Abstract
Background
Clostridioides (Clostridium) difficile colonization is common among infants. Serological sequelae of infant C. difficile colonization are poorly understood.
Methods
In this prospective cohort study of healthy infants, stools serially collected between ages 1-2 and 9-12 months were tested for non-toxigenic and toxigenic C. difficile (TCD). Cultured isolates underwent whole-genome sequencing. Serum collected at 9–12 months underwent measurement of IgA, IgG, and IgM against TCD toxins A and B and neutralizing antibody (NAb) titers against toxin B. For comparison, antitoxin IgG and NAb were measured in cord blood from 50 mothers unrelated to study infants.
Results
Among 32 infants, 16 (50%) were colonized with TCD; 12 were first colonized >1 month before serology measurements. A variety of sequence types were identified, and there was evidence of putative in-home (enrolled siblings) and outpatient clinic transmission. Infants first colonized with TCD >1 month prior had significantly greater serum antitoxin IgA and IgG against toxins A (P = .02 for both) and B (P = .009 and .008, respectively) compared with non–TCD-colonized infants, and greater IgG compared with unrelated cord blood (P = .005). Five of 12 (42%) colonized infants had detectable NAb titers compared with zero non–TCD-colonized infants (P = .02). Breastfeeding was not associated with differences in serological measurements.
Conclusions
TCD colonization is associated with a humoral immune response against toxins A and B, with evidence of toxin B neutralization in vitro. The extent and duration of protection against CDI later in life afforded by natural C. difficile immunization events require further investigation.
Keywords: C difficile, immunity, pediatric, prevention, toxin
Compared with noncolonized infants, infants with toxigenic Clostridioides difficile colonization develop greater levels of Immunoglobin A and Immunoglobin G against toxins A and B and neutralizing antibodies against toxin B, suggesting that infant C. difficile colonization is a natural immunization event.
Clostridioides (Clostridium) difficile is a frequent cause of community-associated and healthcare-associated gastrointestinal infection [1]. C. difficile infection (CDI) is the most common US healthcare-associated infection in adults [2] and is associated with considerable morbidity and mortality [1]. The Centers for Disease Control and Prevention has classified C. difficile among the most significant antibiotic-resistant public health threats that require “urgent and aggressive action” [3]. As such, novel CDI therapeutic and preventive strategies, such as immunologic therapies, have emerged [4].
The adult immune response to C. difficile has been well described [5]. Among hospitalized adults who become colonized with C. difficile, those who subsequently develop CDI have significantly lower serum immunoglobulin (Ig)G against toxin A compared with those who remain asymptomatic [6]. Further, among adults who receive CDI treatment, those who develop recurrent CDI have significantly lower serum IgM against toxins A and B 3 days postinfection and significantly lower serum IgG against toxin A 12 days postinfection [7]. These data suggest that the humoral immune response against C. difficile toxins impacts the risk of CDI and recurrence. As such, bezlotoxumab, a monoclonal antibody against toxin B, has recently been approved for recurrent CDI prevention in adults [8], and vaccines against C. difficile toxins are in clinical development [4].
The immune response to C. difficile in infants and children, however, has been less well studied. Although seropositivity against C. difficile toxins occurs in some infants [9], its association with C. difficile exposure is unknown. Infants comprise a unique population with regard to CDI susceptibility. Although C. difficile colonization in infants is highly prevalent, symptomatic infection in infants and toddlers younger than 12–24 months rarely, if ever, occurs [10]. Although the reason for age-dependent symptomatic CDI susceptibility is unknown, theories include lack of expression of receptors required to bind and internalize toxins A and/or B in the intestine in this age group [11], as well as age-dependent changes in intestinal microbiota [12]. Transplacental transfer of maternal antitoxin antibodies to infants has been proposed [13] but not well studied. The primary objective of this prospective cohort study was to measure the association between infant C. difficile colonization and antitoxin seropositivity. We hypothesized that natural colonization with toxigenic strains of C. difficile during infancy, a developmental period during which infants are seemingly protected against symptomatic infection following C. difficile exposure, is associated with development of a humoral immune response against C. difficile toxins. Secondarily, we aimed to confirm the prevalence and molecular epidemiology of C. difficile colonization in infants [10, 12–15].
