Abstract
Metformin has been used for a long time as an antidiabetic medication for type 2 diabetes. It is used either as a monotherapy or in combination with other antidiabetic medications. The drug came into prominence in diabetes and other conditions with cardiovascular risk after the landmark study of 1995 by the United Kingdom Prospective Diabetes Study which emphasized its importance. However, the drug has been used in experimental trials in various aspects of medicine and pharmacology such as in reproductive medicine, cancer chemotherapy, metabolic diseases, and neurodegenerative diseases. It has been in use in the treatment of polycystic ovarian disease and obesity and is being considered in type 1 diabetes. This study seeks to evaluate the relevance of metformin in cancer management. Different mechanisms have been proposed for its antitumor action which involves the following: (a) the activation of adenosine monophosphate kinase, (b) modulation of adenosine A1 receptor (ADORA), (c) reduction in insulin/insulin growth factors, and (d) the role of metformin in the inhibition of endogenous reactive oxygen species (ROS); and its resultant damage to deoxyribonucleic acid (DNA) molecule is another paramount antitumor mechanism.
1. Introduction
Metformin is one of the widely used oral antidiabetic medications. It belongs to the class called biguanides of which the other members have been withdrawn due to associated lactic acidosis. Metformin has been recognized as a first-line pharmacotherapy in type 2 diabetes management in most guidelines such as American Diabetes Association [1].
The origin of the drug is not well known but has been linked to a plant called Galega officinalis (goat's rue), which is rich in guanidine [2]. The ability of this herbal medicine to reduce blood glucose was shown in 1918. Some of its derivatives except metformin were used to treat diabetes but were withdrawn based on associated toxicities [3]. Metformin came to limelight in 1930 in the search for antimalarial medications where it was discovered to have anti-influenza and antidiabetic properties. Jean Sterne followed this discovery up, and in 1957, the use of metformin to treat diabetes was established. However, the recognition did not continue as metformin was less potent than other biguanides (phenformin and buformin) [4]. Indeed, metformin was only licensed in the USA in 1994. Despite this, metformin was the most widely prescribed oral antidiabetic drug in the USA in 2012 [5].
Metformin acts to lower blood glucose mainly by reducing hepatic gluconeogenesis, hence reducing glucose output from the liver. It is a potent glucose-lowering agent, reducing HbA1c by 10-15 mmol/mol (1.0-1.5%) [6]. The landmark study by the United Kingdom Prospective Diabetes Study (UKPDS) group in 1995 established the long-term cardiovascular benefit of metformin, giving rise to the resurgence in the relevance of metformin in diabetes management [7].
2. Chemical Structure of Metformin
Metformin is a white crystalline compound with a molecular formula of C4H11N5·HCl and a molecular weight of 165.65 g/mol [8]. Metformin hydrochloride is soluble in water but insoluble in acetone and ether. It comes in the following formulations: 500 mg, 850 mg, and 1000 mg and can be taken once daily or twice daily.
The two methyl substituents on metformin are responsible for the low lipophilicity of metformin. The low lipophilicity accounts for the slow passive diffusion across tissues. It requires a transporter SLC22A or multidrug-resistant and toxic compound extrusion type transporters to enter or exit the cells and tissues [9].
3. Pharmacokinetic Profile of Metformin
Metformin is denoted chemically as N,N-dimethylbiguanide. The drug is orally administered up to a maximum daily dose of 2550 mg in divided doses. It is readily absorbed and has a half-life of 1.5-3 hours for the immediate release form and 4-8 hours for the extended release type. The bioavailability is approximately 50-60% in a fasting state which is high despite the low lipophilicity [10]. This is due to the involvement of OCT3 (SLC22A3) transporter molecules [11]. Metformin is not bound to plasma proteins and, thus, has high apparent volume of distribution between 300 and 1000 L after a single dose and is not metabolized [12]. It usually takes about 1-2 days to reach the steady state. The therapeutic plasma level after oral administration of metformin is 0.465-2.5 mg/l which is adequate for diabetes control but much lower than the in vitro concentration required for apoptosis [13].
It is excreted through the kidneys by tubular secretion as the active drug so contraindicated in renal failure [14]. The elimination half-life is 4-8.7 hours.
4. Mechanisms of Action of Metformin as an Anticancer Drug
The mechanisms through which metformin achieves its anti-neoplastic effect include the following.
4.1. Metformin and Mammalian Target of Rapamycin Complex 1
Inhibition of cancer cell growth by suppressing mammalian target of rapamycin complex 1 (mTORC1). mTORC1 is a multiprotein complex which is composed essentially of protein kinase mTOR and scaffolding protein raptor [15]. Adenosine monophosphate protein kinase (AMPK) can directly phosphorylate tuberous sclerosis complex (TSC2) on S1387, thereby promoting its inhibition of mTORC1 [16]. The stimulatory effect of protein synthesis by mTOR emphasizes its role in the metabolism and proliferation of malignant cells [17, 18].
4.2. Activation of Adenosine Monophosphate Protein Kinase (AMPK)
Kahn et al. [19] showed that metformin exhibits its antineoplastic effect by activation of adenosine monophosphate protein kinase (AMPK). This involves direct inhibition of mTORC1 through phosphorylation of S722 and S792 on the mTOR binding raptor [20]. This is similar to the mechanism of the antidiabetic effect of metformin. The latter involves liver kinase B1- (LKB1-) dependent activation of adenosine monophosphate-activated protein kinase (AMPK) as highlighted by Shaw et al. [21].
4.3. Metformin and Inhibition of Generation of Reactive Oxygen Species (ROS)
The ROS signaling pathways are markedly increased in many types of cancers where they give rise to abnormal proliferation and differentiation. Reactive oxygen species include peroxides, superoxides, hydroxyl radicals, singlet oxygen, and alpha oxygen [22]. The role of hydrogen peroxide, a typical example of ROS, is implicated in reversible oxidation of tyrosine phosphatases, tyrosine kinases, and transcription factors [23, 24].
The inhibition of ROS generation is mediated by the action of metformin on complex 1 of the respiratory chain which reduces entry of electron to the chain and eventually ROS production [25, 26]. The concentrations of metformin required to directly inhibit complex 1 molecule is high (20-100 mM) in isolated mitochondria in vitro [26]. However, the in vivo inhibition of complex 1 molecule can be achieved with micromolar concentrations [27]. The explanation is based on the positive charge of metformin which allows slow accumulation within the mitochondrial matrix [28]. The inhibition of endogenous generation of ROS is independent of the AMPKα system [25]. One of the vital targets of ROS-induced cellular damage is the DNA with a consequent structural distortion of its integrity (mutation). Flow cytometry shows that cells pretreated with metformin can reduce ROS levels following paraquat exposure [29].
