Abstract
OBJECTIVES:
To describe social support patterns of gay and bisexual men with prostate cancer (GBMPCa) and how social support is associated with prostate cancer outcomes.
SAMPLE & SETTING:
A cross-sectional online survey with 186 GBMPCa recruited from a national cancer support group network.
METHODS & VARIABLES:
Descriptive statistics of social support and linear regression of social support on general and prostate cancer–specific quality of life (QOL). Social support and health-related QOL were assessed generally and specific to prostate cancer.
RESULTS:
Participants primarily relied on partners or husbands, gay and bisexual friends, chosen family, and men from support groups for support. The most common types of support received were informational and emotional social support. Low general social support was significantly associated with worse prostate cancer symptom bother and general mental QOL. Wanting more social support was significantly associated with worse prostate cancer–specific and general QOL.
IMPLICATIONS FOR NURSING:
Clinicians should be aware of the different social support networks and needs of GBMPCa and refer them to relevant support groups to improve QOL.
Keywords: bisexual men, caregiving, quality of life, homosexuality, prostate cancer, social support
Despite being the most common cancer among men, prostate cancer in gay and bisexual men is a severely under-researched area (Institute of Medicine, 2011; Quinn, Sanchez, et al., 2015; Wender, Sharpe, Westmaas, & Patel, 2015). Sexual minority men (including gay, bisexual, and other men who have sex with men) have higher prevalence of cancer, more risk behaviors, and lower access to health care, including cancer screenings (Boehmer & Case, 2004; Boehmer, Miao, & Ozonoff, 2011; Kamen et al., 2014). Reviews of the scant literature on gay and bisexual men with prostate cancer (GBMPCa) suggest that these men may have worse prostate cancer outcomes than heterosexual men (Rosser, Merengwa, et al., 2016; Ussher et al., 2016). GBMPCa have reported significantly lower health-related quality of life (QOL), masculine self-esteem, and satisfaction with treatment and higher psychological distress, cancer-related distress, and ejaculatory concern than heterosexual men (Ussher et al., 2016; Wassersug, Lyons, Duncan, Dowsett, & Pitts, 2013).
Social support is consistently associated with better prostate cancer outcomes, including QOL, emotional distress, and mortality (Colloca & Colloca, 2016; Du et al., 2012; Jan et al., 2016; Kamen, Mustian, et al., 2015). The most common conceptual framework for social support (Berkman & Glass, 2000) articulates that multiple dimensions of social support (instrumental, informational, appraisal, and emotional support) are provided through one’s social network. Applied to prostate cancer, social support frameworks identify distinct points of support throughout prostate cancer diagnosis, treatment, and survivorship that have been active areas of research among heterosexual men, particularly instrumental support and caregiving after radical prostatectomy surgery and emotional support of treatment side effects (Harden et al., 2013a; Li et al., 2013; Segrin, Badger, & Harrington, 2012; Wootten, Abbott, Farrell, Austin, & Klein, 2014). In other words, the conceptual framework for social support suggests it is multidimensional and is provided via one’s social network.
Of note, GBMPCa may have different social networks to draw on than heterosexual men. Gay and bisexual men are more likely to be single in mid-to-later life (Goldsen et al., 2017) and have less access to spousal support during care-intensive health treatments and recovery periods. In addition, GBMPCa may have a more fluid definition of spouse/partner, and spouses can play more or less intensive support roles throughout prostate cancer treatment (Capistrant et al., 2016). Gay and bisexual men may also rely more on friends and chosen family rather than biologic family for support and care (Shiu, Muraco, & Fredriksen-Goldsen, 2016). Evidence suggests that sexual minority older adults most commonly have a friend-centered or a diverse, child-free type of social network (Erosheva, Kim, Emlet, & Fredriksen-Goldsen, 2016; Kim, Fredriksen-Goldsen, Bryan, & Muraco, 2017). Social support and social capital theories argue that support and resources flow through one’s social network (Berkman & Glass, 2000). Qualitative evidence suggests that support from other GBMPCa is particularly salient because men felt uncomfortable or unable to discuss sexual treatment effects in general support groups for men with prostate cancer (Capistrant et al., 2016). In addition, many men refused or did not seek out support from friends in the week after prostatectomy because of the catheter. This interplay between support provider and type of support is crucial because social support emerges from a social network. Examining the types of people who provide support to GBMPCa is important because differences in social networks may be one potential explanation for lower QOL in GBMPCa (Ussher et al., 2016).
GBMPCa may also have unique social support needs during cancer treatment, particularly with prostate cancer treatment and its side effects (Margolies, 2014). GBMPCa have different support needs about the sexual side effects of prostate cancer treatment. For example, GBMPCa who have had a radical prostatectomy have a particular sadness regarding the loss of ejaculate, which some have argued is distinctly central in gay sex (Mitteldorf, 2005; Wassersug, Westle, & Dowsett, 2017). For GBMPCa engaging in receptive anal sex, changes to sensitivity and bowel function or worries about radiation exposure for their partner have direct implications (Nasser, Cohen, Dauer, & Zelefsky, 2016; Rosser, Capistrant, et al., 2016). A meta-synthesis of qualitative evidence by Matheson et al. (2017) suggests that GBMPCa experience a distinct shift from a sexually active community to celibacy. In addition, GBMPCa experience marginalization and stigma related to support.
The extensive literature on social support and cancer outcomes suggests that social support is associated with better prostate cancer outcomes, including general QOL and prostate cancer–specific treatment outcomes (Carter, Miller, Murphy, Payne, & Bryant-Lukosius, 2014; Jan et al., 2016; LeMasters, Madhavan, Sambamoorthi, & Kurian, 2013). Partnered men have better outcomes, make different treatment decisions, and get more social support than men who are not partnered (Bergman, Gore, Saigal, Kwan, & Litwin, 2009; Chamie et al., 2012; Kamen, Mustian, et al., 2015). Much of the literature on support for men with prostate cancer has focused on spousal support specific to heterosexual relationships (Harden et al., 2013b; McCaughan et al., 2013; Movsas, Yechieli, Movsas, & Darwish-Yassine, 2016). Although considerable evidence exists that GBMPCa have different social networks and support needs than heterosexual men with prostate cancer, no studies have considered the types of social support that GBMPCa receive or how social support is associated with QOL.
To address this gap in the literature on how social support is associated with outcomes and QOL for GBMPCa, the current authors used data from the Restore study, the largest cross-sectional online survey of GBMPCa to date. The authors describe patterns of social support and estimate how social support is associated with prostate cancer–specific and general QOL. Specifically, the authors hypothesized that higher social support would be positively associated with QOL outcomes and that lower social support would be associated with worse QOL.
Methods
Sample
Recruitment for the Restore survey was conducted online via Malecare (www.malecare.org), North America’s largest men’s cancer support group and advocacy organization. Annually, 800–1,000 newly diagnosed GBMPCa join Malecare. Four recruitment emails went out to Malecare members at about 7- to 10-day intervals with a link in the email to the study website where participants were screened for eligibility. Eligible participants were men aged 18 years or older who identified as gay, bisexual, or a man who has sex with men, were English speaking, resided in a U.S. or Canadian zip code, and were diagnosed with and completed treatment for prostate cancer. Active surveillance (also called watchful waiting) was not considered to be treatment, so men on active surveillance were not eligible to participate. Eligible participants were then asked to provide consent, which was implemented as a chunked consent protocol for online surveys. This approach has been used in previous studies and was described by Rosser et al. (2009). Consent was obtained for all individual participants included in this study. Data collection began October 21, 2015, and ended December 31, 2015 (71 days), and 193 valid survey responses were completed. Each participant received a $25 gift card for completing the survey. The University of Minnesota Institutional Review Board approved the study procedures.
Measures and Instruments
Demographics, socioeconomic characteristics, and prostate cancer information:
Demographic questions (i.e., age, race, ethnicity, and education) were adapted from the U.S. Census. The authors characterized race and ethnicity in one dichotomous variable of non-White race or Hispanic White men versus a reference group of non-Hispanic White men. Education was collapsed into three categories (i.e., some college or less, bachelor’s degree, and graduate degree [reference]). Relationship status was assessed by selecting from a group of options which best represented their relationship status (i.e., single, dating, married/in a relationship, widowed/divorced/formerly in a relationship, or refused to answer), which were dichotomized into those currently in relationships and those not in relationships. Medical characteristics, particularly prostate-specific antigen (PSA) level at diagnosis, were asked directly; the authors used PSA at diagnosis as an easily self-reported proxy measure of disease severity. Time since diagnosis was calculated from the self-reported month and year of diagnosis of prostate cancer. A simple mean imputation for those who reported they did not remember their PSA test (22%, n = 41) or their time since diagnosis (3.6%, n = 7) was used. Participants were asked which of nine prostate cancer treatments they under-took, which were collapsed into three groups (i.e., surgery only [reference], radiation only, and systemic/multiple therapy) for analysis.
