Abstract
Background
Direct measurement of tenofovir (TFV) in urine could be an objective measure to monitor adherence to preexposure prophylaxis (PrEP) or TFV-based antiretroviral therapy (ART).
Methods
We conducted a 3-arm randomized, pharmacokinetic study of tenofovir disoproxil fumarate (TDF) 300 mg/emtricitabine (FTC) 200 mg among adults living with human immunodeficiency virus. Participants were randomized to receive controlled TDF/FTC dosing as (1) “perfect” adherence (daily); (2) “moderate” adherence (4 doses/week); or (3) “low” adherence (2 doses/week). We obtained trough spot urine and plasma samples during a 6-week directly observed therapy period and a 4-week washout period. TFV concentrations were compared between adherence arms using 1-way analysis of variance.
Results
Among 28 participants, the median age was 33 years and 16 (57%) were male. Correlation between TFV plasma and urine concentrations was strong (ρ = 0.78; P < .0001). Median (interquartile range) steady-state trough TFV concentrations (ng/mL) for perfect, moderate, and low TDF adherence were 41 (26–52), 16 (14–19), and 4 (3–5) in plasma; and 6480 (3940–14 300), 3405 (2210–5020), and 448 (228–675) in urine. Trough TFV concentrations at steady state were significantly different between the 3 adherence arms for plasma (P < .0001) and urine (P = .0002). Following drug cessation, TFV concentrations persisted longer in urine than plasma samples. Washout urine TFV concentrations and time to undetectable concentrations did not differ between the 3 randomized adherence groups.
Conclusions
Urine TFV concentrations can inform interpretation of novel point-of-care urine-based TFV assays to assess recent TDF adherence.
Clinical Trials Registration
NCT0301260.
Keywords: HIV, preexposure prophylaxis, antiretroviral treatment, tenofovir, directly observed therapy
Measurement of tenofovir directly in urine would be a novel tool to monitor adherence to preexposure prophylaxis or tenofovir-based antiretroviral therapy. Urine concentrations of tenofovir are related to recent adherence of tenofovir disoproxil fumarate in adults.
(See the Editorial Commentary by Castillo-Mancilla on pages 2152–54.)
Daily oral preexposure prophylaxis (PrEP) of tenofovir disoproxil fumarate (TDF) and emtricitabine (FTC) is safe and effective at reducing the risk of human immunodeficiency virus (HIV) acquisition in adults [1–4]. TDF is also recommended by the Word Health Organization as part of first-line antiretroviral therapy (ART), and approximately 70% of people on first-line ART globally are receiving a TDF-based regimen [5]. The efficacy of daily PrEP and ART are highly dependent on sufficient medication adherence to maintain either protective or therapeutic drug concentrations, respectively [6, 7].
The existing tools to monitor ART and PrEP adherence, such as patient self-report and pill counts, have significant limitations [8–11]. Adherence measured via electronic pill bottle monitoring is more accurate than these subjective measures, but is too complex and expensive for routine use [12]. Drug concentrations can be measured by liquid chromatography tandem mass spectrometry (LC-MS/MS) as a more objective measure of PrEP and ART adherence [13], but this is not available for routine testing in resource-limited settings. Novel tools that enable cheaper, real-time adherence monitoring are needed and in development [14–16].
TDF is an oral prodrug that is converted to tenofovir (TFV). Plasma TFV levels have been used to interpret drug adherence in several PrEP trials [7, 17–20]. TFV is excreted by glomerular filtration and active tubular secretion in urine [21], which is a less invasive biological matrix to collect and monitor for recent drug adherence. If urine TFV concentrations are associated with different drug adherence levels [22], then a point-of-care urine TFV assay could provide a simple objective measure of recent TDF adherence [14, 23, 24].
While TFV concentrations in plasma, hair, and dried blood spots have demonstrated relationships with TDF dosing [25–28], no clinical study to date has assessed typical urine TFV concentrations in adults with various levels of controlled and directly observed TDF adherence. Our objectives were to estimate the correlation between urine and plasma TFV concentrations and to determine whether urine TFV levels could distinguish perfect, moderate, and low TDF adherence among adults in a randomized, directly observed pharmacokinetic study.
METHODS
Study Design
We conducted the TARGET (Tenofovir Adherence to Rapidly Guide and Evaluate PrEP and HIV Therapy) study as a single-center, randomized, open-label, pharmacokinetic study in healthy adult volunteers. The clinical trial protocol has been published [29]. In brief, we enrolled participants at Sanpatong Hospital in Chiang Mai, Thailand, from January 2017 to January 2018. Adults aged 18–49 years were eligible if they were HIV uninfected, hepatitis B surface antigen (HBsAg) negative, and had normal renal function (defined as an estimated glomerular filtration rate [eGFR] >60 mL/minute by the Cockcroft-Gault equation) within 14 days of study enrollment. Participants were excluded if they were known to be pregnant, had been using PrEP, or were eligible to receive PrEP.
