Abstract
Although there are myriad binding modes of heterocumulenes to metal centers, the monometallic κ2-ECE (E = O, S, NR) coordination mode has not been reported. Herein, the synthesis, isolation, and physical characterization of Cp2Ti(κ2-tBuNCNtBu) (3) (Cp = cyclopentadienyl, tBu = tert-butyl), a strained 4-membered metallacycle bearing a free carbene, is described. Computational (DFT, CASSCF, QT-AIM, ELF) and solid-state CP-MAS 13C spectroscopic analysis indicate that 3 is best described as a free carbene with partial Ti-Cb bonding that results from Ti-N π-bonding mixing with N-C-N s-bonding of the bent N-C-N framework. Reactivity studies of 3 corroborate its carbene-like nature: protonation with [LutH]I results in formation of a Ti-formamidinate (4), while oxidation with S8 yields a Ti-thioureate (5). Additionally, a related bridged dititanamidocarbene, (Cp2Ti)2(μ-η1,η1-CyNCNCy) (10) (Cy = cyclohexyl) is reported. Taken together, this work suggests that the 2-electron reduction of heterocumulene moieties can allow access to unusual free carbene coordination geometries given the proper stabilizing coordination environment from the reducing transition metal fragment.
Graphical Abstract

Introduction
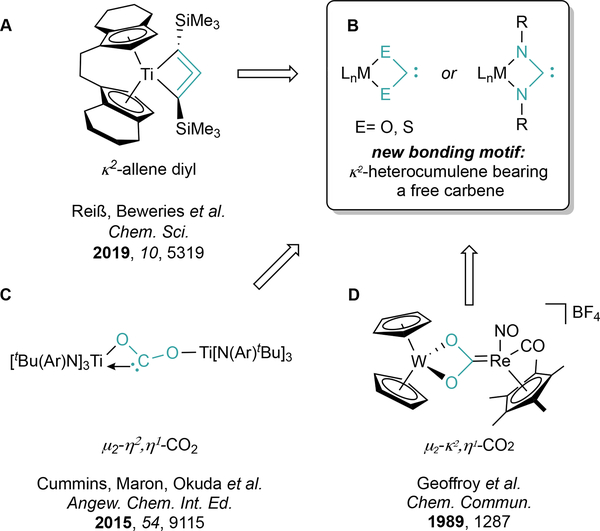
The isolation of unsaturated, strained metallacycles is challenging as they are often reactive, high-energy species.1,2 Nonetheless, group IV metallocenes have been an excellent platform for the isolation of highly strained metallacyclic cores,3–5 such as zirconacyclopentatriene,6 zirconacyclopentyne,7 and hafnacyclopenta-3,4-diene8 complexes, among others. With respect to this endeavor, 4-membered metallacycles are particularly important because of their intermediacy in alkene9 and alkyne10–12 metathesis reactions. Recently, Reiß and Beweries synthesized and described a titanacyclobuta-2,3diene13,14 complex via salt metathesis of [rac-(ebthi)TiCl2] with the deprotonated allene Li2(Me3SiC3SiMe3) (Figure 1a) (rac-(ebthi) = rac-1,2-ethylene-1,1’-bis(tetrahydroindenyl)). This allene-diyl moiety is a model of a decomposition product in alkyne metathesis that arises from deprotonation of the β-C-H in metal-alkylidyne [2+2]-cycloaddition products, though an additional M-β-C interaction is present in similar group VI complexes.15–19
Figure 1.
Examples of unusual binding modes of allenes and heterocumulenes with early transition metals.
While there are several examples of transition-metal complexes bearing the allene-diyl moiety, there are no structurally characterized monometallic examples of heterocumulene (E=C=E, E = O, NR, S, etc) analogues of these 1-metallacyclobuta-2,3-dienes (e.g. complexes of the type M(κ2-ECE), Figure 1b). Indeed, despite the myriad bonding motifs of CO2 to transition metals,20–23 there are no examples of monometallic κ2-ECE bonding, although there are several related examples of κ2-OCO and κ2-SCS-bound structures in bimetallics (Figure 1d).24–32 Similarly, deprotonation of metal-bound formates has resulted in closely related bridging μ-η2,η1-CO2 moieties (Figure 1c).33,34 Thus, isolation and characterization of κ2-ECE complexes could provide new insights and strategies into CO2 activation and further reactivity.
Group 4 κ2-(RNCNR) analogues (e.g. Cp2Ti(κ2-RNCNR), INT2, Figure 2), have been investigated theoretically.35,36 Complexes of this type were thought to be too reactive to isolate;37 however, their intermediacy was proposed by Rosenthal35 in a series of reactions of Cp2Ti(η2-BTMSA) (1) (BTMSA = bis(trimethylsilylacetylene) with various 1,3-disubstituted carbodiimides (Figure 2). These reactions yielded a variety of products stemming from C-N bond cleavage (2a, 2b) or coupling (2b), as well as C-C bond coupling (2d), depending on the nature of the carbodiimide substituents.35,38,39 We were prompted to revisit the reaction of Cp2Ti(η2-BTMSA) with carbodiimides based on a recent report of Ti-carbodiimide adduct intermediacy in Ticatalyzed isocyanide amination.40,41 Herein we report the synthesis, experimental characterization, theoretical analysis, and reactivity of Cp2Ti(κ2-tBuNCNtBu) (3), an isolable example of reactive complex INT2 with free “titana-N-heterocyclic carbene” character, as well as a related bimetallic free carbene, (Cp2Ti)2(μ-η1,η1-CyNCNCy). Complex 3 represents the first example of a structurally characterized monometallic complex with a κ2-bound heterocumulene.
Figure 2.
Left: reactions of Cp2Ti(BTMSA) (1) with substituted carbodiimides to give 2a-d, proposed to stem from reactive intermediate INT2 (Adapted from REF 35). R = tBu, SiMe3, Cy. Right: in this work, revisitation of these reactions under ambient conditions reveals several unusual free carbene complexes of Ti.
Results and Discussion
Reaction of the masked Cp2TiII precursor Cp2Ti(η2-BTMSA) (1) with tBuNCNtBu in pentane at room temperature for 10 minutes led to a color change from brown to deep red. Overnight crystallization of the reaction mixture at −35 °C afforded the C2v-symmetrical product Cp2Ti(κ2-tBuNCNtBu), 3, as a dark red crystalline solid in 92% yield in gram-scale quantities (eq 1). The isolation of 3 was surprising given previous reports that reaction of 1 with tBuNCNtBu yields [Cp2Ti(CN)]4 (2a) (Figure 2), albeit at 60 °C and extended reaction times. 35,38,39 In fact, 3 is stable for months under ambient conditions in an N2 atmosphere.
 |
(1) |
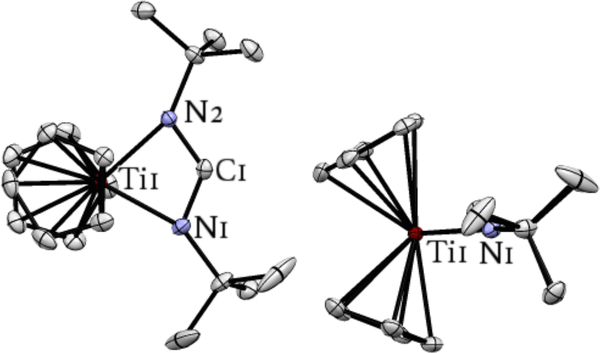
The X-ray crystal structure of 3 is displayed in Figure 3. The structural parameters for 3 are indicative of a system featuring delocalization of π-electron density across the N-C-N atoms of the metallacycle, along with a free singlet carbene, as drawn in eq 1. The C1-N1 (1.3246(13) Å), and C1-N2 (1.3210(13) Å) bond lengths are not far from that of C(sp2)-N(sp2) single bonds (1.355 Å).42 The Ti1-N1 and Ti1-N2 bond lengths are close to that of a typical TiIV-N(pyrrolide) bond,43–45 suggestive of an X-type TiIV-N with little N→Ti π-donation, consistent with the π-electron density of the nitrogen atoms being delocalized across the N-C-N core in order to stabilize the carbene carbon. The Ti1-C1 distance of 2.2674(10) Å is slightly (but significantly) longer than typical Ti-C(sp3) bonds (for example, the average distance of Ti-CH3 in Cp2Ti(CH3)2 is 2.176 Å).46 This Ti1-C1 distance is 0.1 Å longer than Beweries’κ2-allene diyl Ti-C distance (2.178 Å, Figure 1A),13 but significantly shorter than the analogous Ti-C in Rosenthal’s 2c (2.599 Å).35
Figure 3.
Two views of the molecular structure of complex 3. Thermal ellipsoids are drawn at the 50% probability level. Hydrogen atoms are omitted for clarity. Selected bond lengths [Å] and angles [°]: C1-N1 1.3246(13), C1-N2 3210(13), Ti1-N1 2.0012(9), Ti1-N2 2.0057(8), Ti1-C1 2.2674(10), N1-C1-N2 122.66(9), Ti1-N1-C1 83.30(6), Ti1-N2-C1 83.21(6), N1-Ti1-N2 70.80(4).
