Abstract
Background.
During October 2011–December 2012, concurrent with a statewide pertussis outbreak, 443 Bordetella parapertussis infections were reported among Wisconsin residents. We examined clinical features of patients with parapertussis and the effect of antibiotic use for treatment and prevention.
Methods.
Patients with polymerase chain reaction results positive for B. parapertussis reported during October 2011–May 2012 were interviewed regarding presence and durations of pertussis-like symptoms and receipt of azithromycin treatment. Data regarding acute cough illnesses and receipt of azithromycin prophylaxis among parapertussis patient household members (HHMs) were also collected. Using multivariate repeated measures log-binomial regression analysis, we examined associations of treatment receipt by the HHM with the earliest illness onset and prophylaxis receipt among other HHMs with the presence of any secondary cough illnesses in the household.
Results.
Among 218 patients with parapertussis, pertussis-like symptoms were frequently reported. Illness durations were significantly shorter among patients with treatment initiated 0–6 days after cough onset, compared with nonrecipients (median durations: 10 vs 19 days, P = .002). Among 361 HHMs from 120 households, compared with nonrecipients, prompt prophylaxis of HHMs was associated with no secondary cough illnesses (relative risk: 0.16; 95% confidence interval, .04–.69).
Conclusions.
Bordetella parapertussis infection causes pertussis-like illness that might be misclassified as pertussis if B. parapertussis testing is not performed. Prompt treatment might shorten illness duration, and prompt HHM prophylaxis might prevent secondary illnesses. Further study is needed to evaluate antibiotic effectiveness for preventing parapertussis and to determine risks and benefits of antibiotic use.
Keywords: Bordetella parapertussis, Bordetella pertussis, epidemiology
Pertussis (whooping cough) is a highly contagious vaccine-preventable illness caused by infection with Bordetella pertussis. Symptoms include paroxysmal cough, posttussive vomiting, and apnea, and can persist for weeks. Infants too young for vaccination are at greatest risk for pertussis and associated severe disease and complications, including hospitalization and death [1]. In the United States, reported cases of pertussis have increased since the 1990s [2], likely because of multiple factors, including the introduction of polymerase chain reaction (PCR) testing for B. pertussis and waning protection from available pertussis vaccines [3]. Azithromycin or other macrolide antibiotic treatment eliminates B. pertussis from an infected person and might reduce illness duration if received early during illness [4–6].Because of incomplete protection from pertussis vaccination, antibiotic treatment of persons suspected of having pertussis and antibiotic prophylaxis of household members (HHMs) are recommended to prevent B. pertussis transmission [6, 7].
Infection with other Bordetella species (Bordetella parapertussis, rarely Bordetella holmesii or Bordetella bronchiseptica) can also cause pertussis-like illness [1, 8–15], and in the absence of laboratory confirmation might contribute to the number of reported or perceived pertussis cases [15–17]. Bordetella parapertussis infection is increasingly recognized in the United States because of PCR testing but likely remains underrecognized because PCR insertion sequence targets for B. parapertussis (IS1001) and B. pertussis (IS481) are different [18], and many laboratories do not test for IS1001 [19]. No national guidelines exist for antibiotic treatment of patients with parapertussis or prophylaxis of HHMs, but antibiotic susceptibility testing indicates that antibiotics recommended for pertussis might be useful for treating and preventing parapertussis [20, 21]. Because persons with pertussis-like illness are often treated and HHMs receive prophylaxis before PCR results are available and because some states (including Wisconsin) have guidelines for antibiotic management of parapertussis [22, 23], some patients with parapertussis and their HHMs might receive antibiotic treatment and prophylaxis. The effectiveness of these interventions has not been evaluated.
During October 2011–December 2012, concurrent with a statewide outbreak of pertussis, the Wisconsin Division of Public Health (WDPH) received numerous reports from laboratories of B. parapertussis infection. We examined clinical and epidemiologic features of reported parapertussis statewide. In Wood County, where simultaneous outbreaks of parapertussis and pertussis occurred, we compared clinical and epidemiologic features of parapertussis and pertussis cases. Additionally, statewide, we examined the effect of antibiotic treatment on duration of parapertussis illness and the effect of antibiotic treatment and prophylaxis on prevention of cough illnesses among parapertussis patient HHMs.
METHODS
Case Reporting and Definitions
In Wisconsin, B. parapertussis infection is not officially reportable. Among 11 laboratories that report Bordetella results to WDPH, 6 simultaneously test specimens for B. pertussis and B. parapertussis and 3 test for B. parapertussis when requested. In response to increased reporting of B. parapertussis infections since October, on 6 December 2011, WDPH requested all laboratories to report B. parapertussis-positive results.
A clinical case was defined as an acute cough illness in a Wisconsin resident reported as a suspected case of pertussis to WDPH through the Wisconsin Electronic Disease Surveillance System during 1 October 2011–31 December 2012 (parapertussis outbreak period). A case of parapertussis was defined as a clinical case with a specimen positive for only B. parapertussis using PCR (IS1001) or culture. A case of pertussis was defined as a clinical case with a specimen positive for only B. pertussis using PCR (IS481) or culture. A case of parapertussis-pertussis coinfection was defined as a clinical case with PCR or culture results positive for both species.
Data Collection
Attempts were made to interview all patients with positive test results for B. parapertussis or B. parapertussis-B. pertussis coinfection reported during 1 October 2011–31 May 2012 (study interval). Patients, or their parents, were interviewed by local health department or WDPH staff using the parapertussis case report form (CRF). Additionally, in Wood County during the study interval, all patients with B. pertussis-positive test results were administered the parapertussis CRF.
The parapertussis CRF collected patient demographic data, antibiotic treatment history, and presence and durations of symptoms characteristic of pertussis and parapertussis including cough, paroxysmal cough, posttussive vomiting, whoop, apnea, fever, weight loss, cyanosis, sleep disturbance, and sleep disturbance among family members. The CRF also collected data regarding parapertussis patients’ HHMs, including age, onset of acute cough illness, and antibiotic receipt. Vaccination histories were obtained from the Wisconsin Immunization Registry.
Data Analysis
Data were analyzed using SAS version 9.3 (SAS Institute, Inc., Cary, North Carolina). Statewide, clinical characteristics of patients with parapertussis were summarized and stratified by age. Among Wood County patients, characteristics of parapertussis patients and pertussis patients were compared. Comparisons of clinical characteristics between groups on a categorical and continuous scale were conducted using chi-square tests (or Fisher exact tests) and nonparametric Mann–Whitney U tests, respectively.
Because 98% of treatments and 100% of prophylaxes were with azithromycin, we limited our evaluation of antibiotics for parapertussis treatment and prevention to recipients of azithromycin treatment or azithromycin prophylaxis of any duration, compared with nonrecipients who received no antibiotics or received antibiotics not recommended for pertussis.
