Abstract
Herbivory is one of the most globally distributed disturbances affecting carbon (C)-cycling in trees, yet our understanding of how it alters tree C-allocation to different functions such as storage, growth or rhizodeposition is still limited. Prioritized C-allocation to storage replenishment vs growth could explain the fast recovery of C-storage pools frequently observed in growth-reduced defoliated trees. We performed continuous 13C-labeling coupled to clipping to quantify the effects of simulated browsing on the growth, leaf morphology and relative allocation of stored vs recently assimilated C to the growth (bulk biomass) and non-structural carbohydrate (NSC) stores (soluble sugars and starch) of the different organs of two tree species: diffuse-porous (Betula pubescens Ehrh.) and ring-porous (Quercus petraea [Matt.] Liebl.). Carbon-transfers from plants to bulk and rhizosphere soil were also evaluated. Clipped birch and oak trees shifted their C-allocation patterns above-ground as a means to recover from defoliation. However, such increased allocation to current-year stems and leaves did not entail reductions in the allocation to the rhizosphere, which remained unchanged between clipped and control trees of both species. Betula pubescens and Q. petraea showed differences in their vulnerability and recovery strategies to clipping, the ring-porous species being less affected in terms of growth and architecture by clipping than the diffuse-porous. These contrasting patterns could be partly explained by differences in their C cycling after clipping. Defoliated oaks showed a faster recovery of their canopy biomass, which was supported by increased allocation of new C, but associated with large decreases in their fine root biomass. Following clipping, both species recovered NSC pools to a larger extent than growth, but the allocation of 13C-labeled photo-assimilates into storage compounds was not increased as compared with controls. Despite their different response to clipping, our results indicate no preventative allocation into storage occurred during the first year after clipping in either of the species.
Keywords: below-ground allocation, Betula pubescens, carbon (C) allocation, C-storage, δ13C stable isotopes, non-structural carbohydrates, Quercus petraea
Introduction
Trees account for ca 90% of the global biomass of carbon (C) (Körner 2003) and, hence, play a fundamental role in global C dynamics. Carbon-allocation patterns in trees may shift depending on multiple factors, including age (Hartmann et al. 2018), phenology (Palacio et al. 2018), environmental conditions such as water and light availability or temperature (Messier and Nikinmaa 2000, Domisch et al. 2001, Weber et al. 2019), and disturbances (Canham et al. 1994, Van der Heyden and Stock 1995). Carbon-allocation within the tree biomass may determine tree vulnerability to environmental stress and disturbance (Canham et al. 1999, Wiley and Helliker 2012). Furthermore, C-allocation to different functions such as storage, growth, reproduction or rhizodeposition may affect the amount of C cycled and sequestered by trees (Hartmann et al. 2018). Understanding the response of tree C-allocation patterns to different factors may be crucial to predict the response of trees to global change (Körner 2003, Wiley and Helliker 2012).
Herbivory is one of the most globally distributed disturbances affecting C-cycling patterns in trees (Clark et al. 2010). Defoliation by herbivores reduces canopy leaf area causing a decrease in the net C gain of trees by current photosynthesis and altering the balance between C sinks and sources (Trumble et al. 1993). This may lead to important changes in C-allocation patterns, which can influence the environment by changes in below-ground C inputs (Pinkard and Beadle 1998, Eyles et al. 2009). Depending on the severity of damage, the C demands of growing sinks may be supplied temporarily from storage (Van der Heyden and Stock 1995, Pinkard et al. 1998, Quentin et al. 2011), namely non-structural carbohydrates (NSC) and lipids, some of which can be mobilized to support growth or other plant functions (Chapin et al. 1990). Accordingly, several studies have reported a decrease in starch pools after defoliation in deciduous (Canham et al. 1994, Van der Heyden and Stock 1995, Kosola et al. 2001) and evergreen tree species (Ericsson et al. 1985, Fierravanti et al. 2019). However, trees are able to compensate to some degree for loss of foliage by changing allocation patterns (e.g. favoring foliage production), upregulating photosynthesis and changing leaf morphology (Pinkard and Beadle 1998, Fuenzalida et al. 2019). Recovery from light defoliation is considered to rely mainly on current photo-assimilates produced by surviving foliage (Barry et al. 2011) and does not normally cause a significant decrease in NSC pools (Tschaplinski and Blake 1994, Van der Heyden and Stock 1995). In the case of moderate or severe defoliation, decreases in NSC concentrations tend to be transient and of short duration, becoming non-significant over the course of a growing season, while the effects on tree growth seem to be more long-lasting (Palacio et al. 2008, Piper et al. 2015, Puri et al. 2015). Sometimes defoliated trees show even higher NSC concentrations than undefoliated controls (Palacio et al. 2012, Piper et al. 2015).
The differential dynamics of growth vs recovery of NSC pools in defoliated trees have been interpreted in relation to two, non-exclusive processes: (i) a C-sink limitation to growth due to reductions in the numbers of buds, limiting levels of non-C reserves, hormonal changes or allometric adjustments in response to reduced leaf area, leading to surplus-C being allocated to storage (Palacio et al. 2008, 2012, Piper et al. 2015, Puri et al. 2015, Schmid et al. 2017); and (ii) a preventative prioritized C-allocation to storage over growth, ultimately leading to C-limitation (Wiley and Helliker 2012, Wiley et al. 2013, 2017a, Piper et al. 2015, Puri et al. 2015). Preferential allocation of C to NSC over growth has recently been demonstrated in C-starved plants subjected to prolonged shading (Weber et al. 2019) or complete darkness (Weber et al. 2018). Nevertheless, its occurrence in other potentially C-limiting conditions such as defoliation remains equivocal (Wiley et al. 2013, 2017a).
In addition to changes in C-allocation among tree organs, defoliation can induce shifts in C-transfers below-ground, influencing soil microbial communities (Bardgett and Wardle 2003, but see Barto and Rillig 2010) and nutrient cycling (Ayres et al. 2004). Approximately 50% of the C produced by woody plants is allocated below-ground, either directly to the roots, or as rhizodeposition of C exudates from roots to the surrounding soil (Giardina et al. 2005). Defoliation can reduce below-ground C-allocation by enhancing fine root mortality, particularly in trees (Tuomi et al. 1990, Bryant et al. 1993, Vanderklein and Reich 1999, but see Kosola et al. 2001, Endrulat et al. 2016). In contrast, in herbaceous plants, herbivory can increase short-term allocation of C below-ground (Orians et al. 2011), as has also been found for Populus spp. (Babst et al. 2005). Defoliation has been demonstrated to elicit short-term increases in the flux of C to root exudates in grasses (Paterson et al. 2005), while the detection of effects in woody species remains elusive (Ayres et al. 2004, Frost and Hunter 2008). In general, there is a lack of information related to below-ground responses of woody plants to defoliation.
Differences in wood anatomy have also been assumed to entail differences in C-allocation dynamics (Barbaroux and Bréda 2002), with putative consequences on the response of trees to defoliation (Foster 2017). Ring-porous species complete the part of earlywood growth (including large earlywood vessel formation) before bud burst in spring (Dougherty et al. 1979). This phenology is putatively a result of winter embolism of large-diameter vessels, and the need to produce a new set of xylem vessels prior to bud burst to supply newly emerging leaves with water (Lechowicz 1984). Contrastingly, diffuse-porous species have only small xylem vessels and winter embolism has relatively less impact on the hydraulic conductivity of the tree, so leaf expansion can proceed using xylem formed in the previous growing season, without the need to produce new radial stem growth (Lechowicz 1984). The consequence for C-cycling is that ring-porous species show greater seasonal variations in NSC pools and concentrations, and a relatively greater dependence on the remobilization of stored NSC for earlywood growth in spring, than diffuse-porous species (Barbaroux and Bréda 2002, Barbaroux et al. 2003, but see Palacio et al. 2011). It has recently been suggested that these differences in C storage and allocation can underline potential differences between ring-porous and diffuse-porous species in the vulnerability to spring defoliation, the former being more resistant to defoliation owing to their larger C-stores and advanced wood growth phenology (Foster 2017). Nevertheless, to our knowledge, this possibility has never been explored experimentally.
