Abstract
At present, most of the drugs have little effect on the pathological process of rheumatoid arthritis (RA). Analgesia is an important measure in the treatment of RA and is also one of the criteria to determine the therapeutic effects of the disease. Some studies have found that crocin, a kind of Chinese medicine, can effectively alleviate pain sensitization in pain model rats, but the mechanism is not clear. Emerging evidence indicates that crocin may inhibit the metastasis of lung and liver cancer cells from the breast by inhibiting Wnt/β-catenin and the Wnt signaling pathway is closely related to RA. Wnt5a belongs to the Wnt protein family and was previously thought to be involved only in nonclassical Wnt signaling pathways. Recent studies have shown that Wnt5a has both stimulatory and inhibitory effects on the classical Wnt signaling pathway, and so, Wnt5a has attracted increasing attention. This study demonstrated that crocin significantly increased the mechanical thresholds of adjuvant-induced arthritis (AIA) rats, suggesting that crocin can alleviate neuropathic pain. Crocin significantly decreased the levels of pain-related factors and glial activation. Foxy5, activator of Wnt5a, inhibited the above effects of crocin in AIA rats. In addition, intrathecal injection of a Wnt5a inhibitor significantly decreased hyperalgesia in AIA rats. This research shows that crocin may alleviate neuropathic pain in AIA rats by inhibiting the expression of pain-related molecules through the Wnt5a/β-catenin pathway, elucidating the mechanism by which crocin relieves neuropathic pain and provides a new way of thinking for the treatment of AIA pain.
1. Introduction
Rheumatoid arthritis (RA) is characterized by joint pain and deformity caused by chronic degenerative changes in articular cartilage, which seriously affect the quality of life. At present, most drugs for rheumatoid arthritis have little effect on the pathological process of RA. Analgesia is an important measure in the treatment of RA and is also one of the criteria to determine the therapeutic effects of the disease [1, 2].
The Wnt signaling pathway is a complex and ancient signal transduction system within and between cells. It is closely related to many important physiological functions, such as rheumatoid arthritis, stress, injury, repair, immunity, remodeling, and metabolism [3].
Although the Wnt signaling pathway has only recently been studied in RA, it can act on the pathological process of RA in various ways and play an important role in the occurrence and development of RA. The Wnt/β-catenin signaling pathway is a classical Wnt signaling pathway, and it is also the most detailed and important Wnt signaling pathway currently studied. The Wnt/β-catenin pathway can affect the process and prognosis of RA by affecting the proliferation of fibroblast-like synoviocytes and regulating the secretion of inflammatory factors and bone metabolism/bone destruction [4–7].
Wnt proteins can activate classical (β-catenin dependent) or nonclassical (β-catenin independent) Wnt signaling pathways, both of which play important roles in physiological and pathological processes. Wnt5a belongs to the Wnt protein family and was previously thought to be involved only in nonclassical Wnt signaling pathways. Recent studies have shown that Wnt5a has both stimulatory and inhibitory effects on the classical Wnt signaling pathway, and so, Wnt5a has attracted increasing attention [8–10].
During the development of the nervous system, the Wnt protein family plays an important role in regulating axon regeneration and in the pathogenesis of neuropathic pain. Wnt5a plays an important role in central sensitization and neuronal plasticity in acute and chronic pain [11–13].
The expression of Wnt5a and its receptor in the spinal cord is upregulated in spinal nerve ligation (SNL) mice [14], HIV-gp120 [15, 16] or capsaicin-induced pain models [17], and experimental autoimmune encephalomyelitis (EAE) mice. The specific Wnt5a inhibitor Box5 can attenuate the mechanical hyperalgesia induced by CCI [18, 19]; conversely, the Wnt5a activator Foxy5 can promote mechanical hyperalgesia [20].
Activation of the Wnt signaling pathway can stimulate production of the proinflammatory factors TNF-α and IL-1β and exacerbate the progress of neuropathic pain [21–23]. In addition, COX-2, a downstream target molecule of the Wnt5a/β-catenin pathway [24, 25], can affect cell function by regulating the expression of iNOS.
It is known that neuropathic pain and RA pain have similar molecular mechanisms. Emerging evidence suggests that Wnt5a in the spinal cord may play an important role in the regulation of chronic pain and RA. However, it is unclear whether the Wnt5a pathway in the spinal cord plays a role in rheumatoid arthritis pain.
In recent years, Chinese medicine has made some progress in anti-AIA pain and has become one of the hotspots of this research. Crocin is a chemical constituent extracted from saffron (a traditional Chinese medicine). Crocus sativus not only lowers blood pressure, regulates lipids and immunity, and protects the liver and kidney but also has analgesic effects. Its main analgesic component is crocin [26–29].
Crocin is a kind of polyhydroxy flavonoid with anti-inflammatory, antioxidant, and antidepressant effects [26]. In recent years, some studies have found that crocin can effectively alleviate pain sensitization in CCI and STZ model rats [30–34], but the mechanism is not yet clear.
In previous studies of triple-negative breast cancer (TNBC), crocin inhibited the metastasis of lung and liver cancer cells from the breast by inhibiting Wnt/β-catenin and increased the weight, survival rate, and volume of tumors in mice. Crocin reduced metastasis, invasion, and extracellular adhesion of TNBC cells in a dose-dependent manner, and its mechanism was related to the inhibition of the downstream factors FZD7, NEDD9, VIM, and VEGF-a [35, 36].
Combined with the above studies, we hypothesize that crocin plays role against rheumatoid pain by regulating the Wnt5a/β-catenin pathway. In this study, we established an AIA rat model to investigate the effect of crocin on AIA pain and its relationship with Wnt5a/β-catenin and its downstream factors.
2. Materials and Methods
2.1. Materials
SPF male SD rats (8-10 weeks old, weighing 180-220 g) were provided by the Laboratory Animal Center of Xuzhou Medical University. All animal experiments were approved by the Animal Ethics Committee of Xuzhou Medical University. Crocin (Sigma); Von Frey (Institute of Bioengineering, Chinese Academy of Medical Sciences); mouse anti-Wnt5a, β-catenin/COX-2 and iNOS polyclonal antibodies, and sheep anti-mouse antibody (HRP) were obtained from Abcam (UK); ELISA kits (TNF-α, IL-1β (eBioscience, Vienna, Austria)) were used.
