formed from the hydrolysis of ellagitannins, can be obtained from various plant sources, or by chemical synthesis, and is not a unique chemical marker of the pomegranate fruit although methods for its purification from pomegranate peel has been reported [23]. Therefore, beyond ellagic acid alone, pomegranate whole fruit extracts should be standardized to characteristic pomegranate phenolics, such as the ellagitannin isomers, punicalagins A/B, the absence of which may indicate potential adulteration [22]. The use of analytical methods, such as LC-MS/MS, to comprehensively characterize the phenolics in pomegranate extracts is an additional and more definitive measure to ensure the integrity of study materials especially when they are consumed as botanical ingredients in oral dietary supplements for human health benefits.
Pomella® is a patented commercial pomegranate fruit extract that has been studied by our group and others for a wide range of biological effects using in vitro models as well as in animals and human subjects [13, 24–39]. While we have previously reported on the isolation and structure elucidation (by NMR) of twenty-one phenolic compounds, including a new ellagitannin, named pomellatannin, from this pomegranate fruit extract [13], it has not yet been comprehensively characterized by LC-MS/MS. Furthermore, while we have previously reported on the isolation and structure elucidation (by NMR) of fifteen phenolic compounds, including a pair of new ellagitannin isomers, punicatannin A/B, from a pomegranate flower extract [40, 41], it has also not yet been characterized by LC-MS/MS. Therefore, the objectives of this study were to conduct comprehensive phenolic characterization of these two aforementioned pomegranate extracts, using LC-MS/MS, and identify potential unique chemical marker compounds present within them that can be used for their quality control, authentication, and standardization. This is the first reported phenolic characterization of a pomegranate flower extract and of Pomella® using LC-MS/MS.
2. MATERIALS AND METHODS
2.1. Chemicals
Pomegranate (Punica granatum L.) flowers were collected and authenticated by matching their macroscopic and microscopic characteristics to internal monograph and botanical reference standards at Verdure Sciences (Noblesville, IN, USA) as previously reported [40]. The whole pomegranate fruit extract (Pomella®) was provided by Verdure Sciences (Noblesville, IN, USA) and was of the same lot number as the pomegranate fruit extract previously reported by our group to show neuroprotective effects against Alzheimer’s disease in Caenorhabditis elegans and in an aged transgenic Alzheimer’s disease animal model [13, 24]. LC-MS grades of acetonitrile and formic acid were purchased from Sigma-Aldrich (St. Louis, MO, USA).
2.2. Sample preparation
Finely powdered pomegranate flower (1.0 g) was extracted by sonication with methanol (10 mL) for 1 hour under room temperature. Pomella® (100 mg) was dissolved in methanol (10 mL). The solution was store at 4 °C and filtered through a 0.22 μm filter before analyses.
2.3. Liquid Chromatography Mass Spectrometry Conditions
LC-TOF-MS/MS analyses were performed on a SHIMADZU Prominence UFLC system (Marlborough, MA, USA) consisting of two LC-20AD pumps, a DGU-20A degassing unit, SIL-20AC auto sampler, CTO-20AC column oven and CBM-20A communication bus module. Chromatographic separation was performed on a 150 mm × 4.6 mm i.d., 5 μm, Prodigy ODS column (Phenomenex, Torrance, CA, USA). The mobile phase consisted of 0.1% formic acid/acetonitrile (A) and 0.1% aqueous formic acid (B) with a gradient elution of 5% A from 0 to 5 min, 5−12% A from 5 to 12 min, 12−17% A from 12 to 27 min, and 17−35% A from 27 to 45 min. The column temperature was 40 °C, flow rate was 0.50 mL/min, and injection volume was 4.00 μL. Mass spectrometry was performed using a Triple time-of-flight (TOF) 4600 system from Applied Biosystems/MDS Sciex (Framingham, MA, USA) coupled with an electrospray ionization (ESI) interface. Nitrogen was used in all cases. In this study, the parameters were optimized as follows: ESI voltage, −4500 V; nebulizer gas, 40 psi; auxiliary gas, 50 psi; curtain gas, 25 psi; turbo gas temperature, 450 °C; declustering potential, −80V; collision energy, 50 eV. Instrument calibration was carried out according to the manufacturer’s instructions. The mass range was set from m/z 100 to 1150. The data were acquired and processed using Analyst TF 1.7 Software.
3. RESULTS AND DISCUSSION
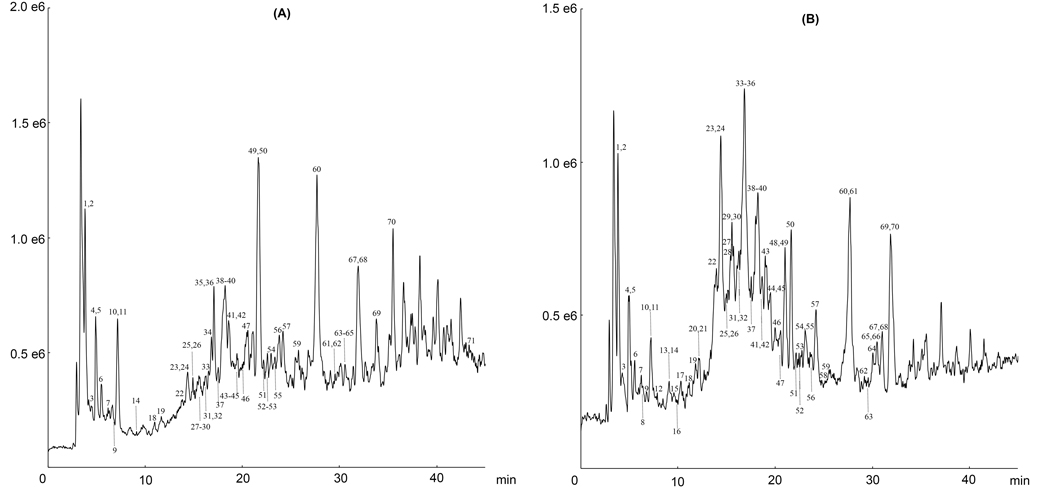
Accurate mass data were acquired in the full scan analysis, and product ion mass data were acquired via the information-dependent acquisition method. A Prodigy ODS column was employed to provide increased chromatographic resolution and the mobile phase contained formic acid (0.1%) which was added to alleviate peak tailing and produce better shapes. The acidic conditions did not appear to significantly affect the ionization efficiency of compounds in negative mode. A total of seventy-one phenolic compounds, including sixty four tannins, were tentatively characterized. The total ion chromatograms profiles are shown in Figure 1. Accurate mass measurements, retention time, formula, errors and main MS/MS product ions for all phenolic compounds are summarized in Table 1. All of the compounds were observed by their [M − H]– ions in negative ESI mode, which was helpful for their elemental composition by accurate mass measurement (Table 1). For ease of discussion, the phenolics are characterized into different sub-categories as described below.
Figure 1.
Total ion chromatograms of pomegranate flower (A) and fruit (B) extracts.
Table 1.
