Abstract
Widespread polybrominated biphenyls (PBBs) contamination occurred in Michigan from 1973 to 1974, when PBBs were accidentally substituted for a nutritional supplement in livestock feed. People who lived in the state were exposed to PBBs via several routes including ingestion, inhalation and skin absorption. PBBs sequestered in lipid-rich matrices such as adipose tissue, are slowly eliminated after entering the human body, and can also be transferred from a mother to her offspring through the placenta and breastfeeding. Due to the long biological half-lives of PBBs, as well as concerns from the exposed community, biomonitoring measurements were conducted from 2012 to 2015. Because of their similar structures, serum PBBs, polychlorinated biphenyls (PCBs), and polybrominated diphenyl ethers (PBDEs) were all measured 40 years after the PBB contamination incident (N=862). The serum PBB-153 levels among the original highly-exposed groups (i.e., chemical workers, the family of chemical workers, and individuals who lived on or received food from the contaminated farms) remains significantly higher than other Michigan residents. Several predictors such as sampling age, sex, and smoking status were significantly associated with the serum levels of some persistent organic pollutants (POPs). Higher average values and also wider ranges of serum POP levels were found in this study compared to the National Health and Nutrition Examination Survey (NHANES), with the most substantial difference in serum PBB-153. This was true for all groups of Michigan residents including those who were not part of the above-described highly-exposed groups. Moreover, the people born after the contamination incident began also have higher serum PBB-153 levels when compared with more recent NHANES data (2010-2014), which suggests potential intergenerational exposure and/or continued environmental exposure following the contamination period.
Keywords: polybrominated biphenyls (PBBs), polychlorinated biphenyls (PCBs), polybrominated diphenyl ethers (PBDEs), persistent organic pollutants (POPs), Michigan PBB Registry, biomonitoring
Graphical abstract
Introduction
Polybrominated biphenyls (PBBs) were widely used as flame retardants in electronic devices, plastics, textiles, furniture, and other consumer products after the increase in fire safety legislation in the 1960s. The total production of PBBs in the United States was approximately 5.9 million kg in the 1970s, and about 88% of the chemicals were produced by the Velsicol Chemical Corporation plant in St. Louis, Michigan (VCC). From July 1973 to May 1974, widespread PBB contamination occurred in Michigan when the commercial PBB mixture “FireMaster” was accidentally added to livestock feed instead of the nutritional supplement “NutriMaster.” An estimated 230 to 450 kg of FireMaster was distributed to the farms and retailers throughout the state of Michigan. The residents of Michigan farms and surrounding communities were exposed to PBBs by consuming contaminated meat, eggs and dairy products (ATSDR, 2004; WHO, 2016).
After the contamination episode was discovered, the Michigan Department of Health established the Michigan Long-Term PBB Study (now called the Michigan PBB Registry) and began enrolling residents in 1976 and 1977. The purpose of the long-term study was to measure baseline serum PBB concentrations, monitor serum levels periodically, conduct surveillance for cancer and other health outcomes, and enroll offspring of women in the registry. The PBB Registry, which has enrolled over 7,000 participants since 1976, is mostly made up of families that lived on contaminated farms, residents who received food from contaminated farms, and workers of VCC and their family members (Carter, 1976; Fries & Kimbrough, 1985; Landrigan et al., 1979).
A baseline exposure assessment found that the workers of VCC and their families had higher serum PBB levels than the farmers or farm food recipients in Michigan (Anderson et al., 1978; Landrigan et al., 1979). Additionally, exposure routes and pathways differed between exposure groups; for example, the workers and the farm residents could be directly exposed to PBBs through inhalation, dermal contact, and ingestion, whereas the family members of the workers could be exposed to PBBs via work-to-home transmission. Moreover, any resident living in Michigan at the time may have knowingly or unknowingly consumed contaminated food purchased at their local grocery (Anderson et al., 1978; WHO, 2016).
High oral PBB exposure was shown to cause thyroid, hepatic, reproductive and development effects and cancers in animal studies (ATSDR, 2004). Higher PBB levels among Michigan residents have also been associated with thyroid dysfunction (Bahn et al, 1980; Jacobson et al., 2017; Curtis et al, 2019a ), breast cancer (Terrell et al., 2016), digestive system cancers and lymphoma (Hoque et al., 1998). Recently, PBBs were reclassified from a possible (Group 2B) to a probable (Group 2A) human carcinogen by the International Agency for Research on Cancer (WHO, 2016). Women who were exposed as children had altered menstrual hormone levels (Howards et al, 2019) and were more likely to have a child with a low Apgar score (Terrell et al, 2015). Recent studies have also revealed epigenetic correlates of PBB-153 exposure (Curtis et al, 2019b, 2019c, 2019d)
PBBs are bioaccumulative, persistent organic pollutants (POPs) with a median human elimination half-life of about 13 years (Blanck et al., 2000b). After entering the human body, PBBs rapidly distribute to lipid-rich tissues while a portion is metabolized and slowly excreted in the feces within a few days (ATSDR, 2004; Robson & Toscano, 2007). To keep a constant equilibrium between matrices, PBBs can mobilize from storage tissues to replace the excreted amount in feces; thus, the stored PBBs are slowly excreted. Therefore, PBB-153 concentrations have been continuously detected in serum samples in the US population, even after the production of PBBs was ceased in 1979 (Sjödin et al., 2008; ATSDR, 2004). Additionally, PBBs can be transferred via the placenta from a mother to her fetus or transferred through breastfeeding (Anderson & Wolff, 2000; Joseph et al., 2009). Several health effects, including earlier age at menarche (Blanck et al., 2000a), and genitourinary conditions (Small et al., 2009) have been found in children of highly exposed mothers. Higher rates of miscarriages have been found in reproductive age daughters of highly exposed mothers (Small et al., 2011).
Due to the persistence and the potential of intergenerational exposure of PBBs, the members of the registry have been expressing their concerns regarding their chemical body burdens and potential health risks. However, limited exposure biomonitoring has been conducted since the 1990s. Therefore, the objective of this study is to evaluate the current serum PBB concentrations for the people in the registry, to evaluate predictors of exposure including sub-populations and demographic factors, and to compare the concentrations in this study to those within the general US population who have participated in the National Health and Nutrition Examination Survey (NHANES). Due to the similarity of the chemical structures and the shared toxicological features, serum concentrations of several other POPs (i.e., polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs)) were also analyzed to provide additional information on the exposure profiles within this population.
Material and Methods
Data collection
In order to recruit participants for PBB follow-up measurements and health updates, we held community meetings throughout Michigan between 2012 and 2015. The members in the registry were invited through mailed invitations and the local community was notified by advertisements in the media. Individuals were eligible to participate in this study if they were: 1) currently participating in the PBB registry; 2) residents in Michigan during the incident (1973-1974); or 3) offspring of people who lived in the state in 1973-1974 (Jacobson et al., 2017). We enrolled 862 people in total for this follow-up study.
Each participant provided a 10-mL sample of blood via venipuncture for exposure assessment, and completed an online general health questionnaire, that collected information on basic demographic characteristics, exposure history and health conditions (missing N=234/862, 27%). The demographic information captured from the questionnaire included sex, age, race, smoking status, and whether the individual or their family members were participants of the original long-term PBB study established by the Michigan Department of Health. The exposure information captured from the questionnaire included self-report of working at the Velsicol Chemical Plant in St. Louis, Michigan (ever worked, family member ever worked), residence during 1973 to 1974 (living on a contaminated farm, non-contaminated farm, not on a farm), contaminated food consumption (ever eaten), and ever receiving information on their PBB level. Participants of reproductive ages (18-56) were invited to complete a second questionnaire with specific questions about their reproductive health, and for women included parity and breastfeeding history (missing N=79/309, females of reproductive age, 26%). Moreover, anthropometric measurements were conducted by study staff, and BMI was calculated from height and weight. However, a substantial proportion of participants did not participate in the physical measurements (missing N=410/862, 48%). This study was reviewed and approved by Emory’s Institutional Review Board.
The participants were further classified into the following exposure groups using their historical PBB Registry identification number or questionnaire data: 1) chemical workers: people who had ever worked in the Velsicol Chemical Plant; 2) family members of former chemical plant workers; 3) farm/food family: people who lived on PBB-contaminated farms or received food from contaminated farms; 4) people born after the incident began: people who were born after July 1973; and 5) other Michigan residents: a combination of people who lived in the St. Louis/Pine River community (the area surrounding the chemical plant), or people who did not fit into the other exposure groups. Additionally, thirty-two people could not be classified into any exposure group because of missing information.
Laboratory analyses
Venous blood samples were collected during April 2012 and May 2015 from the study population. The samples were transported to the lab at Emory University, centrifuged for 30 mins at 3,000 rpm to separate the serum from the whole blood, and then stored at −20 °C until analysis. Each sample (1 mL serum) was spiked with labeled standard solutions, and deproteinated with formic acid. A liquid-liquid extraction and a cleanup process using solid-phase extractions were performed to extract serum PBBs and PCBs (Marder et al., 2016). Serum PBDEs were extracted by a two-stage solid-phase extraction which was developed based on the previous literature and further adjusted to optimize extraction recovery and precision (Hovander et al., 2000; Sandau et al., 2003; Zhang & Rhind, 2011). After extraction and cleanup procedures, these extractants were dried with TurboVap (Zymark; Framingham, MA) and reconstituted with 50 μL toluene (PBB and PCB) or nonane (PBDE) prior to instrument analysis.