The sum of all internal bond angles of the Ti1-N1-C1-N2 plane add up to 359.97°, and the sum of all bond angles around N1 and N2 add to 359.82° and 359.93°, respectively, all of which indicate a high degree of planarity in 3. The N1C1-N2 bond angle of 122.66(9)° is in between the predicted values for singlet (113.1°) and triplet (130.8°) diaminocarbene,47,48 and close to the predicted value for a singlet dianionic diamidocarbene (119.5°) (Figure S41). Typically, increasing the steric profile of N-substituents in diamino carbene compounds leads to larger N-C-N bond angles, while other factors such as ring strain may also contribute to the observed value in 3.47,48 The only other structurally characterized 4-membered N-heterocyclic carbenes, iPr2NP[N(2,6-iPr2Ph)]2C and iPr2NB[N(2,6-iPr2Ph)]2C, have much smaller N-C-N bond angles of 96.72(13)° and 94.02(16)°, respectively, as well as a significant deviation from planarity in the case of the former (sum of bond angles around nitrogen atoms add to 355.1° and 348.2°).49,50
Given the further reactivity of INT2-like structures in previous investigations,35,38,39 steric protection around the carbon atom likely aids in isolation of 3. The percent buried volume (%Vbur)51,52 of 3 is 48.2%, assuming a sphere radius of 3.5 Å and a 2.00 Å distance from the carbene carbon to the center of the sphere.53 In comparison to %Vbur values of carbenes determined from complexes of the type AuCl(NHC), this value far exceeds that of ItBu (39.6%, ItBu = 1,3-tBu2-imidazol-2-ylidene), and is between the values of IPr (45.4%, IPr = 1,3-bis(2,6-iPr2Ph imidazole-2-ylidene) and IPent (49.0%, IPent = 1,3-bis(2,6-dipent-3-yl)imidazole-2-ylidene).54
Complex 3 is diamagnetic, and thus could potentially be described by three limiting electronic states: (A/B) a “titana-NHC” singlet carbene; (C) a closed-shell structure containing a “stretched” Ti-C bond;35 or (D) a TiIII open-shell singlet antiferromagnetically coupled to a C• (Figure 4). Previous DFT calculations (B3LYP/def2-SVP) on various Cp2Ti(κ2-RNCNR) complexes by Jemmis, Schulz, and Rosenthal indicated significant contribution from biradicaloid electronic structures such as D, wherein electron-donating substituents such as tBu in INT2 were proposed to have the strongest Ti-C interaction and the most C character.35
Figure 4.
Possible contributing electronic states for 3.
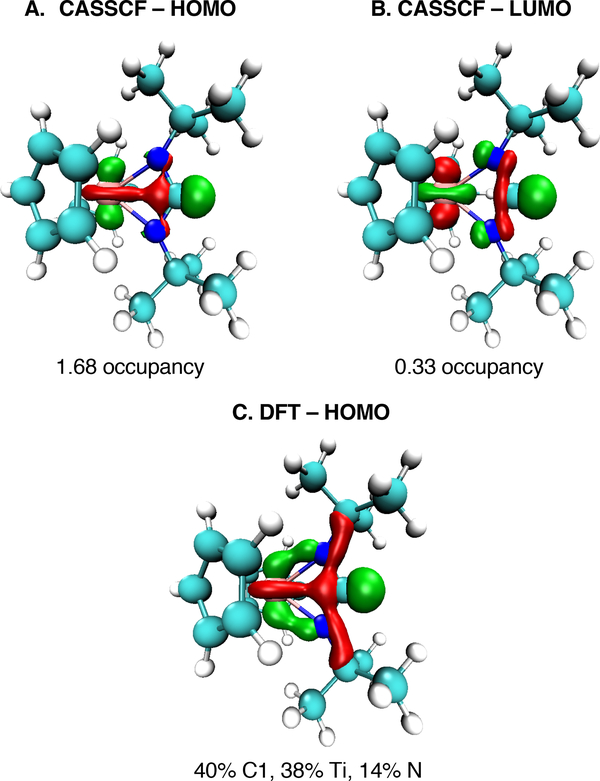
In order to determine the contributions of these three limiting cases, CASSCF calculations were performed on 3. First, we performed geometry optimizations at the DFT level of theory for several exchange-correlation functionals and found that M06-L/6–311G(d,p)55,56 matched the experimentally measured structure very closely (Figure S35). Full computational details and geometries are provided in the Supporting Information. Using that geometry, we performed CAS[14,14] calculations which included all the valence and bonding orbitals for the Ti-N-C-N atoms. The full set of CASSCF orbitals are provided in Figure S36. The HOMO and LUMO CASSCF orbitals are shown in Figure 5a and 5b. The HOMO orbital is primarly the carbon 2py orbital with smaller contributions from the titanium 3dz2 orbital, in an apparent σ interaction with a molecular orbital occupation 1.68. The LUMO orbital has a molecular orbital occupation of 0.33 (β = 0.312),57 with contributions from the same orbitals in an apparent σ* interaction with a nodal plane between the titanium and carbon.
Figure 5.
Top: The HOMO and LUMO from the natural orbital CASSCF calculation on 3. The occupancy is the natural orbital occupancy obtained from the CASSCF density. Bottom: HOMO surface from the DFT (M06-L/6-311G(d,p)) calculation on 3 with NBO-derived molecular orbital coefficients of the natural atomic orbitals.
Mayer Bond Order analysis58 was performed on the CASSCF natural orbitals, revealing a Ti-C bond order of 0.19. This low bond order suggests that electronic state C is an important, but minor, contributor to the overall electronic structure. Next, Natural Bonding Orbital (NBO) analysis was performed. Since NBO requires DFT orbitals, we used the orbitals from our DFT calculations using the M06-L functional. The DFT-calculated HOMO is shown in Figure 5c, and shows relatively good consistency with the CASSCF calculations. NBO analysis was performed to obtain the molecular orbital coefficients of the natural atomic orbitals basis (NAOMO),59 giving significant contributions from Ti, C1, and N in the HOMO (Figure 5, bottom. Full computational details in Table S5). Additionally, NBO analysis gives a 3dz2 occupation of 0.37; previous analysis of Ti complexes indicate that occupations below ~0.4 correspond to TiIV,40,60 suggesting that 3 has more TiIV (A/B/C) character rather than TiIII as in D.
Next, QT-AIM61–65 (Figure 6) and ELF66 (Figure 7) analyses were carried out on the CASSCF wavefunction to get a more specific picture of the bonding paths and localized electron density. (See SI Figure S39 for similar analysis on the M06-L wavefunction). The QT-AIM bonding paths of 3 (Figure 6) are in agreement with the TiIV-diamidocarbene (A/B) structure. The lack of a significant β-carbon interaction in 3 is reminiscent of Beweries’ stable titanacyclobuta-2,3-diene.13 The ELF plot of 3 is bereft of significant electron density between the C and Ti atoms, while localization of electron density is observed behind the C atom outside of the ring, indicative of the carbene lone pair as observed in ELF plots of other free carbenes.13
Figure 6.
Contour plot of the Laplacian of the electron density of 3 in the Ti-N-C-N ring plane. Blue dots represent bond critical points; orange dots represent ring critical points.
Figure 7.
ELF plot of 3 looking at the Ti-N-C-N ring plane.
Taken together, the above calculations indicate contributions from all possible contributing electronic states, and that 3 is best described as a free carbene with A/B being the predominant contributor. This is in contrast to previous calculations (B3LYP/def2-SVP, UBS-B3LYP) which indicated that the open shell singlet structure D is the major contributor;35,36 however, experimental analysis and reactivity agrees that the NHC-like A/B forms dominate (vide infra).
NMR chemical shifts depend on the symmetry and energy of high-lying occupied and low-lying vacant orbitals, and therefore provide additional experimental probes into the identity and accessibility of ground and excited states.67–74 Thus, we decided to further investigate the NMR parameters of 3 to better understand its electronic structure.
The room temperature 13C NMR resonance of the Cp rings in 3 is 106.8 ppm, significantly upfield of 1 (117.8 ppm) and indicative of a very electron-rich Ti center. The N-C-N 13C NMR resonance in 3 is 130.0 ppm in C6D6 (Figure S2). The room temperature value of 130.0 ppm is 9.2 ppm further upfield of the N-C-N resonance of free tBuNCNtBu, which is somewhat surprising given the typical downfield chemical shift observed for singlet NHC carbene carbon atoms.47 Variable temperature solution state 13C NMR spectroscopy demonstrated a change in chemical shift of the N-C-N carbon between 128.5 to 131.6 ppm across a temperature range of 215 to 376 K in C7D8 (Figures S4–S6).
The origin of the unusually shielded 13C resonance in 3 was investigated by measuring all three components of the chemical shift tensor using CP-MAS 13C NMR spectroscopy. The measured solid-state 13C NMR spectrum is shown in Figure 8a. Despite some overlapping spinning sidebands in this spectrum, the carbene signal can be fitted by considering only the sidebands that are not covered by other signals. The corresponding fit is shown below the experimentally measured spectrum in Figure 8a; the corresponding principal components of the shielding tensor are shown in Figure 8b (in parentheses). We further used DFT calculations to calculate the three principal components of the shielding tensor, directly from the crystal structure of 3 (using the hybrid PBE0 functional with a Slater-type triple-ζ basis set in the ADF 2014 code, including relativistic effects through the 2 component zeroth order regular approximation, see SI for details). We compared these results to the calculated shielding tensor of the related N–heterocyclic carbene ItBu. The calculated shielding tensors of 3 and ItBu are shown in Figure 8b. The isotropic chemical shift of the carbene carbon 3 (calculated at 121 ppm) is more shielded as compared to that of ItBu (calculated at 230 ppm). This is mainly because the δ11 and δ33 component of the chemical shift tensor are more shielded in 3, while the δ22 component is fairly similar for both compounds. Importantly, the chemical shift tensor is oriented similarly in both compounds.
Figure 8.
a) Solid-state 13C CP-MAS (3.5 kHz) NMR spectrum (top) of 3 and simulated trace (bottom) of the N-C-N carbon atom. The isotropic signal is marked with an arrow, spinning sidebands are marked with * and (partially) covered spinning sidebands are marked with #. b) Calculated (experimental in parenthesis) chemical shift tensor of the carbene carbon atom 3 (left) and an analogous NHC ItBu (right). Hydrogen atoms are not shown for clarity. c) Contributions of individual natural localized molecular orbitals (NLMOs) to the deshielding of the δ11 component in 3 (left) and the NHC (right). d) Calculated 15N chemical shift tensor of the nitrogen atoms in 3 (left) and ItBu (right).