Prostate cancer–specific and general quality of life:
The authors used the Expanded Prostate Cancer Index Composite (EPIC), a comprehensive and widely used assessment of prostate cancer–specific QOL (Hollenbeck, Dunn, Wei, Montie, & Sanda, 2003; Wei et al., 2002). This 50-item scale measures common prostate cancer side effects: urinary, bowel, sexual, and hormonal. For each group of side effects, the scale measures symptom frequency (how often side effects happen) and perceived bother (how much they affect the respondent’s QOL). For example, the urinary symptom frequency and bother subscale included how often someone has leaked urine, had urinary control issues, or needed diapers in the past four weeks. Participants were also asked to rate how big of a problem these symptoms were in the past four weeks on a five-item Likert-type scale ranging from 1 (no problem) to 5 (big problem). EPIC has acceptable scale and subscale reliability (r ≥ 0.8) and internal consistency (Cronbach alpha ≥ 0.82) (Wei, Dunn, Litwin, Sandler, & Sanda, 2000). The scoring of these four subscales resulted in values from 0–100, with higher values reflecting better prostate cancer–specific QOL.
The SF-12® is a measure of general health-related QOL comprised of two domains: mental and physical health. Each of these domains has multiple subscales. The physical health QOL score includes questions regarding four domains of physical function, extent to which physical health allows an individual to perform important roles in life, extent of pain, and how much energy the individual has. These questions correspond to domains of physical function, role function, physical/bodily pain, and vitality.
The mental health QOL scale includes questions about how the respondent’s mental health affects his ability to fulfill important roles in life, questions about mental health symptoms (e.g., depressive model), and how much mental health inhibits social engagements with friends and family members. These questions correspond to subscales on role function, emotional functioning, mental health functioning, and social functioning.
Two-week test-retest reliability was high for the physical subscale (r = 0.89) and for the mental subscale (r = 0.76) (Ware, Kosinski, & Keller, 1996). The physical components and mental components scores each range from 0–100, with higher values reflecting better QOL. The scores are standardized to an age-normed score based on the U.S. population, where a score of 50 is the average in the U.S. population. Less than 50 is lower than average, and higher than 50 is above average.
General and prostate cancer–specific social support:
General social support was assessed with the seven-item ENRICHD Social Support Inventory (Mitchell et al., 2003), which has high internal reliability (Cronbach alpha = 0.86) and assesses the presence of multiple domains of social support (i.e., instrumental support, appraisal support, informational support, and emotional support). Total scores range from 8–32. Consistent with prior use of this scale, the authors characterized low social support as those with (a) scores of 2 (a little of the time) or less on two or more items regarding presence of social support or (b) scores of 3 (some of the time) or less on two items and a low total score (12 or less) across all items (Collaborative Studies Coordinating Center, 1998).
The authors included four prostate cancer–specific support domains informed by previous qualitative research (Capistrant et al., 2016) and a social support conceptual framework (Berkman & Glass, 2000). First, men reported whether they received individual sources of support for activities of daily living, medical appointment support, health-related care, informational support, and emotional support. The authors reported the proportion of the sample that received each type. Second, the authors calculated the total number of individual types of support to reflect the total amount of support overall. Third, men reported who provided this support (i.e., partner, chosen family, biologic family, friends, support group or other man with prostate cancer, paid caregivers, and other). Fourth, men reported which types of additional support they wanted more of during their prostate cancer treatment (i.e., activities of daily living, health-related care, medical appointment support, informational support, emotional support, talking to another man/men with prostate cancer and support groups, financial support, or none). The authors calculated two variables of the support they wanted: a proportion and a total count. The proportion was men who reported wanting each type of support to capture which types of support were most important to this population, and the total count was the number of types of support a man reported wanting. The goal of the total count was to reflect the extent of unmet need for support overall.
Analysis
The authors excluded men who had any missing key outcome and covariate data (3.6%, n = 7), resulting in a final analytic sample of 186. The authors tested differences in prostate cancer–specific support by prostate cancer treatment and relationships status using chi-square tests. Multivariate-adjusted linear regression models were used to test the hypothesis that social support was related to health-related QOL (Hidalgo & Goodman, 2013). All models were adjusted for key demographic (i.e., age, race, ethnicity, sexual orientation, and relationship status), socioeconomic (i.e., education), and prostate cancer (i.e., PSA at diagnosis, years since treatment, and treatment chosen) variables, which were chosen a priori based on prior research as potential confounding factors of the association between social support and health-related QOL outcomes. Continuous variables (e.g., age, years since diagnosis, PSA test) were mean centered in regression models to facilitate interpretation of the intercept term. Data were analyzed using Stata®, version 14.
Results
The study sample (N = 186) characteristics are presented in Tables 1 and 2, along with the descriptive statistics of the independent (i.e., social support) and dependent (i.e., QOL) variables. The average participant was a 63.5-year-old, gay-identifying, non-Hispanic White man who was married or in a long-term relationship, had a graduate degree, was diagnosed with prostate cancer five years previously, and had surgery or radical prostatectomy treatment. Twenty-three participants in this study (12%) were non-White and/or Hispanic, and 16 men (8.4%) identified their sexual orientation as bisexual. Most men (53%, n = 99) had undertaken surgery as their prostate cancer treatment, and the average time since prostate cancer treatment was 5.8 years. The sexual components score on the prostate cancer–specific QOL was notably low (), and the mental components score for general QOL was also low ().
TABLE 1.
Online Survey Outcomes Regarding Support and QOL (N = 186)
| Characteristic | SD | |
|---|---|---|
| EPIC for prostate cancer-specific QOL | ||
| Hormone | 81.57 | 16.7 |
| Sexual | 44.9 | 21.83 |
| Bowel | 87.38 | 12.53 |
| Urinary | 77.35 | 18.05 |
| SF-12® for general QOL | ||
| Physical components scale | 52.65 | 8.47 |
| Mental components scale | 46.41 | 11.33 |
| Prostate cancer-specific support | ||
| Number of support types received | 2.61 | 1.6 |
| Number of support network types | 2 | 1.73 |
| Number of support types wanted | 1.78 | 1.55 |
| Characteristic | n | % |
| ENRICHD Social Support Inventory | ||
| Low general social support (reference: high general social support) | 67 | 36 |
EPIC—Expanded Prostate Cancer Index Composite; QOL—quality of life
Note. EPIC and SF-12 scores range from 0–100, with higher values indicating better QOL.
TABLE 2.
Sample Characteristics (N = 186)
| Characteristic | SD | |
|---|---|---|
| Age (years) | 63.51 | 8.16 |
| Years since prostate cancer diagnosis | 5.82 | 4.49 |
| Self-reported prostate-specific antigen level | 7.7 | 5.89 |
| Characteristic | n | % |
| Education | ||
| Some college or less | 42 | 23 |
| Bachelor’s degree | 64 | 34 |
| Graduate degree | 80 | 43 |
| Race or ethnicity | ||
| Non-Hispanic White | 163 | 88 |
| Hispanic or non-White | 23 | 12 |
| Relationship status | ||
| Single or unknown | 60 | 32 |
| Dating | 13 | 7 |
| Married or in a relationship | 101 | 54 |
| Widowed, divorced, or formerly in a relationship | 12 | 6 |
| Sexual orientation | ||
| Gay | 170 | 91 |
| Bisexual | 16 | 9 |
| Prostate cancer treatment | ||
| Surgery | 99 | 53 |
| Systemic | 52 | 28 |
| Radiation or brachytherapy | 35 | 19 |
Note. Because of rounding, percentages may not total 100.
About 35% (n = 65) of participants reported low general social support. Respondents reported wanting more emotional support (47%, n = 88) and informational support (38%, n = 70) (see Figure 1). About 58% (n = 108) of respondents reported wanting more support from a support group or other GBMPCa. Respondents also reported wanting more support regarding medical appointments (8%, n = 15), health care (6%, n = 11), and activities of daily living (4%, n = 8).
FIGURE 1. Percentage Prevalence of Additional Support Wanted (N = 186).