We performed continuous 13C-labeling coupled to a clipping experiment to quantify the effects of simulated browsing on the relative allocation of stored and recently assimilated C to growth (bulk biomass) and NSC (soluble sugars [SSs] and starch) of the different organs of two tree species with contrasting wood anatomy: diffuse-porous downy birch (Betula pubescens Ehrh.) and ring-porous sessile oak (Quercus petraea [Matt.] Liebl.). Transfers from the plants to bulk (i.e. not in direct contact with tree roots) and rhizosphere soil were also evaluated. Clipping was selected to mimic the effects of browsing: a major factor hampering the regeneration of native forests worldwide (Hester et al. 2004, Gill 2006). The use of continuous 13C-labeling at close-to-ambient concentrations was chosen as a quantitative mean to separate current from stored C-assimilates, estimate C-allocation to different organs and C-compounds over the course of the growing season and track allocation below-ground, without the potential drawbacks of pulse-chase labeling (see Paterson et al. 2009). We hypothesized that clipping would lead to: (i) increased C-allocation above-ground vs below-ground (i.e. roots and the rhizosphere); (ii) increased allocation of new C into storage; and (iii) reduced growth and C storage, more noticeable in birch than in oak, owing to the ring-porous wood anatomy and subsequent larger storage C-pools of the latter (Foster 2017).
Materials and methods
Experimental set up
The experimental set up was the same as described in Palacio et al. (2011). In brief, in 2007, we applied two clipping treatments: control (unclipped) and clipped (i.e. 66% shoots removal on two consecutive dates: July and September 2007) to 2-year-old sessile oak (Q. petraea [Matt.] Liebl.) and downy birch (B. pubescens Ehrh.) saplings planted in pots at the James Hutton Institute, Aberdeen, UK (57°07′60.0″N 2°09′30.0″W) (Figure 1). In April 2008, before bud burst, five trees of each species and treatment were harvested to account for differences in biomass and NSC allocation in the short-term. At that same time, five extra trees of each species and treatment were moved into a polytunnel with altered δ13C air composition to take part in a continuous δ13C-labeling experiment, while five control trees of each species were left at the greenhouse to serve as ‘ambient’ trees. The aim of the continuous labeling was to separate newly fixed C from ‘old’ C. In August 2008, trees from the C-labeling experiment were harvested to evaluate differences in new C allocation to bulk biomass and NSC (SS and starch) between clipped and control trees of both species one year after clipping (Figure 1). ‘Ambient’ trees were harvested in November 2008 to provide natural abundance δ13C values of the different organs of both species to be used in calculations. Further details of these experimental procedures follow.
Figure 1.

Experimental design with the indication of the main treatments (clipping—red arrows, 13C-labeling), growth measurements (blue arrows) and harvests (black arrows) applied to B. pubescens and Q. petraea saplings.
Clipping experiment
Trees of both study species were lifted from a nursery while dormant (5 April 2007) and planted in 44 l pots filled with gravel at the bottom for drainage and freely drained soil derived from granite and granitic gneiss (Countesswells Association, Glentworth and Muir 1963). At planting, saplings were ~0.3–0.5 m height, a stage considered highly vulnerable to large herbivore browsing in nature (Hester et al. 1996, 2000, Gill 2006). After planting, saplings were moved into an unheated greenhouse and 20 trees of each species were randomly allocated into ‘clipped’ and ‘control’ treatments, leading to 10 replicates per species and treatment combination. Five extra trees per species were allocated to the ‘ambient’ group, which did not receive clipping or 13C-labeling. Trees were numbered and positioned in the greenhouse following a Latin square design. Between April and November 2007, soil was kept moist with tap water without exceeding field capacity and saplings received 0.5 l of a nutrient solution with 3.0 mol N m−3 as NH4NO3, 1.33 mol m−3 Na2 HPO4.12 H2O and 1 mol m−3 K2SO4 once per week, to remove any potential nutrient limitation to growth. A natural photoperiod was used and the greenhouse ventilated to provide temperatures close to ambient. To account for initial tree variability and avoid potential confounding effects on tree growth, morphological measurements (tree height, length, stem diameter and a number of short shoots and long shoots) were taken from every tree at planting and prior to each clipping and harvest.
Clipping was applied as in Palacio et al. (2011) by removing 66% of current-year shoots (two out of every three current-year shoots) in early July and early September 2007, after the first and second flushes of shoot growth were finished (Figure 1). This intensity of damage was selected to reproduce high densities of browsing animals (Speed et al. 2011). In birch, clipping treatments were designed to reproduce browsing damage by red deer or sheep by removing current-year long-shoots (including stems, buds and leaves) up to the maximum stem diameters normally eaten by red deer or sheep (Shipley et al. 1999). In oaks, clipped shoots were selected to ensure a decrease in total tree leaf area of ca 66% owing to the highly variable shoot length and fewer shoots of this species. While the use of clipping to simulate browsing has received some criticism (Baldwin 1990), woody plant responses to well-simulated damage do not differ significantly from responses to real herbivore damage (Bergman 2002, Hester et al. 2004).
Short-term effects of clipping on biomass and carbohydrate allocation
Twenty dormant saplings (five of each species and clipping treatment) were removed from their pots on 2 April 2008 and separated into: 1- and 2-year-old stems (i.e. stems formed in 2007 and 2006, respectively), woody stems (>2 years), coarse roots (>2-mm diameter) and fine roots (<2-mm diameter). Samples were freeze-dried and weighed (ca 0.005 mg) and then milled to a fine powder in a ball mill (Retsch Mixer MM301, Leeds, UK).
Soluble sugars were extracted with 80% (v/v) ethanol and their concentration determined using the phenol-sulfuric method as modified by Buysse and Merckx (1993). Starch and complex sugars remaining in the undissolved pellet after ethanol extractions were reduced (i.e. enzymatically) to glucose and analyzed as described in Palacio et al. (2007).
Continuous 13C labeling experiment
On 1 April 2008, five saplings from each species and clipping treatment combination were transferred to an aluminum and polythene tunnel, 2.4-m wide, 3.0-m long and 2.2-m height (Super 8 Hobby Tunnel, Northernpolytunnels, Colne, UK) as described previously (Palacio et al. 2011; Figure 1). At this time, trees were only just starting to break bud. The polytunnel was supplied with air having CO2 with a depleted 13C-signature (relative to atmospheric CO2), in order to differentiate current (new) from previous (old) plant assimilates (Nogués et al. 2004, Paterson et al. 2009). This was achieved by partially scrubbing CO2 from the air using a CO2-scrubber unit (Texol, Dundee, UK) that reduced the CO2 concentration to 74–103 μmol mol−1. The scrubbed air was then mixed with CO2 from a gas cylinder (BOC, Worsley, UK) with a δ13C-signature of −34.0%, using Brooks 580 s thermal mass flow controllers, interfaced with a Brooks control unit (both Flotech Solutions Ltd, Stockport, UK). Resulting CO2 concentrations inside the polytunnel averaged 332 p.p.m. and had an average δ13C of −21.4%. Temperature inside the polytunnel was checked regularly with a shielded thermometer. On 6 May 2008, we installed a shade mesh intercepting ~30% of the light on top of the polytunnel to reduce warming.
Trees were distributed within the polytunnel following a Latin square design, which was changed in the middle of the experiment. They regularly received 0.5 l of the same nutrient solution described above. We took initial and final tree morphological measurements (as described above) at the beginning and at the end of the 13C-labeling period. On 5 August 2008, 4 months after the beginning of the 13C-labeling, trees inside the polytunnel were harvested for analysis (Figure 1). At this time, leaf senescence was starting and most of the annual growth had been completed. Harvested trees were separated into: current-year (formed in 2008), 1- and 2-year-old stems (formed in 2007 and 2006, respectively), woody stems (>2 years), coarse roots (>2-mm diameter) and fine roots (<2-mm diameter). A 3-year-old branch was clipped off each tree to measure leaf area, individual leaf weight and specific leaf area according to the protocols in Cornelissen et al. (2003). Rhizosphere and bulk soil were harvested from each pot. Rhizosphere soil was collected by separating roots from the soil, gently shaking them and then submerging fine roots in distilled water. Samples were freeze-dried and weighed (ca 0.005 mg). Samples were milled to a fine powder in a ball mill (Retsch Mixer MM301, Leeds, UK) and analyzed for NSC as described above.
Ambient trees
In early November 2008, the five non-clipped, ‘ambient’, saplings of each species were harvested from the greenhouse for analysis of the δ13C at natural abundance in the bulk biomass of the same fractions considered for 13C-labeled trees. Throughout 2008, growth conditions for these trees were similar to those in 2007 (see above). At the time of harvest, trees were shedding their leaves and radial growth had been completed.
Carbon isotope analysis
The 13C signature of samples was measured by continuous flow isotope ratio mass spectrometer (Thermo Finnigan Delta Plusadvantage) interfaced to an elemental analyzer (Thermo FlashEA1112, Thermo Finnigan, Bremen, Germany). Data were expressed as δ13CV-PDB:
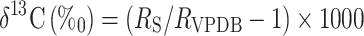 |
where RS and RVPDB are the molecular abundance ratios of carbon isotopes (13C/12C), of the sample and international standard (Vienna Pee Dee Belemnite), respectively. Long-term precision for quality control standards (milled flour) was δ13CV-PDB: −26.0 ± 0.24% (mean ± SD, n = 187). δ13C was measured in bulk plant biomass, bulk soil and rhizosphere soil. Measurements of δ13C in SS and starch followed compound-specific analyses as detailed below.