4.4. Reduction of Serum Levels of Insulin, IGF-1, and IGF-2
Metformin reduces the levels of stimuli that promote cancer cell proliferation [30]. High levels of IGF-1 and IGF-2 are linked to the growth of cancer or with cancer recurrence in cancer survivors [31]. The actions of IGF proteins are mediated by IGF-IR, a transmembrane tyrosine kinase which is structurally related to insulin receptor. The binding of IGF-1 and IGF-2 on IGF-receptors eventually results in the activation of mTOR which enhances cellular proliferation and inhibition of apoptosis [32].
4.5. Inhibition of Chronic Inflammation
Metformin also inhibits chronic inflammation, a process which is an important mechanism in the initiation and promotion of carcinogenesis [33, 34]. Metformin inhibits the initial activation of inflammatory response associated with cellular transformation and cancer stem cell growth. This is related to the inhibition of inflammatory transcription factor, NF-Kb [35].
4.6. Modulation of Adenosine A1 Receptor (ADORA1) Expression
Lan et al. [36] described a new pathway involved in the antineoplastic effect of metformin which involves the modulation of adenosine A1 receptor (ADORA1) expression in human colorectal cancer and breast cancer cells. ADORA1 receptors play essential in the supply of cellular energy. Malignant cells are, therefore, deprived of energy in the course of downregulation of ADORA1 receptors. Metformin treatment appreciably upregulates ADORA1 expression in colorectal cancer cells [37]. The ADORA1-mediated growth inhibition and apoptosis induced by metformin are AMPK-mTOR pathway dependent in human colorectal cancer cells [36].
4.7. Downregulation of Gluconeogenesis in the Mitochondria
The antitumor activity of metformin is related to the downregulation of gluconeogenesis in the mitochondria [21]. Hyperglycaemia modulates various pathways that control cell proliferation, migration, and invasion [38]. Warburg phenomenon refers to rapid glucose uptake and metabolism by cancer cells via a process of aerobic glycolysis for the purpose of generating energy [39]. Therefore, hyperglycaemia provides the necessary adenosine triphosphate (ATP) which is required by cancer cells to proliferate rapidly [38].
The diagrammatic representation of the mechanisms of action of metformin as an anticancer agent is shown in Figure 1.
Figure 1.
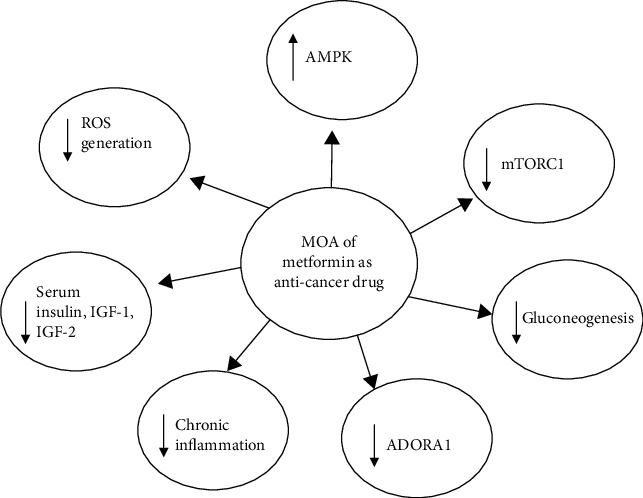
Mechanisms of antineoplastic action of metformin. MOA: mechanism of action; ADORA1: adenosine A1 receptor; AMPK: adenosine monophosphate kinase: ROS: reactive oxygen species; mTORC1: mammalian target of rapamycin complex 1; IGF: insulin-like growth factor; ↓: inhibition; ↑: activation.
5. Cellular Transporters of Metformin
Several transporter proteins are involved in conveying metformin to its intracellular target of action. These transporters, which are usually polyspecific organic cations, are important due to the hydrophilicity of metformin. Transporters are involved in metformin absorption, distribution, and elimination. Several transporters have been described which include OCT1 (SLC22A1), OCT2 (SLC22A2), OCT3 (SLC22A3), MATE1 (SLC47A1), MATE2 (SLC47A2), PMAT (SLC29A4), and OCTN1 (SLC22A4) [40, 41]. Recently, thiamine transporter 2 (THTR-2) (SLC19A3) has been described to play a role in the intestinal absorption and renal reabsorption of metformin.
The cation plasma membrane monoamine transporter (PMAT; SLC29A4) has been implicated in the intestinal absorption of metformin. Others include transporters in the SLC22 family. These include OCT1 (SLC22A1) and OCT3 (SLC22A3), which are expressed in the small intestine. The transporter OCT3 is located at the brush border of the enterocytes, while OCT3 is at the basolateral membrane. Metformin has a large volume of distribution, suggesting a robust tissue uptake. This is facilitated by the transporters OCT3 and OCT1. The pharmacologic actions of metformin are attributed to OCT1 and OCT3.
Organic cation transporter 3 (OCT3) is predominant in human breast cancers. The study done by Cai et al. [42] showed that human breast cancer cell lines which were engineered to express OCT3 achieved >13 times metformin uptake and >4 times antiproliferative effects compared to the control group without OCT3. There was also increased AMPK phosphorylation in the test group.
6. Clinical Trials Using Metformin Hydrochloride in Cancer Therapy
Some clinical trials with metformin are ongoing to ascertain its effect in the treatment of different malignancies. However, some trials have been concluded. The trials involve metformin monotherapy or combination therapy with other chemotherapeutic agents. These are shown in Table 1.
Table 1.
Clinical trials with metformin and other chemotherapeutic agents.
| Cancer type | Phase | Primary outcome | Dosing regimen | Combination | Enrollment number | Clinical trial ref | Completion time |
|---|---|---|---|---|---|---|---|
| Breast cancer [43] | 0 | Changes in Ki67 | Metformin+atorvastatin | Metformin, atorvastatin | 40 | NCT01980823 | 2021 |
| Breast cancer [44] | II | Effects of metformin on AMPK/m/TOR pathway | Metformin 1500 mg daily for 2 weeks before surgery | Metformin monotherapy | 35 | NCT00930579 | 2014 |
| Breast, lung, liver, kidney [45] | I | Effect of metformin+sirolimus on p70S6K | Sirolimus 3 mg daily for 1-7 days. Metformin 500 mg once daily for 8-21 days | Metformin, sirolimus | 64 | NCT02145559 | 2018 |
| Colorectal [46] | II | Disease control rate | FOLFOX+metformin/FOLFIRI+metformin | Metformin, FOLFOX6 | 48 | NCT01926769 | 2014 |
| Prostate [47] | I | DLT assessed at 28days | Enzalutamide PO QD and metformin PO BID | Metformin, enzalutamide | 24 | NCT02339168 | 2021 |
| Pancreas [48] | II | PFS at 12 months | Everolimus+octreotide LAR+metformin | Metformin everolimus, octreotide LAR | 43 | NCT02294006 | 2017 |
| Chronic lymphocytic leukemia [49] | II | Time to treatment failure (assessed 3 months) | Metformin 500 mg PO QD, increased to 500 mg BID after 1week and to 1000 mg BID at week 3 | Metformin monotherapy | 53 | NCT01750567 | 2020 |
| Breast cancer, early stage [50] | III | DFS for a 10-year duration | Arm I: oral metformin HCl QD for 1-4 weeks, then BID afterwards. Treatment for 5 years Arm II: placebo given the same way as metformin in Arm I. |
3649 | NCT01101438 | 2022 |
PFS: progression-free survival; RFS: relapse-free survival; DLT: dose-limiting toxicity; FOLFOX: folinic acid, fluorouracil, oxaliplatin; QD: once daily; BID: two times daily; FOLFIRI: irinotecan, folinic acid, fluorouracil.