2.2. Experimental Method
2.2.1. Animal Model and Intrathecal Catheter [37]
After anesthesia, the interval between the lumbar 4 and lumbar 3 spinous processes was exposed. Two centimeters of a clear cerebral PE-10 catheter (15 cm in length, 10 μl in volume) was inserted through the rupture. An 18G epidural puncture needle was passed through a subcutaneous tunnel in the rats. The other end of the catheter was sent to the back of the neck. Two centimeters of the catheter was exposed and fixed. The external opening was sealed with a lighter to prevent cerebrospinal fluid spillover. Penicillin was injected intramuscularly 3 days after the operation to prevent infection. The rats with spinal cord injury were not included in the experiment. On the second day after the operation, 2% lidocaine hydrochloride (20 μl) was injected through the PE-10 catheter. Paralysis of both hind feet of the rats was soft and recovered in approximately 30 minutes; thus, the intrathecal catheterization was successful.
2.3. AIA Model Establishment [38]
After 7 days of intrathecal catheterization, the rats were anesthetized and intradermally injected with 0.1 ml Freund's complete adjuvant (CFA) (1 mg/ml of heat-inactivated Mycobacterium tuberculosis dissolved in 85% paraffin oil and 15% mannide monooleate; Sigma) to establish the adjuvant arthritis animal. The control group was injected with 0.1 ml saline.
The mechanical withdrawal threshold (MWT) was measured 1 day before and after the operation. The AIA model was considered successful when MWT decreased by more than 40% on the 8th day after the operation compared with the baseline pain threshold.
2.4. Drug Treatment
According to the preexperiment and previous study [33], intraperitoneal injections of crocin at doses of 50 and 100 mg/kg were selected in our study, while the other medicines were intrathecally injected into the different groups. The rats in each group were decapitated and killed after the tests. The ip injections of crocin were performed 30 min before injections of other drugs. The spinal cord tissues of L4-L6 were immediately acquired for subsequent experiments.
2.5. The Degree of Toe Swelling
One day before CFA injection and at different time points after injection, the toe swelling of the rat was detected with a toe volume measuring instrument. The difference between the volume after inflammation at a certain time and the volume before CFA injection was the swelling of the foot at that time degree.
2.6. Von Frey Test
The rats were placed in a transparent box with wire mesh at the bottom. They were allowed to acclimate for 30 minutes before the experiment. Von Frey filaments (0.6, 1, 2, 4, 6, 8, 10, and 15 g) were used to test the pain threshold by the up-down method. The intensity increased slowly and uniformly. If the rats had contractions or foot licking, a positive reaction was observed. At this time, the MWTs were recorded.
2.7. ELISA
The spinal cord tissue of each group was homogenized with a microgrinder. The homogenate was diluted 30 times, and the levels of TNF-α and IL-1β were detected according to ELISA kit instructions.
2.8. Western Blotting to Detect Protein Expression
After boiling for 5 minutes, 80 μg of protein was separated by SDS-PAGE. Then, the proteins were transferred to a PVDF membrane at 150 mA for 3 hours. The membrane was blocked in 3% skimmed milk powder for 1 hour, incubated with primary antibody (all 1 : 1 000) overnight at 4°C, and washed with TBST (5 min × 3 times). After incubation with the secondary antibody at room temperature for 1 hour, TBST was used to wash the membrane (5 min × 3 times), and ECL was added for exposure and development.
2.9. Immunofluorescence Staining
After anesthesia, the rat hearts were exposed rapidly. After ascending aorta intubation, the blood was washed with 200 ml normal saline and then perfused with 4% polyformaldehyde phosphate buffer (0.1 MB, pH 7.4) (200 ml). The L4-5 segments were fixed in 4% paraformaldehyde for 4-6 hours (4°C) and then immersed in 30% sucrose solution at 4°C overnight until they sank. Frozen coronal serial sections were collected in 0.01 PBS. The sections were washed at room temperature 3 times for 5 min, incubated at room temperature with fluorescent sealing fluid for 2 hours, dried, and incubated overnight with polyclonal goat anti-rat primary antibody (Abcam, USA) at 4°C. The sections were washed with PBS 3 times for 5 min, and fluorescent-labeled donkey anti-goat FITC (1 : 100, Biyun Tian) secondary antibody was added in the darkroom and incubated at room temperature in the dark. After 2 hours, the sections were washed with PBS in the darkroom 3 times for 5 min, covered, and fluorescently sealed, and confocal microscopy (TCS-SP2, Leica, Germany) was used to observe and take pictures.
2.10. Statistical Analysis
Data are expressed as the mean ± SEM. SPSS 16.0 statistical software was used to test the difference between groups. ANOVA (Tukey, SNK), two-way ANOVA, and repeated measures ANOVA were employed to test the data, and P < 0.05 indicated that the difference was significant.
3. Results
3.1. The Establishment of AIA Model
There was no significant difference in MWTs between the normal and sham groups (P > 0.05). The MWTs were significantly decreased in AIA rats (P < 0.05, Figure 1(a)). The results showed that the operation induced mechanical hyperalgesia in rats. Along with the course extension, paw swelling of AA rats increased gradually. Paw swelling is still obvious on day 24 (P < 0.05, Figure 1(b)).
Figure 1.
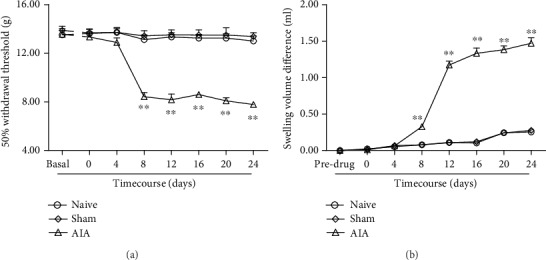
(a) After model establishment, mechanical allodynia was observed in the AIA model. Sham rats did not show a decrease in MWTs. ∗∗P < 0.01vs. day 0 (mean ± SEM, n = 8). (b) The paw swelling of AA rats. ∗∗P < 0.01vs. predrug value in AA rats (mean ± SEM, n = 8). Swelling volume difference = Volume (pre‐CFA injection) − Volume (post‐CFA injection) (mean ± SEM, n = 8).