Prepared serum samples were analyzed for targeted congeners using gas chromatography-tandem mass spectrometry with electron impact ionization in the multiple reaction monitoring mode (GC-MS/MS; 7890A gas chromatograph coupled to an Agilent 7000B tandem mass spectrometer; Agilent Technologies; Santa Clara, CA). PBB (77, 101, 153 and 180), PCB (118, 138, 153, and 180), and PBDE (47, 85, 99, 100, and 153) congeners were selected because they are the major components of the products or the most frequently detected congeners in the environment (Darrow et al., 2016; WHO, 2016). Quantification was performed using isotope dilution calibration with the limits of detection (LOD) ranging from 1-5.6 pg/mL for PBBs, 0.7-1.6 ng/mL for PCBs, and 10-50 ng/mL for PBDEs (Marder et al., 2016; Darrow et al., 2016). LODs were defined as the concentrations at which the S/N ratio of the observed signal was ≥ 3 (Table S1). Solvent-based standard calibration curves were prepared with each concentration expressed in serum equivalent. In each analytical run, one laboratory blank sample and two matrix-based quality control samples were included and analyzed concurrently with unknown samples to ensure proper operation of the methods. The accuracy was 100±20% calculated by two different methods, including the difference between the mean measurements of spiked pooled serum and the expected concentrations and the difference between the values of the NIST certified reference measured from our method and their mean values. The method precision calculated as the relative standard deviation (RSD) was less than 15% at two fortified levels for all analytes with the exception of PCB-138 (RSD=16%). The sample concentrations were adjusted for the background values in laboratory blanks. Invalid data for PCB-118 were obtained on 30 samples due to unstable retention times. For PBDE measurements, they were only conducted on a subset (N=358/862, 42%) of people who were mostly not affiliated with the chemical plant (workers, family members of workers and residents of the surrounding community).
To estimate total blood lipids, we used immunoassay kits to measure total triglyceride (Abnova Corporation) and total cholesterol (Cayman Chemical Company) in serum based on the manufacturer’s instructions. Total serum lipids was calculated using the method published by Phillips et al. (1989). The lipid-adjusted concentrations were serum biomarker concentrations divided by total serum lipids allowing for appropriate unit conversions.
Statistical analyses
Descriptive analyses were performed for the exposure concentrations, including detection frequencies, distribution percentiles, ranges, geometric means (GMs), and 95% confidence intervals were calculated for the individual serum congeners. Summed congener concentrations were calculated both as nanograms per milliliter and in SI units (the summation by molar concentrations). We first examined the frequently detected congeners and correlations between congeners, such as ΣPCB4 (PCB-118, 138, 153 and 180). Three PCB congeners (ΣPCB3: PCB-138, 153 and 180) were summed because they represent approximately 60% of the total PCB exposure and have different degrees of ortho-substitution from PCB-118, which might have different toxicological effects (Barr et al., 2006). ΣPBDE3 was the summation of the three most prevalent congeners, PBDE-47, 99 and 100, in the common technical mixture penta-BDE (La Guardia et al., 2006). PBB-153 was the only PBB congener with a detection frequency above 10%; thus, no summation was conducted for PBBs.
Since the measured concentrations were approximately log-normally distributed, we used log10-transformed values for all statistical analyses. Pearson correlation coefficients were conducted to investigate associations between log10-transformed serum levels. ANOVA was performed to examine the differences of log10-transformed concentrations between different demographic groups and predictors. Multivariable linear regression models were generated for the associations between serum POP levels and exposure groups, adjusting for age, sex and serum lipids (directed acyclic graph in Figure S2). To identify additional predictors of serum POP levels, each variable was added to the base models, which contained age, sex, and serum lipids, separately. We also explored interactions for each variable, and included significant terms in the models. Moreover, we generated separate PBB models for the people born before and after the incident began since the exposure magnitude, route, and the association between serum concentrations and the predictors might vary. In all regression models, the values of the serum lipids were included as a covariate which results in less biased estimates and increased statistical flexibility when compared to the standardized method (serum lipids as a denominator of POP measurements) (Schisterman et al., 2005).
Furthermore, the measured serum levels were compared with those of the US population. The environmental chemical data from NHANES were extracted and evaluated accounting for the weights, primary sampling units, and strata. Since the participants in this study are mostly non-Hispanic whites (94%), we only included the non-Hispanic white data from NHANES in the analyses. The congeners with more than 60% detection frequencies were compared to NHANES data by each demographic characteristic. After 2005, NHANES used a weighted pooled-sample design instead of individual serum samples to improve the sensitivity and reduce the costs. Due to the pooled-sample design, arithmetic means and unadjusted standard errors were calculated for 2011-2014 NHANES instead of GMs and geometric standard deviations (GSD).
Two imputation methods were conducted for the values below LOD. First, simple imputation was applied for GM calculations, using the method reported by the National Report on Human Exposure to Environmental Chemicals (CDC, 2019). This involved imputing the values below the LOD with the LOD divided by the square root of two for the congeners which had detection frequencies ≥ 60%, and with zero for the congeners that were detected in < 60% of the samples. With this method, the GMs are more comparable to the NHANES results. Second, the concentrations below the LOD were imputed based on a lognormal probability distribution where parameters were determined by maximum likelihood estimation. We produced 10 sets of distribution parameters and imputed the measurements below the LOD according to each set of parameters. In regression analyses, this imputation method is expected to generate less biased estimates and nominal confidence intervals than the first approach (Lubin et al., 2004). The statistical analyses were conducted in SAS version 9.4 (SAS, Cary, NC) and R version 3.4.1.
Results
Selected demographic characteristics are presented in Table 1. Frequencies of characteristics between the different populations that had PBB, PCB, or PBDE levels measured were similar, except for exposure group and PBDE levels where it was not measured in chemical workers, measured in only one family member of a chemical worker, and nine other residents. Among the 861 participants who had PBB measured, 245 (30%) participants were born after the incident began, 237 (29%) were people who had lived on PBB-contaminated farms or received food from contaminated farms, and 164 (19%) were chemical workers or family members. About 55% of participants were females and 20-31% were ages 60-88 years when their blood was collected. The average sampling age was 48-50 years. More than 90% of the study participants were non-Hispanic whites, with a small percentage of other races/ethnicities including Black, Latino, other Hispanic, or Native American. For smoking status, 57-66% were non-smokers and 20-27% were past smokers. About 39-40% of participants were obese (BMI ≥ 30 kg/m2), and the average BMI in this study was 29.5 kg/m2.
Table 1.
Demographic and lifestyle characteristics of the Michigan PBB Registry participants with PBB, PCB, and PBDE measurements, 2012-2015 (N=862).
| Characteristics | PBBs (N=861)a |
PCBs (N=832) |
PBDEs (N=358) |
|---|---|---|---|
| N (%) | |||
| Sex | |||
| Female | 478 (55) | 464 (56) | 200 (56) |
| Male | 383 (44) | 368 (44) | 158 (44) |
| Sampling age (year) | |||
| 7-20 | 37 (4.3) | 35 (4.2) | 13 (3.7) |
| 20-29 | 90 (10) | 89 (11) | 37 (10) |
| 30-39 | 114 (13) | 110 (13) | 61 (17) |
| 40-49 | 162 (19) | 155 (19) | 77 (22) |
| 50-59 | 197 (23) | 186 (22) | 97 (27) |
| 60-88 | 258 (30) | 254 (31) | 70 (20) |
| Exposure groups | |||
| Chemical workers | 69 (8.3) | 69 (8.6) | 0 (0) |
| Family members of chemical workers | 95 (11) | 95 (12) | 1 (0) |
| Farm/food family | 237 (29) | 221 (27) | 209 (63) |
| Other residents | 183 (22) | 183 (23) | 9 (2.7) |
| Born after incident began | 245 (30) | 238 (30) | 112 (34) |
| Race/Ethnicities | |||
| Non-Hispanic white | 668 (94) | 648 (94) | 228 (98) |
| Other | 39 (5.5) | 38 (5.5) | 4 (1.7) |
| Smoking status | |||
| Non-smoker | 399 (57) | 386 (57) | 151 (66) |
| Past smoker | 183 (26) | 179 (27) | 45 (20) |
| Current smoker | 115 (16) | 110 (16) | 34 (15) |
| BMI (kg/m2)b | |||
| <18.5 | 3 (0.7) | 3 (0.7) | 2 (1.0) |
| 18.5-24.9 | 124 (28) | 125 (29) | 53 (27) |
| 25.0-29.9 | 142 (31) | 135 (31) | 65 (33) |
| ≥30 | 181 (40) | 169 (39) | 80 (40) |
| Where were you living between 1973 and 1974? | |||
| Not on a farm | 269 (44) | 265 (44) | 30 (19) |
| Quarantined farmc | 72 (12) | 69 (11) | 39 (25) |
| Non-quarantined farm | 88 (14) | 85 (14) | 23 (15) |
| I am not sure | 24 (3.9) | 24 (4.0) | 5 (3.2) |
| Born in 1975 or after | 161 (26) | 160 (27) | 61 (39) |
| Do you know if you ate any foods contaminated with PBB (such as milk, eggs, other dairy products, or meat)? | |||
| Definitely not | 33 (5.4) | 33 (5.5) | 13 (8.4) |
| Definitely | 129 (21) | 124 (21) | 62 (40) |
| Probably | 114 (19) | 113 (19) | 18 (12) |
| I don't know | 334 (55) | 330 (55) | 61 (40) |
| Parity (after incident began) | |||
| 0 | 67 (29) | 65 (29) | 31 (33) |
| 1 | 34 (15) | 34 (15) | 13 (14) |
| 2-6 | 129 (45) | 126 (56) | 49 (53) |
| Breastfeeding (after incident began) | |||
| No | 111 (48) | 109 (48) | 43 (46) |
| Yes | 119 (52) | 116 (52) | 50 (54) |
Abbreviation: N, sample number; %, the percentage of a sample number in the group in relation to the non-missing number.
