Abstract
Background
An increase in invasive meningococcal disease (IMD) serogroup W (IMD-W) cases caused by sequence type-11 clonal complex (cc11) was observed from October 2015 in the Netherlands. We compared the clinical picture and disease outcome of IMD-W cases with other serogroups, adjusting for host characteristics.
Methods
We included IMD cases reported from January 2015 to June 2018 in the Netherlands and assessed clinical manifestation and symptoms at disease onset and calculated case fatality rates (CFRs). We used logistic regression to compare clinical manifestations and mortality of IMD-W with IMD caused by meningococci serogroup B, Y, or C, adjusting for age, gender, and comorbidities.
Results
A total of 565 IMD cases were reported, of which 204 were IMD-W, 270 IMD-B, 63 IMD-Y, and 26 IMD-C. Most IMD-W isolates belonged to cc11 (93%; 175/188). Compared with other serogroups, IMD-W patients were diagnosed more often with septicemia (46%) or pneumonia (12%) and less often with meningitis (17%, P < .001). IMD-W cases presented more often with respiratory symptoms (45%, P < .001); 16% of IMD-W patients presented with diarrhea without IMD-specific symptoms (P = .061). The CFR for IMD-W was 16% (32/199, P < .001). The differences between IMD-W and other serogroups remained after adjusting for age, gender, and comorbidities.
Conclusions
The atypical presentation and severe outcome among IMD-W cases could not be explained by age, gender, and comorbidities. Almost all our IMD-W cases were caused by cc11. More research is needed to identify the bacterial factors involved in clinical presentation and severity of IMD-W cc11.
Keywords: meningococcal infections, Neisseria meningitidis, serogroup W, public health surveillance, Netherlands
Invasive meningococcal disease (IMD) caused by serogroup W clonal complex 11 is associated with higher proportions of septicemia and pneumonia, and a higher case fatality rate than IMD caused by other meningococcal serogroups, after adjusting for age, gender, and comorbidities.
Invasive infections due to the gram-negative bacterium Neisseria meningitidis are usually associated with severe and acute disease presenting as meningitis and/or septicemia and less often as pneumonia, septic arthritis, or subacute meningococcemia [1, 2]. The mortality rate among invasive meningococcal disease (IMD) cases ranges between 5% and 10% in high-income countries [3]. Patients who survive may suffer from serious sequelae, such as cognitive or motor deficits, bilateral hearing loss, seizures, or visual impairment [1, 4].
In Europe, the majority of IMD cases are caused by serogroup B (IMD-B) and C (IMD-C) meningococci. Since the implementation of national meningococcal immunization programs with the use of conjugate serogroup C vaccines (MenC), IMD-C has greatly decreased in Europe [3], which was also observed in the Netherlands [5].
Before 2000, serogroup W meningococcal disease (IMD-W) was only occasionally described. Since 2009/2010, an increase in IMD-W cases has been observed in the United Kingdom [3, 6–8] and outside of Europe, in countries in South America, in Saudi Arabia, and recently in Australia and Canada [8–12].
Annual incidence rates of IMD rose from 0.5/100 000 population in 1960 to 4.5/100 000 in 2001 [5, 13]. Invasive meningococcal disease decreased following implementation of the MenC vaccine in 2002, which was attributed not only to the vaccine-related decline of IMD-C cases but also to a spontaneous reduction in IMD-B cases. In contrast, IMD-W cases have been rare in the Netherlands, with an average of 4 cases per year during the period 2002–2014 [5]. However, since October 2015, a sudden increase in IMD-W was observed, with 80 cases in 2017 (0.5/100 000) [5, 14, 15].
The recent IMD-W increase in the Netherlands and elsewhere is mainly due to the expansion of the hypervirulent sequence type-11 clonal complex (cc11) [6, 7, 15–17], which emerged from a sublineage in South America in 2003 [16] and spread to the United Kingdom in 2009 (so-called original UK strain) [7] and other European countries [16, 18–21]. Since 2013, another sublineage from the South America UK:cc11 strain spread in the United Kingdom [16, 22], which is called the UK 2013 strain sublineage. In the Netherlands, the rise in IMD-W is caused by isolates belonging to the UK 2013 strain [23].
Serogroup W infections have already been described with atypical clinical manifestations such as pneumonia, septic arthritis, or endocarditis [1, 7, 24, 25]. More recently, case reports described IMD-W cases presenting with gastrointestinal symptoms, including abdominal pain and diarrhea [14, 26]. High mortality was observed in a review of 7 IMD-W cases [26] and has been documented in countries with a proportional increase in IMD-W cases [6, 27]. Therefore, the purpose of this study was to compare the clinical picture and the severity of IMD-W cases with IMD cases caused by meningococci with serogroups B, C, and Y. In addition, we assessed if the differences in the clinical picture and the severity of IMD-W cases could be explained by the underlying differences in host characteristics, including comorbidity, age, and gender.
METHODS
The study used an observational cohort design, with data collected prospectively from the Dutch IMD surveillance system from 1 January 2015 to 30 June 2018. Within the surveillance system, patients with a laboratory confirmation of IMD are immediately notified. The Netherlands Reference Laboratory for Bacterial Meningitis (NRLBM) performs typing on isolates of samples from patients with IMD, sent on a voluntary basis from medical microbiology laboratories. All IMD-W isolates were assessed by whole-genome sequencing (WGS), and fine types and genotypes were extracted from the sequences for the whole study period. Invasive meningococcal disease isolates from other serogroups were sequenced from 2017 onward. Diagnostic and typing results were provided by the NRLBM, while pseudonymized clinical and epidemiological data were provided by the Dutch notification system, if available. Data linkage is routinely performed at the National Institute for Public Health (RIVM) since 2003. For the period 2015–2018, 95% of notified cases had an isolate sent to the NRLBM. Demographic data for the Dutch population during the study period were obtained from the Central Bureau of Statistics [28].
Comorbidities were classified as immunocompromised-related and other comorbidities based on the underlying categories of the Dutch notification system. Clinical manifestations were categorized into septicemia/septic shock without meningitis, septicemia/septic shock and meningitis, meningitis only, mild meningococcemia, pneumonia, and septic arthritis. Symptoms at disease onset were categorized into IMD-specific, gastrointestinal, and respiratory symptoms. Gastrointestinal symptoms were further categorized into no diarrhea, diarrhea with IMD-specific symptoms, and diarrhea without IMD-specific symptoms. Data on symptoms and intensive care unit (ICU) admittance were available only since 2017.
