SUMMARY
Fragile X syndrome (FX), the most common inherited form of autism and intellectual disability, is a condition associated with visual perceptual learning deficits. We recently discovered that perceptual experience can encode visual familiarity via persistent low-frequency oscillations in the mouse primary visual cortex (V1). Here, we combine this paradigm with a multifaceted experimental approach to identify neurophysiological impairments of these oscillations in FX mice. Extracellular recordings reveal shorter durations, lower power, and lower frequencies of peak oscillatory activity in FX mice. Directed information analysis of extracellularly recorded spikes reveals differences in functional connectivity from multiple layers in FX mice after the perceptual experience. Channelrhodopsin-2 assisted circuit mapping (CRACM) reveals increased synaptic strength from L5 pyramidal onto L4 fast-spiking cells after experience in wild-type (WT), but not FX, mice. These results suggest differential encoding of visual stimulus familiarity in FX via persistent oscillations and identify circuit connections that may underlie these changes.
Graphical Abstract
In Brief
Kissinger et al. study perceptual experience-dependent visual cortical oscillations in Fragile X mice using extracellular electrophysiology, revealing decreased oscillatory magnitudes and altered temporal profiles in Fragile X mice. Additionally, they perform a cross-layer functional connectivity analysis of these data and channelrhodopsin-2 assisted circuit mapping to identify underlying circuit differences.
INTRODUCTION
Fragile X syndrome (FX) is the most common monogenetic inheritable form of intellectual disability (Gallagher and Hallahan, 2012). FX patients have high comorbidity with autism spectrum disorders (ASDs) (Hall et al., 2008; Harris et al., 2008) and exhibit learning impairments (Berry-Kravis, 2014). Several deficits in perception and learning have been found using visual tasks to assess human individuals with FX, suggesting the presence of neural dysfunction in the visual system associated with the loss of fragile X mental retardation protein (FMRP) expression (Farzin et al., 2008, 2011; Freund and Reiss, 1991; Gallego et al., 2014). Although the precise causes of these impairments are unclear, studies in Fmr1 knockout (KO) mice have revealed diverse neurophysiological phenotypes associated with FMRP loss.
Morphologically, immature, excessive, and unstable dendritic spines were reported from both postmortem patient samples and FX animal models (Portera-Cailliau, 2012). Impaired synaptic plasticity has also been reported, including exaggerated metabotropic glutamate receptor (mGluR)-dependent long-term depression (LTD) (Bhattacharya et al., 2012; Hou et al., 2006; Huber et al., 2002; Nosyreva and Huber, 2006) and deficient cortical long-term potentiation (LTP) (Koga et al., 2015; Larson et al., 2005; Martin et al., 2016). Aberrant persistent activity in the somatosensory cortex and increased excitatory to inhibitory (E/I) ratios were found, consistent with a widely held theme that circuits are hyperexcitable in FX (Gibson et al., 2008; Hays et al., 2011). Recent work supports the notion that an E/I imbalance in autism is driven by decreased inhibition via parvalbumin positive (PV+) interneurons, though this may represent a compensatory mechanism designed to maintain peak depolarization rather than enhance it (Antoine et al., 2019). Two-photon calcium imaging studies have also revealed decreased activity of PV+ interneurons in the primary visual cortex (V1) of adult FX mice, as well as over-synchronized neural activity in the developing somatosensory cortex of young FX mice (Goel et al., 2018; Gonçalves et al., 2013).
These studies have been invaluable for revealing fundamental neurophysiological impairments in individual neurons and defined cortical layers of FX mice. To add to this work and to our understanding of visual impairments in FX, we characterized cross-layer neural activity in V1 of awake Fmr1 KO mice that is modulated by perceptual experience. The recognition of familiar stimuli and their prominent physical features is a critical function of the visual system that is driven by learning. We recently discovered that visually evoked persistent low-frequency oscillations in both single units and local field potentials (LFPs) can encode visual familiarity in V1 of mice (Kissinger et al., 2018). These oscillations emerge after several days of perceptual experience and are not elicited by stimuli with novel spatial frequencies. Here, we ascertained the activity profile of these oscillations in FX mice as a readout of perceptual learning in V1 and sought to identify resulting changes in circuit connectivity and synaptic plasticity in FX mice and wild-type (WT) controls.
We found that these oscillations were lower in power and shorter in duration in FX mice, suggesting attenuated encoding of familiar stimuli in V1. The timing of each oscillatory cycle was also delayed in FX mice, demonstrating that not only the power but also the temporal profile of this familiarity response is altered. Multiple functional connections between cortical layers were weaker in FX mice after perceptual experience, the most prominent of which were connections from multiple layers onto layer 4 (L4) fast-spiking (FS) cells and from L6 FS cells onto L2/3 and L4. Connection-specific changes in synaptic strength elicited by perceptual experience were impaired in FX mice, particularly at the L5 regular-spiking (RS) to L4 FS connection. Together, these results infer connections in V1 that may be involved in visual experience-dependent oscillations and suggest that impairments in these connections may underlie attenuation of the oscillations in FX mice.
RESULTS
Attenuated Visually Evoked Oscillations in LFPs in FX Mice
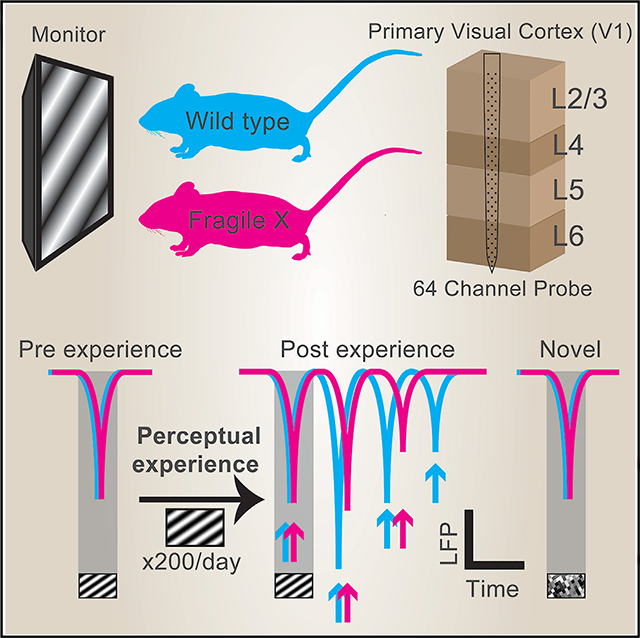
To determine differences in perceptual experience-dependent oscillatory activity in V1 of FX (Fmr1 KO) mice, we subjected awake 2-month-old mice to a passive visual perceptual experience paradigm. Sinusoidal drifting gratings (0.2 s duration; spatial frequency = 0.03 cycles per degree of visual angle [cpd]; temporal frequency = 3 Hz; speed = 100 deg/s) were presented to the mice before and after passively viewing this stimulus 200 times/day over 4 days (inter-trial interval = 8.2 s; Figure 1A). Silicon probes (64 channels) were inserted normal to the surface of binocular V1 to record electrophysiological activity across all cortical layers while mice viewed full-field visual stimuli directly in front of them (Shobe et al., 2015). We simultaneously recorded pupil size and locomotion to assess arousal levels and to control for gain modulatory effects caused by locomotion. Visually evoked potentials (VEPs) were identified from layer 4 of V1 by taking the first and strongest negative deflection in LFPs occurring after visual stimulation. We first analyzed VEPs from trials where the mice did not run (immobile trials) to assess visually evoked activity in FX mice without any confounding effects of locomotion (Figure 1). Visually evoked responses before perceptual experience were comparable between naive wild-type (WT) and FX mice, characterized by a stimulus-locked VEP and occasionally followed by low-power oscillatory activity (Figure 1B). Perceptual experience induced persistent low-frequency oscillatory activity in both WT and FX mice, but these oscillations were significantly lower in amplitude in FX mice at the 2nd and 3rd cycles (Figure 1C). After perceptual experience, a subset of mice was presented a novel checkerboard stimulus that evoked stimulus-locked responses reminiscent of the pre condition. No significant differences in amplitude could be found in responses to novel stimuli between WT and FX mice (Figure 1D). The finding that stimulus-locked VEP amplitudes are comparable between WT and FX mice suggests that feedforward input to L4 is of similar strength. However, the oscillations persisting beyond the stimulus (which are strengthened by experience) are impaired in FX mice.
Figure 1. Attenuated Amplitude of Visually Evoked Oscillations in FX Mice.
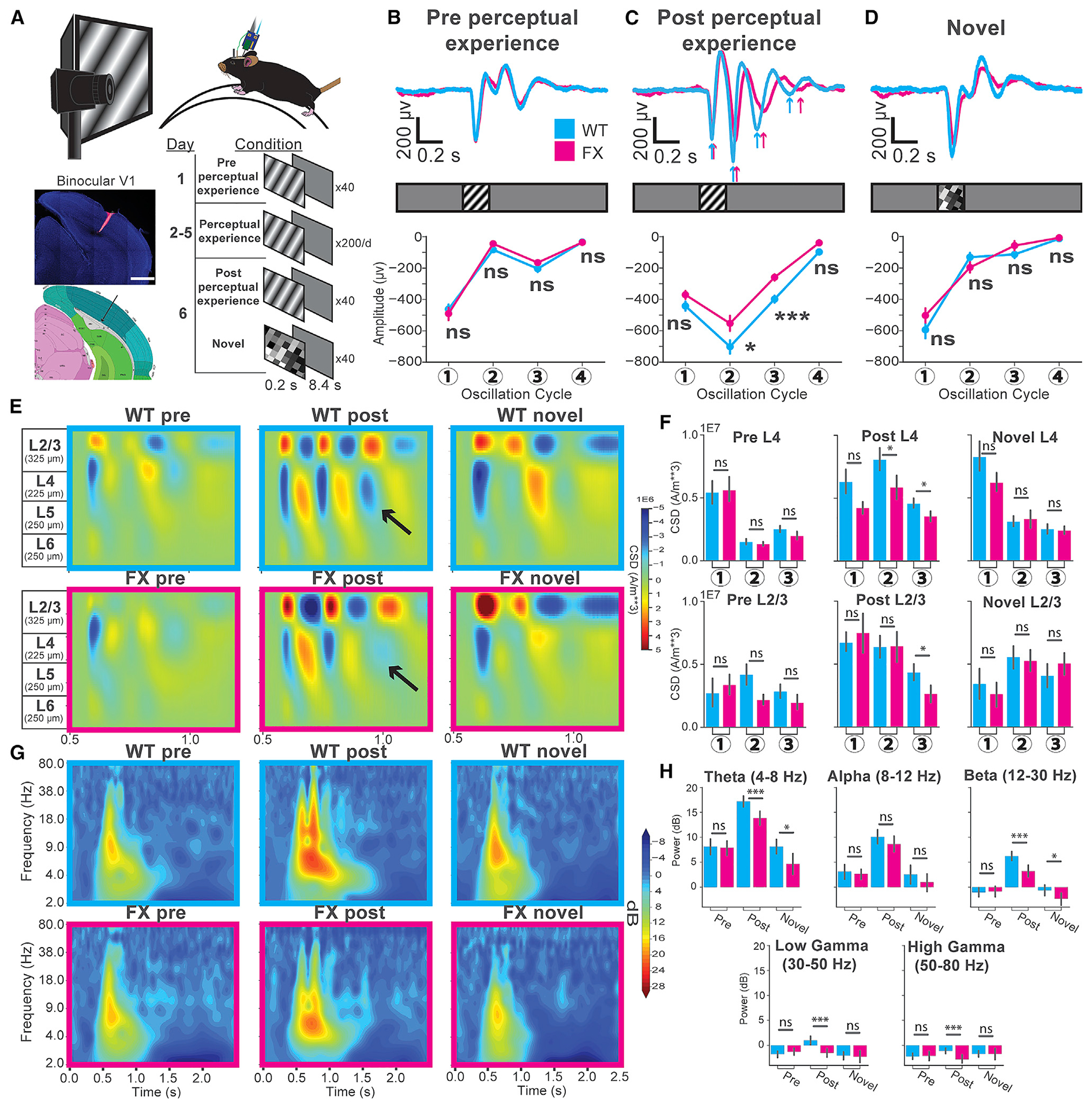
(A) Experimental setup for awake, head-fixed mice. The tiled composite confocal image shows the electrode track in V1 (blue, DAPI; red, Vybrant DiD dye; scale bar, 1,000 μm) compared to the 2019 Allen Institute for Brain Science mouse atlas. Available from https://mouse.brain-map.org/.
(B) Top: layer 4 visually evoked potentials (VEPs) in WT (cyan) and FX (magenta) mice before perceptual experience. Bottom: averaged amplitudes at 4 oscillation cycles. WT n = 37 trial averaged VEPs across 37 mice; FX n = 28 trial averaged VEPs across 28 mice. Linear mixed model (LMM) analysis: genotype: F1, 64 = 0.17; p = 0.681. Oscillation no.: F3,192 = 235.97; p < 1E–4. Genotype/oscillation no. interaction: F3,192 = 1.04; p = 0.376. Least square (LS) means test (Bonferroni corrected): cycle(1): estimate: 0.42; t(240) = 0.32; p = 1.0. (2): estimate: −0.54; t(240) = −1.21; p = 0.88. (3): estimate: −0.39; t(240) = −0.87; p = 1.0. (4): estimate: 0.34; t(240) = 0.76; p = 1.0. Standard error (SE): 0.45. Error bars indicate SEM. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
(C) Post-perceptual experience VEPs. LMM analysis: genotype: F1,66 = 13.34; p = 5E–4. Oscillation no.: F3,198 = 234; p < 1E–4. Genotype/oscillation no. interaction: F3,198 = 2.44; p = 0.06. LS means test (Bonferroni corrected): cycle(1): estimate: estimate: −1.45; t(199.6) = −0.79; t(199.6) = −1.56; p = 0.47. (2): −2.87; p = 0.018. (3): estimate: −2.16; t(199.6) = −4.26; p < 4.0E–4. (4): estimate: −0.80; t(199.6) = −1.59; p = 0.45. SE: 0.51.
(D) Novel VEPs. LMM analysis: genotype: F1,37 = 0.60; p = 0.44. Oscillation no.: F3,111 = 154; p < 1E–4. Genotype/oscillation no. interaction: F 3,111 = 1.04; p = 0.38. LS means test (Bonferroni corrected): cycle(1): estimate: −0.96; t(124.6) = −1.44; p = 0.61. (2): estimate: 2.42E–4; t(124.6) = 0.00; p = 1.0. (3): estimate: 0.29; t(124.6) = 0.44; p = 1.0. (4): estimate: −0.70; t(124.6) = −1.06; p = 1.0. SE: 0.66.
(E) Current source density (CSD) for LFPs across layers from the mice in (B), (C), and (D). The black arrows point to the third current sink in L4.
(F) Quantification of the CSD shown in (E). Mann-Whitney U test WT versus FX: layer 4: pre, sink1: t = 301; p = 0.47. sink2: t = 297; p = 0.44. sink3: t = 224; p = 5.79E–2. Post, sink1: t = 233; p = 0.06. sink2: t = 203; p = 1.72E–2. sink3: t = 221; p = 3.87E–2. Novel, sink1: t = 157; p = 0.20. sink2: t = 183; p = 0.46. sink3: t = 178; p = 0.40. Layer2/3: pre, sink1: t = 237; p = 0.09. sink2: t = 230; p = 0.07. sink3: t = 243; p = 0.11. Post, sink1: t = 267; p = 0.19. sink2: t = 295; p = 0.37. sink3: t = 199; p = . Post L2/3, sink3: t = 199; p = 1.41E–2. Novel, sink1: t = 177; p = 0.39. sink2: t = 180; p = 0.42. sink3: t = 147; p = 0.13.