To investigate the effect of treatment on parapertussis illness duration, we compared characteristics of parapertussis patients by whether and when treatment was initiated.
Among parapertussis patient households, receipt of prophylaxis by a HHM was defined as having received azithromycin before any cough illness onset. In each household, the person with the earliest cough illness onset was considered the primary patient. Using the incubation period of pertussis (7–10 days; range: 4–21 days) [24], we compared attack rates (ARs) of coprimary and secondary cough illness (onsets 0–6 and 7–16 days after primary patient cough onset, respectively) among prophylaxis recipients with ARs among nonrecipients. Secondary cough illness was defined as onset 7–16 (rather than 7–28) days after primary patient cough onset because 81% of households were followed for ≥16 days, and only 57% were followed for ≥28 days.
To investigate effects of treatment and prophylaxis on prevention of secondary cough illnesses among parapertussis patient HHMs, we compared characteristics of households with and without secondary cough illnesses. To examine associations of treatment receipt by the household primary patient and prophylaxis receipt by HHMs with the presence of any secondary cough illnesses, we used multivariate log-binomial regression analysis accounting for repeated measurements (multiple HHMs) within the household on the basis of the generalized estimating equation approach. Potential confounders (HHM age and number of children aged <10 years in the household) were entered into the model individually, but were not included because they did not change associations by ≥10%. The final model included receipt of treatment by the household primary patient (no receipt, initiation <1 week or ≥1 week after cough onset) and receipt of prophylaxis by each HHM (no receipt, initiation <2 weeks or ≥2 weeks after cough onset in the household primary patient). Households with coprimary patients, unknown dates of cough onset, or unknown HHM antibiotic histories were excluded. Sensitivity analyses, adjusting for household follow-up time, produced similar results (data not displayed). Results were summarized as relative risks (RRs) with corresponding 95% confidence intervals (CIs). All P-values were 2-sided; P < .05 was used to define statistical significance. This project was reviewed by the Centers for Disease Control and Prevention and determined to be nonresearch because it was applied public health evaluation and control.
RESULTS
Case Reporting and Inclusion
During the 15-month parapertussis outbreak period, 7022 Bordetella infections were reported to WDPH; 6579 (93.7%) were positive for B. pertussis only; 417 (5.9%) were positive for B. parapertussis only; and 26 (0.4%) were B. parapertussis-B. pertussis coinfections (Figure 1). Fifty-two of Wisconsin’s 72 counties reported B. parapertussis infections among residents.
Figure 1.
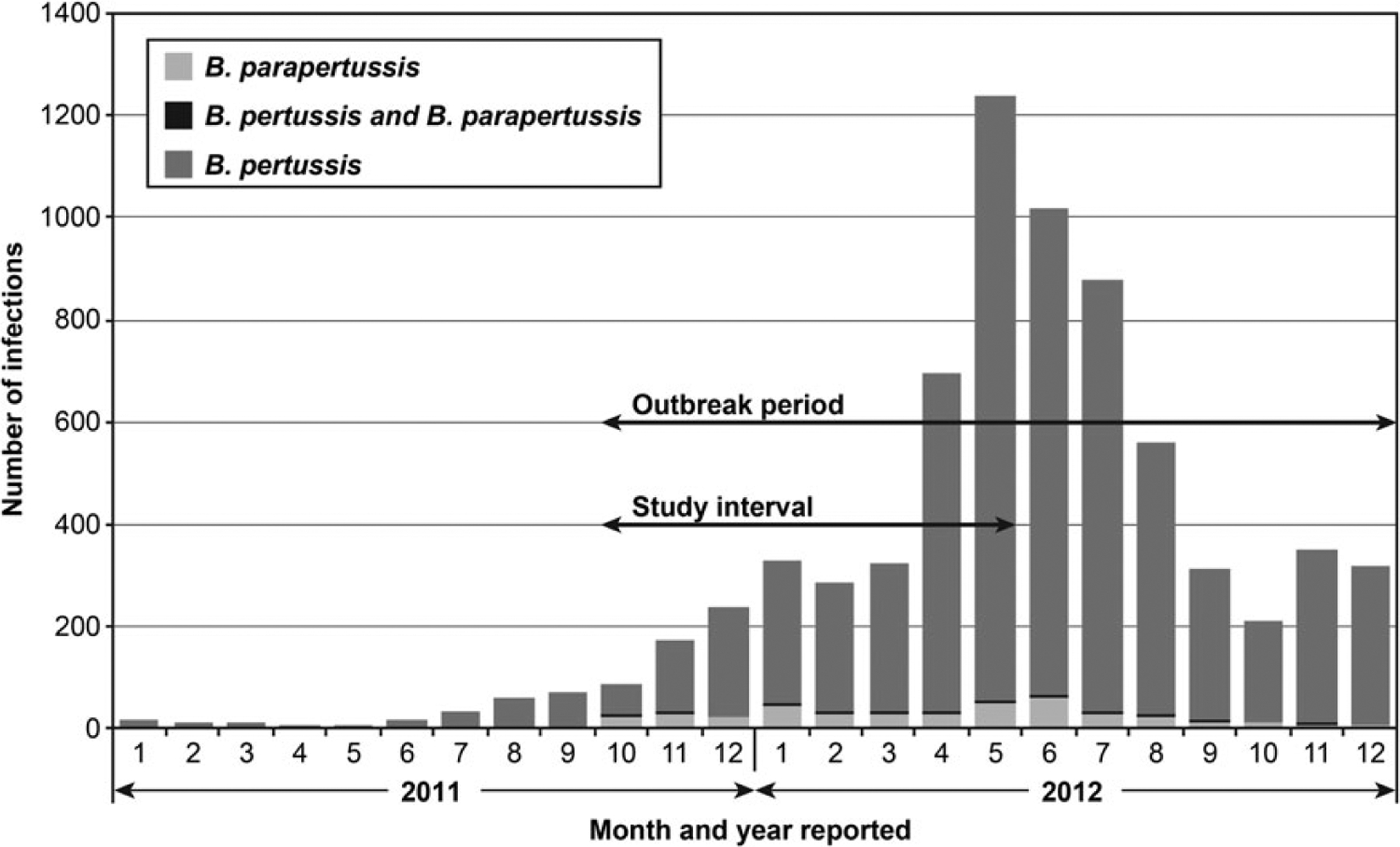
Number of reported cases of Bordetella pertussis and B. parapertussis infections and B. parapertussis-B. pertussis coinfections by month and year of report, Wisconsin. The outbreak period was 1 October 2011–31 December 2012. The study interval was 1 October 2011–31 May 2012.