3.2. Intraperitoneal Injection of Crocin Can Significantly Alleviate AIA-Induced Mechanical Pain
Crocin had no significant effects in the sham group (P > 0.05). Crocin significantly increased the MWTs in AIA rats (P < 0.05, Figure 2). The results showed that crocin may induce analgesic effects in AIA rats.
Figure 2.
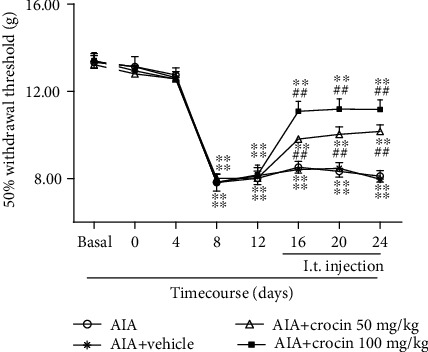
Changes in MWTs in AIA rats after injection of crocin (mean ± SEM, n = 8). ∗∗P < 0.01vs. basal; ##P < 0.01 (AIA+crocin groups vs. AIA+vehicle).
3.3. Crocin Reduces the Expression of Inflammatory Factors and Pain-Related Molecules in the Spinal Dorsal Horn of AIA Rats
ELISA results showed that crocin significantly decreased the levels of TNF-α (P < 0.05, Figure 3(a)) and IL-1β (P < 0.05, Figure 3(b)) in the spinal cords of AIA rats.
Figure 3.
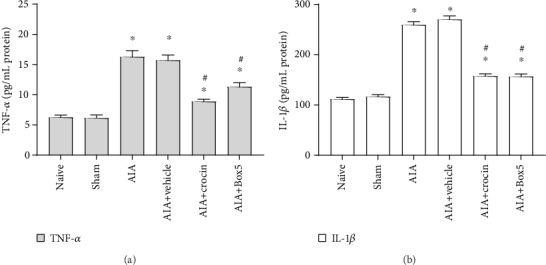
Changes of spinal TNF-α and IL-1β after injection of crocin in AIA rats (mean ± SEM, n = 8). ∗P < 0.05, compared with the sham group; #P < 0.05, compared with the AIA+vehicle group (vs. AIA+crocin group or AIA+Box5 group).
3.4. Crocin Inhibits the Wnt5a/β-Catenin Pathway and Glial Activation
Western blot results showed that upregulation of the Wnt5a/β-catenin pathway and glial cell marker proteins was observed in the spinal cords of AIA rats (Figures 4(a)–4(d)). The use of crocin may decrease expression of the Wnt5a/β-catenin pathway and glial activation in the spinal cords of AIA rats.
Figure 4.
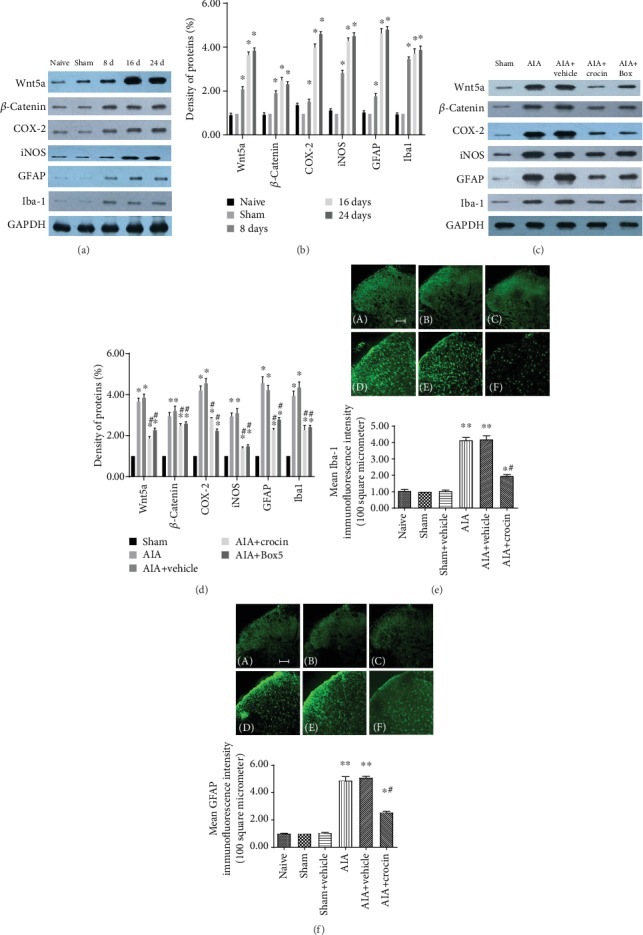
The effect of crocin on expression of the Wnt5a/β-catenin pathway and glial cell activation in AIA rats. (a–d) Western blot results (mean ± SEM, n = 3); ∗P < 0.05, compared with the sham group; #P < 0.05, compared with the AIA+vehicle group (vs. AIA+crocin group). (e, f) AIA model and crocin effects on glial cell activation (mean ± SEM, n = 6); ∗P < 0.05, compared with the sham+vehicle group; #P < 0.05, compared with the AIA+vehicle group (vs. AIA+crocin group).
The levels of Iba-1 and GFAP in the dorsal horn of the spinal cord were significantly higher than those in the control group (Figures 4(e) and 4(f)). Compared with levels in the vehicle group, the increase in GFAP and Iba-1 was abrogated in the crocin group.
3.5. Intrathecal Injection of the Wnt5a Activator (Foxy5) on the Pain Thresholds of Rats
The above data proved that crocin attenuated pain facilitation and the Wnt5a pathway in the spinal dorsal horn of AIA rats. We further observed the intrathecal injection of the Wnt5a activator (Foxy5) on crocin-induced analgesia in AIA rats.