7. Outcome of Some Concluded Clinical Trials
NCT02145559: tried to determine the effect of sirolimus and metformin on solid tumors. The combination was well tolerated, but there were no significant changes in phospho-p70S6K and other pharmacodynamic biomarkers [51].
NCT01926769: studied the effect of FOXFOX6 and metformin/FOLFIRI and metformin on colorectal cancer. The outcome is not yet available.
NCT00930579: evaluated the effect of metformin monotherapy on the AMPK/mTOR pathway in breast tumor. There was no significant variation noted in changes of in Ki67 when metformin arm was compared to control arm (p = 0.47) [52]
7.1. Large Population-Based Studies of Metformin on Specific Cancers
7.1.1. Metformin and Prostate Cancer
Prostate cancer is one of the commonest cancers diagnosed in men. A study demonstrated that out of 87,344 men with 17% of whom were diabetic and on metformin, 22% also had DM but were not on metformin; there was an 18% significant reduction in death risk as well as skeletal related events [53].
The relationship between oxidative stress and prostate cancer has been demonstrated in various clinical studies [54, 55]. The protective effect of some antioxidants in men with cancer of the prostate has also been reported [56]. However, as demonstrated in the SELECT trial and Physicians' Health Study II, some antioxidants such as vitamin E have not shown any protective role against prostate cancers [57, 58].
Studies concerning the use of metformin and the risk of developing prostate carcinoma appear conflicting. While some studies showed that metformin has the ability to reduce the risk of developing prostate cancer, others showed positive association of prostate cancer with metformin administration [59–62] However, in a cohort study by Mergel et al. [63] among 3837 patients, it was noted that the longer duration of metformin treatment after diagnosis of prostate cancer was associated with decline in all-cause mortality.
Xie et al. [64] demonstrated that metformin decreases androgen receptor (AR) and androgen receptor-V7 expression and enhances apoptotic cell death. Based on this property of metformin, it improves the antiprostate activity of abiraterone and enzalutamide combination.
Another study by Kuo et al. [65] showed a significant reduction in prostate cancer risk among 2906 metformin cohort and 2906 nonmetformin cohort who were followed up for 5-10 years (95% CI = 0.49, p = 0.0039).
7.1.2. Liver Cancer
The risk of liver cancer was significantly reduced in type 2 diabetes mellitus patients on metformin compared to those who did not receive metformin. Bhalla et al. [66] showed there was minimal liver tumor activity in mice taking metformin compared to the control group where the tumor growth was significant. One of the proposed mechanisms is the inhibition of lipid synthetic capacity of the liver as lipids promote tumorigenesis. In a meta-analysis by Zhang et al. [67], metformin was associated with 62% reduction in the risk of liver cancer among patients with type 2 diabetes (p < 0.001, OR = 0.38, 95% CI = 0.24-0.59). The risk of hepatocellular cancer was even much more reduced in patients on metformin. Another meta-analysis of 19 studies by Shujuan et al. [68] which involved 550,882 diabetic subjects showed a reduction of liver cancer among metformin users by 48% (OR = 0.52; 95% CI = 0.40-0.68) compared to nonmetformin users. Among 42,217 metformin users, there was a strong inverse association between metformin and liver cancers but was not established for bladder, breast, renal, or pancreatic cancers [69].
7.1.3. Metformin and Colon Cancer
Colon cancer is one of the common cancers in man. Many studies on the effect of metformin on colorectal cancer have been carried out in patients with diabetes. The prognosis of colorectal cancer in diabetic patients tended to be worse [70].
Meng et al. [71] in meta-analysis involving seven cohort studies showed that metformin use in colorectal cancer patients with diabetes improved overall survival, reduced cancer-specific mortality, but was not statistically significant (pooled hazard ratio = 0.75, 95% CI = 0.65-0.87). Mei et al. [72] in a meta-analysis of 6 cohort studies demonstrated that the metformin group had a better overall survival (HR = 0.56, 95% CI = 0.41-0.77) than nonusers. However, this meta-analysis had some limitations such as nonspecification of duration of intake of metformin and the impact of other nondiabetic medications in which participants were taking. The latter, such as additional use of insulin, could present a potent confounder.
Higurashi et al. [73] demonstrated in a randomized control trial that metformin has a chemopreventive effect on sporadic colorectal cancer in patients with high risk of recurrent adenoma. Park et al. [74] showed lower mortality in diabetic patients with colon cancer who were taking metformin than in nonmetformin users [72 (38.9%) versus 107 (46.9%), p = 0.012]. This study further demonstrated that female patients with colon cancer on metformin had lower specific mortality rate than their male counterparts (p = 0.025).
AMPK activation and inhibition of mTOR are the potential mechanisms for the anticolon cancer effect of metformin. However, the role of estrogen receptor ERβ protein expression has been suggested and higher ERβ protein expression was associated with higher survival rate in females than in males [75].
A prospective study which involved 47,351 participants in Northern California with diabetes but not on metformin followed up for 15 years revealed that long-term use (≥5year) was associated with reduced risk of colon cancer in the study population (HR, 0.78; 95% CI, 0.60-1.02) [76]. Further deductions from the study showed that higher cumulative doses of metformin were associated with reduced colorectal cancer risk and switching patients from sulphonylureas to metformin or adding metformin is associated with decreased colon CA risk. Similar findings have been shown by Smiechowski et al. [77].
However, the Rosiglitazone Evaluated for Cardiovascular Outcomes and Regulation of Glycemia in Diabetes (RECORD) trial was not able to demonstrate any significant cancer protective effect of metformin [78].
7.1.4. Metformin and Pancreatic Cancer
Pancreatic cancer mainly refers to cancer affecting the exocrine portion of the pancreas [79]. It is the fourth most common cause of cancer death worldwide. The risk of pancreatic cancer is reduced in diabetic patients on treatment with metformin as shown by Andriulli et al. [80] A meta-analysis of 8 studies which involved 4293 patients with pancreatic cancer coexisting with diabetes showed that among the 2033 patients on metformin, there was a relative survival benefit (hazard ratio, 0.81; 95% confidence interval, 0.70-0.93) [81]. The meta-analysis established that among the study population of 4293 diabetic patients with pancreatic cancer on metformin achieved 19% survival benefit compared to nonmetformin users. However, other researchers have shown contradictory result (Hwang et al. [82] and Reni et al. [83]).