Figure 2 depicts inclusion of study subjects. During the study interval, WDPH received 3371 reports of patients with positive Bordetella PCR results; 218 illnesses met the parapertussis case definition, including 28 Wood County residents. Thirteen illnesses statewide met the coinfection case definition. Additionally, 103 illnesses among Wood County residents met the pertussis case definition.
Figure 2.
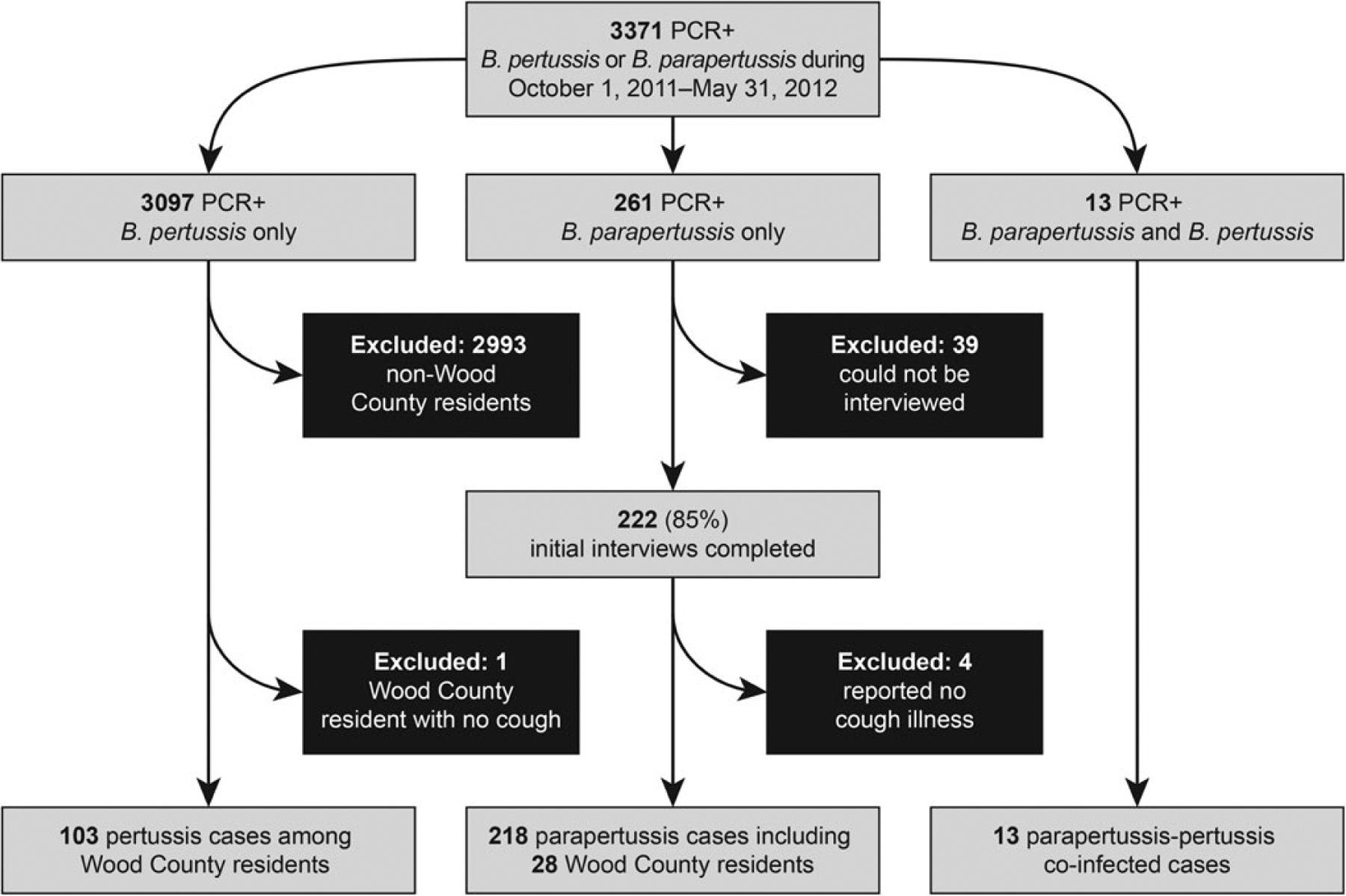
Inclusion of study subjects. During the 8-month study interval, the Wisconsin Division of Public Health received reports of 3371 patients residing in Wisconsin with positive Bordetella polymerase chain reaction (PCR) results. Among these patients, 261 had specimens positive for Bordetella parapertussis only, and 222 (85%) had initial interviews completed. Among the latter, 4 (2%) reported never having a cough and were excluded. In total, 218 illnesses met the parapertussis case definition, including 28 that occurred among Wood County, Wisconsin, residents. Thirteen persons had positive PCR results for both Bordetella pertussis and B. parapertussis; all had initial interviews completed and had illnesses that met the case definition of parapertussis-pertussis coinfection. Additionally, 103 Wood County residents had PCR results positive for B. pertussis with cough onsets during the study interval and were interviewed. A limited number of specimens were confirmed by culture during the study interval. Among 218 patients with parapertussis, 15 had specimens tested by using culture; of these, 5 had culture-results positive for B. parapertussis (2 were Wood County residents). Only 1 of 13 patients with coinfection had a specimen tested by using culture; neither species was isolated. Among 103 Wood County patients with pertussis, 19 had specimens tested by using culture; all results were negative.
During the study interval, 55% (144/261) of B. parapertussis-positive specimens from 261 patients were tested by 2 laboratories that simultaneously test all specimens for B. pertussis and B. parapertussis and consistently report all results. Among Bordetella-positive specimens tested at these 2 laboratories during the study interval, 1,133 (88.2%) were positive for B. pertussis only; 144 (11.2%) were positive for B. parapertussis only; and 8 (0.6%) were positive for both species.
Parapertussis, Statewide
Among 218 patients with parapertussis, median age was 5.6 years (range: 1 month–39 years); 11% were aged <1 year; and 86% were aged <10 years (Table 1). Frequently reported pertussis-like signs and symptoms included paroxysmal cough (60%), posttussive vomiting (31%), whoop (15%), and apnea (9%) (Table 2). Sleep disturbance was reported among 71% of patients and in ≥1 HHM among 55% of households. No seizures or encephalopathies were reported. Two patients (both aged <3 months) were each hospitalized for 3 days. Among 180 (83%) patients followed until all symptoms resolved, the median durations of cough and paroxysmal cough were 15 and 7 days, respectively. Forty-seven percent of patients had illnesses meeting the pertussis clinical case definition. The majority of patients (n = 194; 89%) received an antibiotic recommended for treating pertussis (azithromycin [n = 190, 98%], trimetho-prim/sulfamethoxazole [n = 3], and erythromycin [n = 1]); 8 (4%) received an unknown antibiotic; and 16 (7%) were classified as nonrecipients (no antibiotic treatment [n = 13] or amoxicillin [n = 3]).