Characterization of phenolic compounds in pomegranate flower and fruit extracts by LC-TOF-MS/MS.
| No. | tR | Molecular | HR-ESI(−)-MS | Error | Major MS/MS ions | Identification | Presence |
|
|---|---|---|---|---|---|---|---|---|
| (min) | formula | [M-H]- m/z exptl. (theor.) | (ppm) | Flower | Fruit | |||
| 1 | 3.80 | C20H18O14 | 481.0631 (481.0623) | 1.4983 | 301, 275, 257, 229 | HHDP-hexoside | + | + |
| 2 | 3.92 | C13H16O10 | 331.0678 (331.0670) | 2.2032 | 271, 211, 169, 125 | galloyl-hexoside | + | + |
| 3 | 4.40 | C20H18O14 | 481.0627 (481.0623) | 0.6668 | 301, 275, 257, 229 | HHDP-hexoside | + | + |
| 4 | 4.88 | C20H18O14 | 481.0624 (481.0623) | 0.0431 | 301, 275, 257, 229 | HHDP-hexoside | + | + |
| 5 | 4.99 | C13H16O10 | 331.0680 (331.0670) | 2.8073 | 271, 211, 169, 125 | galloyl-hexoside | + | + |
| 6 | 5.47 | C13H16O10 | 331.0675 (331.0670) | 1.2970 | 271, 211, 169, 125 | galloyl-hexoside | + | + |
| 7 | 6.17 | C13H16O10 | 331.0678 (331.0670) | 2.2032 | 271, 211, 169, 125 | galloyl-hexoside | + | + |
| 8 | 6.36 | C27H22O19 | 649.0684 (649.0682) | 0.2271 | 497, 301, 275, 229, 195 | galloyl-HHDP-glucuronide | - | + |
| 9 | 6.82 | C27H22O18 | 633.0732 (633.0733) | −0.2178 | 481, 463, 421, 301, 275, 257, 249, 229 | galloyl-HHDP-hexoside | + | + |
| 10 | 7.15 | C7H6O5 | 169.0155 (169.0142) | 7.4136 | 125 | gallic acid | + | + |
| 11 | 7.20 | C13H16O10 | 331.0676 (331.0670) | 1.5991 | 271, 211, 169, 125 | galloyl-hexoside | + | + |
| 12 | 7.74 | C34H22O22 | 781.0524 (781.0529) | −0.7637 | 721, 601, 575, 449, 393, 301, 300, 299, 271, 245, 229 | gallagyl-hexoside | - | + |
| 13 | 9.09 | C48H28O30 | 1083.0579 (1083.0592) | −1.2592 | 1065, 807, 781, 763, 721, 601, 575, 549, 341, 301, 300, 299, 275, 273, 271 | HHDP-gallagyl-hexoside* | - | + |
| 14 | 9.10 | C27H22O18 | 633.0737 (633.0733) | 0.5719 | 481, 463, 301, 275, 257, 249, 229 | galloyl-HHDP-hexoside | + | + |
| 15 | 9.60 | C34H24O22 | 783.0684 (783.0686) | −0.3148 | 481, 437, 419, 341, 329, 301, 299, 275, 257, 229 | Bis-HHDP-hexoside | - | + |
| 16 | 9.83 | C27H22O19 | 649.0684 (649.0682) | 0.2271 | 497, 301, 284, 275, 229, 195 | galloyl-HHDP-glucuronide | - | + |
| 17 | 10.31 | C48H28O30 | 1083.0586 (1083.0592) | −0.6129 | 807, 781, 763, 721, 601, 575, 549, 341, 301, 300, 299, 275, 273, 271 | HHDP-gallagyl-hexoside* | - | + |
| 18 | 10.93 | C13H16O10 | 331.0675 (331.0670) | 1.2970 | 169, 125 | galloyl-hexoside | + | + |
| 19 | 11.86 | C34H24O22 | 783.0680 (783.0686) | −0.8257 | 481, 301, 275, 257, 229 | Bis-HHDP-hexoside | + | + |
| 20 | 12.08 | C41H26O26 | 933.0622 (933.0639) | −1.8811 | 781, 763, 721, 601, 575, 549, 449, 393, 301, 300, 299, 271 | galloyl-gallagyl-hexoside | - | + |
| 21 | 12.21 | C15H14O7 | 305.0674 (305.0666) | 2.7313 | 261, 221, 179, 167, 165, 137, 125, 109 | gallocatechin/epigallocatechin | - | + |
| 22 | 13.80 | C27H22O18 | 633.0737 (633.0733) | 0.5719 | 481, 463, 421, 301, 275, 257, 249, 229 | galloyl-HHDP-hexoside | + | + |
| 23 | 14.25 | C21H10O13 | 469.0056 (469.0048) | 1.5687 | 425, 407, 379, 300, 279, 251, 195 | valoneic acid bilactone | + | + |
| 24 | 14.39 | C48H28O30 | 1083.0581 (1083.0592) | −1.0746 | 781, 721, 601, 575, 549, 301, 300, 299, 275, 273, 271 | HHDP-gallagyl-hexoside* | + | + |
| 25 | 14.94 | C27H22O18 | 633.0737 (633.0733) | 0.5719 | 481, 463, 421, 301, 275, 257, 249, 229 | galloyl-HHDP-hexoside | + | + |
| 26 | 15.02 | C20H20O14 | 483.0785 (483.0780) | 0.9743 | 423, 405, 331, 313, 271, 241, 211, 169, 125 | digalloyl-hexoside | + | + |
| 27 | 15.34 | C20H20O14 | 483.0775 (483.0780) | −1.0956 | 423, 331, 313, 271, 211, 169, 125 | digalloyl-hexoside | + | + |
| 28 | 15.39 | C41H28O27 | 951.0735 (951.0745) | −1.0724 | 907, 783, 605, 481, 425, 301, 275, 257, 249, 229 | HHDP-valoneoyl-hexoside | - | + |
| 29 | 15.47 | C27H22O18 | 633.0736 (633.0733) | 0.4139 | 481, 463, 421, 301, 275, 257, 249, 229 | galloyl-HHDP-hexoside | + | + |
| 30 | 15.83 | C34H24O22 | 783.0676 (783.0686) | −1.3365 | 481, 301, 275, 257, 229 | Bis-HHDP-hexoside | + | + |
| 31 | 16.15 | C20H20O14 | 483.0793 (483.0780) | 2.6304 | 423, 331, 313, 271, 211, 169, 125 | digalloyl-hexoside | + | + |
| 32 | 16.44 | C20H20O14 | 483.0785 (483.0780) | 0.9743 | 423, 331, 313, 271, 241, 211, 193, 169, 125 | digalloyl-hexoside | + | + |
| 33 | 16.57 | C27H22O18 | 633.0731 (633.0733) | −0.3758 | 481, 301, 275, 257, 249, 229 | galloyl-HHDP-hexoside | + | + |
| 34 | 16.79 | C48H28O30 | 1083.0587 (1083.0592) | −0.5206 | 781, 721, 601, 575, 549, 301, 300, 299, 275, 273, 271 | HHDP-gallagyl-hexoside* | + | + |
| 35 | 17.06 | C20H20O14 | 483.0785 (483.0780) | 0.9743 | 331, 313, 271, 211, 169, 125 | digalloyl-hexoside | + | + |
| 36 | 17.15 | C34H26O22 | 785.0836 (785.0842) | −0.8873 | 615, 483, 463, 419, 331, 313, 301, 275, 257, 249, 229, 169, 125 | digalloyl-HHDP-hexoside | + | + |
| 37 | 17.53 | C20H20O14 | 483.0787 (483.0780) | 1.3883 | 439, 331, 313, 287, 169, 125 | digalloyl-hexoside | + | + |
| 38 | 17.79 | C20H20O14 | 483.0785 (483.0780) | 0.9743 | 331, 313, 271, 211, 169, 125 | digalloyl-hexoside | + | + |