Study Procedures
At enrollment, eligible participants were randomized to 1 of 3 study arms (1:1:1) to receive a controlled number of doses of TDF/FTC (300/200 mg) in a combination pill (Truvada, Gilead Sciences) for 6 weeks [29]. Participants representing “perfect adherence” received a TDF/FTC tablet once daily. Participants representing “moderate adherence” received a TDF/FTC tablet 4 times per week (Monday, Wednesday, Friday, and Saturday). Participants representing “low adherence” received a TDF/FTC tablet 2 times/week (Monday and Thursday). During this 6-week treatment period, we conducted directly observed therapy (DOT) for all participants to ensure TFV and intracellular TFV-diphosphate (TFV-DP) attained steady-state concentrations in different biological matrices, including whole blood, plasma, urine, oral fluid, red blood cells, and peripheral blood mononuclear cells (PBMCs).
After randomization, all participants started TDF/FTC in the morning of the entry visit. Predose plasma and spot-urine specimens were collected before the second TDF/FTC dose, and on the first day of week 3 and week 5. At week 7, predose plasma and spot-urine specimens were collected and then each patient received their last TDF/FTC tablet. Time-matched plasma and spot urine samples were then collected each morning for the following 7 days of washout. Thereafter, time-matched plasma and spot urine samples were collected up to twice weekly until the end of the 4-week washout period. We evaluated renal and liver function tests for safety evaluations at baseline, week 3, week 5, week 7, and the end of study.
Measurement of Tenofovir Drug Concentrations
All samples were stored at −70°C until analysis. Plasma and urine TFV concentrations were measured using a validated LC-MS/MS assay over the ranges of 3–2500 ng/mL (plasma) and 50–50 000 ng/mL (urine). Each LC-MS/MS assay was validated in accordance with the Clinical Pharmacology Quality Assurance program method validation guidelines [30], which are based on the US Food and Drug Administration Guidance for Industry Bioanalytical Method Validation [31], and required by the Division of AIDS at the US National Institutes of Health [32].
Statistical Analyses
We used Spearman correlation coefficient (r) to estimate the correlation between measured log-transformed TFV concentrations in spot urine and plasma samples collected in the mornings of the lead-in and washout periods. Only time-matched plasma and urine samples with tenofovir concentrations above the lower limit of quantification (LLOQ) were selected for the correlation analysis. Predose TFV concentrations in plasma and urine collected over the 6-week steady state period were compared across the 3 adherence groups using 1-way repeated measures analysis of variance (ANOVA) and for individual days of the washout period using 1-way ANOVA. We performed single imputation of the data when the TFV concentration was detectable but below the LLOQ using half the LLOQ value for each matrix, that is, 1.5 ng/mL for plasma and 25 ng/mL for urine. Intraindividual variability of TFV in plasma and urine at steady state was estimated using the intraindividual coefficient of variation (%CV) across the 3 time points collected per individual at steady state. We used Cox proportional hazard models to estimate the effect of TDF dosing frequency on time to undetectable TFV in urine and plasma during the washout period. All data were collected using REDCap and statistical analyses performed using SAS version 9.4 software (Cary, North Carolina).
Ethical Considerations
Participants were asked to provide written informed consent before study participation. The study protocol and consent documents were approved by ethics committees at the Institute for the Development of Human Research Protections at the Medical Sciences Department, Thai Ministry of Public Health (TARGET/ IHRP); the ethics committee of Sanpatong Hospital (SPT REC 007/60); the Faculty of Associated Medical Sciences, Chiang Mai University (AMSEC-FB-009); and the University of Washington Institutional Review Board in Seattle (STUDY00000058). The study was registered with ClinicalTrials.gov (identifier NCT0301260).
RESULTS
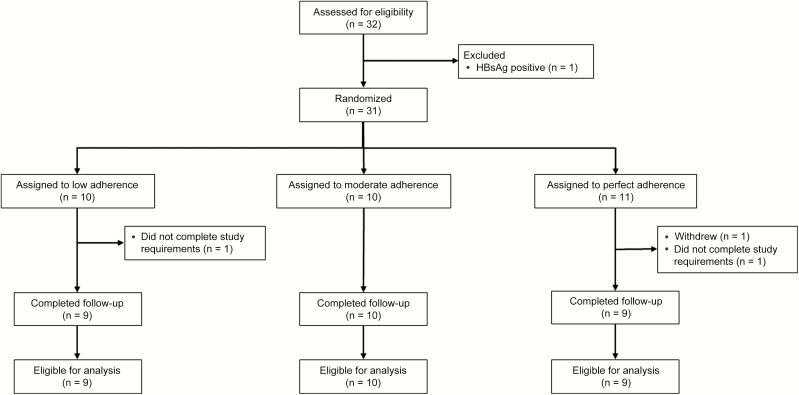
Of 32 adults screened for eligibility, 1 was ineligible due to HBsAg positivity (Figure 1). We enrolled and randomized 31 participants to 1 of the 3 study arms. One participant assigned to the perfect adherence arm withdrew from the study due to the protocol study visit requirements. Two participants (1 in the perfect adherence arm; 1 in the low adherence arm) were not compliant with the drug administration schedule and were excluded from all analyses. Overall, 28 of 30 participants who completed follow-up were included in the final study sample and analyses.
Figure 1.