The numbers of available serum measurements are different for each chemical. The total study population is 862 with one participant having PBDE but not PBB or PCB measurements.
BMI is only available for adults (age ≥ 18).
Farms quarantined because of PBB contamination.
Table 2 (ng/mL serum) and Table S2 (lipid-adjusted ng/g lipid) show the distribution of the targeted chemicals by congeners and summations. Of 861 people, 792 (92%) had detectable serum PBB-153 levels, which was the most frequently detected PBB congener. The four PCB congeners, PCB-118, PCB-138, PCB-153, and PCB-180, were detected in almost 100% of the serum samples with PCB-153 having the highest concentrations. PBDE-47 was the most frequently detected PBDE congener (92%), followed by PBDE-99 (45%) and PBDE-100 (38%). The exposure distributions were right-skewed, especially for serum PBB-153, which had a maximum level 900 times higher than its median. Table 3 shows the Pearson correlation coefficients between the chemicals with detection frequencies ≥ 60%. PCB congeners were highly correlated with each other, with the correlation coefficients ranging from 0.74 to 0.99 (p-values < 0.001). PBB-153 was moderately correlated with the PCB congeners (Pearson correlation coefficients: 0.33-0.56) but was not correlated with PBDE-47.
Table 2.
Distribution of serum PBB, PCB and PBDE congeners (ng/mL) among the Michigan PBB Registry participants.
| Chemical (ng/mL) |
N | %>LOD | P25 | P50 | P75 | P95 | Max | GMa (95% CI) |
|---|---|---|---|---|---|---|---|---|
| Polybrominated biphenyl | ||||||||
| PBB-77 | 861 | 7.4% | <LOD | <LOD | <LOD | 0.01 | 0.34 | - |
| PBB-101 | 861 | 7.9% | <LOD | <LOD | <LOD | 0.01 | 0.60 | - |
| PBB-153 | 861 | 92% | 0.06 | 0.26 | 0.63 | 3.9 | 236 | 0.17 (0.14, 0.10) |
| PBB-180 | 861 | 0.4% | <LOD | <LOD | <LOD | <LOD | 0.31 | - |
| Polychlorinated biphenyl | ||||||||
| PCB-118 | 832 | 100% | 0.03 | 0.06 | 0.11 | 0.29 | 0.89 | 0.06 (0.05, 0.06) |
| PCB-138 | 861 | 100% | 0.09 | 0.21 | 0.40 | 1.1 | 3.3 | 0.20 (0.18, 0.21) |
| PCB-153 | 861 | 100% | 0.11 | 0.25 | 0.45 | 1.1 | 2.7 | 0.22 (0.20, 0.23) |
| PCB-180 | 861 | 100% | 0.08 | 0.19 | 0.34 | 0.75 | 4.9 | 0.16 (0.14, 0.17) |
| ΣPCB4b | 832 | - | 0.34 | 0.74 | 1.3 | 3.1 | 8.1 | 0.64 (0.60, 0.69) |
| ΣPCB4 (pmol/mL) b,c | 832 | - | 0.91 | 2.0 | 3.6 | 8.6 | 22 | 1.8 (1.7, 1.9) |
| ΣPCB3b | 861 | - | 0.30 | 0.67 | 1.2 | 3.0 | 7.6 | 0.58 (0.54, 0.62) |
| ΣPCB3 (pmol/mL) b,c | 861 | - | 0.80 | 1.8 | 3.2 | 8.0 | 21 | 1.6 (1.5, 1.7) |
| Polybrominated diphenyl ether | ||||||||
| PBDE-47 | 358 | 92% | 0.10 | 0.21 | 0.42 | 1.3 | 18 | 0.19 (0.18, 0.21) |
| PBDE-85 | 358 | 2.0% | <LOD | <LOD | <LOD | <LOD | 0.16 | - |
| PBDE-99 | 358 | 45% | <LOD | <LOD | 0.03 | 0.11 | 2.9 | - |
| PBDE-100 | 358 | 38% | <LOD | <LOD | 0.02 | 0.10 | 1.6 | - |
| PBDE-153 | 358 | 5.3% | <LOD | <LOD | <LOD | 0.05 | 0.51 | - |
| ΣPBDE3b | 358 | - | 0.11 | 0.23 | 0.49 | 1.6 | 23 | 0.21 (0.19, 0.23) |
| ΣPBDE3 (pmol/mL) b,c | 358 | - | 0.23 | 0.45 | 0.99 | 3.2 | 45 | 0.43 (0.39, 0.47) |
Abbreviation: N, sample number; LOD, limit of detection; P25, the 25th percentile; GM, geometric mean; CI, confidence interval.
Geometric means were not calculated for congeners with detection frequencies <60% (For the congeners with detection frequencies ≥60%, the values below LOD were replaced by LOD/√2, and the congeners with detection frequencies <60%, were substituted with zero).
ΣPCB4 was calculated by adding PCB-118, 138, 153, 180; ΣPCB3: PCB-138, 153, 180; ΣPBDE3: PBDE-47, 99, 100.
Sums on the molar basis. Molar sums were calculated by dividing the concentration of each congener by its molecular weight, multiplying by 1,000 to convert nmol/mL to pmol/mL, and then summing the congeners.
Table 3.
Pearson correlation coefficients of log10-transformed serum PBB, PCB, and PBDE congeners.
| PBB-153 | PCB-118 | PCB-138 | PCB-153 | PCB-180 | PBDE-47 | |
|---|---|---|---|---|---|---|
| PBB-153 | 1.00 | 0.33* | 0.37* | 0.43* | 0.56* | 0.09 |
| PCB-118 | - | 1.00 | 0.85* | 0.84* | 0.74* | 0.30* |
| PCB-138 | - | - | 1.00 | 0.99* | 0.89* | 0.32* |
| PCB-153 | - | - | - | 1.00 | 0.93* | 0.32* |
| PCB-180 | - | - | - | - | 1.00 | 0.26* |
| PBDE-47 | - | - | - | - | - | 1.00 |
P-value < 0.001.
Table S3 shows unadjusted GMs and GSDs of the exposures by each characteristic. PBB-153 concentrations were significantly different by sex, sampling age, exposure groups, smoking status, residence between 1973 and 1974, ever having eaten contaminated food, parity and breastfeeding in ANOVA analyses. For ΣPCB4 concentrations, sex, sampling age, exposure groups, smoking status, residence between 1973 and 1974, and ever having eaten contaminated food were significantly different. ΣPBDE3 concentrations were significantly different by exposure groups only.
Table 4 shows the associations between exposure groups and serum PBB-153, ΣPCB4, and ΣPBDE3 adjusting for sex, sampling age, and serum lipids. We centered and scaled serum lipids to one standard deviation to observe the effect of serum lipids on serum POP levels. Because serum levels were log10-transformed in the model, we exponentiated the β-coefficients from the models and present the estimates as the multiplicative change in serum POP levels by each unit change of independent variables. For serum PBB-153 concentrations, sampling age was a significant predictor: a one-year increase in age predicted a 3% increase (CI = 2-4%) and a 7% increase (CI = 4-10%) in serum PBB-153 concentrations for those born before and after the incident began, after adjusting for the other covariates. Males tended to have higher serum levels than females, but the effect was only significant for those born before the incident began. The males born before the incident began had 127% (CI 81-183%) higher serum levels than females. More importantly, the exposure groups, including chemical workers, their family members, and farm/food family, were also strong predictors of having higher serum PBB-153 concentrations. The concentrations of chemical workers, their family members, and farm/food family were about 185% (CI = 93-321%), 105% (CI = 49-184%) and 168% (CI = 108-244%) higher than other Michigan residents.
Table 4.
Adjusted associations between log10-transformed serum PBB-153, ΣPCB4, and ΣPBDE3 levels and the main
| Predictors | PBB-153 a,b Born before incident began (N=579) |
PBB-153 a Born after incident began (N=244) |
ΣPCB4
a,b,c (N=801) |
ΣPBDE3
a,b,c (N=352) |
|---|---|---|---|---|
| 10β (95% CI) | 10β (95% CI) | 10β (95% CI) | 10β (95% CI) | |
| Sex | 1.00 | 1.00 | 1.00 | 1.00 |
| Female | 2.27 (1.81, 2.83)* | 1.24 (0.81, 1.89) | 1.41 (0.98, 2.04) | 1.11 (0.86, 1.44) |
| Male | ||||
| Sampling age (year) | 1.03 (1.02, 1.04)* | 1.07 (1.04, 1.10)* | 1.04 (1.04, 1.05)* | 1.01 (1.00, 1.03)* |
| Sampling age × sex | ||||
| Sampling age × female | - | - | 1.00 | - |
| Sampling age × male | 0.99 (0.99, 1.00)* | |||
| Serum lipids (mg/dL)d | 1.14 (1.02, 1.27)* | 0.94 (0.76, 1.16) | 1.02 (0.96, 1.08) | 0.96 (0.83, 1.11) |
| Exposure groups | ||||
| Other residents | 1.00 | 1.00e | ||
| Chemical workers | 2.85 (1.93, 4.21)* | 0.83 (0.65, 1.07) | 1.00e-f | |
| Family of chemical workers | 2.05 (1.49, 2.84)* | - | 1.00 (0.82, 1.22) | - |
| Farm/food family | 2.68 (2.08, 3.44)* | 1.02 (0.88, 1.18) | 1.19 (0.80, 1.76) |
Abbreviation: N, sample number of the model; 10β, exponentiated β-coefficients represent the multiplicative change in serum levels for a one-unit change or comparing to the reference group; CI, confidence interval.