δ13C in NSC were measured by following the procedure in Tcherkez et al. (2003). In brief, leaf powder was suspended with 1 ml of distilled water in an Eppendorf tube (Eppendorf Scientific, Hamburg, Germany). After centrifugation, starch was extracted from the pellet by HCl solubilization. Soluble proteins of the supernatant were heat denatured and precipitated, and SS and organic acids of the protein-free extract were separated by high-performance liquid chromatography. After lyophilization, 200 mg of purified starch were weighted into tin capsules (Courtage Analyze Service, Mont Saint-Aignan, France) for isotope analysis. Determinations of δ13C in NSC were conducted at the Centres Científics i Tecnològics of the University of Barcelona using an elemental analyzer (EA1108, Series 1, Carbo Erba Instrumentazione, Milan, Italy) coupled to an isotope ratio mass spectrometer (Delta C, Finnigan, Mat., Bremen, Germany) operating in continuous flow mode. Data were expressed as indicated above.
Calculations
The proportion of newly assimilated C in the bulk biomass, SS and starch of the different fractions of trees grown in 13C-depleted conditions (FCnew) was calculated using the following equation (Nogués et al. 2004):
 |
where δ13CSample was the isotopic composition in the sample; δ13CAmbient was the natural baseline δ13C value for the bulk biomass, SS or starch of a given fraction of each species calculated from fractions collected from ambient trees in November 2008 (in the case of the bulk biomass) or, in the case of SS and starch, by applying an enrichment of 1.71 and 2.02, respectively, owing to values reported in the literature (Badeck et al. 2005); and δ13CGrass was the average of the δ13C values obtained for Lolium perenne plants that were grown from seed within the polytunnel and hence represented C arising from current assimilation. We assumed that the discrimination against 13C during photosynthesis would be the same in both the grasses and the trees, because the grasses were grown under the same conditions as the trees and had no water stress.
Statistical analyses
We used univariate general linear models (glm) to analyze differences in biomass and NSC-allocation between species, fractions and treatments. Short-term effects of simulated browsing on biomass and NSC-allocation of trees harvested in April 2008 (before the 13C-labeling experiment) and the effects of simulated browsing on tree growth, architecture, leaf morphology, biomass, NSC and % new C-allocation (in bulk biomass, SS and starch) and NSC concentrations in the year after clipping (trees harvested in August 2008) were evaluated by glms with species, fractions and treatments as fixed effects. Treatment effects were further tested within fractions and species using one-way ANOVAs. The initial length (i.e. distance from the base to the tip of the tree) of trees at the beginning of the experiment was included as a covariate in all analyses. Starch and NSC concentrations were angularly transformed to meet normality and homogeneity in variance assumptions. Analyses were done with SPSS Statistics 17.0.
Results
Short-term effects of clipping on biomass and NSC allocation
At the beginning of the labeling experiment (i.e. 7 months after the application of treatments), trees subjected to two successive clipping events were significantly smaller than control trees and showed a significant reduction in the biomass of the youngest shoot cohort, i.e. that formed in 2007 and directly affected by clipping, but larger main stem biomass (particularly in birch; Table 1 and Table S1 available as Supplementary Data at Tree Physiology Online). Trees were still dormant and leafless at this time, and no other significant differences were observed in the allocation to different tree fractions in either of the two species. Differences in allocation to different plant components were quantified between birch and oak trees: birch allocated significantly more biomass to above-ground fractions such as young and main stems, while oak had significantly more biomass in coarse roots (Table 1 and Table S1 available as Supplementary Data at Tree Physiology Online). However, no significant treatment × species interaction was found, indicating that clipping did not lead to a different response in biomass allocation between species in the short-term.
Table 1.
Biomass allocation to the different plant fractions in B. pubescens and Q. petraea trees harvested at the beginning of the δ13C-labeling experiment in April 2008 (i.e. at the beginning of the first growing season after clipping).
| Biomass allocation (% total plant biomass) | B. pubescens | Q. petraea | ||
|---|---|---|---|---|
| Clipped | Control | Clipped | Control | |
| 1-year-old stems | 2.4 (0.3) | 9.8 (1.6)** | 1.3 (0.3) | 4.8 (0.7)** |
| 2-year-old stems | 12.5 (2.8) | 14.3 (1.8) | 11.1 (2.0) | 11.8 (1.5) |
| Main stems | 30.3 (1.8) | 23.8 (2.0)* | 21.7 (1.5) | 18.6 (1.3) |
| Coarse roots | 25.0 (1.9) | 24.8 (2.0) | 39.6 (3.0) | 36.8 (1.6) |
| Fine roots | 16.0 (1.3) | 16.6 (3.7) | 10.0 (1.2) | 13.8 (0.9) |
Data are means and SE (in parentheses). Significant differences between treatments within species are indicated by asterisks: *P < 0.10, **P < 0.05, N = 5.
Clipped trees of both species harvested in April 2008, 7 months after the application of treatments, showed significantly higher starch concentrations in young stems (Tables S1 and S2 available as Supplementary Data at Tree Physiology Online). However, NSC pools were decreased in the youngest shoot cohort, showing the significant effect of clipping on the biomass reduction of this cohort for both species (Tables S1 and S3 available as Supplementary Data at Tree Physiology Online). Starch and SS pools in main stems were significantly larger in clipped trees of both species (Tables S1 and S3 available as Supplementary Data at Tree Physiology Online). This was not due to increased NSC concentrations (Tables S1 and S2 available as Supplementary Data at Tree Physiology Online) but to higher allocation of biomass to main stems in clipped trees (Table 1 and Table S1 available as Supplementary Data at Tree Physiology Online). Overall, birch trees had higher SS concentrations, while oaks showed up to three times higher starch concentrations, particularly in coarse roots (Tables S1 and S2 available as Supplementary Data at Tree Physiology Online).
Changes in growth, architecture, leaf morphology, biomass and NSC allocation 1 year after clipping
Measurements taken at the end of the first year after the application of clipping treatments (trees harvested in August 2008) indicated a lower compensating ability in birch than in oak (Table 2). Although the effect of clipping on tree height was not significant in the general model (F = 2.5, P = 0.134), significant treatment effects arose in birch when both species were analyzed separately, with clipped trees being significantly shorter than controls (Table 2). Clipped B. pubescens trees also had fewer branches, terminal shoots and short shoots than controls, indicating that significant effects on the architecture of clipped trees remained measureable even 1 year after clipping. On the contrary, regrowth of Q. petraea saplings completely compensated for height and branching differences, with clipped trees showing only a marginally significant smaller number of terminal shoots than control trees after 1 year (Table 2). As regards the morphology of leaves, we observed no significant differences in individual leaf area and weight or the SLA of clipped and control trees of both species (Table 2).
Table 2.
Results for architectural: tree height (cm), canopy area (m2), basal stem diameter (mm), number of branches, number of terminal shoots, number of lateral shoots, number of long shoots, number of short shoots; and morphological: individual leaf area (LA; cm2), individual leaf weight (g) and specific leaf area (SLA, cm2 g−1) variables measured in clipped and control saplings of B. pubescens and Q. petraea before harvest in August 2008 (ca 1 year after clipping).
| Measurements | B. pubescens | Q. petraea | ||
|---|---|---|---|---|
| Clipped | Control | Clipped | Control | |
| Tree height (cm) | 130.9 (8.7) | 161.8 (11.3) ** | 124.8 (6.9) | 128.6 (17.4) |
| Canopy area (m2) | 2.9 (0.4) | 2.5 (0.2) | 2.3 (0.4) | 2.6 (0.3) |
| Stem diam (mm) | 15.5 (0.3) | 15.3 (0.5) | 14.3 (0.5) | 14.9 (0.5) |
| No. branches | 12.4 (0.7) | 25.8 (1.7) *** | 7.4 (1.4) | 13.2 (2.9) |
| No. terminal shoots | 15.4 (1.6) | 38.2 (7.5) ** | 6.0 (0.8) | 12.2 (2.8) * |
| No. lateral shoots | 47.4 (6.9) | 61.6 (17.4) | 15.4 (1.9) | 20.8 (5.0) |
| No. long shoots | 62.8 (7.9) | 99.8 (23.9) | 21.4 (1.4) | 33.0 (6.8) |
| No. short shoots | 20.6 (3.1) | 237.0 (59.0) ** | 0.2 (0.2) | 1.4 (0.9) |
| Root:shoot ratio | 0.52 (0.05) | 0.44 (0.07) | 0.57 (0.06) | 0.81 (0.04) ** |
| Ind. LA (cm2) | 10.8 (0.8) | 9.2 (0.4) | 41.2 (5.4) | 40.6 (4.9) |
| Ind. leaf weight (g) | 0.04 (0.00) | 0.03 (0.00) | 0.18 (0.03) | 0.19 (0.02) |
| SLA (cm2 g−1) | 250.1 (11.0) | 273.9 (20.8) | 231.2 (10.0) | 219.9 (10.5) |
Data are means and SE (in parentheses). Significant differences between treatments within species are highlighted in bold. Asterisks indicate the degree of significance: *P < 0.10, **P < 0.05, ***P < 0.001, N = 5.