Vehicle and Foxy5 (10 μg) did not affect the basal MWTs in rats, while Foxy5 (15, 20 μg) may lead to pain facilitation in normal rats. Compared with the effect in the AIA+crocin group, the analgesic effect of crocin (100 mg/kg) was attenuated in the Foxy5 (10 μg)+AIA+crocin group (Figure 5).
Figure 5.
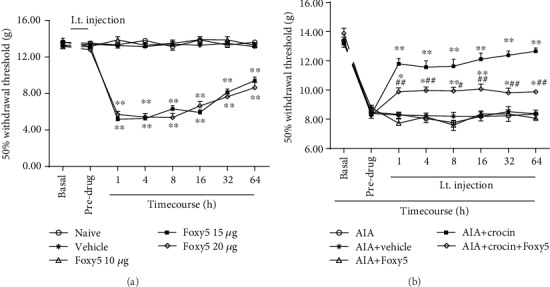
Intrathecal injection of the Wnt5a activator (Foxy5) on the MWTs in rats (mean ± SEM, n = 8). ∗P < 0.05, ∗∗P < 0.01, vs. predrug; #P < 0.05, ##P < 0.01 (AIA+crocin groups vs. AIA+Foxy5).
3.6. Intrathecal Injection of the Wnt5a Inhibitor Can Alleviate Mechanical Hyperalgesia
The above data proved that the Wnt5a activator alleviated crocin-induced analgesia in AIA rats. Then, we observed the effects of the Wnt5a inhibitor (Box5) on the pain thresholds of AIA rats.
Intrathecal injection of Box5 blocked pain hypersensitivity in AIA rats compared with the vehicle group (Figure 6).
Figure 6.
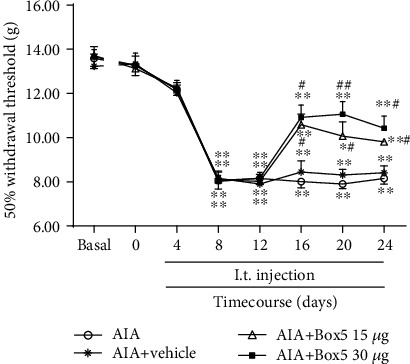
Effects of the Wnt5a inhibitor (Box5) on the MWTs in AIA rats (mean ± SEM, n = 8). ∗P < 0.05, ∗∗P < 0.01vs. basal value; #P < 0.05, ##P < 0.01 (AIA+Box5 groups vs. AIA+vehicle).
3.7. Distribution of Wnt5a/β-Catenin in the Spinal Dorsal Horn of AIA Rats
Neurons, astrocytes, microglial markers (NeuN, GFAP, and Iba-1), and Wnt5a/β-catenin pathway proteins were used for fluorescence double labeling. The expression of Wnt5a/β-catenin was not detected in nearly any of the GFAP and Iba-1 IR cells. However, positive expression of Wnt5a/β-catenin was detected in NeuN IR cells. This suggests that Wnt5a/β-catenin is mainly distributed in the dorsal horn of AIA rats (Figures 7(a) and 7(b)).
Figure 7.
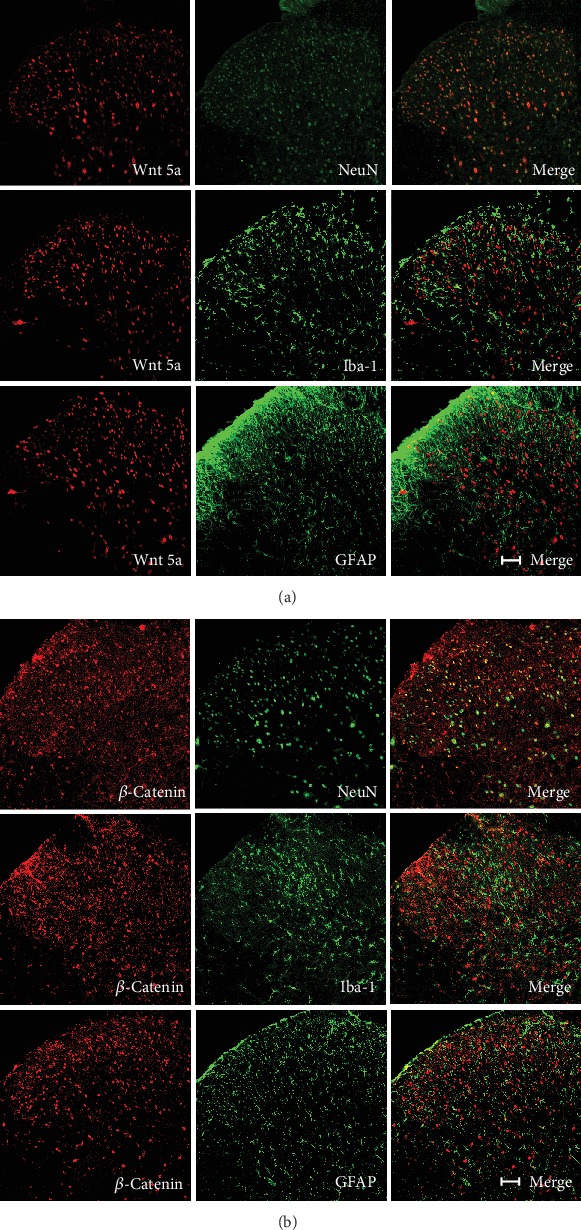
Distribution of the Wnt5a/β-catenin pathway in the spinal dorsal horn of AIA rats (scale bar = 100 μm).
4. Discussion
RA is a chronic, systemic autoimmune disease characterized by symmetry and multijoint inflammation that seriously affects the quality of life [38]. Arthritis-related joint pain leads to limited movement, weakness, severe pain, and even serious impacts on daily life. The application of oral analgesics is limited by inadequate pain relief, severe adverse reactions, and other drug interactions; intra-articular injection of corticosteroids and viscoelastics can relieve pain, but the effects and duration are unstable; the prognosis of arthroscopic debridement is not better than that of placebo or chemical therapy; and joint replacement has a high surgical risk. Therefore, there is an urgent need for a safe and effective method for the treatment of chronic arthritis in AIA. In this study, we found that the mechanical pain threshold of AIA model rats decreased significantly after injection of CFA, which means that the modeling was successful.