The measurements below LOD were imputed by a lognormal probability distribution and maximum likelihood estimation.
PBB-153 and ΣPBDE3 models were adjusted for sex, sampling age, serum lipids, and exposure groups; ΣPCB4 model was adjusted for sex, sampling age, interaction of sex and sampling age, serum lipids, and exposure groups.
ΣPCB4 was calculated by adding PCB-118, 138, 153, 180; ΣPBDE3: PBDE-47, 99, 100.
Serum lipids were centered around the mean and scaled to one standard deviation (SD): 1 SD = 211, 208, 211, 184 mg/dL for the PBB-153 (born before), PBB-153 (born after), ΣPCB4, and ΣPBDE3 models.
The reference group combines the other residents and the people born after the incident began.
The PBDE measurements were not available for chemicals workers (N=0) or family of chemical workers (N=1).
P-value < 0.05.
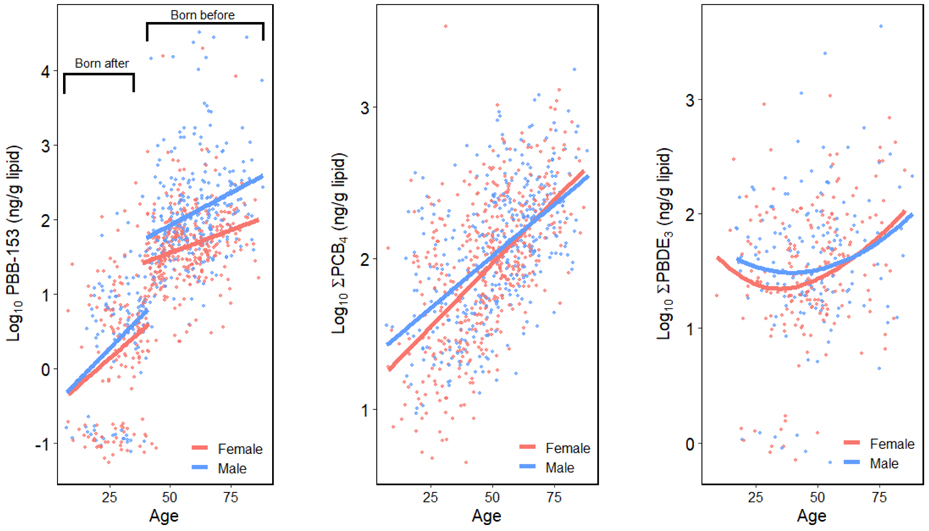
Sampling age was also strongly associated with serum ΣPCB4 and ΣPBDE3. A 4% (CI = 4-5%) and 1% (CI = 0-3%) increase in serum ΣPCB4 and ΣPBDE3 were found with a one-year increase in age after adjusting for sex, serum lipids and exposure groups. A 41% increase (CI = 2-104%) of ΣPCB4 level was found among males but the estimate was not significant. Additionally, a significant interaction effect of age and sex was found in the PCB model, suggesting that PCB concentrations vary by age within strata of sex (Figure 1). The younger male participants tended to have higher ΣPCB4 than the females, whereas the opposite was true among the older participants. The models for individual PCBs (Table S4) are similar to the results of ΣPCB4 although the age where the fitted lines for males and females intersected increased for the higher chlorinated congeners (Figure S1). As shown in Figure 1, the relationship between serum levels and age was different between the three groups of chemicals. Serum PBB-153 and ΣPCB4 concentrations had relatively linear associations with age, whereas a U-shaped association was observed for ΣPBDE3 concentrations. Moreover, the intercept differences for serum PBB-153 among those born before and after the incident began suggested a greater difference of serum levels in these two groups.
Figure 1.
Scatter plots of log10-transformed serum levels and sampling age stratified by sex, including a locally weighted regression fitted line.
The adjusted associations between serum POP levels and the other potential predictors are shown in Table 5. Current smokers had a significant 47% decrease (CI = −68, −10) of serum PBB-153 concentrations than never smokers among the people who were born after the incident. Similar inverse but not statistically significant associations with smoking status were found in the PBDE models. Serum PBB-153 levels were significantly associated with residence between 1973 and 1974: the participants who had lived on quarantined farms had higher serum PBB-153 levels than those who had not lived on a farm. However, no association between serum levels and residence between 1973 and 1974 was found in the PCB and PBDE models. The remaining predictors including race, BMI, and contaminated food consumption were not significantly associated with serum POP levels.
Table 5.
Adjusted associations between log10-transformed serum PBB-153, ΣPCB4, and ΣPBDE3 and other potential
| Predictors | PBB-153a,b Born before incident began (N=616) |
PBB-153a Born after incident began (N=245) |
ΣPCB4
a,b,c (N=832) |
ΣPBDE3
a,b,c (N=358) |
|---|---|---|---|---|
| 10β (95% CI) | 10β (95% CI) | 10β (95% CI) | 10β (95% CI) | |
| Race/Ethnicities | ||||
| Non-Hispanic white | 1.00 | 1.00 | 1.00 | 1.00 |
| Other | 0.89 (0.55, 1.45) | 0.89 (0.35, 2.28) | 0.92 (0.71, 1.20) | 1.59 (0.54, 4.72) |
| Smoking status | ||||
| Non-smoker | 1.00 | 1.00 | 1.00 | 1.00 |
| Past smoker | 0.97 (0.75, 1.25) | 0.61 (0.33, 1.10) | 1.02 (0.88, 1.18) | 0.70 (0.49, 1.02) |
| Current smoker | 1.12 (0.79, 1.58) | 0.53 (0.32, 0.90)* | 1.07 (0.90, 1.26) | 0.76 (0.50, 1.16) |
| BMI (kg/m2)d | 0.98 (0.96, 1.00) | 0.97 (0.94, 1.00) | 0.99 (0.98, 1.01) | 1.00 (0.98, 1.02) |
| Where were you living between 1973 and 1974? | ||||
| Not on a farm | 1.00 | 1.00 | 1.00 | |
| Quarantined farme | 1.56 (1.10, 2.20)* | 0.93 (0.75, 1.15) | 1.17 (0.67, 2.05) | |
| Non-quarantined farm | 1.11 (0.82, 1.51) | -g | 1.03 (0.85, 1.25) | 1.27 (0.68, 2.39) |
| I am not sure | 1.03 (0.56, 1.87) | 1.06 (0.75, 1.48) | 2.32 (0.79, 6.88) | |
| Born in 1975 or after | -f | 0.81 (0.64, 1.04) | 0.88 (0.45, 1.74) | |
| Do you know if you ate any foods contaminated with PBB (such as milk, eggs, other dairy products, or meat)? | ||||
| Definitely not | 1.00 | 1.00 | 1.00 | 1.00 |
| Definitely | 2.44 (0.66, 9.01) | 1.43 (0.19, 10.56) | 0.88 (0.64, 1.21) | 1.12 (0.51, 2.43) |
| Probably | 1.81 (0.49, 6.75) | 0.71 (0.29, 1.77) | 1.01 (0.74, 1.39) | 1.55 (0.66, 3.62) |
| I don't know | 2.01 (0.55, 7.37) | 0.57 (0.29, 1.13) | 1.00 (0.74, 1.33) | 1.15 (0.56, 2.43) |
Abbreviation: N, sample number; 10β exponentiated β-coefficients represent the multiplicative change in serum levels for a one-unit change or comparing to the reference group; CI, confidence interval.
The measurements below LOD were imputed by a lognormal probability distribution and maximum likelihood estimation.
PBB-153 and ΣPBDE3 models were adjusted for sex, sampling age, serum lipids, and exposure groups; ΣPCB4 models were adjusted for sex, sampling age, interaction of sex, and sampling age, serum lipids, and exposure groups. Serum lipids were centered around the mean and scaled to one standard deviation (SD).
ΣPCB4 was calculated by adding PCB-118, 138, 153, 180; ΣPBDE3: PBDE-47, 99, 100.
BMI is only available for adults (age ≥ 18).
Farms quarantined because of PBB contamination.
Not applicable due to the limited sample size for this category (n=1).
Not applicable because most were born in 1975 or after.
P-value < 0.05.