We used Fisher’s exact test to test for statistical significance (P < .05) in categorical variables between the different serogroups. Kruskal-Wallis rank sum test was used to test for an overall difference in age between the serogroups. We performed binomial logistic regression to estimate the association between serogroup and death and multinomial logistic regression to estimate the associations between serogroup and clinical manifestation and symptoms. In the regression analyses, IMD-W cases were compared with IMD cases caused by meningococci with other serogroups (IMD-non-W) to assess the overall difference in characteristics of IMD-W versus IMD-non-W and to ensure adequate sample size. The same regression analyses were performed for IMD-W versus IMD-B only. We focused on IMD-B, as it is the most common serogroup in the Netherlands. We assessed whether the associations changed when we included case characteristics (age, gender, and comorbidity) or disease characteristics (clinical manifestation) in the model. Data management and statistical analysis were conducted using R, version 3.4.3.
RESULTS
Between January 2015 and the end of June 2018, results of 567 IMD cases were reported by the NRLBM. Of those, 2 cases were excluded for missing serogroup information. One IMD-X case and 1 IMD-E case were excluded from the analysis, leaving 563 cases for analysis. Of those, 506 (90%) were diagnosed by culture and 57 (10%) were diagnosed by polymerase chain reaction. A total of 540 cases could be linked to notification data.
Serogroup-specific Number of Cases and Incidence Rates
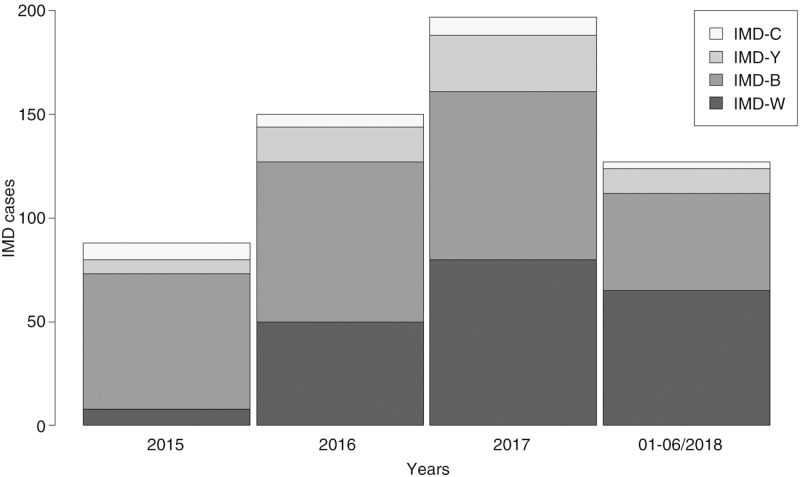
Among the 563 IMD cases, most were due to serogroup B (n = 270, 48%) and W (n = 204, 36%) (Table 1). Invasive meningococcal disease incidence increased significantly from 2015 to the first half of 2018 due to an increase in IMD-W; IMD-B and IMD-Y incidence increased slightly and IMD-C remained stable (Figure 1 and Table 1).
Table 1.
Number and Percentage of Invasive Meningococcal Disease Cases by Serogroup and Year and the Incidence Rate Ratios, the Netherlands, January 2015 to June 2018
| 2015 | 2016 | 2017 | 2018a | 2018a vs 2015a | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Serogroup | n (%) | IR (/100 000) | n (%) | IR (/100 000) | n (%) | IR | n (%) | IR (/100 000) | IRR (95% CI) | P Value |
| W | 9 (10.0) | 0.05 | 50 (33.1) | 0.29 | 80 (40.4) | 0.47 | 65 (50.8) | 0.76 | 16.0 (5.8–43.9) | <.001 |
| B | 65 (72.2) | 0.38 | 77 (51.0) | 0.45 | 81 (40.9) | 0.47 | 47 (36.7) | 0.55 | 1.4 (.9–2.3) | .106 |
| Y | 7 (7.8) | 0.04 | 17 (11.3) | 0.10 | 27 (13.6) | 0.16 | 12 (9.4) | 0.14 | 2.4 (.8–6.7) | .096 |
| C | 8 (8.9) | 0.05 | 6 (4.0) | 0.04 | 9 (4.5) | 0.05 | 3 (2.3) | 0.03 | 0.6 (.1–2.5) | .465 |
| Total | 89 (100) | 0.53 | 150 (100) | 0.88 | 197 (100) | 1.15 | 127 (100) | 1.48 | 2.7(1.9–3.8) | <.001 |
Two cases of IMD-E (one case) and IMD-X (one case) not included in the table.
Abbreviations: CI, confidence interval; IMD, invasive meningococcal disease; IR, incidence rate; IRR, incidence rate ratio.
aData for comparison included January to June 2015 versus January to June 2015.
Figure 1.
Cases with IMD by serogroup and year, the Netherlands, January 2015 to June 2018. Abbreviation: IMD, invasive meningococcal disease.
Clonal Complex Distribution
A total of 329 isolates were assessed by WGS. Of 188 IMD-W isolates, 93% belonged to cc11 (Table 2 and Supplementary Table 1). Another 5% belonged to cc22. One percent of IMD-B isolates and 64% of IMD-C isolates belonged to cc11. Serogroup Y was absent among cc11 isolates. Among IMD-B, the most common cc were cc32 (40%) and cc41/44 (20%). The majority of serogroup Y isolates belonged to cc23 (79%).
Table 2.