METHODS
Setting, Subjects, and Specimens
This prospective cohort study was conducted at the Ann & Robert H. Lurie Children’s Hospital of Chicago and Northwestern University Feinberg School of Medicine. Institutional review boards at both institutions approved this study, and mothers of infant subjects provided informed consent. Healthy full-term infants aged 2 months or younger without previous admission to a neonatal intensive care unit were enrolled through the general pediatrics clinic into this longitudinal observational study between September 2014 and January 2017. Breastfeeding and household exposure data were collected by parent interview at study onset and study completion. Parents were requested to provide infant stool samples at each well-child visit between enrollment and their first routine blood draw (approximately age 2, 4, 6, and 9–12 months). Additional stools were collected at intercurrent sick visits when able. To minimize subject discomfort, serum was collected from infants at age 9–12 months concurrent with venipuncture performed for anemia and lead exposure screening as part of routine preventive care. Evaluable subjects were required to provide serum at 9–12 months. Umbilical cord blood was collected from 50 consecutive full-term deliveries (mothers were unrelated to study infants) at Prentice Women’s Hospital, and serum was extracted and stored at −80°C in preparation for serological studies as described below.
Microbiology and Genomics Analyses
Stools from infants were derived from soiled diapers provided by the parents. Stool was extracted from diapers, aliquoted, and stored at −80°C until ready for batch processing of study-related assays. Thawed infant stool samples underwent several assays to identify toxigenic (TCD) and nontoxigenic C. difficile (NTCD). Stools underwent both glutamate dehydrogenase (GDH; C. difficile common antigen) and toxin A/B enzyme immunoassay (EIA), as well as tcdB (toxin B gene) polymerase chain reaction (PCR) testing. Anaerobic stool culture was performed, as previously described [16]. A single C. difficile colony from each culture was selected for whole-genome sequencing (WGS). Genomic analyses are described in Supplementary Methods. Stools were designated as TCD colonized if they were positive for toxin EIA, tcdB PCR, and/or a cultured C. difficile isolate whose genome contains tcdA or tcdB. Stools were designated as NTCD colonized if they were GDH positive but toxin negative and tcdB PCR negative and/or if a cultured C. difficile isolate did not contain tcdA or tcdB. Thus, an infant could be classified as co-colonized with both TCD (if the stool is tcdB PCR positive) and NTCD (if the C. difficile colony selected from stool culture did not contain tcdA or tcdB). Environmental cultures of the clinic were performed after study completion (Supplementary Methods).
Serological Assays
Serum samples were analyzed for antibody (IgG, IgA, and IgM) concentrations to C. difficile toxins A and B by enzyme-linked immunosorbent assay (ELISA; expressed as arbitrary ELISA units [AU]), as previously described [6, 7, 17, 18]. Serum neutralizing antibody (NAb) titers against toxin B were also determined in serum samples using a high-throughput NAb assay with modification of a previously described procedure [19]. These assays are detailed in Supplementary Methods.
Statistics