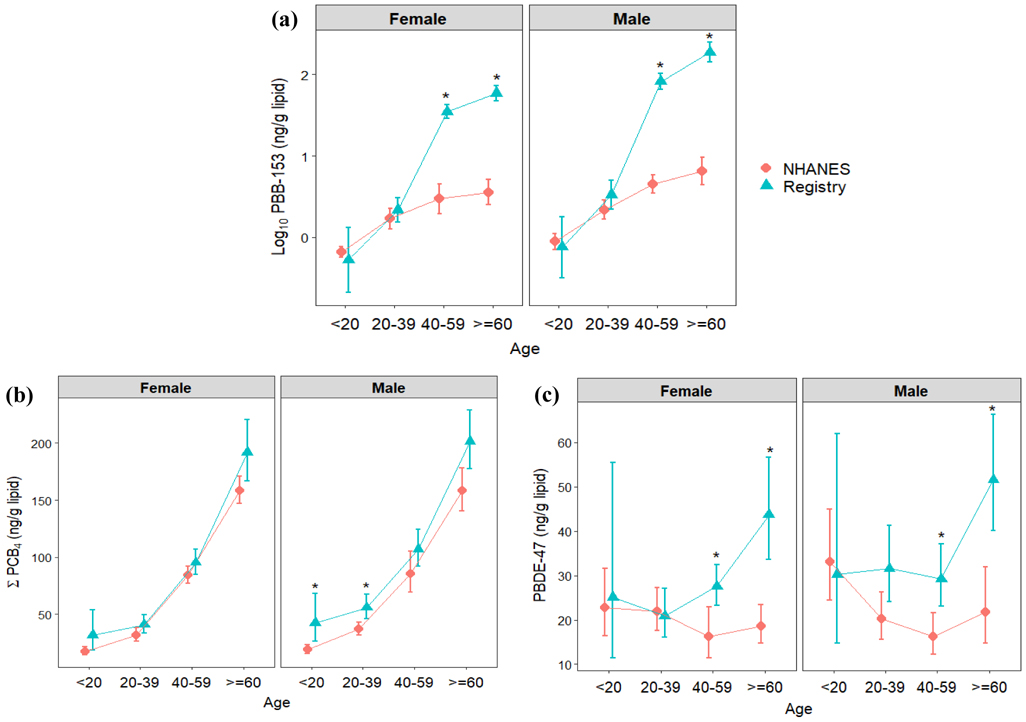
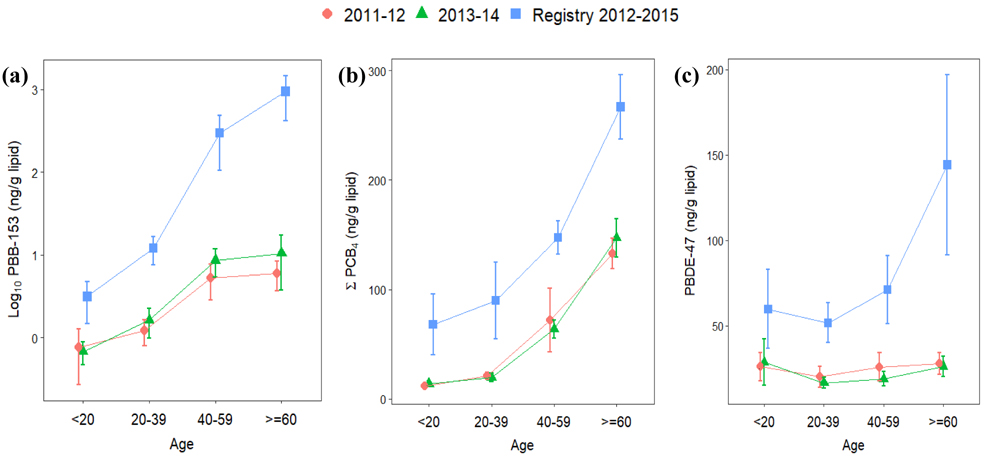
Figures 2 and 3 (and Table S5) show the comparison of GMs between the PBB Registry and 2003-2004 NHANES, and Figure 4 (and Table S6) presents the comparison of arithmetic means between this registry and 2011-2014 NHANES. The GMs of serum PBB-153, individual PCB congeners and PBDE-47 levels in this registry were 10, 1.5-2, and 1.07 times higher than the GMs in NHANES, respectively. Higher PBB-153 concentrations were found for each exposure group than for the NHANES sample; the GMs of chemical workers, their family members, farm/food family, and other Michigan residents were about 80, 23, 35, and 12 times higher than in NHANES. Additionally, the GMs of ΣPCB4 for each exposure group were higher than in NHANES, whereas the GM of PBDE-47 was more comparable between these two populations. For the differences between each demographic group, including sex and age, we found significantly higher serum PBB-153 levels in the PBB Registry than NHANES among those 40 years or older. Although the serum PCB and PBDE levels within the registry were generally higher than NHANES, they were only significantly different for several age groups, ΣPCB4 in males below 40 years old and PBDE-47 for males and females 40 years or older. The arithmetic means in this registry were consistently higher than the pool-designed NHANES concentrations.
Figure 2.
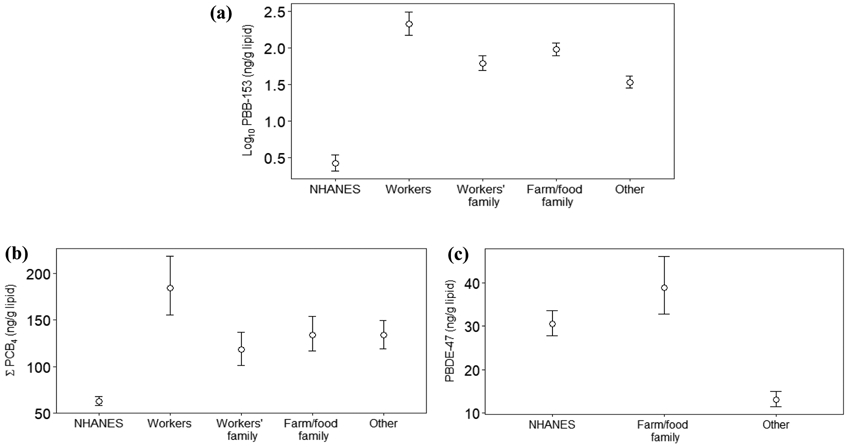
Geometric means and 95% confident intervals of serum levels by exposure groups in the Michigan PBB Registry participants who were born before the incident began (2012-2015) and non-Hispanic whites in NHANES (2003-2004) (Notes: a log10 scale is presented on the y-axis of (a), whereas (b) and (c) are numeric scales)
Figure 3.
Geometric means and 95% confident intervals of serum levels by sex and sampling age in the Michigan PBB Registry participants (2012-2015) and non-Hispanic whites in NHANES individual samples (2003-2004). (Notes: a log10 scale is presented on the y-axis of (a), whereas (b) and (c) are numeric scales; * p-value < 0.05)
Figure 4.
Arithmetic means and 95% confidence intervals of serum levels by sampling age in the Michigan PBB Registry participants (2012-2015) and non-Hispanic whites in NHANES pooled samples (2011-2014). (Notes: a log10 scale is presented on the y-axis of (a), whereas (b) and (c) are numeric scales)
Discussion
In this follow-up study, we measured the concentrations of PBBs, PCBs, and PBDEs in a subset of individuals from the Michigan PBB Registry. This is the first study presenting the levels among different exposure groups 40 years after the PBB contamination incident. Although these chemicals were phased out in the US several decades ago (PBB: 1979; PCB: 1977; PBDE: penta & octaBDE, 2004; decaBDE: 2013), they have been widely detected in different environments and human samples due to their environmental persistence and long biological half-lives (PBB: 13-29 years; PCB: children 3.7-9.1 years, adults: 9.3-14.4 years (WHO, 2016); PBDE-47: 1.8 years (Geyer et al., 2004)). Because of the major PBB exposure in this population, the largest differences in concentrations were observed for PBB-153, which had a maximum level of 236 ng/mL, a 95th percentile of 3.90 ng/mL, and a median of 0.26 ng/mL among those in the PBB registry. PBB concentrations have declined since earlier reports, which is expected given that the high-level contamination source was eliminated and PBB-153 was later phased out. About 2-4 years after the incident, the PBB serum concentrations ranged from <LOD-1,900 ng/mL with a median of 3 ng/mL from 3,639 people who were likely exposed by the incident (Landrigan et al., 1979). Almost 20 years after the incident, the PBB concentrations in 151 women in the PBB registry were found to be in the range of <LOD to 337 ng/mL with a median of 2.16 ng/mL (Terrell et al., 2008).
The positive association between sampling age and serum POP levels has been reported in several cross-sectional studies (Sjödin et al., 2013; Pavuk et al., 2014), and this study and NHANES shows similar results. However, it has also been shown that serum POP concentrations may decrease with age on an individual basis in longitudinal studies due to the reduced exposure after the chemicals were phased out (Nøst et al., 2013; Raffetti et al., 2017). Thus, the positive associations between serum levels and age in this cross-sectional study may be attributable to other factors besides bioaccumulation, such as period effects, cohort effects or metabolic half-lives of the chemicals. The period effects indicate the peak PBB exposure period and the phase-out year for the chemicals, and the cohort effects may reflect generation-specific environmental exposures and dietary habits in this study (Quinn and Wania, 2012; Nøst et al., 2013). As shown in Figure 1, PBB-153 and ΣPCB4 have similar associations with age because they were both phased-out in the 1970s and have longer biological half-lives, whereas ΣPBDE3 has a different association with age because PBDEs were phased-out more recently (from 2004 to 2014) and have shorter biological half-lives. Quinn and Wania’s work suggested that positive associations between POP levels and age in cross-sectional studies might emerge 30 years following the peak exposure for chemicals with half-lives longer than one year, and these are consistent with our findings. A U-shaped association found between PBDE and age was also found in NHANES (Figure 3 & 4) and the other populations, and the higher concentrations among younger age participants might be due to lifestyle and activity differences (Sjödin et al., 2008; Turyk et al., 2010; Lenters et al., 2013).