| 39 | 18.02 | C27H24O18 | 635.0869 (635.0889) | −3.2877 | 483, 465, 423, 313, 295, 271, 235, 211, 193, 169, 125 | trigalloyl-hexoside | + | + |
| 40 | 18.34 | C27H22O18 | 633.0737 (633.0733) | 0.5719 | 481, 463, 421, 301, 275, 257, 249, 229 | galloyl-HHDP-hexoside | + | + |
| 41 | 18.57 | C8H8O5 | 183.0313 (183.0298) | 7.6650 | 168, 140, 124 | methyl gallic acid | + | + |
| 42 | 18.69 | C34H26O23 | 801.0772 (801.0792) | −2.5107 | 649, 499, 347, 301, 275, 229, 195 | digalloyl-HHDP-glucuronide | - | + |
| 43 | 19.04 | C41H28O26 | 935.0781 (935.0796) | −1.6098 | 917, 783, 633, 571, 481, 419, 329, 301, 275, 257, 249, 231, 169, 125 | galloyl-bis-HHDP-hexoside | - | + |
| 44 | 19.27 | C27H22O18 | 633.0730 (633.0733) | −0.5338 | 481, 463, 421, 301, 275, 257, 249, 229 | galloyl-HHDP-hexoside | + | + |
| 45 | 19.43 | C13H8O8 | 291.0152 (291.0146) | 1.9209 | 247, 219, 191 | Brevifolin carboxylic acid | + | + |
| 46 | 19.92 | C27H24O18 | 635.0886 (635.0889) | −0.6109 | 483, 465, 423, 331, 313, 295, 271, 211, 169, 125 | trigalloyl-hexoside | + | + |
| 47 | 20.69 | C34H26O22 | 785.0839 (785.0842) | −0.5022 | 615, 483, 463, 419, 331, 313, 301, 275, 257, 249, 229, 169, 125 | digalloyl-HHDP-hexoside | + | + |
| 48 | 21.00 | C37H28O23 | 839.0926 (839.0948) | −2.6950 | 537, 519, 479, 461, 435, 301, 273, 257, 229 | pomellatannin | - | + |
| 49 | 21.01 | C27H24O18 | 635.0886 (635.0889) | −0.6109 | 483, 465, 313, 169, 125 | trigalloyl-hexoside | + | + |
| 50 | 21.49 | C27H22O18 | 633.0737 (633.0733) | −0.5338 | 481, 463, 421, 301, 275, 257, 249, 229 | galloyl-HHDP-hexoside | + | + |
| 51 | 22.20 | C27H24O18 | 635.0882 (635.0889) | −1.2408 | 483, 465, 313, 211, 169, 125 | trigalloyl-hexoside | + | + |
| 52 | 22.52 | C34H26O22 | 785.0802 (785.0842) | −5.2181 | 615, 483, 463, 419, 331, 313, 301, 275, 257, 249, 229, 169, 125 | digalloyl-HHDP-hexoside | + | + |
| 53 | 22.68 | C21H10O13 | 469.0051 (469.0048) | 0.5026 | 425, 407, 301, 300, 299, 271, 243 | valoneic acid bilactone | + | + |
| 54 | 22.94 | C27H24O18 | 635.0881 (635.0889) | −1.3982 | 483, 465, 313, 295, 271, 211, 169, 125 | trigalloyl-hexoside | + | + |
| 55 | 23.14 | C20H16O13 | 463.0514 (481.0518) | −0.4144 | 301, 300, 257, 229 | ellagic acid-hexoside | + | + |
| 56 | 23.81 | C27H24O18 | 635.0893 (635.0889) | 0.4912 | 483, 465, 423, 331, 313, 295, 271, 221, 211, 169, 125 | trigalloyl-hexoside | + | + |
| 57 | 24.25 | C20H16O13 | 463.0515 (481.0518) | −0.3144 | 301, 300, 257, 229 | ellagic acid-hexoside | + | + |
| 58 | 24.73 | C34H26O22 | 785.0863 (785.0842) | 2.5517 | 615, 483, 463, 419, 331, 313, 301, 275, 257, 249, 229, 169, 125 | digalloyl-HHDP-hexoside | - | + |
| 59 | 25.62 | C27H24O18 | 635.0885 (635.0889) | −0.7684 | 483, 465, 423, 313, 295, 271, 211, 169, 125 | trigalloyl-hexoside | + | + |
| 60 | 27.68 | C41H28O27 | 951.0713 (951.0745) | −3.3855 | 933, 915, 897, 765, 631, 613, 463, 445, 301, 275, 257, 249, 229, 169 | galloyl-HHDP-DHHDP-hexoside | + | + |
| 61 | 29.16 | C19H14O12 | 433.0411 (433.0412) | −0.3458 | 301, 300, 257, 229 | ellagic acid pentoside | + | + |
| 62 | 29.52 | C34H28O22 | 787.0980 (787.0999) | −2.4733 | 635, 617, 483, 465, 447, 423, 313, 295, 249, 211, 169, 125 | tetragalloyl-hexoside | + | + |
| 63 | 30.45 | C19H14O12 | 433.0408 (433.0412) | −1.0386 | 301, 300, 257, 229 | ellagic acid-pentoside | + | + |
| 64 | 30.68 | C34H26O22 | 785.0827 (785.0842) | −2.0337 | 615, 483, 463, 419, 331, 313, 301, 275, 257, 249, 229, 169, 125 | digalloyl-HHDP-hexoside | + | + |
| 65 | 30.89 | C34H28O22 | 787.0971 (787.0999) | −3.6167 | 635, 617, 573, 465, 313, 295, 211, 169, 125 | tetragalloyl-hexoside | + | + |
| 66 | 31.05 | C20H16O12 | 447.0571 (447.0568) | 0.4476 | 301, 300, 257, 229 | ellagic acid-deoxyhexoside | - | + |
| 67 | 31.82 | C14H6O8 | 300.9986 (300.9989) | −1.2986 | 284, 257, 245, 229, 201, 185 | ellagic acid | + | + |
| 68 | 32.23 | C34H28O22 | 787.0972 (787.0999) | −2.7467 | 617, 465, 449, 423, 313, 295, 169, 125 | tetragalloyl-hexoside | + | + |
| 69 | 33.97 | C41H30O26 | 937.0930 (937.0952) | −2.4067 | 785, 767, 635, 617, 597, 483, 465, 445, 419, 313, 301, 275, 257, 249, 229, 169, 125 | trigalloyl-HHDP-hexoside | + | + |
| 70 | 35.61 | C41H32O26 | 939.1076 (939.1109) | −3.5197 | 787, 769, 635, 617, 599, 465, 447, 431, 313, 295, 169, 125 | pentagalloyl-hexoside | + | + |
| 71 | 43.33 | C43H34O28 | 997.1123 (997.1163) | −4.0966 | 845, 827, 695, 615, 525, 463, 445, 381, 301, 257, 229 | punicatannin A/B | + | - |
punicalagin isomers
3.1. Characterization of galloyl hexosides
Compounds 2 (tR = 3.92 min), 5 (tR = 4.99 min), 6 (tR = 5.47 min), 7 (tR = 6.17 min), 11 (tR = 7.20 min) and 18 (tR = 10.93 min) all gave the same [M − H]– ions at m/z 331, with molecular formula C13H16O10 provided by TOF-MS. Their MS/MS fragmentation ions at m/z 125 (typical fragment ion for gallic acid due to loss of CO2) and m/z 169 [M − H − 162]− indicated the existence of a galloyl moiety and the loss of a hexose moiety, respectively. Thus, compounds 2, 5, 6, 7, 11 and 18 were tentatively identified as galloyl-hexosides.
