CONSPECTUS
While lipids were first appreciated as a critical hydrophobic barrier, our understanding of their roles at the cellular and organismal levels continues to grow. Not only are they important independent operators, providing a platform for both static and dynamic organization and communication within the cell, they also exert significant effects via the chemical modification of proteins. Addition of a lipid post-translational modification (PTM) alters protein hydrophobicity and behavior, with distinct consequences for subcellular trafficking, localization, intra- and intermolecular interactions, and stability. One of the most abundant and widespread protein lipidation events is S-acylation, installation of a long-chain lipid to the thiol of a cysteine side chain through a thioester linkage. S-acylation is often referred to as S-palmitoylation, due to the prevalence of palmitate as the lipid modification. Unlike many lipid PTMs, S-acylation is enzymatically reversible, enabling the cell to tune proteome-wide properties through dynamic alterations in protein lipidation status. While much has been uncovered about the molecular effects of S-acylation and its implications for physiology, current biochemical and chemical methods only assess substrate lipidation levels or steady-state levels of enzyme activity. Yet, the writer protein acyl transferases (PATs) and eraser acyl protein thioesterases (APTs) are dynamically active, responsible for sometimes-rapid changes in S-palmitoylation status of target proteins. Thus, to understand the full scope, significance, and subtlety of S-deacylation and its regulation in the cell, it is necessary to observe the timing and cellular geography of regulatory enzyme activities.
In this Account, we review the chemical tools developed by our group to selectively visualize and perturb the activity of APTs in live cells, highlighting the biological insights gained from their application. To visualize APT activity, we masked fluorogenic molecules with thioacylated, peptide-based APT substrate mimetics; APT activity and thus thiol deprotection releases a fluorescent product in the turn-on depalmitoylation probes (DPPs), while in ratiometric depalmitoylation probes (RDPs), the emission of the parent fluorophore is altered. Application of these probes in live cells reveals that APT activity is sensitive to cell signaling events and metabolic disturbances. Additionally, as indicated above, the location of regulatory enzymes is critical in lipid signaling and one organelle of particular interest - due to its role in maintaining cellular homeostasis and its legion of lipidated proteins - is the mitochondria. Therefore, we developed a class of spatially-constrained mitoDPPs to visualize mitochondrial APT activity, as well as a selective inhibitor of mitochondrial deacylation activity, mitoFP. With these tools, we identify two mitochondrial S-depalmitoylases and connect mitochondrial S-depalmitoylation to redox buffering capacity. Moreover, some of the changes in activity observed are specific to the mitochondria, confirming spatial as well as temporal regulation of eraser protein activity. Overall, this chemical toolkit for S-depalmitoylase activity - imaging reagents and a targeted inhibitor - will continue to illuminate the regulatory mechanisms and roles of S-depalmitoylation within the complex homeostatic networks of the cell.
Graphical Abstract

INTRODUCTION
United by a hydrophobic nature and an indispensability across biological systems, lipids are nonetheless remarkably diverse in form and versatile in function. Enclosing both cells and their internal organelles, lipids - phospholipids, interspersed with cholesterol and glycolipids - compartmentalize cellular operations and provide a fluid organizational interface, while also enabling the regulation and maintenance of a homeostatic environment. Intracellularly, lipids - mainly triglycerides and steryl esters - exist in lipid droplets and provide pools of mobilizable energy, as epitomized macroscopically by the energy reservoirs distinct in humped ungulates1. In addition, lipids are also dynamic and potent secondary signaling messengers; prostaglandins, endocannabinoids, leukotrienes, phosphatidylinositol-phosphates, and diacylglycerol are all lipid-derived, transient signaling molecules2.
In their above roles, lipids frequently interact with proteins, assisting in structure maintenance, organelle targeting, and signaling complex assembly. However, lipids can also directly modulate protein function, namely as post-translational modifications (PTMs). The resulting increase in target hydrophobicity has significant functional consequences, including, but not limited to, changes in membrane association, protein-protein interactions, subcellular localization, protein stability, and enzyme activity3. At least six types of lipids are covalently tethered to proteins via nucleophilic moieties of at least five amino acids4. Such modifications commonly utilize thioether (cysteine S-prenylation), amide (glycine N-myristoylation), and ester (serine O-octanoylation) linkages; generally, the resultant bond stability renders them irreversible. It was only recently discovered that the ester-linked Wnt serine O-palmitoleoylation is enzymatically reversible5. Similarly, a growing body of evidence suggests that certain HDAC and Sirtuin family members possess Nε-lysine deacylase activity3. In contrast, the well-known lability and enzymatic reversibility of the thioester linkage of cysteine S-palmitoylation makes it a key regulator of protein function, with increasingly known implications for physiology and disease6–8. Our lab has initiated a program aimed at developing and applying chemical technologies to discover processes regulating S-palmitoylation.
This Account summarizes our progress in developing chemical tools, from activity-based imaging reagents to a spatially-constrained inhibitor, for the study of the eraser enzymes of S-palmitoylation, as well as the biological discoveries, including enzyme discovery, enzyme (re)annotation, and temporally dynamic APT activities, resulting from their application.
PROTEIN CYSTEINE ACYLATION (S-PALMITOYLATION)
Within the family of protein lipid modifications, S-palmitoylation - the incorporation of a long chain fatty acid on a cysteine residue (commonly the 16C palmitate, though C14 and C18:0/1/2 modifications have also been observed) - is conspicuous for the enzymatic regulation of both addition and removal. Protein acyltransferases (PATs) catalyze acyl group addition, while acyl protein thioesterases (APTs) cleave the resultant thioester bond. DHHC-domain containing PATs, of which 23 are known in mammalian systems, and APTs, members of the metabolic serine hydrolase (mSH) superfamily and including APT1/2, PPT1, and ABHD17A/B/C, moderate the S-palmitoylation and thus subcellular trafficking, localization, and stability of numerous soluble and integral membrane proteins9–13. Recent reviews present comprehensive lists of palmitoylated proteins, from scaffolding and signaling proteins to ion channels and receptors, highlighting the broad physiological consequences of S-palmitoylation in areas including synaptic transmission, the innate immune response, GPCR and tyrosine kinase signaling, and transcription factor function (Figure 1)3,4. Particularly striking in these enumerations is how crucial regulated cycles of acylation and deacylation are for target function and the maintenance of cellular homeostasis. For example, the immuno-metabolic glycoprotein CD36, a scavenger receptor and fatty acid translocase, requires palmitoylation by DHHC4 and DHHC5 for its transport to and continued presence at the plasma membrane, respectively14. Disruption of CD36 palmitoylation alters fatty acid uptake, JNK/NF-κB signaling, and even AMPK-activated fatty acid β-oxidation, with implications for non-alcoholic steatohepatitis14,15. Treatment with palmostatin B (PalmB), an S-depalmitoylase inhibitor, can mitigate the loss of membrane-localized CD36 observed in DHHC5 knockdown cells, underscoring the importance of both writer and eraser activity in modulating S-palmitoylation-dependent protein function14.