Consolidated Standards of Reporting Trials diagram of recruitment, randomization, follow-up, and analysis for the Tenofovir Adherence to Rapidly Guide and Evaluate Preexposure Prophylaxis and Human Immunodeficiency Virus Therapy study. Abbreviation: HBsAg, hepatitis B surface antigen.
Among 28 participants, 16 (57%) were male, the median age was 33 (interquartile range [IQR], 28–40) years, and median body mass index was 23.3 (IQR, 20.0–24.8) kg/m2 [2]. Median eGFR was 108 (IQR, 94–119) mL/minute. Between the 3 study arms, the moderate adherence arm had more male participants (8/10 [80%]) compared with the low adherence arm (3/9 [33%]) (Table 1). There were no substantial differences in mean clinical measures or hematologic and chemistry laboratory parameters between the 3 study arms. Participants in the moderate adherence arm had a median eGFR of 126 (IQR, 98–132), indicating slightly better renal function. During the study, 1 person acquired a dengue infection, and there were no other safety concerns or significant adverse events.
Table 1.
Baseline Characteristics of the Tenofovir Adherence to Rapidly Guide and Evaluate Preexposure Prophylaxis and Human Immunodeficiency Virus Therapy (TARGET) Study Participants (N = 28)
| Adherence Arm | |||
|---|---|---|---|
| Characteristic | Low (n = 9) |
Moderate (n = 10) |
Perfect (n = 9) |
| Sociodemographic and clinical characteristics | |||
| Male, no. (%) | 3 (33) | 8 (80) | 5 (56) |
| Age, y | 38 (27–40) | 32 (28–33) | 34 (31–39) |
| Body mass index, kg/m2 | 23.1 (20.2–28.4) | 24.0 (22.1–25.1) | 20.2 (19.1–24.3) |
| Laboratory measures | |||
| White blood cells, cells/μL | 6700 (5200–7700) | 7950 (5800–8800) | 6500 (5900–6900) |
| Hemoglobin, g/dL | 13.3 (13.3–15.0) | 14.5 (14.4–15.2) | 12.4 (11.9–13.2) |
| eGFR (Cockcroft-Gault equation) | 108 (102–115) | 124 (98–132) | 91 (87–108) |
| Total bilirubin, mg/dL | 0.58 (0.47–0.79) | 0.84 (0.51–1.10) | 0.68 (0.35–0.76) |
| AST, U/L | 23 (21–24) | 26 (21–34) | 23 (21–24) |
| ALT, U/L | 17 (12–27) | 31.5 (20–46) | 16 (11–21) |
Data are presented as median (interquartile range) unless otherwise indicated.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; eGFR, estimated glomerular filtration rate.
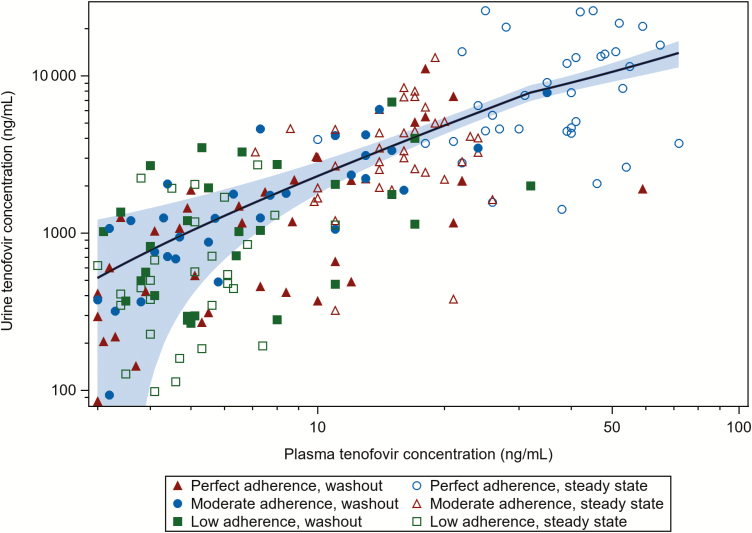
The correlation between quantifiable tenofovir concentrations in plasma and spot urine samples was strong (n = 199; Spearman r = 0.78; P < .0001) (Figure 2). Plasma and urine were correlated both during the steady-state period (n = 105; r = 0.76; P < .0001) and during the drug washout period (n = 94; r = 0.65; P < .0001).
Figure 2.
Correlation of quantifiable spot urine and plasma tenofovir concentrations (ng/mL) (n = 199). A locally estimated scatterplot smoothing curve fit to quantified tenofovir concentrations and 95% confidence interval within the shaded area.