Compounds 26 (tR = 15.02 min), 27 (tR = 15.34 min), 31 (tR = 16.15 min), 32 (tR = 16.44 min), 35 (tR = 17.06 min), 37 (tR = 17.53 min) and 38 (tR = 17.79 min) were tentatively identified as digalloyl-hexosides based on their [M − H]− ions at m/z 483 and MS/MS fragment ions at m/z 331 [M − H − 152]− due to the loss of a galloyl group, and m/z 169 [M − H − 152 − 162]− due to the loss of a hexose moiety. The typical MS/MS fragmentation ions of gallic acid at m/z 169 and m/z 125 were also observed.
Compounds 39, 46, 49, 51, 54, 56, and 59, which eluted at retention times (tR) of 18.02, 19.92, 21.01, 22.20, 22.94, 23.81 and 25.62 min, respectively, were tentatively identified as isomers of trigalloyl-hexosides. These compounds all had identical m/z 635 [M − H]− ions with MS/MS product ions at m/z 483 [M − H − 152]− (loss of galloyl moiety), m/z 465 [M − H − 170]− (loss of gallic acid), and m/z 313 [M − H − 170 − 152]− (sequential losses of a gallic acid and a galloyl moiety). They also exhibited typical galloyl moiety fragment ions at m/z 125 and m/z 169.
Compounds 62 (tR = 29.52 min), 65 (tR = 30.89 min) and 68 (tR = 32.23 min) all had the same [M − H]– ions at m/z 787. Based on the MS/MS daughter ions formed by sequential losses of gallic acid/galloyl moieties at m/z 617 [M − H − 170]−, m/z 465 [M − H − 170 − 152]− and m/z 313 [M − H − 170 − 152 − 152]−, as well as the typical daughter ions at m/z 169 and m/z 125, compounds 62, 65, and 68 were tentatively identified as isomers of tetragalloyl-hexoside. Compound 70 (tR = 35.61 min) displayed [M − H]– ions at m/z 939 and was tentatively identified as a pentagalloyl-hexoside based on the MS/MS daughter ions formed by sequential losses of gallic acid/galloyl moieties at m/z 787 [M − H − 152]−, m/z 635 [M − H − 152 − 152]−, m/z 465 [M − H − 152 − 152 − 170]− and m/z 313 [M − H − 152 − 152 − 170 − 152]−, as well as the typical daughter ions at m/z 169 and m/z 125 indicating the existence of galloyl moiety.
3.2. Characterization of hexahydroxydiphenoyl (HHDP) derivative tannins
Compounds 1 (tR = 3.80 min), 3 (tR = 4.40 min) and 4 (tR = 4.88 min) all had the same [M − H]– ions at m/z 481, with a molecular formula of C20H18O14 provided by TOF-MS. Their MS/MS fragmentation ion at m/z 301 [M − H − 180]− suggested the loss of a hexose moiety. The daughter ions at m/z 275, 257 and 229 were also observed, suggesting the formation of ellagic acid and supporting the existence of a hexahydroxydiphenoyl (HHDP) moiety. Thus, compounds 1, 3, and 4 were tentatively identified as HHDP-hexosides. Compounds 15 (tR = 9.60 min), 19 (tR = 11.86 min), and 30 (tR = 15.83 min) had the same [M − H]– ion at m/z 783. The product ions at m/z 481 [M − H − 302]− and m/z 301 [M − H − 302 − 180]− indicated the loss of a HHDP group and a hexose. Diagnostic daughter ions at m/z 301, 275, 257, and 229 suggested the formation of ellagic acid and confirmed the presence of the HHDP moiety. Therefore, compounds 15, 19, and 30 were tentatively identified as isomers of bis-HHDP-hexoside.
Compounds 9, 14, 22, 25, 29, 33, 40, 44, and 50, which eluted at tR 6.82, 9.10, 13.80, 14.94, 15.47, 16.57, 18.34, 19.27 and 21.49 min, respectively, were identified as galloyl-HHDP-hexosides due to their deprotonated ions [M − H]– ion at m/z 633 with MS/MS fragment ions of m/z 481 (loss of a galloyl moiety [M − H − 152]−, HHDP-hexoside), m/z 463 (loss of a gallic acid [M − H − 170]−), and m/z 301 (loss of galloyl hexose [M − H – 152 − 180]−). Diagnostic daughter ions at m/z 301, 275, 257 and 229 suggested the formation of ellagic acid and the existence of the HHDP moiety. Compounds 36 (tR = 17.15 min), 47 (tR = 20.69 min), 52 (tR = 22.52 min), 58 (tR = 24.73 min) and 64 (tR = 30.68 min), had the same [M − H]– ion at m/z 785 and were identified as digalloyl-HHDP-hexosides. In the MS/MS spectra, diagnostic daughter ions at m/z 301, 275, 257, and 229 suggested the formation of ellagic acid and the existence of the HHDP moiety. The fragment ion at m/z 483 ([M − H − 302]−, loss of 302 Da) was due to the loss of the HHDP moiety. Fragment ions at m/z 615 ([M − H − 170]−, loss of a gallic acid) and m/z 463 ([M − H – 170 − 152]−, loss of a gallic acid and a galloyl moiety), as well as the diagnostic daughter ions at m/z 169 and 125, indicated the existence of two galloyl moieties. The fragment ion at m/z 331 [M − H − 302 − 152]− was due to the loss of HHDP and a galloyl moiety, and the fragment ion at m/z 313 [M − H − 302 − 170]− was due to the loss of HHDP and gallic acid. Compound 69 (tR = 33.97 min), with an [M − H]– ion at m/z 937, was identified as trigalloyl-HHDP-hexoside. Its typical fragment ions at m/z 785 [M − H – 152]−, m/z 635 [M − H – 302]−, and m/z 483 [M − H – 152 – 302]−, represented digalloyl-HHDP-hexoside, trigalloyl-hexoside and digalloyl-hexoside, respectively. It also gave fragment ions at m/z 767 [M − H – gallic acid]−, m/z 597 [M − H – gallic acid – gallic acid]−, m/z 445 [M − H – gallic acid – gallic acid – galloyl]−. Compound 43 (tR = 19.04 min) had a [M − H]– ion at m/z 935 and was tentatively identified as galloyl-bis-HHDP-hexoside. It gave typical MS/MS fragment ions at m/z 783 ([M − H − 152]−), m/z 633 ([M − H − 302]−) and m/z 481 ([M − H − 152 − 302]−) suggesting bis-HHDP-hexoside, galloyl-HHDP-hexoside, and HHDP-hexoside, respectively. It also gave diagnostic MS/MS fragment ions at m/z 301, 257, 169 and 125, representing HHDP and galloyl moieties.