Case Characteristics, Clinical Manifestation, Symptoms at Disease Onset, and Severity of Invasive Meningococcal Disease Cases by Serogroup, the Netherlands, January 2015 to June 2018
| W | B | Y | C | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | P Valuea | |
| Age | <.001 | ||||||||||
| Median (range), y | 56 (0–97) | 17 (0–96) | 70 (0–95) | 52 (0–93) | 33 (0–97) | <.001 | |||||
| <10 y | 18 | 8.9 | 108 | 40.9 | 1 | 1.6 | 3 | 11.5 | 130 | 23.2 | |
| 10–19 y | 29 | 14.3 | 53 | 19.3 | 7 | 11.1 | 2 | 7.7 | 91 | 16.2 | |
| 20–64 y | 85 | 41.9 | 73 | 27.4 | 14 | 22.2 | 11 | 42.3 | 183 | 32.6 | |
| ≥65 y | 71 | 35.0 | 35 | 13.0 | 41 | 65.1 | 10 | 38.5 | 157 | 28.0 | |
| Genderb | .029 | ||||||||||
| Female | 134 | 66.0 | 143 | 53.0 | 37 | 58.7 | 13 | 50.0 | 327 | 58.2 | |
| Comorbidities | .009 | ||||||||||
| No | 123 | 74.5 | 165 | 86.4 | 31 | 66.0 | 16 | 84.2 | 335 | 79.6 | |
| Yes, immunocompromised | 19 | 11.5 | 9 | 4.7 | 8 | 17.0 | 1 | 5.3 | 37 | 8.6 | |
| Yes, other | 23 | 13.9 | 17 | 8.9 | 8 | 17.0 | 2 | 10.5 | 50 | 11.9 | |
| MenC vaccination | <.001 | ||||||||||
| Yes | 44 | 27.7 | 96 | 45.9 | 6 | 13.6 | 2 | 11.1 | 148 | 34.4 | |
| No | 115 | 72.3 | 113 | 54.1 | 38 | 86.4 | 16 | 88.9 | 282 | 65.6 | |
| Clinical manifestations | <.001 | ||||||||||
| Septicemia without meningitis | 86 | 45.5 | 63 | 24.7 | 19 | 35.2 | 7 | 28.0 | 175 | 33.5 | |
| Septicemia and meningitis | 11 | 5.8 | 25 | 9.8 | 7 | 13.0 | 1 | 4.0 | 44 | 8.4 | |
| Meningitis without septicemia | 32 | 16.9 | 144 | 56.5 | 12 | 22.2 | 8 | 32.0 | 196 | 37.5 | |
| Mild, without S/M | 17 | 9.0 | 14 | 5.5 | 3 | 5.6 | 5 | 20.0 | 39 | 7.5 | |
| Pneumonia, without S/M | 23 | 12.2 | 4 | 1.6 | 10 | 18.5 | 1 | 4.0 | 38 | 7.3 | |
| Arthritis, without S/M | 9 | 4.8 | 2 | 0.8 | 1 | 1.9 | 3 | 12.0 | 15 | 2.9 | |
| Other | 11 | 5.8 | 3 | 1.2 | 2 | 3.7 | 0 | 0.0 | 16 | 3.1 | |
| Symptomsc | |||||||||||
| Gastrointestinal | .056 | ||||||||||
| No | 81 | 56.6 | 76 | 57.1 | 23 | 62.2 | 11 | 78.6 | 191 | 58.4 | |
| Yes, without diarrhea | 35 | 24.5 | 46 | 34.6 | 9 | 24.3 | 1 | 7.1 | 91 | 27.8 | |
| Yes, with diarrhea | 27 | 18.9 | 11 | 8.3 | 5 | 13.5 | 2 | 14.3 | 45 | 13.8 | |
| Diarrhea | .061 | ||||||||||
| No | 113 | 80.7 | 114 | 91.2 | 31 | 88.6 | 10 | 83.3 | 268 | 85.9 | |
| Yes, with IMD-specific symptomsd | 5 | 3.6 | 5 | 4.0 | 0 | 0.0 | 1 | 8.3 | 11 | 3.5 | |
| Yes, without IMD-specific symptomsd | 22 | 15.7 | 6 | 4.8 | 4 | 11.4 | 1 | 8.3 | 33 | 10.6 | |
| Respiratory | <.001 | ||||||||||
| No | 79 | 55.2 | 115 | 84.6 | 25 | 67.6 | 11 | 78.6 | 230 | 69.7 | |
| Yes | 64 | 44.8 | 21 | 15.4 | 12 | 32.4 | 3 | 21.4 | 100 | 30.3 | |
| Death | |||||||||||
| No | 167 | 83.9 | 253 | 96.2 | 54 | 93.1 | 24 | 96.0 | 439 | 91.3 | |
| Yes | 32 | 16.1 | 10 | 3.8 | 4 | 6.9 | 1 | 4.0 | 47 | 8.7 | |
| ICUc | .160 | ||||||||||
| No | 74 | 58.3 | 76 | 63.9 | 14 | 46.7 | 9 | 81.8 | 173 | 60.3 | |
| Yes | 53 | 41.7 | 43 | 36.1 | 16 | 53.3 | 2 | 18.2 | 114 | 39.7 | |
| Clonal complexe | |||||||||||
| cc11 | 175 | 93.1 | 1 | 1.0 | 0 | 0.0 | 7 | 63.6 | 183 | 55.6 | |
| cc213 | 0 | 0.0 | 16 | 16.0 | 0 | 0.0 | 0 | 0.0 | 16 | 4.9 | |
| cc22 | 9 | 4.8 | 0 | 0.0 | 5 | 15.2 | 0 | 0.0 | 14 | 4.3 | |
| cc23 | 0 | 0.0 | 0 | 0.0 | 26 | 78.8 | 0 | 0.0 | 26 | 7.9 | |
| cc269 | 0 | 0.0 | 12 | 12.0 | 0 | 0.0 | 0 | 0.0 | 12 | 3.6 | |
| cc32 | 0 | 0.0 | 42 | 42.0 | 0 | 0.0 | 0 | 0.0 | 39 | 11.9 | |
| cc41/44 | 0 | 0.0 | 19 | 19.0 | 0 | 0.0 | 1 | 9.1 | 20 | 6.1 | |
| Other cc | 4 | 2.1 | 10 | 10.0 | 2 | 6.1 | 3 | 27.3 | 19 | 5.8 | |
Data are from January to June. Two cases of IMD-E (1 case) and IMD-X (1 case) are not included in the table. “Total” includes cases of serogroup W, B, Y, and C.
Abbreviations: ICU, intensive care unit; IMD, invasive meningococcal disease; S/M, septicemia or meningitis.
a P values for difference between variables and serogroups (W, B, Y, and C).
bOne case with missing information on gender.
cData available only since 2017.
dIMD-specific symptoms: petechiae and/or neck stiffness.
eClonal complex data based on isolates for which clonal complex was known; Data for IMD-W based on the full study period January 2015–June 2018; data for IMD-B, IMD-Y, and IMD-C based on the time period January 2017–June 2018.