Table 1.
Demographic Characteristics of Study Patients, by Type of Bordetella Infection—Wisconsin, 1 October 2011–31 May 2012
| Wisconsina | Wood Countyb | |||
|---|---|---|---|---|
| Characteristic | Parapertussis No. (%) (n = 218) | Coinfection No. (%) (n = 13) | Parapertussis No. (%) (n = 28) | Pertussis No. (%) (n = 103) |
| Age (yrs) | ||||
| <1 | 25 (11) | 1 (8) | 2 (7) | 3 (3) |
| 1–4 | 69 (32) | 2 (15) | 11 (39) | 7 (7) |
| 5–9 | 93 (43) | 3 (23) | 11 (39) | 20 (19) |
| 10–14 | 22 (10) | 4 (31) | 3 (11) | 49 (48) |
| 15–20 | 6 (3) | 2 (15) | 0 (0) | 17 (16) |
| ≥21 | 3 (1) | 1 (8) | 1 (4) | 7 (7) |
| Median (IQR)c | 5.6 (3.4–7.7) | 10.7 (5.5–14.5) | 5.3 (3.5–7.9) | 11.9 (9.2–14.6) |
| Sex | ||||
| Male | 121 (56) | 7 (54) | 14 (50) | 48 (47) |
| Female | 97 (44) | 6 (46) | 14 (50) | 55 (53) |
| Raced | ||||
| White | 156 (94) | 11 (92) | 13 (87) | 40 (95) |
| Black | 4 (2) | 0 (0) | 0 (0) | 0 (0) |
| Other | 6 (4) | 1 (8) | 2 (13) | 2 (5) |
| Ethnicityd | ||||
| Hispanic | 12 (8) | 0 (0) | 2 (14) | 0 (0) |
| Non-Hispanic | 146 (92) | 12 (100) | 12 (86) | 42 (100) |
Coinfection: Test results positive for both Bordetella parapertussis and Bordetella pertussis.
Abbreviation: IQR, interquartile range.
2010 US Census Bureau report for Wisconsin population of approximately 5.68 million persons.
2010 U.S. Census Bureau reports for Wood County, Wisconsin, population of approximately 74 000 persons.
Statewide, parapertussis patients were significantly younger than patients with coinfection (Mann–Whitney U test, P = .010). Among Wood County patients, parapertussis patients were significantly younger than pertussis patients (Mann–Whitney U test, P < .001).
Percentages exclude patients with missing information.
Table 2.
Clinical Features of Patients With Parapertussis, by Age—Wisconsin, 1 October 2011–31 May 2012
| Clinical Feature | All Ages No. (%)a (n = 218) | Aged <1 y No. (%)a (n = 25) | Aged 1–4 y No. (%)a (n = 69) | Aged 5–9 y No. (%)a (n = 93) | Aged ≥10 y No. (%)a (n = 31) | P Valueb |
|---|---|---|---|---|---|---|
| Cough | 218 (100) | 25 (100) | 69 (100) | 93 (100) | 31 (100) | … |
| Cough duration (days), median (IQR) | 15 (10–26) | 15 (10–27) | 16 (9–28) | 15 (10–26) | 15 (10–24) | .527 |
| Paroxysmal cough | 131 (60) | 14 (56) | 47 (68) | 53 (57) | 17 (55) | .931 |
| Paroxysmal cough duration (days), median (IQR) | 7 (4–11) | 5 (3–8) | 7 (5–9) | 7 (3–12) | 8 (6–12) | .074 |
| Posttussive vomiting | 67 (31) | 11 (44) | 23 (33) | 28 (30) | 5 (16) | .035c |
| Whoop | 33 (15) | 5 (20) | 11 (16) | 11 (12) | 6 (19) | 1.000 |
| Apnea | 19 (9) | 4 (16) | 9 (13) | 4 (4) | 2 (6) | .047c |
| Sleep disturbance in patient | 154 (71) | 19 (76) | 52 (75) | 65 (70) | 18 (58) | .208 |
| Sleep disturbance in any household memberd | 106 (55) | 13 (57) | 39 (64) | 39 (48) | 15 (54) | .833 |
| Cyanosisd | 2 (1) | 1 (4) | 1 (2) | 0 (0) | 0 (0) | .462 |
| Feverd | 51 (25) | 10 (43) | 16 (24) | 19 (22) | 6 (20) | .079 |
| Weight lossd | 15 (8) | 1 (4) | 5 (9) | 7 (9) | 2 (7) | 1.000 |
| Hospitalized | 2 (1) | 2 (8) | 0 (0) | 0 (0) | 0 (0) | .184 |
| Met pertussis clinical case definitione | 103 (47) | 9 (36) | 36 (52) | 46 (49) | 12 (39) | .835 |
| Received recommended antibioticf | 194 (89) | 22 (88) | 61 (88) | 82 (88) | 29 (94) | .579 |
| Days from cough onset to antibiotic receipt, median (IQR) | 7 (4–13) | 7 (4–15) | 7 (4–11) | 6 (3–12) | 7 (3–14) | .677 |
Abbreviation: IQR, interquartile range.
All data are presented as number and percentage except for the cough and paroxysmal cough durations, which are presented as median and IQR.
Difference among patients aged <1 year and patients aged ≥10 years were calculated using χ2 or Fisher exact test for differences in categorical variables or Mann–Whitney U test for differences in continuous variables.
P-value of Cochran–Armitage test for trend with age.
Percentages exclude patients with missing information.
Illnesses were classified by whether they met the Council of State and Territorial Epidemiologists clinical case definition of pertussis (cough illness ≥14 days with ≥1 of the following: whoop, paroxysms, or posttussive vomiting) used during 2011–2012 [25].
Recommended antibiotic treatment includes azithromycin, erythromycin, clarithromycin, or trimethoprim-sulfamethoxazole.
Presence of paroxysmal cough, whoop and duration of cough were similar across all age groups (Table 2). Reports of posttussive vomiting and apnea decreased with increasing patient age.
Coinfection, Statewide
Coinfected patients (n = 13) were older (median age: 10.7 years; range: 7 months–55 years) than parapertussis patients (Table 1). Clinical features among coinfected patients are presented in Supplementary Table 1. Frequencies of reported pertussis-like symptoms were similar among coinfected patients and parapertussis patients, but durations of cough and paroxysmal cough were longer among coinfected patients.