(G) Time-frequency spectrograms from the layer 4 VEPs (from B, C, and D) for each condition.
(H) Oscillatory power across various frequency bands of the layer 4 VEPs. Mann-Whitney U test; pre: theta: t = 503, p = 0.33; alpha: t = 462, p = 0.16; beta: t = 525, p = 0.44; low gamma, t = 470, p = 0.19; high gamma, t = 508, p = 0.35. Post: theta: t = 304, p = 4.62E–4; alpha: t = 472, p = 0.10; beta: t = 270, p = 9.53E–5; low gamma: t = 278, p = 1.4E–4; high gamma t = 305, p = 4.83E–4. Novel: theta: t = 105, p = 0.01; alpha: t = 160, p = 0.22; beta: t = 122, p = 0.03; low gamma: t = 174, p = 0.36.
See also Figures S7 and S8.
We estimated the translaminar currents underlying the oscillations across the cortical depth by performing current source density (CSD) analysis on the averaged LFPs across mice for each stimulus condition (Aizenman et al., 1996; Łeski et al., 2007; Mitzdorf, 1985; Pettersen et al., 2006; Figures 1E and 1F). Consistent with our previous findings, oscillatory activity was most prominently displayed in the superficial layers (L4 and L2/3), though it was observable across the cortical depth (Kissinger et al., 2018). The L4 current sinks were significantly stronger in WT mice after perceptual experience at the second and third oscillation cycles, with the third sink barely distinguishable in FX mice (Figures 1E and 1F, black arrows). The corresponding sinks in L2/3 at cycles 2 and 3 appeared stronger in FX mice but did not reach statistical significance. However, the L2/3 sink at cycle 3, similar to L4, was significantly attenuated in FX mice compared to WT. No significant differences in CSD could be found between WT and FX mice in either the pre or novel conditions (Figures 1E and 1F). To reveal any frequency-band-specific differences in the persistent (occurring after the stimulus; 0.7–1.2 s) oscillations between WT and FX mice, we quantified oscillatory power relative to the baseline period across a range of frequencies. This time-frequency analysis revealed no significant differences in the power of any frequency bands before perceptual experience between WT and FX mice (Figures 1G and 1H). However, the theta (4–8 Hz), beta (12–30 Hz), low gamma (30–50 Hz), and high gamma (50–80 Hz) frequency bands showed significantly lower power in FX mice after perceptual experience (Figures 1G and 1H). Although alpha (8–12 Hz) power was also lower in FX mice compared to WT, this decrease did not reach statistical significance. Mild differences were found in response to novel stimuli, with significantly higher theta and beta power in WT compared to FX mice (Figures 1G and 1H). These results suggest that, although familiarity to the experienced visual stimulus is encoded in FX mice, it is done so less efficiently. In particular, attenuation in the theta band is consistent with the reported impairments in working memory in FX patients, while attenuation in the beta band is suggestive of impairments in feedback connections that may drive this oscillatory activity (Ethridge et al., 2017; Lee et al., 2005; Lovelace et al., 2018; Munir et al., 2000).
Attenuated Visually Evoked Oscillations in Single Units across Multiple Cortical Layers in FX Mice
To reveal layer- or cell-type-specific differences in oscillatory activity in FX mice, we recorded single-unit activity across all cortical layers of V1 before and after perceptual experience (Figure 2A). Given the prior and recent literature demonstrating reduced inhibition in the cortex of FX mice and other autism models (Antoine et al., 2019; Gibson et al., 2008; Goel et al., 2018), we segregated units into putative RS or FS neurons to understand the relative contributions of each in the expression of perceptual experience-dependent oscillatory activity (Figure S1).
Figure 2. Decreased Duration of Visually Evoked Oscillations in FX Mice.
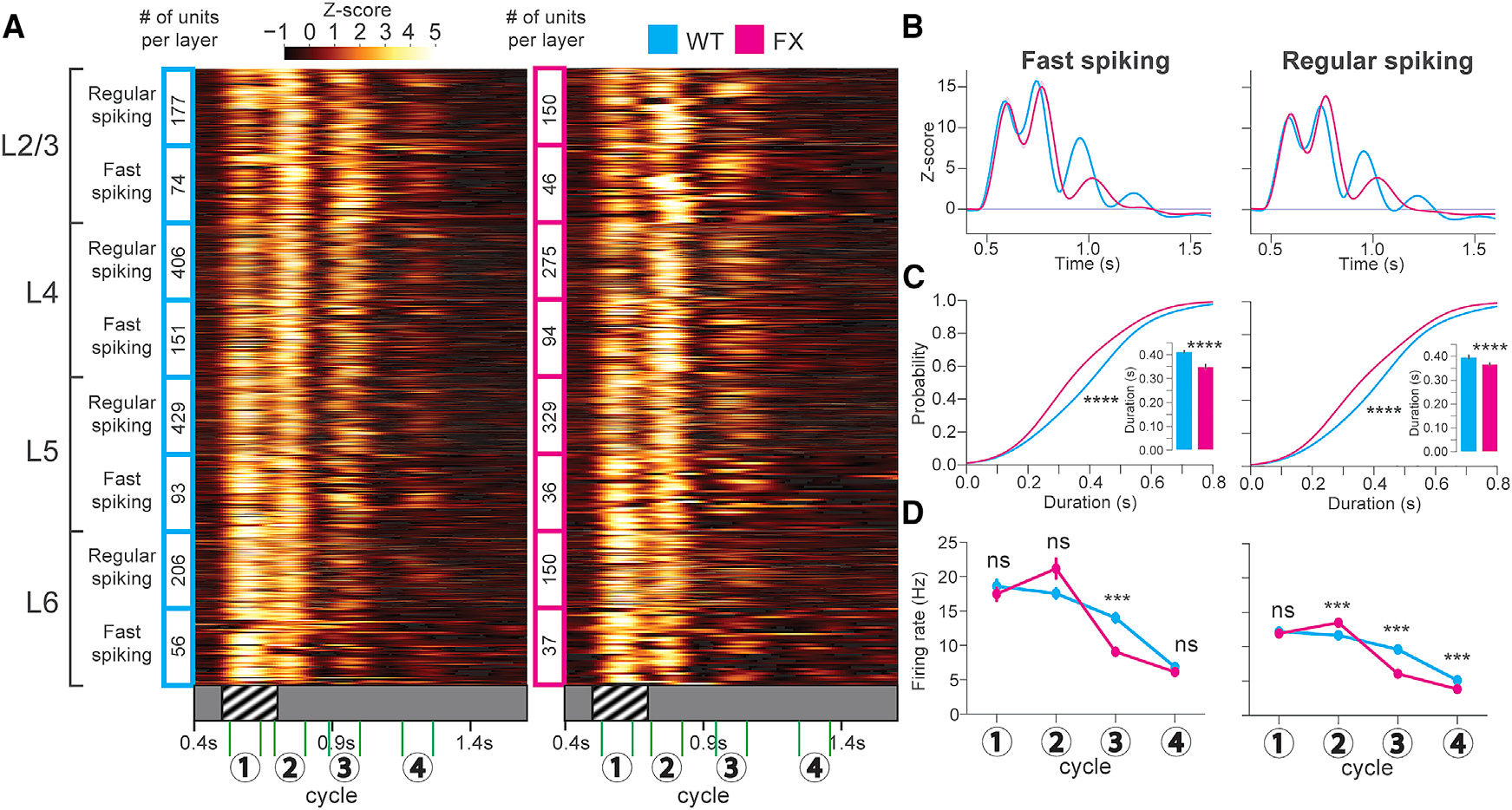
(A) Heatmaps showing the Z scored firing rates for oscillatory units from WT (cyan, 31 mice) and FX (magenta, 25 mice) mice after perceptual experience across all layers of V1. The numbers 1–4 indicate the timings of 4 cycles of the oscillation.
(B) Population (across all layers) Z score line plots.
(C) Cumulative distributions of oscillation duration. Inset: mean duration bar graphs are shown. FS cells: 2-sample Kolmogorov-Smirnov (KS) test of duration CDFs; WT versus FX duration: D(566) = 0.30; p = 3.81E–11. Welch’s t test of mean duration: t(566) = 3.73; p = 2.1E–4 (units after peak detection: WT FS n = 362; FX FS n = 206). RS cells: 2-sample KS test of duration CDFs; WT versus FX duration: D(1,993) = 0.27, p = 1.35E–33. Welch’s t test of mean duration: t(1,993) = 6.65; p = 3.77E–11 (units after peak detection: WT RS n = 1,152; FX RS n = 843). Error bars indicate SEM. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
(D) Mean firing rates at 4 oscillation cycles between WT and FX. LMM analysis for FS cells after perceptual experience: genotype: F1,585 = 1.0; p = 0.32. Oscillation no.: F3,1755 = 472; p < 1E–4. Genotype/oscillation no. interaction: F3,1755 = 27.2; p < 1E–4. LS means test (Bonferroni corrected): cycle(1): estimate: −1.24E–2; t(1,036) = −0.15; p = 1.0. (2): estimate: 0.19; t(1,036) = 2.37; p = 0.07. (3): estimate: −0.41; t(1,036) = −5.08; p < 4E–4. (4): estimate: −4.54E–2; t(1,036) = −0.56; p = 1.0. SE: 8.13E–2. LMM analysis for RS cells after perceptual experience: genotype: F1,2120 = 25.5; p < 1E–4. Oscillation no.: F3,6,360 = 1,902.23; p = 1E–4. Genotype/oscillation no. interaction: F3,6360 = 141.13; p < 1E–4. LS means test (Bonferroni corrected): cycle(1): estimate: −4.05E 2; t(4,333) = 1.34; p = 0.72. (2): estimate: −0.145; t(4,333) = 4.81; p < 4E–4. (3): estimate: −0.41, t(4,333) = 13.7; p < 4E–4. (4): estimate: −0.19; t(4,333) = −6.33; p < 4E–4. SE: 3.02E–2.
See also Figures S1–S8 and Table S1.
As we discovered previously, the most prominent readout of visual perceptual experience is the transition from a primarily stimulus-locked response to one that is oscillatory and persists for 3–5 (or in rare cases 6) distinct cycles after visual stimulation. To capture this change, we estimated oscillation duration using a peak detection algorithm on the Z score time series of each unit (Figure 2B). Before perceptual experience, differences in the mean duration of oscillatory activity were found between WT and FX mice in 3 cell types: RS in L4 and L6 and FS in L4 (Figures S2 and S3). After perceptual experience, we found significant differences in the oscillation duration of 7 cell types: RS and FS neurons in L2/3, L4, and L5 and RS in L6. These decreased durations were evident across the whole population in both male (Figure 2) and homozygous female FX mice (Figures S4A and S4B) as well as in subpopulations of units in different layers (Figures S2 and S3). Specifically, there was less prominent engagement at oscillatory cycles 3 and 4 for FX mice compared to WT (Figures 2B, S2, and S3). This is seen quantitatively as significantly decreased durations (i.e., less persistence) in FX mice compared to the WT at both the population level and in L2/3 and L4 (Figures 2C and S2A–S2D). WT mice also showed higher mean firing rates at the 3rd and 4th cycle in pyramidal cells across the population and in L2/3, L4, and L5 as well as at cycle 3 in FS cells in L2/3 and L4 (Figures 2D and S5; Table S1). L5 RS units also displayed significantly shorter mean oscillation durations in FX compared to WT mice, but no significant differences were found in L5 FS or L6 FS or RS cells (Figures S3B–S3D). Presentation of a novel stimulus to a subset of these animals elicited primarily stimulus-locked responses comparable to those seen before perceptual experience (Figure S6). Although the responses were qualitatively not oscillatory, peak detection revealed significant differences in the mean duration between WT and FX in L2/3 RS, L4 FS, and L6 RS cells, likely due to the engagement of cycle 3 for subsets of these units (Figures S6B, S6C, and S6H).
In summary, these results reveal visual experience-dependent oscillations that are less persistent in FX mice, particularly in L2/3 and L4. Consistent with our CSD analysis, these results also demonstrate that the oscillations are more prominent in the single-unit activity of the superficial compared to deep cortical layers.
Decreased Peak Response Frequency of Visually Evoked Oscillations in FX Mice
Across the full population of recorded units as well as VEPs, we noticed delays in the peak response magnitudes in FX compared to WT mice after perceptual experience (Figure 3A). This delay was observed in single-unit activity across all layers and for both RS and FS cells (see Figures S2 and S3 line plots). Raster plots from individual mice in these populations revealed this delay in FX mice robustly across trials, particularly at the later oscillation cycles, where the delay progressively increased (Figure 3B). The distributions of peak times for each cycle across these unit populations also displayed this trend (Figures 3C and 3D, top). We then quantified the mean frequency across these oscillatory cycles between WT and FX mice using the peak responses detected on the Z score time series for each unit. Only units with at least 3 distinct oscillatory cycles were considered, such that the period and frequency of peak oscillatory activity in a set of comparable units between WT and FX mice could be determined. Among these units, a significant decrease was seen in the frequency of the peak oscillatory activity in FX compared to WT mice (Figure 3C). There was overlap in the distributions of oscillation frequency for individual units between the two groups, yet the oscillations were lower in frequency in FX mice on average by ~0.68 Hz. Both the distributions of peak times for each cycle and distributions of oscillation frequency from WT and FX female mice trended toward those seen in males, though they did not reach statistical significance in this smaller dataset (Figures S4C and S4D). Quantification of the oscillation frequency for LFP recordings yielded similar results (Figure 3D). These results are consistent with other studies, which have shown that the resonance frequency at which pyramidal cells respond to sinusoidal current injections is decreased by a similar amount in FX mice (Kalmbach et al., 2015; Zhang et al., 2014). We may be seeing the consequences of this resonance frequency decrease but at the scale of neural ensembles evoked by visual stimulation in awake animals.
Figure 3. Decreased Frequency of Peak Oscillatory Activity in FX Mice.