Case Characteristics
Patients with IMD-W were older than IMD-B cases (median, 56 vs 17 years), but younger than IMD-Y patients (median, 70 years) (Table 2). Most IMD-B cases occurred among the youngest age group less than 10 years (41%), while most IMD-W cases occurred in the age group 20–64 years (42%, P < .001) (Supplementary Figure 1).
The proportion of females was higher for IMD-W (66%) compared with the other serogroups (P = .029) (Table 2).
The number of patients with (immunocompromising) comorbidities was higher among IMD-W (26%) and IMD-Y (34%) cases compared with IMD-B (14%) and IMD-C (16%, P = .009) cases (Table 2). The differences between IMD-W and IMD-non-W in having (immunocompromising) comorbidities disappeared after adjusting for age (adjusted odds ratio [aOR], 0.9; 95% confidence interval [CI], .5–1.6). Thirty-four percent (148/430) of the study population with known MenC vaccination status were vaccinated (Table 2).
Clinical Manifestations and Symptoms
Cases with IMD-W were more often diagnosed with septicemia/septic shock without meningitis (46%) and less often with meningitis without septicemia/septic shock (17%) compared with other serogroups (P < .001) (Table 2). This difference remained after adjusting for age, gender, and comorbidities (Supplementary Table 2). Additionally, IMD-W cases presented more often with pneumonia (12%) or septic arthritis (4.8%) compared with IMD-B cases (pneumonia: 1.6%, P < .001; septic arthritis: 0.8%, P = .011), which also remained significant after adjusting for host characteristics (Supplementary Table 2). Cases with IMD-Y showed a clinical manifestation distribution similar to IMD-W cases, while IMD-C cases were comparable to IMD-B cases, although numbers were small.
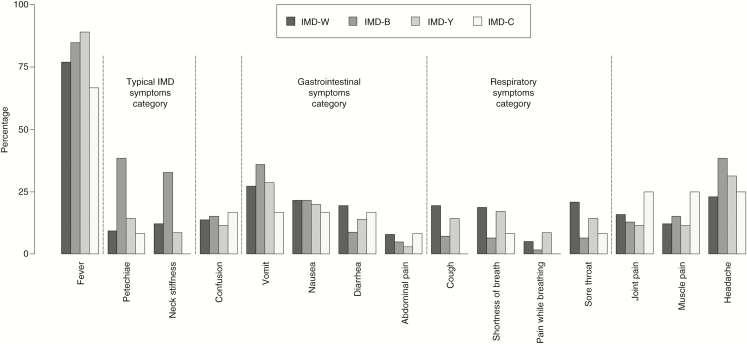
The proportion of cases presenting with typical IMD symptoms, such as petechiae and neck stiffness, was highest among IMD-B patients (59%) and relatively low among IMD-W (19%), and IMD-Y (20%) patients (Figure 2). Patients with IMD-W and IMD-Y more often had symptoms such as diarrhea or abdominal pain, as well as cough, shortness of breath, pain while breathing, or a sore throat compared with IMD-B patients.
Figure 2.
Proportion of IMD cases with specific presenting symptoms (most common ones) by serogroup, the Netherlands, January 2017 to June 2018. Total numbers of IMD cases with information on symptoms varied on serogroups and symptoms: IMD-W, n = 140–142; IMD-B, n = 125–135; IMD-Y, n = 35–36; IMD-C, n = 12–13. Abbreviation: IMD, invasive meningococcal disease.
There was no significant difference between serogroups in having at least 1 gastrointestinal symptom at clinical presentation (P = .408). However, IMD-W cases more often had gastrointestinal symptoms with diarrhea (IMD-W, 19%) compared with IMD-B cases (8.3%, P < .018) (Table 2). Cases with IMD-W more often (16%) had diarrhea without IMD-specific symptoms compared with IMD-B cases (4.8%, P < .012). For IMD-Y, 4 of 35 (11%) cases presented with diarrhea without IMD-specific symptoms. The association between serogroup and diarrhea without IMD-specific symptoms remained significant after adjusting for host characteristics (IMD-W vs IMD-non-W: crude odds ratio [OR], 2.7; 95% CI, 1.3–5.9; aOR, 2.6; 95% CI, 1.1–5.8). Similar estimates were obtained when comparing IMD-W with IMD-B instead of IMD-non-W, even though significance was not achieved due to the smaller sample size (Supplementary Table 3).
The proportion of patients with at least 1 respiratory symptom was also higher among IMD-W (45%) and IMD-Y (32%) compared with IMD-B (15%) patients (P < 0.001). IMD-W remained associated with having more respiratory symptoms compared with IMD-non-W cases after adjusting for host characteristics (aOR, 3.2; 95% CI, 1.9–5.6) (Supplementary Table 4).
Severity: ICU Admittance and Case Fatality Rate
No difference in ICU admission was observed between the serogroups (P = 0.160) (Table 2). Of 486 patients with IMD, 47 (8.7%) died (Table 3). The overall IMD-W CFR was 16% (32/199), which is significantly higher than for IMD-B with 3.8% (10/263, P < .001). Cases with IMD-Y had a CFR of 6.9% (4/58), which was not statistically different from the CFR of IMD-W cases (P = .087). Among IMD-C patients, 1 of 25 (4%) died (P = .139). The CFR among IMD-W cases was highest in the age group 10–19 years (7/29; 24%).
Table 3.