7.1.5. Metformin and Breast Cancer
Some studies have reported decreased breast cancer incidence and/or mortality in patients with diabetes who are receiving metformin relative to those receiving other antidiabetic drugs [84, 85]. Breast cancer like other cancers arises when cells lose the ability to halt the process of mitosis, in addition to resistance to apoptotic cell death. High levels of phosphatidylinositol-3-kinase (PI3K)/Akt and mammalian target of rapamycin (mTOR) signaling molecules are expressed by breast cancer cells which impair their ability to undergo apoptosis [86].
Metformin inhibits the inflammatory pathways which are induced by hyperglycaemia and insulin resistance. This indirectly acts by deactivating AMPK pathways, thereby allowing the anticancer effects of metformin to be exhibited [21]. Metformin also works synergistically with chemotherapeutic agents and reduces the development of resistance of breast cancer to them [87].
The mechanism of metformin action in breast cancer is not limited to AMPK pathways. Metformin also induces cell cycle arrest, giving rise to sub-G1 populations and activating apoptotic pathways through downregulation of differentiated embryo chondrocyte 1 (DEC1) and p53 [88]. Administration of metformin has also been shown to cause an increase in intracellular ROS by disruption of the mitochondrial electron transport chain and reduction in the mitochondrial membrane potential [89]. Metformin exhibits proapoptotic effects and promotes cell cycle arrest via increased oxidative stress, as well as AMPK and FOXO3a activation [88].
A retrospective study carried out among Taiwanese women with type 2 DM showed that among 285,087 nonmetformin users, 9322 (2.10%) developed breast cancer (Ca) while among 191,195 metformin users, 2412 participants (1.26%) had breast cancer [90]. Thus, metformin use is associated with a decreased risk of breast cancer. Another retrospective study by Aksoy et al. [91] showed that among 784 breast cancer patients, metformin users had better clinicopathological properties compared to metformin users (p = 0.03).
Implicated in the molecular mechanism of metformin in breast CA is the inhibition of STAT3 phosphorylation in triple-negative and HER2-positive breast cancer [92]. The effect is that of inhibition of cellular proliferation and induction of apoptosis. This is mediated by reduction in the phosphorylation of Tyr 705 and Ser727 [93].
The antineoplastic action of metformin has also been related to its ability to increase miR-26b and a reduction in visfatin levels [94]. Sheikhpou [95] postulated that an increase in visfatin level is linked to malignant characteristic and adverse prognosis in breast cancer. This is mediated through c-Abl and STAT3 oncoproteins.
8. Metformin as Adjuvant to Standard Chemotherapy/Radiotherapy
Tumor cells often times develop multidrug resistance to many chemotherapeutic agents in use. Conversely, metformin may prevent multidrug resistance in breast cancer [96]. Metformin may also resensitize cancer cells to some standard chemotherapeutic agents which they were initially sensitive to. Liu et al. [96] and Qu et al. [97] demonstrated that metformin alters the features of multidrug resistance and resensitizes cells to 5-fluorouracil, adriamycin, and paclitaxel through AMPK and mTOR pathways in breast cancer.
Metformin also blocks the pathways for nicotinamide adenine dinucleotide (NAD+) regeneration [87]. Cell death eventually results in the depletion of NAD+.
Metformin also improves the sensitivity of cancer cells to radiation therapy [98]. The drug may cause superoxide generation by the activation of pyruvate dehydrogenase and α-ketoglutarate dehydrogenase complexes through NADH-dependent mechanism [89].. ROS causes structural damage to DNA of the tumor cells. Samsuri et al. [99] showed that patients with squamous cancer of the oesophagus who received radiotherapy and metformin had improved outcomes.
Metformin is also in phase I/II clinical trial with cisplatin and external beam radiation therapy in participants with stage III-IV head and neck squamous cell cancer [100]. This is actively ongoing in 3 locations at the United States of America. Metformin hydrochloride functions by blocking some enzymes required for cell growth. Cisplatin works by either killing the cells or stopping them from dividing by forming DNA adducts and preventing DNA repairs [101]. External beam radiation kills tumor cells and shrinks tumors.
9. Limitations/Challenges
The dose of metformin used in vitro is very high, usually between 10 and 40 mM (1650-6600 mg/l) and up to 100 mM in isolated mitochondria which is much greater than the therapeutic plasma levels (0.465-2.5 mg/l) in humans [13, 102]. Therefore, the challenge is how to achieve such therapeutic dose in the serum without toxic effects. However, a recent study has shown that concentration of 10 mmol/l can be used in vitro to inhibit cancer proliferation but higher concentrations may be required to achieve apoptosis [103]. The cell culture media used to achieve optimal condition for tumor cells contain high concentrations of growth factors, hormones, and glucose which are nonphysiological. Dowling et al. [104] attributed the elevated metformin dose required in vitro to high concentrations of the culture media constituents
The in vitro studies were conducted outside the confines of the human body; therefore, the notable effects of metformin may not have relevance in clinical settings. The focus should be on how to obtain culture media with physiological concentration of growth factors and glucose, so that the effects of metformin noted on isolated tumor cells can be extrapolated to humans [104]
10. Is Metformin Carcinogenic?
Metformin contains a compound called N-nitrosodimethylamine (NDMA). At an average daily intake of greater than 96 nanograms, NDMA possesses carcinogenic property [105]. This led to a recent evaluation of NDMA levels in different versions of metformin by the Food and Drug Administration (FDA). However, the findings from laboratory evaluation showed that FDA-approved metformin products do not contain dangerous levels of NDMA [106].
11. Conclusion
Metformin is well-known for its use in the treatment of patients with diabetes mellitus. Several molecular properties such as the inhibition of reactive oxygen species, mTORC1. ADORA and activation of AMPK have suggested its utility as an antitumor agent. A few clinical trials have been conducted to investigate its use in some tumors, but results have not been encouraging, though other trials are still ongoing. Several population studies have suggested a protective effect of metformin in the cancer of the breast, colon, pancreas, prostate, and liver. However, these have not clearly demonstrated a role for metformin as either a chemotherapeutic agent or adjuvant therapy. As the results of ongoing clinical trials are awaited, the authors suggest that further investigational research may focus on validating these findings with the aim of metformin use in cancer chemotherapy even in nondiabetic patients also. Furthermore, correlating the metformin dose used in clinical medicine with the concentration used in vitro may enhance the clinical utility of metformin in neoplasms.
Acknowledgments
The work was supported by means of contributions from the personal funds of all the authors. There was no external funding and no institutional or employer support.