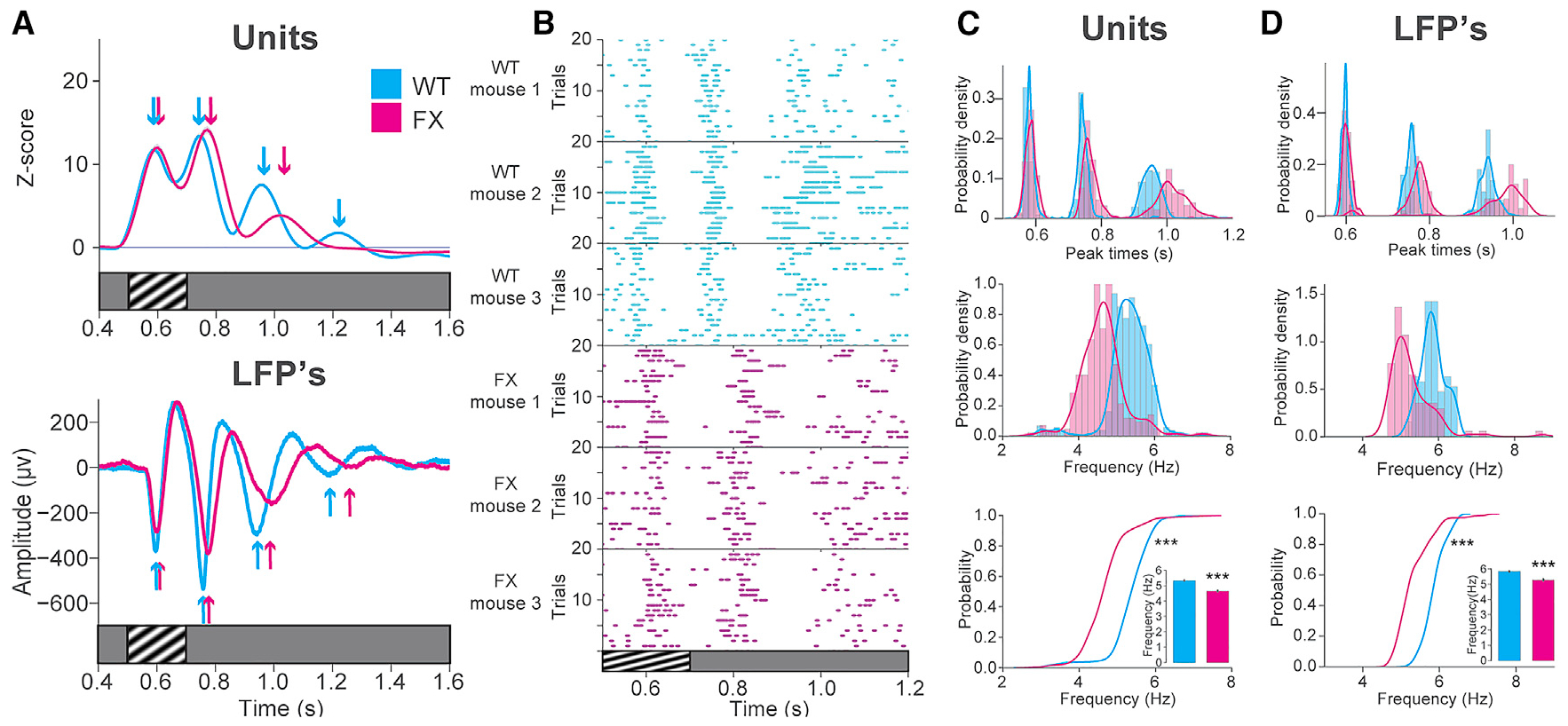
(A) Z scored firing rate for all visually excited units recorded after experience (across all layers) in WT (cyan, 31 mice; n = 1,592 units) and FX (magenta; 25 mice; n = 1,117 units) mice. Arrows indicate the peak response times.
(B) Raster plots of individual units from three WT or FX mice.
(C) Top: distributions of peak times across WT and FX unit populations at 3 oscillation cycles. Middle: distributions of oscillation frequency are shown. Bottom: cumulative distributions of oscillation frequency and mean oscillation frequency (inset) are shown. 2-sample KS test, WT versus FX frequency: D(934) = 0.65; p = 4.36E–73. Welch’s t test: t(934) = 16.25; p = 1.87E–47. WT mean frequency = 5.3307 Hz; FX mean frequency = 4.6484 Hz; difference = 0.6823 Hz (units after peak detection: WT: n = 668; FX: n = 268). Error bars indicate SEM. *p < 0.05; **p < 0.01; ***p < 0.001.
(D) Top: distributions of peak times across WT and FX LFPs at 3 oscillation cycles. Middle: distributions of oscillation frequency are shown. Bottom: cumulative distributions of oscillation frequency and mean oscillation frequency (inset) are shown. 2-sample KS test, WT versus FX frequency: D(200) = 0.66; p = 2.15E 20. Welch’s t test: t(200) = 8.16; p = 2.22E–13. WT mean frequency = 5.8484 Hz; FX mean frequency = 5.2841 Hz; difference = 0.5643 Hz.
Decreased Habituation of Visually Evoked Pupil Dynamics in FX Mice
Anxiety, hyperactivity, and hyperarousal are defining characteristics of FX. To determine the arousal state of WT and FX mice, we simultaneously recorded pupil size and locomotion during our recordings. Before the perceptual experience, both WT and FX mice increased their run speed shortly after viewing visual stimuli (Figure S7A). However, no obvious stimulus-evoked running occurred in the post or novel conditions, nor could significant differences be found in the mean running speed or percentage of mobile trials between WT and FX mice (Figures S7A and S7B). Although this observation differs from reports of FX hyperactivity in an open field setting (Dolan et al., 2013; Kramvis et al., 2013), it is consistent with a lack of locomotion differences found in head-fixed FX mice (Goel et al., 2018). On the other hand, pupil diameter is an indirect measurement of locus coeruleus activity and serves as a more robust measure of arousal. Although locomotion is often reported to increase pupil size, the dynamics of that relationship are not necessarily inextricably linked. Within each stimulus condition and within each genotype, the percent increase in pupil size relative to the baseline period was larger for mobile trials, though the response dynamics were different between measurements of pupil size and locomotion (Figure S7D). Consistent with our previous findings, both WT and FX mice displayed a sustained pupillary surprise response when viewing a stimulus for the first time (pre and novel; Kissinger et al., 2018). Although this “arousal” response was larger in FX than WT, no significant differences were found during mobile or immobile trials (Figures S7D–S7F). After perceptual experience, this arousal response was attenuated in both WT and FX mice. However, it remained significantly larger in FX compared to WT mice, suggesting decreased experience-dependent habituation to the stimulus. Arousal and locomotion are also known to have profound effects on cortical activity, both decreasing the power of spontaneous low-frequency oscillations in LFPs and exerting a gain modulation to increase firing rates (Niell and Stryker, 2010; Polack et al., 2013; Reimer et al., 2014; Vinck et al., 2015). Consistent with these findings, locomotion decreased the power of all frequency bands during the oscillations and increased firing rates compared to immobile trials in WT and FX mice (Figure S8).
Functional Connectivity Changes across Layers in V1 of FX Mice after Perceptual Experience
As demonstrated above, we observed attenuation of the visually evoked oscillations in multiple layers in FX mice relative to WT controls. Using the subpopulations of units in each layer, we sought to systematically characterize the influence of neural activity between layers to ascertain their relative contributions to visually evoked oscillatory activity in WT and FX mice. There are several methodologies available to infer functional connectivity from extracellularly recorded spikes, which can be based on pairwise rate correlations or the timing of individual spikes relative to each other. Toward this goal, we performed directed information analysis, a non-parametric generalization of Granger causality (Quinn et al., 2011, 2015). Broadly, it estimates how robustly the spike times from one or more parent units are predictive of the spike times of a single recipient unit, allowing one to model more complex dynamics than what could be achieved solely using pairwise correlations. This technique can determine not only the strength of that functional connectivity but also its directionality when the cortical layer of each unit is considered. This analysis cannot assess whether two units are directly connected, and cells just outside of the recorded tissue volume likely exert an influence through common inputs or feedback connections. However, it does provide a snapshot of functional connectivity in V1, much of which should be a result of connections that span the recorded tissue column. Directed information analysis was performed across all cortical layers and neural subtypes in WT and FX mice under each stimulus condition during the post-stimulus oscillatory period (0.7–1.5 s). In some cases, single-parent units were the most predictive of activity in the recipient unit (Figure S9A), although in others, sets of parents were optimal (Figure S9B). In an attempt to minimize the influence of indirect connections, the predictive power of parent-unit activity was considered up to 10 ms prior to the recipient unit (Markov order 10). To capture longer latency direct connections or indirect connections, we performed a concurrent analysis with a Markov order of 30. Connectivity matrices were then constructed for each condition, and the FX connectivity matrices were subtracted from their corresponding WT matrix to detect differences across genotypes (Figure 4). Before perceptual experience, we observed significantly decreased functional connectivity in multiple connections in WT compared to FX, including in the L5 RS → L4 FS, L4 RS → L4 RS, L2/3 RS → L5 FS, and L2/3 FS → L5 RS putative connections (Figure 4A). The results also trended toward stronger connections in FX with a Markov order of 30, though the L5 RS → L2/3 RS connection was strong in WT in this case (Figure S10A). After perceptual experience, we noticed dramatic increases in functional connectivity between all layers in both WT and FX mice relative to the pre-experience condition (Figure 4B). Comparing WT and FX mice, one of the most striking differences observed in the functional connectivity matrices was a decrease in connection strengths from multiple layers onto L4 FS cells in FX mice. Many of these connections were weaker in FX mice, with significant differences occurring at the inferred L2/3 FS/L4 FS (Markov 10 only), L4 FS → L4 FS, L4 RS → L4 FS, L5 RS → L4 FS, and L6 FS → L4 FS connections (Figures 4B and S10B). There was also significantly stronger connectivity in WT compared to FX from L6 RS → L6 FS (Markov 10; Figure 4B) and L6 FS → L4 FS (Markov 10 and 30), L6 FS → L4 RS (Markov 30), and L6 FS → L2/3 RS (Markov 30) cells, which suggest weaker connections in FX known to be involved in cross-cortical inhibition (Bortone et al., 2014; Olsen et al., 2012). On the other hand, many connections onto L2/3 RS cells were significantly stronger in FX, including the L2/3 RS → L2/3 RS (Markov 10), L4 RS → L2/3 RS (Markov 10), and L5 RS → L2/3 RS (Markov 30) connections (Figures 4B and S10B). Directed information matrices for the novel condition displayed decreased functional connectivity across the layers compared to the post-experience condition but were not identical to the matrices in the pre-experience condition (contrast Figure 4C with Figure 4A). Three functional connections were stronger for FX mice in the novel condition, including the L5 RS → L5 RS, L6 RS → L5 RS (Markov 10 only), and L6 RS → L6 RS connections (Figures 4C and S10C). Together, these results provide a snapshot of functional connectivity changes after perceptual experience that promote persistent visually evoked oscillations and identify connections in FX mice that might underlie impairments in these oscillations.
Figure 4. Layer- and Cell-Type-Specific Changes in Functional Connectivity in WT and FX Mice.
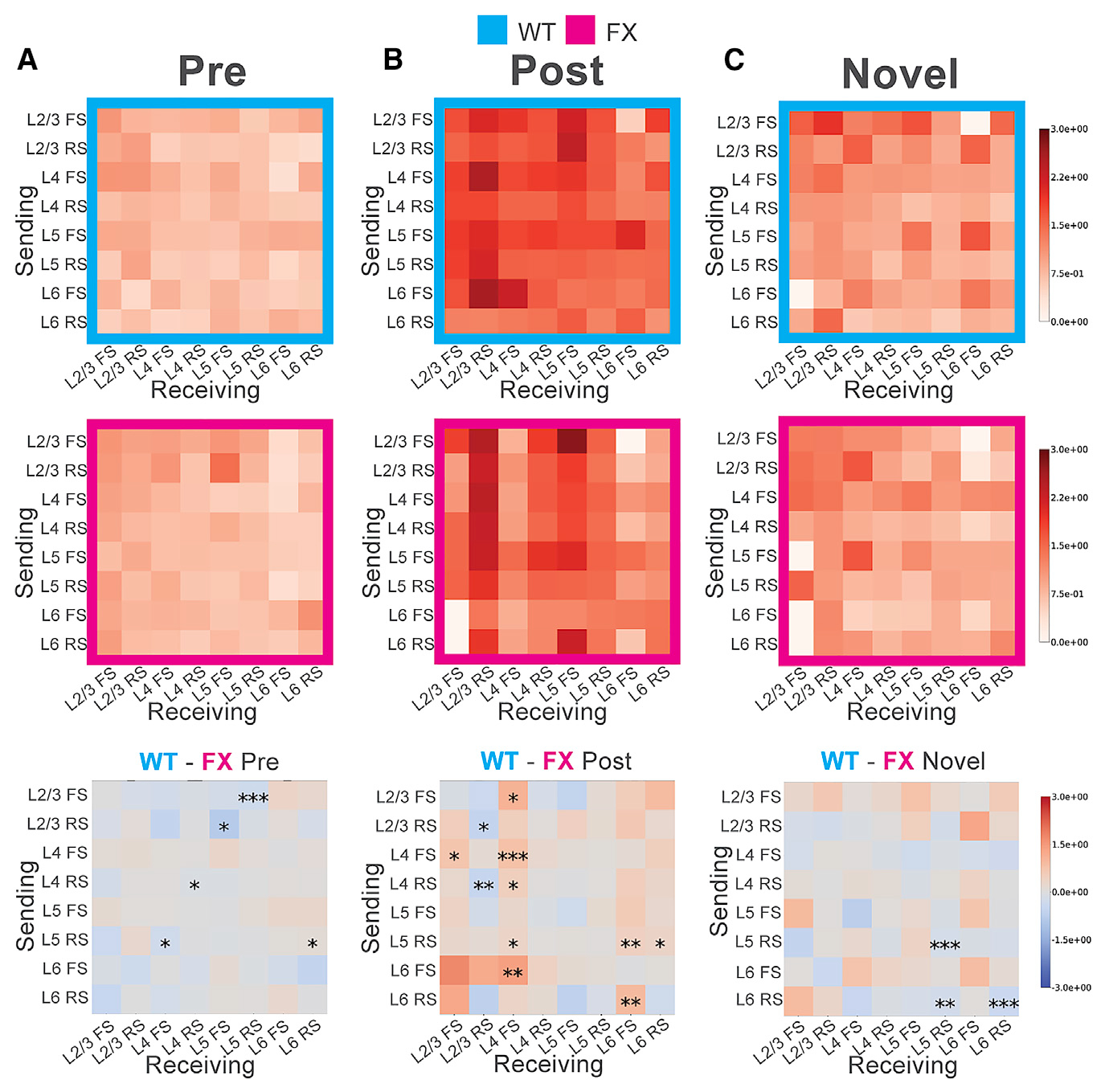
(A) Functional connectivity (normalized directed information) pre-perceptual experience for WT (cyan) and FX (magenta) mice, where a Markov order of 10 ms was used to compute directed information values. Darker colors indicate stronger (more predictive) connections. The vertical axis indicates cells in different layers sending information, although the horizontal axis indicates cells receiving that information. Bottom: difference between the WT and FX heatmaps is shown. Monte Carlo simulations (10E6 runs) were used to approximate the permutation test for each square in each difference matrix above. Significance levels: *p < 0.1; **p < 0.05; ***p < 0.01.
(B) Functional connectivity post-perceptual experience.
(C) Functional connectivity in response to novel stimuli.
See also Figures S9 and S10.