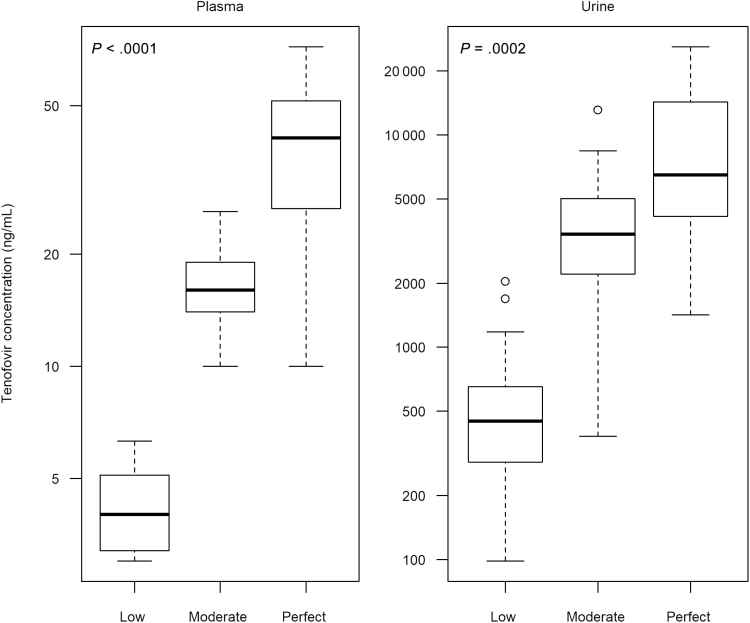
All steady-state spot urine TFV concentrations were detectable, while 6 of 78 (8%) plasma samples were detectable but below the LLOQ. Comparing across the 3 groups there was a significant difference in plasma (P < .0001) and urine (P = .0002) predose concentrations at steady state (Figure 3). For plasma TFV levels, the median steady-state trough concentrations were 41.0 (IQR, 26.0–52.0) ng/mL in the perfect adherence arm, 16.0 (IQR, 14.0–19.0) ng/mL in the moderate adherence arm, and 4.0 (IQR, 3.0–5.1) ng/mL in the low adherence arm. Measurable plasma TFV concentrations in the low adherence arm (range, 3.0–6.3 ng/mL) did not overlap with concentrations observed in the moderate (range, 10.0–26.0 ng/mL) or perfect (range, 10.0–72.0 ng/mL) adherence arms. Concentrations of TFV in the moderate arm fully overlapped with the lower quartile of the perfect arm (10.0–26.0 ng/mL). Intraindividual variability of TFV in plasma samples was 9%–21% across the 3 groups.
Figure 3.
Trough tenofovir concentrations in plasma and spot urine by randomized adherence arm (“low” = 2 doses/week, sampled 96 hours after dosing; “moderate” = 4 doses/week, 48 hours after dosing; “perfect” = 7 doses/week, 24 hours after dosing) at steady state. The thick center line in each box plot indicates the median, top of the box marks the 75th percentile, bottom of the box marks the 25th percentile, and whiskers mark upper and lower bounds excluding outliers. Outliers are defined as values 1.5 times the interquartile range above the upper quartile or below the lower quartile. Six plasma tenofovir concentrations that were detectable but below the lower limit of quantification (3.0 ng/mL) in the low adherence group were assigned a value of 1.5 ng/mL.
The median steady-state trough TFV concentrations in urine were 6480 (IQR, 3940–14 300) ng/mL, 3405 (IQR, 2210–5020) ng/mL, and 448 (IQR, 228–675) ng/mL in the perfect, moderate, and low adherence arms, respectively. In urine, 17 of 27 (63%) trough TFV concentrations in the low adherence arm overlapped with the bottom quartile of TFV concentrations observed in the moderate arm (minimum, 380 ng/mL; 25th percentile, 2210 ng/mL). Similarly, 2 high outlying TFV concentrations in the low adherence arm (1690 and 2040 ng/mL) were within the range observed in the perfect adherence arm (minimum, 1420 ng/mL). Intraindividual variability of TFV in urine samples was 40%–52% across the 3 adherence groups.
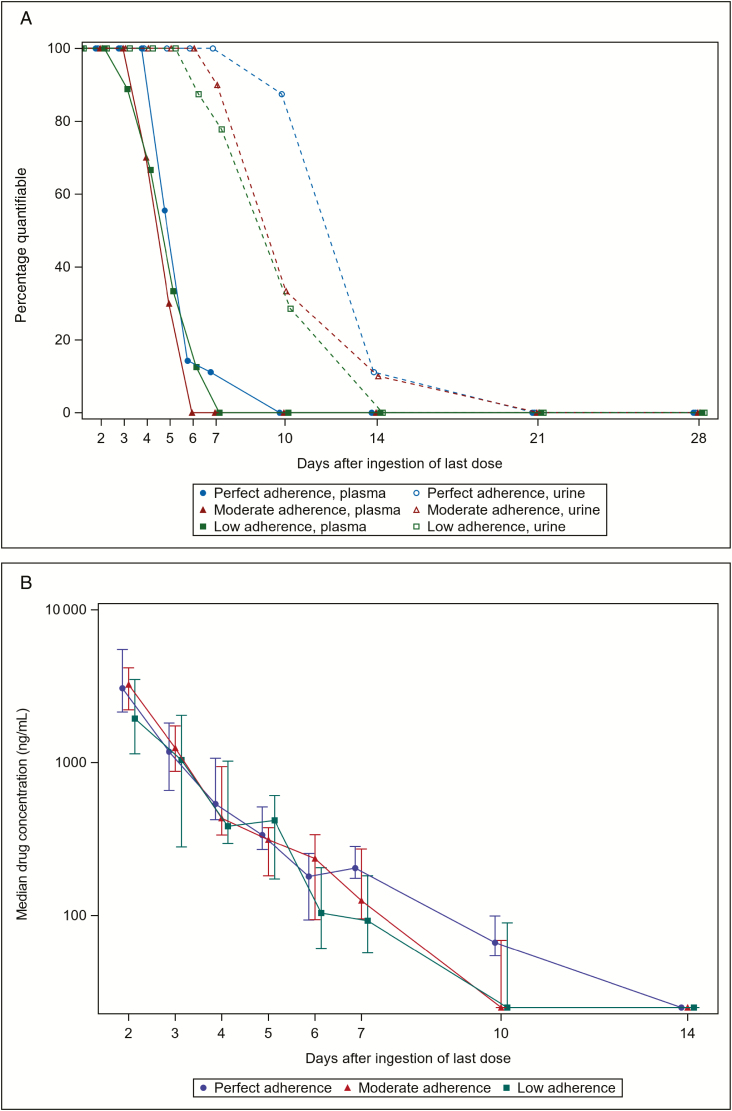
After stopping TDF/FTC, plasma TFV levels remained detectable (ie, >3.0 ng/mL) in all participants for 2, 3, and 4 days in the low, moderate, and perfect adherence arms, respectively (Figure 4A). Two days after ingesting the last TDF dose, the median plasma TFV concentration was 18 (IQR, 12–21) ng/mL in the perfect adherence arm, 13 (IQR, 12–15) ng/mL in the moderate adherence arm, and 11 (IQR, 5–15) ng/mL in the low adherence arm, which were marginal statistical differences across the 3 adherence arms (P = .045). Four days after the last TDF dose, median plasma TFV concentration was 4.7 (IQR, 3.9–5.1) ng/mL in the perfect adherence arm, 3.7 (IQR, 1.5–4.4) ng/mL in the moderate adherence arm, and 3.5 (IQR, 1.5–4.9) ng/mL in the low adherence arm, with no statistical difference between arms (P = .196). Plasma TFV was no longer detectable above 3.0 ng/mL in any participants at 6, 7, and 10 days in the low, moderate, and perfect adherence arms, respectively. Time to undetectable TFV concentration in plasma did not differ statistically between the 3 randomized adherence groups (P = .533). Compared to the perfect adherence arm, there was no difference with the low adherence arm (hazard ratio [HR], 1.52; P = .393) or moderate adherence arm (HR, 1.67; P = .284).