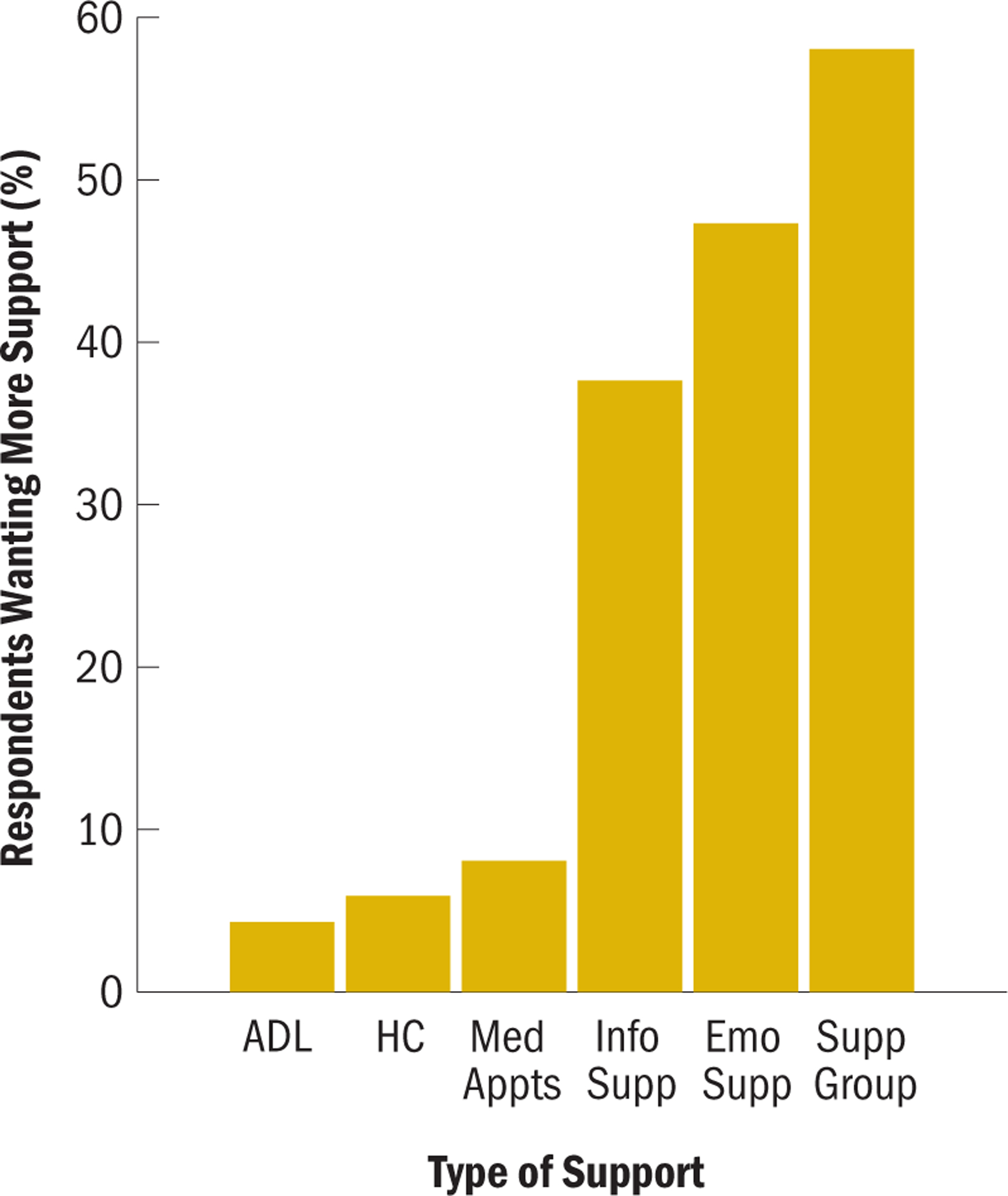
ADL—activities of daily living; emo supp—emotional support; HC—health-related care; info supp—informational support; med appts—medical appointments; supp group—other men with prostate cancer or a support group
Regarding sources of support, about 48% (n = 89) of the participants reported receiving support from another man with prostate cancer or a support group. About 46% (n = 86) of the participants reported getting support from a partner, which is consistent with the marital status of the sample. Forty percent (n = 74) of men reported getting support from chosen family, but only 34% (n = 63) received support from biologic family members. About 22% (n = 41) reported receiving support from a friend. Three percent (n = 6) of respondents said that they had paid support, and 6% (n = 11) reported having another type of support.
The authors’ hypothesis that social support was positively associated with QOL was supported by survey responses. Low general social support was associated with lower QOL compared to men with moderate or high general social support (see Tables 3 and 4). Specifically, a man with low general social support had, on average, 11.63 points lower hormonal QOL (p < 0.001), 5.2 points lower bowel QOL (p < 0.05), and 8.63 points lower mental QOL (p < 0.001) compared to a man with moderate or high general social support.
TABLE 3.
Beta Coefficients and 95% CI of Association Between Low General Social Support and Health-Related Quality-of-Life Outcomes Using EPIC Subscales
| Hormonal | Sexual | Bowel | Urinary | |||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Coeff | 95% CI | Coeff | 95% CI | Coeff | 95% CI | Coeff | 95% CI |
| Low general social support | 10.51*** | [−15.62, −5.39] | −2.06 | [−8.84, 4.72] | −6.12** | [−10.01, −2.23] | −6.88* | [−12.78, −0.98] |
| Age (centered at mean) | −0.01 | [−0.33, 0.32] | −0.52* | [−0.95, −0.08] | 0.14 | [−0.11, 0.39] | −0.21 | [−0.59, 0.17] |
| Some college or less (ref: graduate degree) | −2.63 | [−8.63, 3.37] | −3.26 | [−11.21, 4.7] | 0.21 | [−4.35, 4.78] | −0.85 | [−7.77, 6.08] |
| Bachelor’s degree (ref: graduate degree) | 0.89 | [−0.44, 6.22] | 1.77 | [−5.3, 8.83] | 2.39 | [−1.67, 6.44] | 3.03 | [−3.12, 9.18] |
| Non-Hispanic White (ref: Hispanic or non-White) | 6.7 | [−0.55, 13.96] | 5.3 | [−4.31, 14.91] | 1.66 | [−3.86, 7.18] | 4.4 | [−3.97, 12.76] |
| In a relationship (ref: single) | −4.06 | [−9.17, 1.05] | 1.29 | [−5.48, 8.06] | −4.3* | [−8.18, −0.41] | −2.88 | [−8.77, 3.01] |
| Gay (ref: bisexual) | −4.06 | [−12.48, 4.36] | −0.97 | [−12.13, 10.19] | 7.81* | [1.4, 14.21] | 1.64 | [−8.08, 11.35] |
| Systemic treatment (ref: surgery) | −10.37*** | [−16.08, −4.67] | −14.61*** | [−22.17, −7.06] | −8.45*** | [−12.79, −4.12] | −2.32 | [−8.89, 4.26] |
| Radiation or brachytherapy (ref: surgery) | −5.38 | [−11.72, 0.97] | 0.45 | [−7.96, 8.86] | −7.39** | [−12.22, −2.56] | 3.68 | [−3.64, 11] |
| Years since diagnosis (centered at mean) | −0.22 | [−0.79, 0.35] | 0.22 | [−0.54, 0.97] | −0.16 | [−0.6, 0.27] | −0.02 | [−0.68, 0.63] |
| PSA level at diagnosis (centered at mean) | 0.03 | [−0.38, 0.45] | 0.24 | [−0.3, 0.79] | 0.03 | [−0.28, 0.35] | −0.09 | [−0.57, 0.39] |
| Intercept | 89.7*** | [78.58, 100.83] | 45.23*** | [30.48, 59.98] | 86.23*** | [77.77, 94.7] | 75.41*** | [62.58, 88.25] |
p < 0.05;
p < 0.01;
p < 0.001
CI—confidence interval; coeff—beta coefficient; EPIC—Expanded Prostate Cancer Index Composite; PSA—prostate-specific antigen; ref—reference group for categorical/binary variables
Note. Intercept represents referent participant, a 63.5-year-old, non-Hispanic White, married, gay man who had surgery and is 5.8 years since diagnosis, with a PSA level at diagnosis of 7.6 ng/ml and high social support.
TABLE 4.
Beta Coefficients and 95% CI of Association Between Low General Social Support and Health-Related Quality-of-Life Outcomes Using SF-12® Subscales
| Physical Components Score | Mental Components Score | |||
|---|---|---|---|---|
| Characteristic | Coeff | 95% CI | Coeff | 95% CI |
| Low general social support | −0.59 | [−3.29, 2.1] | −8.41*** | [−11.69, −5.14] |
| Age (centered at mean) | −0.3*** | [−0.47, −0.12] | 0.42*** | [0.21, 0.63] |
| Some college or less (ref: graduate degree) | −2.17 | [−5.33, 1] | −0.79 | [−4.63, 3.05] |
| Bachelor’s degree (ref: graduate degree) | 0.19 | [−2.62, 3] | 1.61 | [−1.8, 5.02] |
| Non-Hispanic White (ref: Hispanic or non-White) | 1.26 | [−2.56, 5.08] | −1.68 | [−6.32, 2.95] |
| In a relationship (ref: single) | −1.21 | [−3.9, 1.48] | −0.52 | [−3.79, 2.74] |
| Gay (ref: bisexual) | −0.18 | [−4.61, 4.26] | −0.67 | [−6.06, 4.71] |
| Systemic treatment (ref: surgery) | −3.43* | [−6.43, −0.42] | −3.9* | [−7.55, −0.25] |
| Radiation or brachytherapy (ref: surgery) | −0.98 | [−4.32, 2.37] | −5.34* | [−9.4, −1.28] |
| Years since diagnosis (centered at mean) | −0.13 | [−0.43, 0.17] | −0.06 | [−0.43, 0.3] |
| PSA level at diagnosis (centered at mean) | 0.07 | [−0.14, 0.29] | −0.11 | [−0.38, 0.15] |
| Intercept | 54.13*** | [48.27, 60] | 53.54*** | [46.42, 60.65] |
p < 0.05;
p < 0.01;
p < 0.001
CI—confidence interval; coeff—beta coefficient; PSA—prostate-specific antigen; ref—reference group for categorical/binary variables
Note. Intercept represents referent participant, a 63.5-year-old, non-Hispanic White, married, gay man who had surgery and is 5.8 years since diagnosis, with a PSA level at diagnosis of 7.6 ng/ml and high social support.