Figure 1.

Overview of S-palmitoylation and its broad relevance in cell and organismal physiology.
Our understanding of the function and regulation of protein S-palmitoylation has progressed in parallel with the development of chemical and biochemical methods to assay and perturb protein lipidation (Figure 2). Although posited in 1979 and validated in cell culture in 1980, it was in the twenty-first century, with the advent of chemical and biochemical methods to assess protein S-palmitoylation, that knowledge acquisition in the field accelerated16,17. With acyl-biotin exchange (ABE), which exploits the lability of the thioester bond to substitute an enrichment handle for the acyl group, substrate palmitoylation can be observed in a cysteine-specific manner, while the stoichiometry of acylation can be assessed with a homologous method, acyl-PEG exchange (APE)18,19. Metabolic labeling, once requiring radiolabeled fatty acids and immunoprecipitation, now utilizes fatty acid analogues chemically functionalized with ω-alkynes or azides suitable for biorthogonal Cu(I)-catalyzed “click” chemistry20–22. This offers a more sensitive, fatty acid-specific method for detecting acylated proteins23. Small molecule inhibitors disrupt the steady-state acylation/deacylation cycle, allowing assessment of target phenotypes. Commonly used as a pan-DHHC inhibitor is 2-bromopalmitate (2BP), although its application (and any ensuring observations) are complicated by evidence that it additionally inhibits lipid metabolism and, by some accounts, the eraser APTs.24–27 Palmostatin B/M target APTs and serine hydrolases more broadly, while inhibitors specific for two principle S-depalmitoylases, APT1 and APT2, enable greater precision24–26. Activity-based protein profiling (ABPP), in which target enzymes are labeled in an active-site directed manner, has been applied to the mSH family (which encompasses APTs) and to the PATs in order to broadly profile the enzymes and their functions across cell states28–30. However, S-palmitoylation is dynamic, with an enzymatically-regulated, sometimes-rapid flux in the level of modification. The existing proteomic methods only provide information about total populations at a single, defined point, offering no insight into the timing and cellular geography of regulatory writer and eraser activities.
Figure 2.

Chronological progression of chemical and biochemical methods to assess and probe protein lipidation in the decades since its initial observation.
APT ACTIVITY-BASED IMAGING PROBES
The S-palmitoylation of a given protein - and its subsequent effects - is regulated by the balance of DHHC and APT activities. Understanding what in turn regulates these enzyme modulators of S-palmitoylation status is crucial for elucidating the function and consequences of dynamic S-palmitoylation in the broader cellular milieu. Lipidation-based changes in small molecule or peptide substrate trafficking can be used to monitor the global balance between S-palmitoylation and S-depalmitoylation activities, but cannot reveal independent regulatory mechanisms of writers and erasers31,32. Recent temporal analysis of S-palmitoylation using the lipase inhibitor hexadecylfluorophosphonate (HDFP) emphasizes the role of APTs in particular in regulating dynamic, rather than steady-state, S-palmitoylation33; controlled depalmitoylase activity is also critical for maintaining the native regulatory and signaling activities of substrates such as the scaffold protein Scribble and the GTPase Ras26,32,34. Towards the understanding of APT dynamic regulation, our group has developed a suite of chemical tools to selectively measure APT activities in live cells. Both our turn-on and ratiometric probes feature thioacylated, peptide-based APT substrates that convert thioesterase activity into an optical change in a pendant fluorophore.
Turn-on probes to visualize APT activity
We established depalmitoylation probes (DPPs) as a class of molecular imaging agents to monitor the level of S-deacylase activity in live cells (Figure 3). These enzyme-activated, fluorogenic probes feature a readily-modifiable rhodol-based parent fluorophore whose constitutive fluorescence is masked by a synthetic APT substrate - a peptide featuring a lipidated cysteine residue (Figure 3 and Table 1)35. S-deacylase activity generates a free thiol, and then thiol-based cleavage of the carbamate linker, promoted by a five-membered ring transition state, releases a fluorescent product - thereby providing a readout of S-deacylase activity (Figure 3). Our initial probe, DPP-1, demonstrated that a palmitoylated (C16) cysteine functioned effectively as both a masking group and an enzyme recognition moiety, with APT1/2 incubation producing fluorescent signal in vitro. However, the C16 lipid limited water solubility and therefore application in live cells; in DPP-2, substituting a C8 lipid chain improved solubility and utility in live cells. In DPP-5, inclusion of a succinylated piperazine further improved solubility and, in fact, enabled modification of the masking cysteine with the highly hydrophobic, but natural, C16 lipid substrate once again36. These paired improvements in solubility and enzyme specificity render DPP-5 an excellent tool for visualizing global S-depalmitoylase activity in vitro and in vivo. To generate the target-specific deacylase probe DPP-3, we masked the DPP-2 framework with a Cys-Lys group. This dipeptide mimics the APT1 substrate H-Ras37; thus, DPP-3 provides more sensitive visualization of APT1 versus APT2 activity in live cells.
Figure 3.

Overview of Depalmitoylation Probes (DPPs). (A) Schematic representing the mechanism of DPP fluorescence upon deacylase activity. Thiol acyl modification (R2), the peptide substrate (R1), or the xanthene scaffold (R3) can be varied (see Table 1) to modulate the physical and biological properties of DPPs. (B) In vitro characterization of DPP-2 (1 μM) in buffer (20 mM HEPES, 150 mM NaCl, pH 7.4) with or without 50 nM APT1 (λex 490/20 nm; λem 545/20 nm). (C) Incubation of HEK293T cells with DPP-2 (1 μM) results in a robust fluorescence signal that is abrogated by PalmB (5 μM), validating that DPPs respond to enzymatic S-deacylation in live cells. 50 μm scale bar shown.
Table 1.