Figure 4.
Proportion of plasma and urine samples with tenofovir (TFV) levels above the limit of quantification (A), and median TFV concentration in urine (B), for liquid chromatography–tandem mass spectrometry during the drug washout period. A, Plasma is indicated with a solid line and urine with a dotted line. Threshold of detection was <3 ng/mL for plasma and <50 ng/mL for urine. Sample size of 7, 7, and 8 in the perfect, moderate, and low adherence arms, respectively, on day 6. B, Interquartile range is represented by a vertical bar for each day and each adherence arm. Sample size of 7, 7, and 8 in the perfect, moderate, and low adherence arms, respectively, on day 6. Single imputation was performed for samples with detectable TFV concentrations but below the lower limit of quantification (50 ng/mL) using 25 ng/mL.
Detectability of urine TFV concentrations during the washout period differed between the 3 different adherence arms, but did not reach statistical significance in time-to-event analysis (P = .404); there was no difference between the low adherence arm (HR, 1.77; P = .233) or moderate adherence arm (HR, 1.71; P = .254), when compared to the perfect adherence arm (Figure 4A). Two days after ingesting the last TDF dose, median urine TFV concentration was 3060 (IQR, 2140–5500) ng/mL in the perfect adherence arm, 3240 (IQR, 2220–4170) ng/mL in the moderate adherence arm, and 1940 (IQR, 1140–3490) ng/mL in the low adherence arm, which was not significantly different (P = .337). Four days after the last TDF dose, median urine TFV concentration was 537 (IQR, 424–1070) ng/mL in the perfect adherence arm, 433 (IQR, 336–943) ng/mL in the moderate adherence arm, and 384 (IQR, 297–1020) ng/mL in the low adherence arm, with no difference between the 3 adherence groups (P = .786). Urine TFV levels were similar after stopping TDF/FTC in the perfect, moderate, and low adherence arms (Figure 4B). Urine TFV was no longer detectable (>50 ng/mL) at 14 days among participants in the low adherence arm, but remained detectable in 1 participant in each of the moderate and perfect adherence arms at this time point. All participants had undetectable TFV concentrations in spot urine samples 21 days after stopping TDF/FTC.
DISCUSSION
In this randomized controlled directly observed pharmacokinetic trial, urine TFV concentrations correlated with plasma TFV collected during a range of dosing frequencies both during steady-state and following drug cessation in adults receiving TDF/FTC. Predose spot urine and plasma TFV concentrations were statistically significantly different among the 3 adherence arms at steady-state. TFV remained detectable in plasma and urine for several days during a drug washout period; however, after the last TDF/FTC dose, urine TFV concentrations during the following washout period did not differ between the 3 randomized adherence groups. This suggests that TFV urine concentrations testing could provide useful information about recent adherence rather than specific patterns of recent adherence due to its sensitivity to recent TDF intake. Taken together, our results suggest that plasma and spot urine TFV samples are suitable for objectively determining recent adherence to PrEP and TFV-based ART, and are matrices to inform the interpretation of recently developed point-of-care immunoassays [15, 16].