There were no significant differences in biomass allocation to leaves and coarse roots of both species in trees harvested one year after clipping, but clipped trees of both birch and oak allocated significantly more biomass to current-year stems than control trees (Figure 2). In oak, clipped trees showed more biomass allocation to the main stems and a sharp decrease in biomass allocation to fine roots, which were reduced by 45% in relation to control trees (Figure 2). Such reduction in fine root biomass led to a significant decrease in the root: shoot ratio of clipped oaks, not observed in birch (Table 2). At the end of the first growing season after clipping, the significant reduction of shoots formed in 2007 observed at the beginning of the C-labeling experiment in both species (Table 1) was only noticeable in birch, and differences between treatments in oak were no longer significant (Figure 2). There were no significant differences in total plant biomass between treatments, either in the general model (F = 3.1, P = 0.098) or when species were analyzed separately (F = 2.3, P = 0.169 and F = 1.3, P = 0.280 in birch and oak, respectively).
Figure 2.

Biomass (BM) allocation to different tree fractions in clipped and control trees of B. pubescens and Q. petraea harvested in August 2008 (ca 1 year after clipping). Average values are shown for each fraction. L = leaves, 0St = current year stems, 1St = 1-year-old stems, 2St = 2-year-old stems, MS = main stems, CR = coarse roots, FR = fine roots. Significant differences between treatments within species are indicated by asterisks: *P < 0.10, **P < 0.05.
Differences between control and clipped trees in NSC pools of different plant fractions mimicked results for biomass allocation. Higher SS (and also starch in the case of oak) pools were found in current-year stems of clipped trees of both species (Figure 3). Contrastingly, NSC pools were lower in 1-year-old stems of clipped birch trees (Figure 3). Clipped oak trees showed also a trend for higher net allocation of SS to main stems, while both starch and SS pools were significantly reduced in their fine roots (Figure 3).
Figure 3.

Differences between clipped (gray bars) and control (black bars) B. pubescens and Q. petraea saplings in the allocation of SS and starch pools to different plant fractions 1 year after clipping (trees harvested in August 2008). Asterisks denote significant differences between treatments within a given species at α = 0.10 (*) and α = 0.05 (**).
The differences observed in NSC pool allocation were not driven by changes in NSC concentrations, which remained similar for both SS and starch across treatments and fractions, except for SS concentrations in the leaves of clipped birch trees, which were lower than those of control trees (Table 3). In any case, the lower SS concentrations of the leaves of clipped birch trees did not result in significantly different SS pools between control and clipped birch trees (Figure 3 and Table 3).
Table 3.
Soluble sugar and starch concentrations in the different fractions of clipped (B) and control (C) B. pubescens and Q. petraea trees harvested in August 2008 (ca 1 year after clipping).
| B. pubescens | Q. petraea | |||||||
|---|---|---|---|---|---|---|---|---|
| SS (mg g−1) | Starch (mg g−1) | SS (mg g−1) | Starch (mg g−1) | |||||
| B | C | B | C | B | C | B | C | |
| L | 65.2 (3.6) | 79.1* (4.0) | 69.2 (6.8) | 68.0 (3.9) | 56.7 (4.8) | 50.8 (3.5) | 55.0 (9.6) | 57.7 (5.1) |
| 0-St | 37.9 (1.7) | 38.9 (1.4) | 40.3 (2.6) | 42.9 (3.7) | 20.8 (1.1) | 21.1 (0.9) | 70.2 (8.8) | 81.2 (15.9) |
| 1-St | 33.4 (3.9) | 37.9 (2.5) | 36.3 (2.1) | 44.9 (6.3) | 22.4 (2.2) | 22.2 (1.0) | 68.2 (10.0) | 68.3 (10.4) |
| 2-St | 30.9 (2.4) | 33.0 (2.0) | 43.5 (3.1) | 51.6 (9.3) | 21.3 (2.2) | 19.6 (2.3) | 73.6 (13.4) | 90.9 (10.9) |
| MS | 23.8 (2.1) | 26.3 (1.3) | 54.8 (6.4) | 43.6 (3.0) | 20.0 (2.0) | 16.5 (0.7) | 107.0 (10.1) | 121.3 (14.0) |
| CR | 28.1 (2.5) | 30.3 (2.7) | 182.2 (14.4) | 147.6 (12.7) | 27.9 (3.3) | 24.3 (1.1) | 270.3 (6.4) | 266.8 (9.9) |
| FR | 23.4 (1.7) | 25.3 (1.6) | 85.8 (5.2) | 75.0 (8.5) | 22.0 (1.5) | 19.2 (1.1) | 65.7 (5.8) | 66.7 (5.6) |
L = leaves, 0-St = current year stems, 1-St = 1-year-old stems, 2-St = 2-year-old stems, MS = main stems, CR = coarse roots, FR = fine roots. Data are means and SE (in parentheses). Significant differences between treatments within a given species at α = 0.05 are indicated by an asterisk.
Changes in the net allocation of newly fixed C to different plant fractions and the soil during the recovery of clipped birch and oak trees
The two species showed very different responses in their δ13C isotopic composition after labeling and clipping (Table S4 available as Supplementary Data at Tree Physiology Online). In general, birch allocated significantly more new C to biomass than oak, pointing to a lower reliance on C-stores and a higher C-fixing ability of birch than oak (Figure 4 and Table 4). Across species and treatments, the fractions receiving proportionally more newly fixed C were rapidly growing ones, including, in descending order, leaves, current-year stems, young (1- and 2-year-old) stems and fine roots (Figure 4). Despite being winter deciduous, the leaves of both species were mainly built on newly fixed C, which accounted for 80% of the bulk leaf biomass in birch and over 65% in oak (Figure 4).
Figure 4.

Differences between clipped (gray bars) and control (black bars) B. pubescens and Q. petraea saplings in the allocation of newly fixed C to the different plant organs 1 year after clipping (trees harvested in August 2008). Asterisks denote significant differences between treatments within a given species at α = 0.05.
Table 4.
Summary statistics of glm analysis showing the effects of the different factors included in the full model on the allocation of new C to bulk biomass (BM), SS and starch to the different fractions of B. pubescens and Q. petraea saplings subjected to different clipping treatments and harvested in August 2008 (ca 1 year after clipping).
| Model term | Bulk BM | SS | Starch |
|---|---|---|---|
| Initial length | 20.4 (<0.001) | 12.3 (0.001) | 0.2 (0.641) |
| Species | 170.7 (<0.001) | 212.1 (<0.001) | 105.2 (<0.001) |
| Treatment | 12.0 (0.001) | 1.9 (0.175) | 0.1 (0.814) |
| Fraction | 155.6 (<0.001) | 43.2 (<0.001) | 33.8 (<0.001) |
| Species * Treatment | 17.6 (<0.001) | 1.5 (0.225) | 10.0 (0.002) |
| Species * Fraction | 2.8 (0.013) | 1.2 (0.314) | 6.6 (<0.001) |
| Treatment * Fraction | 2.5 (0.027) | 0.3 (0.957) | 2.7 (0.017) |
| Species * Treatment * Fraction | 0.8 (0.562) | 0.9 (0.505) | 2.5 (0.025) |
F-rations along with P-values (in parentheses) are shown. Significant differences at α = 0.05 are highlighted in bold.
Clipped trees allocated significantly more newly fixed C to the different fractions than controls (Table 4 and Figure 4), but results were very different depending on the species (Figure 4 and Table S5 available as Supplementary Data at Tree Physiology Online). This explains why all interaction terms in the general model were significant (Table 4). In general, clipped trees tended to allocate more new C into fast growing fractions like young stems and fine roots than control trees (Figure 4 and Table S5 available as Supplementary Data at Tree Physiology Online), but when both species were analyzed separately, effects were only significant in oak (Figure 4). In the year after clipping, clipped Q. petraea trees allocated significantly more new C than controls to all fractions but the main stems (Figure 4). Contrastingly, birch trees showed no differences in the allocation of new C to different fractions between control and clipped trees, except for the main stems, where control trees received a larger proportion of new C (Figure 4).