Parapertussis and Pertussis, Wood County
Among Wood County patients, parapertussis patients were younger than pertussis patients (median age: 5.3 vs 11.9 years; P < .001) (Table 1). Pertussis-like symptoms were reported frequently among parapertussis and pertussis patients, including paroxysmal cough (75% and 76%), posttussive vomiting (29% and 35%), whoop (29% and 15%), and sleep disturbance (78% and 77%), respectively (Table 3). Among the 82% of parapertussis and 98% of pertussis patients followed until all symptoms resolved, median cough duration was longer among pertussis patients than parapertussis patients (28 vs 14 days; P = .004) (Table 3). Similarly, duration of paroxysmal cough was longer among pertussis patients. However, percentages of patients with illnesses meeting the pertussis clinical case definition did not differ significantly between parapertussis and pertussis patients (61% vs 74%; P = .177).
Table 3.
Clinical Features of Patients With Parapertussis and Patients With Pertussis, by Age—Wood County, Wisconsin, 1 October 2011–31 May 2012
| All Ages | Aged <5 y | Aged 5–10 y | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Clinical Feature | Parapertussis No. (%)a (n = 28) | Pertussis No. (%)a (n = 103) | P Valueb | Parapertussis No. (%)a (n = 13) | Pertussis No. (%)a (n = 10) | P Valueb | Parapertussis No. (%)a (n = 13) | Pertussis No. (%)a (n = 32) | P Valueb |
| Cough duration (days), median (IQR) | 14 (11–28) | 28 (17–40) | .004 | 14 (7–25) | 31 (20–41) | .045 | 14 (12–37) | 30 (17–35) | .265 |
| Paroxysmal cough | 21 (75) | 78 (76) | .937 | 10 (77) | 8 (80) | 1.000 | 10 (77) | 21 (66) | .724 |
| Paroxysmal cough duration (days), median (IQR) | 6 (4–7) | 16 (8–30) | <.001 | 7 (5–7) | 12 (7–18) | .254 | 5 (3–7) | 14 (8–18) | .022 |
| Posttussive vomiting | 8 (29) | 36 (35) | .603 | 5 (38) | 7 (70) | .231 | 3 (23) | 9 (28) | 1.000 |
| Whoop | 8 (29) | 15 (15) | .078 | 3 (23) | 2 (20) | 1.000 | 4 (31) | 3 (9) | .075 |
| Apnea | 2 (7) | 12 (12) | .512 | 2 (15) | 5 (50) | .074 | 0 (0) | 1 (3) | 1.000 |
| Sleep disturbance in patientc | 21 (78) | 78 (77) | .952 | 9 (75) | 8 (80) | 1.000 | 11 (85) | 25 (78) | 1.000 |
| Sleep disturbance in any household memberc | 14 (54) | 22 (56) | .839 | 5 (45) | 2 (100) | .462 | 8 (62) | 9 (69) | 1.000 |
| Hospitalized | 0 (0) | 2 (2) | 1.000 | 0 (0) | 2 (20) | .178 | 0 (0) | 0 (0) | … |
| Met pertussis clinical case definitiond | 17 (61) | 76 (74) | .177 | 8 (62) | 8 (80) | .405 | 8 (62) | 23 (72) | .502 |
| Up-to-date for age with pertussis vaccinations, patients aged 3 mo-10 yc,e | 24 (96) | 34 (89) | .640 | 11 (92) | 4 (67) | .245 | 13 (100) | 30 (94) | 1.000 |
Abbreviation: IQR, interquartile range.
All data are presented as number and percentage except for the cough and paroxysmal cough durations which are presented as median and IQR.
P-value of χ2 or Fisher exact test for difference in presence of symptoms by type of infection or Mann–Whitney U test for difference in symptom duration by type of infection.
Percentages exclude patients with missing information.
Illnesses were classified by whether they met the Council of State and Territorial Epidemiologists clinical case definition of pertussis (cough illness ≥14 days with ≥1 of the following: whoop, paroxysms, or posttussive vomiting) used during 2011–2012 [25].
Patients aged 3 months–10 years were categorized as up-to-date for age with pertussis vaccinations on the basis of the Advisory Committee on Immunization Practices recommended vaccination schedule [26].
When stratified by age group, similar trends were observed; frequencies of pertussis-like symptoms were not significantly different among patients with parapertussis and pertussis, and durations of cough and paroxysmal cough tended to be longer among pertussis patients (Table 3).
Antibiotics for Treatment, Statewide
Among parapertussis patients, 81% (154/190) of azithromycin treatment recipients and 88% (14/16) of nonrecipients were followed until coughing resolved. Among 150 (97%) treatment recipients with known initiation dates, 74 (49%), 42 (28%), and 34 (23%) received antibiotics 0–6, 7–13, and ≥14 days after cough onset, respectively. Patient ages were similar among treatment recipients and nonrecipients (Table 4). Cough illnesses were shorter among patients treated 0–6 days after cough onset, compared with nonrecipients (P = .002) and patients treated 7–13 and ≥14 days after cough onset (Table 4). Neither of the hospitalized patients received antibiotics 0–6 days after cough onset.
Table 4.
Duration of Cough Illness Among Patients With Parapertussis, by Timing of the Initiation of Azithromycin Treatment—Wisconsin, 1 October 2011–31 May 2012
| Initiation of Azithromycin Treatment | |||||
|---|---|---|---|---|---|
| Feature | Nonrecipients (n = 14) | 0–6 d After Cough Onset (n = 74) | 7–13 d After Cough Onset (n = 42) | ≥14 d After Cough Onset (n = 34) | P Valuea |
| Age (yrs), median (IQR) | 5 (4–6) | 5 (3–8) | 5 (3–7) | 5 (3–7) | .996 |
| Duration of cough illness (days), median (IQR) | 19 (14–29) | 10 (8–15) | 16 (14–25) | 26 (24–38) | .002b |
| Hospitalized, n (%) | 0 (0) | 0 (0) | 2 (5) | 0 (0) | … |
Abbreviation: IQR, interquartile range.
P-value of Mann–Whitney U test for the difference between initiation of azithromycin treatment 0–6 days after cough onset vs nonrecipients.
P-value of Mann–Whitney U test for differences in duration of cough illness by timing of initiation of azithromycin treatment: 0–6 days vs 7–13 days (P <.001); 0–6 days vs ≥14 days (P < .001); 7–13 days vs ≥14 days (P = .001).