The TARGET study is novel for being the first randomized controlled pharmacokinetic study to describe both urine and plasma TFV levels among adults both during and after controlled steady-state levels of TDF/FTC adherence. For plasma TFV levels, the median steady-state TFV plasma trough concentration with daily dosing was 41.0 ng/mL, which is consistent with other studies that have evaluated plasma TFV concentrations with controlled adherence. In the HIV Prevention Trials Network (HPTN) 066 study [26], 2 of the 4 arms studied were similar to the “perfect” and “low” adherence arms assessed in the TARGET study, and predose serum TFV concentrations of 52 ng/mL and 3.6 ng/mL were reported following daily and twice weekly dosing of TDF, respectively, concentrations that are comparable with those observed in our study. No other reported study has measured urine TFV concentrations following controlled TDF dosing, although a study among 10 adults following a single dose of TDF/FTC found that TFV remained detectable in urine for at least 7 days [23]. In our study, all adults receiving daily TDF/FTC had detectable urine TFV concentrations 7 days after cessation. The urine collection in TARGET extended beyond 7 days and in 2 adults urine TFV concentrations remained detectable 2 weeks after stopping TDF/FTC.
The clinical pharmacokinetics of TFV in various biological specimens under controlled directly observed adherence conditions can help define the target thresholds, or adherence “benchmarks,” for the clinical interpretation of novel point-of-care tests. The introduction of scalable TFV-based real-time tests will allow for rapid identification of people struggling with PrEP or ART adherence, allowing for the implementation of targeted adherence interventions. Improving adherence to both PrEP and ART will help prevent HIV transmission, while also preserving an important drug in the efforts to end the HIV/AIDS epidemic.
Urine levels of TFV represent a metric of short-term adherence. While difference in predose TFV concentrations were observed at steady state in both plasma and urine when comparing the 3 randomized adherence groups considerable overlap existed, particularly between the moderate and perfect groups. There was no significant difference in urine concentrations following drug cessation. These results demonstrate that spot urine TFV testing could be influenced by a “white coat” effect, whereby someone ingests TDF shortly prior to testing. Thus, it is important to be cautious when considering target thresholds assessing patterns of adherence based on urine TFV concentrations due to the risk of misclassifying the true adherence of patients.
Drug molecules with a long half-life that achieve steady state over several days or weeks are preferable to assess cumulative exposure and drug adherence pattern [21, 33]. TFV-DP has an intracellular half-life in PBMCs of between 2 and 6 days and measurement of TFV-DP in PBMCs was initially adopted as a marker of tenofovir-based PrEP adherence, and a TFV-DP concentration of 16 fmol/10×6 PBMCs was associated with a 90% reduction in risk of HIV acquisition [6, 25, 34–37]. A subsequent DOT study of TDF showed that TFV-DP concentrations in PBMCs attained from 2, 4, and 7 doses per week corresponded to an HIV risk reduction of 76%, 96%, and 99%, respectively. TFV-DP was later found to have a longer half-life of approximately 17 days in red blood cells of dried blood spots (DBSs) offering a more convenient sample matrix compared to PBMCs for assessment of cumulative drug exposure [21, 25]. Initial modeling studies proposed a range of TFV-DP concentrations in DBS samples associated with different levels of TDF adherence—that is, <2, 2–3, 4–6, and 7 doses per week—and these TFV-DP concentrations were strongly associated with HIV incidence among men who have sex with men/transgender women receiving PrEP [35]. These TFV-DP levels in DBS samples associated with different degrees of adherence were recently confirmed in a DOT study [34, 35].
While the detection of TFV in plasma and TFV-DP in DBSs have been proposed as objective measures of recent and cumulative drug adherence, respectively, the use of more accessible biological samples, such as fingerprick blood, urine, or oral fluid samples [38], is preferable for real-time drug testing for adherence. Moreover, measurement of TFV in plasma and TFV-DP in DBSs via LC-MS/MS requires expensive equipment that is often limited to specialized laboratories in resource-rich settings. Our group and others are working on novel assays to determine recent adherence to TDF by detecting TFV in more accessible clinical samples such as urine. The TARGET data presented suggest that it will be challenging to identify clear thresholds of TFV urine concentrations that can clearly classify differences adherence patterns like TFV-DP measurement in DBS samples, but TFV urine concentrations can provide useful information about recent TDF intake. Urine concentration data from TARGET have informed an adherence cutoff for a recently developed point-of-care TFV immunoassay developed by our group [16]. Using a mixed-effects interval regression model, a urine concentration >1500 ng/mL correctly classified 98% of adults who had taken a 300-mg dose of TDF within the last 24 hours. High specificity (94%) and sensitivity (99%) of the immunoassay to detect urine TFV concentrations above and below this threshold were also observed when compared with the gold standard LC-MS/MS assay [16]. However, urine testing is not without limitations as individual physiological differences, as well as the volume of water intake, can potentially affect urine drug concentrations.