Catalogue of current DPP and RDP family members.
| R1 | R2 | R3 | localization | in vitro? | in cellulo? | |
|---|---|---|---|---|---|---|
| DPP-1 | Me | −C15H31 |  |
———— | ✓ | × |
| DPP-2 | Me | −C7H15 |  |
cytoplasm | ✓ | ✓ |
| DPP-3 | LysC(O)OMe | −C7H15 |  |
cytoplasm | ✓ | ✓ |
| DPP-4 | Me | −C15H31 |  |
———— | ✓ | × |
| DPP-5 | Me | −C15H31 |  |
cytoplasm | ✓ | ✓ |
| mitoDPP-2 | Me | −C7H15 |  |
mitochondria | ✓ | ✓ |
| mitoDPP-3 | LysC(O)OMe | −C7H15 |  |
mitochondria | ✓ | ✓ |
| RDP-1 | Me | −C7H15 | ———— | cytoplasm | ✓ | ✓ |
| RDP-2 | LysC(O)OMe | −C7H15 | ———— | cytoplasm | ✓ | ✓ |
Ratiometric probes to visualize APT activity
As turn-on probes, DPPs can be challenging to use in heterogeneous samples, since fluorescent signal intensity reflects not only S-deacylase activity but also probe uptake and distribution. To address this, we developed ratiometric depalmitoylation probes (RDPs), whose aminocoumarin fluorophore’s emission is altered, rather than generated, by S-deacylase activity (Table 1)38. In RDP-1, an α/β unsaturated cyanoacetamide links the fluorophore to a S-octanoyl cysteine. Upon APT S-deacylation, intramolecular Michael addition by the free thiol disrupts conjugation of the π-system, blue-shifting the fluorophore emission (Figure 4). The ratio of deprotected (λem = 470 nm) to protected (λem = 575 nm) RDP-1 permits normalization of fluorescent signal to probe concentration; indeed, we successfully used RDP-1 to monitor S-deacylase activity in colon organoids, a variegated, three-dimensional ex vivo tissue model38. RDP-2, an RDP-1 analogue, possesses a C-terminal Lys residue akin to that of DPP-3, improving its engagement with APT1 over APT2 (Table 1). Thus, RDPs are capable of assaying APT activity more quantitatively in complex samples.
Figure 4.

Overview of Ratiometric Depalmitoylation Probes (RDPs). (A) Schematic of RDP design and the mechanism of blue-shifted emission upon deacylase activity. (B) UV-Vis absorption spectrum of 15 μM RDP-1 (see Table 1) and deacylated RDP-1 in buffer (20 mM HEPES, 150 mM NaCl, 0.1% Triton X-100, pH 7.4). (C) Cells loaded with RDP-1 (1 μM) after treatment with either DMSO carrier or PalmB (20 μM) and then analyzed by ratiometric imaging, demonstrating the blue-shift in RDP-1 fluorescent signal upon enzymatic S-deacylation in live cells. 20 μm scale bar shown.
CHEMICAL TOOLS TO PROBE MITOCHONDRIAL APTS
Recent expansion of the S-palmitoylome catalogue by proteomic-based approaches indicates that S-palmitoylated proteins are present across a range of cellular organelles, including the lysosome, nucleus, endoplasmic reticulum, and mitochondria7. Intriguingly, in the mitochondria, there are a suite of S-palmitoylated proteins known to be relevant in energy homeostasis, apoptosis, and redox signaling39–41. As it is generally accepted that the colocalization of DHHCs and APTs with their substrates partially regulates palmitoylation/depalmitoylation events, we sought to investigate if mitochondrial S-depalmitoylation is enzymatic, and if so, to identify the regulatory proteins involved.
Mitochondrial-targeted probes to visualize subcellular APT activity
Determining the presence of regulatory S-palmitoylation proteins in the mitochondria required DPPs that would report not only the level, but also the location, of S-depalmitoylase activity in live cells. Therefore, we developed a class of DPPs that are spatially constrained to the mitochondria, therefore reflecting S-depalmitoylase activity only in this compartment42,43. The first of these probes, mitoDPP-2 (Table 1), features the lipophilic cation triphenylphosphonium (TPP), linked to the rhodol via a piperazine moiety. The electron gradient across the inner mitochondrial membrane drives the delivery to and accumulation of TPP-linked compounds in the mitochondria44. Like its parent probe DPP-2, mitoDPP-2 has an S-octanoyl cysteine substrate (Table 1) and, upon APT activity, demonstrates an enhancement in fluorescence congruent with a shift in the equilibrium from the xanthene-like structure to the fully-conjugated lactone-open structure (Figure 3). When using mitoDPP-2 for live cell imaging, we observed robust deacylase activity in the mitochondria. Chemical inhibition of S-depalmitoylase activity with PalmB in both live cells and respiring mitochondria confirmed that mitoDPP-2 reports enzymatic activity, not non-specific hydrolysis of the probe thioester in the alkaline mitochondrial environment42. In our second mitochondrial-targeted DPP, mitoDPP-3, we again added a proximal lysine to slightly increase APT1 engagement (Table 1) and therefore assay putative APT1 activity in the mitochondria with greater sensitivity, as described below.
MitoFP: A mitochondrial-targeted APT inhibitor
After observing S-depalmitoylase activity in the mitochondria, we were keen to determine the protein substrates and functional consequences of S-depalmitoylase activity in this organelle. Pharmacological inhibition of APTs by the pan-depalmitoylase inhibitor PalmB is unsuitable for selective interrogation of mitochondrial S-depalmitoylation, as it inhibits S-depalmitoylation throughout the cell. Genetic manipulations, such as overexpression, knockdown, and knockout, are similarly non-selective, as they alter target protein levels throughout the cell, disrupting function globally not locally. We therefore developed a chemical tool to selectively perturb S-depalmitoylation in mitochondria: the spatially-constrained mitochondrial pan-APT inhibitor, mitoFP (Figure 5A). The reactivity of mitoFP relies on a serine-reactive electrophilic war-head, a fluorophosphonate group, present in known lipase inhibitor hexadecylfluorophosphonate (HDFP) (Figure 5A)45. Other key features include a C8 lipid, which is long enough to interact with lipid-binding mSHs, but short enough not to affect cellular permeability adversely35,42, and a TPP moiety for targeted delivery to the mitochondria. Live cell DPP imaging in two cell lines confirms that mitoFP inhibits mitochondrial APTs (Figure 5B,C) while the activity of cytosolic APTs remains unperturbed, even at concentrations as high as 5 μM (Figure 5D,E). Using mitoFP in conjunction with chemo-proteomic approaches46, we can now assess the effect(s) of altering the mitochondrial S-palmitoylome, identify targeted proteins, and ascertain the biological significance of mitochondrial APTs.
Figure 5.