To account for the time required to develop an antibody response, C. difficile–colonized infants were grouped based on whether the first colonization event was detected at least 1 month prior to serum collection. Proportions were compared between groups using 2-tailed Fisher’s exact test, and medians were compared between groups using the nonparametric Wilcoxon rank sum test. We examined the association of TCD colonization with either the highest quartile of ELISA units of IgG against toxins A and B (compared with the lowest 3 quartiles) or the presence of detectable NAb titers against toxin B by calculating risk ratios (or risk difference if risk was zero in a group) and using a 2-tailed Fisher’s exact test. Two-sided P values <.05 were considered statistically significant. Analyses were performed using Stata/IC statistical software, version 12.1 (StataCorp).
RESULTS
Infant C. difficile Colonization
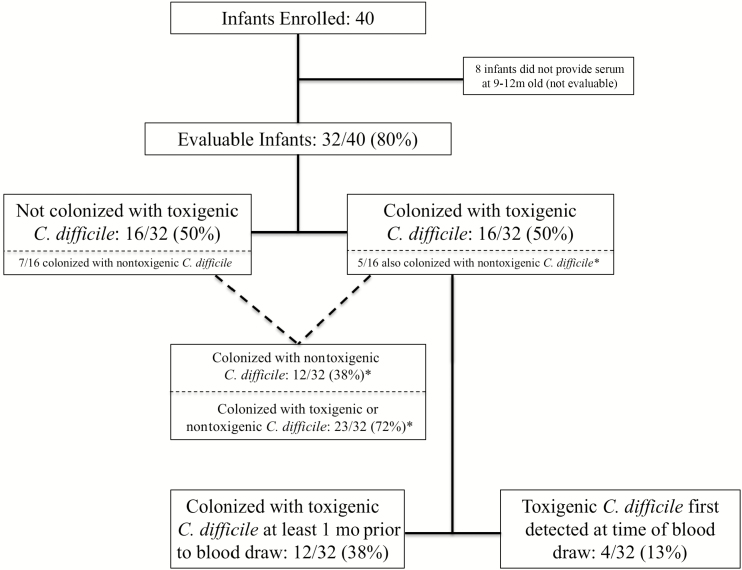
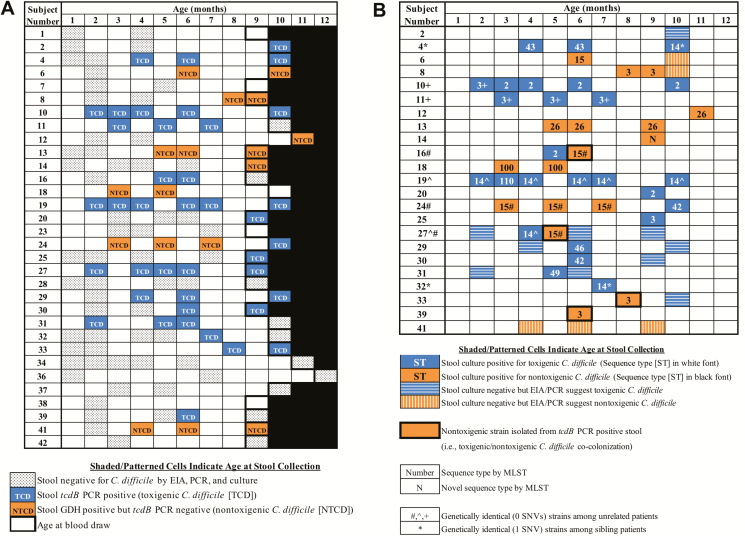
Forty infants were enrolled, and 32 (80%) evaluable subjects provided serum at 9–12 months of age (Figure 1). Subject demographic characteristics are listed in Table 1. Figure 2A illustrates C. difficile assay results from the 124 stools collected from 32 evaluable infants. In total, 23 (72%) infants were colonized with C. difficile, 16 (50%) with TCD, and 12 (38%) with NTCD; 5 infants were colonized with both TCD and NTCD during the study period. Nine of 16 (56%) TCD-colonized infants had at least 1 toxin EIA-positive stool during the study. Of the 16 TCD-colonized infants, 12 (75%) were colonized at least 1 month prior to the blood draw. Of the 23 infants colonized with either TCD or NTCD, 18 (78%) were colonized at least 1 month prior to the blood draw.
Figure 1.
Subject flow chart. *Five infants were colonized with both TCD and NTCD during the study period, including 3 infants with evidence of TCD and NTCD in the same stool sample (i.e., stool was tcdB PCR positive but NTCD was isolated from culture). Abbreviations: NTCD, nontoxigenic Clostridioides difficile; PCR, polymerase chain reaction; TCD, toxigenic Clostridioides difficile.
Table 1.
Infant Demographics and Characteristics and Univariate Analysis of Factors Associated With Clostridioides difficile Colonization
| Characteristic | All Infants (N = 32) | Infants Without TCD Colonization (n = 16) | Infants With TCD Colonization (n = 16) | P Value |
|---|---|---|---|---|
| Male sex, no. (%) | 12 (38) | 8 (50) | 12 (75) | .27 |
| Hispanic or Latino ethnicity, no. (%) | 15 (47) | 8 (50) | 7 (44) | 1 |
| Born by vaginal delivery, no. (%) | 27 (84) | 14 (88) | 13 (81) | 1 |
| Breastfeeding, no. (%) | 17 (53) | 9 (56) | 8 (50) | 1 |
| Stopped breastfeeding before study blood draw, no. (%) | 11/17 (65) | 4/9 (44) | 7/8 (88) | .18 |
| Median (IQR) age stopped breastfeeding, mo | 4 (3, 6) | 4.5 (3, 6) | 4 (3, 7) | .69 |
| Youngest age of other children in household, no. (%) | .52 | |||
| <1 y | 0 | 0 | 0 | |
| 1–2 y | 7 (22) | 5 (31) | 2 (13) | |
| 3–7 y | 12 (38) | 7 (44) | 5 (31) | |
| 8–12 y | 3 (9) | 1 (6) | 2 (13) | |
| 13–18 y | 4 (13) | 1 (6) | 3 (19) | |
| No other children in household | 6 (19) | 2 (13) | 4 (25) | |
| Daycare with other children, no. (%) | 2 (6) | 0 (0) | 2 (13) | .48 |
| Median (IQR) no. of stools collected from each infant | 4 (3, 5) | 3 (2, 5) | 4 (4, 5) | .02 |
| C. difficile colonization before 9–12 months old,a no. (%) | 23 (72) | … | … | … |
| TCD colonization | 16 (50) | … | … | |
| TCD colonization (at least 1 month prior to 9–12 months old) | 12 (38) | … | … | |
| NTCD colonization | 12 (38) | … | … | |
| Median (IQR) age at first TCD colonization, mo | … | … | 5 (2, 9) | … |
| Median (IQR) duration of TCD colonization, mo | … | … | 3 (1, 7) | … |
Abbreviations: IQR, interquartile range; NTCD, nontoxigenic Clostridioides difficile; PCR, polymerase chain reaction; TCD, toxigenic Clostridioides difficile.
aFive infants were colonized with both TCD and NTCD during the study period, including 3 infants co-colonized with TCD and NTCD (ie, stool was tcdB PCR positive but NTCD was isolated from culture).