Compounds 8 (tR = 6.36 min) and 16 (tR = 9.83 min) had the same [M − H]– ions at m/z 649. The product ions at m/z 497 [M − H − 152]− were due to the loss of a galloyl moiety. Diagnostic daughter ions at m/z 301, 275 and 229 suggested the formation of ellagic acid and the existence of HHDP. The product ions at m/z 301 [M − H − 152 − 196]− indicated the loss of a galloyl moiety and a glucuronic acid. Thus, compounds 8 and 16 were tentatively identified as galloyl-HHDP-glucuronides. Compound 42 (tR = 18.69 min) was identified as digalloyl-HHDP glucuronide due to their deprotonated [M − H]– ion at m/z 801 with MS/MS fragment ions of m/z 499 (loss of HHDP [M − H − 302]−), m/z 649 (loss of a galloyl moiety [M − H − 152]−), m/z 347 (loss of a galloyl moiety and HHDP [M − H − 152 − 302]−), and m/z 301 (loss of digalloyl glucuronic acid [M − H − 152 − 152 − 196]−).
Compounds 13, 17, 24, and 34, which eluted at tR 9.09, 10.31, 14.39 and 16.79 min, respectively, exhibited [M − H]− ions at m/z 1083. Gil et al. [42] reported a quasimolecular ion at m/z 1083 [M − H]− in accordance with punicalagin (glucose + gallagyl + hexahydroxydiphenoyl), a characteristic ellagitannin found in pomegranate. Punicalagin is known to form fragments at m/z 301 (residue of ellagic acid), m/z 601 (residue of gallagic moiety) and m/z 781 (loss of ellagic acid). Compounds 13, 17, 24 and 34 all gave typical MS/MS fragment ions at m/z 301, m/z 601 and m/z 781, and thus, they were tentatively identified as HHDP-gallagyl-hexosides (punicalagin isomers) which is consistent with a previous report of four punicalagin isomers being detected in pomegranate [16].
Compound 12 (tR = 7.74 min) had a [M − H]– ion at m/z 781 and the diagnostic fragment ion at m/z 601 indicated the loss of a gallagyl moiety. Fragment ions at m/z 300 and m/z 299 were observed, due to the quinoidic and radical products of ellagic acid after the fission of the gallagic acid moiety [18]. The fragment ion at m/z 601 [M − H − 180]− indicated the loss of a hexose moiety. Therefore, compound 12 was tentatively identified as gallagyl-hexoside. Compound 20 (tR = 12.08 min) had a [M − H]– ion at m/z 933 with a daughter ion of m/z 781 equivalent to the [M − H]– ion of compound 12. The release of this ion was caused by the loss of a galloyl moiety. The typical fragment ions at m/z 601, m/z 300 and m/z 299 of compound 12 were all observed for compound 20. Thus, compound 20 was tentatively identified as galloyl-gallagyl-hexoside.
Compounds 28 (tR = 15.39 min) and 60 (tR = 27.68 min) had the identical [M − H]– ions at m/z 951. Compound 28 showed fragment ions at m/z 907 [M − H − CO2]− and m/z 783 [M − H − CO2 − 124]−, indicating the existence of a valoneoyl group [43]. Further fragment ions at m/z 605 [M − H − CO2 − HHDP]−, m/z 481 (HHDP-hexoside) and m/z 301 suggested that compound 28 was HHDP-valoneoyl glucoside. Compound 60 gave dehydrated fragment ions at m/z 933, m/z 915 and m/z 897, and showed additional loss of ellagic acid fragment ions at m/z 631 and m/z 613. Therefore, based on these fragmentation patterns and by comparison to literature [18], compound 60 was tentatively identified as galloyl-HHDP-DHHDP (dehydrohexahydroxydiphenoyl) -hexoside.
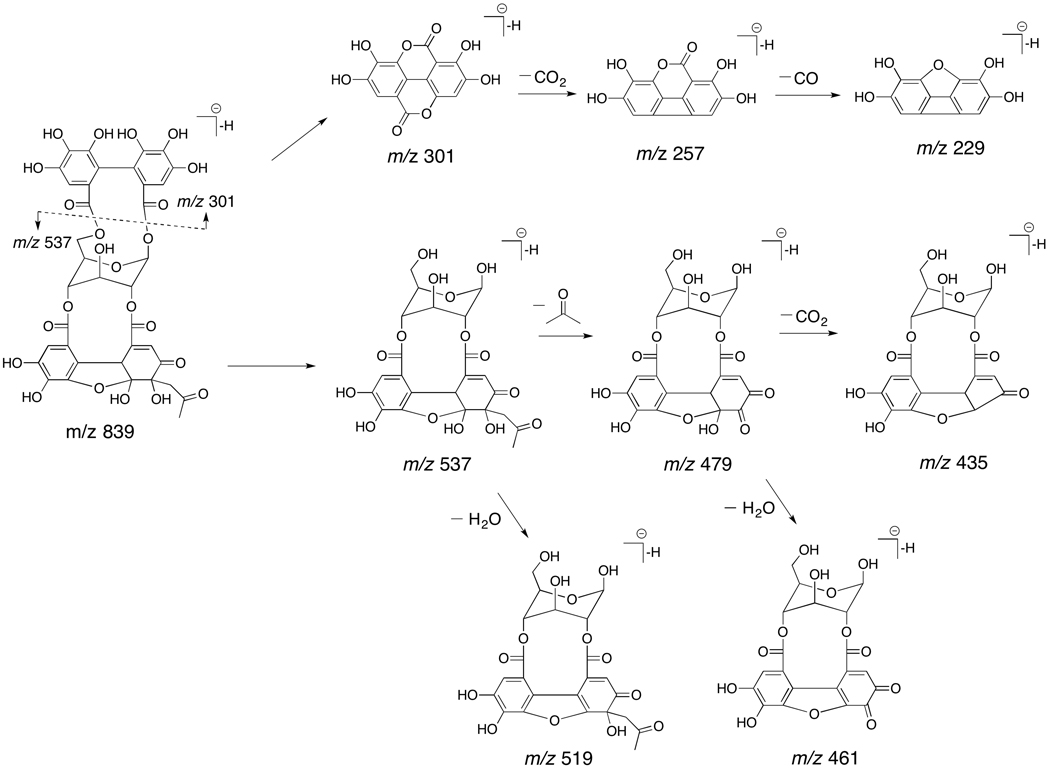
Compound 48 (tR = 21.00 min) was tentatively identified as pomellatannin, a new tannin previously isolated and identified (by NMR) from Pomella®) [13], due to its quasimolecular ion at m/z 839 and MS/MS fragment ions. Figure 2 shows the proposed fragmentation pathways of pomellatannin. In its MS/MS spectra, fragment ions at m/z 537, 519, 479, 461, 435, 301, 257 and 229 were observed. The diagnostic product ions at m/z 301, 257 and 229 confirmed the existence of the HHDP group. The MS/MS ions at m/z 537, 479 and 435 were produced by the subsequent losses of a HHDP group (302 Da), an acetone (58 Da), and carbon dioxide (44 Da) from the precursor ion at m/z 839. The fragment ions at m/z 519 and 461 were dehydrated products.