Overview of mitoFP. (A) Chemical structure and salient features of mitoFP, a selective, mitochondrial-targeted APT inhibitor. (B) Fluorescence microscopy images of HepG2 cells demonstrating inhibition of mitochondrial APTs by mitoFP, as assessed by mitoDPP-2. (C) Quantification of mitoDPP-2 signal shown in (B). (D) Fluorescence microscopy images of HepG2 cells demonstrating no inhibition of cytosolic APTs by mitoFP, as assessed by DPP-2. (E) Quantification of DPP-2 signal shown in (D). 20 μm scale bar shown.
Discovery of mitochondrial APTs
With the development of chemical tools to selectively visualize and perturb S-depalmitoylation in the mitochondria, we can now explore the regulation and role of mitochondrial S-palmitoylation. Knowledge of mitochondrial S-palmitoylation is currently nascent; increased understanding of eraser dynamics and functional consequences will contribute to the growth of this field. Here we summarize our recent discovery of two mitochondrial S-depalmitoylases and our questions and progress in probing their function.
APT1 is a mitochondrial S-depalmitoylase
APT 1 is a S-depalmitoylase with known roles in cell division and signaling. Since its discovery in 1998, it has primarily been considered to be cytoplasmic11,47, with fluorescent protein or epitope-tagged APT1 indicating additional localization to the Golgi and plasma membranes37,48. However, fluorescence microscopy using mitoDPP-3 - our APTI-selective probe - with RNAi-mediated APT1 knockdown, along with immunofluorescence imaging and Western blotting of cytoplasmic and mitochondrial cellular fractions, establish that APT1 is in fact also localized to mitochondria; the dominant cytoplasmic localization observed previously is likely an artifact of bulky fluorescent tags42. Our finding of APT1 in the mitochondria marks it as the first identified regulator of mitochondrial S-depalmitoylation and redefines our perspective on its activity and function in the cell. Therefore, we have now begun to investigate the broader physiological role of its S-depalmitoylase activity in mitochondria and to identify novel protein substrates. Additionally, as known APT1 substrate proteins reside outside the mitochondria, it is intriguing to consider where and how pools of APT1 are maintained within cells3,4.
ABHD10 is a mitochondrial S-deacylase
Although APT1 is primarily localized to the mitochondria, it is not solely responsible for the totality of S-depalmitoylation activity reported by the mitoDPPs, hinting at the presence of other regulatory erasers42. As APTs are members of the mSH superfamily, we looked to this family to identify novel mitochondrial S-depalmitoylases49. From an in cellulo screen with DPP-2 and overexpressed mSH proteins emerged ABHD10, an α/β-hydrolase domain-containing protein that is putatively resident in the mitochondria50. At the time, there were no reports of its endogenous substrates, although it had been annotated to deglucuronidate mycophenolic acid acylglucuronide (AcMPAG) and probenecid acyl glucuronide (PRAG) in the human liver51,52. We used DPP-s and mitoDPP-2 for biochemical and fluorescence microscopy-based cell assays, respectively, to confirm that ABHD10 possesses S-deacylase activity (Figure 6A,B)53. For structurally informed insights into the function of ABHD10, we resolved the X-ray crystal structure (1.66 A) of the mouse protein (Figure 6C), which shares ~78% identity with its human counterpart. Comparison with APT1 crystal structure54 reveals structural similarities, such as a long hydrophobic pocket, but also that the active site serine residue in ABHD10 is less accessible53. This observation could potentially account for the lower catalytic turnover, relative to APT1, that was observed in vitro, despite the strong DPP signal with ABHD10 overexpression observed in cellulo (Figure 6A,B)53. Intriguingly, this lower catalytic turnover could also suggest a more expansive S-palmitoylation regulatory network, with adaptor proteins or other PTMs enhancing ABHD10 reactivity in the cellular environment. Finally, the discovery of ABHD10 as a mitochondrial S-depalmitoylase is certainly significant for cellular physiology, as we have shown that it regulates mitochondrial redox buffering capacity and annotated PRDX5 as its first natural target53.
Figure 6.

ABHD10 is a mitochondrial APT. (A) Fluorescence microscopy images of HEK293T cells demonstrating ABHD10 overexpression (OE) enhances mitoDPP-2 signal. Scale bar: 20 μm. (B) Quantification of mitoDPP-2 signal shown in (A). (C) Crystal structure of mitochondrial presequence-cleaved murine ABHD10, highlighting the catalytic triad (PDB: 6NY9).
DYNAMIC APT ACTIVITIES
It has been long-appreciated that cycles of acylation and deacylation govern the localization, sorting, and function of modified proteins - on timescales ranging from minutes to days - and that cell stimuli and physiological states can modulate the dynamics of palmitate turnover. However, without the ability to monitor enzyme activity, it was unclear how and if such dynamics were regulated at the S-depalmitoylase level. Our probes - DPPs and RDPs - reveal that APT activity is sensitive to cellular signaling events, evincing rapid dynamics in the face of extrinsic and intrinsic stimuli. Moreover, under some circumstances, the changes in activity observed are also spatially specific, confirming spatiotemporal regulation of eraser protein activity.
APTs respond to cell signaling
It is well-established that a host of proteins involved in cell signaling events are S-palmitoylated, with dynamic S-palmitoylation resulting from stimulation and occurring with the kinetics required for signal transduction. For example, stimulation of the β-adrenergic receptor results in an increase of Gas and Gai palmitoylation on a timescale congruent with cAMP production, while the activation of the Fas receptor sparks a rapid, transient increase in the palmitoylation of the Src family kinase, Lck, that parallels the activation timing of downstream effectors Zap70 and PLC-γ155,56. However, it is the small GTPase Ras that has one of the best-characterized dynamic palmitoylation cycles; if S-depalmitoylase activity contributes to signaling regulation, it would certainly be evident along the EGFR-Ras signaling pathway. Indeed, in serum-starved cells, which interestingly possess elevated basal S-depalmitoylase activity, stimulation with epidermal growth factor (EGF) results in a diminishment of DPP-3 signal and therefore an inhibition or relocalization of S-depalmitoylation activity (Figure 7A,B)35. S-depalmitoylase dynamics have been discovered along other signaling pathways as well; using DPP-3, Witze et al. observed that in Wnt5a-stimulated cells, the S-depalmitoylase activity of APT1 increased, reducing the palmitoylation of the downstream effector and cell adhesion protein MCAM57. Notably, this change in activity was shown to be concurrent with and dependent upon induction of APT1 phosphorylation - a clear example of PTM cross-talk and co-regulation. Thus, not only is S-palmitoylation clearly a signal-regulated modification, its regulation arises from modulation of S-depalmitoylase activity.