Crocin, as a typical flavonoid, has antiviral, hypoglycemic, hypotensive, immunomodulatory, and cardiovascular protective activities [39–41]. In recent years, studies have shown that crocin has protective effects against alcohol-induced neurological damage in mice. Crocin has been found to increase the pain threshold in models [30–34], but its mechanism is not well studied.
This study demonstrated that crocin significantly increased the mechanical thresholds of AIA rats, suggesting that crocin can alleviate neuropathic pain in AIA rats. Crocin significantly decreased the levels of pain-related factors (Wnt5a and β-catenin, TNF-α and IL-1β) and glial activation. Foxy5 (an activator of Wnt5a) inhibited the above effects of crocin in AIA rats. In addition, intrathecal injection of a Wnt5a inhibitor significantly decreased hyperalgesia in AIA rats.
It is known that expression levels of the inflammatory factors TNF-α and IL-1β are significantly increased in the chronic sciatic nerve constriction injury model [42, 43], which is consistent with the present results. A high concentration of TNF-α in the central nervous system can be regarded as neurotoxic and can induce the production of oxygen free radicals in the central nervous system. Previous studies also proved that inhibition of inflammatory signaling pathways can effectively alleviate neuropathic pain [44–49].
Wnt5a has been reported to play an important role in the inflammatory response. It can upregulate the expression of many important proinflammatory factors and inflammatory mediators, including interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor alpha (TNF-α) [44]. Zhang et al. proved that the expression of inflammatory cytokines in the spinal cord increased after CCI surgery, and activation of the Wnt/β-catenin pathway induced by Wnt agonists could also increase the expression of inflammatory cytokines in the spinal cord [42]. Li et al. observed that Wnt5a played an important role in the regulation of TNF-α and IL-1β expression levels by culturing mixed neurons [43]. The previously mentioned studies were consistent with the present results. Therefore, we proved for the first time that crocin alleviates AIA pain in rats by inhibiting Wnt5a/β-catenin and the downstream inflammatory pathway, but the specific mechanism requires further experimental study.
The Wnt signaling pathway is involved in the regulation of chronic pain, which may be related to spinal dorsal horn neuroinflammation. Marchetti and Pluchino showed that the Wnt signaling pathway participates in the production of the inflammatory response in the central nervous system mediated by glial cell modification [50]. Halleskog et al. showed that many Wnt receptor proteins are expressed in microglia (such as N13 cells and primary mouse microglia). Another experiment showed that exogenous Wnt proteins activate classical or nonclassical Wnt signaling pathways in microglia. Inhibitors of the Wnt/β-catenin signaling pathway administered by intrathecal injection significantly reduced PSL-induced abnormal pain and abnormal activation of glial cells in the spinal dorsal horn [51], suggesting that the Wnt signaling pathway is closely related to abnormal activation of glial cells in neuropathic pain. Activation of microglia induced by neuroinflammation and subsequent release of a series of proinflammatory cytokines plays an important role in inducing hyperalgesia through central sensitization [52–54].
Moreover, COX-2, a target molecule downstream of the Wnt/β-catenin pathway, can affect cell function by regulating the expression of iNOS. NO is a new neurotransmitter and inflammatory mediator that is catalyzed by nitric oxide synthase. It can be categorized as endothelial nitric oxide synthase (eNOS/NOS3), neuronal NOS (nNOS/NOS1), and inducible NOS (iNOS/NOS2). NO can also enhance the sensitivity of pain perception and transmission. Excessive concentrations of NO can lead to increased pain and inflammatory responses in the spinal cord center [55–57]. Animal experiments showed that COX-2, as a pain-related gene, was overexpressed in a neuropathic pain model. Therefore, selective COX-2 inhibitors can effectively inhibit the development of neuropathic pain [58–62].
Our results showed that crocin significantly decreased the expression levels of iNOS and COX-2 in the spinal cords of AIA rats. These results suggest that crocin may reduce the expression of the inflammatory factors and pain-related molecules iNOS and COX-2 in the spinal cord of AIA rats by inhibiting the Wnt/β-catenin pathway.
In conclusion, the results showed that crocin may alleviate neuropathic pain in AIA rats by inhibiting the expression of pain-related molecules through the Wnt5a/β-catenin pathway. This study elucidates the mechanism by which crocin alleviates neuropathic pain and provides a new way of thinking for the treatment of AIA pain.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31100801 and 81200858).
Contributor Information
Qin Yin, Email: yq3204@sina.com.
Wei Cheng, Email: cwmztt@163.com.
Data Availability
The data used to support the findings of the present research are available from the corresponding authors once requested.
Disclosure
Jin-Feng Wang and Hai-Jun Xu claim the equal status of the first author.
Conflicts of Interest
The authors have no conflict of interests to declare.