Synaptic Connectivity from L5 to L4 RS and FS Neurons following Visual Experience in FX Mice
To directly measure the V1 microcircuit and changes in its plasticity resulting from perceptual experience, we conducted channelrhodopsin-assisted circuit mapping (CRACM) on brain slice preparations (Hooks et al., 2013; Petreanu et al., 2007). Other circuit mapping methods, including paired recordings and glutamate uncaging, have limitations. Paired recordings can measure connectivity between neurons within only 200 mm of each other reliably, as the probability of connectivity decays exponentially with increasing distance, making it unlikely to measure interlaminar connections. Similarly, glutamate uncaging cannot provide the cell type specificity needed to test the connectivity changes observed in our in vivo data. Considering that one of the consistent differences in cross-layer functional connectivity in V1 between experienced WT and FX mice occurred at the L5 RS to L4 FS connection, we measured the connectivity of L4 and L5 neurons receiving projections from L5 excitatory neurons in naive and visually experienced animals. We bred homozygote Thy1-ChR2-YFP male mice with heterozygote Fmr1 KO female mice to perform these experiments. First, we validated that the ChR2 expression levels are not affected by the Fmr1 KO genotype. There was no significant difference in evoked action potential frequency at a series of light intensity steps between WT and FX agematched littermates (Figure S11A). To study the effect of the FX genotype on visual experience-induced circuit plasticity, WT and FX littermates with a Thy1-ChR2-YFP background were pseudo-randomly assigned to either naive or experienced groups while the experimenter was blinded to the genotype during data collection. The trained group was subjected to the same habituation and visual perceptual experience as described for the in vivo recordings. Acute brain slices were made for CRACM on the day after the end of the perceptual experience.
We performed whole-cell patch clamp recordings of L4 neurons in V1 on acute brain slices from 4 groups of animals: WT naive; FX naive; WT post-experience; and FX post-experience (Figure 5A). To measure local L5 to L4 synaptic strength, we optically stimulated individual cells in a 10 by 10 grid (0.67 mm by 0.67 mm) covering a square area from L2/3 to L5 of V1 using a light patterned illuminator (Figure 5B). For each 10-ms light pulse at each pixel, excitatory post-synaptic currents (EPSCs) were recorded under voltage clamp at −70 mV. All CRACM recordings were conducted with the presence of 10 μM tetrodotoxin (TTX) and 50 μM 4-aminopyridine (4-AP) to block action potentials and thus block multi-synaptic responses to the stimulation. Based on the current-voltage curve measured from step-current injections, there is a bimodal distribution of low and high cell impedance, corresponding to RS excitatory neurons and FS interneurons, respectively (Figures S11B–S11D). For a subpopulation of recorded cells, evoked action potentials were recorded before applying TTX/4-AP. In some recordings, fluorescent dye (Alexa Fluor 594 Hydrazide) was added to the pipette internal solution to allow for the subsequent reconstruction of cell morphology. There were consistent and expected correlations between impedance and action potential waveform, cell body morphology under differential interference contrast (DIC) optics, and whole-cell morphology following reconstruction that corresponds to cell types (Figures 5C–5G and 5K–5O). We found that there was no significant difference in impedance among RS cells (Figure S11C) and among FS cells (Figure S11D) between the WT and FX groups. Perceptual experience had no effect on cell impedance, as expected (Figure S11E).
Figure 5. Visual Perceptual Experience Induced Cell-type-Specific Circuit Connectivity Changes from L5 to L4 in V1 of WT and FX Mice.
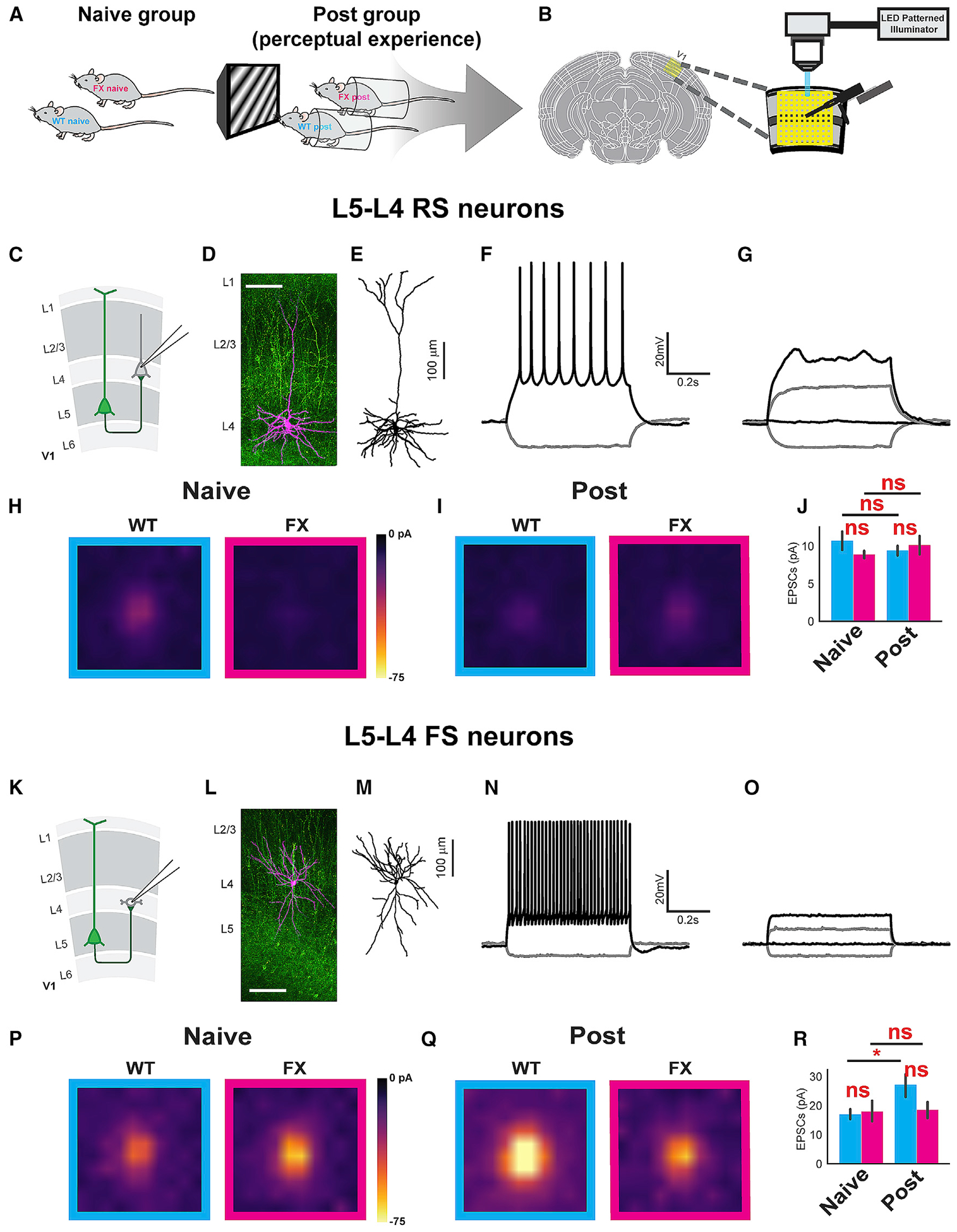
(A) Experimental groups.
(B) Acute visual cortical slices CRACM setup.
(C) Illustration of L5 to L4 RS neuron projections.
(D and L) Example tiled composite confocal image of mapped neurons (magenta) filled with Alexa Fluor 568 Hydrazide (scale bar, 100 mm). The green color indicates ChR2-YFP-positive neurons and processes.
(E and M) Traced neuron from (D) and (L) showing the morphology of specific neuronal types.
(F and N) Step current injection traces showing action potentials corresponding to the neurons shown in (D) and (L). (G and O) Step current injection traces with application of TTX/4AP mix corresponding to the neurons shown in (D) and (L).
(H and I) The averaged CRACM maps from L4 RS neurons receiving L5 projections in naive (H) and post (I) WT (cyan) and FX (magenta) animals. WT naive L4 RS: 11 mice, 32 cells. FX naive L4 RS: 11 mice, 39 cells. WT post L4 RS: 10 mice, 33 cells. FX post L4 RS: 10 mice, 39 cells.
(J) Bar graphs showing the averaged EPSC amplitudes for each group ± SEM. Significance is reported from two-way ANOVA followed by Tukey’s honest significant difference (HSD) tests to compare the mean Box-Cox transformed EPSCs (two-way ANOVA; no significant interaction was found between genotype and perceptual experience: F = 0.03; p = 0.85. Main effects after removing the interaction term: genotype: F = 0.24; p = 0.63. Perceptual experience: F = 0.22; p = 0.64. Tukey’s post hoc: WT naive versus FX naive: p = 0.66; WT post versus FX post: p = 0.83; WT naive versus WT post: p = 0.87; FX naive versus FX post: p = 0.64).
(K) L5 to L4 FS neurons projection illustration.
(P and Q) The averaged CRACM map from L4 FS neurons receiving L5 projections in naive (P) and post (Q) WT (cyan) and FX (magenta) animals. WT naive L4 FS: 11 mice, 17 cells. FX naive L4 FS: 11 mice, 11 cells. WT post L4 FS: 10 mice, 15 cells. FX post L4 FS: 10 mice, 7 cells.
(R) Bar graphs showing the averaged EPSC amplitudes for each group ± SEM. Significance is reported from two-way ANOVA followed by Tukey’s HSD tests to compare the mean log-transformed EPSCs (two-way ANOVA: no significant interaction was found between the main effects of genotype and perceptual experience: F = 0.91; p = 0.35. Main effects after removing the interaction term: genotype: F = 0.85; p = 0.36. Perceptual experience: F = 5.28; p = 0.02. Tukey’s post hoc: WT naive versus FX naive: p = 0.81; WT post versus FX post: p = 0.20; WT naive versus WT post: p = 1.79E–2; FX naive versus FX post: p = 0.63).
See also Figure S11.
To determine any differences in connection strength at the L5-L4 RS connection between WT and FX mice, we first created averaged CRACM maps for each experimental group. Only mild differences were observable in the heatmaps (Figures 5H and 5I). We then averaged the CRACM values for each cell to estimate the global differences in connection strength in accordance with established methodology (Shepherd et al., 2003). These averaged values were then Box-Cox transformed to obtain a normal distribution of EPSCs across cells and compared with a two-way ANOVA and post hoc Tukey test. Neither the main effects of perceptual experience or genotype had a significant effect on the means of the transformed averaged EPSCs (Figure 5J). Next, we examined connectivity changes in the L5-L4 FS connection. The averaged CRACM heatmaps appeared hotter post-visual experience compared to naive for WT, but not FX, mice (Figures 5P and 5Q). This observation was confirmed by quantifying the mean of the loge transformed averaged EPSC amplitudes in this connection, which revealed significantly increased synaptic strength after perceptual experience in WT, but not FX, mice relative to the naive state (Figure 5R). However, no significant differences between the mean EPSC values could be found in direct comparisons between WT and FX in either the naive or the post-experience conditions. The potentiation of L5 to L4 FS cells in WT animals after perceptual experience relative to pre and the lack of this change in FX animals largely agree with directed information analysis for this particular connection.
Synaptic Connectivity from L5 to L5 RS and IB Neurons following Visual Experience in FX Mice
We next conducted CRACM measurements on L5 to L5 local connectivity in WT and FX mice, either with or without visual perceptual experience. Putative FS interneurons (identified as described above) were discarded from the analysis due to low cell counts (0–2 cells per group). The remaining putative L5 excitatory neurons were divided into intrinsically bursting (IB) and RS neurons (Figures 6A–6E and 6I–6M) based on input resistance, sag ratio, and the presence/absence of a compensatory current after a depolarizing current step (Figures S11F–S11M). Consistent with the literature, L5 IB cells had lower input resistance and higher sag ratio, as well as a prominent compensatory current after depolarization compared to RS cells (Kasper et al., 1994). Cell types determined by these three parameters were consistent in each of the patched cells and confirmed by cell morphology when available. Because IB cells and RS cells have distinctive projection targets (Kasper et al., 1994) and may contribute to the oscillations in different ways, we analyzed the connectivity of these two groups of cells separately. The averaged CRACM heatmaps for the L5 to L5 RS connection appeared hotter for FX compared to WT in the naive state and for FX in the naive state compared to the post-experience state (Figures 6F and 6G). Although no significant differences could be found in direct comparisons between WT and FX in this connection, a significant depression was observed after perceptual experience in FX mice (Figure 6H). L5 IB cells receiving L5 recurrent projections yielded hotter connectivity heatmaps in both WT and FX compared to the L5-L5 RS connection but did not suggest any difference in connectivity between the experimental groups (Figures 6N and 6O). No significant differences could be found in the mean EPSC strengths of any comparison for this connection (Figure 6P).
Figure 6. Visual Experience Induced Cell-Type-Specific Local L5 Circuit Connectivity Changes.
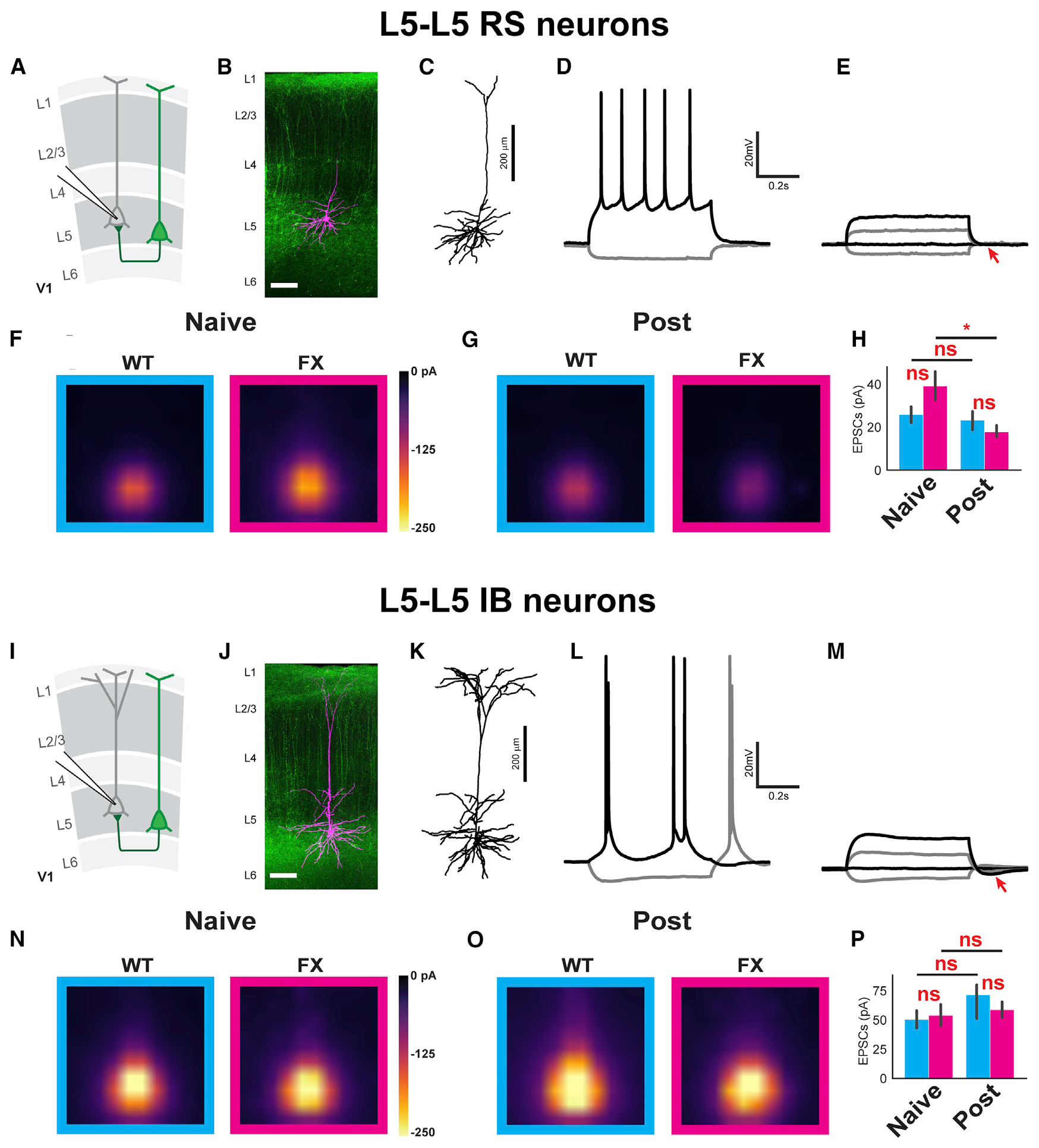
(A and I) Illustration of L5 to L5 regular spiking pyramidal neuron projections (A) and L5 to L5 intrinsically bursting neuron projections (I).