Men who wanted more types of social support had, on average, lower QOL. For every additional type of social support a GBMPCa wanted, he had, on average, 1.86 points lower hormonal QOL (p < 0.05), 2.82 points lower sexual QOL (p < 0.05), 1.25 points lower bowel QOL (p < 0.05), and 1.13 points lower general mental QOL (p < 0.05) after controlling for demographic, socioeconomic, and prostate cancer variables (see Tables 5 and 6). For example, a man who wanted three types of additional support (i.e., social support, emotional support, and informational support) had, on average, significantly lower hormonal, sexual, bowel, and general mental QOL than someone who wanted two types of additional support (i.e., social support and emotional support).
TABLE 5.
Beta Coefficients and 95% CI of Association Between Additional Prostate Cancer Social Support Wanted and Health-Related Quality-of-Life Outcomes Using EPIC Subscales
| Hormonal | Sexual | Bowel | Urinary | |||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Coeff | 95% CI | Coeff | 95% CI | Coeff | 95% CI | Coeff | 95% CI |
| Additional prostate cancer support wanted | −1.65* | [−3.22, −0.09] | −2.7** | [−4.67, −0.72] | −1.28* | [−2.45, −0.11] | −1.72 | [−3.48, 0.04] |
| Age (centered at mean) | 0.02 | [−0.32, 0.36] | −0.56* | [−0.99, −0.13] | 0.15 | [−0.1, 0.4] | −0.2 | [−0.58, 0.18] |
| Some college or less (ref: graduate degree) | −2.15 | [−8.35, 4.04] | −3.36 | [−11.15, 4.43] | 0.46 | [−4.16, 5.09] | −0.59 | [−7.53, 6.36] |
| Bachelor’s degree (ref: graduate degree) | 0.97 | [−4.54, 6.49] | 1.24 | [−5.7, 8.18] | 2.36 | [−1.75, 6.48] | 2.94 | [−3.24, 9.12] |
| Non-Hispanic White (ref: Hispanic or non-White) | 6.03 | [−1.45, 13.52] | 5.95 | [−3.47, 15.37] | 1.37 | [−4.22, 6.96] | 4.17 | [−4.22, 12.56] |
| In a relationship (ref: single) | −0.95 | [−5.91, 4.01] | 0.92 | [−5.32, 7.17] | −2.61 | [−6.32, 1.09] | −1.1 | [−6.67, 4.46] |
| Gay (ref: bisexual) | −5.17 | [−13.84, 3.51] | −0.51 | [−11.42, 10.41] | 7.25* | [0.78, 13.73] | 1.09 | [−8.63, 10.82] |
| Systemic treatment (ref: surgery) | −10.67*** | [−16.57, −4.78] | −14.19*** | [−21.61, −6.78] | −8.57*** | [−12.97, −4.17] | −2.38 | [−8.99, 4.22] |
| Radiation or brachytherapy (ref: surgery) | −5.74 | [−12.31, 0.84] | −0.28 | [−8.55, 7.99] | −7.68** | [−12.59, −2.78] | 3.27 | [−4.1, 10.63] |
| Years since diagnosis (centered at mean) | −0.26 | [−0.85, 0.32] | 0.2 | [−0.54, 0.94] | −0.19 | [−0.63, 0.25] | −0.06 | [−0.71, 0.6] |
| PSA level at diagnosis | −0.03 | [−0.46, 0.4] | 0.16 | [−0.38, 0.7] | −0.01 | [−0.33, 0.31] | −0.15 | [−0.63, 0.33] |
| Intercept | 88.54*** | [76.93, 100.14] | 48.7*** | [34.1, 63.29] | 86.04*** | [77.38, 94.7] | 75.64*** | [62.63, 88.65] |
p < 0.05;
p < 0.01;
p < 0.001
CI—confidence interval; coeff—beta coefficient; EPIC—Expanded Prostate Cancer Index Composite; PSA—prostate-specific antigen; ref—reference group for categorical/binary variables
Note. Intercept represents referent participant, a 63.5-year-old, non-Hispanic White, married, gay man who had surgery and is 5.8 years since diagnosis, with a PSA level at diagnosis of 7.6 ng/ml and high social support.
TABLE 6.
Beta Coefficients and 95% CI of Association Between Additional Prostate Cancer Social Support Wanted and Health-Related Quality-of-Life Outcomes Using SF-12® Subscales
| Physical Components Score | Mental Components Score | |||
|---|---|---|---|---|
| Characteristic | Coeff | 95% CI | Coeff | 95% CI |
| Additional prostate cancer support wanted | −0.17 | [−0.97, 0.63] | −1.13* | [−2.15, −0.1] |
| Age (centered at mean) | −0.3*** | [−0.47, −0.12] | 0.45*** | [0.23, 0.67] |
| Some college or less (ref: graduate degree) | −2.15 | [−5.31, 1.01] | −0.39 | [−4.45, 3.66] |
| Bachelor’s degree (ref: graduate degree) | 0.18 | [−2.63, 2.99] | 1.73 | [−1.88, 5.33] |
| Non-Hispanic White (ref: Hispanic or non-White) | 1.25 | [−2.57, 5.06] | −2.29 | [−7.18, 2.61] |
| In a relationship (ref: single) | −1.07 | [−3.6, 1.46] | 2.05 | [−1.2, 5.3] |
| Gay (ref: bisexual) | −0.22 | [−4.64, 4.21] | −1.62 | [−7.29, 4.06] |
| Systemic treatment (ref: surgery) | −3.43* | [−6.44, −0.42] | −4.18* | [−8.04, −0.33] |
| Radiation or brachytherapy (ref: surgery) | −1.02 | [−4.37, 2.33] | −5.57* | [−9.87, −1.27] |
| Years since diagnosis (centered at mean) | −0.14 | [−0.44, 0.16] | −0.1 | [−0.48, 0.29] |
| PSA level at diagnosis | 0.07 | [−0.15, 0.29] | −0.15 | [−0.43, 0.13] |
| Intercept | 54.18*** | [48.26, 60.1] | 52.29*** | [44.7, 59.89] |
p < 0.05;
p < 0.01;
p < 0.001
CI—confidence interval; coeff—beta coefficient; PSA—prostate-specific antigen; ref—reference group for categorical/binary variables
Note. Intercept represents referent participant, a 63.5-year-old, non-Hispanic White, married, gay man who had surgery and is 5.8 years since diagnosis, with a PSA level at diagnosis of 7.6 ng/ml and high social support.
In supplemental analyses, the current authors note that men who received more types of support and had a broader social network of supporters had higher QOL. Men who received more types of prostate cancer–specific social support had slightly higher hormonal QOL. For every additional type of support a man received, he had, on average, 2.17 points higher hormonal QOL (p < 0.05) (see Tables 7 and 8). In other words, a man who had help with activities of daily living and emotional support had, on average, 2.17 points higher hormonal QOL than someone who only had emotional support, controlling for demographic, socioeconomic, and prostate cancer variables in the model. Similarly, men who had a more diverse social support network had higher QOL (see Tables 9 and 10). However, this association was only statistically significant for sexual and physical QOL. For example, a man who received help from three types of supporters (i.e., a partner, chosen family, and support group members) had, on average, 2.12 points higher sexual QOL (p < 0.05) and 0.87 points higher physical QOL (p < 0.05) than a man who had two types of supporters in his network (i.e., a partner and support group members).
TABLE 7.