Figure 2.
A, Clostridioides difficile assay results from 124 serial stool specimens collected from 32 infants. Shaded/patterned cells indicate age at stool collection; blank cells indicate no stool was collected at that age. B, Molecular epidemiology of 43 C. difficile isolates collected from 23 colonized infants. Abbreviations: EIA, enzyme immunoassay; MLST, multilocus sequence typing; PCR, polymerase chain reaction; SNV, single nucleotide variant.
Molecular Epidemiology
Infant C. difficile colonization molecular epidemiology is illustrated in Figure 2B. Of 57 stools from 23 infants that were positive by GDH EIA and/or tcdB PCR, 43 (75%) were culture positive. All isolates were successfully sequenced, assembled, and typed by in silico multilocus sequence typing. Phylogenetic analysis demonstrated that strains colonizing infants clustered with strains previously identified to cause CDI in older children at our pediatric medical center between 2011 and 2013 [16, 20, 21] (Supplementary Figure S1). Genetically identical (0–1 single nucleotide variants) isolates were identified among 2 pairs and a trio of unrelated infants and 1 pair of siblings (25-month difference in age) enrolled in this study (Figure 2B). Because this suggested potential transmission within the clinic (or the home in the case of the siblings), environmental cultures of the general pediatrics clinic were obtained and all were negative. Additional details are described in Supplementary Results.
Serological Responses to C. difficile Colonization
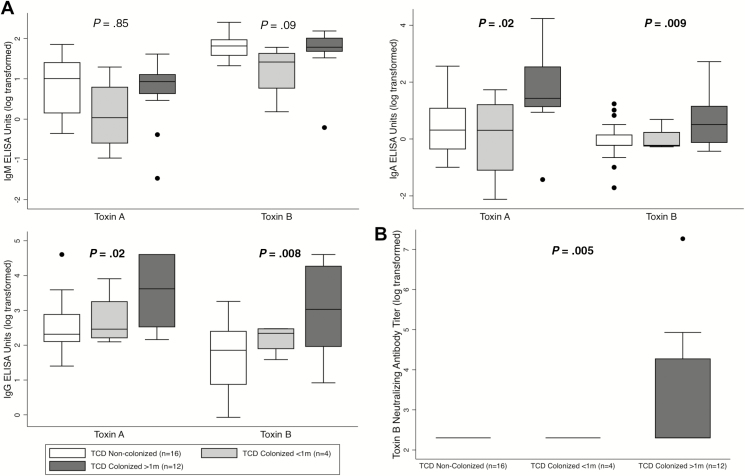
At 9–12 months of age, IgG and IgA against toxins A and B (Figure 3A), as well as toxin B NAb titers (Figure 3B) were significantly higher among the 12 TCD-colonized infants (whose first colonization event was at least 1 month prior) compared with 16 non–TCD-colonized infants. TCD colonization at least 1 month prior was associated with measurement of IgG against toxins A and B in the top quartile of the cohort (toxin A: risk ratio [RR], 5.0; 95% confidence interval [CI], 1.2–20.9; P = .04; toxin B: RR, 5.0; 95% CI, 1.2–20.9; P = .04), but this association was not noted for IgA (toxin A: RR, 1.7; 95% CI, 0.5–5.5; P = .66; toxin B: RR, 2.8; 95% CI, 0.8–9.6; P = .21). Five of 12 (42%) TCD-colonized infants had detectable NAb titers, but none of the 16 non–TCD-colonized infants had detectable NAb titers (risk difference, 42%; 95% CI, 19–68%; P = .02). Differences between TCD-colonized infants with and without detectable NAb titers are summarized in Table 2. Detectable NAb titers did not correlate with sequence types of the colonizing strains (Supplementary Table S1).
Figure 3.
A, Association between TCD colonization and serum IgM (top left), IgA (top right), and IgG (bottom left) against toxins A and B. B Association between TCD colonization and toxin B NAb titers (bottom right). Data are expressed as AU (A) or NAb titers (B) on a natural logarithmic scale. Boxes delineate the median and IQR, whiskers delineate the upper and lower adjacent values (within 1.5 × IQR), and isolated data points are outliers. Reported P values are for comparisons between non–TCD-colonized infants and infants with TCD colonization for at least 1 month’s duration. Bolded P values are statistically significant. Abbreviations: ELISA, enzyme-linked immunosorbent assay; Ig, immunoglobulin; IQR, interquartile range; Nab, neutralizing antibody; TCD, toxigenic Clostridioides difficile.