(B and J) Example tiled composite confocal images of mapped neurons (magenta) filled with Alexa Fluor 568 Hydrazide (scale bars represent 100 μm). The green color indicates ChR2-YFP-positive neurons and processes.
(C and K) Traced neuron from (B) and (J) showing the morphology of specific neuronal types corresponding to (B) and (J).
(D and L) Step current injection traces showing action potentials corresponding to the neurons shown in (B) and (J).
(E and M) Step current injection traces in the presence of TTX/4-AP corresponding to the neurons shown in (B) and (J). The arrow points to the absence/presence of compensatory potential after depolarizing current injection.
(F and G) The averaged CRACM map from L5 RS neurons receiving L5 local projections in naive (F) and post (G) (cyan) and FX (magenta) animals. WT naive L5 RS: 11 mice, 29 cells. FX naive L5 RS: 11 mice, 36 cells. WT post L5 RS: 10 mice, 32 cells. FX post L5 RS: 10 mice, 31 cells.
(H) Bar graphs showing the averaged EPSC amplitudes for each group ± SEM. Significance is reported from two-way ANOVA followed by Tukey’s HSD tests to compare the mean Box-Cox transformed EPSCs. Two-way ANOVA, no significant interaction was found between genotype and perceptual experience: F = 1.24; p = 0.26. Main effects after removing the interaction term: genotype: F = 0.04; p = 0.83. Perceptual experience: F = 5.65; p = 0.02. Tukey’s post hoc: WT naive versus FX naive: p = 0.56; WT post versus FX post: p = 0.30; WT naive versus WT post: p = 0.37; FX naive versus FX post: p = 3.46E–2.
(N and O) The averaged CRACM map from L5 intrinsically bursting (IB) neurons receiving L5 local projections in naive (N) and post-WT (O) (cyan) and Fmr1 KO (magenta) animals. WT naive L5 IB: 11 mice, 19 cells. FX naive L5 IB: 11 mice, 17 cells. WT post L5 IB: 10 mice, 13 cells. FX post L5 IB: 10 mice, 12 cells.
(P) Bar graphs showing the averaged EPSC amplitudes for each group ± SEM. Significance is reported from two-way ANOVA followed by Tukey’s HSD tests to compare the mean raw EPSCs (two-way ANOVA, no significant interaction was found between genotype and perceptual experience: F = 0.6885; p = 0.41. Main effects after removing the interaction term: genotype: F = 0.11; p = 0.74. Perceptual experience: F = 1.88; p = 0.18. Tukey’s post hoc: WT naive versus FX naive: p = 0.77 WT post versus FX post: p = 0.45; WT naive versus WT post: p = 0.16; FX naive versus FX post: p = 0.71).
See also Figure S11.
DISCUSSION
Attenuation of a Neural Correlate of Visual Familiarity in FX Mice
In this study of a mouse FX model, visual perceptual experience resulted in decreased amplitudes of oscillatory visually evoked potentials in FX mice relative to WT. Considering that LFPs are primarily driven by synaptic activity within the local tissue volume, this attenuation in FX mice may be a result of impaired synaptic plasticity at the connections that drive the oscillation. Decreased theta (4–8 Hz), beta (12–30 Hz), low gamma (30–50 Hz), and high gamma (50–80 Hz) baseline normalized power was also observed in FX compared to WT mice, with alpha power decreased, but not reaching significance. This complex oscillatory profile may point to impairments in a diverse group of recurrent connections in FX mice. The most well-characterized driver of oscillatory activity is the thalamocortical system. Thalamocortical recurrent connectivity is known to drive cortical oscillations over many frequency ranges, the most prominent of which include the alpha (Bollimunta et al., 2011), beta (Bastos et al., 2014), and gamma (McAfee et al., 2018) bands during awake states. Therefore, it is feasible that perceptual experience strengthens thalamocortical recurrent connectivity and that this is impaired in FX mice. Theta, the predominant frequency observed in the oscillations described here, is not a typical thalamocortical generated oscillation in awake states. Rather, thalamocortical theta falls within the lower range of spindle oscillations occurring during sleeping states or absence seizures (Crunelli and Leresche, 2002; Fogerson and Huguenard, 2016). The possibility also remains that the oscillation is generated locally within V1 or is a result of strengthened feedback connections from higher order cortical areas. Different frequency bands have been attributed to the directionality of information flow between visual cortical areas, with theta and gamma associated with feedforward information, although alpha and beta are associated with feedback (Bastos et al., 2015; Michalareas et al., 2016; Richter et al., 2017; van Kerkoerle et al., 2014). Therefore, the differences in oscillatory activity we have observed in V1 of FX may arise from both thalamocortical and interareal influences, though future studies will be necessary to elucidate these possibilities.
The duration of this oscillatory activity was also decreased on average in FX mice compared to WT. Although we still do not fully understand what determines oscillation duration, we can hypothesize that longer durations are representative of better encoding of the visual information. If indeed top-down, local, or thalamocortical feedback connections are important for driving the oscillations, stronger plasticity in these recurrent connections might allow the oscillations to persist longer in the absence of feedforward input. It may also be true that the oscillations are not simply a neural signature of familiarity but represent an active encoding process of that familiarity. As has been demonstrated in the hippocampus, an oscillation can be used to promote cellt-ype-specific synaptic plasticity (Zarnadze et al., 2016). A common LTP protocol based on an oscillatory stimulus, known as theta burst stimulation, is also known to strengthen synaptic connections (Larson et al., 1986). By analogy, visually evoked oscillations in the cortex may generate a strengthening of synaptic connections that are activated by a familiar stimulus. If the frequency of this oscillatory activity decreases too much, then this visual information may be encoded less efficiently. In turn, the stimulus may resist becoming familiar, despite repeated exposure during the perceptual experience. Although we do not know why the frequency of peak oscillatory activity is decreased in FX mice, we speculate that it may depend on the resonance properties of neurons involved in the oscillatory circuit. Resonance is the frequency at which neurons optimally respond to oscillatory current input and is determined by their passive membrane and channel properties (Hutcheon and Yarom, 2000). Resonance depends on hyperpolarization-activated cyclic nucleotide-gated channel 1 (HCN1), which is expressed extensively in apical dendrites and drives nonselective cation currents after hyperpolarizing input (Narayanan and Johnston, 2007; Zemankovics et al., 2010). L5 pyramidal neurons, driven by optogenetic stimulation of PV+ interneurons in the neocortex, exhibit HCN1-dependent theta resonance (Stark et al., 2013). Decreased resonance frequency has been observed in the primary somatosensory cortex (S1) and the prefrontal cortex of FX mice compared to WT controls (Kalmbach et al., 2015; Zhang et al., 2014). The intrinsic properties of the HCN1 channels in S1 appear normal in FX mice, but their expression levels are significantly reduced in FX mice, which may account for the resonance frequency shift (Zhang et al., 2014). Our results reveal both attenuated oscillatory power together with an altered temporal profile, though whether and how these results are related to each other remain unclear.
Impaired Connectivity in V1 after Perceptual Experience in FX Mice
Directed information, a non-parametric measure of Granger causality, can be used to estimate the functional connectivity between different groups of neurons (Quinn et al., 2011). Performing this analysis between all cortical layers and between RS and FS cells has allowed us to ascertain the cortical wide changes in functional connectivity induced by perceptual experience, as well as the key differences in that connectivity between WT and FX mice. The largest number of significant differences between WT and FX occurred at functional connections from multiple layers and cell types onto L4 FS cells. Previous work has demonstrated impaired excitatory drive onto inhibitory cells in L4 of somatosensory cortex in FX mice (Gibson et al., 2008). Reduced visually evoked activity in PV cells has also been observed in FX mice, and increasing that activity with designer receptors exclusively activated by designer drugs (DREADDs) has been shown to increase performance on a visual discrimination task (Goel et al., 2018). This decreased inhibition in FX mice has long been thought to underlie the cortical hyperexcitability seen in FX, though recent work has suggested that this E/I imbalance is representative of a compensatory mechanism to preserve the peak depolarization of neurons rather than increase it (Antoine et al., 2019).
Another significant difference in functional connectivity was observed from L6 FS cells onto L4 FS cells. Excitatory cells in L6 have been shown to inhibit the activity of all other layers of V1, suppressing them primarily via intracortical connections but also through indirect suppression of the dorsolateral geniculate nucleus (dLGN) via the thalamic reticular nucleus (Olsen et al., 2012). Interestingly, the intracortical inhibition driven by these L6 excitatory cells occurs at least in part due to their activation of FS inhibitory cells in L6 that send axonal arborizations across all other layers (Bortone et al., 2014). Consistent with these ideas, we also observed significantly stronger functional connectivity from L6 RS cells onto these L6 FS cells in WT compared to FX. These differences were not observed in the pre-experience or novel conditions.
Although L4 interneurons are well known for their role in feedforward inhibition of thalamocortical input, less is understood about the role of intracortical feedback connections onto these cells. Within the context of our study, we can only speculate as to why the L5 RS to L4 FS connection may be important for the propagation of visually evoked oscillatory activity. A computational model offers one possibility, where an IB pyramidal cell can form connections onto a FS interneuron, which then connects to a RS cell that closes the loop by connecting back onto the IB cell and forming an oscillator (Visser and Van Gils, 2014). One feature of this model is the generation of bursts of action potentials within the recurrently connected IB cells, which is dependent on the Ih current. Another feature of the model is the feedback connection between the L5 IB cells and the FS interneurons. The weakening of this connection leads to the abolishment of the theta oscillation within the network, consistent with our in vivo functional connectivity and in vitro synaptic connectivity measurements.
FMRP is a transcriptional master switch that is upstream of many synaptic-plasticity-related proteins (Niere et al., 2012; Sidorov et al., 2013). The absence of FMRP leads to exaggerated mGluR-dependent LTD in the hippocampus and cortex and an elevated threshold for LTP (Huber et al., 2002; Yun and Trommer, 2011). Here, we systematically measured synaptic connections within the V1 microcircuit with and without perceptual experience in WT and FX mice. We observed a general shift toward depression (smaller magnitude of potentiation and/or larger magnitude of depression) in FX compared to WT mice after perceptual experience. This finding is consistent with the previous observations of enhanced LTD and decreased LTP in FX mice (Huber et al., 2002; Lauterborn et al., 2007; Yun and Trommer, 2011).
Measuring visual cortical circuit connectivity before and after visual experience has allowed us to identify the synaptic connections that changed after perceptual experience and verify connectivity differences identified in the in vivo spiking data. We have identified strengthening of the intracortical connections from L5 pyramidal cells onto FS inhibitory interneurons in L4 after perceptual experience in WT, but not FX, mice. As described previously, computational modeling suggests that the strengthening of excitatory to inhibitory connections may be critical for the generation and propagation of low-frequency oscillations in a cortical circuit (Visser and Van Gils, 2014). The lack of strengthening of the L5 RS to L4 FS connection in FX mice may partially explain the weaker and shorter oscillations observed after visual experience in these mice. Interestingly, following visual experience, synaptic strengths in V1 are not uniformly strengthened to the same magnitude. Some remained stable although others slightly weakened (Figures 5 and 6). The differential plasticity at different synapses of the circuit may be important for achieving this oscillatory behavior.
Given its expression in dendrites, the cell body, and axons and its role as a translational regulator, loss of FMRP is expected to have diverse consequences on neural activity (Christie et al., 2009; Darnell et al., 2011). Altered expression of proteins involved in synaptic plasticity may lead to an altered profile of synaptic weights within the microcircuit and, consequently, weaker oscillations, although the frequency shift may be mediated by reduced HCN1 expression and impaired dendritic Ih function. Our work suggests that the identification of impairments in experience-dependent neural activity at both large scales across cortical layers and in the local microcircuitry may be critical for understanding the pathophysiology of FX in sensory systems. Furthermore, this work has strengthened our understanding of the circuit mechanisms driving the visually evoked oscillations themselves, allowing us to better use this visual perceptual experience paradigm to study models of intellectual disability or diseases impacting visual learning and memory.
STAR★METHODS
LEAD CONTACT AND MATERIALS AVAILABILITY
While no unique reagents were generated during this study, requests regarding resources, reagents, and any additional information should be directed to the lead contact, Alexander A Chubykin (Chubykin@purdue.edu). The address for correspondence is 915 W State St, West Lafayette, IN 47907, Department of Biological Sciences, Purdue University
EXPERIMENTAL MODEL AND SUBJECT DETAILS
All animal procedures in this study were approved by Purdue Animal Care and Use Committee (PACUC, protocol number 1408001112). Adult B6.129P2-Fmr1tm1Cgr/J (Fmr1 KO, JAX Stock No. 003025), B6.Cg-Tg(Thy1-COP4/EYFP)18Gfng/J (Thy1-ChR2-YFP, JAX Stock No. 007612), and wild-type (WT) C57/BL6 mice (all strains obtained from JAX) were used as breeders. For in vivo extracellular recordings, P60-P65 littermate-controlled WT and Fmr1 KO male or female (homozygous) mice were used. For in vitro CRACM experiments, P35 to P39 littermate-controlled WT and Fmr1 KO male mice in the background of heterozygous Thy1-ChR2-YFP were used. These mice were generated from breeding homozygous Thy1-ChR2-YFP females with Fmr1 KO males.
METHOD DETAILS
Surgical Procedures
The surgical procedures for head-fixed mice follow those previously described. To summarize: Littermate matched C57BL/6 or Fmr1 KO mice were selected for surgery at ~P55. A head post was implanted, and a headcap formed with metabond™ bone cement under 1.5% inhaled isoflurane anesthesia. The coordinates of the binocular visual cortex (from lambda: AP 0.8 mm, ML ± 3.2 mm) were marked using Neurostar™ stereodrive software. After a day of recovery, awake mice began habituation to the head-fixation apparatus for a minimum of 4 days (90 min/day). For mice that were habituated on a treadmill instead of a stationary (tube) set up, at least 6 days were allowed to ensure they had learned to control the wheel. The apparatus for stationary animals consists of an immobile tube that secures the mouse on a raised platform 16.51 cm directly in front of and centered on a 47.63 cm × 26.99 cm monitor screen and a bar to hold the surgically implanted head post. The apparatus is the same for the mobile setup, with the exception that the animal can freely move on a 6” diameter vertical treadmill. The majority of the mice in this study were recorded on the treadmill, but 7 WT and 7 FX were included from the stationary set up and were considered immobile as it was not possible for them to run. On the first day of habituation, some mice displayed signs of struggle in order to escape head fixation in the stationary setup. By the third or fourth day of habituation and during recording sessions, we did not observe these behaviors. Mice also exhibited grooming behavior by the third or fourth day of habituation. On the treadmill, many mice moved cautiously the first 3 days of habituation but could typically run well by day 5 or 6 when they chose to. On the recording day (~P60), a craniotomy was made above the visual cortex of a single hemisphere during ~5min of inhaled anesthesia (1.5% isoflurane) in the stereotaxic apparatus. Mice were then head fixed to the experimental apparatus, and a 64 channel silicon electrode was inserted normal to the surface of the binocular area of the primary visual cortex (AP: 0.8 mm, ML: +/− 3.2 mm, DV +1.0 mm from Lambda). 30 mins was allowed for the animals to fully awaken from anesthesia before recording.