In conclusion, urine TFV concentrations correlate with plasma TFV levels and can be used to determine recent TDF adherence among adults receiving PrEP or tenofovir-based ART. The information generated from this clinical study is helpful to determine how TFV concentrations in various biological specimens may distinguish different levels of recent TDF adherence, which in turn can be applied to interpret drug level monitoring tests. Real-time drug testing and simple, low-cost tools to identify poor PrEP and/or ART adherence would be valuable to help maintain the success of global HIV treatment and prevention programs.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. P. K. D., J. M. B., and T. R. C. conceived the concept of the study. R. W. K. and P. B. conducted the statistical analyses. All authors reviewed and approved the final manuscript.
Disclaimer. None of the funders had any role in study design, data collection, or analysis. The results and interpretation presented here do not necessarily reflect the views of the study funders.
Financial support. This work was supported by research grants from the National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH) (grant numbers R21AI127200 and R01AI143340). Gilead Sciences donated Truvada.
Potential conflicts of interest. J. M. B. has received personal fees from Gilead Sciences, Merck, and Janssen, and grants from the NIH, Centers for Disease Control and Prevention, US Agency for International Development, and Bill & Melinda Gates Foundation. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Baeten JM, Donnell D, Ndase P, et al. Partners PrEP Study Team. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 2012; 367:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Choopanya K, Martin M, Suntharasamai P, et al. Bangkok Tenofovir Study Group. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2013; 381:2083–90. [DOI] [PubMed] [Google Scholar]
- 3. Grant RM, Lama JR, Anderson PL, et al. iPrEx Study Team. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010; 363:2587–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thigpen MC, Kebaabetswe PM, Paxton LA, et al. TDF2 Study Group. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med 2012; 367:423–34. [DOI] [PubMed] [Google Scholar]
- 5. World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection 2016 Recommendations for a public health approach Available at: http://www.who.int/hiv/pub/arv/arv-2016/en/. Accessed 14 January 2017. [PubMed]
- 6. Anderson PL, Glidden DV, Liu A, et al. iPrEx Study Team. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med 2012; 4:151ra125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Donnell D, Baeten JM, Bumpus NN, et al. HIV protective efficacy and correlates of tenofovir blood concentrations in a clinical trial of PrEP for HIV prevention. J Acquir Immune Defic Syndr 2014; 66:340–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fogarty L, Roter D, Larson S, Burke J, Gillespie J, Levy R. Patient adherence to HIV medication regimens: a review of published and abstract reports. Patient Educ Couns 2002; 46:93–108. [DOI] [PubMed] [Google Scholar]
- 9. Bärnighausen T, Chaiyachati K, Chimbindi N, Peoples A, Haberer J, Newell ML. Interventions to increase antiretroviral adherence in sub-Saharan Africa: a systematic review of evaluation studies. Lancet Infect Dis 2011; 11:942–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kalichman SC, Amaral C, Swetsze C, et al. Monthly unannounced pill counts for monitoring HIV treatment adherence: tests for self-monitoring and reactivity effects. HIV Clin Trials 2010; 11:325–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Biressaw S, Abegaz WE, Abebe M, Taye WA, Belay M. Adherence to antiretroviral therapy and associated factors among HIV infected children in Ethiopia: unannounced home-based pill count versus caregivers’ report. BMC Pediatr 2013; 13:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Checchi KD, Huybrechts KF, Avorn J, Kesselheim AS. Electronic medication packaging devices and medication adherence: a systematic review. JAMA 2014; 312:1237–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Koss CA, Hosek SG, Bacchetti P, et al. Comparison of measures of adherence to human immunodeficiency virus preexposure prophylaxis among adolescent and young men who have sex with men in the United States. Clin Infect Dis 2018; 66:213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Anderson PL. What can urine tell us about medication adherence? EClinicalMedicine 2018; 2–3. doi:101016/jeclinm201809003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gandhi M, Bacchetti P, Rodrigues WC, et al. Development and validation of an immunoassay for tenofovir in urine as a real-time metric of antiretroviral adherence. EClinicalMedicine 2018; 2-3:22–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gandhi M, Bacchetti P, Spinelli MA, et al. Validation of a urine tenofovir immunoassay for adherence monitoring to PrEP and ART and establishing the cut-off for a point-of-care test. J Acquir Immune Defic Syndr 2019; 81:72–7. doi:10.1097/QAI.0000000000001971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Van Damme L, Corneli A, Ahmed K, et al. FEM-PrEP Study Group. Preexposure prophylaxis for HIV infection among African women. N Engl J Med 2012; 367:411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Marrazzo JM, Ramjee G, Richardson BA, et al. VOICE Study Team. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med 2015; 372:509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Musinguzi N, Muganzi CD, Boum Y 2nd, et al. Partners PrEP Ancillary Adherence Study Team. Comparison of subjective and objective adherence measures for preexposure prophylaxis against HIV infection among serodiscordant couples in East Africa. AIDS 2016; 30:1121–9. [DOI] [PubMed] [Google Scholar]