Table 2.
Univariate Analysis of Factors Associated With Detectable Neutralizing Antibody Titers Against Toxin B in Children With Toxigenic Clostridioides difficile Colonization for at Least 1 Month’s Duration
| Characteristic | Detectable Toxin B NAb (n = 5) | Nondetectable Toxin B NAb (n = 7) | P Value |
|---|---|---|---|
| Male sex, no. (%) | 3 (60) | 6 (86) | .52 |
| Hispanic or Latino ethnicity, no. (%) | 2 (40) | 4 (57) | 1 |
| Breastfeeding, no. (%) | 2 (40) | 6 (86) | .22 |
| Stopped breastfeeding before study blood draw, no. (%) | 2/2 (100) | 5/6 (83) | 1 |
| Median age stopped breastfeeding, mo | 7 | 3 | .11 |
| Median age at first detected TCD colonization, mo | 4 | 4 | .75 |
| Median duration of detectable TCD colonization, mo | 5 | 5 | .62 |
| Median stool tcdB PCR cycle thresholda | 23.9 | 25.4 | .42 |
| At least 1 toxin EIA-positive stool, no. (%) | 4 (80) | 5 (71) | 1 |
| Median antitoxin B IgG (AU) | 43.0 | 7.8 | .09 |
| Median antitoxin B IgA (AU) | 2.4 | 0.9 | .02 |
N = 12.
Abbreviations: AU, arbitrary ELISA units; EIA, enzyme immunoassay; Ig, immunoglobulin; IQR, interquartile range; Nab, neutralizing antibody; NTCD, nontoxigenic Clostridioides difficile; PCR, polymerase chain reaction; TCD, toxigenic Clostridioides difficile.
aThe single lowest stool tcdB PCR cycle threshold value measured per patient included in this analysis.
Antibody responses did not significantly differ between breastfed and nonbreastfed infants (median IgA against toxins A [P = .43] or B [P = .83]; median IgM against toxins A [P = .51] or B [P = .35]). Median IgG against both toxins A (11.2 AU vs 18.8 AU, P = .06) and B (6.5 AU vs 11.0 AU, P = .26) was lower among breastfed infants but not statistically significant. Breastfeeding was not associated with a difference in proportion of infants with detectable NAb titers (40% vs 56%, P = .65).
Detection of toxin by EIA in stool of TCD-colonized infants was associated with a significantly higher level of IgA and IgG against both toxins A and B and higher NAb titers compared with non–TCD-colonized infants. There was a non–statistically significant trend toward higher levels of antitoxin IgA and IgG, and higher NAb titers, in toxin EIA-positive infants compared with toxin EIA-negative, tcdB PCR-positive colonized infants (Supplementary Figures S2 and S3).
To assess the potential impact of transplacental transfer of maternal IgG against toxins A and B on infant serological measurements, IgG in infant serum was compared with IgG in cord blood collected from 50 consecutive deliveries of mothers who were not related to study infants. Cord blood IgG against both toxins A and B was similar to non-TCD-colonized infant sera but significantly lower than sera from infants with TCD colonization for at least 1 month duration (Supplementary Figure S4). Neutralizing antibody titers were detected in 9 of 50 (18%) cord blood samples, and a detectable NAb titer was associated with greater median antitoxin B IgG (19.1 AU vs 7.5 AU, P < .001).
To assess infant humoral immune response against C. difficile nontoxin antigens, we measured infant antibody responses against nontoxin antigens using a cell lysate preparation from an NTCD strain isolated in this study (ST-26). Median ELISA units of IgM, IgG, and IgA against nontoxin antigens were similar between 18 infants with TCD and/or NTCD colonization for at least 1 month duration and 9 infants with neither TCD nor NTCD colonization (Supplementary Figure S5).