Figure 7.
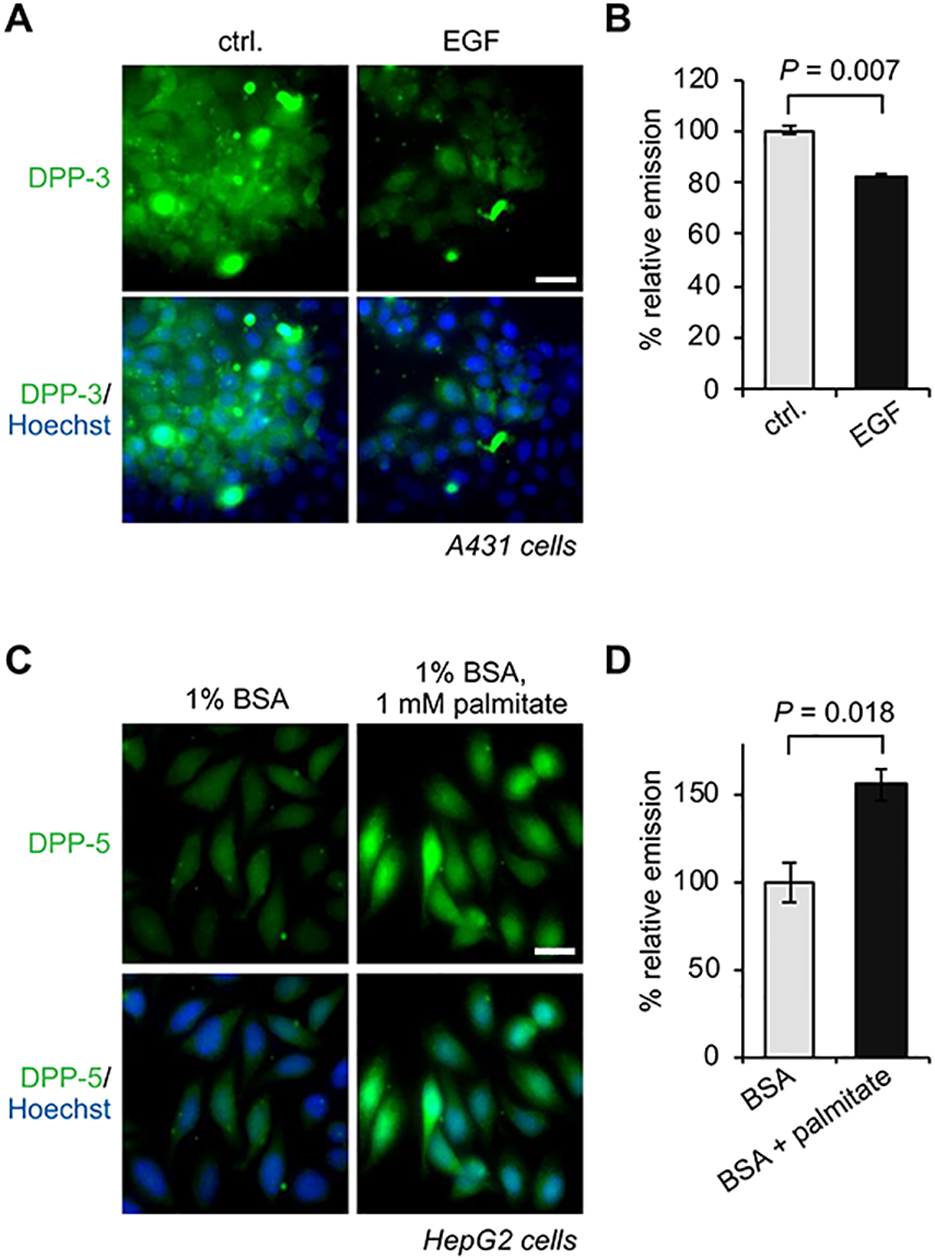
APT activities respond dynamically to growth factors and lipid stress. (A) Fluorescence microscopy images of A431 cells demonstrating EGF stimulation deactivates APTs, as assessed by DPP-3. 20 μm scale bar shown. (B) Quantification of DPP-3 signal shown in (A). (C) Fluorescence microscopy images of HepG2 cells demonstrating exogenous palmitate activates APTs, as assessed by DPP-5. 20 μm scale bar shown. (D) Quantification of DPP-5 signal shown in (C).
APTs respond to lipid homeostasis
Maintenance of lipid homeostasis is essential at both the cellular and organismal level. Imbalances in uptake, consumption, and degradation, as seen in obesity and metabolic syndrome, can result in high levels of circulating free fatty acids. In cell culture, this is modeled by the administration of a bolus of palmitate. Palmitate treatment is known to induce ER stress, which in turn has been correlated with overall increases in the level of S-palmitoylation58. The mechanisms for this effect and therefore regulation of S-palmitoylation regulatory proteins are not yet known. Using DPP-5, we found that APT activity is enhanced in response to the increase in cellular lipid levels (Figure 7C,B)36. In contrast, RDP-1 readout indicates a decrease in APT activity38. As these probes diverge in molecular structure and in lipid chain length (C16 and C8, respectively), they could be distributed differently in the cell; this difference in signal could then suggest that APT activity is spatially regulated. This observation also indicates that caution is needed when interpreting the directionality of changes as measured by activity probes, especially if spatial dynamics are a contributing mechanism. That is, if activity is increasing in one locale, it may be decreasing in another, and so the when and how of a measurement are critical considerations when describing activity dynamics. In addition to bolus treatment of palmitate, alterations in APT activity upon perturbation of local lipid pools mediated by various acyl-CoA thioesterase (ACOT) proteins further underscore the tightly controlled spatial regulation of S-depalmitoylation42.
SUMMARY and OUTLOOK
In the past two decades, there has been rapid progress in deciphering the consequences of S-palmitoylation at the molecular and cellular level in variety of eukaryotes, including microbes, plants, and animals7. This expansion of knowledge has been propelled by the development of methods to observe changes in substrate S-palmitoylation levels and visualize steady-state enzyme activity. However, S-palmitoylation is a highly dynamic lipid modification, and so to understand the nuances of its regulation, new approaches are required to directly assay its writer and eraser protein activity. In this Account, we reviewed the development of a chemical toolkit to investigate the erasers of S-palmitoylation, APTs, in live cells. Collectively, these probes - DPPs, RDPs, mitoDPPs, and mitoFP - enable a more discriminating view of APTs and allowed us to begin to discern the subcellular organization and geographic regulation of S-depalmitoylase activities.