Since chemical shift is a property that is directly related to the symmetry and energy of frontier molecular orbitals,75 the differing δ11 and δ33 components indicate different electronic structures between 3 and ItBu. Thus, we performed a Natural Chemical Shielding (NCS) analysis, that allows for decomposition of the individual components of the shielding tensor into contributions of individual natural localized molecular orbitals (NLMOs), corresponding to bonds and lone-pairs.76–78 The large deshielding of the δ11 component of ItBu is due to a high-lying lone-pair on C in combination with a low-lying vacant p-orbital on the same atom; in addition, the bonding s(C–N) orbitals also contribute to deshielding by coupling to the low-lying vacant p-orbital (Figure 8c, right). In 3, the contributions of the lone-pair on carbon to the deshielding of δ11 is similar compared to the NHC; however the bonding s(C–N) orbitals contribute significantly less, because of the wider N–C–N angle. The much more shielded δ33 component in 3, which is oriented perpendicular to the metallacycle-plane, has a more complex origin (vide infra). Notably, a similar remarkable shielding along the same direction is observed in metallacyclobutanes that engage in the olefin metathesis reaction.79
In addition to the unusually shielded 13C chemical shift of the N-C-N ring, the 15N chemical shift of 3 is quite deshielded, appearing at 246.1 ppm (Figure S3) compared to ItBu (calculated at 218 ppm). Thus, we further investigated the 15N chemical shift tensors of the nitrogen atoms in 3 and ItBu. Figure 8d shows the calculated chemical shift tensors of both compounds. In ItBu, the most deshielded component (δ11) is in the plane of the molecule perpendicular to the N– C(carbene) bond, δ22 is oriented along the N–C(carbene) bond, and the most shielded δ33 component is oriented perpendicular to the ring, along the direction of the nitrogen lone-pair. The two most deshielded components (δ11 and δ22) are directionally perpendicular to the N–C π-bond, indicative of a low-lying vacant π*(N–C) orbital.
In comparison, the orientation of the 15N chemical shift tensor in 3 is significantly different: here, the most shielded δ33 component is in the plane of the ring, while δ11 and δ22 point out of the ring plane (with an angle of roughly 45° with respect to the plane). As a result, upon going from ItBu to 3 the deshielding in the plane perpendicular to the N–C π-bond (the metallacycle plane) decreases, and the overall deshielding perpendicular to the ring-plane increases. This suggests that upon going from ItBu to 3, the N–C π-interaction perpendicular to the ring weakens, while a π-interaction between the Ti and N in the plane of the ring develops. The metallacyclic bonding in 3 (Figure 9) is thus characterized by two perpendicular partial p-interactions: the π(N–C) interaction perpendicular to the ring as is seen in the classical NHC bonding picture (not drawn), and one π(Ti–N) interaction in the plane of the ring (π(Ti–N) + σ(N-C), maroon). This π(Ti–N) interaction constitutes the bonding between an empty d-orbital on Ti pointing into the ring and the N-C-N σ-bond mixed with the Cβ lone pair. This bonding and deshielding pattern is analogous to metallacyclobutanes that engage in olefin metathesis (e.g. Cp2Ti(C3H6)).48 In these compounds, Cα also shows a large deshielding perpendicular to the metallacycle plane that arises from the magnetic coupling of a high-lying occupied orbital of σ(M–Cα) symmetry in combination with a low-lying vacant M-Cα π* orbital pointing into the ring in the direction of Cβ. Thus, it appears somewhat general that the presence of M-Eα π-bonding and the resulting magnetic coupling to the σ framework is responsible for both a large deshielding on the α-atom and a large shielding on the β-atom in the direction perpendicular to the ring. Thus, the NMR tensor analysis agrees with the computational description of the Ti free carbene, and also describes the partial Ti-Cβ interaction seen in the CASSCF analysis of 3 (Figure 5). In fact, this interaction can be clearly seen in the HOMO and LUMO of 3 (Figure 5a and 5b), which have both π/π*(Ti–N) symmetry and σ(N–C) symmetry.
Figure 9.
Qualitative molecular orbital diagram of the Cp2Ti2+ fragment interacting with the dianionic carbodiimide fragment, showing key in-ring orbital interactions, including the in-ring π-interaction between Ti and the a1-symmetric N– C bonding orbitals (maroon) that is responsible for the unusual 13C and 15N chemical shifts of the ring.
Based on the above spectroscopic and computational analysis, the best descriptor of 3 is that of an unusual free TiIVdiamidocarbene, in which the σ(N–C) bonds interact further with the metal via a bond of π-symmetry. This is the first example of an unsupported κ2-bound heterocumulene, as well as the first example of a 4-membered metal-containing free N-heterocyclic carbene, although several metal-containing carbene80–82 and metal-containing N-heterocyclic carbene coordination complexes have been reported.83–89
Having established a theoretical basis for the carbene-like character of 3, we next explored sundry reactions with 3 for experimental comparison. First, protonation reactions were carried out, demonstrating that the free carbene is capable of 2-electron Brønsted acid/base chemistry (Figure 10, top). Reaction of 3 with 0.5 equiv or 1.0 equiv of [LutH]+I- ([LutH]+I- = lutidinium iodide) in C6H6 resulted in the formation of the formamidinate TiIII complex 4 in 47% yield as a crystalline solid with a characteristic color of trivalent Ti. The solution state magnetic moment of 4 was determined to be 1.65(3) μB (Evans’ method, C6D6, 25 °C), which is near the spin-only value of 1.73 μB for an S = ½ TiIII ground state. The X-ray crystal structure of 4 is presented in Figure 10 (bottom right). The hydrogen atom H1 on the N-C-N carbon atom of 4 was located on the difference map, unambigiously identifying 4 as the TiIII formamidinate. Complex 4 is a rare example of a mononuclear Ti(III) formamidinate complex,37 though several Ti(III) amidinate and guanidinate complexes have been isolated.90–96 Complex 4 can be oxidized with excess AgOTf, yielding 4-OTf. The formamidinate C-H can be identified in diamagnetic 4-OTf by both 1H NMR spectroscopy and X-ray crystallography (Figure 10, bottom left), providing further evidence for the formamidinate structure of 4.
Figure 10.
Top: Acid/base and oxidation/reduction reactions of 3. Bottom: Molecular structures of complexes 4 (bottom right) and 4-OTf (bottom left). Thermal ellipsoids are drawn at the 50% probability level. Hydrogen atoms are omitted for clarity, except for H1 in both 4 and 4-OTf, which was located on the difference map in both structures. Selected bond lengths [Å] and angles [°] for 4: C1-N1 1.356(3), C1-N2 1.265(3), Ti1-N1 2.0626(17), Ti1-N2 2.2148(18), N1-C1N2 116.28(17). 4-OTf: C1-N1 1.324(2), C1-N2 1.327(2), Ti1-N1 2.0765(15), Ti1-N2 2.0721(16), N1-C1-N2 114.35(17).
Formation of 4, the product of formal addition of H• to 3, is surprising given the expected 2-electron chemistry of a singlet carbene. However, this reaction likely proceeds first through protonation to yield [Cp2Ti(κ2-tBuNCHNtBu)]+I- (4-I). Protonated 4-I can then be reduced by another equiv of 3, leading to a maximum yield of 50% of 4 along with free tBuNCNtBu and presumably [Cp2TiI]2, along with several unidentified decomposition products. Supporting this general pathway, maximum yields of 47% of 4 are obtained regardless of whether 0.5 equiv or 1.0 equiv [LutH]+I- are used. Further, treatment of 4-OTf with one equiv of 3 results in reduction of 4-OTf and formation of 4, consistent with the proposed reactivity of transient 4-I.
Deprotonation of 4-OTf by the strong, bulky base NaHMDS results in reformation of the free carbene 3, demonstrating that the dehydrohalogenation of transition metal formamidinate halide or pseudohalide ligands may be a viable alternative route to free carbene complexes of the type M(κ2-RNCNR). The regeneration of 3 via deprotonation of 4-OTf is somewhat reminiscent of some recent examples of formate deprotonation, although a free κ2-CO2 complex analogous to 3 has not been observed.33,34
2-Electron oxidation of 3 with 0.125 equiv S8 yields the thioureate complex 5, in accordance with typical reactions of free N-heterocyclic carbenes with S8 (Figure 11).97,98 Complex 5 is the only example of a crystallographically characterized Ti thioureate. Unfortunately, attempts to synthesize heterobimetallic complexes via coordination of the carbene of 3 have been unsuccessful.99
Figure 11.
Formation of 5 from 3 and 0.125 equiv S8. Right: molecular structure of complex 5. Thermal ellipsoids are drawn at the 50% probability level. Hydrogen atoms are omitted for clarity. Selected bond lengths [Å] and angles [°]: C1-N1 1.364(2), C1-N2 1.3616(19), Ti1-N1 2.0379(13), Ti1-N2 2.0369(13), C1-S1 1.7180(16), N1-C1-N2 108.12(13).
Coordination of tBuNCNtBu to Ti is reversible. Reaction of 3 with various other oxidants results in displacement of tBuNCNtBu and the formation of TiIV complexes 6–9 (Figure 12), in analogy to substitution chemistry seen with 1. Of note is the reaction of 3 with 2 equiv TEMPO, which results in an unusual ring-slipped η5,η1-Cp2Ti(TEMPO)2 (7).
Figure 12.
Reactions of 3 with methyl iodide (top left), 2 equiv TEMPO (top right), diphenyldisulfide (bottom left), and azobenzene (bottom right).
Sterics likely plays a primary factor in the stabilization of 3, so we next revisited the reaction of 1 with the smaller 1,3-dicyclohexylcarbodiimide. Reaction of 2 equiv 1 with 1 equiv 1,3-dicyclohexylcarbodiimide in pentane yielded (Cp2Ti)2(μ-η1,η1-(CyNCNCy)) (10), which crystallized from the reaction mixture within 10 minutes at room temperature (Figure 13). Complex 10 is paramagnetic (μeff = 1.98(3) μB, Evans’ method, C6D6, 25 °C).
Figure 13.