Figure 2.
The proposed fragmentation pathways of pomellatannin.
Compound 71 (tR = 43.33 min) was tentatively identified as punicatannin A/B, two new compounds previously isolated and identified (by NMR) from pomegranate flowers [40]. These are two stereoisomers which cannot be distinguished by mass spectrometry and therefore, compound 71 was identified as punicatannin A/B due to its quasimolecular ion at m/z 997 and the MS/MS fragment ions. Figure 3 shows the proposed fragmentation pathways of punicatannin A/B. In its MS/MS spectra, fragment ions at m/z 845, 827, 695, 615, 525, 463, 301, 257 and 229 can be observed. The diagnostic product ions at m/z 301, 257 and 229 clearly supported the existence of the HHDP group. The MS/MS ions at m/z 695 and 525 were produced by the subsequent losses of a HHDP group (302 Da), and a gallic acid (170 Da) from the precursor ion at m/z 997. The MS/MS ions at m/z 615 and 463 were produced by the subsequent losses of a benzofuran carboxylic acid moiety (382 Da), and a galloyl moiety (152 Da) from the precursor ion at m/z 997. The MS/MS ions at m/z 845, 827 and 381 were produced by loss of a galloyl, gallic acid and galloyl-HHDP-hexosyl moiety, respectively, from the precursor ion at m/z 997.
Figure 3.
The proposed fragmentation pathways of punicatannin A/B.
3.3. Characterization of other ellagic acid derivatives
Compounds 55 (tR = 23.14 min), and 57 (tR = 24.25 min) all had the same [M − H]– ions at m/z 463, with molecular formula of C20H16O13 provided by TOF-MS. Their MS/MS fragmentation ions at m/z 301, 300, 257, and 229 indicated the existence of an ellagic acid moiety and the loss of a hexosyl moiety. Thus, compounds 55 and 57 were tentatively identified as ellagic acid-hexosides. Compounds 61 (tR = 29.16 min), and 63 (tR = 30.45 min) had the same [M − H]– ions at m/z 433, with a molecular formula of C19H14O12 provided by TOF-MS. The MS/MS fragmentation ions at m/z 301, 300, 257 and 229 indicated the existence of ellagic acid moiety and the loss of a pentosyl moiety. Thus, compounds 61 and 63 were tentatively identified as ellagic acid-pentosides. Compounds 66 (tR = 31.05 min) had a [M − H]– ion at m/z 447, with a molecular formula of C20H16O12 provided by TOF-MS. The MS/MS fragmentation ions at m/z 301, 300, 257, and 229 indicated the existence of an ellagic acid moiety and the loss of deoxyhexosyl moiety. Thus, compound 66 was tentatively identified as ellagic acid-deoxyhexoside.
3.4. Characterization of other phenolic compounds
Compound 10 (tR = 7.15 min) exhibited an [M − H]– ion at m/z 169.0155, with a molecular formula of C7H6O5 provided by TOF-MS. In the MS/MS spectra, it gave a characteristic product ion at m/z 125 due to the loss of CO2 (44 Da). Thus, compound 10 was tentatively identified as gallic acid. Compound 41 (tR = 18.57 min) was tentatively identified as methyl-gallic acid due to the [M − H]– ion at m/z 183 and fragment ions at m/z 168 [M – H – •CH3]– [44], m/z 140 [M – H – •CH3 – CO]– and m/z 124 [M – H – •CH3 – CO2]–. Compound 67 (tR = 31.82 min) had a [M − H]– ion at m/z 300.9986, with a molecular formula of C14H6O8 provided by TOF-MS. In its MS/MS spectrum, the characteristic fragment ions at m/z 257, 229, 201 and 185 were observed, which is coincident with ellagic acid [18]. Therefore, compound 67 was identified as ellagic acid.
Compound 45 (tR = 19.43 min) had a [M − H]– ion at m/z 291.0152, with a molecular formula of C13H8O8 provided by TOF-MS. It exhibited a decarboxylation fragment ion at m/z 247 [M − H − 44]–. According to the literature, it was tentatively identified as brevifolin carboxylic acid [14]. Compounds 23 (tR = 14.25 min) and 53 (tR = 22.68 min) had the same [M − H]– ions at m/z 469. By comparison to the literature [19], these two compounds were tentatively identified as isomers of valoneic acid bilactone. Their fragment ion at m/z 425 and m/z 407 were produced due to sequential decarboxylation [M − H − 44]– and dehydration of valoneic acid bilactone.
Compound 21 (tR = 12.21 min) had a [M − H]– ion at m/z 305 and was tentatively identified as gallocatechin/epigallocatechin by comparing the typical fragment ions at m/z 261, 221, 179, 167, 165, 137, 125 and 109 with the reported of diastereoisomers of gallocatechin (gallocatechin and epigallocatechin) [45].
3.5. LC-TOF-MS/MS comparison of pomegranate flower and fruit extracts
As shown in Table 1, LC-TOF-MS/MS analyses revealed that the pomegranate flower extract was very similar to the pomegranate fruit extract and contained the vast majority of the identified compounds except for compounds 8, 12, 13, 15, 16, 17, 20, 21, 28, 42, 43, 48, 58, and 66. Notably, the pomegranate fruit extract contained tannin-glucuronides which were not detected in the pomegranate flower extract. In addition, the pomegranate fruit extract contained only two punicalagin isomers while four punicalagin isomers were detected in the flower extract. Lastly, the previously reported new compounds, pomellatannin [13] and punicatannins A/B [40], were identified as unique chemical markers in the pomegranate fruit and flower extracts, respectively. However, further studies using a larger number of samples of these respective extracts, produced under different conditions, to confirm the presence/absence of these aforementioned compounds, are warranted.
4. CONCLUDING REMARKS
In summary, a reliable method employing LC-TOF-MS/MS was developed for the unequivocal identification of phenolic compounds in a commercial pomegranate fruit extract (Pomella®) and a pomegranate flower extract. The data accumulated from the current study will aid in the quality control, authentication, and standardization of these botanical ingredients to evaluate their potential health benefits in planned pre-clinical and clinical studies which will be pursued in the future. In addition, the phenolic characterization, using the LC-TOF-MS/MS methods developed herein, of various commercial pomegranate botanical dietary supplements, obtained from different manufacturers, will be pursued by our group in the future.
ACKNOWLEDGEMENTS
Research reported in this publication was made possible by the use of equipment and services available through the RI-INBRE Centralized Research Core Facility which is supported by the Institutional Development Award (IDeA) Network for Biomedical Research Excellence from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM103430.
Non-standard abbreviations:
- HHDP
hexahydroxydiphenoyl
- DHHDP
dehydrohexahydroxydiphenoyl
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors have declared no conflicts of interest.