References
- 1.Dreher M., Kosz M., Schwarting A. Physical activity, exercise and nutrition in rheumatism: adjuvant treatment options for inflammatory-rheumatic diseases. Der Orthopäde. 2019;48(11):917–926. doi: 10.1007/s00132-019-03808-4. [DOI] [PubMed] [Google Scholar]
- 2.Zhou S., Zou H., Chen G., Huang G. Synthesis and biological activities of chemical drugs for the treatment of rheumatoid arthritis. Topics in Current Chemistry. 2019;377(5):p. 28. doi: 10.1007/s41061-019-0252-5. [DOI] [PubMed] [Google Scholar]
- 3.Brunt L., Scholpp S. The function of endocytosis in Wnt signaling. Cellular and Molecular Life Sciences. 2018;75(5):785–795. doi: 10.1007/s00018-017-2654-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Sousa L. M., Dos Santos Alves J. M., da Silva Martins C., Pereira K. M. A., Goes P., Gondim D. V. Immunoexpression of canonical Wnt and NF-κB signaling pathways in the temporomandibular joint of arthritic rats. Inflammation Research. 2019;68(10):889–900. doi: 10.1007/s00011-019-01274-4. [DOI] [PubMed] [Google Scholar]
- 5.Li G. Q., Fang Y. X., Liu Y., et al. MALAT1-driven inhibition of Wnt signal impedes proliferation and inflammation in fibroblast-like synoviocytes through CTNNB1 promoter methylation in rheumatoid arthritis. Human Gene Therapy. 2019;30(8):1008–1022. doi: 10.1089/hum.2018.212. [DOI] [PubMed] [Google Scholar]
- 6.Yu M., Guo Y., Zhang P., et al. Increased circulating Wnt5a protein in patients with rheumatoid arthritis-associated interstitial pneumonia (RA-ILD) Immunobiology. 2019;224(4):551–559. doi: 10.1016/j.imbio.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 7.Liang J. J., Li H. R., Chen Y., et al. Diallyl Trisulfide can induce fibroblast-like synovial apoptosis and has a therapeutic effect on collagen-induced arthritis in mice via blocking NF-κB and Wnt pathways. International Immunopharmacology. 2019;71:132–138. doi: 10.1016/j.intimp.2019.03.024. [DOI] [PubMed] [Google Scholar]
- 8.Pashirzad M., Shafiee M., Rahmani F., et al. Role of Wnt5a in the pathogenesis of inflammatory diseases. Journal of Cellular Physiology. 2017;232(7):1611–1616. doi: 10.1002/jcp.25687. [DOI] [PubMed] [Google Scholar]
- 9.Zeng R., Huang J., Zhong M. Z., et al. Multiple roles of WNT5A in breast cancer. Medical Science Monitor. 2016;22:5058–5067. doi: 10.12659/msm.902022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asem M., Buechler S., Wates R., Miller D., Stack M. Wnt5a signaling in cancer. Cancers. 2016;8(9):p. 79. doi: 10.3390/cancers8090079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rong Y., Liu W., Zhou Z., et al. Harpagide inhibits neuronal apoptosis and promotes axonal regeneration after spinal cord injury in rats by activating the Wnt/β-catenin signaling pathway. Brain Research Bulletin. 2019;148:91–99. doi: 10.1016/j.brainresbull.2019.03.014. [DOI] [PubMed] [Google Scholar]
- 12.Li X., Fan C., Xiao Z., et al. A collagen microchannel scaffold carrying paclitaxel-liposomes induces neuronal differentiation of neural stem cells through Wnt/β-catenin signaling for spinal cord injury repair. Biomaterials. 2018;183:114–127. doi: 10.1016/j.biomaterials.2018.08.037. [DOI] [PubMed] [Google Scholar]
- 13.Strand N. S., Hoi K. K., Phan T. M. T., Ray C. A., Berndt J. D., Moon R. T. Wnt/β-catenin signaling promotes regeneration after adult zebrafish spinal cord injury. Biochemical and Biophysical Research Communications. 2016;477(4):952–956. doi: 10.1016/j.bbrc.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 14.Tang J., Ji Q., Jin L., Tian M., Zhang L. D., Liu X. Y. Secreted frizzled-related protein 1 regulates the progression of neuropathic pain in mice following spinal nerve ligation. Journal of Cellular Physiology. 2018;233(8):5815–5822. doi: 10.1002/jcp.26358. [DOI] [PubMed] [Google Scholar]
- 15.Yuan S. B., Ji G., Li B., Andersson T., Neugebauer V., Tang S. J. A Wnt5a signaling pathway in the pathogenesis of HIV-1 gp120-induced pain. Pain. 2015;156(7):1311–1319. doi: 10.1097/j.pain.0000000000000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanda H., Kanao M., Liu S., et al. HSV vector-mediated GAD67 suppresses neuropathic pain induced by perineural HIV gp120 in rats through inhibition of ROS and Wnt5a. Gene Therapy. 2016;23(4):340–348. doi: 10.1038/gt.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi Y., Yuan S., Li B., et al. Regulation of Wnt signaling by nociceptive input in animal models. Molecular Pain. 2012;8:1744–8069. doi: 10.1186/1744-8069-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuan S., Shi Y., Tang S. J. Wnt signaling in the pathogenesis of multiple sclerosis-associated chronic pain. Journal of Neuroimmune Pharmacology. 2012;7(4):904–913. doi: 10.1007/s11481-012-9370-3. [DOI] [PubMed] [Google Scholar]
- 19.Wang J., Zhang S., Li L., Zhang L. Involvement of Wnt5a within the cerebrospinal fluid-contacting nucleus in nerve injury-induced neuropathic pain. The International Journal of Neuroscience. 2015;125(2):147–153. doi: 10.3109/00207454.2014.915399. [DOI] [PubMed] [Google Scholar]
- 20.Zhu A., Shen L., Xu L., Chen W., Huang Y. Wnt5a mediates chronic post-thoracotomy pain by regulating non-canonical pathways, nerve regeneration, and inflammation in rats. Cellular Signalling. 2018;44:51–61. doi: 10.1016/j.cellsig.2018.01.017. [DOI] [PubMed] [Google Scholar]