Reactions of Cp2Ti(BTMSA) (1) with 1,3-dicyclohexylcarbodiimide.
The reaction of 1 and 1,3-dicyclohexylcarbodiimide had been previously reported to yield dinuclear amidinate-like 2c in hexanes (Figure 13).36 C6D6 solutions of 10 (~0.017 M) stored at room temperature in an inert atmosphere slowly convert to yellow needles of 2c over a period of approximately 4 days, demonstrating the intermediacy of 10 prior to formation of 2c.
The X-ray structure of 10 is presented in Figure 14. Three similar independent molecules were found within the asymmetric unit, one of which is displayed. The C-N bonds of the carbodiimide fragment in 10 are significantly elongated to > 1.3 Å, similar to the degree of elongation seen in 3. The N1C1-N2 bond angle is approximately 137°, and the Ti-N bond lengths of >2.0 Å are in the range of typical TiIV-N X-type bonds that lack N→Ti π-donation.43–45 The average Ti1-C1 and Ti2-C1 bond distances are 2.310(31) Å and 2.307(23) Å, respectively, both of which exceed the Ti-C distance in 3 (2.2674(10) Å). Additionally, the Ti1-N1-C1 bonding plane intersects the Ti2-N2-C1 bonding plane at a dihedral angle of 38.43(10.11)°, instead of the expected 90° for Ti atoms independently interacting with the orthogonal π bonds of an RN=C=NR heterocumulene. These structural features indicate that 10 is best described as a free dititanamidocarbene similar to the dimetalloxycarbene proposed by Cummins in Ti-promoted CO2 coupling.33
Figure 14.
Two views of one of the three molecules of 10 in the asymmetric unit. Thermal ellipsoids are drawn at the 50% probability level. Hydrogen atoms are omitted for clarity. Selected bond lengths [Å] and angles [°] are an average of the three molecules in the asymmetric unit: N1-C1 1.313(2), N2-C1 1.317(4), Ti1-N1 2.042(19), Ti2-N2 2.043(15), Ti1-C1 2.310(31), Ti2-C1 2.307(23), N1-C1-N2 136.6(1.9).
Only two other examples of Ti2(μ-C(NR)2) complexes have been structurally characterized, both of which are within structured TiIII/TiIII bimetallic motifs with a μ-η2,η2-C(NR)2 bonding pattern.100,101 Both complexes demonstrated similar extents of activation of the carbodiimide moiety in the solid state as observed in 10, but have shorter Ti-C distances (approx. 2.1 Å); however, due to the rigid bimetallic frameworks, direct structural comparison between these structures is difficult.
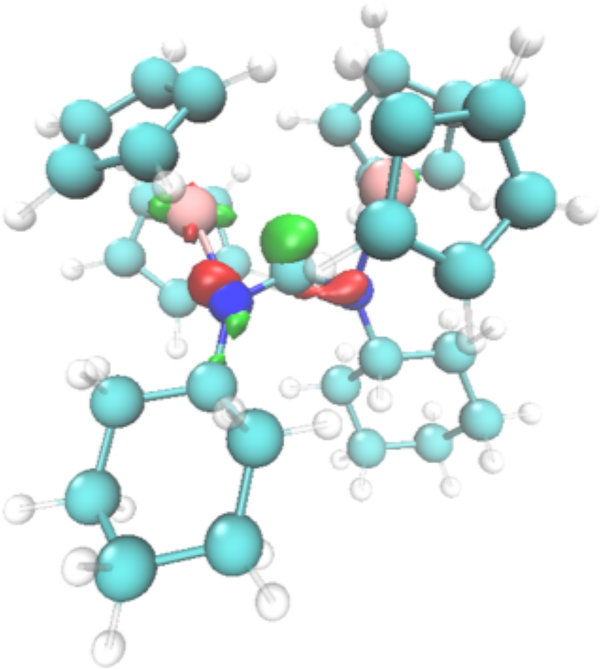
DFT calculations were next used to further investigate the structure of 10. The structure was optimized for both the singlet state (Figure S38) and the triplet state (Figure 15). The free energy of the triplet state was 32.9 kcal/mol lower than the singlet state, and structural parameters of the triplet state matched well with the experimental crystal structure. NBO analysis of 10 and comparative studies of related TiIII complexes further corroborate a TiIII2 triplet structure on the basis of 3dz2 occupation of values (Table S8). Much like in 3, the free carbene in 10 can be clearly seen in the HOMO-4 surface.
Figure 15.
DFT-Optimized (M06/6-311G(d,p)) unrestricted triplet structure of 10 showing the free carbene HOMO-4 surface.
Conclusions
In conclusion, reaction of Cp2Ti(BTMSA) (1) with sterically encumbered tBuNCNtBu results in the formation of the free carbene complex Cp2Ti(κ2-tBuNCNtBu) (3), which contains the first example of a κ2-bound heterocumulene supported by a single metal center. Both experimental solid-state NMR and theoretical analysis of this unusual metallacycle indicate that the free carbene is further stabilized via a Ti-NCN π-bonding interaction in the plane of the metallacycle. Similar reaction of CyNCNCy with 1 results in the formation of another free carbene complex, (Cp2Ti)2(μ-η2,η2-C(NC6H11)2) (10), which contains another surprising heterocumulene bonding motif: a free dititanamidocarbene. These unique molecules should provide an experimental and theoretical platform for the continued investigation of new modes of metalheterocumulene bonding and reactivity. Further, initial reactivity of the carbene moiety of 3 has been demonstrated, hinting at the rich potential of these types of molecules.
Experimental Section
General Considerations
All chemical manipulations were carried out in a glovebox under a nitrogen atmosphere. Cp2Ti(BTMSA)102 was prepared following the literature procedure. CH3I (Sigma Aldrich) and 1,3-di-tert-butylcarbodiimide (TCI) were freeze-pump-thaw degassed three times, brought into the glovebox, and passed through dried basic alumina prior to use. Azobenzene (TCI America) was purified via flash chromatography (hexanes), finely ground, and dried in vacuo prior to use. 2,2,6,6-Tetramethyl-1-piperidine 1-oxyl (Sigma-Aldrich) was sublimed at 25 °C onto a −78 °C cold finger under dynamic vacuum prior to use. Ph2S2 (Sigma-Aldrich), S8 (Sigma-Aldrich), and 1,3-dicyclohexyl-carbodiimide (Fluka) were dried in vacuo overnight prior to use and stored in the glovebox. AgOTf (Sigma-Aldrich) and NaHMDS (Millipore-Sigma) were stored in the glovebox and used as received. 2,6-Lutidinium iodide was prepared following the literature procedure,103 and stored in the glovebox. C6D6, C7D8, and CDCl3 were dried via vacuum transfer from CaH2 followed by filtration through dried basic alumina and stored in the glovebox prior to use. Benzene, pentane, tetrahydrofuran, acetonitrile, dichloromethane, and toluene were dried on a Pure Process Technology solvent purification system.
1H, 13C, 19F, and 1H15N HMBC NMR spectra were recorded on Bruker Avance 400 MHz spectrometers. Chemical shifts are reported with respect to residual protio-solvent impurity for 1H (s, 7.26 ppm for CHCl3; s, 7.16 ppm for C6D5H; p, 2.08 ppm for C7D7H), solvent carbons for 13C (t, 77.16 ppm for CDCl3; t 128.08 ppm for C6D6; s, 137.48 for ispo carbon of C7D8), CF3COOH for 19F (s, −76.6 ppm in CDCl3), and N,NMe2NPh (referenced to NH3 at 0 ppm) for 15N (s, 49.5 ppm in C6D6).104 Evans’ method measurements were performed following previously outlined procedures.105 Elemental analyses were performed at Midwest Microlabs. Despite repeated attempts, acceptable elemental analyses for complexes 4-OTf, 5, and 6 were not obtained.
X-ray: General Procedure
X-ray data were collected using a Bruker Photon II CMOS diffractometer for data collection at 100(2) or 125(2) K using Mo Kα radiation (normal parabolic mirrors). The data intensity was corrected for absorption and decay (SADABS).106 Final cell constants were obtained from least-squares fits of all measured reflections and the structure was solved and refined using SHELXL-2014/7.39.107 All nonhydrogen atoms were refined with anisotropic displacement parameters. Details regarding refined data and cell parameters are available in Tables S1–S3. CCDC entries 1923125, 1975943, 1975944, 1975945, 1975961, 1975969, and 1976006 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html, Cambridge Crystallographic Data Center, 12 Union Road, Cambridge CB2 1EZ, United Kingdom, fax: (+44) 1223–336-033, or e-mail: deposit@ccdc.cam.ac.uk. Computational details as well as information related to CP-MAS 13C NMR spectroscopy can be found within the Supporting Information of this manuscript.
Synthesis of Cp2Ti(κ2-tBuNCtNBu) (3)
Cp2Ti(BTMSA) (1) (2.03 g, 5.74 mmol, 1 equiv.) was added to a 20 mL vial and dissolved in 15 mL pentane. Then, 1,3-di-tert-butylcarbodiimide (888 mg, 5.74 mmol, 1 equiv.) in 1 mL pentane was slowly added. The vial was sealed with a Teflon cap and allowed to stand at room temperature for 10 min while a rapid color change from light brown to red was observed. The reaction mixture was placed at −35 °C overnight to afford the title complex as a dark red crystalline solid (1.75 g, 92%). The title product was isolated via decanting the mother liquor and drying the resulting solid in vacuo.