Serogroup-specific Case Fatality Rate by Case Characteristics, Clinical Manifestation, and Symptoms at Disease Onset Among Invasive Meningococcal Disease Cases, the Netherlands, January 2015 to June 2018
| W | B | Y | C | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CFR | n | CFR | n | CFR | n | CFR | n | CFR | n | P Valuea | |
| Age | .216 | ||||||||||
| <10 y | 11.1 | 2/18 | 3.8 | 4/104 | 0.0 | 0/1 | 0.0 | 0/3 | 4.8 | 6/126 | |
| 10–19 y | 24.1 | 7/29 | 3.8 | 2/52 | 28.6 | 2/7 | 0.0 | 0/2 | 12.2 | 11/90 | |
| 20–64 y | 17.3 | 14/81 | 4.2 | 3/72 | 7.7 | 1/13 | 0.0 | 0/10 | 10.2 | 18/176 | |
| ≥65 y | 13.8 | 9/65 | 2.9 | 1/34 | 2.7 | 1/37 | 90.0 | 1/10 | 8.2 | 12/146 | |
| Genderb | .964 | ||||||||||
| Males | 16.4 | 11/67 | 5.0 | 6/121 | 11.5 | 3/26 | 0.0 | 0/12 | 8.8 | 20/226 | |
| Females | 16.5 | 21/127 | 2.8 | 4/142 | 3.1 | 1/32 | 7.7 | 1/12 | 8.6 | 27/314 | |
| Comorbidities | .163 | ||||||||||
| No | 18.0 | 22/122 | 3.7 | 6/163 | 6.5 | 2/31 | 0.0 | 0/16 | 9.0 | 30/332 | |
| Yes, immunocompromised | 21.1 | 4/19 | 33.3 | 3/9 | 0.0 | 0/8 | 0.0 | 0/1 | 19.4 | 7/36 | |
| Yes, other | 13.0 | 3/23 | 0.0 | 0/17 | 12.5 | 1/8 | 0.0 | 0/2 | 8.0 | 4/50 | |
| Clinical manifestations | <.001 | ||||||||||
| Septicemia without meningitis | 27.1 | 23/85 | 9.5 | 6/63 | 10.5 | 2/19 | 14.3 | 1/7 | 18.4 | 32/174 | |
| Septicemia and meningitis | 36.4 | 4/11 | 8.0 | 2/25 | 28.6 | 2/7 | 0.0 | 0/1 | 18.2 | 8/44 | |
| Meningitis without septicemia | 3.1 | 1/32 | 1.4 | 2/142 | 0.0 | 0/12 | 0.0 | 0/8 | 1.5 | 3/194 | |
| Other | 6.2 | 4/65 | 0.0 | 0/30 | 0.0 | 0/19 | 0.0 | 0/9 | 3.3 | 4/123 | |
| Symptomsc: diarrhea | <.001 | ||||||||||
| No | 10.6 | 12/113 | 6.2 | 7/112 | 3.2 | 1/31 | 0.0 | 0/10 | 7.5 | 20/266 | |
| Yes, with IMD-specific symptomsd | 40.0 | 2/5 | 0.0 | 0/5 | NaN | 0/0 | 0.0 | 0/1 | 18.2 | 2/11 | |
| Yes, without IMD-specific symptomsd | 52.4 | 11/21 | 0.0 | 0/6 | 0.0 | 0/4 | 0.0 | 0/1 | 34.4 | 11/32 | |
Data are from January to June. Two cases of IMD-E (1 case) and IMD-X (1 case) are not included in the table. Total includes cases of serogroup W, B, Y, and C.
Abbreviations: CFR, case fatality rate; IMD, invasive meningococcal disease; NaN, not a number (dividing by zero is undefined).
a P values for the difference between variables and CFR among cases from all serogroups.
bOne case with missing information on gender.
cData available only since 2017.
dIMD-specific symptoms: petechiae and/or neck stiffness.
Case fatality rates were highest among patients with septicemia/septic shock with or without meningitis (P < .001), which was observed among all serogroups. The CFR was higher in patients presenting with diarrhea without IMD-specific symptoms (11/32; 34%) compared with those with diarrhea and IMD-specific symptoms (2/11; 18%) and patients without diarrhea (20/266; 7.5%; P < .001). The CFR among IMD-W patients with diarrhea without IMD-specific symptoms was 52% (11/21).
IMD-W was associated with a higher CFR compared with IMD-non-W, after adjusting for age, gender, and comorbidities (aOR, 4.5; 95% CI, 2.2–9.8). This association was still significant when additionally adjusting for clinical manifestation (fully aOR, 4.3; 95% CI, 2.0–10.2) (Table 4). Findings were similar when IMD-W was compared with IMD-B (fully aOR, 4.4; 95% CI, 1.8–11.8).
Table 4.
Crude and Adjusted Odds Ratios From Logistic Regression Analysis for the Association Between Serogroup, Case Characteristics, Clinical Manifestation, and Death Among Invasive Meningococcal Disease Cases, the Netherlands, January 2015 to June 2018
| Crude | Adjusted | |||
|---|---|---|---|---|
| Variables | OR (95% CI) | P | OR (95% CI) | P |
| Serogroup | ||||
| Non-Wa | Reference | |||
| W | 4.2 (2.3–8.2) | <.001 | 4.3 (2.0–10.2) | <.001 |
| Age | ||||
| 0–9 y | Reference | |||
| 10–19 y | 2.8 (1.0–8.4) | .052 | 1.4 (.4–4.9) | .551 |
| 20–64 y | 2.3 (.9–6.4) | .093 | 0.8 (.3–2.7) | .729 |
| ≥65 y | 1.7 (.7–5.1) | .283 | 0.4 (.1–1.6) | .197 |
| Gender | ||||
| Male | Reference | |||
| Female | 1.0 (.5–1.8) | .910 | 0.9 (.4–2.1) | .878 |
| Comorbidities | ||||
| No | Reference | |||
| Yes, immunocompromised | 2.4 (.9–5.8) | .055 | 2.5 (.8–7.5) | .114 |
| Yes, other | 0.9 (.3–2.4) | .811 | 1.3 (.3–4.2) | .685 |
| Clinical manifestations | ||||
| Meningitis without septicemia | Reference | |||
| Septicemia without meningitis | 14.3 (5.0–61) | <.001 | 9.3 (3.0–41) | <.001 |
| Septicemia and meningitis | 14.1 (3.9–67) | <.001 | 11.3 (2.8–57) | .001 |
| Other | 2.1 (.5–11) | .325 | 1.1 (.2–6.1) | .952 |
For 2018 only until June 2018.
Abbreviations: CI, confidence interval; IMD, invasive meningococcal disease; OR, odds ratio.
aNon-W includes cases of serogroup B, Y, and C.
DISCUSSION
Cases with IMD-W were associated with more septicemia and pneumonia, and less meningitis, independent of age, gender and comorbidities. Cases with IMD-W presented more often with respiratory symptoms and diarrhea without IMD-specific symptoms. Cases with IMD-W were similar to IMD-Y cases on host characteristics and clinical picture compared with IMD-B cases. The IMD-W CFR was high (16%) and was associated with 68% of all IMD fatalities. Serogroup W meningococcal disease was associated with a higher mortality even after adjustment for age, gender, and comorbidities. The CFR was highest among IMD-W patients presenting with diarrhea without IMD-specific symptoms.