Yet our understanding of the regulation of S-depalmitoylation and its biological consequences remains incomplete, particularly at the level of individual APTs. Addressing this gap will require rationally-designed activity-based probes and inhibitors to selectively target each APT. Amara et. al. recently unveiled a fluorescently quenched, S-palmitoylated peptide library-based high-throughput approach to screen peptide substrates of the parasite depalmitoylase TgPPT1 and human APT1/2 in vitro59. The resulting dataset reveals the biochemical specificities of the profiled APTs and could provide a starting point for targeted probe and inhibitor development. Additionally, connecting cell culture models to organismal physiology is critical for understanding the implications of S-palmitoylation. The current collection of green light-emitting DPP and RDP probes lack utility in murine models due to poor tissue penetration and relatively high background fluorescence from tissues. Therefore, we are currently expanding our DPP toolkit to include next-generation fluorescent probes with red-shifted and enhanced emission qualities for use in tissues.
Visualization of APT dynamics using DPPs and RDPs presents a step forward in integrating S-palmitoylation dynamics into the broader signaling and homeostatic networks of the cell. We are now working to detail and expound upon the molecular and mechanistic framework of dynamic S-depalmitoylation along cell signaling pathways relevant for human health and disease. The discovery of APT1 and ABHD10 as the first two members of the mitochondrial APT family, stemming from the application of mitoDPPs and mitoFP, has opened new avenues to explore relevance of S-palmitoylation in mitochondrial biology. Although incipient, the potential for connections between lipid signaling and the metabolically-active mitochondria is intriguing and worthy of deeper investigation. Finally, our work to-date has focused entirely on the erasers of S-palmitoylation, the APTs - but full understanding of S-palmitoylation homeostasis requires methods to monitor and perturb both directions of the dynamic equilibrium. Indeed, we anticipate that development of better chemical technologies for the study of DHHCs will be the next thrust of our research program.
Acknowledgements
This work was supported by the University of Chicago and the National Institute of General Medical Sciences of the National Institutes of Health (R35 GM119840). We thank Dr. Somayeh Ahmadiantehrani for assistance editing this manuscript.
Biographies
Saara-Anne Azizi received her B.A. in Biological Chemistry and Classical Languages & Literature from Dartmouth College and her M.Phil. in Chemistry from the University of Cambridge, before landing at the University of Chicago via the Medical Scientist Training Program (MSTP). In the Dickinson group, she is working to map cross-talk between palmitoylation and other PTMs in the context of physiologically-relevant cell signaling events.
Rahul S. Kathayat obtained his B.S. and M.S. degrees in Chemistry from Hindu College and Indian Institute of Technology Bombay (IITB), respectively. He earned his Ph.D. in Organic Chemistry in 2014 at University of Zurich, Switzerland with Professor Nathaniel S. Finney, where he investigated new mechanisms for fluorescence-based sensing of metal ions. The same year, he moved to the University of Chicago to pursue his postdoctoral research with Professor Bryan C. Dickinson, where his current research interests involve the design and synthesis of chemical probes to study cysteine S-palmitoylation.
Bryan C. Dickinson earned his B.S. in Biochemistry from the University of Maryland, College Park and his Ph.D. in Chemistry from the University of California at Berkeley for work performed with Professor Christopher Chang on developing small molecule fluorescent probes for the detection of hydrogen peroxide in living systems. Then, as a Jane Coffin Childs Memorial postdoctoral fellow with Professor David Liu at Harvard University, he developed new methods to rapidly evolve proteins to perform novel functions. Bryan joined the faculty at the University of Chicago in the Department of Chemistry in the Summer of 2014 and was promoted to Associate Professor in 2019. The Dickinson Group employs synthetic organic chemistry, molecular evolution, and protein design to develop molecular technologies to study chemistry in living systems. The group’s current primary research interests include: 1) how lipid modifications on proteins are controlled and regulate cell signaling; 2) developing new evolution technologies to reprogram and control biomolecular interactions; and 3) developing new biotechnologies to understand and exploit epitranscriptomic regulation.
Footnotes
Competing interests
B.C.D. and R.S.K. have a patent on the DPPs.
References
- (1).Jackson CL; Walch L; Verbavatz JM Lipids and Their Trafficking: An Integral Part of Cellular Organization. Dev Cell 2016, 39, 139–153. [DOI] [PubMed] [Google Scholar]
- (2).Wymann MP; Schneiter R Lipid signalling in disease. Nat Rev Mol Cell Biol 2008, 9, 162–176. [DOI] [PubMed] [Google Scholar]
- (3).Jiang H; Zhang X; Chen X; Aramsangtienchai P; Tong Z; Lin H Protein Lipidation: Occurrence, Mechanisms, Biological Functions, and Enabling Technologies. Chem Rev 2018, 118, 919–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Chen B; Sun Y; Niu J; Jarugumilli GK; Wu X Protein Lipidation in Cell Signaling and Diseases: Function, Regulation, and Therapeutic Opportunities. Cell Chem Biol 2018, 25, 817–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Kakugawa S; Langton PF; Zebisch M; Howell S; Chang TH; Liu Y; Feizi T; Bineva G; O’Reilly N; Snijders AP; Jones EY; Vincent JP Notum deacylates Wnt proteins to suppress signalling activity. Nature 2015, 519, 187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Linder ME; Deschenes RJ Palmitoylation: policing protein stability and traffic. Nat Rev Mol Cell Biol 2007, 8, 74–84. [DOI] [PubMed] [Google Scholar]