Alternative synthesis of Cp2Ti(κ2-tBuNCtNBu) (3)
Complex 4-OTf (59.4 mg, 0.124 mmol, 1 equiv.) was suspended in THF (2 mL) in a 20 mL vial containing a stir bar and cooled to −35 °C. Then, NaHMDS (23.9 mg, 0.124 mmol, 1 equiv.) in THF (4 mL) was slowly added. The reaction was allowed to warm to room temperature and stirred for 90 min. Volatiles were removed in vacuo, and the dark residue was suspended in pentane (~6 mL), filtered through a plug of Celite, and dried in vacuo. Recrystallization from pentane at −35 °C overnight afforded the title complex as a dark red crystalline solid, which was isolated via decanting the mother liquor and drying the resulting solid in vacuo (15.7 mg, 38%). 1H NMR (400 MHz, C6D6, 25 °C, δ, ppm): 5.26 (s, 10H, Cp-H), 1.40 (s, 18H, tBu-H). 13C NMR (101 MHz, C6D6, 25 °C, δ, ppm): 130.0, 106.8, 58.9, 33.1. 15N NMR (41 MHz, C6D6, 25 °C, δ, ppm): 246.1. Elemental analysis for 3 reproducibly gave values consistent with an additional oxygen atom added to the title complex (calcd, found; for C19H28N2Ti·O): C (65.52, 65.27), H (8.10, 8.05), N (8.04, 7.95).
Synthesis of Cp2TiIII(tBuNC(H)NtBu) (4)
Complex 3 (200 mg, 0.602 mmol, 1 equiv.) and 2,6-lutidinium iodide (142 mg, 0.602 mmol, 1 equiv.) were added to a 20 mL vial containing a Teflon stir bar and suspended in 7 mL C6H6. The reaction was sealed with a Teflon cap and stirred for 2 h at room temperature. Volatiles were removed in vacuo, leaving a dark brown residue that was extracted with pentane to give a deep blue extract that was filtered through a plug of Celite (~10 mL pentane, product was extracted until filtrate was colorless). The filtrate was concentrated in vacuo to give a deep blue crystalline solid (93.4 mg, 47%). 4 can be recrystallized from minimal pentane at −35 °C over several days to afford crystals suitable for X-ray diffraction.
Synthesis of Cp2TiIII(tBuNC(H)NtBu) (4) with 0.5 equiv lutidinium iodide. 3
(200 mg, 0.602 mmol, 1 equiv.) and 2,6-lutidinium iodide (71.0 mg, 0.301 mmol, 0.5 equiv.) were added to a 20 mL vial, and the reaction was set up under identical reaction conditions to the above procedure, affording 92.0 mg of the title complex (47%). 1H NMR (400 MHz, C6D6, 25 °C, δ, ppm): 3.3 (br s). Evans’ Method (C6D6, 25 °C): μeff = 1.65(3) μB (triplicate). Elemental analysis (calcd, found; for C19H29N2Ti): C (68.47, 68.60), H (8.77, 9.26), N (8.40, 8.46).
Alternative synthesis of Cp2TiIII(tBuNC(H)NtBu) (4)
Complex 4-OTf (35.1 mg, 0.0726 mmol, 1 equiv.) and 3 (24.8 mg, 0.0726 mmol, 1 equiv.) were added to a 20 mL vial containing a stir bar, and suspended in C6H6 (2 mL). The vial was sealed with a Teflon cap and the reaction was stirred for 30 min at room temperature. During the reaction, the color changed from red (heterogeneous) to deep blue-green (homogeneous). Volatiles were removed in vacuo and the residue was suspended in pentane (~5 mL), filtered through a plug of Celite, and dried in vacuo to afford the title complex, in the presence of some unidentified impurities (25.5 mg). The impurities may be a result of decomposition stemming from a complex containing the constituents of Cp2Ti(OTf) that apparently have similar solubility properties to 5.
Synthesis of Cp2Ti(tBuNC(H)NtBu)(OTf) (4-OTf)
Complex 4 (70.8 mg, 0.212 mmol, 1 equiv.) and AgOTf (109.5 mg, 0.425 mmol, 2 equiv.) were added to a 20 mL vial containing a Teflon stir bar and suspended in 3 mL CH3CN. The reaction was sealed with a Teflon cap and stirred for 30 min at room temperature, over which time the color changed from light blue to red along with precipitation of Ag0. Volatiles were removed in vacuo, leaving a red-brown residue that was suspended in CH2Cl2 (~8 mL, extracted until filtrate was colorless) and filtered through a plug of Celite. The extract was concentrated in vacuo, redissolved in minimal CH2Cl2, layered with an equal volume pentane, and placed at −35 °C overnight to afford a dark red crystalline solid. The solid was collected on a glass frit, rinsed with Et2O (5 × 20 mL), and dried in vacuo to afford 4-OTf (52.1 mg, 51%). 1H NMR (400 MHz, CDCl3, 25 °C, δ, ppm): 7.46 (s, 1H, N-CH-N), 6.88 (s, 10H, Cp-H), 1.29 (s, 18H, tBu-H). 13C NMR (101 MHz, CDCl3, 25 °C, δ, ppm): 134.7, 122.4, 58.6, 32.4. DEPT-90 NMR (101 MHz, CDCl3, 25 °C, δ, ppm): 134.7, 122.4. 19F NMR (376 MHz, CDCl3, 25 °C, δ, ppm): −76.9 (s, -O3SCF3).
Synthesis of Cp2Ti(κ2-tBuNC(S)NtBu) (5)
Complex 3 (50.8 mg, 0.150 mmol, 1 equiv.) and S8 (4.7 mg, 0.019 mmol, 0.125 equiv.) were added to a 20 mL vial containing a Teflon stir bar, dissolved in 4 mL C6H6, and stirred at 25 °C for 2 h. A slow color change from dark red to brown-yellow was observed over the course of the reaction. After 2 h, the reaction mixture was dried in vacuo. The residue was dissolved in a minimum of C7H8 (approximately 6 mL), then filtered through Celite and dried in vacuo once more. The crude material was recrystallized from C7H8/pentane at −35 °C overnight, affording 5 as tan needles (39.6 mg, 72%). 1H NMR (400 MHz, C6D6, 25 °C, δ, ppm): 5.98 (s, 10H, Cp-H), 1.56 (br s, 18H, tBu-H). 13C NMR (101 MHz, C6D6, 25 °C, δ, ppm): 162.9, 117.9, 60.9, 31.6.
Synthesis of Cp2Ti(Me)I (6)
Complex 3 (50.0 mg, 0.150 mmol, 1 equiv.) and CH3I (22.3 mg, 0.150 mmol, 1 equiv.) were added to a 20 mL vial containing a Teflon stir bar, dissolved in 1 mL C6H6, and stirred at 40 °C for 16 h. A slow color change from dark red to brown-orange was observed over the course of the reaction. After 16 h, the reaction mixture was dried in vacuo. The residue was dissolved in a minimum of C7H8 (approximately 4 mL), then filtered through Celite and dried in vacuo once more, affording 6 as an orange solid (34.1 mg, 71%). The 1H NMR was matched to that in the literature.108 1H NMR (400 MHz, C6D6, 25 °C, δ, ppm): 5.86 (s, 10H, Cp-H), −0.08 (s, 3H, -CH3).
Synthesis of Cp2Ti(TEMPO)2 (7)
Complex 3 (50.9 mg, 0.150 mmol, 1 equiv.) and 2,2,6,6-tetramethylpiperidin-1yl)oxyl (TEMPO) (47.8 mg, 0.150 mmol, 2 equiv.) were added to a 20 mL vial containing a Teflon stir bar, dissolved in 2 mL C6H6, and stirred at 25 °C for 27 h. A slow color change from dark red to dark red-orange was observed over the course of the reaction. After 27 h, the reaction mixture was dried in vacuo. The residue was dissolved in a minimum of pentane (approximately 10 mL), then filtered through Celite and dried in vacuo once more, affording a mixture of the title complex and a trace of residual 3. The resulting solid was rinsed with 3 × 1 mL pentane to remove residual 3, yielding 7 as an orange solid (25.9 mg, 35%).
Alternative synthesis of Cp2Ti(TEMPO)2 (7)
Complex 1 (300 mg, 0.287 mmol, 1 equiv.) and 2,2,6,6tetramethylpiperidin-1-yl)oxyl (TEMPO) (90.2 mg, 0.574 mmol, 2 equiv.) were added to a 20 mL vial containing a Teflon stir bar, dissolved in 6 mL C6H6, and stirred at 25 °C for 1 h. A rapid color change from light brown to orange was observed immediately. After 1 h, the reaction mixture was dried in vacuo. The residue was dissolved in a minimum of pentane (approximately 10 mL), then filtered through Celite and dried in vacuo once more, affording 7 as a somewhat sticky orange solid (139 mg, 99%). Analytically pure material is more easily obtained via this alternative route than the synthetic route above. Crystalline samples of 7 were afforded by placing a saturated pentane solution of 7 at −35 °C for approximately one week. 1H NMR (400 MHz, C6D6, 25 °C, δ, ppm): 6.33 (s, 10H, Cp-H), 1.49–1.43 (m, 8H, -NC(Me)2CH2CH2), 1.33–1.27 (m, 4H, NC(Me)2CH2CH2), 1.26 (s, 24H, -CH3). 13C NMR (101 MHz, C6D6, 25 °C, δ, ppm): 117.2, 61.3, 41.3, 27.2 (br), 17.0.
Synthesis of Cp2Ti(SPh)2 (8)
Complex 3 (50.5 mg, 0.150 mmol, 1 equiv.) and Ph2S2 (31.8 mg, 0.150 mmol, 1 equiv.) were added to a 20 mL vial containing a Teflon stir bar, dissolved in 2 mL C6H6, and stirred at 40 °C for 17 h. A slow color change from dark red to deep purple was observed over the course of the reaction. After 17 h, the reaction mixture was cooled to room temperature and filtered through a plug of Celite, frozen at −35 °C, and lyophilized in vacuo, affording the title complex as a dark powder (47.2 mg, 94% pure with a 6% impurity of unreacted Ph2S2, 74% corrected yield). The 1H NMR spectrum was matched to that of the literature complex.109 1H NMR (400 MHz, C6D6, 25 °C, δ, ppm): 7.89 (d, J = 7.6 Hz, 4H, Ar-H), 7.16 (m, 4H, Ar-H, overlapping with residual C6D5H), 6.98 (t, J = 7.2 Hz, 2H, Ar-H), 5.68 (s, 10H, Cp-H).