Conflicts of Interest
The authors declare that they have no conflict of interest
References
- 1.American Diabetes Association (ADA) Standards of medical care in diabetes. Diabetes Care. 2017;40:1–142. [Google Scholar]
- 2.Bailey C. J., Day C. Metformin: its botanical background. Practical Diabetes International. 2004;21(3):115–117. doi: 10.1002/pdi.606. [DOI] [Google Scholar]
- 3.Watanabe C. K. Studies in the metabolic changes induced by THE administration of guanidine bases. The Journal of Biochemistry. 1922;1(2):195–200. doi: 10.1093/oxfordjournals.jbchem.a125363. [DOI] [Google Scholar]
- 4.Stearne J. Du nouveau dans les antidiabetiques. La NN dimethylamineguanylguanide (N.N.D. G) Maroc Médical. 1957;36:1295–1296. [Google Scholar]
- 5.Hampp C., Borders-Hemphill V., Moeny D. G., Wysowski D. K. Use of antidiabetic drugs in the U.S., 2003-2012. Diabetes Care. 2014;37(5):1367–1374. doi: 10.2337/dc13-2289. [DOI] [PubMed] [Google Scholar]
- 6.Marshall S. M. 60 years of metformin use: a glance at the past and a look to the future. Diabetologia. 2017;60(9):1561–1565. doi: 10.1007/s00125-017-4343-y. [DOI] [PubMed] [Google Scholar]
- 7.King P., Peacock I., Donnelly R. The UK Prospective Diabetes Study (UKPDS): clinical and therapeutic implications for type 2 diabetes. British Journal of Clinical Pharmacology. 1999;48(5):643–648. doi: 10.1046/j.1365-2125.1999.00092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sirtori C. R., Franceschini G., Galli-Kienle M., et al. Disposition of metformin(N, N- dimethylbiguanide) in man. Clinical Pharmacology & Therapeutics. 1978;24(6):683–693. doi: 10.1002/cpt1978246683. [DOI] [PubMed] [Google Scholar]
- 9.Liang X., Giacomini K. M. Transporters involved in metformin pharmacokinetics and treatment response. Journal of Pharmaceutical Sciences. 2017;106(9):2245–2250. doi: 10.1016/j.xphs.2017.04.078. [DOI] [PubMed] [Google Scholar]
- 10.Hundal R. S., Inzucchi S. E. Metformin: new understandings, new uses. Drugs. 2003;63(18):1879–1894. doi: 10.2165/00003495-200363180-00001. [DOI] [PubMed] [Google Scholar]
- 11.Shirasaka Y., Lee N., Zha W., Wagner D., Wang J. Involvement of organic cation transporter 3 (Oct3/Slc22a3) in the bioavailability and pharmacokinetics of antidiabetic metformin in mice. Drug Metabolism and Pharmacokinetics. 2016;31(5):385–388. doi: 10.1016/j.dmpk.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hardie D. G. AMP-activated protein kinase as a drug target. Annual Review of Pharmacology and Toxicology. 2007;47(1):185–210. doi: 10.1146/annurev.pharmtox.47.120505.105304. [DOI] [PubMed] [Google Scholar]
- 13.He L., Wondisford F. Metformin action: concentrations matter. Cell Metabolism. 2015;21(2):159–162. doi: 10.1016/j.cmet.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Nolte M. S. Pancreatic hormones & Anti diabetic drugs. In: Katzung B. G., Masters S. B., Trevor A. J., editors. Basic and Clinical Pharmacology. 11th. New York, NY: McGrawHill Companies Inc; 2012. pp. 759–784. [Google Scholar]
- 15.Laplante M., Sabatini D. M. mTOR signaling in growth control and disease. Cell. 2012;149(2):274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pezze P. D., Ruf S., Sonntag A. G., et al. A systems study reveals concurrent activation of AMPK and mTOR by amino acids. Nature Communications. 2016;7(1):p. 13254. doi: 10.1038/ncomms13254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Viollet B., Guigas B., Garcia N. S., Leclerc J., Foretz M., Andreelli F. Cellular and molecular mechanisms of metformin: an overview. Clinical Science. 2012;122(6):253–270. doi: 10.1042/CS20110386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dowling R. J. O., Zakikhani M., Fantus I. G., Pollak M., Sonenberg N. Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Research. 2007;67(22):10804–10812. doi: 10.1158/0008-5472.CAN-07-2310. [DOI] [PubMed] [Google Scholar]
- 19.Kahn B. B., Alquier T., Carling D., Hardie D. G. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metabolism. 2005;1(1):15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 20.Gwinn D. M., Shackelford D. B., Egan D. F., et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Molecular Cell. 2008;30(2):214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaw R. J., Lamia K. A., Vasquez D., et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310(5754):1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dix M. Everything you should know about oxidative stress. 2017. https://www.healthline.com.