Beta Coefficients and 95% CI of Association Between Number of Prostate Cancer–Specific Social Support Types and Health-Related Quality-of-Life Outcomes Using EPIC Subscales
| Hormonal | Sexual | Bowel | Urinary | |||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Coeff | 95% CI | Coeff | 95% CI | Coeff | 95% CI | Coeff | 95% CI |
| Number of social support types | 2.02* | [0.31, 3.72] | 0.56 | [−1.63, 2.75] | −0.13 | [−1.42, 1.17] | 1.23 | [−0.69, 3.16] |
| Age (centered at mean) | 0.1 | [−0.24, 0.44] | −0.49* | [−0.93, −0.06] | 0.17 | [−0.08, 0.43] | −0.14 | [−0.52, 0.24] |
| Some college or less (ref: graduate degree) | −1.33 | [−7.53, 4.88] | −2.94 | [−10.93, 5.04] | 0.52 | [−4.18, 5.23] | −0.02 | [−7.04, 6.99] |
| Bachelor’s degree (ref: graduate degree) | 1.3 | [−4.19, 6.78] | 1.84 | [−5.22, 8.9] | 2.66 | [−1.5, 6.83] | 3.3 | [−2.91, 9.51] |
| Non-Hispanic White (ref: Hispanic or non-White) | 5.54 | [−1.91, 12.99] | 5.07 | [−4.51, 14.66] | 0.95 | [−4.7, 6.6] | 3.63 | [−4.79, 12.06] |
| In a relationship (ref: single) | −1.68 | [−6.73, 3.37] | 1.64 | [−4.86, 8.14] | −2 | [−5.83, 1.83] | −1.26 | [−6.97, 4.46] |
| Gay (ref: bisexual) | −4.05 | [−12.79, 4.69] | −0.83 | [−12.08, 10.42] | 6.79* | [0.16, 13.42] | 1.57 | [−8.32, 11.46] |
| Systemic treatment (ref: surgery) | −9.13** | [−15.21, −3.06] | −14.21*** | [−22.04, −6.39] | −8.94*** | [−13.55, −4.33] | −1.58 | [−8.46, 5.29] |
| Radiation or brachytherapy (ref: surgery) | −2.63 | [−9.54, 4.28] | 1.21 | [−7.68, 10.1] | −7.5** | [−12.74, −2.25] | 5.36 | [−2.46, 13.18] |
| Years since diagnosis (centered at mean) | −0.22 | [−0.8, 0.37] | 0.22 | [−0.53, 0.98] | −0.19 | [−0.64, 0.26] | −0.02 | [−0.69, 0.64] |
| PSA level at diagnosis | 0.04 | [−0.39, 0.47] | 0.25 | [−0.3, 0.79] | 0.03 | [−0.3, 0.35] | −0.09 | [−0.57, 0.4] |
| Intercept | 78.95*** | [66.18, 91.72] | 42.54*** | [26.11, 58.97] | 84.49*** | [74.81, 94.18] | 68.68*** | [54.23, 83.12] |
p < 0.05;
p < 0.01;
p < 0.001
CI—confidence interval; coeff—beta coefficient; EPIC—Expanded Prostate Cancer Index Composite; PSA—prostate-specific antigen; ref—reference group for categorical/binary variables
Note. Intercept represents referent participant, a 63.5-year-old, non-Hispanic White, married, gay man who had surgery and is 5.8 years since diagnosis, with a PSA level at diagnosis of 7.6 ng/ml and high social support.
TABLE 8.
Beta Coefficients and 95% CI of Association Between Number of Prostate Cancer–Specific Social Support Types and Health-Related Quality-of-Life Outcomes Using SF-12® Subscales
| Physical Components Score | Mental Components Score | |||
|---|---|---|---|---|
| Characteristic | Coeff | 95% CI | Coeff | 95% CI |
| Number of social support types | 0.68 | [−0.19, 1.54] | 0.98 | [−0.14, 2.11] |
| Age (centered at mean) | −0.28** | [−0.45, −0.11] | 0.49*** | [0.27, 0.72] |
| Some college or less (ref: graduate degree) | −1.9 | [−5.05, 1.25] | 0.04 | [−4.05, 4.13] |
| Bachelor’s degree (ref: graduate degree) | 0.2 | [−2.59, 2.99] | 1.96 | [−1.66, 5.57] |
| Non-Hispanic White (ref: Hispanic or non-White) | 1.21 | [−2.58, 4.99] | −2.63 | [−7.54, 2.28] |
| In a relationship (ref: single) | −1.47 | [−4.04, 1.1] | 1.83 | [−1.5, 5.15] |
| Gay (ref: bisexual) | 0.27 | [−4.18, 4.71] | −1.16 | [−6.93, 4.6] |
| Systemic treatment (ref: surgery) | −2.84 | [−5.93, 0.25] | −3.5 | [−7.5, 0.51] |
| Radiation or brachytherapy (ref: surgery) | −0.08 | [−3.6, 3.43] | −3.97 | [−8.53, 0.59] |
| Years since diagnosis (centered at mean) | −0.12 | [−0.42, 0.18] | −0.08 | [−0.46, 0.31] |
| PSA level at diagnosis | 0.08 | [−0.14, 0.3] | −0.11 | [−0.39, 0.17] |
| Intercept | 51.57*** | [45.08, 58.06] | 47.12*** | [38.7, 55.54] |
p < 0.05;
p < 0.01;
p < 0.001
CI—confidence interval; coeff—beta coefficient; PSA—prostate-specific antigen; ref—reference group for categorical/binary variables
Note. Intercept represents referent participant, a 63.5-year-old, non-Hispanic White, married, gay man who had surgery and is 5.8 years since diagnosis, with a PSA level at diagnosis of 7.6 ng/ml and high social support.
TABLE 9.
Beta Coefficients and 95% CI of Association Between Number of Prostate Cancer–Specific Social Network Types and Health-Related Quality-of-Life Outcomes Using EPIC Subscales
| Hormonal | Sexual | Bowel | Urinary | |||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Coeff | 95% CI | Coeff | 95% CI | Coeff | 95% CI | Coeff | 95% CI |
| Number of social network types | 1.18 | [−0.27, 2.62] | 2.09* | [0.26, 3.91] | 0.03 | [−1.06, 1.12] | 0.67 | [−0.96, 2.3] |
| Age (centered at mean) | 0.07 | [−0.27, 0.41] | −0.47* | [−0.9, −0.05] | 0.18 | [−0.08, 0.43] | −0.16 | [−0.54, 0.22] |
| Some college or less (ref: graduate degree) | −1.63 | [−7.88, 4.61] | −2.46 | [−10.32, 5.41] | 0.58 | [−4.12, 5.27] | −0.23 | [−7.25, 6.8] |
| Bachelor’s degree (ref: graduate degree) | 1.7 | [−3.85, 7.25] | 2.47 | [−4.52, 9.45] | 2.67 | [−1.51, 6.84] | 3.53 | [−2.71, 9.78] |
| Non-Hispanic White (ref: Hispanic or non-White) | 5.78 | [−1.73, 13.3] | 5.58 | [−3.88, 15.04] | 0.96 | [−4.7, 6.61] | 3.77 | [−4.69, 12.23] |
| In a relationship (ref: single) | −1.32 | [−6.44, 3.79] | 0.16 | [−6.27, 6.6] | −2.11 | [−5.96, 1.73] | −1 | [−6.75, 4.76] |
| Gay (ref: bisexual) | −5.44 | [−14.15, 3.27] | −0.92 | [−11.89, 10.05] | 6.89* | [0.34, 13.45] | 0.72 | [−9.09, 10.52] |
| Systemic treatment (ref: surgery) | −10.95*** | [−16.86, −5.03] | −14.64*** | [−22.09, −7.19] | −8.82*** | [−13.27, −4.37] | −2.7 | [−9.35, 3.96] |
| Radiation or brachytherapy (ref: surgery) | −5.59 | [−12.19, 1.01] | −0.08 | [−8.39, 8.23] | −7.34** | [−12.31, −2.37] | 3.57 | [−3.86, 10.99] |
| Years since diagnosis (centered at mean) | −0.23 | [−0.82, 0.36] | 0.27 | [−0.48, 1.01] | −0.19 | [−0.63, 0.26] | −0.03 | [−0.7, 0.63] |
| PSA level at diagnosis | 0.05 | [−0.38, 0.48] | 0.29 | [−0.25, 0.83] | 0.03 | [−0.3, 0.35] | −0.08 | [−0.57, 0.4] |
| Intercept | 83.65*** | [71.9, 95.4] | 40.4*** | [25.61, 55.2] | 84*** | [75.16, 92.84] | 71.64*** | [58.42, 84.87] |
p < 0.05;
p < 0.01;
p < 0.001
CI—confidence interval; coeff—beta coefficient; EPIC—Expanded Prostate Cancer Index Composite; PSA—prostate-specific antigen; ref—reference group for categorical/binary variables
Note. Intercept represents referent participant, a 63.5-year-old, non-Hispanic White, married, gay man who had surgery and is 5.8 years since diagnosis, with a PSA level at diagnosis of 7.6 ng/ml and high social support.
TABLE 10.
Beta Coefficients and 95% CI of Association Between Number of Prostate Cancer–Specific Social Network Types and Health-Related Quality-of-Life Outcomes Using SF-12® Subscales
| Physical Components Score | Mental Components Score | |||
|---|---|---|---|---|
| Characteristic | Coeff | 95% CI | Coeff | 95% CI |
| Number of social network types | 0.86* | [0.14, 1.59] | 0.71 | [−0.24, 1.66] |
| Age (centered at mean) | −0.28** | [−0.45, −0.11] | 0.48*** | [0.26, 0.7] |
| Some college or less (ref: graduate degree) | −1.85 | [−4.97, 1.27] | −0.07 | [−4.16, 4.03] |
| Bachelor’s degree (ref: graduate degree) | 0.47 | [−2.3, 3.24] | 2.19 | [−1.44, 5.83] |
| Non-Hispanic White (ref: Hispanic or non-White) | 1.41 | [−2.35, 5.16] | −2.48 | [−7.41, 2.45] |
| In a relationship (ref: single) | −1.77 | [−4.33, 0.78] | 1.88 | [−1.48, 5.23] |
| Gay (ref: bisexual) | −0.12 | [−4.47, 4.23] | −1.82 | [−7.53, 3.9] |
| Systemic treatment (ref: surgery) | −3.42* | [−6.38, −0.47] | −4.38* | [−8.25, −0.5] |
| Radiation or brachytherapy (ref: surgery) | −1.2 | [−4.5, 2.1] | −5.45* | [−9.78, −1.12] |
| Years since diagnosis (centered at mean) | −0.11 | [−0.41, 0.18] | −0.08 | [−0.47, 0.31] |
| PSA level at diagnosis | 0.09 | [−0.12, 0.31] | −0.1 | [−0.39, 0.18] |
| Intercept | 52.23*** | [46.35, 58.1] | 49.14*** | [41.44, 56.85] |
p < 0.05;
p < 0.01;
p < 0.001
CI—confidence interval; coeff—beta coefficient; PSA—prostate-specific antigen; ref—reference group for categorical/binary variables
Note. Intercept represents referent participant, a 63.5-year-old, non-Hispanic White, married, gay man who had surgery and is 5.8 years since diagnosis, with a PSA level at diagnosis of 7.6 ng/ml and high social support.