Synthesis of Cp2Ti(PhNNPh) (9)
Complex 3 (50.1 mg, 0.150 mmol, 1 equiv.) and PhNNPh (27.6 mg, 0.150 mmol, 1 equiv.) were added to a 20 mL vial containing a Teflon stir bar, dissolved in 3 mL C6H6, and stirred at 40 °C for 17 h. A slow color change from dark red to black was observed over the course of the reaction. After 17 h, the reaction mixture was dried in vacuo. The residue was dissolved in a minimum of pentane (approximately 10 mL), then filtered through Celite and cooled to −35 °C overnight, affording the title complex as a black crystalline solid with some precipitated powder also present. The free crystalline solid was removed from the precipitated residue (16.5 mg, 97% pure with a 3% impurity of pentane, 30% corrected yield). Although the title complex is known,110 1H and 13C NMR data have not been presented in the literature previously. 1H NMR (400 MHz, C6D6, 25 °C, δ, ppm): 7.23 (app br s, 4H, Ar-H), 6.89 (t, J = 7.3 Hz, 2H, Ar-H), 6.60 (app br s, 4H, Ar-H), 5.78 (s, 10H, Cp-H). 13C NMR (101 MHz, C6D6, 25 °C, δ, ppm): 158.7, 129.1, 122.0, 112.9. The ipso carbon of the azobenzene moiety could not be found, possibly due to broadening from neighboring quadrupolar nitrogen atoms.
Synthesis of (Cp2Ti)2(μ-η2,η2-C(NC6H11)2) (10)
Complex 1 (201 mg, 0.577 mmol, 2 equiv.) and 1,3-dicyclohexylcarbodiimide (63.1 mg, 0.305 mmol, 1 equiv.) were charged to a 20 mL scintillation vial and dissolved in 6 mL pentane. The solution changed to dark red and the formation of a dark red-black crystalline solid was observed immediately. Precipitation was allowed to continue at room temperature for 10 min. The reaction was dried in vacuo, and the resulting solid was collected on a sintered glass frit, rinsed with pentane (3 × 3 mL), and dried once more in vacuo to afford the title complex as a dark red-black powder (105 mg, 65%). The precipitated crystalline solid was reproducibly suitable for single crystal X-ray diffraction. 1H NMR (400 MHz, C6D6, 25 °C, δ, ppm): 37.9, 3.4, 2.7, 1.5, 0.9, 0.8, −10.4. Evans’ Method (C6D6, 25 °C): μeff = 1.98(3) μB (average of 6 runs with 2 batches of material). Elemental analysis for 10 gave values consistent with two additional oxygen atoms added to the title complex (one per Ti atom) (calcd, found; for C33H42N2Ti2·O2): C (66.68, 67.00), H (7.12, 7.57), N (4.71, 5.06).
Supplementary Material
ACKNOWLEDGMENT
James T. Moore, Brendan J. Graziano, and Dr. Victor G. Young Jr. (UMN) are thanked for crystallographic assistance.
Funding Sources
Financial support was provided by the National Institutes of Health (1R35GM119457), and the Alfred P. Sloan Foundation (I.A.T. is a 2017 Sloan Fellow). C.P.G. acknowledges the Scholarship Fund of the Swiss Chemical Industry for funding. Instrumentation for the University of Minnesota Chemistry NMR facility was supported from a grant through the National Institutes of Health (S10OD011952). X-ray diffraction experiments were performed with a diffractometer purchased through a grant from NSF/MRI (1229400) and the University of Minnesota. We acknowledge the Minnesota Supercomputing Institute (MSI) at the University of Minnesota and the National Energy Research Scientific Computing Center (NERSC), a DOE Office of Science User Facility supported by the Office of Science of the U.S. Department of Energy under contract no. DE-AC0205CH11231, for providing resources that contributed to the results reported within this paper.
Footnotes
ASSOCIATED CONTENT
Complete experimental procedures, computational methods, spectroscopic methods, and associated data(PDF)
REFERENCES
- (1).Johnson RP Strained Cyclic Cumulenes. Chem. Rev 1989, 89, 1111–1124. [Google Scholar]
- (2).Daoust KJ; Hernandez SM; Konrad KM; Mackie ID; Winstanley J Jr.; Johnson RP Strain Estimates for Small-Ring Cyclic Allenes and Butatrienes. J. Org. Chem 2006, 71, 5708–5714. [DOI] [PubMed] [Google Scholar]
- (3).Rosenthal U Recent Synthetic and Catalytic Applications of Group 4 Metallocene Bis(Trimethylsilyl)Acetylene Complexes. Eur. J. Inorg. Chem 2019, 895–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Rosenthal U; Burlakov VV; Bach MA; Beweries T Five-Membered Metallacycles of Titanium and Zirconium - Attractive Compounds for Organometallic Chemistry and Catalysis. Chem. Soc. Rev. 2007, 36, 719–728. [DOI] [PubMed] [Google Scholar]
- (5).Beweries T; Haehnel M; Rosenthal U Recent Advances in the Chemistry of Heterometallacycles of Group 4 Metallocenes. Catal. Sci. Technol 2013, 3, 18–28. [Google Scholar]
- (6).Rosenthal U; Ohff A; Baumann W; Kempe R; Tillack A; Burlakov VV Synthesis and Structure of the Smallest Cyclic Cumulene; Reaction of 1,3Diynes with Zirconocene Complexes. Angew. Chem. Int. Ed. Engl 1994, 33, 1605–1607. [Google Scholar]
- (7).Suzuki N; Nishiura M; Wakatsuki Y Isolation and Structural Characterization of 1Zirconacyclopent-3-Yne, Five-Membered Cyclic Alkynes. Science. 2002, 295, 660–663. [DOI] [PubMed] [Google Scholar]
- (8).Ugolotti J; Dierker G; Kehr G; Fröhlich R; Grimme S; Erker G Five-Membered Metallacyclic Allenoids: Synthesis and Structure of Remarkably Stable Strongly Distorted Cyclic Allene Derivatives. Angew. Chem. Int. Ed 2008, 47, 2622–2625. [DOI] [PubMed] [Google Scholar]
- (9).Grubbs RH; O’Leary DJ Handbook of Metathsis, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2015; Vol. 2 (Applications in Organic Synthesis; ). [Google Scholar]
- (10).Wu X; Tamm M Recent Advances in the Development of Alkyne Metathesis Catalysts. Beilstein J. Org. Chem 2011, 7, 82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Ehrhorn H; Tamm M Well-Defined Alkyne Metathesis Catalysts: Developments and Recent Applications. Chem. - A Eur. J 2019, 25, 3190–3208. [DOI] [PubMed] [Google Scholar]
- (12).Fürstner A Alkyne Metathesis on the Rise. Angew. Chemie - Int. Ed 2013, 52, 2794–2819. [DOI] [PubMed] [Google Scholar]
- (13).Reiß F; Reiß M; Bresien J; Spannenberg A; Jiao H; Baumann W; Arndt P; Beweries T 1-Titanacyclobuta-2,3-Diene - an Elusive Four-Membered Cyclic Allene. Chem. Sci 2019, 10, 5319–5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Rosenthal U A Ghost Trapped: Realization of the 1-Titanacyclobuta-2,3-Diene as the First Four-Membered Group 4 Metallacycloallene. Eur. J. Inorg. Chem 2019, 3456–3461. [Google Scholar]
- (15).Mccullough LG; Listemann ML; Schrock RR; Churchill MR; Ziller JW Why Terminal Alkynes Cannot Be Metathesized. Preparation and Crystal Structure of a Deprotonated Tungstenacyclobutadiene Complex, W(η5C5H5)[C3(CMe3)2]Cl. J. Am Chem. Soc 1983, 105, 6729–6730. [Google Scholar]
- (16).Churchill MR; Ziller JW Crystal Structure of a Deprotonated Tungstenacyclobutadiene Complex, (η5-C5H5)W[C3(CMe3)2]Cl; Characterization of an η3-RCCCR Ligand. J. Organomet. Chem 1985, 281, 237–248. [Google Scholar]
- (17).Ehrhorn H; Bockfeld D; Freytag M; Bannenberg T; Kefalidis CE; Maron L; Tamm M Studies on Molybdena- and Tungstenacyclobutadiene Complexes Supported by Fluoroalkoxy Ligands as Intermediates of Alkyne Metathesis. Organometallics 2019, 38, 1627–1639. [Google Scholar]
- (18).Heppekausen J; Stade R; Kondoh A; Seidel G; Goddard R; Fürstner A Optimized Synthesis, Structural Investigations, Ligand Tuning and Synthetic Evaluation of Silyloxy-Based Alkyne Metathesis Catalysts. Chem. Eur. J 2012, 18, 10281–10299. [DOI] [PubMed] [Google Scholar]
- (19).McCullough, Laughlin G; Schrock Richard, R.; Dewan JC; Murdzek JC Preparation of Trialkoxymolybdenum(VI) Alkylidyne Complexes, Their Reactions with Acetylenes, and the X-Ray Structure of Mo[C3(CMe3)2][OCH(CF3)2]2(C5H5N)2. J. Am Chem. Soc 1985, 107, 5987–5998. [Google Scholar]
- (20).Gibson DH The Organometallic Chemistry of Carbon Dioxide. Chem. Rev 1996, 96, 2063–2095. [DOI] [PubMed] [Google Scholar]