Antibiotics for Prevention, Statewide
The 218 parapertussis patients resided among 210 households; interviews were conducted with persons from 156 (74%) households, and 478 nonindex patient HHMs were identified. Antibiotic prophylaxis was received by 27% (131/478) of HHMs; all prophylaxes were with azithromycin. Among HHMs aged 1–10 years, ARs of coprimary and secondary cough illnesses were significantly lower among prophylaxis recipients, compared with nonrecipients (Table 5). Among HHMs aged >10 years, ARs of secondary cough illness were significantly lower among prophylaxis recipients. Additionally, among 12 HHMs aged <1 year, ARs were lower among prophylaxis recipients.
Table 5.
Attack Rates of Coprimary and Secondary Cough Illnesses Among Household Members of Patients With Parapertussis, by Receipt of Azithromycin Prophylaxis and Age of Household Members—Wisconsin, 1 October 2011–31 May 2012
| Azithromycin Prophylaxis Received | |||||||
|---|---|---|---|---|---|---|---|
| Household Member Age Category | Household Members No. | Cough Illness Type Occurring Among Household Members | Yes | No | |||
| No. | Attack Rate (%) | No. | Attack Rate (%) | P Valuea | |||
| All ages | 478 | 131 | 347 | ||||
| Coprimaryb | 1/131 (1) | 39/347 (11) | <.001 | ||||
| Secondaryc | 0/130 (0) | 36/308 (12) | <.001 | ||||
| Aged >10 y | 317 | 90 | 227 | ||||
| Coprimaryb | 1/90 (1) | 8/227 (4) | .45 | ||||
| Secondaryc | 0/89 (0) | 14/219 (6) | .013 | ||||
| Aged 1–10 y | 149 | 36 | 113 | ||||
| Coprimaryb | 0/36 (0) | 29/113 (26) | <.001 | ||||
| Secondaryc | 0/36 (0) | 20/84 (24) | <.001 | ||||
| Aged <1 y | 12 | 5 | 7 | ||||
| Coprimaryb | 0/5 (0) | 2/7 (29) | .470 | ||||
| Secondaryc | 0/5 (0) | 2/5 (40) | .444 | ||||
The primary patient in each household was excluded from these analyses.
P-value of Fisher exact test for difference in attack rate by receipt of azithromycin prophylaxis.
Coprimary illness is defined as cough onset 0–6 days after cough onset in the household primary patient.
Secondary illness is defined as cough onset 7–16 days after cough onset in the household primary patient. Attack rate calculations exclude from the denominator all persons with coprimary illnesses because persons with coprimary illnesses were not at risk for having a secondary illness.
Households with and without secondary illnesses were similar regarding the number of HHMs aged <10 years, duration of follow-up, and treatment receipt by the household primary patient (Table 6). Prophylaxis receipt by HHMs was more frequent among households with no secondary cough illnesses (P = .086). Treatment (P = .046) and prophylaxis (P < .001) occurred significantly earlier during the primary patients’ cough illnesses among households without secondary illnesses.
Table 6.
Univariate and Multivariate Analyses of Household and Household Member Characteristics Associated With Secondary Cough Illnesses in the Household — Wisconsin, 1 October 2011–31 May 2012
| Univariate Analysis | |||
|---|---|---|---|
| Household Member had a Secondarya Cough Illness | |||
| Characteristic | No 101 Households With 300 Nonprimary Patient Household Members | Yes 19 Households With 61 Nonprimary Patient Household Members | P Valueb |
| Number of members in household aged <10 y, median (IQR) | 2 (1–2) | 2 (2–2) | .156 |
| Days household was followed, median (IQR) | 29 (18–46) | 32 (20–62) | .424 |
| Azithromycin treatment received by household primary patient, No.(%) | 84 (83) | 15 (79) | .742 |
| Days from cough onset to initiation of azithromycin treatment by household primary patient, median (IQR) | 7 (4–12) | 14 (8–15) | .046 |
| Azithromycin prophylaxis received by household members, No. (%) | 93 (31) | 12 (20) | .086 |
| Days from cough onset in household primary patient to initiation of azithromycin prophylaxis by household member, median (IQR) | 8 (6–13) | 15 (15–17) | <.001 |
| Multivariate Analysis | |||
| n (%) | n (%) | Adjustedc RR (95% CI) | |
| Azithromycin treatment of household primary patient | |||
| None | 17 (17) | 4 (21) | Reference |
| Initiated <1 wk after cough onset | 39 (39) | 3 (16) | 0.60 (.14–2.46) |
| Initiated ≥1 wk after cough onset | 45 (45) | 12 (63) | 1.31 (.46–3.70) |
| Azithromycin prophylaxis of individual household member | |||
| None | 211 (70) | 49 (80) | Reference |
| Initiated <2 wks after cough onset in household primary patient | 70 (23) | 2 (3) | 0.16 (.04–.69) |
| Initiated ≥2 wk after cough onset in household primary patient | 19 (6) | 10 (16) | 1.40 (.66–2.99) |
Abbreviations: CI, confidence interval; IQR, interquartile range; RR, relative risk.
Secondary cough illness is defined as cough onset in a household member 7–16 days after cough onset in the household primary patient.
P-value of χ2 test or Fisher exact test for categorical variables and Mann–Whitney U test for continuous variables.
Adjusted relative risk of secondary cough illness in the household, adjusted for azithromycin prophylaxis of household members, azithromycin treatment of household primary patients, and for repeated measurements within each household.
In multivariate analysis, compared with no prophylaxis, prophylaxis receipt by HHMs <2 weeks after primary patient cough onset was significantly associated with no secondary cough illnesses among HHMs (RR: 0.16; 95% CI, .04–.69) (Table 6). Treatment receipt by the primary patient <1 week after cough onset was also associated with having no secondary cough illnesses among HHMs, but the association was not statistically significant (RR: 0.60; 95% CI, .14–2.46).
DISCUSSION
The number of B. parapertussis infections observed in Wisconsin during October 2011–December 2012 (n = 443) is the largest reported in the United States. Observations of B. parapertussis infections [1, 16, 27–29], including a mixed outbreak of B. pertussis, B. parapertussis, and B. holmseii infections in Ohio during 2010–2011 [28], have been reported recently in the United States, likely because of increased use of PCR testing to detect B. parapertussis. Despite increased testing, the burden of B. parapertussis infection in the United States is challenging to measure because testing that differentiates Bordetella species is not universal [18, 19]. Among specimens tested simultaneously for B. pertussis and B. parapertussis, we observed 11.2% of specimens positive for Bordetella were positive for B. parapertussis only, and an additional 0.6% were positive for B. parapertussis and B. pertussis. This percentage of Bordetella specimens positive for B. parapertussis is similar to previous observations in Wisconsin (culture: 11.9%; PCR: 14.3% [Supplementary Table 2]) and in other states (range: 10%–14.9%) [16, 27, 28], which indicates that infection with B. parapertussis is endemic in the United States and will be identified when testing for B. parapertussis is routinely conducted.