The rhizosphere soil collected underneath both study species showed more 13C-depleted values as compared with the bulk soil (F = 118.6, P < 0.001; Figure 5), indicating that plant roots significantly altered the δ13C signature of the soil in direct contact with them. δ13C values of the rhizosphere soil collected underneath birch trees were more depleted than those of oak, potentially indicating a larger amount of labeled-C transferred to the rhizosphere in the former species (F = 6.5, P = 0.016; Figure 5). However, no significant clipping effects were observed in the δ13C signature of bulk and rhizosphere soil in both species (Figure 5). This indicates that the loss of newly fixed C from roots was similar in clipped and control treatments of both species.
Figure 5.
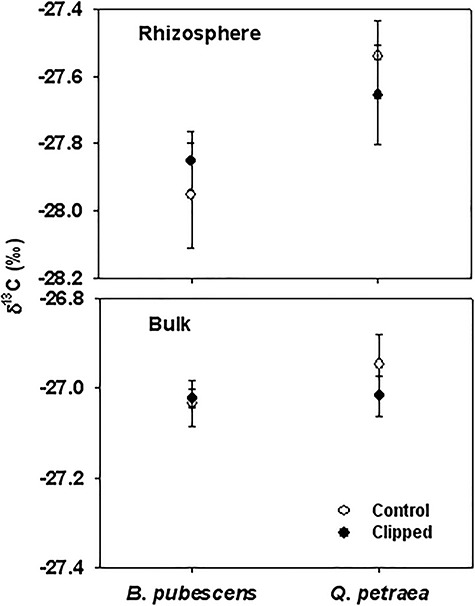
Isotopic composition (δ13C; %) of the rhizosphere and bulk soil collected underneath control (white dots) and clipped (black dots) B. pubescens and Q. petraea trees harvested in August 2008 (ca 1 year after clipping). No significant differences between treatments were detected at α = 0.05.
Differences in the allocation of new C to NSC between clipped and control trees
Birch allocated more new C to SS than oak in all fractions and also significantly more new C to starch, particularly in fast turnover fractions such as leaves, young stems and fine-roots, while both species showed similar new C-allocation to starch in coarse roots and main stems (Table 4, Figure 6 and Table S6 available as Supplementary Data at Tree Physiology Online). In general, clipping had no significant effect on the allocation of newly fixed C to storage (both SS and starch) during the next season after clipping (Table 4). However, in the case of the allocation to starch, the response varied depending on the species and the fraction, and consequently, the interaction terms between species and treatment and the full interaction term were significant (Table 4). Simplified models run separately per species and fraction indicated that, in birch, clipped trees allocated significantly less C to starch in leaves and main stems (the latter marginally significant only) and marginally higher C to SS in current-year stems than control ones (Figure 6). Contrastingly, clipped oaks showed a trend for higher allocation of newly fixed C to starch in current-year and main stems (Figure 6), but results were only marginally significant.
Figure 6.

Differences between control (black bars) and clipped (gray bars) B. pubescens and Q. petraea saplings in the allocation of newly fixed C to SS and starch to different plant fractions on the year after clipping (trees harvested in August 2008). Asterisks denote significant differences between treatments within a given species at α = 0.10 (*) and α = 0.05 (**).
Discussion
In accordance with our first hypothesis, our results indicate that clipped birch and oak trees shifted their C-allocation patterns above-ground as a means to recover from defoliation. However, contrary to our expectations, such increased allocation to current-year stems and leaves did not entail reductions in the allocation to the rhizosphere. As initially expected, B. pubescens and Q. petraea showed differences in their vulnerability and recovery strategies to clipping, the ring-porous species being less vulnerable than the diffuse-porous. These contrasting patterns could be explained by differences in their C cycling after clipping. Defoliated oaks showed a more efficient recovery of their canopy, which was supported by a larger allocation of new C into biomass, particularly above-ground. However, this was associated with large decreases in the fine root biomass of clipped oak trees. Although in both species clipped trees recovered NSC pools faster than growth, the allocation of 13C-labeled photo-assimilates into starch and SS was not increased as compared to controls. This indicates that, contrary to our second hypothesis, no preferential allocation into NSC occurred during the first year after clipping.
Trees recovered from defoliation by increasing C allocation above-ground but maintaining allocation to the rhizosphere
Clipping led to a rapid reduction in tree growth (Palacio et al. 2011) and also in biomass and NSC pools in current-year stems (the fraction directly affected by clipping treatments). Over the course of the first year after clipping, trees managed to recover initial differences in main stem diameter, total plant biomass and, in the case of oaks, also tree height (Table 2). This was mainly a result of shifting allocation above-ground, with increased allocation to current-year stems (almost double that of control trees in oak) and producing similar leaf biomass to controls (Figure 2). In oak, these results were consistent for both biomass allocation as a whole and when the proportion of new C allocated to bulk biomass was considered (Figure 4). The ability of trees to recover from defoliation by increased above-ground allocation is a well-known phenomenon (Eyles et al. 2009, Quentin et al. 2011). Such increased allocation may be achieved by a combination of shifts in architecture, leaf morphology and C-allocation patterns (Eyles et al. 2009).
The removal of apical buds by browsing, clipping or defoliating insects frequently leads to a decrease in apical dominance (due to changes in auxin fluxes; Teichmann and Muhr 2015) with subsequent increases in lateral branch growth (Haukioja et al. 1990, Wilson 1993). Clipped trees in our study showed a lower number of terminal shoots, but increased branching was not detected in either of the two study species. Clipped oaks showed similar lateral branch numbers to control trees, while clipped birch trees had fewer lateral branches than controls. However, birch trees showed a dramatic decrease in the number of short shoots produced after clipping (Table 2; Palacio et al. 2011), which may be a direct consequence of the decreased apical dominance after clipping (Haukioja et al. 1990). In addition, increases in the proportion of leaves per bud (Millard et al. 2001) or in the foliage to wood ratio (Mizumachi et al. 2004) have been reported as mechanisms to increase above-ground allocation in clipped trees. This was not the case in the trees included in this experiment, which showed similar leaf biomass to control trees but increased current-year stem biomass. It seems, therefore, that clipped trees in our experiment maximized the recovery of the canopy, increasing investment into new stems while keeping a similar allocation to foliage. In clipped birch trees, the decrease in short shoot number (likely in favor of long shoot development) may be a mechanism to recover canopy spread and renewal of bud numbers since, in this species, renewal buds are mostly borne in the long shoots (MacDonald et al. 1984).
Several previous studies have detected shifts in leaf morphology after defoliation to compensate losses in the C-assimilating capacity of the canopy, frequently leading to increased individual leaf area and weight and increased SLA (Trumble et al. 1993, Millard et al. 2001, Quentin et al. 2011, Piper et al. 2015, Fuenzalida et al. 2019). We did not detect any significant differences in leaf morphology between clipped and control trees of either study species. Discrepancies with previous studies may be related to differences in the type of disturbance applied and in the duration of experiments. For example, Trumble et al. (1993), Piper et al. (2015) and Fuenzalida et al. (2019) evaluated responses after defoliating insects or treatments simulating defoliation by arthropods, which may elicit a very different response by trees than clipping (Haukioja et al. 1990). Furthermore, Millard et al. (2001) and Quentin et al. (2011) applied clipping in spring and measured the effects on leaf morphology at the end of the same growing season, while in our study, effects on leaf morphology were recorded at the end of the next growing season after clipping, i.e. a much longer duration. Similarly, Eyles et al. (2009) carried out a shorter duration experiment and did not detect any significant effects on individual leaf area of Eucalyptus globulus 5 months after 40% defoliation.
Our results show that the increased above-ground allocation of clipped trees was largely supported by currently fixed (new) C, particularly in oak (Figure 4). Increases in photosynthetic rate have been repeatedly reported in defoliated trees (e.g. Pinkard et al. 1998, Vanderklein and Reich 1999). Although we did not measure photosynthetic rates in our study, clipped oak trees showed higher new C-allocation to bulk biomass, compatible with increased C-fixing ability and with decreased respiratory losses (see below). In both control and clipped trees of both species, new C was preferentially allocated to actively growing fractions such as leaves, young stems and fine roots.