Discussion
It is well known that TCD colonization frequently occurs in infants [10, 12–15]. Our data support these previous findings; we identified TCD colonization in half of the infants. It is also known that seropositivity against C. difficile toxins A and B occurs in some infants [9], but its association with C. difficile exposure was previously unknown. We identified an association between infant TCD colonization and a serological response against toxins A and B, as well as toxin B neutralizing activity. Thus, our data suggest that natural immunization against C. difficile toxins A and B occurs in infants following TCD colonization. These data strongly support the hypothesis previously presented by Jangi and Lamont [10], who suggested that TCD colonization in infancy is associated with an antitoxin serological response. Although transplacental transfer of maternal antitoxin antibodies to infants has been proposed to occur [13], our data suggest that the infant humoral immune response to C. difficile colonization is primarily responsible for the observed serological findings rather than passive immunization from the mother. For example, we observed significantly higher levels of antitoxin IgG in infants with a history of TCD colonization compared with cord blood from a cohort of unrelated mothers; this finding would be strengthened if confirmed in a cohort of infant–mother pairs. More importantly, we observed infant antitoxin IgA seropositivity following TCD colonization, but IgA is not commonly detected in high levels in cord blood [22] (unless fetal infection occurs in utero). The lack of association between breastfeeding and infant serological responses further supports our conclusion that TCD colonization is responsible for the observed infant serological findings.
For reasons yet to be definitively determined, unlike older children and adults, infants seem to be innately protected from clinical disease caused by C. difficile toxins [10]. Our study supports this well-established observation, as we did not observe a CDI-like illness in any infants in our cohort despite frequent TCD colonization and detection of toxin in stool. Although toxin was not detected in all infants with TCD colonization, toxin detection was associated with a trend toward higher serum antitoxin levels. While this suggests a potential dose-dependent antitoxin serological response, further investigation is required.
Protection against CDI provided by an antitoxin humoral immune response has been well established in adults [5–7], and this knowledge has guided the development of passive and active immunization strategies for CDI prevention [4]. Identification of NAb activity in infants following C. difficile colonization is particularly important given the protection against CDI recurrence in adults following receipt of bezlotoxumab, a monoclonal antibody that neutralizes toxin B [8]. The extent and duration of CDI protection later in life provided by an antitoxin humoral immune response following TCD colonization in infancy remains unknown. Published pediatric CDI epidemiologic data [23, 24] support our hypothesis that natural immunization to toxins A and B occurs in infants following TCD colonization and may protect against CDI until the late teenage years. There is a bimodal distribution of CDI incidence in childhood, with peaks in early childhood and in the late teenage years [23, 24]. The CDI incidence peak in early childhood may be related to a large proportion of susceptible toddlers who did not experience TCD colonization in infancy; the decline of CDI incidence in school-age children may be related to widespread protection against CDI induced by either infant TCD colonization or CDI in early childhood, and a late teenage CDI incidence peak may be related to waning immunity. However, this hypothesis requires further investigation. Further understanding of the association between C. difficile immunity acquired in infancy/childhood and subsequent CDI prevention is important because of rising CDI incidence in children [25] and because infants may be a source of C. difficile exposure in adults. Infant exposure is a risk factor for community-associated CDI in adults [26] and putative transmission from infants to adults with CDI has been described [15].
Similar to prior studies [12–15], there were a wide variety of TCD/NTCD strains identified in our cohort, and infants often had persistent colonization with the same sequence type. Using WGS, we confirmed persistent colonization with genetically identical strains. While some infants demonstrated strain variation over time, it is unclear if host or pathogen characteristics are predominantly responsible for strain persistence or clearance. In general, TCD strains colonizing infants were phylogenetically similar to strains causing CDI in our previously published pediatric cohort [16, 20, 21]. Of note, epidemic binary toxin-producing strains (eg, BI/NAP1/027 and BK/078) were not identified in our infant cohort and were very uncommon in our previously published cohort of children with CDI [16]. The relatedness of colonizing infant strains and those causing CDI is similar to a prior study in the United Kingdom that identified genetically related strains leading to both infant C. difficile colonization and CDI in adults [15]. The authors suggested that community reservoirs have yet to be identified. In the present study, genetically identical isolates were identified among siblings (25-month age difference). This most likely suggests household transmission, but there may be other shared exposures outside of the home. Genetically identical isolates were identified in 2 pairs and a trio of unrelated infants, suggesting potential outpatient clinic transmission. These infants did not receive care on the same day or within the same clinic room within 1 month of each other. Although clinic environmental cultures were all negative for C. difficile, these were performed post hoc 1–2 years after these putative transmission events. Although routine cleaning of our outpatient clinic is done using a nonsporicidal quaternary ammonium disinfectant, our data do not yet support routine cleaning of clinics with a sporicidal disinfectant.