- 21.Yuan S., Shi Y., Guo K., Tang S. J. Nucleoside reverse transcriptase inhibitors (NRTIs) induce pathological pain through Wnt5a-mediated neuroinflammation in aging mice. Journal of Neuroimmune Pharmacology. 2018;13(2):230–236. doi: 10.1007/s11481-018-9777-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu T., Zhang J., Geng M., Tang S. J., Zhang W., Shu J. Nucleoside reverse transcriptase inhibitors (NRTIs) induce proinflammatory cytokines in the CNS via Wnt5a signaling. Scientific Reports. 2017;7(1):p. 4117. doi: 10.1038/s41598-017-03446-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi S., Man Z., Li W., Sun S., Zhang W. Silencing of Wnt5a prevents interleukin-1β-induced collagen type II degradation in rat chondrocytes. Experimental and Therapeutic Medicine. 2016;12(5):3161–3166. doi: 10.3892/etm.2016.3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang L., Chu Y., Wang Y., et al. siRNA-mediated silencing of Wnt5a regulates inflammatory responses in atherosclerosis through the MAPK/NF-κB pathways. International Journal of Molecular Medicine. 2014;34(4):1147–1152. doi: 10.3892/ijmm.2014.1860. [DOI] [PubMed] [Google Scholar]
- 25.Briolay A., Lencel P., Bessueille L., Caverzasio J., Buchet R., Magne D. Autocrine stimulation of osteoblast activity by Wnt5a in response to TNF-α in human mesenchymal stem cells. Biochemical and Biophysical Research Communications. 2013;430(3):1072–1077. doi: 10.1016/j.bbrc.2012.12.036. [DOI] [PubMed] [Google Scholar]
- 26.Korani S., Korani M., Sathyapalan T., Sahebkar A. Therapeutic effects of Crocin in autoimmune diseases: a review. BioFactors. 2019;45(6):835–843. doi: 10.1002/biof.1557. [DOI] [PubMed] [Google Scholar]
- 27.Li L., Zhang H., Jin S., Liu C. Effects of crocin on inflammatory activities in human fibroblast-like synoviocytes and collagen-induced arthritis in mice. Immunologic Research. 2018;66(3):406–413. doi: 10.1007/s12026-018-8999-2. [DOI] [PubMed] [Google Scholar]
- 28.Li X., Jiang C., Zhu W. Crocin reduces the inflammation response in rheumatoid arthritis. Bioscience, Biotechnology, and Biochemistry. 2017;81(5):891–898. doi: 10.1080/09168451.2016.1263145. [DOI] [PubMed] [Google Scholar]
- 29.Liu W., Sun Y., Cheng Z., Guo Y., Liu P., Wen Y. Crocin exerts anti-inflammatory and anti-arthritic effects on type II collagen-induced arthritis in rats. Pharmaceutical Biology. 2018;56(1):209–216. doi: 10.1080/13880209.2018.1448874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Safakhah H. A., Taghavi T., Rashidy-Pour A., et al. Effects of saffron (Crocus sativus L.) stigma extract and its active constituent crocin on neuropathic pain responses in a rat model of chronic constriction injury. Iranian Journal of Pharmaceutical Research. 2016;15(1):253–261. [PMC free article] [PubMed] [Google Scholar]
- 31.Farshid A. A., Tamaddonfard E. Histopathological and behavioral evaluations of the effects of crocin, safranal and insulin on diabetic peripheral neuropathy in rats. Avicenna Journal of Phytomedicine. 2015;5(5):469–478. [PMC free article] [PubMed] [Google Scholar]
- 32.Hosseinzadeh H., Modaghegh M. H., Saffari Z. Crocus sativus L. (saffron) extract and its active constituents (crocin and safranal) on ischemia-reperfusion in rat skeletal muscle. Evidence-based Complementary and Alternative Medicine. 2009;6(3):350. doi: 10.1093/ecam/nem125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tamaddonfard E., Farshid A. A., Eghdami K., Samadi F., Erfanparast A. Comparison of the effects of crocin, safranal and diclofenac on local inflammation and inflammatory pain responses induced by carrageenan in rats. Pharmacological Reports. 2013;65(5):1272–1280. doi: 10.1016/s1734-1140(13)71485-3. [DOI] [PubMed] [Google Scholar]
- 34.Lei M., Guo C., Hua L., et al. Crocin attenuates joint pain and muscle dysfunction in osteoarthritis rat. Inflammation. 2017;40(6):2086–2093. doi: 10.1007/s10753-017-0648-8. [DOI] [PubMed] [Google Scholar]
- 35.Arzi L., Farahi A., Jafarzadeh N., Riazi G., Sadeghizadeh M., Hoshyar R. Inhibitory effect of crocin on metastasis of triple-negative breast cancer by interfering with Wnt/β-catenin pathway in murine model. DNA and Cell Biology. 2018;37(12):1068–1075. doi: 10.1089/dna.2018.4351. [DOI] [PubMed] [Google Scholar]
- 36.Arzi L., Riazi G., Sadeghizadeh M., Hoshyar R., Jafarzadeh N. A comparative study on anti-invasion, antimigration, and antiadhesion effects of the bioactive carotenoids of saffron on 4T1 breast cancer cells through their effects on Wnt/β-catenin pathway genes. DNA and Cell Biology. 2018;37(8):697–707. doi: 10.1089/dna.2018.4248. [DOI] [PubMed] [Google Scholar]
- 37.Yin Q., Lu F. F., Zhao Y., et al. Resveratrol facilitates pain attenuation in a rat model of neuropathic pain through the activation of spinal Sirt1. Regional Anesthesia and Pain Medicine. 2013;38(2):93–99. doi: 10.1097/AAP.0b013e3182795b23. [DOI] [PubMed] [Google Scholar]
- 38.Luo J.-G., Zhao X.-L., Xu W.-C., et al. Activation of spinal NF-κB/p65 contributes to peripheral inflammation and hyperalgesia in rat adjuvant-induced arthritis. Arthritis & Rhematology. 2014;66(4):896–906. doi: 10.1002/art.38328. [DOI] [PubMed] [Google Scholar]
- 39.Motaghinejad M., Safari S., Feizipour S., Sadr S. Crocin may be useful to prevent or treatment of alcohol induced neurodegeneration and neurobehavioral sequels via modulation of CREB/BDNF and Akt/GSK signaling pathway. Medical Hypotheses. 2019;124:21–25. doi: 10.1016/j.mehy.2019.01.017. [DOI] [PubMed] [Google Scholar]
- 40.Yang H., Li X., Liu Y., et al. Crocin improves the endothelial function regulated by Kca3.1 through ERK and Akt signaling pathways. Cellular Physiology and Biochemistry. 2018;46(2):765–780. doi: 10.1159/000488735. [DOI] [PubMed] [Google Scholar]