- 23.Rhee S. G., Chang T. S., Bae Y. S., Lee S. R., Kang S. W. Hydrogen peroxide as intracellular messenger, production, target and elimination. In: Bhattacharya J., editor. Cell signaling in vascular inflammation. Totowa, NJ: Human Press Inc; 2005. pp. 191–202. [Google Scholar]
- 24.Lennicke C., Rahn J., Lichtenfels R., Wessjohann L. A., Seliger B. Hydrogen peroxide- production, fate and role in redox signaling of tumor cells. Cell Communication and Signaling. 2015;13(1) doi: 10.1186/s12964-015-0118-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Algire C., Moiseeva O., Deschenes-Simard X., et al. Metformin Reduces Endogenous Reactive Oxygen Species and Associated DNA Damage. Cancer Prevention Research. 2012;5(4):536–543. doi: 10.1158/1940-6207.capr-11-0536. [DOI] [PubMed] [Google Scholar]
- 26.Vial G., Detaille D., Guigas B. Role of mitochondria in the mechanism(s) of action of metformin. Frontiers in Endocrinology. 2019;10 doi: 10.3389/fendo.2019.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wessels B., Ciapaite J., van den Broek N. M. A., Nicolay K., Prompers J. J. Metformin impairs mitochondrial function in skeletal muscle of both lean and diabetic rats in a Dose-Dependent manner. PLoS One. 2014;9(6, article e100525) doi: 10.1371/journal.pone.0100525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bridges H. R., Jones A. J., Pollak M. N., Hirst J. Effects of metformin and other biguanides on oxidative phosphorylation in mitochondria. The Biochemical Journal. 2014;462(3):475–487. doi: 10.1042/BJ20140620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aljofan M., Riethmacher D. Anticancer activity of metformin: a systematic review of the literature. Future Science OA. 2019;5(8):p. FSO410. doi: 10.2144/fsoa-2019-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goodwin P. J., Pritchard K. I., Ennis M., Clemons M., Graham M., Fantus I. G. Insulin-lowering effects of metformin in women with early breast cancer. Clinical Breast Cancer. 2008;8(6):501–505. doi: 10.3816/CBC.2008.n.060. [DOI] [PubMed] [Google Scholar]
- 31.Pollak M. The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nature Reviews Cancer. 2012;12(3):159–169. doi: 10.1038/nrc3215. [DOI] [PubMed] [Google Scholar]
- 32.Navarro M., Baserga R. Limited redundancy of survival signals from the type 1 insulin-like growth factor receptor. Endocrinology. 2001;142(3):1073–1081. doi: 10.1210/endo.142.3.7991. [DOI] [PubMed] [Google Scholar]
- 33.Hirsch H. A., Iliopoulos D., Struhl K. Metformin inhibits the inflammatory response associated with cellular transformation and cancer stem cell growth. Proceedings of the National Academy of Sciences. 2013;110(3):972–977. doi: 10.1073/pnas.1221055110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dong J., Peng H., Yang X., et al. Metformin mediated microRNA-7 upregulation inhibits growth, migration, and invasion of non-small cell lung cancer A549 cells. Anti-Cancer Drugs. 2020;31(4):345–352. doi: 10.1097/CAD.0000000000000875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sultuybek G. K., Soydas T., Yenmis G. NF‐κB as the mediator of metformin's effect on ageing and ageing‐related diseases. Clinical and Experimental Pharmacology and Physiology. 2019;46(5):413–422. doi: 10.1111/1440-1681.13073. [DOI] [PubMed] [Google Scholar]
- 36.Lan B., Zhang J., Zhang P., et al. Metformin suppresses CRC growth by inducing apoptosis via ADORA1. Frontiers in Bioscience. 2017;22(2):248–257. doi: 10.2741/4484. [DOI] [PubMed] [Google Scholar]
- 37.Saito M., Yaguchi T., Yasuda Y., Nakano T., Nishizaki T. Adenosine suppresses CW2 human colonic cancer growth by inducing apoptosis via A1 adenosine receptors. Cancer Letters. 2010;290(2):211–215. doi: 10.1016/j.canlet.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 38.Li W., Zhang X., Sang H., et al. Effects of hyperglycemia on the progression of tumor diseases. Journal of Experimental & Clinical Cancer Research. 2019;38(1):p. 327. doi: 10.1186/s13046-019-1309-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liberti M. V., Locasale J. W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends in Biochemical Sciences. 2006;41(3):211–218. doi: 10.1016/j.tibs.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hilgendorf C., Ahlin G., Seithel A., Artursson P., Ungell A.-L., Karlsson J. Expression of thirty six drug transporter genes in human intestine, liver, kidney and organotypic cell lines. Drug Metabolism and Disposition. 2007;35(8):1333–1340. doi: 10.1124/dmd.107.014902. [DOI] [PubMed] [Google Scholar]
- 41.Zhou M., Xia L., Wang J. Metformin transport by a newly cloned proton-stimulated organic cation transporter (plasma membrane monoamine transporter) expressed in human intestine. Drug Metabolism and Disposition. 2007;35(10):1956–1962. doi: 10.1124/dmd.107.015495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cai H., Zhang Y., Han T. K., Everett R. S., Thakker D. R. Cation-selective transporters are critical to the AMPK-mediated antiproliferative effects of metformin in human breast cancer cells. International Journal of Cancer. 2015;138(9):2281–2292. doi: 10.1002/ijc.29965. [DOI] [PubMed] [Google Scholar]
- 43.Kalinsky K. Pre-surgical trial of the combination of metformin and atorvastatin in newly diagnosed operable breast cancer. Estimated study completion 2021 phase 0. 2021, NCT01980823.
- 44.Hershman D. L. Phase II presurgical intervention study for evaluating the effect of metformin on breast cancer proliferation. Estimated study completion date: 2014. 2014, NCT00930579.
- 45.Sharma M. A pharmacodynamic study of of sirolimus and metformin in patients with advanced solid tumors. Completion of the study: 2018. 2018, NCT02145559. [DOI] [PMC free article] [PubMed]
- 46.Ahn H. K. A phase II study to determine the safety and efficacy of second line treatment with metformin and chemotherapy (FOLFOX6 or FOFIRI) in the second line treatment of advanced colorectal cancer (actual completion date 2014) 2014, NCT01926769.
- 47.Evans C. Enzalutamide and metformin hydrochloride in treating patients with hormone resistant prostate cancer, Completion date: 2021. 2021, NCT02339168.
- 48.Yu X.-J. A phase II, randomized, double-blind, placebo controlled study to evaluate the efficacy and safety of the combination of gemcitabine and metformin in treating patients with pancreatic cancer after curative resection. 2017, NCT02294006.
- 49.Malek S. A phase II pilot study of metformin therapy with relapsed chronic lymphocytic leukemia and untreated CLL patients with genomic deletion 11q. Completion date 2020. 2020, NCT01750567.
- 50.Parulekar W., Chen B. E., Elliott C., et al. A phase III randomized trial of metformin versus placebo on recurrence and survival in early-stage breast cancer (BC)(NCIC Clinical Trials Group MA. 32) Journal of Clinical Oncology. 2011;29(15_supplement):p. TPS103. doi: 10.1200/jco.2011.29.15_suppl.tps103. 2020, NCT01101438. [DOI] [Google Scholar]
- 51.Sehdev A., Zha Y., Karrison T. G., et al. A pharmacodynamic study of sirolimus and metformin in patients with advanced solid tumors. Journal of Clinical Oncology. 2017;35(15_suppl):p. TPS11628. doi: 10.1200/jco.2017.35.15_suppl.tps11628. NCT02145559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kalinsky K., Crew K. D., Refice S., et al. Presurgical trial of metformin in overweight and obese patients with newly diagnosed breast cancer. Cancer Investigation. 2014;32(4):150–157. doi: 10.3109/07357907.2014.889706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Richards K. A., Liou J. I., Cryns V. L., Downs T. M., Abel E. J., Jarrard D. F. Metformin use is associated with improved survival for patients with advanced prostate Cancer on androgen deprivation therapy. The Journal of Urology. 2018;200(6):1256–1263. doi: 10.1016/j.juro.2018.06.031. [DOI] [PubMed] [Google Scholar]
- 54.Khandrika L., Kumar B., Koul S., Maroni P., Koul H. K. Oxidative stress in prostate cancer. Cancer Letters. 2009;282(2):125–136. doi: 10.1016/j.canlet.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reuter S., Gupta S. C., Chaturvedi M. M., Aggarwal B. B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radical Biology and Medicine. 2010;49(11):1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Y., Cui R., Xiao Y., Fang J., Xu Q. Effect of carotene and lycopene on the risk of prostate cancer: a systematic review and Dose-Response meta-analysis of observational studies. PLoS One. 2015;10(9, article e0137427) doi: 10.1371/journal.pone.0137427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Klein E. A., Thompson I. M., Tangen C. M., et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2011;306(14):1549–1556. doi: 10.1001/jama.2011.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gaziano J. M., Glynn R. J., Christen W. G., et al. Vitamins E and C in the prevention of prostate and total cancer in Men. JAMA. 2009;301(1):52–62. doi: 10.1001/jama.2008.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sayyid R. K., Fleshner N. E. Potential role for metformin in urologic oncology. Investigative and Clinical Urology. 2016;57(3):157–164. doi: 10.4111/icu.2016.57.3.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yu H., Yin L., Jiang X., et al. Effect of metformin on cancer risk and treatment outcome of prostate cancer: a meta-analysis of epidemiological observational studies. PLoS One. 2014;9(12, article e116327) doi: 10.1371/journal.pone.0116327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stopsack K. H., Ziehr D. R., Rider J. R., Giovannucci E. L. Metformin and prostate cancer mortality: a meta-analysis. Cancer Causes & Control. 2016;27(1):105–113. doi: 10.1007/s10552-015-0687-0. [DOI] [PubMed] [Google Scholar]
- 62.Raval A. D., Thakker D., Vyas A., Salkini M., Madhavan S., Sambamoorthi U. Impact of metformin on clinical outcomes among men with prostate cancer: a systematic review and meta-analysis. Prostate Cancer and Prostatic Diseases. 2015;18(2):110–121. doi: 10.1038/pcan.2014.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Margel D., Urbach D. R., Lipscombe L. L., et al. Metformin use and All-Cause and prostate Cancer–Specific mortality among men with diabetes. Journal of Clinical Oncology. 2012;31(25):3069–3075. doi: 10.1200/jco.2012.46.7043. [DOI] [PubMed] [Google Scholar]
- 64.Xie Y., Wawng L., Hussain A. Metformin enhances the anti-prostate cancer activity of abiraterone and enzalutamide. 2016.