Serogroup-specific differences in the epidemiology of IMD were recently described in a study comparing 77 IMD-W cases with other serogroups (n = 872) in France in 2015–2016 [19]. Comparable to our findings, IMD-W (and IMD-Y) occurred more often in older age groups compared with IMD-B and -C. This shift toward a broader age distribution was previously described in England and Australia [7, 9, 17].
Ladhani and colleagues [7] analyzed clinical characteristics among 129 IMD-W cases, notified in England from 2010 to 2013. Half of the IMD-W cases presented with septicemia, which is comparable to our data and data from France [19]. Stoof and colleagues [29], analyzing 879 IMD cases from medical records in the Netherlands in 1999–2011, found that the small number of IMD-W patients (n = 16) presented more often with clinical manifestations such as pneumonia or arthritis compared with IMD-B and IMD-C patients, as we observed from 2015 to 2018.
Recent reports about atypical IMD-W presentations [7, 14, 30, 31] include a case report of gastrointestinal symptoms among IMD-W patients in England [26]. Guiddir and colleagues [31] investigated 105 patients with IMD with early abdominal presentations in France from 1991 to 2016; unlike the association described here between IMD-W and gastrointestinal symptoms [31], we did not find a serogroup-specific difference in overall gastrointestinal symptoms. However, IMD-W cases presented more often with diarrhea without IMD-typical symptoms compared with IMD-non-W cases. We further evaluated if the higher number of cases with diarrhea reflects the higher number of cases with septicemia among IMD-W cases. However, IMD-W cases with septicemia still presented more often (31%; 21/68) with diarrhea compared with IMD-B cases with septicemia (11%; 4/37).
Several studies observed high CFRs among IMD-W patients [7, 26, 27, 32]. A higher age-adjusted CFR among IMD-W cases was also seen in France [19]. A higher CFR could be explained by the higher proportion of septicemia among IMD-W patients, which is known to be associated with a higher mortality [2]. Our study, however, confirms the high IMD-W CFR, even after adjusting for age, gender, comorbidities, and clinical manifestation. Additionally, we observed an association between presenting with diarrhea without IMD-specific symptoms and death after adjusting for case characteristics. A higher CFR (24%) among patients with IMD with abdominal symptoms was also described by Guiddir et al [31]. The elevated IMD-W CFR with atypical symptoms could be attributed to a delay among patients seeking healthcare and the challenge of accurately diagnosing patients with symptoms not specific for IMD. In our data, the median time difference between date of symptom onset and date of laboratory confirmation was indeed greater among cases with diarrhea (median, 5 days) compared with those without diarrhea (median, 4 days).
As previously mentioned, the current increase in IMD-W in several European countries is mainly caused by cc11 [6, 7, 15–17], which is also seen in our data.
Clonal complex shows a strong association with serogroup. Ninety-six percent of cc11 isolates are found among IMD-W isolates. Likewise, all cc23 isolates were of serogroup Y and all cc32 isolates were of serogroup B. Looking at the association between different cc and host characteristics, cc11 mirrors the overall IMD-W picture. The association between cc and severity was also reflected in the overall picture based on the serogroups; however, due to the lower numbers, some associations were no longer significant. We have no indication that the severity increased when looking at cc11 versus other cc strains (neither non-cc11 nor other hypervirulent strains such as cc32 or cc4144) compared with looking at IMD-W versus other serogroups.
In general, irrespective of the serogroup, cc11 infections are associated with more severe disease in animal models and in patients. Meningococci belonging to cc11 have also been described of being highly virulent in mouse infection models by Deghmane and colleagues [33] as well as by Lancelotti and colleagues [34].
A high CFR or severity among IMD-C cc11 versus other cc strains was described in several studies [33, 35–38]. This was also observed in studies comparing cc11 of serogroup B with other IMD-B cc strains [39]. In contrast, Stoof and colleagues [29] were unable to show an association between cc11 infection and severity of disease. Hong and colleagues [22] compared the CFR between IMD-W cc11 and IMD-W non-cc11 cases. While they found a higher CFR among W:cc11 cases, this difference was not statistically significant. Guiddir and colleagues[15] investigated the association between cc and abdominal presentations of IMD cases. They found that cc11 (belonging to IMD-C as well as IMD-W) was the predominant cc (45%) among patients with IMD with abdominal presentations.
These results emphasize that the course of IMD due to cc11 is often more severe than that caused by isolates of other cc strains. Compared with IMD caused by more historical C:cc11 isolates [32] and W:cc11 isolates (Anglo-French Hajj strain) [22], the IMD course caused by recent W:cc11 isolates (original UK strain and UK 2013 strain) seems to be even more severe. It is possible that more recent W:cc11 isolates have, over time, acquired genetic material contributing to higher virulence. Comparisons of WGS and association with patient data should be performed to resolve this question.
A limitation of our study is a missing clear definition of IMD-attributable death in our surveillance data, which may have led to some underreporting of deaths. This is most likely independent of serogroup and therefore does not influence our analysis. The strength of our analysis is the prospectively collected data and the completeness of the serogroup-specific information. A comparison between (clinical) IMD cases notified within the surveillance system and those reported by the NRLBM indicated high concordance of ~90% [13]. Additionally, we were able to include and analyze IMD serogroup–specific symptoms at disease onset in our analysis as this was added to the national IMD notification questionnaire in 2017.
Because of the recent increase in the numbers and severity of IMD-W cases, a tetravalent conjugate vaccine, which gives protection against 4 serogroups (MenACWY), was introduced in the United Kingdom in 2015 [40]. The vaccine is recommended in other European countries, including Austria, Greece, and Italy [41]. The Netherlands introduced MenACWY vaccination in May 2018 as part of the national immunization program for children at 14 months of age, replacing MenC [15, 42]. In addition, MenACWY vaccination is being offered to 13- to 14-year-olds as of October 2018; in 2019, the campaign was extended to 15- to 18-year-olds.