We found higher serum PBB-153 levels for males, which has also been seen in NHANES (Sjödin et al., 2008), but this sex-specific difference was not significantly found in the model that included those born after the incident. This finding could be due to the potential occupational PBB exposure among males. The exponentiated sex coefficient reduced from 2.34 (CI = 1.88-2.92) to 2.27 (CI = 1.81-2.83) after including exposure groups in the model for those born before the incident began [model not shown], indicating that the sex-specific difference of serum levels can partially be attributed to exposure groups. Moreover, the males in this registry might have been exposed to higher PBBs in each exposure group. For example, the males in the chemical worker group might have worked more closely with the chemicals than the females; similarly, the male farmers might have handled contaminated feed more often than the females. However, the interaction between exposure groups and sex was not significant in the PBB-153 models, which could be partially attributed to the small sample size of females in the chemical worker group (N=11). Males also tended to have higher overall PCB levels than females, but a one-year increase in age was associated with higher serum PCB among females than males. These results suggest that older females had higher PCB levels than older males, which was also found in a previous study (Pavuk et al., 2014). We observed congener-specific sex differences for PCB levels among the older participants: females had higher serum levels of the lower chlorinated PCB congener (i.e., PCB-118), whereas males had higher concentrations of the higher chlorinated PCB congener (i.e., PCB-180). These effects might be related to the differences in the biological half-lives of PCBs (Salihovic et al., 2012). The differences between serum POP levels and sex is also likely attributable to lactation, pregnancy history among females, the difference in body physiology and food choices (Salihovic et al., 2012).
The serum PBB-153 concentrations were significantly higher among chemical workers, family members of chemical workers, and farm/food family than other Michigan residents in the adjusted models. Differences between exposure groups were also shown in previous studies around the 1980s. Landrigan et al. (1979) showed that workers and their families had higher median serum PBB levels (4.5 ng/mL) than the people who lived on quarantined farms (4 ng/mL), followed by low-level farm residents (2 ng/mL), and control participants and volunteers (1 ng/mL) (N=3,639). Stross et al. (1981) reported arithmetic means of 48 ng/mL, 14 ng/mL and 2 ng/mL for chemical workers, farmers, and the staff in the Michigan Department of Public Health (N=60), respectively. In our study, we found median PBB-153 levels of 0.76 ng/mL, 0.44 ng/mL, 0.47 ng/mL, and 0.26 ng/mL for chemical workers, family members of chemical workers, farm/food family and other Michigan residents, respectively. These results show that PBB-153 serum levels remain relatively high among the original highly-exposed groups. In addition, Blanck et al., (2000) showed that the people with higher initial PBB levels had slower decay rates and longer PBB biological half-lives, which may partially explain the difference between the exposure groups. It is also important to note that even Michigan residents not belonging to one of the highly-exposed groups, had higher levels of PBB indicating widespread distribution of contaminated farm products.
We observed negative associations between serum POP levels and BMI after adjusting for sex, sampling age and serum lipids, although a borderline significance was only found in the PBB-153 models and non-significance was found in the PCB and PDBE models. Inverse associations have been found in some biomonitoring studies suggesting the dilution of serum POP concentrations due to higher BMI (Chevrier et al., 2000; Barr et al., 2006; Darrow et al., 2016; Lenters et al., 2013). However, the relationship between serum POP levels and BMI has been inconsistent in cross-sectional studies, which might relate to different age groups being studied, cohort effects (obesity epidemic) and period effects (time after the peak exposure) (Wood et al., 2016). Smoking was also negatively associated with serum PBB-153 in the born after model, and the negative effect might be due to the faster PBB decay rates among smokers than non-smokers (Terrell et al., 2008). However, inconsistent or insignificant effects of smoking were found in the other models. Previous research suggests that smoking can lead to bioaccumulation of PCBs through affecting p-450 cytochrome oxidase system but some other metabolizing action may be triggered when smoking frequencies increase; thus, inconsistent associations might be reported frequently due to the lack of data on smoking amount and variability of individual metabolism (Moon et al., 2017).
For the self-reported exposure question, residence between 1973-1974, the PBB-153 concentrations were significantly higher in the people who said they lived on a quarantined farm (10β=1.56, CI = 1.10-2.20) compared to those who were not living on a farm, which suggests that the contaminated farms were a major exposure source in this study population. Moreover, the people who reported whether they consumed contaminated food products, had higher though not significant serum PBB-153 levels than those who reported no consumption of contaminated food products. The fact that the effects were only observed in the PBB models but not PCB or PBDE suggests that contaminated food consumption contributes to PBB exposure in this population. However, adjusted or stratified analyses including parity and breastfeeding were not conducted due to the small sample sizes within parity and breastfeeding strata.
Our results show generally higher average values and also wider ranges of serum POP levels than NHANES among different demographic groups. Serum PBB-153 had the most substantial difference between the PBB registry and NHANES. About 60% of the PBB-153 concentrations in our study are higher than the 95th percentile in 2003-2004 NHANES. Although the people born after the incident began were suspected to have no direct exposure to PBBs, their serum PBB-153 concentrations were higher than similarly aged participants in the more recent NHANES data (from ages 7 to 41 years old in Figure 4). In contrast to the PBB exposure, which was the result of the industrial accident, PCB and PBDE exposures are more likely to be chronic and continuous. The higher concentrations of serum PCBs and PBDE-47 in this registry might relate to the historical chemical production, higher environmental pollution or the consumption of contaminated fish or meat within this geographic area (ATSDR, 2000; ASTDR, 2017). Moreover, a greater increase was observed for PBDE-47 among the older participants in this study than NHANES, which could be due to the differences in predominant exposure pathways between older and younger participants. Although household dust is considered the predominant exposure route for PBDE, a recent study in California found continuing increases in PBDE levels in older adults during a time period when PBDE in household dust was declining. This suggests that biomagnification in the food chain and food ingestion may have a greater impact on older individuals (Hurley et al., 2017). Since higher contamination in the food chain was found in the Great Lakes basin area, greater concentration differences might be found among the older than the younger population.
Although low levels of PBB-153 were found in the general population after the chemical was phased out, the population near the contaminated farms and the original chemical plant may have still had low continuing exposure. According to the superfund site evaluation of the US Environmental Protection Agency, the human exposure has not been under control within the area, which means an unsafe level of contamination has been detected at the Velsicol Chemical Corp St. Louis, Michigan Superfund site (the main plant site that produced PBBs), and people could be exposed to the hazardous chemicals, such as dichlorodiphenyltrichloroethane (DDT), PBBs, hexabromobenzene, 1,2-dibromo-3-chloropropane and chlorobenzene (EPA, 2019). From 2013 to 2014, a study showed that the ambient levels of PBBs increased when the distance from the Velsicol Superfund site decreased (Peverly et al., 2014). The sediment PBB-153 concentrations also decreased from the Pine River (surrounding the plant) to the upper reaches of the Saginaw River (downstream of the Pine River) (Yun et al., 2008).
Our results highlight several profound impacts of this historical contamination. The people who were directly exposed continue to have significantly higher chemical body burdens over 40 years after the incident. These lipophilic chemicals can sustainably be stored in lipid-rich tissues and be gradually released into the bloodstream; thus, the tissues storing POPs constitute internal sources resulting in continuous exposure (La Merrill et al., 2012). It is evident that PBBs can transfer intergenerationally through the placenta or breastfeeding. One-third of PBB concentrations in maternal blood were detected in umbilical cord blood, and the concentrations detected in breast milk were 1000 times higher than maternal blood (Jacobson, 1984; Joseph et al., 2009). Thus, the fetuses or infants of exposed mothers can not only have substantial exposure but greater health risks because of the exposure during developmental periods (Small et al., 2009; Blanck et al., 2000a; Small et al., 2011; Terrell et al., 2015). Even when the offspring were not directly exposed to the chemicals, the epigenetic marks associated with environmental exposure may be transmitted to the next generation (Anway et al., 2005; Curtis et al, 2018). Moreover, even low exposure might be concerning due to the non-monotonic response and the low-dose effects of POPs (Li et al., 2007; Vandenberg et al., 2012; Kim et al., 2014). Taken together, we suggest that continuing exposure assessment, as well as health surveillance, are needed for the people in this registry, even long after the major contamination period ended.
Our study is limited in several ways. First, the small number of individuals below 20 years old led to wider confidence intervals and unstable point estimates for serum POP levels within this subgroup when compared to NHANES data. Second, there is the potential for selection bias. The people who knew they had higher PBB levels might have been more likely to participate in this study. While these may have little impact on the internal validity of the associations between serum PBB levels and the predictors, the point estimates (i.e., geometric and arithmetic means) may be biased upward, and the biases might affect the comparisons with NHANES. Although the information on the participation rate among different exposure groups was not available, only 18% (N=113/624) of participants recalled receiving information about their previous PBB levels. As a sensitivity analysis, we excluded the people who knew their previous PBB levels, and the point estimates of current PBB-153 serum level were somewhat lower than the entire study sample but still higher than NHANES (data not shown). Moreover, potential survival bias might also exist in this study population because PBB exposure has been associated with major cancers such as digestive system cancers, lymphoma, and breast cancer (Hoque et al., 1998, Terrell et al, 2016), and those surviving to participate 40 years later may bias the point estimates downward. Lastly, we were unable to examine how parity and breastfeeding associated with serum POP levels due to the small sample size of women with this information. The other potential predictors for serum POP levels, such as the amount of fish consumption, hours spent indoors, frequent use of household electronics, and other environmental determinants were not available for consideration.