- (21).Gibson DH Carbon Dioxide Coordination Chemistry: Metal Complexes and Surface-Bound Species. What Relationships? Coord. Chem. Rev 1999, 185–186, 335–355. [Google Scholar]
- (22).Paparo A; Okuda J Carbon Dioxide Complexes: Bonding Modes and Synthetic Methods. Coord. Chem. Rev 2017, 334, 136–149. [Google Scholar]
- (23).Yin X; Moss JR Recent Developments in the Activation of Carbon Dioxide by Metal Complexes. Coord. Chem. Rev 1999, 181, 27–59. [Google Scholar]
- (24).Gade LH; Memmler H; Kauper U; Schneider A; Fabre S; Bezougli I; Lutz M; Galka C; Scowen IJ; McPartlin M Cooperative Reactivity of Early-Late Heterodinuclear Transition Metal Complexes with Polar Organic Substrates. Chem. - A Eur. J 2000, 6, 692–708. [DOI] [PubMed] [Google Scholar]
- (25).Lisy JM; Dobrzynski ED; Angelici RJ; Clardy J A Platinum(II) Complex Containing a Metallodithiocarboxylate Ligand. J. Am. Chem. Soc 1975, 97, 656–657. [Google Scholar]
- (26).Yoo C; Lee Y Carbon Dioxide Binding at a Ni/Fe Center: Synthesis and Characterization of Ni(η1CO2-κC) and Ni-μ-CO2-ΚC:Κ2O,O′-Fe. Chem. Sci 2017, 8, 600–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Pilato RS; Geoffroy GL; Rheingold AL Net [2+2] Cycloaddition of the Metal-Oxo Bonds of Cp2M=O (Cp = C5H5; M = Mo, W) across the Carbon-Oxygen Bond of Carbonyl Ligands to Form μ2, η3-CO2 Complexes. J. Chem. Soc., Chem. Commun 1989, 1287–1288. [Google Scholar]
- (28).Schneck F; Ahrens J; Finger M; Stückl AC; Würtele C; Schwarzer D; Schneider S The Elusive Abnormal CO2 Insertion Enabled by MetalLigand Cooperative Photochemical Selectivity Inversion. Nat. Commun 2018, 9, 1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Mandal SK; Krause JA; Orchin M The Preparation and X-Ray Crystal Structure of the First Homobimetallic μ(η2-O,O′:η1-C) Carbon Dioxide Complex: (Dppp)(CO)2ReO2CRe(CO)3(Dppp). Polyhedron 1993, 12, 1423–1425. [Google Scholar]
- (30).Gibson DH; Mehta JM; Ye M; Richardson JF; Mashuta MS Synthesis and Characterization of Rhenium Metallocarboxylates. Organometallics 1994, 13, 1070–1072. [Google Scholar]
- (31).Senn DR; Gladysz JA; Emerson K; Larsen RD Synthesis, Structure, and Reactivity of Transition-Metal/Main-Group-Metal Bridging Carboxylate Complexes of the Formula (η5C5C5)Re(NO)(PPh3(CO2MLn) (M = Li, K, Ge, Sn, Pb). Inorg. Chem. 1987, 26 (17), 2737–2739. [Google Scholar]
- (32).Tso CT; Cutler AR Heterobimetallic μ(η1-C: η2-O,O’) Carbon Dioxide and μ(η1-C,O) Formaldehyde Complexes Cp(NO)(CO)Re–C(O)O–Zr(Cl)Cp2 and Cp(NO)(CO)Re–CH2O– Zr(Cl)Cp2. J. Am. Chem. Soc 1986, 108, 6069–6071. [DOI] [PubMed] [Google Scholar]
- (33).Paparo A; Silvia JS; Kefalidis CE; Spaniol TP; Maron L; Okuda J; Cummins CC A Dimetalloxycarbene Bonding Mode and Reductive Coupling Mechanism for Oxalate Formation from CO2. Angew. Chem. Int. Ed 2015, 54, 9115–9119. [DOI] [PubMed] [Google Scholar]
- (34).Zimmermann P; Hoof S; Braun-Cula B; Herwig C; Limberg C A Biomimetic Nickel Complex with a Reduced CO2 Ligand Generated by Formate Deprotonation and Its Behaviour towards CO2. Angew. Chem. Int. Ed 2018, 57, 7230–7233. [DOI] [PubMed] [Google Scholar]
- (35).Haehnel M; Ruhmann M; Theilmann O; Roy S; Beweries T; Arndt P; Spannenberg A; Villinger A; Jemmis ED; Schulz A; Rosenthal U Reactions of Titanocene Bis(Trimethylsilyl)Acetylene Complexes with Carbodiimides: An Experimental and Theoretical Study of Complexation versus C-N Bond Activation. J. Am. Chem. Soc 2012, 134, 15979–15991. [DOI] [PubMed] [Google Scholar]
- (36).Roy S; Jemmis ED; Schulz A; Beweries T; Rosenthal U Theoretical Evidence of the Stabilization of an Unusual Four-Membered Metallacycloallene by a Transition-Metal Fragment. Angew. Chem. Int. Ed 2012, 51, 5347–5350. [DOI] [PubMed] [Google Scholar]
- (37).Haehnel M; Schubert K; Becker L; Arndt P; Spannenberg A; Rosenthal U Reactions of Group 4 Metallocenes with N,N’-Diphenylformamidine – Hydrogen Generation versus Oxidative Addition. Z. Anorg. Allg. Chem 2014, 640, 2532–2536. [Google Scholar]
- (38).Kaleta K; Ruhmann M; Theilmann O; Beweries T; Roy S; Arndt P; Villinger A; Jemmis ED; Schulz A; Rosenthal U Reactions of Group 4 Metallocene Alkyne Complexes with Carbodiimides: Experimental and Theoretical Studies of the Structure and Bonding of Five-Membered Hetero-Metallacycloallenes. J. Am. Chem. Soc 2011, 133, 5463–5473. [DOI] [PubMed] [Google Scholar]
- (39).Theilmann O; Ruhmann M; Villinger A; Schulz A; Seidel WW; Kaleta K; Beweries T; Arndt P; Rosenthal U [Cp2TiIII(NCy)2C-TiIIICp2]: A Transient Titanocene Carbene Complex? Angew. Chem. Int. Ed 2010, 49, 9282–9285. [DOI] [PubMed] [Google Scholar]
- (40).Beaumier EP; Mcgreal ME; Pancoast AR; Wilson RH; Moore JT; Graziano BJ; Goodpaster JD; Tonks IA Carbodiimide Synthesis via Ti-Catalyzed Nitrene Transfer from Diazenes to Isocyanides. ACS Catal. 2019, 9, 11753–11762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Beaumier EP; Pearce AJ; See XY; Tonks IA Modern Applications of Low-Valent Early Transition Metals in Synthesis and Catalysis. Nat. Rev. Chem 2019, 3, 15–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Allen FH; Kennard O; Watson DG; Brammer L; Orpen AG; Taylor R Tables of Bond Lengths Determined by X-Ray and Neutron Diffraction. Part 1. Bond Lengths in Organic Compounds. J. Chem. Soc. Perkin Trans 2 1987, S1–S19. [Google Scholar]
- (43).Dunn PL; Reath AH; Clouston LJ; Young VGY Jr.; Tonks IA Homo- and Heteroleptic Group 4 2-(Diphenylphosphino)Pyrrolide Complexes: Synthesis, Coordination Chemistry and Solution State Dynamics. Polyhedron 2014, 84, 111–119. [Google Scholar]
- (44).Harris SA; Ciszewski JT; Odom AL Titanium Η1-Pyrrolyl Complexes: Electronic and Structural Characteristics Imposed by the N,NDi(Pyrrolyl-α-Methyl)-N-Methylamine (Dpma) Ligand. Inorg. Chem 2001, 40, 1987–1988. [DOI] [PubMed] [Google Scholar]
- (45).Li Y; Shi Y; Odom AL Titanium Hydrazido and Imido Complexes: Synthesis, Structure, Reactivity, and Relevance to Alkyne Hydroamination. J. Am. Chem. Soc 2004, 126, 1794–1803. [DOI] [PubMed] [Google Scholar]
- (46).Thewalt U; Wöhrle T Die Struktur von Cp2TiMe2. J. Organomet. Chem 1994, 464, C17–C19. [Google Scholar]
- (47).Bourissou D; Guerret O; Gabbaï FP; Bertrand G Stable Carbenes. Chem. Rev 2000, 100, 30–91. [DOI] [PubMed] [Google Scholar]
- (48).Heinemann C; Thiel W Ab Initio Study on the Stability of Diaminocarbenes. Chem. Phys. Lett 1994, 217, 11–16. [Google Scholar]
- (49).Despagnet-Ayoub E; Grubbs RH A Stable Four-Membered N-Heterocyclic Carbene. J. Am. Chem. Soc 2004, 126, 10198–10199. [DOI] [PubMed] [Google Scholar]
- (50).Ishida Y; Donnadieu B; Bertrand G Stable Four-π-Electron, Four-Membered Heterocyclic Cations and Carbenes. Proc. Natl. Acad. Sci 2006, 103, 13585–13588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Falivene L; Cao Z; Petta A; Serra L; Poater A; Oliva R; Scarano V; Cavallo L Towards the Online Computer-Aided Design of Catalytic Pockets. Nat. Chem 2019, 11, 872–879. [DOI] [PubMed] [Google Scholar]
- (52).Poater A; Cosenza B; Correa A; Giudice S; Ragone F; Scarano V; Cavallo L SambVca: A Web Application for the Calculation of the Buried Volume of N-Heterocyclic Carbene Ligands. Eur. J. Inorg. Chem 2009, 2009, 1759–1766. [Google Scholar]
- (53).Clavier H; Nolan SP Percent Buried Volume for Phosphine and N-Heterocyclic Carbene Ligands: Steric Properties in Organometallic Chemistry. Chem. Commun 2010, 46, 841–861. [DOI] [PubMed] [Google Scholar]
- (54).Gómez-Suárez A; Nelson DJ; Nolan SP Quantifying and Understanding the Steric Properties of N-Heterocyclic Carbenes. Chem. Commun 2017, 53, 2650–2660. [DOI] [PubMed] [Google Scholar]
- (55).Zhao Y; Truhlar DG The M06 Suite of Density Functionals for Main Group Thermochemistry, Thermochemical Kinetics, Noncovalent Interactions, Excited States, and Transition Elements: Two New Functionals and Systematic Testing of Four M06-Class Functionals and 12 Other Function. Theor. Chem. Acc 2008, 120, 215–241. [Google Scholar]