Visual Stimulation
Open source python based psychology software (PsychoPy) was used to present visual stimuli. Control gray screen was created using the color space “gray.” The mean luminance of the monitor was 73 cd/m2. After a day of recovery, mice began habituation to the head-fixation apparatus. During habituation, mice viewed a control gray screen for 90mins per day. Mice were shown single 0.2 s sinusoidal drifting gratings (spatial frequency (SF) = 0.03 cycles per degree of visual angle, temporal frequency (TF) = 3 Hz, speed = 100 deg/s, oriented and drifting at an angle of 150 degrees) for 20 trials in pre-experience recordings for experiments in the immobile setup, or 40 trials for experiments on the treadmill. Gray screen was presented for 0.5 s before stimulus onset to serve as a baseline with a total recording time of 2.5 s for each trial, with an inter-trial interval of 8 s. Mice were then trained to the same stimulus for 4 days. Animals were trained to this stimuli 200 times in 30 mins each day for 4 days. Post-experience recordings included the same visual stimulation paradigm as pre-experience, but with the addition of a novel stimulus in the form of a checkerboard for a subset of mice.
Data Acquisition and Python Packages
Recordings were made using 64 channel silicon probes, 1.05 mm in length, with channels separated 25–50 μm vertically and 16–20 μm horizontally (Shobe et al., 2015). Recordings were made in sets of 20 trials for stationary mice or 40 trials for mice recorded on the treadmill, 4.0 s in duration (later cut to 2.5 s per trial). Raw traces and bandpass filtered units (300 to 6000 Hz) were digitized at 30 kHz and acquired with OpenEphys acquisition hardware and software. Local field potentials (LFP’s) were filtered (1–300 Hz) from raw traces post hoc. All data were plotted and analyzed with jupyter notebook using custom Python code written in our laboratory. Open source data analysis libraries including Pandas, Scipy, Matplotlib, Seaborn, and sklearn were used to analyze and plot the data. Pupillometry recordings were acquired with a Thorlabs DCC1545M camera positioned approximately 28.5 cm away from the mouse eye, while the pupil was illuminated with infrared light. The videos were analyzed post hoc.
LFP analysis
Raw traces were down sampled to 1 kHz and manually inspected for artifacts before further analysis. A notch filter was applied to remove 60 Hz noise. LFPs were compared between animals by taking the first and strongest trial averaged (20 trials for stationary mice or 40 trials subdivided into mobile or immobile trials for treadmill mice) visually evoked potential (VEP) elicited after visual stimulation (putative L4 VEPs) from each of the 3 channel columns of the silicon probe, which were then averaged to obtain a single LFP recording per animal. Because the probes were inserted normal to the surface of the cortex, we could ascertain the current source density (CSD) profile of visually evoked responses. CSD analysis was performed on the trial averaged VEPs across the cortical depth using the spline iCSD method in python on a single column of channels on the 64 Ch probe (Aizenman et al., 1996; Łeski et al., 2007; Mitzdorf, 1985; Pettersen et al., 2006). To determine the average amplitudes of the trial averaged VEPs between mice, 4 windows of time were used to capture local minima (VEP1: 0.53–0.63 s, VEP2: 0.73–0.83 s, VEP3: 0.93–1.03 s, VEP4:1.15–1.25 s) corresponding to the VEP timings observed in our recordings. Time-frequency analysis was performed by using complex wavelet convolution on trial averaged L4 VEPs across mice (Cohen, 2015). We used a series of complex wavelets to extract power and phase at each sample point. We used 40 frequencies across a logarithmic range from 2 to 80 Hz, with the number of cycles of the wavelet ranging from 3 to 10 for an optimal time-frequency precision tradeoff. Power was dB normalized to the baseline period. The mean power was then calculated across the Theta (4–8 Hz), Alpha (8–12 Hz), Beta (12–30 Hz), Low Gamma (30–50 Hz), and High Gamma (50–80 Hz) frequency ranges for each animal, and was then used to compare between groups.
Spike detection and sorting
We used Kilosort, a template based clustering algorithm implemented in MATLAB, to detect and sort spikes from raw binary data (Pachitariu et al., 2016). We used the default Kilosort parameters, but set a threshold of 6 SD for spike detection and initialized the templates from data. Clusters were further manually inspected using the Phy template GUI, and several criteria were used to determine the quality of units to be used for further analysis (Rossant et al., 2016) as previously described (Kissinger et al., 2018).
Single unit analysis
Peristimulus time histograms (PSTHs) of single unit activity were computed using 10 ms bins and smoothed with a Gaussian Kernel (width = 100 ms). PSTHs for immobile and mobile trials were created separately, where at least 10 mobile trials had to be present for those trials to be included in the mobile PSTH. All recordings had at least 10 immobile trials. For heatmaps, z-scores were calculated by normalizing to the mean firing rate (FR) across all time (z = (FR – mean FR)/SD. FR). For population time course line plots, z-scores were calculated by normalizing FR to the baseline period (0–0.5 s)(z = (FR – mean baseline FR)/SD baseline FR). To quantify the duration of oscillations in single units, we applied a peak detection algorithm on the z-transformed PSTH with the following criteria: 1) The minimum peak height must be at least 1.5 SD from baseline, 2) the first peak must be within 100 ms of the stimulus onset, 3) peaks must be within 200 ms from one another. The timing of visual stimulus onset (0.5 s) was then subtracted from the timing of the last detectable peak to ascertain oscillation duration. Units were grouped into different clusters using K-Means, an unsupervised clustering algorithm from the sklearn Python package. The input matrix was the PSTH zscore of single units 0 to 1.2 s after the onset of visual stimulation (the timing window where visually evoked oscillations are observed). We used simple 2-cluster unbiased k-means clustering to separate units into those that were strongly excited during the oscillatory period and those that were not. All units with waveform trough to peak times less than 0.45 were considered putative FS neurons, resulting in ~20%–23% of the visually excited units recorded for each condition being sorted into this category. FX mice had 3%–4% fewer FS cells on average for each condition, though this was not significant.
Directed Information Analysis
Layer-Wise Connection Strength Estimation:
We used a statistical procedure to infer putative connections between units. Connection strength was measured with regularized directed information based on parametric regression models. Those unit-level connection strengths were then aggregated into layer-wise connection strengths.
Spike Train Preprocessing:
For each recording, we only used spikes that occurred between 700 ms (end of stimulus onset) and 1500 ms for each trial to capture the persistent oscillatory period. Time was discretized into 1 ms bins, resulting in binary-valued spike trains for each unit.
Partially-Exhaustive Search for Putative Connections Between Units:
To identify putative connections between units, for each unit Y we used a partially-exhaustive search over all sets of candidate pre-synaptic units from the same recording. If a recording contained few units, then for each unit Y, all sets of up to four other units were examined as sets of candidate pre-synaptic units for Y. Otherwise, all sets of cardinality one were examined, and for cardinality k with 2%k%4, 10,000 sets of candidate pre-synaptic units with that cardinality were selected based on the best candidates from the k = 1 and k-1 searches.
Regressions:
To assess the statistical fit of each set of candidate pre-synaptic units for each unit Y, we modeled Y’s activity with a parametric model. The activity Y(t) was regressed using several exogenous variables. Two exogenous variables were for Y’s own past, Y(t-1)+Y(t-2), specified to capture the refractory period, and Y(t-3)+…+Y(t-10) to capture self-dependence. One exogenous variable, X(t-1)+ …+X(t-10), was used for each unit X in the set of candidate pre-synaptic units. Lastly, a constant was used as an offset. The Markov order of 10 ms and choice of exogenous variables were selected ad hoc to balance model simplicity, accuracy, and computational burden. To demonstrate that 10ms is not too limiting of a Markov order, in the supplementary material we include an analysis using a Markov order of 30 ms and obtain comparable results.
For regression, a generalized linear model using the logit link function (logistic regression) was used. For a given unit Y, a set of candidate pre-synaptic units, and a time t, the likelihood of the binary variable Y(t) given the column vector of exogenous variables x and row vector of parameters θ was modeled as
| (1) |
Regression was performed using the GLM fit function for the binomial family in the Statsmodels (v0.9.0) package for Python (v3.7.0) to find maximum likelihood estimates for the parameter vector θ. The time periods [700 ms, 1500 ms] for all the trials were used together for regression. Periods of time with no spikes from Y or candidate pre-synaptic units and for which there were no spikes in the 100 ms prior were not included, to mitigate data imbalance.
Directed Information Calculation:
To measure the strength of candidate connections, we used regularized directed information. Directed information is an information theoretic quantity that measures in bits how well the past of one (or more) time-series predicts the future of another (Marko, 1973). It is a non-parametric generalization of Granger causality (Granger, 1969) using expected cumulative regret with the log-loss function (Quinn et al., 2015). It was independently discovered as transfer entropy (Schreiber, 2000).
For a time-series Y(t) and a (possibly vector-valued) time-series X(t), the directed information from X to Y under joint distribution P(X(1),…,X(T),Y(1),…,Y(T)) with a Markov-order one model is
| (2) |
The argument in the sum is also the conditional mutual informationl(X(t – 1); Y(t)|Y(t – 1))
For every recorded unit Y and set of candidate pre-synaptic units, we estimated the directed information. To do so, we used the maximum likelihood estimate of the parameter vector θ for the Markov-order 10 ms model described above and took the empirical average as an approximation to the statistical average. The empirical average is known to converge to the statistical average for stationary conditional distributions (Quinn et al., 2011).
Minimum Description Length:
To avoid over-fitting and to compare candidate sets of pre-synaptic units with different cardinalities, we regularized the directed information estimates. We used the minimum description length (MDL) complexity penalty for parametric models (Grünwald, 2007). Regularizers for parametric models assist in comparing models with different numbers of parameters and mitigate over-fitting. They balance low model error (low negative log likelihood) and using few parameters (low model complexity). Using MDL, the total complexity of a Markov-order one model Pθ of time-series Y conditioned on its past and the past of time-series X and parameterized by θ is
where |θ| is the number of parameters. We seek the model with the overall lowest complexity. We can compare how well any set X of candidate pre-synaptic units performs compared to the null model (with Y(t) only depending on Y’s past) by taking the difference between total complexities, yielding that we seek to maximize
where θX and θ0 are the parameter vectors of the candidate and null models, respectively. A value of zero entails that the improvement in modeling the data is the same as would be expected due to overfitting. For each unit Y, this quantity was computed for all sets X of candidate pre-synaptic units using the fitted Markov-order 10 ms models. The set X of candidate pre-synaptic units with the largest value greater than zero was selected as having putative pre-synaptic connections to Y. The values were then normalized by the complexity of Y alone to yield a percentage of how much of unit Y’s randomness was reduced by conditioning on the past of X.
Layer-wise Connection Strength Estimation
After computing connection strengths between units and selecting putative connections, we then assigned connection strengths between layers as follows. For each unit Y, we evenly divided the regularized, normalized directed information among Y’s putative presynaptic units. Thus, each putative connection between a pair of units had a corresponding value for strength. We then aggregated the weights based on layers of the pre-synaptic and post-synaptic units in the putative connections. Thus, for every ordered pair of layers, there was a vector of connection weights from the putative connections between units from these layers.
To assess the overall differences between wild-type and Fragile-X type mice due to perceptual experience, we first computed the median connection weights between each ordered pair of layers. For each ordered pair of layers, we then took the difference between the median weight for those layers using wild-type post-experience data and the median weight for those layers using Fragile-X type post-experience data.
Significance Testing:
Significance was tested using Monte Carlo approximations for two-sided permutation tests. One million iterations were used for every pair of layers for each heatmap in Figure 4. For each iteration, each putative edge from a unit in the pre-synaptic layer to a unit in the post-synaptic layer was relabeled as wild-type or Fragile-X using proportions of possible edges between the corresponding ordered pair of layers. The number of possible edges was calculated based on the number of units in each layer in each WT and FX post-experience recording and the in-degree limit used in the search for putative connections. For each iteration, the medians of non-zero edge strengths were calculated for wild-type and Fragile-X edges, and the difference of the median computed. Monte Carlo approximations were used because some layer-layer pairs had no or few inferred edges in any wild-type or any Fragile-X recordings, precluding the use of standard tests like the Wilcoxon rank-sum test. The Monte Carlo approximations were computed in Python. We then corrected the p values by controlling the false discovery rate using the Benjamini-Hochberg procedure, implemented in the Statsmodels package (Benjamini and Hochberg, 1995). Since there were eight layers, there were 64 layer-layer pairs. With three settings, pre-experience, post-experience, and post-experience with novel stimuli, there were 3*64 = 192 simultaneous hypothesis tests.
Cross Correlation Analysis of Unit Pairs
Cross-correlation analysis is a simple, interpretable procedure to examine statistical correlation between pairs of spike trains over time. It allows for researchers to visually inspect and quantify how likely one unit will have an action potential a short period after another unit does. With appropriate normalization of spiking coincidences between the pair, significance of the observed peaks (for excitation) or troughs (for inhibition) can be measured. We followed standard cross-correlation methods (Dupret et al., 2013). We used 50 ms windows before and after pre-synaptic action potentials, with 1 ms bins. For each pre-synaptic action potential, we counted the number of post-synaptic action potentials within 50 ms before and afterward. We then normalized by the number of pre-synaptic action potentials. We subtracted a baseline rate measured by averaging the 30–50 ms before and after the pre-synaptic action potential. We then identified the highest peak 1–3 ms after the pre-synaptic action potential. The peak height above baseline was interpreted as the spike transmission probability. A significance threshold was set for 3 standard deviations from the baseline.
Pupillometry and Locomotion Analysis
All recordings of mouse pupil size were analyzed post hoc using custom programs written in Python and utilizing the open source computer vision library OpenCV. We imaged the mouse eye at 400 × 300 pixels at 20 Hz with an infrared (IR) camera and lens (Thorlabs). The eye was illuminated with an 850 nm IR LED. (CMVision IR30). Acquired videos were analyzed using the OpenCV library in Python. For each video, the region of interest that only included eye boundaries was selected. For each frame, we first performed image histogram equalization to improve the contrast of the images followed by a Gaussian blur. To perform image segmentation and separate the pupil from the rest of the eye, we applied binary image thresholding. The morphological transformation function morphologyEx was used to remove noise. This was achieved by first using erosion that removes white noise, followed by dilation to restore the original object boundaries, effectively removing white noise. Then we identified the pupil contours by using the function ‘findContours’ with a mode (RETR_TREE) and method (CHAIN_APPROX_SIMPLE). We used a minimum enclosing circle to define the pupil and to remove edge artifacts caused by whiskers and the IR illumination. Following these procedures, pupil area was extracted. If the eye was not sufficiently illuminated, we could not properly track the pupil and had to exclude those recordings from analysis. All recordings of pupil diameter were baseline normalized and reported as a % change from baseline. Locomotion was acquired using a rotary encoder (U.S. Digital) attached to a custom made treadmill. TTL squarewave outputs from the rotary enoder were sent to both an Arduino board and the OpenEphys data acquisition board. Inputs to Arduino from the rotary encoder were processed at 40Hz. Mobile trials (running faster than 0.5 cm/s from 0.5 to 1.0 s in a trial) were segregated from immobile trials to reveal any differences in stimulus-evoked running, and to determine the influence of locomotion on pupil size.