Our results provide additional evidence that B. parapertussis infection can cause pertussis-like illness [8–13]. Our results also demonstrate the duration of parapertussis illness and the presence of paroxysmal cough were generally similar among all age groups. Other pertussis-like signs and symptoms and hospitalization were most common among infants. Considering occur-rences of B. parapertussis bacteremia among 2 children with underlying medical conditions [30], these findings underscore the importance of treating and preventing B. parapertussis infections, especially among infants and other populations at increased risk for severe disease.
Results of antibiotic susceptibility studies indicate the same antibiotics recommended for treating and preventing pertussis might be useful for treating and preventing parapertussis [20, 21]. Our results indicate that azithromycin treatment early during parapertussis illness might reduce the duration of illness. Furthermore, our results indicate that prompt prophylaxis of HHMs and prompt treatment of parapertussis patients might prevent secondary cough illnesses among parapertussis patients’ HHMs. Wisconsin is among a limited number of states [22] with guidelines for managing persons with B. parapertussis infection and recommends antibiotic treatment of infected persons and prophylaxis of contacts aged <6 months and all HHMs if an infant aged <6 months is in the household [23]. Although our results provide support for the effectiveness of these interventions, controlled studies are needed to evaluate the effectiveness of these interventions and determine risks for antibiotic use vs benefits of preventing illness among infants.
Although shorter in duration, parapertussis illnesses were similar to illnesses caused by B. pertussis and B. parapertussis-B. pertussis coinfection. The similarity in clinical presentation of these infections is important for the perception and measurement of pertussis vaccine effectiveness because available pertussis vaccines provide little or no protection from illnesses caused by B. parapertussis [31, 32] or B. holmesii [15]. Consequently, parapertussis cases misclassified as pertussis might be perceived as vaccine failures [17].
The younger age among parapertussis patients, compared with pertussis patients, has been observed previously [16, 27–29] and might be a result of selection bias because older persons with a mild illness might not seek care or testing. Others have suggested the age difference might be because acellular pertussis vaccination of young children provides protection from B. pertussis, but might increase their susceptibility to B. parapertussis [27, 33].
Our study has several limitations. Because culture is now rarely used, few infections were culture confirmed, and studies to characterize strains were not conducted. Regarding Bordetella differentiation, only 1 Wisconsin laboratory (Wisconsin State Laboratory of Hygiene [WSLH]) uses a PCR that differentiates between B. pertussis, B. parapertussis, and B. holmesii. However, of 8505 specimens tested by WSLH during 2012–2013, none was positive for B. holmesii. Our estimate of the relative occurrence of B. parapertussis and B. pertussis infections was based on patients with positive PCR results and thus may not reflect the true relative occurrence of parapertussis compared with pertussis. Because our study was observational, it is possible factors associated with antibiotic receipt and development of cough illness might confound our results. For example, households accepting antibiotics might have been more likely to use other preventive measures that were not measured. Additionally, because PCR testing among symptomatic HHMs was uncommon, the proportion of HHMs with secondary cough illness caused by B. parpertussis is unknown. Controlled studies are needed to evaluate azithromycin effectiveness to treat and prevent parapertussis.
The Ohio [15, 28] and Wisconsin outbreaks demonstrate the potential for cocirculation of Bordetella species and the importance of testing patients with pertussis-like illness using tests that differentiate B. pertussis, B. parapertussis, and B. holmesii. A PCR test that differentiates between these species has been developed and is used by many public health laboratories [18, 34] and can be used by any laboratory testing for Bordetella. Although empiric management of patients presenting with pertussis-like illness might be effective, when a patient infected with B. parapertussis is tested for B. pertussis only, the negative result might lead to unnecessary testing for non-Bordetella etiologies or ineffective treatments. Differentiation of Bordetella species can confirm diagnoses, permit assessment of treatments, and facilitate species-specific studies of disease burden and more accurate determination of pertussis vaccine effectiveness.
Supplementary Material
Acknowledgments.
We thank the staff of Wisconsin’s local health departments and the Wisconsin Division of Public Health Surveillance and Outbreak Support team for their case investigation and data collection efforts, staff of the Wisconsin Electronic Disease Surveillance System for their rapid creation of the online parapertussis case report form and technical assistance, Mary Wedig of the Wisconsin State Laboratory of Hygiene for providing historical data regarding detection of Bordetella, and all of the laboratories that reported Bordetella parapertussis and Bordetella pertussis infections to the Wisconsin Division of Public Health.
Financial support. This work was partially supported by funding from Sanofi Pasteur, Swiftwater, Pennsylvania (grant IND 8502, study M5A16).
Potential conflicts of interest. R. K., J. C. E., R. A. A., and J. H. C. received funding support from Sanofi-Pasteur, Swiftwater, Pennsylvania (Grant IND 8502, Study M5A16) for a study separate from the present work. J. P. D. is the coordinating investigator of a grant from Sanofi-Pasteur (Grant IND 8502, Study M5A16) but does not receive funding support. All other authors report no potential conflicts.