- 20. Van Damme L, Corneli A. Antiretroviral preexposure prophylaxis for HIV prevention. N Engl J Med 2013; 368:84. [DOI] [PubMed] [Google Scholar]
- 21. Kearney BP, Flaherty JF, Shah J. Tenofovir disoproxil fumarate: clinical pharmacology and pharmacokinetics. Clin Pharmacokinet 2004; 43:595–612. [DOI] [PubMed] [Google Scholar]
- 22. Landovitz RJ, Beymer M, Kofron R, et al. Plasma tenofovir levels to support adherence to TDF/FTC preexposure prophylaxis for HIV prevention in MSM in Los Angeles, California. J Acquir Immune Defic Syndr 2017; 76:501–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Koenig HC, Mounzer K, Daughtridge GW, et al. Urine assay for tenofovir to monitor adherence in real time to tenofovir disoproxil fumarate/emtricitabine as pre-exposure prophylaxis. HIV Med 2017; 18:412–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lalley-Chareczko L, Clark D, Conyngham C, et al. Delivery of TDF/FTC for pre-exposure prophylaxis to prevent HIV-1 acquisition in young adult men who have sex with men and transgender women of color using a urine adherence assay. J Acquir Immune Defic Syndr 2018; 79:173–8. [DOI] [PubMed] [Google Scholar]
- 25. Castillo-Mancilla JR, Zheng JH, Rower JE, et al. Tenofovir, emtricitabine, and tenofovir diphosphate in dried blood spots for determining recent and cumulative drug exposure. AIDS Res Hum Retroviruses 2013; 29:384–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hendrix CW, Andrade A, Bumpus NN, et al. Dose frequency ranging pharmacokinetic study of tenofovir-emtricitabine after directly observed dosing in healthy volunteers to establish adherence benchmarks (HPTN 066). AIDS Res Hum Retroviruses 2016; 32:32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zheng JH, Guida LA, Rower C, et al. Quantitation of tenofovir and emtricitabine in dried blood spots (DBS) with LC-MS/MS. J Pharm Biomed Anal 2014; 88:144–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu A, Yang Q, Huang Y, et al. Strong relationship between dose and tenofovir level in hair: a novel method of monitoring adherence to pre-exposure prophylaxis (PrEP). PLoS One 2014; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cressey TR, Siriprakaisil O, Klinbuayaem V, et al. A randomized clinical pharmacokinetic trial of tenofovir in blood, plasma and urine in adults with perfect, moderate and low PrEP adherence: the TARGET study. BMC Infect Dis 2017; 17:496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. University of Buffalo. Clinical pharmacology quality assurance and quality control (CPQA) g uidelines for chromatographic method development and validation based on (and including) US FDA guidelines dated May 2001 Version 4.0 reviewed/revised: February 14, 2012 Buffalo, NY: University at Buffalo. Contract Number: HHSN272200800019C. [Google Scholar]
- 31. US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Center for Veterinary Medicine. May 2001. Guidance for industry: bioanalytical method validation Available at: https://wwwfdagov/downloads/Drugs/Guidance/ucm070107pdf. Accessed 21 May 2019.
- 32. DiFrancesco R, Tooley K, Rosenkranz SL, et al. Clinical pharmacology quality assurance for HIV and related infectious diseases research. Clin Pharmacol Ther 2013; 93:479–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hazra R, Balis FM, Tullio AN, et al. Single-dose and steady-state pharmacokinetics of tenofovir disoproxil fumarate in human immunodeficiency virus-infected children. Antimicrob Agents Chemother 2004; 48:124–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Anderson PL, Liu AY, Castillo-Mancilla JR, et al. Intracellular tenofovir-diphosphate and emtricitabine-triphosphate in dried blood spots following directly observed therapy. Antimicrob Agents Chemother 2017; 62. doi:10.1128/AAC.01710-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Grant RM, Anderson PL, McMahan V, et al. iPrEx Study Team. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis 2014; 14:820–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hawkins T, Veikley W, St Claire RL 3rd, Guyer B, Clark N, Kearney BP. Intracellular pharmacokinetics of tenofovir diphosphate, carbovir triphosphate, and lamivudine triphosphate in patients receiving triple-nucleoside regimens. J Acquir Immune Defic Syndr 2005; 39:406–11. [DOI] [PubMed] [Google Scholar]
- 37. Louissaint NA, Cao YJ, Skipper PL, et al. Single dose pharmacokinetics of oral tenofovir in plasma, peripheral blood mononuclear cells, colonic tissue, and vaginal tissue. AIDS Res Hum Retroviruses 2013; 29:1443–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. de Lastours V, Fonsart J, Burlacu R, Gourmel B, Molina JM. Concentrations of tenofovir and emtricitabine in saliva: implications for preexposure prophylaxis of oral HIV acquisition. Antimicrob Agents Chemother 2011; 55: 4905–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gilead Sciences. TRUVADA (emtricitabine/tenofovir disoproxil fumarate) tablets. Foster City, CA: Gilead Sciences, 2016. [Google Scholar]