Ex vivo acute cortical slice preparation
Animals were euthanized the next day after the last day of visual perceptual experience (between P35 and P39). A cocktail of ketamine (100mg/kg body weight) and xylazine (16mg/kg body weight) was intraperitoneally (IP) injected to anesthetize the animal. Then, the animal was trans-cardially perfused with chilled high-sucrose dissection buffer (HSDC) containing (in mM) 75 sucrose, 10 glucose, 87 NaCl, 2.5 KCl, 1.25 NaH2PO4, 25 NaHCO3, 0.5 CaCl2, 7 MgCl2, and 1.3 ascorbic acid. The brain was quickly removed from the skull, the cortices were cut into blocks and super-glued onto the vibratome (Leica VT1000) stage. Brain slices were cut at 300mm thickness in ice-cold HSDB and then transferred into a holding chamber in 32°C artificial cerebral-spinal fluid (ACSF) containing (in mM): 124 NaCl, 3.5 KCl, 1 CaCl2, 0.8 MgCl2, 1.23 NaH2PO4, 26 NaHCO3, and 10 glucose. Brain slices were initially incubated at 32°C for 30min then at room temperature (about 25°C) for 1h or until used for recording. Brain slices were kept for up to 7h after slicing. All HSDB and ACSF used in the above described procedures were aerated with a gas mixture containing 95% oxygen and 5% carbon dioxide to maintain the pH at around 7.4 and oxygen saturation.
Whole-cell patch clamp recording
Whole-cell patch-clamp recording electrodes were pulled from filamented borosilicate glass capillaries (BF150–86-10, Sutter Instruments) using a micropipette puller (P-97, Sutter Instruments) to a resistance of 3.5–7.9 MΩ. The glass electrodes were filled with an internal solution containing (in mM): 20 KCl, 100 K-gluconate, 10 HEPES, 4 MgATP, 0.3 Na2GTP, 7 phosphocreatine, and 0.2% biocytin with pH adjusted to 7.4 and osmolarity adjusted to 300 mOsm. In some experiments, a small amount of 4% w/v Alexa Fluor 594 (A-10438, ThermoFisher Scientific) dissolved in internal solution was back-loaded into the glass electrode to label the patched cell. This dye loading method was described in previous literature. The whole-cell patching procedure was conducted using an image-guided automatic in vitro patching system (Autopatcher IG) developed in our lab (Wu and Chubykin, 2017; Wu et al., 2016). The patch clamp recording signal was amplified (Multiclamp 700B) and digitized at 20 kHz sampling rate (Digidata 1550A, Molecular Devices) before being saved to the computer. All raw traces were low-pass filtered at 10 kHz before further analysis.
Channelrhodopsin-assisted Circuit Mapping (CRACM)
CRACM was conducted in acute ex vivo visual cortical slices on a patch-clamp electrophysiology rig. Light stimulation was generated by a high-power LED (470 nm, 50 W, Mightex). The stimulation pattern was generated by an LED patterned illuminator (Polygon 400, Mightex) and projected onto the brain slice via a 10x objective lens (Avants et al., 2015). The total area scanned for each map is 0.67 mm by 0.67 mm, which is divided into 10×10 grid. Each stimulus (one pixel) is 10 ms in length, with a 2 s inter-stimulus interval. The power coming out of the objective was 0.3 mW for a 10 ms stimulus, as measured by a digital power meter (Thorlabs PM121D). The stimulation sequence was a pre-defined pseudo-random sequence, which avoids surrounding inhibition from scanning in sequence. All CRACM recordings were conducted under voltage-clamp mode with a −70 mV holding potential. LED stimulation patterns were designed and controlled with the manufacture’s software. Stimulation and recording were synchronized by the patch-clamp digitizer. Cell averaged CRACM maps were constructed by averaging the pixel values between cells in each experimental group, with the soma positioned in the center of each map.
Perfusions, Histology and Imaging
Mice were perfused with 1xPBS, followed by 4% PFA. After extraction, the brain was stored in 4% PFA for 24–48 hr before making 100 μm thick coronal sections on a vibrating microtome (1000 Plus, Vibratome). Some slices were counter stained (free floating) with DAPI in PBS. Slices were mounted onto glass slides with anti-fading mounting media containing n-propyl-gallate and sealed with transparent nail polish. The electrode track was then visualized by light microscopy (VWR) to verify the electrode placement in V1, according to landmarks shown in a mouse brain reference atlas (Neurostar stereotaxic mouse brain atlas, Allen mouse brain atlas). Acute brain slices from ex vivo patch clamp recordings were fixed in 4%PFA for 30min to 1h then made into microscopic slides. Images were obtained with confocal microscopy (Zeiss 710).
QUANTIFICATION AND STATISTICAL ANALYSIS
The statistical details for each experiment can be found in the figure legends. Much of our statistical analysis was performed in Python. We used two sample Kolmogorov-Smirnov (KS) tests in Python (Scipy library) to compare the distributions of oscillation durations among populations of single units. These tests were also used to compare the distributions of the peak response oscillation frequency among single units. To account for unequal variance and unequal numbers of units between comparisons, we used a Welch’s two sample t test in Python to compare the mean oscillation durations between two unit populations. The Welch’s test was also used to compare firing rates at different oscillatory cycles between WT and FX mice, and the mean frequency of peak oscillatory activity among single units. A Mann-Whitney-U test was used to compare the mean baseline normalized power of different frequency bands from LFP recordings, as well as the raw amplitudes of responses at different oscillatory cycles. The mean firing rates of units during mobile or immobile trials were compared using the Wilcoxon-signed rank test. We also analyzed the cumulative distributions of single values averaged across the entire CRACM map for each cell, but this did not yield significance for any comparison when performing a Kolmogorov-Smirnov test. To compare the average EPSC strength (bar graphs in Figures 5 and 6), all EPSCs across the CRACM map for each cell were averaged into single values for comparison between experimental groups. If these data were not normally distributed, we then applied either a log transformation or Box-Cox transformation (Lambda = −1.3614 for L5-L4RS, −0.245 for L5-L5 RS) and performed a two way ANOVA followed by the Tukey test in python. We also performed non-parametric tests (Mann-Whitney U test) for these comparisons and obtained similar results. We have also performed averaging of the top 10 largest values to acquire one value for each CRACM heatmap for these comparisons and obtained similar results. We used a combined D’Agostino and Pearson’s normality test from Python SciPy library to test the normality of a distribution. We used a repeated-measures ANOVA in MATLAB to compare optogenetic input-output curves and I-V curves between genotypes. To compare the LFP amplitudes or firing rates at four individual oscillation cycles between WT and FX, we used linear mixed models (LMM) using SAS software followed by the testing of the least-squares (LS) means of fixed effects. Our model contained genotype and cycles as fixed effects along with a mouse/unit as a random effect. Since LMM is a linear model, it shares similar assumptions as ANOVA. We used SAS to fit the model to our data. We tried different transformations of our dependent variable and found that taking the rectified LFP and firing rate to the power of 0.4 gave us the best results (normal residuals). For post hoc tests, we used simple effect comparisons using least-squares means of fixed effects to test for differences between groups at each cycle. P values were then manually corrected by the Bonferroni method.
DATA AND CODE AVAILABILITY
Data and code are available from the corresponding author on a reasonable request.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, Peptides, and Recombinant Proteins | ||
| Monosodium Phosphate | Santa Cruz | sc-202342 |
| Sodium Bicarbonate | DOT scientific, Inc | DSS22060 |
| Dextrose | DOT scientific, Inc | 7203B |
| Sodium Chloride | DOT scientific, Inc | DSS23020 |
| Calcium Chloride | DOT scientific, Inc | DSC20010 |
| Magnesium Chloride | DOT scientific, Inc | DSM24000 |
| Potassium Chloride | DOT scientific, Inc | DSP41000 |
| Alexa Fluor 594 Hydrazide | ThermoFisher Scientific | A10438 |
| Phosphocreatine | Sigma-Aldrich | P7936 |
| GTP | Sigma-Aldrich | G8877 |
| ATP | Sigma-Aldrich | A9187 |
| Ascorbic Acid | DOT scientific, Inc | DSA50040 |
| HEPES | Sigma-Aldrich | H3375 |
| Experimental Models: Organisms/Strains | ||
| C57BL/6 | The Jackson Laboratory | C57BL/6 |
| Fmr1 KO | The Jackson Laboratory | Fmr1 KO |
| Software and Algorithms | ||
| Python | Python | Version 2.7 |
| Anaconda Distribution | Anaconda Inc. | Anaconda for Python 2.7 |
| MATLAB | Mathworks | MATLAB |
| SAS | SAS Institute | SAS |
| Other | ||
| C&B-Metabond® Quick! Cement System | Parkell | S380 |
| ZIP Kicker CA accelerator | ZAP Glue | #PT-27 |
| LOCTITE super glue ultragel control | LOCTITE | SKU #688626 (Home Depot) |
| Animal Temperature Controller | World Precision Instruments (WPI) | ATC-2000 |
| Motorized Stereotax | Neurostar | Single robot stereotax |
| Isoflurane Vaporizer | Parkland Scientific | V3000PK |
| Micromanipulator | Scientifica | PatchStar Micromanipulator with PatchPad |
| Stereo Microscope | AmScope | SM-4TZ-144A |
| Aluminum Breadboard 12” × 18” × 1/2,” 1/4”−20Taps | Thorlabs | MB1218 |
| Miniature V-Clamp, 0.42” Long, M4 Tapped Hole | Thorlabs | VH1/M |
| Ø1.25” Studded Pedestal Base Adaptor, 1/4”−20 Thread | Thorlabs | BE1 |
| Short Clamping Fork, 1.24” Counterbored Slot, 1/4”−20 Captive Screw | Thorlabs | CF125C |
| Swivel Post Clamp, 360° Continuously Adjustable | Thorlabs | SWC |
| Ø1/2” Optical Post, SS, 8–32 Setscrew, 1/4”−20 Tap, L = 6” | Thorlabs | TR6 |
| Ø1/2” Optical Post, SS, 8–32 Setscrew, 1/4”−20 Tap, L = 3” | Thorlabs | TR3 |
| Large V-Clamp with PM4 Clamping Arm, 2.5” Long | Thorlabs | VC3 |
| Ø1” Pedestal Pillar Post, 1/4”−20 Taps, L = 3” | Thorlabs | RS3P |
| Adaptor with External 8–32 Threads and External 1/4”- 20 Threads | Thorlabs | AP8E25E |
| Rotary encoder | U.S. Digital | H5 ball bearing optical shaft encoder |
| Acrylic disk, 6” diameter, 1/8” thick | Amazon | Acrylic disk, 6” diameter, 1/8” thick |
| 64 Channel Silicon Probe | Masmanidis Lab, UCLA | 64D |
| Acquisition Board | OpenEphys | Acquisition Board |
| 128 Channel Amplifier Board | Intan Technologies | RHD2000 |
| Arduino Board | Arduino | A000066 |
| I/O Board | OpenEphys | I/O Board |
| Electroplating Board | Intan Technologies | RHD2000 Electroplating Board |
| Interface Cable | Intan Technologies | RHD2000 SPI interface Cable |
| Camera | Thorlabs | DCC1545M |
| Camera Lens | Thorlabs | MVL50M23 |
| Infrared Illuminator | Towallmark | Towallmark Crazy Cart 48-LED CCTV Ir Infrared Night Vision Illuminator |
| Patch Clamp Digitizer | Molecular Devices | Multiclamp 700B |
| Patch Clamp Amplifier | Molecular Devices | Digidata 1550A |
| Patch Software Suite | Molecular Devices | pCLAMP 10 |
| Capillary Glass Micropipette | Sutter Instruments | BF150-86-10 |
| Micropipette Puller | Sutter Instruments | P-97 |
| Vibratome | Leica | VT1000 |
| LED patterned Illuminator | Mightex | Polygon 400 |
Highlights.
Experience-dependent oscillations are reduced in magnitude and duration in FX mice
A frequency shift is found in oscillations from FX mice
Functional connections onto L4 FS cells are weaker in FX mice following experience
L5 Pyr to L4 FS synapse is strengthened after experience in WT, but not in FX, mice
ACKNOWLEDGMENTS
We are grateful for the financial support from the National Institutes of Health (NIH) grant R01 MH116500. We thank Dr. Sotiris Masmanidis for the provided silicon probes. We thank Purdue Statistical Consulting Service for the advice on statistical analysis.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information can be found online at https://doi.org/10.1016/j.celrep.2020.03.050.
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- Aizenman CD, Kirkwood A, and Bear MF (1996). A current source density analysis of evoked responses in slices of adult rat visual cortex: implications for the regulation of long-term potentiation. Cereb. Cortex 6, 751–758. [DOI] [PubMed] [Google Scholar]
- Antoine MW, Langberg T, Schnepel P, and Feldman DE (2019). Increased excitation-inhibition ratio stabilizes synapse and circuit excitability in four autism mouse models. Neuron 101, 648–661.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants BW, Murphy DB, Dapello JA, and Robinson JT (2015). NeuroPG: open source software for optical pattern generation and data acquisition. Front. Neuroeng 8, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos AM, Briggs F, Alitto HJ, Mangun GR, and Usrey WM (2014). Simultaneous recordings from the primary visual cortex and lateral geniculate nucleus reveal rhythmic interactions and a cortical source for g-band oscillations. J. Neurosci 34, 7639–7644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos AM, Vezoli J, Bosman CA, Schoffelen JM, Oostenveld R, Dowdall JR, De Weerd P, Kennedy H, and Fries P (2015). Visual areas exert feedforward and feedback influences through distinct frequency channels. Neuron 85, 390–401. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, and Hochberg Y (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57, 289–300. [Google Scholar]
- Berry-Kravis E (2014). Mechanism-based treatments in neurodevelopmental disorders: fragile X syndrome. Pediatr. Neurol 50, 297–302. [DOI] [PubMed] [Google Scholar]
- Bhattacharya A, Kaphzan H, Alvarez-Dieppa AC, Murphy JP, Pierre P, and Klann E (2012). Genetic removal of p70 S6 kinase 1 corrects molecular, synaptic, and behavioral phenotypes in fragile X syndrome mice. Neuron 76, 325–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollimunta A, Mo J, Schroeder CE, and Ding M (2011). Neuronal mechanisms and attentional modulation of corticothalamic a oscillations. J. Neurosci 31, 4935–4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortone DS, Olsen SR, and Scanziani M (2014). Translaminar inhibitory cells recruited by layer 6 corticothalamic neurons suppress visual cortex. Neuron 82, 474–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie SB, Akins MR, Schwob JE, and Fallon JR (2009). The FXG: a presynaptic fragile X granule expressed in a subset of developing brain circuits. J. Neurosci 29, 1514–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX (2015). Analyzing Neural Time Series Data: Theory and Practice (MIT).