Although clipping induced increased C-allocation above-ground, our results showed no significant effects of clipping on net deposition to soil in either of the two species analyzed. Frost and Hunter (2008) obtained similar results in red oak (Frost and Hunter 2008) and suggested that rhizodeposition might be a tightly controlled process buffered against damage-induced shifts in C-allocation. Both species had a significant effect on the δ13C isotopic composition of the soil, indicated by the depletion in 13C detected in rhizosphere vs bulk soils. Such an effect was larger in birch than oak, likely in relation to the larger C-fixing ability of the former. Consequently, trees were able to impose changes in the C dynamics of soils, but such effects were not modified by clipping.
Betula pubescens trees were more severely affected than oaks by clipping
In agreement with the predictions by Foster (2017), the ring-porous species, Q. petraea, was more efficient in recovering the biomass lost by clipping than the diffuse-porous, B. pubescens. Clipped birch trees showed lower height and altered architecture (reduced number of branches, terminal and lateral shoots) as compared with controls. Also, while biomass losses in stems formed in 2007 (the cohort directly affected by clipping treatments) were no longer significant at the end of the 2008 growing season in oaks, they were still noticeable in birch. Foster (2017) hypothesized that ring-porous species would be more resistant to spring defoliation than diffuse-porous ones owing to their earlier wood phenology and increased C-stores (Dougherty et al. 1979, Barbaroux and Bréda 2002, Barbaroux et al. 2003). We did not measure wood phenology in this study, but it seems likely that this might have had an effect on the differential responses of both species. In our experiment, clipping consisted of shoot removal in early July and early September 2007, after the first and second flushes of shoot growth were finished. If the differences in wood growth phenology between ring-porous and diffuse-porous adult trees can be applied to saplings, oaks would be expected to have started wood growth at least 2 weeks prior to the first defoliation event, while birch trees would be just starting (Foster 2017). Foster (2017) further hypothesized that such differences in damage due to wood growth phenology would entail a differential decrease in NSC stores, which would be more severely decreased in diffuse-porous trees and further exacerbated by their lower C-storage capacity. Our results do not confirm this prediction, since both species were equally able to recover NSC pools to the same level as controls in the same year as clipping (except for the shoot cohort directly affected by treatments). Similar fast recovery of NSC stores in defoliated trees has been previously reported (Palacio et al. 2008, Piper et al. 2015, Puri et al. 2015).
Instead, our results show that the differential recovery ability of birch and oak trees after clipping could be, at least partly, explained by the different effects of clipping on their C-cycling, including differences in C-allocation. In accordance with previous studies, oaks showed a larger reliance on storage than birch trees to support new growth (Barbaroux et al. 2003, but see Palacio et al. 2011). This increased ability to re-mobilize C-stores could have been crucial to support the re-growth of clipped oaks, at least initially. Several previous studies have reported a positive relationship between NSC storage and the re-growth ability of defoliated trees (Kays and Canham 1991, Luostarinen and Kauppi 2005, Fierravanti et al. 2019). However, clipped oak trees invested significantly less ‘old’ C (and proportionally more ‘new’ C) in their new growth than control trees (Figure 4). Consequently, while clipped oaks recovered to a larger extent than clipped birch trees in our experiment, this was not linked to increased total NSC remobilization as measured at the end of the growing season.
The increased allocation of new C in clipped oaks to support re-growth as compared with controls illustrates a shift in C cycling after damage resulting in significantly more new C being allocated to most fractions in clipped vs control oaks, an effect not observed in birch. Such a change can be the result of increased C-fixation, but also of decreased losses by respiration or rhizosphere allocation. Differences in C-allocation to the rhizosphere were not significant among treatments (see above). However, we cannot rule out the possibility that the increased allocation of new C assimilates in oak was (at least partly) due to reduced respiratory losses, particularly in relation to the drastic reduction in fine root biomass detected in clipped trees of this species. Accordingly, the increased investment into above-ground components in oak was associated with a reduced production of fine roots of 45% during the year after clipping. These changes were not observed in birch, which maintained similar biomass allocation below-ground between control and clipped trees. Increased fine root mortality is a frequently reported process in defoliated trees (Tuomi et al. 1990, Vanderklein and Reich 1999, Frost and Hunter 2008, but see Kosola et al. 2001, Endrulat et al. 2016). Tuomi et al. (1990) suggested that the reductions in fine root biomass after defoliation could vary largely depending on the relative root biomass, the degree of reserve depletion and the compensatory C gain of trees. All these three factors likely differed in the two study species, which could explain their contrasting response. Oaks have relatively high root: shoot ratios (Shaw 1974), as was the case also in this experiment (Table 1). This means an increased non-productive biomass to support during re-growth (Tuomi et al. 1990). The higher storage pools may not have been sufficient to recover above-ground losses and maintain a large root biomass in oak (Tuomi et al. 1990).
Clipping did not result in preferential C-allocation to storage in the long-term
Our study did not detect temporal differences in NSC concentrations in the different organs of clipped and control trees throughout the first year of recovery, but NSC concentrations of clipped trees of both species reached similar levels to those of control trees by the end of the first growing season. This indicates that decreases in NSC stores due to canopy re-growth, if any, were short-lived and fully compensated within less than one year. Similar results have been previously reported in the literature (e.g. Palacio et al. 2012, Wiley et al. 2013, Puri et al. 2015). In our experiment, the replenishment of stores was likely supported by an increased C-fixing ability in clipped trees, particularly in oak, as denoted by their higher new C-allocation to bulk biomass.
Despite NSC concentrations of clipped trees being rapidly restored to even higher levels than control trees (Tables S2 and S3 available as Supplementary Data at Tree Physiology Online), we did not detect a significant increase in new C-allocation to storage in clipped trees in the first year after clipping. The only significant effect of clipping on new C-allocation was a reduction in allocation to SS in leaves of clipped birches. Wiley et al. (2013, 2017b) suggested that the growth of defoliated trees would be largely limited by C-availability, first by the decrease in leaf area directly related to defoliation and then by a prioritized allocation to storage over growth to secure tree survival under future potential defoliation events. They argued that the fact that NSC stores were replenished to control levels did not necessarily mean tree growth was not limited by C-availability, since prioritized allocation to storage over growth could still proceed (Wiley and Helliker 2012). Two recent experiments have experimentally demonstrated that NSC concentrations can be maintained to control levels over periods of C-limitation by preferential allocation of C into storage, calling for a cautious use of NSC concentrations to predict the C-status of trees (Weber et al. 2018, 2019). In both experiments, trees subjected to low or no illumination were progressively C-deprived, reaching minimum SS and starch thresholds below which tree survival was impaired. In both cases, re-illumination resulted in a period of reduced growth and refilling of NSC stores up to a certain threshold. These results indicate that prioritized allocation to storage over growth does occur in C-starved trees (with very low NSC levels) and that such prioritization is arrested once a certain level of recovery of NSC is achieved.
In contrast to the experiments in Weber et al. (2018, 2019), our trees were not C-starved. We did not find the very significant depletion of NSC reported by these previous studies (Weber et al. 2018, 2019). Consequently, our trees probably did not prioritize allocation to NSC over growth. Starch and SS concentrations of defoliated trees are normally not depleted below the C-starvation thresholds detected by Weber et al. (2018, 2019), even after severe treatments (e.g. Kays and Canham 1991, Vanderklein and Reich 1999, Palacio et al. 2012, but see Kosola et al. 2001, Puri et al. 2015). This seems to indicate that C-limitation after defoliation in trees is normally short-lived and of low magnitude.
Conclusions
Our results show that clipping triggers a shift in biomass allocation above-ground favoring the recovery of the canopy in both oak and birch trees. However, such a shift does not entail a decrease in C-allocation to the rhizosphere, which seems to be a tightly regulated process. Future research on the mechanisms behind such tight regulation would greatly contribute to our understanding of the effects of defoliation on tree C-cycling and its impact on below-ground processes. The observed differences in the recovery strategies of the two study species could have potential implications for their vulnerability under different browsing frequencies. The ring-porous species, Q. petraea, showed a faster recovery of its canopy after clipping than the diffuse-porous, B. pubescens. However, this came at the cost of a marked decrease in the fine root biomass of oak, which raises questions on the potential consequences for the nutrition of the tree and its vulnerability to sustained browsing over longer time periods. Finally, despite the different effect of clipping on the C-allocation of the study species, none of them increased new C-allocation to storage one year after damage. This indicates that clipping does not entail a sustained preventative allocation of C into storage in the long-term.
Data and materials availability
The authors agree to make experimental data and materials available to third party academic researchers upon reasonable request.
Authors’ contributions
S.P., P.M., A.J.H. and E.P. designed the study. S.P., E.P. and P.M. implemented continuous δ13C- labeling. S.P., E.P., P.M., M.M., G.L., S.N. and A.A.-R. ran carbohydrate and stable isotope analyses. S.P., P.M. and S.N. analyzed data. All authors interpreted results. S.P. wrote the manuscript, receiving revisions from all authors.