This study has some limitations. Although, to our knowledge, this is the largest cohort study to assess C. difficile serological responses in infants, the sample size was relatively modest. Despite this limitation, statistical significance was demonstrated for the primary outcome measure, which is the association between colonization and antitoxin antibody measurements. Baseline serological measurements and additional serological measurements beyond our 1-year observational period would more precisely delineate C. difficile immunity dynamics in the pediatric population. Although the longitudinal design permitted evaluation of colonization dynamics, some transient colonization events may have been missed. Stools were frozen and thawed prior to microbiologic assays, which may have reduced the recovery of C. difficile in some samples. However, prior data suggest that the impact of freezing on PCR and culture is likely minimal [27]. Furthermore, because only 1 colony was selected from culture for WGS, co-colonization with multiple sequence types may have been missed. However, because TCD colonization was determined by highly sensitive commercial tcdB PCR positivity [28], TCD-colonized and non–TCD-colonized infants were reliably classified. Nontoxin antigen serological responses were characterized using a crude nontoxin antigen preparation, and this assay may cross-react with other non–C. difficile antigens. Because antibodies against nontoxin antigens, as opposed to antitoxin antibodies, may prevent C. difficile colonization, identification of immunogenic nontoxin antigens in infants is an important area of future investigation.
In conclusion, C. difficile colonization is common in infants and TCD colonization is associated with seropositivity against toxins A and B and NAb titers against toxin B. While these data suggest that this humoral response in TCD-colonized infants is associated with toxin B neutralization in vitro, additional studies are required to determine the extent and duration of protection against CDI afforded by this natural C. difficile immunization event during childhood. This additional knowledge would guide our understanding of the potential public health value of identifying children as a priority population for C. difficile vaccine clinical trials.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. None of the study funding sources contributed to the study design; data collection, analysis, or interpretation; or the writing of or decision to publish this manuscript. The corresponding author had access to all study data and had the final responsibility for the decision to submit for publication.
Financial support. This work was supported by grants from Lurie Children’s Hospital (Pediatric Physician-Scientist Research Career Development Award to L. K. K.), the Gerber Foundation (Novice Researcher Award to L. K. K.), the American Cancer Society (grant number MRSG-13-220-01 to E. A. O.), the National Institute of Allergy and Infectious Diseases (grant numbers K23 AI123525 to L. K. K., K24 AI104831 to A. R. H., U01 AI24290 to T. S. and M. E. C., and R01 AI116596, U19 AI109776, and R01 AI132711 to C. P. K.), and the National Institute of Diabetes and Digestive and Kidney Diseases (grant number P30 DK56338 to T. S.) at the National Institutes of Health. Research reported in this publication was supported, in part, by the National Institutes of Health’s National Center for Advancing Translational Sciences, grant number UL1TR001422.
Potential conflicts of interest. L. K. K. was a scientific advisor for Actelion and Synthetic Biologics, received research supplies from Alere, and received research grants from Merck and Cubist. D. N. G. holds patents for the prevention of C. difficile infection; is a consultant for Sanofi Pasteur, DaVolterra, MGB Biopharma, Matrivax, Medpace, and Pfizer; and an advisory board member of Merck, Rebiotix, Summit, and Actelion. T. S. holds patents for the diagnosis and prevention of C. difficile infection and received research funding from Merck, Nivalis, Cubist, Mead Johnson, Rebiotix, BioFire, and Assembly BioSciences and served on the advisory board for Rebiotix and BioFire. C. P. K. has served as a consultant for Facile Therapeutics, First Light Biosciences, and Finch; as an advisory board member for Artugen, Matrivax, Merck, Sanofi Pasteur, and Vedanta; and received research grants from Institut Merieux, the National Institutes of Health–National Institute of Allergy and Infectious Diseases, and Merck. E. A. O. has served as an advisory board member for Gladius Pharmaceuticals. X. C. has served as a consultant for Artugen. A. R. H. has served as a consultant and scientific advisor for Microbiotix. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med 2015; 372:825–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Magill SS, Edwards JR, Bamberg W, et al. ; Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team. Multistate point-prevalence survey of health care-associated infections. N Engl J Med 2014; 370:1198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. US Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Available at: http://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf. Accessed 21 January 2019.