- Crunelli V, and Leresche N (2002). Childhood absence epilepsy: genes, channels, neurons and networks. Nat. Rev. Neurosci 3, 371–382. [DOI] [PubMed] [Google Scholar]
- Darnell JC, Van Driesche SJ, Zhang C, Hung KY, Mele A, Fraser CE, Stone EF, Chen C, Fak JJ, Chi SW, et al. (2011). FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell 146, 247–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan BM, Duron SG, Campbell DA, Vollrath B, Shankaranarayana Rao BS, Ko HY, Lin GG, Govindarajan A, Choi SY, and Tonegawa S (2013). Rescue of fragile X syndrome phenotypes in Fmr1 KO mice by the small-molecule PAK inhibitor FRAX486. Proc. Natl. Acad. Sci. USA 110, 5671–5676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupret D, O’Neill J, and Csicsvari J (2013). Dynamic reconfiguration of hippocampal interneuron circuits during spatial learning. Neuron 78, 166–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethridge LE, White SP, Mosconi MW, Wang J, Pedapati EV, Erickson CA, Byerly MJ, and Sweeney JA (2017). Neural synchronization deficits linked to cortical hyper-excitability and auditory hypersensitivity in fragile X syndrome. Mol. Autism 8, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzin F, Whitney D, Hagerman RJ, and Rivera SM (2008). Contrast detection in infants with fragile X syndrome. Vision Res. 48, 1471–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzin F, Rivera SM, and Whitney D (2011). Resolution of spatial and temporal visual attention in infants with fragile X syndrome. Brain 134, 3355–3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogerson PM, and Huguenard JR (2016). Tapping the brakes: cellular and synaptic mechanisms that regulate thalamic oscillations. Neuron 92, 687–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund LS, and Reiss AL (1991). Cognitive profiles associated with the fra(X) syndrome in males and females. Am. J. Med. Genet 38, 542–547. [DOI] [PubMed] [Google Scholar]
- Gallagher A, and Hallahan B (2012). Fragile X-associated disorders: a clinical overview. J. Neurol 259, 401–413. [DOI] [PubMed] [Google Scholar]
- Gallego PK, Burris JL, and Rivera SM (2014). Visual motion processing deficits in infants with the fragile X premutation. J. Neurodev. Disord 6, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson JR, Bartley AF, Hays SA, and Huber KM (2008). Imbalance of neocortical excitation and inhibition and altered UP states reflect network hyperexcitability in the mouse model of fragile X syndrome. J. Neurophysiol 100, 2615–2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel A, Cantu DA, Guilfoyle J, Chaudhari GR, Newadkar A, Todisco B, de Alba D, Kourdougli N, Schmitt LM, Pedapati E, et al. (2018). Impaired perceptual learning in a mouse model of Fragile X syndrome is mediated by parvalbumin neuron dysfunction and is reversible. Nat. Neurosci 21, 1404–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves JT, Anstey JE, Golshani P, and Portera-Cailliau C (2013). Circuit level defects in the developing neocortex of Fragile X mice. Nat. Neurosci 16, 903–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger CWJ (1969). Investigating causal relations by econometric models and cross-spectral methods. Econometrica 37, 424–438. [Google Scholar]
- Grünwald PD (2007). The Minimum Description Length Principle (MIT).
- Hall SS, Lightbody AA, and Reiss AL (2008). Compulsive, self-injurious, and autistic behavior in children and adolescents with fragile X syndrome. Am. J. Ment. Retard 113, 44–53. [DOI] [PubMed] [Google Scholar]
- Harris SW, Hessl D, Goodlin-Jones B, Ferranti J, Bacalman S, Barbato I, Tassone F, Hagerman PJ, Herman H, and Hagerman RJ (2008). Autism profiles of males with fragile X syndrome. Am. J. Ment. Retard 113, 427–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays SA, Huber KM, and Gibson JR (2011). Altered neocortical rhythmic activity states in Fmr1 KO mice are due to enhanced mGluR5 signaling and involve changes in excitatory circuitry. J. Neurosci 31, 14223–14234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooks BM, Mao T, Gutnisky DA, Yamawaki N, Svoboda K, and Shepherd GM (2013). Organization of cortical and thalamic input to pyramidal neurons in mouse motor cortex. J. Neurosci 33, 748–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L, Antion MD, Hu D, Spencer CM, Paylor R, and Klann E (2006). Dynamic translational and proteasomal regulation of fragile X mental retardation protein controls mGluR-dependent long-term depression. Neuron 51, 441–454. [DOI] [PubMed] [Google Scholar]
- Huber KM, Gallagher SM, Warren ST, and Bear MF (2002). Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc. Natl. Acad. Sci. USA 99, 7746–7750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheon B, and Yarom Y (2000). Resonance, oscillation and the intrinsic frequency preferences of neurons. Trends Neurosci. 23, 216–222. [DOI] [PubMed] [Google Scholar]
- Kalmbach BE, Johnston D, and Brager DH (2015). Cell-type specific channelopathies in the prefrontal cortex of the fmr1-/y mouse model of Fragile X syndrome. eNeuro 2, ENEURO.0114–15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper EM, Larkman AU, Lübke J, and Blakemore C (1994). Pyramidal neurons in layer 5 of the rat visual cortex. I. Correlation among cell morphology, intrinsic electrophysiological properties, and axon targets. J. Comp. Neurol 339, 459–474. [DOI] [PubMed] [Google Scholar]
- Kissinger ST, Pak A, Tang Y, Masmanidis SC, and Chubykin AA (2018). Oscillatory encoding of visual stimulus familiarity. J. Neurosci 38, 6223–6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga K, Liu MG, Qiu S, Song Q, O’Den G, Chen T, and Zhuo M (2015). Impaired presynaptic long-term potentiation in the anterior cingulate cortex of Fmr1 knock-out mice. J. Neurosci 35, 2033–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramvis I, Mansvelder HD, Loos M, and Meredith R (2013). Hyperactivity, perseveration and increased responding during attentional rule acquisition in the Fragile X mouse model. Front. Behav. Neurosci 7, 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson J, Wong D, and Lynch G (1986). Patterned stimulation at the theta frequency is optimal for the induction of hippocampal long-term potentiation. Brain Res. 368, 347–350. [DOI] [PubMed] [Google Scholar]
- Larson J, Jessen RE, Kim D, Fine AK, and du Hoffmann J (2005). Age-dependent and selective impairment of long-term potentiation in the anterior piriform cortex of mice lacking the fragile X mental retardation protein. J. Neurosci 25, 9460–9469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauterborn JC, Rex CS, Kramár E, Chen LY, Pandyarajan V, Lynch G, and Gall CM (2007). Brain-derived neurotrophic factor rescues synaptic plasticity in a mouse model of fragile X syndrome. J. Neurosci 27, 10685–10694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Simpson GV, Logothetis NK, and Rainer G (2005). Phase locking of single neuron activity to theta oscillations during working memory in monkey extrastriate visual cortex. Neuron 45, 147–156. [DOI] [PubMed] [Google Scholar]
- Łeski S, Wójcik DK, Tereszczuk J, Swiejkowski DA, Kublik E, and Wróbel A (2007). Inverse current-source density method in 3D: reconstruction fidelity, boundary effects, and influence of distant sources. Neuroinformatics 5, 207–222. [DOI] [PubMed] [Google Scholar]
- Lovelace JW, Ethell IM, Binder DK, and Razak KA (2018). Translation-relevant EEG phenotypes in a mouse model of Fragile X Syndrome. Neurobiol. Dis 115, 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marko H (1973). The bidirectional communication theory - a generalization of information theory. IEEE Trans. Commun 21, 1345–1351. [Google Scholar]
- Martin HGS, Lassalle O, Brown JT, and Manzoni OJ (2016). Age-dependent long-term potentiation deficits in the prefrontal cortex of the Fmr1 knockout mouse model of Fragile X syndrome. Cereb. Cortex 26, 2084–2092. [DOI] [PubMed] [Google Scholar]
- McAfee SS, Liu Y, Dhamala M, and Heck DH (2018). Thalamocortical communication in the awake mouse visual system involves phase synchronization and rhythmic spike synchrony at high gamma frequencies. Front. Neurosci 12, 837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalareas G, Vezoli J, van Pelt S, Schoffelen JM, Kennedy H, and Fries P (2016). Alpha-beta and gamma rhythms subserve feedback and feedforward influences among human visual cortical areas. Neuron 89, 384–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitzdorf U (1985). Current source-density method and application in cat cerebral cortex: investigation of evoked potentials and EEG phenomena. Physiol. Rev 65, 37–100. [DOI] [PubMed] [Google Scholar]
- Munir F, Cornish KM, and Wilding J (2000). Nature of the working memory deficit in fragile-X syndrome. Brain Cogn. 44, 387–401. [DOI] [PubMed] [Google Scholar]
- Narayanan R, and Johnston D (2007). Long-term potentiation in rat hippocampal neurons is accompanied by spatially widespread changes in intrinsic oscillatory dynamics and excitability. Neuron 56, 1061–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niell CM, and Stryker MP (2010). Modulation of visual responses by behavioral state in mouse visual cortex. Neuron 65, 472–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niere F, Wilkerson JR, and Huber KM (2012). Evidence for a fragile X mental retardation protein-mediated translational switch in metabotropic glutamate receptor-triggered Arc translation and long-term depression. J. Neurosci 32, 5924–5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosyreva ED, and Huber KM (2006). Metabotropic receptor-dependent long-term depression persists in the absence of protein synthesis in the mouse model of fragile X syndrome. J. Neurophysiol 95, 3291–3295. [DOI] [PubMed] [Google Scholar]
- Olsen SR, Bortone DS, Adesnik H, and Scanziani M (2012). Gain control by layer six in cortical circuits of vision. Nature 483, 47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachitariu M, Steinmetz N, Kadir S, Carandini M, and Kenneth DH (2016). Kilosort: realtime spike-sorting for extracellular electrophysiology with hundreds of channels. bioRxiv 10.1101/061481. [DOI] [Google Scholar]
- Petreanu L, Huber D, Sobczyk A, and Svoboda K (2007). Channelrhodopsin-2-assisted circuit mapping of long-range callosal projections. Nat. Neurosci 10, 663–668. [DOI] [PubMed] [Google Scholar]
- Pettersen KH, Devor A, Ulbert I, Dale AM, and Einevoll GT (2006). Current-source density estimation based on inversion of electrostatic forward solution: effects of finite extent of neuronal activity and conductivity discontinuities. J. Neurosci. Methods 154, 116–133. [DOI] [PubMed] [Google Scholar]
- Polack PO, Friedman J, and Golshani P (2013). Cellular mechanisms of brain state-dependent gain modulation in visual cortex. Nat. Neurosci 16, 1331–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portera-Cailliau C (2012). Which comes first in fragile X syndrome, dendritic spine dysgenesis or defects in circuit plasticity? Neuroscientist 18, 28–44.21551076 [Google Scholar]
- Quinn CJ, Coleman TP, Kiyavash N, and Hatsopoulos NG (2011). Estimating the directed information to infer causal relationships in ensemble neural spike train recordings. J. Comput. Neurosci 30, 17–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn CJ, Kiyavash N, and Coleman TP (2015). Directed information graphs. IEEE Trans. Inf. Theory 61, 6887–6909. [Google Scholar]
- Reimer J, Froudarakis E, Cadwell CR, Yatsenko D, Denfield GH, and Tolias AS (2014). Pupil fluctuations track fast switching of cortical states during quiet wakefulness. Neuron 84, 355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter CG, Thompson WH, Bosman CA, and Fries P (2017). Top-down beta enhances bottom-up gamma. J. Neurosci 37, 6698–6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossant C, Kadir SN, Goodman DFM, Schulman J, Hunter MLD, Saleem AB, Grosmark A, Belluscio M, Denfield GH, Ecker AS, et al. (2016). Spike sorting for large, dense electrode arrays. Nat. Neurosci 19, 634–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber T (2000). Measuring information transfer. Phys. Rev. Lett 85, 461–464. [DOI] [PubMed] [Google Scholar]
- Shepherd GM, Pologruto TA, and Svoboda K (2003). Circuit analysis of experience-dependent plasticity in the developing rat barrel cortex. Neuron 38, 277–289. [DOI] [PubMed] [Google Scholar]
- Shobe JL, Claar LD, Parhami S, Bakhurin KI, and Masmanidis SC (2015). Brain activity mapping at multiple scales with silicon microprobes containing 1,024 electrodes. J. Neurophysiol 114, 2043–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidorov MS, Auerbach BD, and Bear MF (2013). Fragile X mental retardation protein and synaptic plasticity. Mol. Brain 6, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark E, Eichler R, Roux L, Fujisawa S, Rotstein HG, and Buzsáki G (2013). Inhibition-induced theta resonance in cortical circuits. Neuron 80, 1263–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kerkoerle T, Self MW, Dagnino B, Gariel-Mathis MA, Poort J, van der Togt C, and Roelfsema PR (2014). Alpha and gamma oscillations characterize feedback and feedforward processing in monkey visual cortex. Proc. Natl. Acad. Sci. USA 111, 14332–14341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinck M, Batista-Brito R, Knoblich U, and Cardin JA (2015). Arousal and locomotion make distinct contributions to cortical activity patterns and visual encoding. Neuron 86, 740–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser S, and Van Gils SA (2014). Lumping Izhikevich neurons. EPJ Nonlinear Biomed. Phys 2, 6. [Google Scholar]
- Wu Q, and Chubykin AA (2017). Application of automated image-guided patch clamp for the study of neurons in brain slices. J. Vis. Exp, 56010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Kolb I, Callahan BM, Su Z, Stoy W, Kodandaramaiah SB, Neve R, Zeng H, Boyden ES, Forest CR, and Chubykin AA (2016). Integration of autopatching with automated pipette and cell detection in vitro. J. Neurophysiol 116, 1564–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun SH, and Trommer BL (2011). Fragile X mice: reduced long-term potentiation and N-Methyl-D-Aspartate receptor-mediated neurotransmission in dentate gyrus. J. Neurosci. Res 89, 176–182. [DOI] [PubMed] [Google Scholar]
- Zarnadze S, Bäuerle P, Santos-Torres J, Böhm C, Schmitz D, Geiger JR, Dugladze T, and Gloveli T (2016). Cell-specific synaptic plasticity induced by network oscillations. eLife 5, e14912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemankovics R, Káli S, Paulsen O, Freund TF, and Hájos N (2010). Differences in subthreshold resonance of hippocampal pyramidal cells and interneurons: the role of h-current and passive membrane characteristics. J. Physiol 588, 2109–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Bonnan A, Bony G, Ferezou I, Pietropaolo S, Ginger M, Sans N, Rossier J, Oostra B, LeMasson G, and Frick A (2014). Dendritic channelopathies contribute to neocortical and sensory hyperexcitability in Fmr1(-/y) mice. Nat. Neurosci 17, 1701–1709. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and code are available from the corresponding author on a reasonable request.