Supplementary Material
Acknowledgments
Thanks to Raul Castillo, Richard Gwatkin and Shona Osborne for their help in running the experiment, to Elena Lahoz and Elena Royo for carbohydrate analyses and to two anonymous reviewers for useful comments on an earlier version of the manuscript. Publication fees were covered by the CSIC Open Access Publication Support Initiative through its Unit of Information Resources for Research (URICI).
Conflict of interest
None declared.
Funding
This work was supported by the Ministerio de Cienciae Innovación, Spain through a MEC-SEUI postdoctoral fellowship, a Ramón y Cajal fellowship (RYC-2013-14164) and project CGL2015-71360-P to S.P.; and by the Scottish Government’s Rural and Environmental Research and Analysis Directorate through funds to E.P., A.J.H. and P.M.
References
- Ayres E, Heath J, Possell M, Black HIJ, Kerstiens G, Bardgett RD (2004) Tree physiological responses to above-ground herbivory directly modify below-ground processes of soil carbon and nitrogen cycling. Ecol Lett 7:469–479. [Google Scholar]
- Babst BA, Ferrieri RA, Gray DW, Lerdau M, Schlyer DJ, Schueller M, Thorpe MR, Orians CM (2005) Jasmonic acid induces rapid changes in carbon transport and partitioning in Populus. New Phytol 167:63–72. [DOI] [PubMed] [Google Scholar]
- Badeck FW, Tcherkez G, Nogués S, Piel C, Ghashghaie J (2005) Post-photosynthetic fractionation of stable carbon isotopes between plant organs—a widespread phenomenon. Rapid Commun Mass Spectrom 19:1381–1391. [DOI] [PubMed] [Google Scholar]
- Baldwin IT. (1990) Herbivory simulations in ecological research. Trends Ecol Evol 5:91–93. [DOI] [PubMed] [Google Scholar]
- Barbaroux C, Bréda N (2002) Contrasting distribution and seasonal dynamics of carbohydrate reserves in stem wood of adult ring-porous sessile oak and diffuse-porous beech trees. Tree Physiol 22:1201–1210. [DOI] [PubMed] [Google Scholar]
- Barbaroux C, Bréda N, Dufrêne E (2003) Distribution of above-ground and below-ground carbohydrate reserves in adult trees of two contrasting broad-leaved species (Quercus petraea and Fagus sylvatica). New Phytol 157:605–615. [DOI] [PubMed] [Google Scholar]
- Bardgett RD, Wardle DA (2003) Herbivore-mediated linkages between aboveground and belowground communities. Ecology 84:2258–2268. [Google Scholar]
- Barry KM, Quentin A, Eyles A, Pinkard EA (2011) Consequences of resource limitation for recovery from repeated defoliation in Eucalyptus globulus Labilladière. Tree Physiol 32:24–35. [DOI] [PubMed] [Google Scholar]
- Barto EK, Rillig MC (2010) Does herbivory really suppress mycorrhiza? A meta-analysis. J Ecol 98:745–753. [Google Scholar]
- Bergman M. (2002) Can saliva from moose, Alces alces, affect growth responses in the sallow, Salix caprea? Oikos 96:164–168. [Google Scholar]
- Bryant JP, Reichardt PB, Clausen TP, Werner RA (1993) Effects of mineral nutrition on delayed inducible resistance in Alaska paper birch. Ecology 74:2072–2084. [Google Scholar]
- Buysse J, Merckx R (1993) An improved colorimetric method to quantify sugar content of plant tissue. J Exp Bot 44:1627–1629. [Google Scholar]
- Canham CD, Kobe RK, Latty EF, Chazdon RL (1999) Interspecific and intraspecific variation in tree seedling survival: effects of allocation to roots versus carbohydrate reserves. Oecologia 121:1–11. [DOI] [PubMed] [Google Scholar]
- Canham CD, Mcaninch JB, Wood DM (1994) Effects of the frequency, timing, and intensity of simulated browsing on growth and mortality of tree seedlings. Can J Forest Res 24:817–825. [Google Scholar]
- Chapin FS, Schulze ED, Mooney HA (1990) The ecology and economics of storage in plants. Annu Rev Ecol Syst 21:423–447. [Google Scholar]
- Clark KL, Skowronski N, Hom J (2010) Invasive insects impact forest carbon dynamics. Glob Chang Biol 16:88–101. [Google Scholar]
- Cornelissen JHC, Lavorel S, Garnier E et al. (2003) A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust J Bot 51:335–380. [Google Scholar]
- Domisch T, Finér L, Lehto T (2001) Effects of soil temperature on biomass and carbohydrate allocation in scots pine (Pinus sylvestris) seedlings at the beginning of the growing season. Tree Physiol 21:465–472. [DOI] [PubMed] [Google Scholar]
- Dougherty PM, Teskey RO, Phelps JE, Hinckley TM (1979) Net photosynthesis and early growth trends of a dominant white oak (Quercus-alba L). Plant Physiol 64:930–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endrulat T, Buchmann N, Brunner IB (2016) Carbon allocation into different fine-root classes of young Abies alba trees is affected more by phenology than by simulated browsing. PLoS One 11:e0154687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericsson A, Hellqvist C, Langstrom B, Larsson S, Tenow O (1985) Effects on growth of simulated and induced shoot pruning by Tomicus piniperda as related to carbohydrate and nitrogen dynamics in scots pine. J Appl Ecol 22:105–124. [Google Scholar]
- Eyles A, Pinkard EA, Mohammed C (2009) Shifts in biomass and resource allocation patterns following defoliation in Eucalyptus globulus growing with varying water and nutrient supplies. Tree Physiol 29:753–764. [DOI] [PubMed] [Google Scholar]
- Fierravanti A, Rossi S, Kneeshaw D, De Grandpré L, Deslauriers A (2019) Low non-structural carbon accumulation in spring reduces growth and increases mortality in conifers defoliated by spruce budworm. Front Forest Global Change 2:15. [Google Scholar]
- Foster JR. (2017) Xylem traits, leaf longevity and growth phenology predict growth and mortality response to defoliation in northern temperate forests. Tree Physiol 37:1151–1165. [DOI] [PubMed] [Google Scholar]
- Frost CJ, Hunter MD (2008) Herbivore-induced shifts in carbon and nitrogen allocation in red oak seedlings. New Phytol 178:835–845. [DOI] [PubMed] [Google Scholar]
- Fuenzalida TI, Hernández-Moreno Á, Piper FI (2019) Secondary leaves of an outbreak-adapted tree species are both more resource acquisitive and more herbivore resistant than primary leaves. Tree Physiol 39:1499–1511. [DOI] [PubMed] [Google Scholar]
- Giardina CP, Coleman MD, Hancock JE, King JS, Lilleskov EA, Loya WM, Pregitzer KS, Ryan MG, Trettin CC (2005) The response of belowground carbon allocation in forests to global change. Springer, Dordrecht, The Netherlands, pp 119–154. [Google Scholar]
- Gill RMA. (2006) The influence of large herbivores on tree recruitment and forest dynamics In: Danell K, Bergström R, Duncan P, Pastor J (eds) Large herbivore ecology and ecosystem dynamics. Cambridge University Press, Cambridge, pp 170–202. [Google Scholar]
- Glentworth R, Muir JW (1963) The soils of the country round Aberdeen, Inverurie and Frazerburgh. In Memoirs of the Soil Survey of Great Britain. Macaulay Institute of Soil Research, Edinburgh; HMSO. [Google Scholar]
- Hartmann H, Adams HD, Hammond WM, Hoch G, Landhäusser SM, Wiley E, Zaehle S (2018) Identifying differences in carbohydrate dynamics of seedlings and mature trees to improve carbon allocation in models for trees and forests. Environ Exp Bot 152:7–18. [Google Scholar]
- Haukioja E, Ruohomaki K, Senn J, Suomela J, Walls M (1990) Consequences of herbivory in the mountain birch (Betula pubescens ssp tortuosa)—importance of the functional organization of the tree. Oecologia 82:238–247. [DOI] [PubMed] [Google Scholar]
- Hester AJ, Mitchell FJG, Kirby KJ (1996) Effects of season and intensity of sheep grazing on tree regeneration in a British upland woodland. For Ecol Manage 88:99–106. [Google Scholar]
- Hester AJ, Edenius L, Buttenschon RM, Kuiters AT (2000) Interactions between forests and herbivores: the role of controlled grazing experiments. Forestry 73:381–391. [Google Scholar]
- Hester AJ, Millard P, Baillie GJ, Wendler R (2004) How does timing of browsing affect above- and below-ground growth of Betula pendula, Pinus sylvestris and Sorbus aucuparia? Oikos 105:536–550. [Google Scholar]