Footnotes
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Mattoo S, Cherry JD. Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Clin Microbiol Rev 2005; 18:326–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clark TA. Changing pertussis epidemiology: everything old is new again. J Infect Dis 2014; 209:978–81. [DOI] [PubMed] [Google Scholar]
- 3.Cherry JD. Epidemic pertussis in 2012—the resurgence of a vaccine-preventable disease. N Engl J Med 2012; 367:785–7. [DOI] [PubMed] [Google Scholar]
- 4.Bortolussi R, Miller B, Ledwith M, Halperin S. Clinical course of pertussis in immunized children. Pediatr Infect Dis J 1995; 14:870–4. [DOI] [PubMed] [Google Scholar]
- 5.Farizo KM, Cochi SL, Zell ER, Brink EW, Wassilak SG, Patriarca PA. Epidemiological features of pertussis in the United States, 1980–1989. Clin Infect Dis 1992; 14:708–19. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Recommended antimicrobial agents for the treatment and postexposure prophylaxis of pertussis: 2005 CDC guidelines. MMWR Recomm Rep 2005; 54(RR–14):1–16. [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Pertussis (whooping cough). Outbreaks. Postexposure antimicrobial prophylaxis. 2013. Available at: http://www.cdc.gov/pertussis/outbreaks/PEP.html. Accessed 20 February 2015. [Google Scholar]
- 8.Bergfors E, Trollfors B, Taranger J, Lagergard T, Sundh V, Zackrisson G. Parapertussis and pertussis: differences and similarities in incidence, clinical course, and antibody responses. Int J Infect Dis 1999; 3:140–6. [DOI] [PubMed] [Google Scholar]
- 9.He Q, Viljanen MK, Arvilommi H, Aittanen B, Mertsola J. Whooping cough caused by Bordetella pertussis and Bordetella parapertussis in an immunized population. JAMA 1998; 280:635–7. [DOI] [PubMed] [Google Scholar]
- 10.Heininger U, Stehr K, Schmitt-Grohe S, et al. Clinical characteristics of illness caused by Bordetella parapertussis compared with illness caused by Bordetella pertussis. Pediatr Infect Dis J 1994; 13:306–9. [DOI] [PubMed] [Google Scholar]
- 11.Liese JG, Renner C, Stojanov S, Belohradsky BH. Clinical and epidemiological picture of B. pertussis and B. parapertussis infections after introduction of acellular pertussis vaccines. Arch Dis Child 2003; 88:684–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mastrantonio P, Stefanelli P, Giuliano M, et al. Bordetella parapertussis infection in children: epidemiology, clinical symptoms, and molecular characteristics of isolates. J Clin Microbiol 1998; 36:999–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wirsing von Konig CH, Finger H. Role of pertussis toxin in causing symptoms of Bordetella parapertussis infection. Eur J Clin Microbiol Infect Dis 1994; 13:455–8. [DOI] [PubMed] [Google Scholar]
- 14.Yih WK, Silva EA, Ida J, Harrington N, Lett SM, George H. Bordetella holmesii-like organisms isolated from Massachusetts patients with pertussis-like symptoms. Emerg Infect Dis 1999; 5:441–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodgers L, Martin SW, Cohn A, et al. Epidemiologic and laboratory features of a large outbreak of pertussis-like illnesses associated with cocirculating Bordetella holmesii and Bordetella pertussis—Ohio, 2010–2011. Clin Infect Dis 2013; 56:322–31. [DOI] [PubMed] [Google Scholar]
- 16.Cherry JD, Seaton BL. Patterns of Bordetella parapertussis respiratory illnesses: 2008–2010. Clin Infect Dis 2012; 54:534–7. [DOI] [PubMed] [Google Scholar]
- 17.Cherry JD. Why do pertussis vaccines fail? Pediatrics 2012; 129:968–70. [DOI] [PubMed] [Google Scholar]
- 18.Williams MM, Taylor TH Jr, Warshauer DM, Martin MD, Valley AM, Tondella ML. Harmonization of Bordetella pertussis real-time PCR diagnostics in the United States in 2012. J Clin Microbiol 2015; 53:118–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tatti KM, Martin SW, Boney KO, Brown K, Clark TA, Tondella ML. Qualitative assessment of pertussis diagnostics in United States laboratories. Pediatr Infect Dis J 2013; 32:942–5. [DOI] [PubMed] [Google Scholar]
- 20.Hoppe JE, Bryskier A. In vitro susceptibilities of Bordetella pertussis and Bordetella parapertussis to two ketolides (HMR 3004 and HMR 3647), four macrolides (azithromycin, clarithromycin, erythromycin A, and roxithromycin), and two ansamycins (rifampin and rifapentine). Antimicrob Agents Chemother 1998; 42:965–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mortensen JE, Rodgers GL. In vitro activity of gemifloxacin and other antimicrobial agents against isolates of Bordetella pertussis and Bordetella parapertussis. J Antimicrob Chemother 2000; 45(suppl 1):47–9. [DOI] [PubMed] [Google Scholar]
- 22.Bak RY, Coronado F, Cohn A, Liang JL, Clark TA, Martin SW. Parapertussis: a new look at an overlooked disease In: 44th National Immunization Conference. Atlanta, GA, 2010. Available at: https://cdc.confex.com/cdc/nic2010/webprogram/Paper22593.html. Accessed 20 February 2015. [Google Scholar]
- 23.Wisconsin Department of Health Services. Pertussis whooping cough. 2015. Available at: http://www.dhs.wisconsin.gov/immunization/pertussis.htm. Accessed 20 February 2015. [Google Scholar]
- 24.Centers for Disease Control and Prevention. Epidemiology and Prevention of Vaccine-Preventable Diseases. 12 ed. Washington, DC: Public Health Foundation, 2012. [Google Scholar]
- 25.Centers for Disease Control and Prevention. Case definitions for infectious conditions under public health surveillance. MMWR Recomm Rep 1997; 46(RR–10):1–55. [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention. Recommended immunization schedules for persons aged 0 through 18 years—United States, 2012. MMWR Morb Mortal Wkly Rep 2012; 61:1–4. [PubMed] [Google Scholar]
- 27.Lavine J, Broutin H, Harvill ET, Bjornstad ON. Imperfect vaccine-induced immunity and whooping cough transmission to infants. Vaccine 2010; 29:11–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spicer KB, Salamon D, Cummins C, Leber A, Rodgers LE, Marcon MJ. Occurrence of 3 Bordetella species during an outbreak of cough illness in Ohio: epidemiology, clinical features, laboratory findings and antimicrobial susceptibility. Pediatr Infect Dis J 2014; 33:e162–7. [DOI] [PubMed] [Google Scholar]
- 29.Theofiles AG, Cunningham SA, Chia N, et al. Pertussis outbreak, southeastern Minnesota, 2012. Mayo Clin Proc 2014; 89:1378–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wallihan R, Selvarangan R, Marcon M, Koranyi K, Spicer K, Jackson MA. Bordetella parapertussis bacteremia: two case reports. Pediatr Infect Dis J 2013; 32:796–8. [DOI] [PubMed] [Google Scholar]
- 31.Heininger U, Stehr K, Christenson P, Cherry JD. Evidence of efficacy of the Lederle/Takeda acellular pertussis component diphtheria and tetanus toxoids and pertussis vaccine but not the Lederle whole-cell component diphtheria and tetanus toxoids and pertussis vaccine against Bordetella parapertussis infection. Clin Infect Dis 1999; 28:602–4. [DOI] [PubMed] [Google Scholar]
- 32.Stehr K, Cherry JD, Heininger U, et al. A comparative efficacy trial in Germany in infants who received either the Lederle/Takeda acellular pertussis component DTP (DTaP) vaccine, the Lederle whole-cell component DTP vaccine, or DT vaccine. Pediatrics 1998; 101(1 Pt 1):1–11. [DOI] [PubMed] [Google Scholar]
- 33.Long GH, Karanikas AT, Harvill ET, Read AF, Hudson PJ. Acellular pertussis vaccination facilitates Bordetella parapertussis infection in a rodent model of bordetellosis. Proc Biol Sci 2010; 277:2017–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tatti KM, Sparks KN, Boney KO, Tondella ML. Novel multitarget real-time PCR assay for rapid detection of Bordetella species in clinical specimens. J Clin Microbiol 2011; 49:4059–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