- 65.Kuo Y. J., Sung F. C., Hsieh P. F., Chang H. P., Wu K. L., Wu H. C. Metformin reduces prostate cancer risk among men with benign prostatic hyperplasia: a nationwide population-based cohort study. Cancer Medicine. 2019;8(5):2514–2523. doi: 10.1002/cam4.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bhalla K., Hwang B. J., Dewi R. E., et al. Metformin prevents liver tumorigenesis by inhibiting pathways driving hepatic lipogenesis. Cancer Prevention Research. 2012;5(4):544–552. doi: 10.1158/1940-6207.CAPR-11-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang Z.-J., Zheng Z. J., Shi R., Su Q., Jiang Q., Kip K. E. Metformin for liver cancer prevention in patients with type 2 diabetes: a systematic review and meta-analysis. The Journal of Clinical Endocrinology & Metabolism. 2012;97(7):2347–2353. doi: 10.1210/jc.2012-1267. [DOI] [PubMed] [Google Scholar]
- 68.Shujuan M., Yixiang Z., Yanni X., Pencheng Z., Hongzhuan T. Meta-analysis of studies using metformin as a reducer for liver cancer risk in diabetic patients. Medicine. 2017;96, article e6888 doi: 10.1097/MD.0000000000006888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Murff H. J., Roumie C. L., Greevy R. A., et al. Metformin use and incidence cancer risk: evidence for a selective protective effect against liver cancer. Cancer Causes & Control. 2018;29(9):823–832. doi: 10.1007/s10552-018-1058-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Millis K. T., Bellows C. F., Hoffman A. E., Kelly T. N., Gagliardi G. Diabetes mellitus and colorectal cancer prognosis: a meta-analysis. Diseases of the Colon and Rectum. 2013;11:1304–1319. doi: 10.1097/DCR.0b013e3182a479f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Meng F., Song L., Wang W. Metformin Improves Overall Survival of Colorectal Cancer Patients with Diabetes: A Meta-Analysis. Journal of Diabetes Research. 2017;2017:8. doi: 10.1155/2017/5063239.5063239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mei Z. B., Zhang Z. J., Liu C. Y., et al. Survival benefits of metformin for colorectal cancer patients with diabetes: a systematic review and Meta-Analysis. PLoS One. 2014;9(3, article e91818) doi: 10.1371/journal.pone.0091818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Higurashi T., Hosono K., Takahashi H., et al. Metformin for chemoprevention of metachronous colorectal adenoma or polyps in post-polypectomy patients without diabetes: a multicentre double-blind, placebo-controlled, randomised phase 3 trial. The Lancet Oncology. 2016;17(4):475–483. doi: 10.1016/S1470-2045(15)00565-3. [DOI] [PubMed] [Google Scholar]
- 74.Park J. W., Lee J. H., Park Y. H., et al. Sex-dependent difference in the effect of metformin on colorectal cancer-specific mortality of diabetic colorectal cancer patients. World Journal of Gastroenterology. 2017;23(28):5196–5205. doi: 10.3748/wjg.v23.i28.5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Press O. A., Zhang W., Gordon M. A., et al. Gender-related survival differences associated with polymorphic variants of estrogen receptor-β (ERβ) in patients with metastatic colon cancer. Pharmacogenomics. 2011;11(5):375–382. doi: 10.1038/tpj.2010.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bradley M. C., Ferrara A., Achacoso N., Ehrlich S. F., Quesenberry C. P., Jr., Habel L. A. A cohort study of metformin and colorectal cancer risk among patients with diabetes mellitus. Cancer Epidemiology Biomarkers & Prevention. 2018;27(5):525–530. doi: 10.1158/1055-9965.epi-17-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Smiechowski B., Azoulay L., Yin H., Pollak M. N., Suissa S. The use of metformin and colorectal cancer incidence in patients with type II diabetes mellitus. Cancer Epidemiology, Biomarkers & Prevention. 2010;22(10):1877–1883. doi: 10.1158/1055-9965.epi-13-0196. [DOI] [PubMed] [Google Scholar]
- 78.Home P. D., Kahn S. E., Jones N. P., et al. Experience of malignancies with oral glucose-lowering drugs in the randomised controlled ADOPT (A Diabetes Outcome Progression Trial) and RECORD (Rosiglitazone Evaluated for Cardiovascular Outcomes and Regulation of Glycaemia in Diabetes) clinical trials. Diabetologia. 2010;53(9):1838–1845. doi: 10.1007/s00125-010-1804-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Siegel R., Naishadham D., Jemal A. Cancer statistics, 2012. Cancer Journal for Clinicians. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 80.Andriulli A., Festa V., Botteri E., et al. Neoadjuvant/preoperative gemcitabine for patients with localized pancreatic cancer. A meta-analysis of prospective studies. Annals of Surgical Oncology. 2012;19(5):1644–1662. doi: 10.1245/s10434-011-2110-8. [DOI] [PubMed] [Google Scholar]
- 81.Xin W., Fang L., Fang Q., Zheng X., Huang P. Effects of metformin on survival outcomes of pancreatic cancer patients with diabetes: a meta-analysis. Molecular and Clinical Oncology. 2018;8(3):483–488. doi: 10.3892/mco.2017.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hwang A. L., Haynes K., Hwang W. T., Yang Y. X. Metformin and survival in pancreatic cancer: a retrospective cohort study. Pancreas. 2013;42(7):1054–1059. doi: 10.1097/MPA.0b013e3182965a3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Reni M., Dugnani E., Cereda S., et al. (Ir)relevance of metformin treatment in patients with metastatic pancreatic cancer: an open-label, randomized phase II trial. Clinical Cancer Research. 2016;22(5):1076–1085. doi: 10.1158/1078-0432.CCR-15-1722. [DOI] [PubMed] [Google Scholar]