- 4. Kociolek LK, Gerding DN. Breakthroughs in the treatment and prevention of Clostridium difficile infection. Nat Rev Gastroenterol Hepatol 2016; 13:150–60. [DOI] [PubMed] [Google Scholar]
- 5. Kelly CP, Kyne L. The host immune response to Clostridium difficile. J Med Microbiol 2011; 60:1070–9. [DOI] [PubMed] [Google Scholar]
- 6. Kyne L, Warny M, Qamar A, Kelly CP. Asymptomatic carriage of Clostridium difficile and serum levels of IgG antibody against toxin A. N Engl J Med 2000; 342:390–7. [DOI] [PubMed] [Google Scholar]
- 7. Kyne L, Warny M, Qamar A, Kelly CP. Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea. Lancet 2001; 357:189–93. [DOI] [PubMed] [Google Scholar]
- 8. Wilcox MH, Gerding DN, Poxton IR, et al. ; MODIFY I and MODIFY II Investigators. Bezlotoxumab for prevention of recurrent Clostridium difficile infection. N Engl J Med 2017; 376:305–17. [DOI] [PubMed] [Google Scholar]
- 9. Viscidi R, Laughon BE, Yolken R, et al. Serum antibody response to toxins A and B of Clostridium difficile. J Infect Dis 1983; 148:93–100. [DOI] [PubMed] [Google Scholar]
- 10. Jangi S, Lamont JT. Asymptomatic colonization by Clostridium difficile in infants: implications for disease in later life. J Pediatr Gastroenterol Nutr 2010; 51:2–7. [DOI] [PubMed] [Google Scholar]
- 11. Eglow R, Pothoulakis C, Itzkowitz S, et al. Diminished Clostridium difficile toxin A sensitivity in newborn rabbit ileum is associated with decreased toxin A receptor. J Clin Invest 1992; 90:822–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rousseau C, Levenez F, Fouqueray C, Doré J, Collignon A, Lepage P. Clostridium difficile colonization in early infancy is accompanied by changes in intestinal microbiota composition. J Clin Microbiol 2011; 49:858–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rousseau C, Lemée L, Le Monnier A, Poilane I, Pons JL, Collignon A. Prevalence and diversity of Clostridium difficile strains in infants. J Med Microbiol 2011; 60:1112–8. [DOI] [PubMed] [Google Scholar]
- 14. Rousseau C, Poilane I, De Pontual L, Maherault AC, Le Monnier A, Collignon A. Clostridium difficile carriage in healthy infants in the community: a potential reservoir for pathogenic strains. Clin Infect Dis 2012; 55:1209–15. [DOI] [PubMed] [Google Scholar]
- 15. Stoesser N, Eyre DW, Quan TP, et al. ; Modernising Medical Microbiology Informatics Group (MMMIG). Epidemiology of Clostridium difficile in infants in Oxfordshire, UK: risk factors for colonization and carriage, and genetic overlap with regional C. difficile infection strains. PLoS One 2017; 12:e0182307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kociolek LK, Patel SJ, Shulman ST, Gerding DN. Molecular epidemiology of Clostridium difficile infections in children: a retrospective cohort study. Infect Control Hosp Epidemiol 2015; 36:445–51. [DOI] [PubMed] [Google Scholar]
- 17. Hourigan SK, Chirumamilla SR, Ross T, et al. Clostridium difficile carriage and serum antitoxin responses in children with inflammatory bowel disease. Inflamm Bowel Dis 2013; 19:2744–52. [DOI] [PubMed] [Google Scholar]
- 18. Hughes M, Qazi T, Berg A, et al. Host immune response to Clostridium difficile infection in inflammatory bowel disease patients. Inflamm Bowel Dis 2016; 22:853–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xie J, Zorman J, Indrawati L, et al. Development and optimization of a novel assay to measure neutralizing antibodies against Clostridium difficile toxins. Clin Vaccine Immunol 2013; 20:517–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kociolek LK, Gerding DN, Espinosa RO, Patel SJ, Shulman ST, Ozer EA. Clostridium difficile whole genome sequencing reveals limited transmission among symptomatic children: a single-center analysis. Clin Infect Dis 2018; 67:229–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kociolek LK, Gerding DN, Hecht DW, Ozer EA. Comparative genomics analysis of Clostridium difficile epidemic strain DH/NAP11/106. Microbes Infect 2018; 20:245–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ownby DR, McCullough J, Johnson CC, Peterson EL. Evaluation of IgA measurements as a method for detecting maternal blood contamination of cord blood samples. Pediatr Allergy Immunol 1996; 7:125–9. [DOI] [PubMed] [Google Scholar]
- 23. Khanna S, Baddour LM, Huskins WC, et al. The epidemiology of Clostridium difficile infection in children: a population-based study. Clin Infect Dis 2013; 56:1401–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wendt JM, Cohen JA, Mu Y, et al. Clostridium difficile infection among children across diverse US geographic locations. Pediatrics 2014; 133:651–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tamma PD, Sandora TJ. Clostridium difficile infection in children: current state and unanswered questions. J Pediatric Infect Dis Soc 2012; 1:230–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chitnis AS, Holzbauer SM, Belflower RM, et al. Epidemiology of community-associated Clostridium difficile infection, 2009 through 2011. JAMA Intern Med 2013; 173:1359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Freeman J, Wilcox MH. The effects of storage conditions on viability of Clostridium difficile vegetative cells and spores and toxin activity in human faeces. J Clin Pathol 2003; 56:126–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shin S, Kim M, Kim M, et al. Evaluation of the Xpert Clostridium difficile assay for the diagnosis of Clostridium difficile infection. Ann Lab Med 2012; 32:355–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.