In conclusion, our study demonstrates that the current increase in IMD-W incidence in the Netherlands, caused by a cc11 strain, is associated with a different clinical picture and a higher severity compared with IMD due to other serogroups, which is not explained by age, gender, comorbidities, and clinical presentation. More research is needed to identify the bacterial characteristics associated with clinical presentation and severity of IMD-W cc11. Our results underline the importance of the recent MenACWY vaccination implementation in the Netherlands, in order to prevent a severe and rapidly progressing disease with an atypical presentation, and may inform vaccination policy in other countries facing a similar challenge.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank Anneke Westerhof from the National Institute for Public Health and the Environment (RIVM) for data management within the Dutch Invasive Meningococcal Disease surveillance system. Furthermore, they acknowledge the work of the laboratory personnel of the Netherlands Reference Laboratory for Bacterial Meningitis. This publication made use of the Neisseria Multi Locus Sequence Typing website (https://pubmlst.org/neisseria/) developed by Keith Jolley and sited at the University of Oxford [43]. The development of this site was funded by the Wellcome Trust and the European Union. The authors gratefully acknowledge the contributions of Lisa Hansen and her useful and constructive suggestions on this paper.
Financial support. This work was funded by the National Institute of Public Health, the Netherlands.
Potential conflicts of interest. A. v. d. E. received a grant for an investigator-initiated project, “Epidemiology of invasive pneumococcal disease” (IIR WI173197), and fees paid to the institution for consultancy activities for GlaxoSmithKline (GSK) and for participation in advisory boards for Pfizer and GSK. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Pace D, Pollard AJ. Meningococcal disease: clinical presentation and sequelae. Vaccine 2012; 30(Suppl 2):B3–9. [DOI] [PubMed] [Google Scholar]
- 2. Rosenstein NE, Perkins BA, Stephens DS, Popovic T, Hughes JM. Meningococcal disease. N Engl J Med 2001; 344:1378–88. [DOI] [PubMed] [Google Scholar]
- 3. Pelton SI. The global evolution of meningococcal epidemiology following the introduction of meningococcal vaccines. J Adolesc Health 2016; 59:S3–S11. [DOI] [PubMed] [Google Scholar]
- 4. Edmond K, Clark A, Korczak VS, Sanderson C, Griffiths UK, Rudan I. Global and regional risk of disabling sequelae from bacterial meningitis: a systematic review and meta-analysis. Lancet Infect Dis 2010; 10:317–28. [DOI] [PubMed] [Google Scholar]
- 5. National Institute for Public Health and the Environment (RIVM). RIVM report. In: Schurink-van ‘t Klooster TM, de Melker HE, eds.. The National Immunisation Programme in the Netherlands: surveillance and developments in 2015–2016. Bilthoven, The Netherlands: National Institute for Public Health and the Environment (RIVM), 2016. [Google Scholar]
- 6. Araya P, Fernández J, Del Canto F, et al. Neisseria meningitidis ST-11 clonal complex, Chile 2012. Emerg Infect Dis 2015; 21:339–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ladhani SN, Beebeejaun K, Lucidarme J, et al. Increase in endemic Neisseria meningitidis capsular group W sequence type 11 complex associated with severe invasive disease in England and Wales. Clin Infect Dis 2015; 60:578–85. [DOI] [PubMed] [Google Scholar]
- 8. Abad R, López EL, Debbag R, Vázquez JA. Serogroup W meningococcal disease: global spread and current affect on the Southern Cone in Latin America. Epidemiol Infect 2014; 142:2461–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Australian Government Department of Health. Invasive meningococcal disease. National surveillance report 2018. Available at: https://www.health.gov.au/internet/main/publishing.nsf/Content/5FEABC4B495BDEC1CA25807D001327FA/$File/31-Mar18-IMD-Surveillance-report.pdf. Accessed 12 October 2018.
- 10. Moreno G, López D, Vergara N, Gallegos D, Advis MF, Loayza S. [Clinical characterization of cases with meningococcal disease by W135 group in Chile, 2012.] Rev Chilena Infectol 2013; 30:350–60. [DOI] [PubMed] [Google Scholar]
- 11. Mustapha MM, Marsh JW, Harrison LH. Global epidemiology of capsular group W meningococcal disease (1970-2015): Multifocal emergence and persistence of hypervirulent sequence type (ST)-11 clonal complex. Vaccine 2016; 34:1515–23. [DOI] [PubMed] [Google Scholar]
- 12. Tsang R, Hoang L, Tyrrell GJ, et al. Increase in Neisseria meningitidis serogroup W invasive disease in Canada: 2009-2016. Can Commun Dis Rep 2017; 43:144–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bijlsma MW, Bekker V, Brouwer MC, Spanjaard L, van de Beek D, van der Ende A. Epidemiology of invasive meningococcal disease in the Netherlands, 1960-2012: an analysis of national surveillance data. Lancet Infect Dis 2014; 14:805–12. [DOI] [PubMed] [Google Scholar]
- 14. Wunderink HF, Vlasveld IN, Knol MJ, van der Ende A, van Essen EHR, Kuijper EJ. [Gastrointestinal symptoms with meningococcal infection: emergence of Neisseria meningitidis serogroup W.] Ned Tijdschr Geneeskd 2017; 161:D1456. [PubMed] [Google Scholar]
- 15. Knol MJ, Ruijs WL, Antonise-Kamp L, de Melker HE, van der Ende A. Implementation of MenACWY vaccination because of ongoing increase in serogroup W invasive meningococcal disease, the Netherlands, 2018. Euro Surveill 2018; 23:2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lucidarme J, Scott KJ, Ure R, et al. An international invasive meningococcal disease outbreak due to a novel and rapidly expanding serogroup W strain, Scotland and Sweden, July to August 2015. Euro Surveill 2016; 21:15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Carville KS, Stevens K, Sohail A, et al. Increase in meningococcal serogroup W disease, Victoria, Australia, 2013-2015. Emerg Infect Dis 2016; 22:1785–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Abad R, Vázquez JA. Early evidence of expanding W ST-11 CC meningococcal incidence in Spain. J Infect 2016; 73:296–7. [DOI] [PubMed] [Google Scholar]