Conclusion
This is the first comprehensive report of the current serum concentrations of PBBs, PCBs, and PBDEs among different exposure groups in the PBB Registry 40 years after the contamination incident. We found serum PBB-153 levels among the highly-exposed groups (chemical workers, family members of chemical workers and farm/food family) remain higher than in among other Michigan residents after several decades. Even those without documented exposure to PBB still have higher concentrations than the general US population. For the people born after the incident began, the serum concentrations were also higher than in the US population when compared to those of similar age ranges, which may suggest the transplacental/breastfeeding exposure potentially coupled with continued environmental exposure in the state. Higher serum PCB and PBDE levels were also found in this registry compared to the general US population, within certain demographic groups, which may relate to the generally higher environmental exposures of POPs in this geographic area.
Supplementary Material
Highlight.
The people in the Michigan PBB Registry still have higher serum PBB-153 levels compared to the general US population 40 years after the contamination incident.
The people born after the contamination incident began also have higher average serum PBB-153 levels compared to the general US population (of the same age group and similar sampling year).
Serum PBB-153 levels among the former chemical workers, family of former chemical workers, and people who lived on PBB-contaminated farms or received food from contaminated farms remain higher than other Michigan residents. All groups of Michigan residents tested have higher PBB-153 serum levels than the US population as a whole.
Some demographic groups within the PBB Registry have higher serum PCB and PBDE levels compared to the general US population.
Acknowledgments
We are grateful to the members of the Michigan PBB Registry for their participation and engagement with the research studies over the past 40 years, to the Michigan Department of Health and Human Services, which had the foresight to create the registry, and to our community partners (PBB Citizen Advisory Board, Pine River Superfund Citizen Task Force, and the Mid-Michigan District Health Department) who continue to provide guidance and insight to the Michigan PBB Research Team.
This work was supported by the National Institute of Environmental Health Sciences (NIEHS) research grants [R01ES012014, R01ES025775, R24ES028528, and R21ES023927]; NIEHS training grant [T32ES012870]; NIEHS center grant [P30ES019776]; and also the US Centers for Disease Control and Prevention.
Abbreviation footnote
- GM
geometric mean
- GSD
geometric standard deviation
- LOD
limits of detection
- NHANES
the National Health and Nutrition Examination Survey
- PBB
polybrominated biphenyl
- PBDE
polybrominated diphenyl ether
- PCB
polychlorinated biphenyl
- POP
persistent organic pollutant
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
✓ The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Agency for Toxic Substance and Disease Registry (ATSDR). Toxicological profile for polybrominated biphenyl (2004). Available from: https://www.atsdr.cdc.gov/toxprofiles/tp68.pdf. [PubMed]
- Agency for Toxic Substance and Disease Registry (ATSDR). Toxicological profile for polychlorinated biphenyls (2000). Available from: https://www.atsdr.cdc.gov/ToxProfiles/tp17.pdf. [PubMed]
- Agency for Toxic Substance and Disease Registry (ATSDR). Toxicological profile for polybrominated diphenyl ethers (2017). Available from: https://www.atsdr.cdc.gov/toxprofiles/tp207.pdf. [PubMed]
- Anderson HA, & Wolff MS (2000). Environmental contaminants in human milk. Journal of Exposure Science and Environmental Epidemiology, 10(S6), 755. [DOI] [PubMed] [Google Scholar]
- Anderson HA, Wolff MS, Fischbein A, & Selikoff IJ (1978). Investigation of the health status of Michigan chemical corporation employees. Environmental health perspectives, 23, 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahn AK, Mills JL, Snyder PJ, Gann PH, Houten L, Bialik O, Hollmann L, & Utiger RD (1980). Hypothyroidism in workers exposed to polybrominated biphenyls. New England Journal of Medicine, 302(1), 31–33. [DOI] [PubMed] [Google Scholar]
- Barr DB, Weihe P, Davis MD, Needham LL, & Grandjean P (2006). Serum polychlorinated biphenyl and organochlorine insecticide concentrations in a Faroese birth cohort. Chemosphere, 62(7), 1167–1182. [DOI] [PubMed] [Google Scholar]
- Blanck HM, Marcus M, Tolbert PE, Rubin C, Henderson AK, Hertzberg VS, Zhang RH & Cameron L (2000a). Age at menarche and tanner stage in girls exposed in utero and postnatally to polybrominated biphenyl. Epidemiology, 641–647. [DOI] [PubMed] [Google Scholar]
- Blanck HM, Marcus M, Hertzberg V, Tolbert PE, Rubin C, Henderson AK, & Zhang RH (2000b). Determinants of polybrominated biphenyl serum decay among women in the Michigan PBB cohort. Environmental Health Perspectives, 108(2), 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter LJ (1976). Michigan's PBB incident: Chemical mix-up leads to disaster. Science, 192(4236): 240–243. [DOI] [PubMed] [Google Scholar]
- Centers for Diseases Control and Prevention (CDC). Fourth National Report on Human Exposure to Environmental Chemicals (2019). Available from: https://www.cdc.gov/exposurereport/pdf/FourthReport_UpdatedTables_Volume1_Jan2019-508.pdf.
- Chevrier J, Dewailly E, Ayotte P, Mauriege P, Despres JP, & Tremblay A (2000). Body weight loss increases plasma and adipose tissue concentrations of potentially toxic pollutants in obese individuals. International journal of obesity, 24(10), 1272. [DOI] [PubMed] [Google Scholar]
- Curtis SW, Terrell ML, Jacobson MH, Cobb DO, Jiang VS, Neblett MF, Gerkowicz SA, Spencer JB, Marder ME, Barr DB, Conneely KN, Smith AK, & Marcus M (2019a). Thyroid hormone levels associate with exposure to polychlorinated biphenyls and polybrominated biphenyls in adults exposed as children. Environmental Health, 18(1), 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis SW, Cobb DO, Kilaru V, Terrell ML, Kennedy EM, Marder ME, Barr DB, Marsit CJ, Marcus M, Conneely KN, & Smith AK (2019b). Exposure to polybrominated biphenyl (PBB) associates with genome-wide DNA methylation differences in peripheral blood. Epigenetics, 14(1), 52–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis SW, Cobb DO, Kilaru V, Terrell ML, Marder ME, Barr DB, Marsit CJ, Marcus M, Conneely KN, & Smith AK (2019c). Exposure to polybrominated biphenyl and stochastic epigenetic mutations: application of a novel epigenetic approach to environmental exposure in the Michigan polybrominated biphenyl registry. Epigenetics, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis SW, Cobb DO, Kilaru V, Terrell ML, Marder ME, Barr DB, Marsit CJ, Marcus M, Conneely KN, & Smith AK (2019d). Environmental exposure to polybrominated biphenyl (PBB) associates with an increased rate of biological aging. Aging (Albany NY), 11(15), 5498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis SW, Conneely KN, Marder ME, Terrell ML, Marcus M, & Smith AK (2018). Intergenerational effects of endocrine-disrupting compounds: a review of the Michigan polybrominated biphenyl registry. Epigenomics, 10(6), 845–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrow LA, Jacobson MH, Preston EV, Lee GE, Panuwet P, Hunter RE Jr, Marder ME, Marcus M & Barr DB (2016). Predictors of serum polybrominated diphenyl ether (PBDE) concentrations among children aged 1–5 years. Environmental science & technology, 51(1), 645–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries GF, & Kimbrough RD (1985). The PBB episode in Michigan: an overall appraisal. CRC critical reviews in toxicology, 16(2), 105–156. [DOI] [PubMed] [Google Scholar]
- Geyer HJ, Schramm KW, Darnerud PO, Aune M, Feicht EA, Fried KW, Henkelmann B, Lenoir D, Schmid P & McDonald TA (2004). Terminal elimination half-lives of the brominated flame retardants TBBPA, HBCD, and lower brominated PBDEs in humans. Organohalogen compounds, 66(2004), 3820–3825. [Google Scholar]
- Howards PP, Terrell ML, Jacobson MH, Taylor KC, Kesner JS, Meadows JW, Spencer JB, Manatunga AK, Marcus M Polybrominated Biphenyl Exposure and Menstrual Cycle Function. Epidemiology. 2019. 30(5):687–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoque A, Sigurdson AJ, Burau KD, Humphrey HE, Hess KR, & Sweeney AM (1998). Cancer among a Michigan cohort exposed to polybrominated biphenyls in 1973. Epidemiology, 373–378. [PubMed] [Google Scholar]
- Hovander L, Athanasiadou M, Asplund L, Jensen S, & Wehler EK (2000). Extraction and cleanup methods for analysis of phenolic and neutral organohalogens in plasma. Journal of analytical toxicology, 24(8), 696–703. [DOI] [PubMed] [Google Scholar]
- Hurley S, Goldberg D, Nelson DO, Guo W, Wang Y, Baek HG, Park J, Petreas M., Bernstein L., Anton-Culver H., & Reynolds P. (2017). Temporal evaluation of Polybrominated Diphenyl Ether (PBDE) serum levels in middle-aged and older California women, 2011–2015. Environmental science & technology, 51(8), 4697–4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson MH, Darrow LA, Barr DB, Howards PP, Lyles RH, Terrell ML, Smith AK, Conneely KN, Marder ME & Marcus M (2017). Serum polybrominated biphenyls (PBBs) and polychlorinated biphenyls (PCBs) and thyroid function among Michigan adults several decades after the 1973–1974 PBB contamination of livestock feed. Environmental health perspectives, 125(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson JL, Fein GG, Jacobson SW, Schwartz PM, & Dowler JK (1984). The transfer of polychlorinated biphenyls (PCBs) and polybrominated biphenyls (PBBs) across the human placenta and into maternal milk. American Journal of Public Health, 74(4), 378–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph AD, Terrell ML, Small CM, Cameron LL, & Marcus M (2009). Assessing intergenerational transfer of a brominated flame retardant. Journal of Environmental Monitoring, 11(4), 802–807. [DOI] [PubMed] [Google Scholar]