Discussion
In this cross-sectional study of 186 GBMPCa, the authors found evidence that lower social support was associated with lower general and prostate cancer–specific QOL. These patterns were most evident for men with low general social support and men who wanted additional support. Men with low general social support had worse prostate cancer–specific QOL and mental health. Similarly, wanting more prostate cancer–specific social support was associated with lower prostate cancer–specific and general QOL. Conversely, having more types of social support in one’s network was associated with better QOL. This finding supports the idea from the conceptual model that social support operates through the social network and supports other work that suggests that gay and bisexual men may have wider-ranging sources of support than heterosexual men. The current findings connect these ideas of social networks and support with evidence that they are associated with differences in QOL for GBMPCa.
These results are consistent with extensive literature on social relationships and health (Berkman & Glass, 2000; Umberson, Crosnoe, & Reczek, 2010), particularly that socially isolated individuals have worse health than those with more social integration and support. For prostate cancer specifically, others have found that lower social support is associated with higher risk of mortality (Jan et al., 2016) and worse QOL (Colloca & Colloca, 2016). The current findings regarding the relatively high prevalence of support received from chosen family and friends versus biologic family is consistent with other work on support and caregiving for gender and sexual minorities (Gabrielson, Holston, & Dyck, 2014), including lesbians with cancer and older adults who identify as a sexual minority.
The specific outcomes and findings of the hormonal scale of the EPIC measure and the mental components scale may be a function of social support, with these outcomes reflecting similar domains. For example, the hormonal scale items include questions related to changes in mood that may result in that subscale being more associated with psychosocial exposures (e.g., social support). The sexual EPIC subscale was also associated with social support, particularly more types of social support and wanting more social support. In addition to the general effect of prostate cancer treatment on sexual functioning experienced by all men, GBMPCa face unique challenges, including the loss of the prostate as a site for sexual pleasure in anal sex (Rosser, Capistrant, et al., 2016), loss of ejaculate (Mitteldorf, 2005), persistent rectal irritation or pain sufficient to prevent receptive anal sex (Goldstone, 2005; Hart et al., 2014), and erections too weak for insertive sex. The authors’ results suggest that having more diversity of social support providers was associated with higher sexual QOL and that wanting more support was associated with lower sexual QOL. Having more support providers, including support groups or talking to other gay and bisexual men, may help GBMPCa adjust to the changes in sexual function associated with prostate cancer treatment, particularly those changes that are unique to gay and bisexual men.
Although support groups and supportive interventions have been an active area of cancer practice and research, interventions have rarely been conducted among gay and bisexual men. The current findings that GBMPCa wanted more informational support is consistent with qualitative evidence that the experience of receiving advice during prostate cancer support groups for gay and bisexual men informed prostate cancer treatment decisions (Capistrant et al., 2016). Evidence from research on lesbians with breast cancer and older adults who identify as a sexual minority report similar findings, particularly a strong preference and unmet need for sexual minority–specific support groups and community services (Wandrey, Qualls, & Mosack, 2016). Because of findings that low social support and wanting more support were associated with lower QOL, clinicians treating GBMPCa should consider referral to local GBMPCa groups where available and/or to online groups to meet this need for more support. Given the effects of prostate cancer treatment on sexual functioning, many GBMPCa feel alienated rather than supported in groups where most survivors are heterosexual (Capistrant et al., 2016). Support from other gay and bisexual men seems particularly relevant as a source of emotional and informational support. Cancer centers and clinical organizations that facilitate prostate cancer support groups should consider offering tailored groups for GBMPCa or resources to connect these men.
The salience of friends and chosen family has implications for research and clinical practice. Additional research is needed to establish systematic measures of social support networks and support types that are specific to cancer and inclusive of the diversity of support for sexual minorities, including chosen family (Kamen, Smith-Stoner, Heckler, Flannery, & Margolies, 2015). Future studies should examine the dyadic relationship between partners’ roles and QOL (Umberson, Thomeer, Kroeger, Lodge, & Xu, 2015; Umberson, Thomeer, Reczek, & Donnelly, 2016), as well as the efficacy of social support interventions tailored to GBMPCa (Badger et al., 2011). Clinical teams working with GBMPCa should acknowledge and support the diversity of support providers, particularly those who are considered chosen family. Other research has identified the importance of friends and chosen family for broader groups of patients with cancer who identify as a sexual minority (Kamen, Smith-Stoner, et al., 2015).
These results are in line with previous work that suggests GBMPCa have low mental and physical health and well-being. Although the current study design did not include heterosexual men to test formal differences between gay and bisexual men and heterosexual men with prostate cancer, Ussher et al. (2016) found that GBMPCa had lower QOL compared to heterosexual men with prostate cancer (Ussher et al., 2016). The current study findings that social support was associated with worse QOL outcomes may be one mechanism for such a difference between gay and bisexual men and heterosexual men with prostate cancer. Future research should investigate this more explicitly to document prevalence and identify mechanisms of health disparities for GBMPCa (Quinn, Sanchez, et al., 2015; Wender et al., 2015).
Strengths and Limitations
The current study had some limitations. First, the measures of prostate cancer–specific support (e.g., support received and wanted, the network of support providers) were not scales that have been tested for reliability or validity and should be interpreted with caution. Because this is a new area of research, no measures exist with the specificity of prostate cancer–specific support and the networks of gay and bisexual men. However, these measures resulted from previous qualitative work with GBMPCa, so they are likely measuring relevant domains. In addition, the strong correspondence between these new measures and existing, validated measures of general social support (e.g., the ENRICHD Social Support Inventory) offsets these concerns. Second, the study design involved a convenience sample of gay and bisexual men in North America recruited from an online social support website with limited generalizability beyond White, educated men in the United States and Canada who have access to the Internet. Third, in online research where the researchers never meet the participants in person, unique risks of participant fidelity and fraud exist. The authors used established de-duplication and cross-validation methods to reject 57% of submitted surveys as suspect and to minimize the bias this threat might bring to the results. Fourth, the authors used self-report measures. The nature of Internet-based survey research made it impractical to use objective assessments or medical record extractions, which are easier to implement in clinic-based research. For example, PSA levels prior to treatment were ascertained and self-reported, which may be subject to recall bias based on the duration of time since treatment. Fifth, although the authors adjusted for several demographic, socioeconomic, and prostate cancer–specific variables that, based on prior literature, may confound the relationship between social support and low QOL, residual confounding may exist from other variables. Lastly, the cross-sectional design yields associations. The authors caution against making causal claims (e.g., that low social support results in lower QOL) from these results.
However, this study also has many strengths. This is the largest survey of GBMPCa conducted to date. The survey covered about 15 domains, and its comprehensiveness facilitates addressing significant gaps in the quantitative data on social determinants of QOL and prostate cancer outcomes among sexual minority men. Most of the measures used to assess social support and QOL were longstanding, widely used scales, which enhance the internal validity of the findings and facilitate comparisons to other literature. The new questions assessing prostate cancer–specific support (e.g., support received and wanted, the network of support providers) were informed by previous qualitative research and offer a level of depth and detail about the kinds and sources of support gay and bisexual men have and want throughout their experiences with prostate cancer.
Implications for Nursing Practice
Oncology nurses play important roles to encourage and sustain social support and are uniquely suited to address these needs among GBMPCa (Carter et al., 2014; Cohen, Ferrell, Vrabel, Visovsky, & Schaefer, 2010; Kamen, Smith-Stoner, et al., 2015). Sexual minority patients report feeling unsure whether or how to disclose sexual orientation with their providers, and they feel isolated as a result (Baughman, Clark, & Boehmer, 2017). Nurses may be unaware of a patient’s sexual orientation (Boehmer & Case, 2004; Quinn, Schabath, Sanchez, Sutton, & Green, 2015). One approach to identifying whether a patient may want referrals to resources specific to sexual minorities is to take a sexual history (Rosser et al., 2017), which would offer an opportunity to understand a patient’s support needs while gathering clinically relevant information for monitoring prostate cancer outcomes. During hospital discharge, outpatient visits to cancer centers for treatment, and myriad other clinical encounters, oncology nurses may come into contact with social networks of GBMPCa, which may include friends and chosen family as support providers more often than the typical support network of biologic family. Oncology nurses should be cognizant of these differences in the support networks of GBMPCa. Given the reported importance of support from GBMPCa, nurses may also take direct steps to develop or refer to existing networks of other GBMPCa. Outside of the clinical encounters, nurses might consider the extent to which support groups for GBMPCa are available within the medical center or region. If none are available, nurses might consider starting one with a medical social worker or other professional trained in group facilitation or referring patients to online support resources, such as Malecare.