REFERENCES
- [1].Dwyer JT, Coates PM, Smith MJ, Dietary supplements: regulatory challenges and research resources. Nutrients 2018, 10, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Duan S, Qi W, Zhang S, Huang K, Yuan D, Ultra high performance liquid chromatography coupled with electrospray ionization/quadrupole time-of-flight mass spectrometry for the rapid analysis of constituents in the traditional Chinese medicine formula Wu Ji Bai Feng Pill. J. Sep. Sci 2017, 40, 3977–3986. [DOI] [PubMed] [Google Scholar]
- [3].Liu L, Zhang J, Zheng B, Guan Y, Wang L, Chen L, Cai W, Rapid characterization of chlorogenic acids in Duhaldea nervosa based on ultra-high-performance liquid chromatography-linear trap quadrupole-Orbitrap-mass spectrometry and mass spectral trees similarity filter technique. J. Sep. Sci 2018, 41, 1764–1774. [DOI] [PubMed] [Google Scholar]
- [4].Sommella E, Pagano F, Salviati E, Chieppa M, Bertamino A, Manfra M, Sala M, Novellino E, Campiglia P, Chemical profiling of bioactive constituents in hop cones and pellets extracts by online comprehensive two-dimensional liquid chromatography with tandem mass spectrometry and direct infusion Fourier transform ion cyclotron resonance mass spectrometry. J. Sep. Sci 2018, 41, 1548–1557. [DOI] [PubMed] [Google Scholar]
- [5].Wang T-M, Liu J, Yi T, Zhai Y-J, Zhang H, Chen H-B, Cai S-Q, Kang T-G, Zhao Z-Z, Multiconstituent identification in root, branch, and leaf extracts of Juglans mandshurica using ultra high performance liquid chromatography with quadrupole time-of-flight mass spectrometry. J. Sep. Sci 2017, 40, 3440–3452. [DOI] [PubMed] [Google Scholar]
- [6].BenSaad LA, Kim KH, Quah CC, Kim WR, Shahimi M, Anti-inflammatory potential of ellagic acid, gallic acid and punicalagin A&B isolated from Punica granatum. BMC Complement. Altern. Med 2017, 17, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cardellina JH, Blumenthal M, Adulteration of pomegranate products-A review of the evidence. HerbalGram 2016, 112, 62–69. [Google Scholar]
- [8].Jasuja ND, Saxena R, Chandra S, Sharma R, Pharmacological characterization and beneficial uses of Punica granatum. Asian J. Plant Sci 2012, 11, 251–267. [Google Scholar]
- [9].Jurenka JS, Therapeutic applications of pomegranate (Punica granatum L.): a review. Altern. Med. Rev 2008, 13, 128–144. [PubMed] [Google Scholar]
- [10].Katz SR, Newman RA, Lansky EP, Punica granatum: Heuristic treatment for diabetes mellitus. J. Med. Food 2007, 10, 213–217. [DOI] [PubMed] [Google Scholar]
- [11].Liu C, Cai D, Zhang L, Tang W, Yan R, Guo H, Chen X, Identification of hydrolyzable tannins (punicalagin, punicalin and geraniin) as novel inhibitors of hepatitis B virus covalently closed circular DNA. Antiviral Res. 2016, 134, 97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Miguel MG, Neves MA, Antunus MD, Pomegranate (Punica granatum L.): A medicinal plant with myriad biological properties – A short review. J. Med. Plants Res 2010, 4, 2836–2847. [Google Scholar]
- [13].Yuan T, Ma H, Liu W, Niesen DB, Shah N, Crews R, Rose KN, Vattem DA, Seeram NP, Pomegranate’s neuroprotective effects against Alzheimer’s Disease are mediated by urolithins, its ellagitannin-gut microbial derived metabolites. ACS Chem. Neurosci 2016, 7, 26–33. [DOI] [PubMed] [Google Scholar]
- [14].Huang TH, Peng G, Kota BP, Li GQ, Yamahara J, Roufogalis BD, Li Y, Anti-diabetic action of Punica granatum flower extract: activation of PPAR-gamma and identification of an active component. Toxicol. Appl. Pharmacol 2005, 207, 160–169. [DOI] [PubMed] [Google Scholar]
- [15].Abdulla R, Mansur S, Lai H, Ubul A, Sun G, Huang G, Aisa HA, Qualitative analysis of polyphenols in macroporous resin pretreated pomegranate husk extract by HPLC-QTOF-MS. Phytochem. Anal 2017, 28, 465–473. [DOI] [PubMed] [Google Scholar]
- [16].Ambigaipalan P, de Camargo AC, Shahidi F, Phenolic compounds of pomegranate byproducts (Outer Skin, Mesocarp, Divider Membrane) and Their Antioxidant Activities. J. Agric. Food Chem 2016, 64, 6584–6604. [DOI] [PubMed] [Google Scholar]
- [17].Ambigaipalan P, de Camargo AC, Shahidi F, Identification of phenolic antioxidants and bioactives of pomegranate seeds following juice extraction using HPLC-DAD-ESI-MS(n). Food Chem. 2017, 221, 1883–1894. [DOI] [PubMed] [Google Scholar]
- [18].Fischer UA, Carle R, Kammerer DR, Identification and quantification of phenolic compounds from pomegranate (Punica granatum L.) peel, mesocarp, aril and differently produced juices by HPLC-DAD-ESI/MS(n). Food Chem. 2011, 127, 807–821. [DOI] [PubMed] [Google Scholar]
- [19].Garcia-Villalba R, Espin JC, Aaby K, Alasalvar C, Heinonen M, Jacobs G, Voorspoels S, Koivumaki T, Kroon PA, Pelvan E, Saha S, Tomas-Barberan FA, Validated method for the characterization and quantification of extractable and nonextractable ellagitannins after acid hydrolysis in pomegranate fruits, juices, and extracts. J. Agric. Food Chem 2015, 63, 6555–6566. [DOI] [PubMed] [Google Scholar]
- [20].Mena P, Calani L, Dall’Asta C, Galaverna G, Garcia-Viguera C, Bruni R, Crozier A, Del Rio D, Rapid and comprehensive evaluation of (poly)phenolic compounds in pomegranate (Punica granatum L.) juice by UHPLC-MSn. Molecules 2012, 17, 14821–14840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Young JE, Pan Z, Teh HE, Menon V, Modereger B, Pesek JJ, Matyska MT, Dao L, Takeoka G, Phenolic composition of pomegranate peel extracts using an liquid chromatography-mass spectrometry approach with silica hydride columns. J. Sep. Sci 2017, 40, 1449–1456. [DOI] [PubMed] [Google Scholar]
- [22].Pomegranate Laboratory Guidance Document Issued by ABC-AHP-NCNPR Botanical Adulterants Prevention Program http://cms.herbalgram.org/BAP/LGD/PomegranateLabGuidanceDocument.html?ts=1524491933&signature=401360847083cf82ab4d8dfcc8457d8e&ts=1524539882&signature=f1c1c910771f41ef760947d5c933c211 (accessed May 1, 2018).