- Kim YR, Harden FA, Toms LML, & Norman RE (2014). Health consequences of exposure to brominated flame retardants: a systematic review. Chemosphere, 106, 1–19. [DOI] [PubMed] [Google Scholar]
- La Guardia MJ, Hale RC, & Harvey E (2006). Detailed polybrominated diphenyl ether (PBDE) congener composition of the widely used penta-, octa-, and deca-PBDE technical flame-retardant mixtures. Environmental science & technology, 40(20), 6247–6254. [DOI] [PubMed] [Google Scholar]
- La Merrill M, Emond C, Kim MJ, Antignac JP, Le Bizec B, Clément K, Barouki R, & Barouki R (2012). Toxicological function of adipose tissue: focus on persistent organic pollutants. Environmental health perspectives, 121(2), 162–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landrigan PJ, Wilcox KR Jr, Silva J Jr, Humphrey HE, Kauffman C, & Heath CW Jr (1979). Cohort study of Michigan residents exposed to polybrominated biphenyls: epidemiologic and immunologic findings. Annals of the New York Academy of Sciences, 320(1), 284–294. [DOI] [PubMed] [Google Scholar]
- Lenters V, Thomsen C, Smit LA, Jönsson BA, Pedersen HS, Ludwicki JK, Zviezdai V, Piersma AH, Toft G, Bonde JP, Becher G, Vermeulen R & Becher G (2013). Serum concentrations of polybrominated diphenyl ethers (PBDEs) and a polybrominated biphenyl (PBB) in men from Greenland, Poland and Ukraine. Environment international, 61, 8–16. [DOI] [PubMed] [Google Scholar]
- Li L, Andersen ME, Heber S, & Zhang Q (2007). Non-monotonic dose–response relationship in steroid hormone receptor-mediated gene expression. Journal of molecular endocrinology, 38(5), 569–585. [DOI] [PubMed] [Google Scholar]
- Lubin JH, Colt JS, Camann D, Davis S, Cerhan JR, Severson RK, Bernstein L, & Hartge P (2004). Epidemiologic evaluation of measurement data in the presence of detection limits. Environmental health perspectives, 112(17), 1691–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder ME, Panuwet P, Hunter RE, Ryan PB, Marcus M, & Barr DB (2016). Quantification of polybrominated and polychlorinated biphenyls in human matrices by isotope-dilution gas chromatography–tandem mass spectrometry. Journal of analytical toxicology, 40(7), 511–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon HJ, Lim JE, & Jee SH (2017). Association between serum concentrations of persistent organic pollutants and smoking in Koreans: A cross-sectional study. Journal of epidemiology, 27(2), 63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nøst TH, Breivik K, Fuskevåg OM, Nieboer E, Odland JØ, & Sandanger TM (2013). Persistent organic pollutants in Norwegian men from 1979 to 2007: intraindividual changes, age–period–cohort effects, and model predictions. Environmental health perspectives, 121(11–12), 1292–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuk M, Olson JR, Wattigney WA, Dutton ND, Sjödin A, Shelton C, … Cash J. (2014). Predictors of serum polychlorinated biphenyl concentrations in Anniston residents. Science of the Total Environment, 496, 624–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peverly AA, Salamova A, & Hites RA (2014). Air is still contaminated 40 years after the Michigan chemical plant disaster in St. Louis, Michigan. Environmental science & technology, 48(19), 11154–11160. [DOI] [PubMed] [Google Scholar]
- Phillips DL, Pirkle JL, Burse VW, Bernert JT, Henderson LO, & Needham LL (1989). Chlorinated hydrocarbon levels in human serum: effects of fasting and feeding. Archives of environmental contamination and toxicology, 18(4), 495–500. [DOI] [PubMed] [Google Scholar]
- Quinn CL, & Wania F (2012). Understanding differences in the body burden–age relationships of bioaccumulating contaminants based on population cross sections versus individuals. Environmental health perspectives, 120(4), 554–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffetti E, Speziani F, Donato F, Leonardi L, Orizio G, Scarcella C, Apostoli P, & Magoni M (2017). Temporal trends of polychlorinated biphenyls serum levels in subjects living in a highly polluted area from 2003 to 2015: a follow-up study. International journal of hygiene and environmental health, 220(2), 461–467. [DOI] [PubMed] [Google Scholar]
- Robson MG, & Toscano WA (Eds.). (2007). Risk assessment for environmental health (Vol. 2). John Wiley & Sons. [Google Scholar]
- Salihovic S, Lampa E, Lindström G, Lind L, Lind PM, & van Bavel B (2012). Circulating levels of persistent organic pollutants (POPs) among elderly men and women from Sweden: results from the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS). Environment international, 44, 59–67. [DOI] [PubMed] [Google Scholar]
- Sandau CD, Sjödin A, Davis MD, Barr JR, Maggio VL, Waterman AL, … Patterson DG (2003). Comprehensive solid-phase extraction method for persistent organic pollutants. Validation and application to the analysis of persistent chlorinated pesticides. Analytical chemistry, 75(1), 71–77. [DOI] [PubMed] [Google Scholar]
- Sjödin A, Jones RS, Caudill SP, Wong LY, Turner WE, & Calafat AM (2013). Polybrominated diphenyl ethers, polychlorinated biphenyls, and persistent pesticides in serum from the National Health and Nutrition Examination Survey: 2003–2008. Environmental science & technology, 48(1), 753–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjödin A, Wong LY, Jones RS, Park A, Zhang Y, Hodge C, DiPietro E, McClure C, Turner W, Needham LL & Patterson DG (2008). Serum concentrations of polybrominated diphenyl ethers (PBDEs) and polybrominated biphenyl (PBB) in the United States population: 2003–2004. Environmental science & technology, 42(4), 1377–1384. [DOI] [PubMed] [Google Scholar]
- Small CM, DeCaro JJ, Terrell ML, Dominguez C, Cameron LL, Wirth J, & Marcus M (2009). Maternal exposure to a brominated flame retardant and genitourinary conditions in male offspring. Environmental health perspectives, 117(7), 1175–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small CM, Murray D, Terrell ML, & Marcus M (2011). Reproductive outcomes among women exposed to a brominated flame retardant in utero. Archives of environmental & occupational health, 66(4), 201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stross JK, Smokler IA, Isbister J, & Wilcox KR (1981). The human health effects of exposure to polybrominated biphenyls. Toxicology and applied pharmacology, 58, 145–150. [DOI] [PubMed] [Google Scholar]
- Terrell ML, Hartnett KP, Lim H, Wirth J, & Marcus M (2015). Maternal exposure to brominated flame retardants and infant Apgar scores. Chemosphere, 118, 178–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrell ML, Manatunga AK, Small CM, Cameron LL, Wirth J, Blanck HM, Lyles RH, & Marcus M (2008). A decay model for assessing polybrominated biphenyl exposure among women in the Michigan Long-Term PBB Study. Journal of Exposure Science and Environmental Epidemiology, 18(4), 410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrell ML, Rosenblatt KA, Wirth J, Cameron LL, & Marcus M (2016). Breast cancer among women in Michigan following exposure to brominated flame retardants. Occup Environ Med, 73(8), 564–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turyk ME, Anderson HA, Steenport D, Buelow C, Imm P, & Knobeloch L (2010). Longitudinal biomonitoring for polybrominated diphenyl ethers (PBDEs) in residents of the Great Lakes basin. Chemosphere, 81(4), 517–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Environmental Protection Agency (EPA). Superfund site: Velsicol Chemical Corp. St. Louis, Michigan: (2019). Available from: https://cumulis.epa.gov/supercpad/SiteProfiles/index.cfm?fuseaction=second.Healthenv&id=0502194 [Google Scholar]
- Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR Jr, Lee DH, … Zoeller RT (2012). Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocrine reviews, 33(3), 378–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SA, Xu F, Armitage JM, & Wania F (2016). Unravelling the relationship between body mass index and polychlorinated biphenyl concentrations using a mechanistic model. Environmental Science & Technology, 50(18), 10055–10064. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO). (2016). IARC monographs on the evaluation of carcinogenic risks to humans Polychlorinated Biphenyls and Polybrominated Biphenyls. Vol. 107. [PMC free article] [PubMed] [Google Scholar]
- Yun SH, Addink R, McCabe JM, Ostaszewski A, Mackenzie-Taylor D, Taylor AB, & Kannan K (2008). Polybrominated diphenyl ethers and polybrominated biphenyls in sediment and floodplain soils of the Saginaw River watershed, Michigan, USA. Archives of environmental contamination and toxicology, 55(1), 1–10. [DOI] [PubMed] [Google Scholar]
- Zhang Z, & Rhind SM (2011). Optimized determination of polybrominated diphenyl ethers and polychlorinated biphenyls in sheep serum by solid-phase extraction–gas chromatography–mass spectrometry. Talanta, 84(2), 487–493. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.