- 41.Feidantsis K., Mellidis K., Galatou E., Sinakos Z., Lazou A. Treatment with crocin improves cardiac dysfunction by normalizing autophagy and inhibiting apoptosis in STZ-induced diabetic cardiomyopathy. Nutrition, Metabolism, and Cardiovascular Diseases. 2018;28(9):952–961. doi: 10.1016/j.numecd.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Y. K., Huang Z. J., Liu S., Liu Y. P., Song A. A., Song X. J. WNT signaling underlies the pathogenesis of neuropathic pain in rodents. The Journal of Clinical Investigation. 2013;123(5):2268–2286. doi: 10.1172/JCI65364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li B., Zhong L., Yang X., Andersson T., Huang M., Tang S. J. WNT5A signaling contributes to Aβ-induced neuroinflammation and neurotoxicity. PLoS One. 2011;6(8, article e22920) doi: 10.1371/journal.pone.0022920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nong X., Lan Y. Picroside II attenuates CCI-induced neuropathic pain in rats by inhibiting spinal reactive astrocyte-mediated neuroinflammation through the NF-κB pathway. Neurochemical Research. 2018;43(5):1058–1066. doi: 10.1007/s11064-018-2518-7. [DOI] [PubMed] [Google Scholar]
- 45.Tang R., Lin Y. M., Liu H. X., Wang E. S. Neuroprotective effect of docosahexaenoic acid in rat traumatic brain injury model via regulation of TLR4/NF-kappa B signaling pathway. The International Journal of Biochemistry & Cell Biology. 2018;99:64–71. doi: 10.1016/j.biocel.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 46.Gopalsamy B., Farouk A. A. O., Tengku Mohamad T. A. S., Sulaiman M. R., Perimal E. K. Antiallodynic and antihyperalgesic activities of zerumbone via the suppression of IL-1β, IL-6, and TNF-α in a mouse model of neuropathic pain. Journal of Pain Research. 2017;10:2605–2619. doi: 10.2147/JPR.S143024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang M. T., Wang B., Jia Y. N., et al. Neuroprotective effect of liquiritin against neuropathic pain induced by chronic constriction injury of the sciatic nerve in mice. Biomedicine & Pharmacotherapy. 2017;95:186–198. doi: 10.1016/j.biopha.2017.07.167. [DOI] [PubMed] [Google Scholar]
- 48.Zhou J., Wang J., Li W., Wang C., Wu L., Zhang J. Paeoniflorin attenuates the neuroinflammatory response in a rat model of chronic constriction injury. Molecular Medicine Reports. 2017;15(5):3179–3185. doi: 10.3892/mmr.2017.6371. [DOI] [PubMed] [Google Scholar]
- 49.Tao L., Ding Q., Gao C., Sun X. Resveratrol attenuates neuropathic pain through balancing pro-inflammatory and anti-inflammatory cytokines release in mice. International Immunopharmacology. 2016;34:165–172. doi: 10.1016/j.intimp.2016.02.033. [DOI] [PubMed] [Google Scholar]
- 50.Marchetti B., Pluchino S. Wnt your brain be inflamed? Yes, it Wnt! Trends in Molecular Medicine. 2013;19(3):144–156. doi: 10.1016/j.molmed.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Halleskog C., Mulder J., Dahlström J., et al. WNT signaling in activated microglia is proinflammatory. Glia. 2011;59(1):119–131. doi: 10.1002/glia.21081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brifault C., Kwon H., Campana W. M., Gonias S. L. LRP1 deficiency in microglia blocks neuro-inflammation in the spinal dorsal horn and neuropathic pain processing. Glia. 2019;67(6):1210–1224. doi: 10.1002/glia.23599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang D., Couture R., Hong Y. Activated microglia in the spinal cord underlies diabetic neuropathic pain. European Journal of Pharmacology. 2014;728:59–66. doi: 10.1016/j.ejphar.2014.01.057. [DOI] [PubMed] [Google Scholar]
- 54.Padi S. S., Kulkarni S. K. Minocycline prevents the development of neuropathic pain, but not acute pain: possible anti-inflammatory and antioxidant mechanisms. European Journal of Pharmacology. 2008;601(1-3):79–87. doi: 10.1016/j.ejphar.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 55.Vellucci R., Terenzi R., Kanis J. A., et al. Understanding osteoporotic pain and its pharmacological treatment. Osteoporosis International. 2018;29(7):1477–1491. doi: 10.1007/s00198-018-4476-y. [DOI] [PubMed] [Google Scholar]
- 56.Setia S., Nehru B., Sanyal S. N. The PI3K/Akt pathway in colitis associated colon cancer and its chemoprevention with celecoxib, a cox-2 selective inhibitor. Biomedicine & Pharmacotherapy. 2014;68(6):721–727. doi: 10.1016/j.biopha.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 57.Zhang Q. Y., Wang L., Song Z. Y., Qu X. J. Knockdown of type I insulin-like growth factor receptor inhibits human colorectal cancer cell growth and downstream PI3K/Akt, WNT/β-catenin signal pathways. Biomedicine & Pharmacotherapy. 2015;73:12–18. doi: 10.1016/j.biopha.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 58.Farghaly H. S. M., Elbadr M. M., Ahmed M. A., Abdelhaffez A. S. Effect of single and repeated administration of amitriptyline on neuropathic pain model in rats: focus on glutamatergic and upstream nitrergic systems. Life Sciences. 2019;233:p. 116752. doi: 10.1016/j.lfs.2019.116752. [DOI] [PubMed] [Google Scholar]
- 59.Liu M., Gao L., Zhang N. Berberine reduces neuroglia activation and inflammation in streptozotocin-induced diabetic mice. International Journal of Immunopathology and Pharmacology. 2019;33 doi: 10.1177/2058738419866379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yin Y., Park H., Lee S. Y., et al. Analgesic effect of toll-like receptor 4 antagonistic peptide 2 on mechanical allodynia induced with spinal nerve ligation in rats. Experimental Neurobiology. 2019;28(3):352–361. doi: 10.5607/en.2019.28.3.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kimura S., Kontani H. Demonstration of antiallodynic effects of the cyclooxygenase-2 inhibitor meloxicam on established diabetic neuropathic pain in mice. Journal of Pharmacological Sciences. 2009;110(2):213–217. doi: 10.1254/jphs.09006sc. [DOI] [PubMed] [Google Scholar]
- 62.Lee J. Y., Choi H. Y., Park C. S., et al. Inhibition of COX-2 alleviates lumbar spinal stenosis-induced chronic mechanical allodynia in rats. International Immunopharmacology. 2019;75, article 105738 doi: 10.1016/j.intimp.2019.105738. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of the present research are available from the corresponding authors once requested.