- (7).Blanc M; David F; Abrami L; Migliozzi D; Armand F; Burgi J; van der Goot FG SwissPalm: Protein Palmitoylation database. F1000Res 2015, 4, 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Lanyon-Hogg T; Faronato M; Serwa RA; Tate EW Dynamic Protein Acylation: New Substrates, Mechanisms, and Drug Targets. Trends Biochem Sci 2017, 42, 566–581. [DOI] [PubMed] [Google Scholar]
- (9).Gottlieb CD; Linder ME Structure and function of DHHC protein S-acyltransferases. Biochem Soc Trans 2017, 45, 923–928. [DOI] [PubMed] [Google Scholar]
- (10).Won SJ; Cheung See Kit M; Martin BR Protein depalmitoylases. Crit Rev Biochem Mol Biol 2018, 53, 83–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Duncan JA; Gilman AG A cytoplasmic acyl-protein thioesterase that removes palmitate from G protein alpha subunits and p21(RAS). J Biol Chem 1998, 273, 15830–15837. [DOI] [PubMed] [Google Scholar]
- (12).Lin DT; Conibear E ABHD17 proteins are novel protein depalmitoylases that regulate N-Ras palmitate turnover and subcellular localization. Elife 2015, 4, e11306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Yokoi N; Fukata Y; Sekiya A; Murakami T; Kobayashi K; Fukata M Identification of PSD-95 Depalmitoylating Enzymes. J Neurosci 2016, 36, 6431–6444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Wang J; Hao JW; Wang X; Guo H; Sun HH; Lai XY; Liu LY; Zhu M; Wang HY; Li YF; Yu LY; Xie C; Wang HR; Mo W; Zhou HM; Chen S; Liang G; Zhao TJ DHHC4 and DHHC5 Facilitate Fatty Acid Uptake by Palmitoylating and Targeting CD36 to the Plasma Membrane. Cell Rep 2019, 26, 209–221 e205. [DOI] [PubMed] [Google Scholar]
- (15).Zhao L; Zhang C; Luo X; Wang P; Zhou W; Zhong S; Xie Y; Jiang Y; Yang P; Tang R; Pan Q; Hall AR; Luong TV; Fan J; Varghese Z; Moorhead JF; Pinzani M; Chen Y; Ruan XZ CD36 palmitoylation disrupts free fatty acid metabolism and promotes tissue inflammation in non-alcoholic steatohepatitis. J Hepatol 2018, 69, 705–717. [DOI] [PubMed] [Google Scholar]
- (16).Schmidt MF; Bracha M; Schlesinger MJ Evidence for covalent attachment of fatty acids to Sindbis virus glycoproteins. Proc Natl Acad Sci U S A 1979, 76, 1687–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Schlesinger MJ; Magee AI; Schmidt MF Fatty acid acylation of proteins in cultured cells. J Biol Chem 1980, 255, 10021–10024. [PubMed] [Google Scholar]
- (18).Drisdel RC; Green WN Labeling and quantifying sites of protein palmitoylation. Biotechniques 2004, 36, 276–285. [DOI] [PubMed] [Google Scholar]
- (19).Percher A; Ramakrishnan S; Thinon E; Yuan X; Yount JS; Hang HC Mass-tag labeling reveals site-specific and endogenous levels of protein S-fatty acylation. Proc Natl Acad Sci U S A 2016, 113, 4302–4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Hang HC; Geutjes EJ; Grotenbreg G; Pollington AM; Bijlmakers MJ; Ploegh HL Chemical probes for the rapid detection of Fatty-acylated proteins in Mammalian cells. J Am Chem Soc 2007, 129, 2744–2745. [DOI] [PubMed] [Google Scholar]
- (21).Martin BR; Cravatt BF Large-scale profiling of protein palmitoylation in mammalian cells. Nat Methods 2009, 6, 135–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Hannoush RN; Arenas-Ramirez N Imaging the lipidome: omega-alkynyl fatty acids for detection and cellular visualization of lipid-modified proteins. ACS Chem Biol 2009, 4, 581–587. [DOI] [PubMed] [Google Scholar]
- (23).Gao X; Hannoush RN A Decade of Click Chemistry in Protein Palmitoylation: Impact on Discovery and New Biology. Cell Chem Biol 2018, 25, 236–246. [DOI] [PubMed] [Google Scholar]
- (24).Webb Y; Hermida-Matsumoto L; Resh MD Inhibition of protein palmitoylation, raft localization, and T cell signaling by 2-bromopalmitate and polyunsaturated fatty acids. J Biol Chem 2000, 275, 261–270. [DOI] [PubMed] [Google Scholar]
- (25).Hedberg C; Dekker FJ; Rusch M; Renner S; Wetzel S; Vartak N; Gerding-Reimers C; Bon RS; Bastiaens PI; Waldmann H Development of highly potent inhibitors of the Ras-targeting human acyl protein thioesterases based on substrate similarity design. Angew Chem Int Ed Engl 2011, 50, 9832–9837. [DOI] [PubMed] [Google Scholar]
- (26).Dekker FJ; Rocks O; Vartak N; Menninger S; Hedberg C; Balamurugan R; Wetzel S; Renner S; Gerauer M; Scholermann B; Rusch M; Kramer JW; Rauh D; Coates GW; Brunsveld L; Bastiaens PI; Waldmann H Small-molecule inhibition of APT1 affects Ras localization and signaling. Nat Chem Biol 2010, 6, 449–456. [DOI] [PubMed] [Google Scholar]
- (27).Pedro MP; Vilcaes AA; Tomatis VM; Oliveira RG; Gomez GA; Daniotti JL 2-Bromopalmitate reduces protein deacylation by inhibition of acyl-protein thioesterase enzymatic activities. PLoS One 2013, 8, e75232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Zheng B; DeRan M; Li X; Liao X; Fukata M; Wu X 2-Bromopalmitate analogues as activity-based probes to explore palmitoyl acyltransferases. J Am Chem Soc 2013, 135, 7082–7085. [DOI] [PubMed] [Google Scholar]
- (29).Zheng B; Zhu S; Wu X Clickable analogue of cerulenin as chemical probe to explore protein palmitoylation. ACS Chem Biol 2015, 10, 115–121. [DOI] [PubMed] [Google Scholar]
- (30).Kidd D; Liu Y; Cravatt BF Profiling serine hydrolase activities in complex proteomes. Biochemistry 2001, 40, 4005–4015. [DOI] [PubMed] [Google Scholar]
- (31).Rocks O; Peyker A; Kahms M; Verveer PJ; Koerner C; Lumbierres M; Kuhlmann J; Waldmann H; Wittinghofer A; Bastiaens PI An acylation cycle regulates localization and activity of palmitoylated Ras isoforms. Science 2005, 307, 1746–1752. [DOI] [PubMed] [Google Scholar]
- (32).Rocks O; Gerauer M; Vartak N; Koch S; Huang ZP; Pechlivanis M; Kuhlmann J; Brunsveld L; Chandra A; Ellinger B; Waldmann H; Bastiaens PI The palmitoylation machinery is a spatially organizing system for peripheral membrane proteins. Cell 2010, 141, 458–471. [DOI] [PubMed] [Google Scholar]