Conclusion
This novel study among GBMPCa, an understudied population for cancer research, found that low social support was associated with lower QOL. Most men expressed wanting more social support, specifically and most commonly from other GBMPCa. In addition, men who reported wanting more support had lower QOL than those who did not want more support. This gap suggests that programs need to offer social support, particularly emotional and informational support. Such programs should be tailored to the needs of sexual minority men if disparities in outcomes between gay and bisexual men and heterosexual men with prostate cancer are to be addressed effectively.
KNOWLEDGE TRANSLATION.
Compared to studies of heterosexual men with prostate cancer (PCa), gay and bisexual men with PCa rely on different social support networks, such as chosen family, friends, and other gay and bisexual men with PCa.
Gay and bisexual men with PCa who reported wanting more social support had lower mental health–related quality of life and lower quality of life related to PCa symptoms.
Gay and bisexual men with PCa reported high demand for additional support from other gay and bisexual men with PCa.
Acknowledgments
This research was funded by grants from the National Cancer Institute (R21CA182041, R01CA218657, principal investigator [PI]: Rosser) and via an institutional research grant to the University of Minnesota from the American Cancer Society (PI: Capistrant). During the writing of this article, Konety was supported by research grants from Genomic Health, FKD Pharmaceuticals, and Genentech, and has previously consulted for OPKO Health. Mitteldorf has previously participated in advisory or review activities for Astellas Pharmaceuticals.
Contributor Information
Benjamin D. Capistrant, School for Social Work at Smith College in Northampton, MA;.
Lindsey Lesher, Department of Epidemiology and Community Health,.
Nidhi Kohli, Department of Educational Psychology,.
Enyinnaya N. Merengwa, School of Public Health,.
Badrinath Konety, Department of Urology, all at the University of Minnesota in Minneapolis;.
Darryl Mitteldorf, Malecare in New York, NY;.
William G. West, College of Liberal Arts.
B.R. Simon Rosser, School of Public Health, both at the University of Minnesota in Minneapolis..
REFERENCES
- Badger TA, Segrin C, Figueredo AJ, Harrington J, Sheppard K, Passalacqua S, … Bishop M (2011). Psychosocial interventions to improve QOL in prostate cancer survivors and their intimate or family partners. Quality of Life Research, 20, 833–844. 10.1007/s11136-010-9822-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baughman A, Clark MA, & Boehmer U (2017). Experiences and concerns of lesbian, gay, or bisexual survivors of colorectal cancer. Oncology Nursing Forum, 44, 350–357. 10.1188/17.ONF.350-357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman J, Gore JL, Saigal CS, Kwan L, & Litwin MS (2009). Partnership and outcomes in men with prostate cancer. Cancer, 115, 4688–4694. 10.1002/cncr.24544 [DOI] [PubMed] [Google Scholar]
- Berkman LF, & Glass TA (2000). Social integration, social networks, social support, and health In Berkman LF, & Kawachi I (Eds.), Social epidemiology (pp. 137–173). New York, NY: Oxford University Press. [Google Scholar]
- Boehmer U, & Case P (2004). Physicians don’t ask, sometimes patients tell: Disclosure of sexual orientation among women with breast carcinoma. Cancer, 101, 1882–1889. 10.1002/cncr.20563 [DOI] [PubMed] [Google Scholar]
- Boehmer U, Miao X, & Ozonoff A (2011). Cancer survivorship and sexual orientation. Cancer, 117, 3796–3804. 10.1002/cncr.25950 [DOI] [PubMed] [Google Scholar]
- Capistrant BD, Torres B, Merengwa E, West WG, Mitteldorf D, & Rosser BR (2016). Caregiving and social support for gay and bisexual men with prostate cancer. Psycho-Oncology, 25, 1329–1336. 10.1002/pon.4249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter N, Miller PA, Murphy BR, Payne VJ, & Bryant-Lukosius D (2014). Healthcare providers’ perspectives of the supportive care needs of men with advanced prostate cancer. Oncology Nursing Forum, 41, 421–430. 10.1188/14.ONF.421-430 [DOI] [PubMed] [Google Scholar]
- Chamie K, Kwan L, Connor SE, Zavala M, Labo J, & Litwin MS (2012). The impact of social networks and partnership status on treatment choice in men with localized prostate cancer. BJU International, 109, 1006–1012. 10.1111/j.1464-410X.2011.10515.x [DOI] [PubMed] [Google Scholar]
- Cohen MZ, Ferrell BR, Vrabel M, Visovsky C, & Schaefer B (2010). What does it mean to be an oncology nurse? Reex-amining the life cycle concepts. Oncology Nursing Forum, 37, 561–570. 10.1188/10.ONF.561-570 [DOI] [PubMed] [Google Scholar]
- Collaborative Studies Coordinating Center. (1998). ENRICHD protocol [v.7.0]. Retrieved from http://www.cscc.unc.edu/enrichd/protocol/ENRICHDProtocol072103.pdf
- Colloca G, & Colloca P (2016). The effects of social support on health-related quality of life of patients with metastatic prostate cancer. Journal of Cancer Education, 31, 244–252. 10.1007/s13187-015-0884-2 [DOI] [PubMed] [Google Scholar]
- Du KL, Bae K, Movsas B, Yan Y, Bryan C, & Bruner DW (2012). Impact of marital status and race on outcomes of patients enrolled in Radiation Therapy Oncology Group prostate cancer trials. Supportive Care in Cancer, 20, 1317–1325. 10.1007/s00520-011-1219-4 [DOI] [PubMed] [Google Scholar]
- Erosheva EA, Kim HJ, Emlet C, & Fredriksen-Goldsen KI (2016). Social networks of lesbian, gay, bisexual, and transgender older adults. Research on Aging, 38, 98–123. 10.1177/0164027515581859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrielson ML, Holston EC, & Dyck MJ (2014). Are they family or friends? Social support instrument reliability in studying older lesbians. Journal of Homosexuality, 61, 1589–1604. 10.1080/00918369.2014.944050 [DOI] [PubMed] [Google Scholar]
- Goldsen J, Bryan AE, Kim HJ, Muraco A, Jen S, & Fredriksen-Goldsen KI (2017). Who says I do: The changing context of marriage and health and quality of life for LGBT older adults. Gerontologist, 57(Suppl. 1), S50–S62. 10.1093/geront/gnw174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstone SE (2005). The ups and downs of gay sex after prostate cancer treatment In Perlman G, & Drescher J (Eds.), A gay man’s guide to prostate cancer (pp. 43–55). Binghampton, NY: Haworth Medical Press. [Google Scholar]
- Harden JK, Sanda MG, Wei JT, Yarandi H, Hembroff L, Hardy J, & Northouse LL (2013a). Partners’ long-term appraisal of their caregiving experience, marital satisfaction, sexual satisfaction, and quality of life 2 years after prostate cancer treatment. Cancer Nursing, 36, 104–113. 10.1097/NCC.0b013e3182567c03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden JK, Sanda MG, Wei JT, Yarandi HN, Hembroff L, Hardy J, & Northouse L (2013b). Survivorship after prostate cancer treatment: Spouses’ quality of life at 36 months. Oncology Nursing Forum, 40, 567–573. 10.1188/13.ONF.567-573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart TL, Coon DW, Kowalkowski MA, Zhang K, Hersom JI, Goltz HH, … Latini DM (2014). Changes in sexual roles and quality of life for gay men after prostate cancer: Challenges for sexual health providers. Journal of Sexual Medicine, 11, 2308–2317. 10.1111/jsm.12598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo B, & Goodman M (2013). Multivariate or multivariable regression? American Journal of Public Health, 103, 39–40. 10.2105/AJPH.2012.300897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenbeck BK, Dunn RL, Wei JT, Montie JE, & Sanda MG (2003). Determinants of long-term sexual health outcome after radical prostatectomy measured by a validated instrument. Journal of Urology, 169, 1453–1457. 10.1097/01.ju.0000056737.40872.56 [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. (2011). The health of lesbian, gay, bisexual and transgender people: Building a foundation for a better understanding. Washington, DC: National Academies Press. [PubMed] [Google Scholar]
- Jan M, Bonn SE, Sjölander A, Wiklund F, Stattin P, Holmberg E, … Bälter K (2016). The roles of stress and social support in prostate cancer mortality. Scandinavian Journal of Urology, 50, 47–55. 10.3109/21681805.2015.1079796 [DOI] [PubMed] [Google Scholar]