- Kays JS, Canham CD (1991) Effects of time and frequency of cutting on hardwood root reserves and sprout growth. Forest Sci 37:524–539. [Google Scholar]
- Körner C. (2003) Carbon limitation in trees. J Ecol 91:4–17. [Google Scholar]
- Kosola KR, Dickmann DI, Paul EA, Parry D (2001) Repeated insect defoliation effects on growth, nitrogen acquisition, carbohydrates, and root demography of poplars. Oecologia 129:65–74. [DOI] [PubMed] [Google Scholar]
- Lechowicz MJ. (1984) Why do temperate deciduous trees leaf out at different times? Adaptation and ecology of forest communities. Am Nat 124:821–842. [Google Scholar]
- Luostarinen K, Kauppi A (2005) Effects of coppicing on the root and stump carbohydrate dynamics in birches. New Forests 29:289–303. [Google Scholar]
- MacDonald AD, Mothersill DH, Caesar JC (1984) Shoot development in Betula papyrifera. III. Long-shoot organogenesis. Can J Bot 62:437–445. [Google Scholar]
- Messier C, Nikinmaa E (2000) Effects of light availability and sapling size on the growth, biomass allocation, and crown morphology of understory sugar maple, yellow birch, and beech. Ecoscience 7:345–356. [Google Scholar]
- Millard P, Hester A, Wendler R, Baillie G (2001) Interspecific defoliation responses of trees depends on sites of winter nitrogen storage. Funct Ecol 15:535–543. [Google Scholar]
- Mizumachi E, Osawa N, Akiyama R, Tokuchi N (2004) The effects of herbivory and soil fertility on the growth patterns of Quercus serrata and Q. crispula saplings at the shoot and individual levels. Popul Ecol 46:203–211. [Google Scholar]
- Nogués S, Tcherkez G, Cornic G, Ghashghaie J (2004) Respiratory carbon metabolism following illumination in intact French bean leaves using 13C/12C isotope labeling. Plant Physiol 136:3245–3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orians CM, Thorn A, Gomez S (2011) Herbivore-induced resource sequestration in plants: why bother? Oecologia 167:1–9. [DOI] [PubMed] [Google Scholar]
- Palacio S, Camarero JJ, Maestro M, Alla AQ, Lahoz E, Montserrat-Martí G (2018) Are storage and tree growth related? Seasonal nutrient and carbohydrate dynamics in evergreen and deciduous Mediterranean oaks. Trees Struct Funct 32:777–790. [Google Scholar]
- Palacio S, Maestro M, Montserrat-Martí G (2007) Seasonal dynamics of non-structural carbohydrates in two species of Mediterranean sub-shrubs with different leaf phenology. Environ Exp Bot 59:34–42. [Google Scholar]
- Palacio S, Hester AJ, Maestro M, Millard P (2008) Browsed Betula pubescens trees are not carbon-limited. Funct Ecol 22:808–815. [Google Scholar]
- Palacio S, Paterson E, Sim A, Hester AJ, Millard P (2011) Browsing affects intra-ring carbon allocation in species with contrasting wood anatomy. Tree Physiol 31:150–159. [DOI] [PubMed] [Google Scholar]
- Palacio S, Hernández R, Maestro-Martínez M, Camarero JJ (2012) Fast replenishment of initial carbon stores after defoliation by the pine processionary moth and its relationship to the re-growth ability of trees. Trees Struct Funct 26:1627–1640. [Google Scholar]
- Paterson E, Thornton B, Midwood AJ, Sim A (2005) Defoliation alters the relative contributions of recent and non-recent assimilate to root exudation from Festuca rubra. Plant Cell Environ 28:1525–1533. [Google Scholar]
- Paterson E, Midwood AJ, Millard P (2009) Through the eye of the needle: a review of isotope approaches to quantify microbial processes mediating soil carbon balance. New Phytol 184:19–33. [DOI] [PubMed] [Google Scholar]
- Pinkard EA, Beadle CL (1998) Regulation of photosynthesis in Eucalyptus nitens (Deane and Maiden) Maiden following green pruning. Trees Struct Funct 12:366–376. [Google Scholar]
- Pinkard EA, Beadle CL, Davidson NJ, Battaglia M (1998) Photosynthetic responses of Eucalyptus nitens (Deane and Maiden) Maiden to green pruning. Trees Struct Funct 12:119–129. [Google Scholar]
- Piper FI, Gundale MJ, Fajardo A (2015) Extreme defoliation reduces tree growth but not C and N storage in a winter-deciduous species. Ann Bot 115:1093–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri E, Hoch G, Körner C (2015) Defoliation reduces growth but not carbon reserves in Mediterranean Pinus pinaster trees. Trees Struct Funct 29:1187–1196. [Google Scholar]
- Quentin AG, Beadle CL, O'Grady AP, Pinkard EA (2011) Effects of partial defoliation on closed canopy Eucalyptus globulus Labilladière: growth, biomass allocation and carbohydrates. For Ecol Manage 261:695–702. [Google Scholar]
- Schmid S, Palacio S, Hoch G (2017) Growth reduction after defoliation is independent of CO2 supply in deciduous and evergreen young oaks. New Phytol 214:1479–1490. [DOI] [PubMed] [Google Scholar]
- Shaw MW. (1974) The reproductive characteristics of oaks In: British oak: Its history and natural history. Botanical Society of the British Isles, Brighton, pp 162–181. [Google Scholar]
- Shipley LA, Illius AW, Danell K, Hobbs NT, Spalinger DE (1999) Predicting bite size selection of mammalian herbivores: a test of a general model of diet optimization. Oikos 84:55–68. [Google Scholar]
- Speed JDM, Austrheim G, Hester AJ, Mysterud A (2011) Growth limitation of mountain birch caused by sheep browsing at the altitudinal treeline. For Ecol Manage 261:1344–1352. [Google Scholar]
- Tcherkez G, Nogués S, Bleton J, Cornic G, Badeck F, Ghashghaie J (2003) Metabolic origin of carbon isotope composition of leaf dark-respired CO2 in French bean. Plant Physiol 131:237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichmann T, Muhr M (2015) Shaping plant architecture. Front Plant Sci 6:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumble JT, Kolodny-Hirsch DM, Ting IP (1993) Plant compensation for arthropod herbivory. Annu Rev Entomol 38:93–119. [Google Scholar]
- Tschaplinski TJ, Blake TJ (1994) Carbohydrate mobilization following shoot defoliation and decapitation in hybrid poplar. Tree Physiol 14:141–151. [DOI] [PubMed] [Google Scholar]
- Tuomi J, Niemelä P, Sirén S (1990) The Panglossian paradigm and delayed inducible accumulation of foliar phenolics in mountain birch. Oikos 59:399–410. [Google Scholar]
- Van der Heyden F, Stock WD (1995) Non-structural carbohydrate allocation following different frequencies of simulated browsing in three semiarid shrubs. Oecologia 102:238–245. [DOI] [PubMed] [Google Scholar]
- Vanderklein DW, Reich PB (1999) The effect of defoliation intensity and history on photosynthesis, growth and carbon reserves of two conifers with contrasting leaf lifespans and growth habits. New Phytol 144:121–132. [Google Scholar]
- Weber R, Schwendener A, Schmid S, Lambert S, Wiley E, Landhäusser SM, Hartmann H, Hoch G (2018) Living on next to nothing: tree seedlings can survive weeks with very low carbohydrate concentrations. New Phytol 218:107–118. [DOI] [PubMed] [Google Scholar]
- Weber R, Gessler A, Hoch G (2019) High carbon storage in carbon-limited trees. New Phytol 222:171–182. [DOI] [PubMed] [Google Scholar]
- Wiley E, Helliker B (2012) A re-evaluation of carbon storage in trees lends greater support for carbon limitation to growth. New Phytol 195:285–289. [DOI] [PubMed] [Google Scholar]
- Wiley E, Huepenbecker S, Casper BB, Helliker BR (2013) The effects of defoliation on carbon allocation: can carbon limitation reduce growth in favour of storage? Tree Physiol 33:1216–1228. [DOI] [PubMed] [Google Scholar]
- Wiley E, Casper BB, Helliker BR (2017a) Recovery following defoliation involves shifts in allocation that favour storage and reproduction over radial growth in black oak. J Ecol 105:412–424. [Google Scholar]
- Wiley E, Hoch G, Landhäusser SM (2017b) Dying piece by piece: carbohydrate dynamics in aspen (Populus tremuloides) seedlings under severe carbon stress. J Exp Bot 68:5221–5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson BF. (1993) Compensatory shoot growth in young black birch and red maple trees. Can J For Res 23:302–306. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