- 84.Calip G. S., Yu O., Elmore J. G., Boudreau D. M. Comparative safety of diabetes medications and risk of incident invasive breast cancer: a population-based cohort study. Cancer Causes & Control. 2016;27(5):709–720. doi: 10.1007/s10552-016-0744-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gong Z., Aragaki A. K., Chlebowski R. T., et al. Diabetes, metformin and incidence of and death from invasive cancer in postmenopausal women: results from the women's health initiative. International Journal of Cancer. 2016;138(8):1915–1927. doi: 10.1002/ijc.29944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Porta C., Paglino C., Mosca A. Targeting PI3K/Akt/mTOR signaling in cancer. Frontiers in Oncology. 2014;4:p. 64. doi: 10.3389/fonc.2014.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Roshan M. H. K., Shing Y. K., Pace N. P. Metformin as an adjuvant in breast cancer treatment. Sage open Medicine. 2019;7:p. 205031211986511. doi: 10.1177/2050312119865114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li S.-M. H., Liu S.-T., Chang Y.-L., Ho C.-L., Huang S.-M. Metformin causes cancer cell death through downregulation of p53-dependent differentiated embryo chondrocyte 1. Journal of Biomedical Science. 2018;25(1):p. 81. doi: 10.1186/s12929-018-0478-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Murphy M. P. How mitochondria produce reactive oxygen species. The Biochemical Journal. 2009;417(1):1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tseng C. H. Metformin may reduce breast cancer risk in Taiwanese women with type 2 diabetes. Breast Cancer Research and Treatment. 2014;145(3):785–790. doi: 10.1007/s10549-014-2985-8. [DOI] [PubMed] [Google Scholar]
- 91.Aksoy S., Sendur M. A. N., Altundag K. Demographic and clinico-pathological characteristics in patients with invasive breast cancer receiving metformin. Medical Oncology. 2013;30(2):p. 590. doi: 10.1007/s12032-013-0590-z. [DOI] [PubMed] [Google Scholar]
- 92.Deng X.-S., Wang S., Deng A., et al. Metformin targets STAT3 to inhibit cell growth and induce apoptosis in triple negative breast cancers. Cell Cycle. 2012;11(2):367–376. doi: 10.4161/cc.11.2.18813. [DOI] [PubMed] [Google Scholar]
- 93.Zhu P., Davis M., Blackwelder A. J., et al. Metformin selectively targets tumor-initiating cells in ErbB2-overexpressing breast cancer models. Cancer Prevention Research. 2014;7(2):199–210. doi: 10.1158/1940-6207.CAPR-13-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu X.-X., Li X.-J., Zhang B., et al. MicroRNA-26b is underexpressed in human breast cancer and induces cell apoptosis by targeting SLC7A11. FEBS Letters. 2011;585(9):1363–1367. doi: 10.1016/j.febslet.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 95.Sheikpou R. Visfatin and its role in breast cancer. Middle East Journal of Cancer. 2017;8:171–177. [Google Scholar]
- 96.Liu B., Fan Z., Edgerton S. M., Yang X., Lind S. E., Thor A. D. Potent anti-proliferative effects of metformin on trastuzumab-resistant breast cancer cells via inhibition of erbB2/IGF-1 receptor interactions. Cell Cycle. 2011;10(17):2959–2966. doi: 10.4161/cc.10.17.16359. [DOI] [PubMed] [Google Scholar]
- 97.Qu C., Zhang W., Zheng G., Zhang Z., Yin J., He Z. Metformin reverses multidrug resistance and epithelial–mesenchymal transition (EMT) via activating AMP-activated protein kinase (AMPK) in human breast cancer cells. Molecular and Cellular Biochemistry. 2014;386(1-2):63–71. doi: 10.1007/s11010-013-1845-x. [DOI] [PubMed] [Google Scholar]
- 98.Rao M., Gao C., Guo M., Law B. Y. K., Xu Y. Effects of metformin treatment on radiotherapy efficacy in patients with cancer and diabetes: a systematic review and meta-analysis. Cancer Management and Research. 2018;Volume 10:4881–4890. doi: 10.2147/CMAR.S174535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Samsuri N. A., Leech M., Marignol L. Metformin and improved treatment outcomes in radiation therapy – A review. Cancer Treatment Reviews. 2017;55:150–162. doi: 10.1016/j.ctrv.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 100.Sandulache V. Metformin hydrochloride, cisplatin and external beam radiation therapy in treating participants with stage III-IV Head and Neck squamous cell cancer.
- 101.Aldossary S. A. Review on pharmacology of cisplatin: clinical use, toxicity and mechanism of resistance of cisplatin. Biomedical and Pharmacology Journal. 2019;12(1):07–15. doi: 10.13005/bpj/1608. [DOI] [Google Scholar]
- 102.Stambolic V., Woodget J. R., Fanyus I. G., Pritchard K. J., Goodwin P. J. Utility of metformin in breast cancer treatment, is neoangiogenesis a risk factor? Breast Cancer Research and Treatment. 2009;114(2, article 15):387–389. doi: 10.1007/s10549-008-0015-4. [DOI] [PubMed] [Google Scholar]
- 103.Sena P., Mancini S., Benincasa M., Mariani F., Palumbo C., Roncucci L. Metformin induces apoptosis and alters cellular responses to oxidative stress in Ht29 colon cancer cells: preliminary findings. International Journal of Molecular Sciences. 2018;19(5):p. 1478. doi: 10.3390/ijms19051478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dowling R. J. O., Niraula S., Stambolic V., Goodwin P. J. Metformin in cancer: translational challenges. Journal of Molecular Endocrinology. 2012;48(3):R31–R43. doi: 10.1530/JME-12-0007. [DOI] [PubMed] [Google Scholar]
- 105.Koenig D. FDA Investigating Metformin for Possible Carcinogen- Medscape. 2019.
- 106.Preidt R. 2019. FDA testing levels of carcinogen in diabetes drug, metformin. https://www.usnews.com. [Google Scholar]