- [23].Zhang H, Zhao S, Zhang L, Han B, Yao X, Chen W, Hu Y, Preparation of ellagic acid molecularly imprinted polymeric microspheres based on distillation-precipitation polymerization for the efficient purification of a crude extract. J. Sep. Sci 2016, 39, 3098–3104. [DOI] [PubMed] [Google Scholar]
- [24].Ahmed AH, Subaiea GM, Eid A, Li L, Seeram NP, Zawia NH, Pomegranate extract modulates processing of amyloid-beta precursor protein in an aged Alzheimer’s disease animal model. Curr. Alzheimer. Res 2014, 11, 834–843. [PubMed] [Google Scholar]
- [25].Bhadbhade SJ, Acharya AB, Rodrigues SV, Thakur SL, The antiplaque efficacy of pomegranate mouthrinse. Quintessence Int. 2011, 42, 29–36. [PubMed] [Google Scholar]
- [26].Dai Z, Nair V, Khan M, Ciolino HP, Pomegranate extract inhibits the proliferation and viability of MMTV-Wnt-1 mouse mammary cancer stem cells in vitro. Oncol. Rep 2010, 24, 1087–1091. [PubMed] [Google Scholar]
- [27].DaSilva NA, Nahar PP, Ma H, Eid A, Wei Z, Meschwitz S, Zawia NH, Slitt AL, Seeram NP, Pomegranate ellagitannin-gut microbial-derived metabolites, urolithins, inhibit neuroinflammation in vitro. Nutr. Neurosci DOI: 10.1080/1028415X.2017.1360558. [DOI] [PubMed] [Google Scholar]
- [28].DiSilvestro RA, DiSilvestro DJ, DiSilvestro DJ, Pomegranate extract mouth rinsing effects on saliva measures relevant to gingivitis risk. Phytother. Res 2009, 23, 1123–1127. [DOI] [PubMed] [Google Scholar]
- [29].Goyal R, Nagtilak S, Thawani V, Pathania M, Jindal S, An antioxidative effect of Punica granatum (pomegranate) on biochemical parameters in patients with myocardial infarction: a double blind placebo controlled trial. Eur. J. Biomed. Pharm. Sci 2016, 3, 662–667. [Google Scholar]
- [30].Goyal R, Thawani V, Nagtilak S, Pathania M, Jindal S, Antioxidative effect of Punica granatum (pomegranate) on biochemical parameters in patients with diabetes mellitus (type 2) and myocardial infarction: a double blind placebo controlled trial. Int. J. Adv. Res 2016, 4, 857–864. [Google Scholar]
- [31].Gumus M, Tekin R, Firat U, Onder A, Kapan M, Boyuk A, Aldemir M, Kilinc C, The effects of pomegranate on bacterial translocation in rats with obstructive jaundice. Eur. Rev. Med. Pharmacol. Sci 2013, 17, 1488–1494. [PubMed] [Google Scholar]
- [32].Jean-Gilles D, Li L, Vaidyanathan VG, King R, Cho B, Worthen DR, Chichester CO 3rd, Seeram NP, Inhibitory effects of polyphenol punicalagin on type-II collagen degradation in vitro and inflammation in vivo. Chem. Biol. Interact 2013, 205, 90–99. [DOI] [PubMed] [Google Scholar]
- [33].Liu W, Ma H, DaSilva NA, Rose KN, Johnson SL, Zhang L, Wan C, Dain JA, Seeram NP, Development of a neuroprotective potential algorithm for medicinal plants. Neurochem. Int 2016, 100, 164–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Liu W, Ma H, Frost L, Yuan T, Dain JA, Seeram NP, Pomegranate phenolics inhibit formation of advanced glycation endproducts by scavenging reactive carbonyl species. Food Funct. 2014, 5, 2996–3004. [DOI] [PubMed] [Google Scholar]
- [35].Mertens-Talcott SU, Jilma-Stohlawetz P, Rios J, Hingorani L, Derendorf H, Absorption, metabolism, and antioxidant effects of pomegranate (Punica granatum L.) polyphenols after ingestion of a standardized extract in healthy human volunteers. J. Agric. Food Chem 2006, 54, 8956–8961. [DOI] [PubMed] [Google Scholar]
- [36].Molva C, Baysal AH, Evaluation of bioactivity of pomegranate fruit extract against Alicyclobacillus acidoterrestris DSM 3922 vegetative cells and spores in apple juice. LWT - Food Sci. Tech 2015, 62, 989–995. [Google Scholar]
- [37].Nair V, Dai Z, Khan M, Ciolino HP, Pomegranate extract induces cell cycle arrest and alters cellular phenotype of human pancreatic cancer cells. Anticancer Res. 2011, 31, 2699–2704. [PubMed] [Google Scholar]
- [38].Pacheco-Palencia LA, Noratto G, Hingorani L, Talcott ST, Mertens-Talcott SU, Protective effects of standardized pomegranate (Punica granatum L.) polyphenolic extract in ultraviolet-irradiated human skin fibroblasts. J. Agric. Food. Chem 2008, 56, 8434–8441. [DOI] [PubMed] [Google Scholar]
- [39].Patel C, Dadhaniya P, Hingorani L, Soni MG, Safety assessment of pomegranate fruit extract: acute and subchronic toxicity studies. Food Chem. Toxicol 2008, 46, 2728–2735. [DOI] [PubMed] [Google Scholar]
- [40].Yuan T, Ding Y, Wan C, Li L, Xu J, Liu K, Slitt A, Ferreira D, Khan IA, Seeram NP, Antidiabetic ellagitannins from pomegranate flowers: inhibition of alpha-glucosidase and lipogenic gene expression. Org. Lett 2012, 14, 5358–5361. [DOI] [PubMed] [Google Scholar]
- [41].Yuan T, Wan C, Ma H, Seeram NP, New phenolics from the flowers of Punica granatum and their in vitro alpha-glucosidase inhibitory activities. Planta Med. 2013, 79, 1674–1679. [DOI] [PubMed] [Google Scholar]
- [42].Gil MI, Tomas-Barberan FA, Hess-Pierce B, Holcroft DM, Kader AA, Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J. Agric. Food. Chem 2000, 48, 4581–4589. [DOI] [PubMed] [Google Scholar]
- [43].Gu D, Yang Y, Bakri M, Chen Q, Xin X, Aisa HA, A LC/QTOF-MS/MS application to investigate chemical compositions in a fraction with protein tyrosine phosphatase 1B inhibitory activity from Rosa rugosa flowers. Phytochem. Anal 2013, 24, 661–670. [DOI] [PubMed] [Google Scholar]
- [44].Ersan S, Guclu Ustundag O, Carle R, Schweiggert RM, Identification of phenolic compounds in red and green pistachio (Pistacia vera L.) hulls (exo- and mesocarp) by HPLC-DAD-ESI-(HR)-MS(n). J. Agric. Food. Chem 2016, 64, 5334–5344. [DOI] [PubMed] [Google Scholar]
- [45].Hamed AI, Al-Ayed AS, Moldoch J, Piacente S, Oleszek W, Stochmal A, Profiles analysis of proanthocyanidins in the argun nut (Medemia argun--an ancient Egyptian palm) by LC-ESI-MS/MS. J. Mass Spectrom 2014, 49, 306–315. [DOI] [PubMed] [Google Scholar]