- (33).Won SJ; Martin BR Temporal Profiling Establishes a Dynamic S-Palmitoylation Cycle. ACS Chem Biol 2018, 13, 1560–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Hernandez JL; Davda D; Cheung See Kit M; Majmudar JD; Won SJ; Gang M; Pasupuleti SC; Choi AI; Bartkowiak CM; Martin BR APT2 Inhibition Restores Scribble Localization and S-Palmitoylation in Snail-Transformed Cells. Cell Chem Biol 2017, 24, 87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Kathayat RS; Elvira PD; Dickinson BC A fluorescent probe for cysteine depalmitoylation reveals dynamic APT signaling. Nat Chem Biol 2017, 13, 150–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Qiu T; Kathayat RS; Cao Y; Beck MW; Dickinson BC A Fluorescent Probe with Improved Water Solubility Permits the Analysis of Protein S-Depalmitoylation Activity in Live Cells. Biochemistry 2018, 57, 221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Kong E; Peng S; Chandra G; Sarkar C; Zhang Z; Bagh MB; Mukherjee AB Dynamic palmitoylation links cytosol-membrane shuttling of acyl-protein thioesterase-1 and acyl-protein thioesterase-2 with that of proto-oncogene H-ras product and growth-associated protein-43. J Biol Chem 2013, 288, 9112–9125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Beck MW; Kathayat RS; Cham CM; Chang EB; Dickinson BC Michael addition-based probes for ratiometric fluorescence imaging of protein S-depalmitoylases in live cells and tissues. Chem Sci 2017, 8, 7588–7592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Kostiuk MA; Corvi MM; Keller BO; Plummer G; Prescher JA; Hangauer MJ; Bertozzi CR; Rajaiah G; Falck JR; Berthiaume LG Identification of palmitoylated mitochondrial proteins using a bio-orthogonal azido-palmitate analogue. FASEB J 2008, 22, 721–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Frohlich M; Dejanovic B; Kashkar H; Schwarz G; Nussberger S S-palmitoylation represents a novel mechanism regulating the mitochondrial targeting of BAX and initiation of apoptosis. Cell Death Dis 2014, 5, e1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Thinon E; Fernandez JP; Molina H; Hang HC Selective Enrichment and Direct Analysis of Protein S-Palmitoylation Sites. J Proteome Res 2018, 17, 1907–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Kathayat RS; Cao Y; Elvira PD; Sandoz PA; Zaballa ME; Springer MZ; Drake LE; Macleod KF; van der Goot FG; Dickinson BC Active and dynamic mitochondrial S-depalmitoylation revealed by targeted fluorescent probes. Nat Commun 2018, 9, 334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Kathayat RS; Dickinson BC Measuring S-Depalmitoylation Activity In Vitro and In Live Cells with Fluorescent Probes. Methods Mol Biol 2019, 2009, 99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Murphy MP Targeting lipophilic cations to mitochondria. Biochim Biophys Acta 2008, 1777, 1028–1031. [DOI] [PubMed] [Google Scholar]
- (45).Martin BR; Wang C; Adibekian A; Tully SE; Cravatt BF Global profiling of dynamic protein palmitoylation. Nat Methods 2011, 9, 84–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Wan J; Roth AF; Bailey AO; Davis NG Palmitoylated proteins: purification and identification. Nat Protoc 2007, 2, 1573–1584. [DOI] [PubMed] [Google Scholar]
- (47).Sugimoto H; Hayashi H; Yamashita S Purification, cDNA cloning, and regulation of lysophospholipase from rat liver. J Biol Chem 1996, 271, 7705–7711. [DOI] [PubMed] [Google Scholar]
- (48).Vartak N; Papke B; Grecco HE; Rossmannek L; Waldmann H; Hedberg C; Bastiaens PI The autodepalmitoylating activity of APT maintains the spatial organization of palmitoylated membrane proteins. Biophys J 2014, 106, 93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Long JZ; Cravatt BF The metabolic serine hydrolases and their functions in mammalian physiology and disease. Chem Rev 2011, 111, 6022–6063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Rhee HW; Zou P; Udeshi ND; Martell JD; Mootha VK; Carr SA; Ting AY Proteomic mapping of mitochondria in living cells via spatially restricted enzymatic tagging. Science 2013, 339, 1328–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Iwamura A; Fukami T; Higuchi R; Nakajima M; Yokoi T Human alpha/beta hydrolase domain containing 10 (ABHD10) is responsible enzyme for deglucuronidation of mycophenolic acid acyl-glucuronide in liver. J Biol Chem 2012, 287, 9240–9249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Ito Y; Fukami T; Yokoi T; Nakajima M An orphan esterase ABHD10 modulates probenecid acyl glucuronidation in human liver. Drug Metab Dispos 2014, 42, 2109–2116. [DOI] [PubMed] [Google Scholar]
- (53).Cao Y, Qiu T, Kathayat RS, Azizi S -A., Thorne, A.K., Ahn, D., Fukata, Y., Fukata, M., Rice, P. A., Dickinson, B.C. ABHD10 regulates mitochondrial redox homeostasis through S-depalmitoylation of PRDX5. Nat Chem Biol 2019, Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Won SJ; Davda D; Labby KJ; Hwang SY; Pricer R; Majmudar JD; Armacost KA; Rodriguez LA; Rodriguez CL; Chong FS; Torossian KA; Palakurthi J; Hur ES; Meagher JL; Brooks CL 3rd; Stuckey JA; Martin BR Molecular Mechanism for Isoform-Selective Inhibition of Acyl Protein Thioesterases 1 and 2 (APT1 and APT2). ACS Chem Biol 2016, 11, 3374–3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Adachi N; Hess DT; McLaughlin P; Stamler JS S-Palmitoylation of a Novel Site in the beta2-Adrenergic Receptor Associated with a Novel Intracellular Itinerary. J Biol Chem 2016, 291, 20232–20246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Akimzhanov AM; Boehning D Rapid and transient palmitoylation of the tyrosine kinase Lck mediates Fas signaling. Proc Natl Acad Sci U S A 2015, 112, 11876–11880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Sadeghi RS; Kulej K; Kathayat RS; Garcia BA; Dickinson BC; Brady DC; Witze ES Wnt5a signaling induced phosphorylation increases APT1 activity and promotes melanoma metastatic behavior. Elife 2018, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Baldwin AC; Green CD; Olson LK; Moxley MA; Corbett JA A role for aberrant protein palmitoylation in FFA-induced ER stress and beta-cell death. Am J Physiol Endocrinol Metab 2012, 302, E1390–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Amara N; Foe IT; Onguka O; Garland M; Bogyo M Synthetic Fluorogenic Peptides Reveal Dynamic Substrate Specificity of Depalmitoylases. Cell Chem Biol 2019, 26, 35–47 e37. [DOI] [PMC free article] [PubMed] [Google Scholar]