- 19. Hong E, Barret AS, Terrade A, et al. Clonal replacement and expansion among invasive meningococcal isolates of serogroup W in France. J Infect 2017; 76:149–58. [DOI] [PubMed] [Google Scholar]
- 20. Whittaker R, Dias JG, Ramliden M, et al. ; ECDC Network Members for Invasive Meningococcal Disease. The epidemiology of invasive meningococcal disease in EU/EEA countries, 2004-2014. Vaccine 2017; 35:2034–41. [DOI] [PubMed] [Google Scholar]
- 21. European Centre for Disease Prevention and Control. Invasive meningococcal disease. In: ECDC. Annual epidemiological report for 2016. Stockholm: ECDC; 2018. [Google Scholar]
- 22. Lucidarme J, Hill DM, Bratcher HB, et al. Genomic resolution of an aggressive, widespread, diverse and expanding meningococcal serogroup B, C and W lineage. J Infect 2015; 71:544–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Knol MJ, Hahné SJM, Lucidarme J, et al. Temporal associations between national outbreaks of meningococcal serogroup W and C disease in the Netherlands and England: an observational cohort study. Lancet Public Health 2017; 2:e473–82. [DOI] [PubMed] [Google Scholar]
- 24. Gaschignard J, Levy C, Deghmane AE, et al. Invasive serogroup W meningococcal disease in children: a national survey from 2001 to 2008 in France. Pediatr Infect Dis J 2013; 32:798–800. [DOI] [PubMed] [Google Scholar]
- 25. Vienne P, Ducos-Galand M, Guiyoule A, et al. The role of particular strains of Neisseria meningitidis in meningococcal arthritis, pericarditis, and pneumonia. Clin Infect Dis 2003; 37:1639–42. [DOI] [PubMed] [Google Scholar]
- 26. Campbell H, Parikh SR, Borrow R, Kaczmarski E, Ramsay ME, Ladhani SN.. Presentation with gastrointestinal symptoms and high case fatality associated with group W meningococcal disease (MenW) in teenagers, England, July 2015 to January 2016. Euro Surveill 2016; 21:11–4. [DOI] [PubMed] [Google Scholar]
- 27. Martin NV, Ong KS, Howden BP, et al. ; Communicable Diseases Network Australia MenW Working Group. Rise in invasive serogroup W meningococcal disease in Australia 2013-2015. Commun Dis Intell Q Rep 2016; 40:E454–9. [DOI] [PubMed] [Google Scholar]
- 28. Central Bureau of Statistics. StatLine databank. 2018. Den Haag/Heerlen/Bonaire: Centraal Bureau voor de Statistiek. Available at: http://statline.cbs.nl/StatWeb Accessed 06 August 2018. [Google Scholar]
- 29. Stoof SP, Rodenburg GD, Knol MJ, et al. Disease burden of invasive meningococcal disease in the Netherlands between June 1999 and June 2011: a subjective role for serogroup and clonal complex. Clin Infect Dis 2015; 61:1281–92. [DOI] [PubMed] [Google Scholar]
- 30. Russcher A, Fanoy E, van Olden GDJ, Graafland AD, van der Ende A, Knol MJ. Necrotising fasciitis as atypical presentation of infection with emerging Neisseria meningitidis serogroup W (MenW) clonal complex 11, the Netherlands, March 2017. Euro Surveill 2017; 22:10–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guiddir T, Gros M, Hong E, et al. Unusual initial abdominal presentations of invasive meningococcal disease. Clin Infect Dis 2018; 67:1220–7. [DOI] [PubMed] [Google Scholar]
- 32. Valenzuela MT, Moreno G, Vaquero A, et al. [Emergence of W135 meningococcal serogroup in Chile during 2012.] Rev Med Chil 2013; 141:959–67. [DOI] [PubMed] [Google Scholar]
- 33. Deghmane AE, Parent du Chatelet I, Szatanik M, et al. Emergence of new virulent Neisseria meningitidis serogroup C sequence type 11 isolates in France. J Infect Dis 2010; 202:247–50. [DOI] [PubMed] [Google Scholar]
- 34. Lancellotti M, Guiyoule A, Ruckly C, Hong E, Alonso JM, Taha MK. Conserved virulence of C to B capsule switched Neisseria meningitidis clinical isolates belonging to ET-37/ST-11 clonal complex. Microbes Infect 2006; 8:191–6. [DOI] [PubMed] [Google Scholar]
- 35. Waśko I, Hryniewicz W, Skoczyńska A. Significance of meningococcal hyperinvasive clonal complexes and their influence on vaccines development. Pol J Microbiol 2015; 64:313–21. [DOI] [PubMed] [Google Scholar]
- 36. Stefanelli P, Miglietta A, Pezzotti P et al. Increased incidence of invasive meningococcal disease of serogroup C/clonal complex 11, Tuscany, Italy, 2015 to 2016. Euro Surveill 2016; 21:6–10. [DOI] [PubMed] [Google Scholar]
- 37. Heckenberg SG, de Gans J, Brouwer MC, et al. Clinical features, outcome, and meningococcal genotype in 258 adults with meningococcal meningitis: a prospective cohort study. Medicine (Baltimore) 2008; 87:185–92. [DOI] [PubMed] [Google Scholar]
- 38. Smith I, Caugant DA, Høiby EA, Wentzel-Larsen T, Halstensen A. High case-fatality rates of meningococcal disease in Western Norway caused by serogroup C strains belonging to both sequence type (ST)-32 and ST-11 complexes, 1985-2002. Epidemiol Infect 2006; 134:1195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stefanelli P, Fazio C, Vacca P, et al. An outbreak of severe invasive meningococcal disease due to a capsular switched Neisseria meningitidis hypervirulent strain B:cc11. Clin Microbiol Infect 2018; 25:111.e1-111.e4. [DOI] [PubMed] [Google Scholar]
- 40. Campbell H, Saliba V, Borrow R, Ramsay M, Ladhani SN.. Targeted vaccination of teenagers following continued rapid endemic expansion of a single meningococcal group W clone (sequence type 11 clonal complex), United Kingdom 2015. 2015; 20:21188. [DOI] [PubMed] [Google Scholar]
- 41. European Centre for Disease Prevention and Control (ECDC)., E.C.f.D.P.a.C. Vaccine scheduler. Meningococcal disease: recommended vaccinations. 2018, ECDC; [2005–2018]. Available at: https://vaccine-schedule.ecdc.europa.eu/Scheduler/ByDisease?SelectedDiseaseId=48&SelectedCountryIdByDisease=-1 Accessed 29 October 2018. [Google Scholar]
- 42. National Institute for Public Health and the Environment (RIVM). Meningococcal disease in the Netherlands. Bilthoven, The Netherlands: National Institute for Public Health and the Environment (RIVM),2017. Report No.: 2017-0031. [Google Scholar]
- 43. Jolley KA, Maiden MC. BIGSdb: Scalable analysis of bacterial genome variation at the population level. BMC Bioinformatics 2010; 11:595. doi:10.1186/1471-2105-11-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.