- Kamen C, Mustian KM, Heckler C, Janelsins MC, Peppone LJ, Mohile S, … Morrow GR (2015). The association between partner support and psychological distress among prostate cancer survivors in a nationwide study. Journal of Cancer Survivorship, 9, 492–499. 10.1007/s11764-015-0425-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamen C, Palesh O, Gerry AA, Andrykowski MA, Heckler C, Mohile S, … Mustian K (2014). Disparities in health risk behavior and psychological distress among gay versus heterosexual male cancer survivors. LGBT Health, 1, 86–92. 10.1089/lgbt.2013.0022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamen CS, Smith-Stoner M, Heckler CE, Flannery M, & Margolies L (2015). Social support, self-rated health, and lesbian, gay, bisexual, and transgender identity disclosure to cancer care providers. Oncology Nursing Forum, 42, 44–51. 10.1188/15.ONF.44-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Fredriksen-Goldsen KI, Bryan AE, & Muraco A (2017). Social network types and mental health among LGBT older adults. Gerontologist, 57(Suppl. 1), S84–S94. 10.1093/geront/gnw169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMasters T, Madhavan S, Sambamoorthi U, & Kurian S (2013). A population-based study comparing HRQoL among breast, prostate, and colorectal cancer survivors to propensity score matched controls, by cancer type, and gender. Psycho-Oncology, 22, 2270–2282. 10.1002/pon.3288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Zeliadt SB, Hall IJ, Smith JL, Ekwueme DU, Moinpour CM, … Ramsey SD (2013). Burden among partner caregivers of patients diagnosed with localized prostate cancer within 1 year after diagnosis: An economic perspective. Supportive Care in Cancer, 21, 3461–3469. 10.1007/s00520-013-1931-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolies L (2014). The psychosocial needs of lesbian, gay, bisexual, or transgender patients with cancer. Clinical Journal of Oncology Nursing, 18, 462–464. 10.1188/14.CJON.462-464 [DOI] [PubMed] [Google Scholar]
- Matheson L, Watson EK, Nayoan J, Wagland R, Glaser A, Gavin A, … Rivas C (2017). A qualitative metasynthesis exploring the impact of prostate cancer and its management on younger, unpartnered and gay men. European Journal of Cancer Care, 26(6), e12676 10.1111/ecc.12676 [DOI] [PubMed] [Google Scholar]
- McCaughan E, Prue G, McSorley O, Northouse L, Schafenacker A, & Parahoo K (2013). A randomized controlled trial of a self-management psychosocial intervention for men with prostate cancer and their partners: A study protocol. Journal of Advanced Nursing, 69, 2572–2583. 10.1111/jan.12132 [DOI] [PubMed] [Google Scholar]
- Mitchell PH, Powell L, Blumenthal J, Norten J, Ironson G, Pitula CR, … Berkman LF (2003). A short social support measure for patients recovering from myocardial infarction: The ENRICHD social support inventory. Journal of Cardiopulmonary Rehabilitation, 23, 398–403. [DOI] [PubMed] [Google Scholar]
- Mitteldorf D (2005). Psychotherapy with gay prostate cancer patients. Journal of Gay and Lesbian Psychotherapy, 9, 56–67. 10.1300/J236v09n01_05 [DOI] [Google Scholar]
- Movsas TZ, Yechieli R, Movsas B, & Darwish-Yassine M (2016). Partner’s perspective on long-term sexual dysfunction after prostate cancer treatment. American Journal of Clinical Oncology, 39, 276–279. 10.1097/coc.0000000000000067 [DOI] [PubMed] [Google Scholar]
- Nasser NJ, Cohen GN, Dauer LT, & Zelefsky MJ (2016). Radiation safety of receptive anal intercourse with prostate cancer patients treated with low-dose-rate brachytherapy. Brachytherapy, 15, 420–425. 10.1016/j.brachy.2016.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn GP, Sanchez JA, Sutton SK, Vadaparampil ST, Nguyen GT, Green BL, … Schabath MB (2015). Cancer and lesbian, gay, bisexual, transgender/transsexual, and queer/questioning (LGBTQ) populations. CA: A Cancer Journal for Clinicians, 65, 384–400. 10.3322/caac.21288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn GP, Schabath MB, Sanchez JA, Sutton SK, & Green BL (2015). The importance of disclosure: Lesbian, gay, bisexual, transgender/transsexual, queer/questioning, and intersex individuals and the cancer continuum. Cancer, 121, 1160–1163. 10.1002/cncr.29203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosser BRS, Capistrant BD, Torres MB, Konety B, Merengwa E, Mitteldorf D, & West W (2016). The effects of radical prostatectomy on gay and bisexual men’s sexual functioning and behavior: Qualitative results from the Restore study. Sexual and Relationship Therapy, 31, 432–445. 10.1080/14681994.2016.1217985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosser BRS, Gurak L, Horvath KJ, Oakes JM, Konstan J, & Danilenko GP (2009). The challenges of ensuring participant consent in internet-based sex studies: A case study of the men’s internet sex (MINTS-I and II) studies. Journal of Computer-Mediated Communication, 14, 606–626. 10.1111/j.1083-6101.2009.01455.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosser BRS, Kohli N, Lesher L, Capistrant BD, Dewit J, Kilian G, … West W (2017). What gay and bisexual men treated for prostate cancer are offered and attempt as sexual rehabilitation for prostate cancer: Results from the Restore Study. Urology Practice. Advance online publication. 10.1016/j.urpr.2017.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosser BRS, Merengwa E, Capistrant BD, Iantaffi A, Kilian G, Kohli N, … West W (2016). Prostate cancer in gay, bisexual, and other men who have sex with men: A review. LGBT Health, 3, 32–41. 10.1089/lgbt.2015.0092 [DOI] [Google Scholar]
- Segrin C, Badger TA, & Harrington J (2012). Interdependent psychological quality of life in dyads adjusting to prostate cancer. Health Psychology, 31, 70–79. 10.1037/a0025394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiu C, Muraco A, & Fredriksen-Goldsen K (2016). Invisible care: Friend and partner care among older lesbian, gay, bisexual, and transgender (LGBT) adults. Journal of the Society for Social Work and Research, 7, 527–546. 10.1086/687325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umberson D, Crosnoe R, & Reczek C (2010). Social relationships and health behavior across life course. Annual Review of Sociology, 36, 139–157. 10.1146/annurev-soc-070308-120011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umberson D, Thomeer MB, Kroeger RA, Lodge AC, & Xu M (2015). Challenges and opportunities for research on same-sex relationships. Journal of Marriage and Family, 77, 96–111. 10.1111/jomf.12155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umberson D, Thomeer MB, Reczek C, & Donnelly R (2016). Physical illness in gay, lesbian, and heterosexual marriages: Gendered dyadic experiences. Journal of Health and Social Behavior, 57, 517–531. 10.1177/0022146516671570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ussher JM, Perz J, Kellett A, Chambers S, Latini D, Davis ID, … Williams S (2016). Health-related quality of life, psychological distress, and sexual changes following prostate cancer: A comparison of gay and bisexual men with heterosexual men. Journal of Sexual Medicine, 13, 425–434. 10.1016/j.jsxm.2015.12.026 [DOI] [PubMed] [Google Scholar]
- Wandrey RL, Qualls WD, & Mosack KE (2016). Are main-stream support services meeting the needs of sexual minority women with breast cancer? An exploration of the perspectives and experiences of users of an online support forum. Journal of Gay and Lesbian Social Services, 28, 336–348. 10.1080/10538720.2016.1221783 [DOI] [Google Scholar]
- Ware J Jr., Kosinski M, & Keller SD (1996). A 12-item short-form health survey: Construction of scales and preliminary tests of reliability and validity. Medical Care, 34, 220–233. [DOI] [PubMed] [Google Scholar]
- Wassersug RJ, Lyons A, Duncan D, Dowsett GW, & Pitts M (2013). Diagnostic and outcome differences between heterosexual and nonheterosexual men treated for prostate cancer. Urology, 82, 565–571. 10.1016/j.urology.2013.04.022 [DOI] [PubMed] [Google Scholar]
- Wassersug RJ, Westle A, & Dowsett GW (2017). Men’s sexual and relational adaptations to erectile dysfunction after prostate cancer treatment. International Journal of Sexual Health, 29, 69–79. 10.1080/19317611.2016.1204403 [DOI] [Google Scholar]
- Wei JT, Dunn RL, Litwin MS, Sandler HM, & Sanda MG (2000). Development and validation of the Expanded Prostate Cancer Index Composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology, 56, 899–905. [DOI] [PubMed] [Google Scholar]
- Wei JT, Dunn RL, Sandler HM, McLaughlin PW, Montie JE, Litwin MS, … Sanda MG (2002). Comprehensive comparison of health-related quality of life after contemporary therapies for localized prostate cancer. Journal of Clinical Oncology, 20, 557–566. 10.1200/JCO.2002.20.2.557 [DOI] [PubMed] [Google Scholar]
- Wender R, Sharpe KB, Westmaas JL, & Patel AV (2015). The American Cancer Society’s approach to addressing the cancer burden in the LGBT community. LGBT Health, 3, 15–18. 10.1089/lgbt.2015.0089 [DOI] [PubMed] [Google Scholar]
- Wootten AC, Abbott JM, Farrell A, Austin DW, & Klein B (2014). Psychosocial interventions to support partners of men with prostate cancer: A systematic and critical review of the literature. Journal of Cancer Survivorship, 8, 472–484. 10.1007/s11764-014-0361-7 [DOI] [PubMed] [Google Scholar]