- (56).Krishnan R; Binkley JS; Seeger R; Pople JA Self-Consistent Molecular Orbital Methods. XX. A Basis Set for Correlated Wave Functions. J. Chem. Phys 1980, 72, 650–654. [Google Scholar]
- (57).Miliordos E; Ruedenberg K; Xantheas SS Unusual Inorganic Biradicals: A Theoretical Analysis. Angew. Chemie 2013, 125, 5848–5851. [DOI] [PubMed] [Google Scholar]
- (58).Mayer I Charge, Bond Order and Valence in the AB Initio SCF Theory. Chem. Phys. Lett. 1983, 97, 270–274. [Google Scholar]
- (59).Reed AE; Weinstock RB; Weinhold F Natural Population Analysis. J. Chem. Phys. 1985, 83, 735–746. [Google Scholar]
- (60).Davis-Gilbert ZW; Wen X; Goodpaster JD; Tonks IA Mechanism of Ti-Catalyzed Oxidative Nitrene Transfer in [2 + 2 + 1] Pyrrole Synthesis from Alkynes and Azobenzene. J. Am. Chem. Soc 2018, 140, 7267–7281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Bader RFW Atoms in Molecules. Acc. Chem. Res 1985, 18, 9–15. [Google Scholar]
- (62).Bader RFW A Quantum Theory of Molecular Structure and Its Applications. Chem. Rev 1991, 91, 893–928. [Google Scholar]
- (63).Bader RFW The Quantum Mechanical Basis of Conceptual Chemistry. Monatsh. Chem 2005, 136, 819–854. [Google Scholar]
- (64).Bader RWF Atoms in Molecules: A Quantum Theory; Oxford University Press, 1994. [Google Scholar]
- (65).Lu T; Chen F Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem 2012, 33, 580–592. [DOI] [PubMed] [Google Scholar]
- (66).Silvi B; Savin A Classification of Chemical Bonds Based on Topological Analysis of Electron Localization Functions. Nature 1994, 371, 683–686. [Google Scholar]
- (67).Widdifield, Cory M; Schurko RW Understanding Chemical Shielding Tensors Using Group Theory, MO Analysis, and Modern Density Functional Theory. Concepts Magn. Reson. Part A 2009, 34A, 191–123. [Google Scholar]
- (68).Pyykkö P Calculation of NMR and EPR Parameters In Theory of NMR Parameters. From Ramsey to Relativity, 1953–1983; Kaupp M, Bühl M, Malkin VG, Eds.; Wiley-VCH: Weinheim, 2004; pp 7–19. [Google Scholar]
- (69).Kaupp M Interpretation of NMR Chemical Shifts In Calculation of NMR and EPR Parameters; Kaupp M, Bühl M, Malkin VG, Eds.; Wiley-VCH: Weinheim, 2004; pp 293–306. [Google Scholar]
- (70).Salzmann R; Kaupp M; McMahon MT; Oldfield E Solid-State Nuclear Magnetic Resonance Spectroscopic and Quantum Chemical Investigation of 13C and 17O Chemical Shift Tensors, 17O Nuclear Quadrupole Coupling Tensors, and Bonding in Transition-Metal Carbonyl Complexes and Clusters. J. Am. Chem. Soc 1998, 120, 4771–4783. [Google Scholar]
- (71).Sutter K; Autschbach J Computational Study and Molecular Orbital Analysis of NMR Shielding, Spin-Spin Coupling, and Electric Field Gradients of Azido Platinum Complexes. J. Am. Chem. Soc 2012, 134, 13374–13385. [DOI] [PubMed] [Google Scholar]
- (72).Vummaleti SVC; Nelson DJ; Poater A; Gómez-Suárez A; Cordes DB; Slawin AMZ; Nolan SP; Cavallo L What Can NMR Spectroscopy of Selenoureas and Phosphinidenes Teach Us about the π-Accepting Abilities of N-Heterocyclic Carbenes? Chem. Sci 2015, 6, 1895–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Viesser RV; Ducati LC; Tormena CF; Autschbach J The Unexpected Roles of σ and π Orbitals in Electron Donor and Acceptor Group Effects on the 13C NMR Chemical Shifts in Substituted Benzenes. Chem. Sci 2017, 8, 6570–6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Staun SL; Sergentu D-C; Wu G; Autschbach J; Hayton TW Use of 15N NMR Spectroscopy to Probe Covalency in a Thorium Nitride. Chem. Sci 2019, 10, 6431–6436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Gordon CP; Raynaud C; Andersen RA; Copéret C; Eisenstein O Carbon-13 NMR Chemical Shift: A Descriptor for Electronic Structure and Reactivity of Organometallic Compounds. Acc. Chem. Res 2019, 52, 2278–2289. [DOI] [PubMed] [Google Scholar]
- (76).Bohmann JA; Weinhold F; Farrar TC Natural Chemical Shielding Analysis of Nuclear Magnetic Resonance Shielding Tensors from Gauge-Including Atomic Orbital Calculations. J. Chem. Phys 1997, 107, 1173–1184. [Google Scholar]
- (77).Autschbach J Analyzing NMR Shielding Tensors Calculated with Two-Component Relativistic Methods Using Spin-Free Localized Molecular Orbitals. J. Chem. Phys 2008, 128, 164112. [DOI] [PubMed] [Google Scholar]
- (78).Autschbach J; Zheng S Analyzing Pt Chemical Shifts Calculated from Relativistic Density Functional Theory Using Localized Orbitals: The Role of Pt 5d Lone Pairs. Magn. Reson. Chem 2008, 46, S45–S55. [DOI] [PubMed] [Google Scholar]
- (79).Gordon CP; Yamamoto K; Liao W-C; Allouche F; Andersen RA; Copéret C; Raynaud C; Eisenstein O Metathesis Activity Encoded in the Metallacyclobutane Carbon-13 NMR Chemical Shift Tensors. ACS Cent. Sci 2017, 3, 759–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (80).Takemoto S; Ohata J; Umetani K; Yamaguchi M; Matsuzaka H A Diruthenium μ-Carbido Complex That Shows Singlet-Carbene-like Reactivity. J. Am. Chem. Soc 2014, 136, 15889–15892. [DOI] [PubMed] [Google Scholar]
- (81).Takemoto S; Tsujita M; Matsuzaka H Diruthenium Carbido Complexes as N-Heterocyclic Carbene Like C-Donor Ligands to Group 11 Metals. Organometallics 2017, 36, 3686–3691. [Google Scholar]
- (82).Barnett HJ; Hill AF A Dirhoda-Heterocyclic Carbene. Angew. Chem. Int. Ed 2020, 59, 4274–4277. [DOI] [PubMed] [Google Scholar]
- (83).Ruiz J; García L; Perandones BF; Vivanco M A Fischer Carbene within an Arduengo Carbene. Angew. Chem. Int. Ed 2011, 50, 3010–3012. [DOI] [PubMed] [Google Scholar]
- (84).Ruiz J; García L; Mejuto C; Vivanco M; Díaz MR; García-Granda S Strong Electron-Donating Metalla-N-Heterocyclic Carbenes. Chem. Commun 2014, 50, 2129–2132. [DOI] [PubMed] [Google Scholar]
- (85).Tskhovrebov AG; Luzyanin KV; Dolgushin FM; Guedes da Silva MFC; Pombeiro AJL; Kukushkin VY Novel Reactivity Mode of Metal Diaminocarbenes : Palladium(II)-Mediated Coupling between Acyclic Diaminocarbenes and Isonitriles Leading to Dinuclear Species. Organometallics 2011, 30, 3362–3370. [Google Scholar]
- (86).Khramov DM; Rosen EL; Lynch VM; Bielawski CW Diaminocarbene[3]Ferrocenophanes and Their Transition-Metal Complexes. Angew. Chemie - Int. Ed 2008, 47, 2267–2270. [DOI] [PubMed] [Google Scholar]
- (87).Siemeling U; Färber C; Leibold M; Bruhn C; Mücke P; Winter RF; Sarkar B; Von Hopffgarten M; Frenking G Six-Membered N-Heterocyclic Carbenes with a 1,1′-Ferrocenediyl Backbone: Bulky Ligands with Strong Electron-Donor Capacity and Unusual Non-Innocent Character. Eur. J. Inorg. Chem 2009, 2009, 4607–4612. [Google Scholar]
- (88).Hildebrandt B; Frank W; Ganter C A Cationic N-Heterocyclic Carbene with an Organometallic Backbone: Synthesis and Reactivity. Organometallics 2011, 30, 3483–3486. [Google Scholar]
- (89).Siemeling U Singlet Carbenes Derived from Ferrocene and Closely Related Sandwich Complexes. Eur. J. Inorg. Chem 2012, 2012, 3523–3536. [Google Scholar]
- (90).Aguilar-Caldero JR; Murillo J; Gomez-Torres A; Saucedo C; Jordan A; Metta-Magaña AJ; Pink M; Fortier S Redox Character and Small Molecule Reactivity of a Masked Titanium(II) Synthon. Organometallics 2020, 39, 295–311. [Google Scholar]
- (91).Trunkely EF; Epshteyn A; Zavalij PY; Sita LR Synthesis, Structural Characterization, and Preliminary Reactivity Profile of a Series of Monocyclopentadienyl, Monoacetamidinate Titanium(III) Alkyl Complexes Bearing β-Hydrogens. Organometallics 2010, 29, 6587–6593. [Google Scholar]
- (92).Wasslen YA; Tois E; Haukka S; Kreisel KA; Yap GPA; Halls MD; Barry ST A Family of Heteroleptic Titanium Guanidinates: Synthesis, Thermolysis, and Surface Reactivity. Inorg. Chem 2010, 49, 1976–1982. [DOI] [PubMed] [Google Scholar]
- (93).Lim BS; Rahtu A; Park J-S; Gordon RG Synthesis and Characterization of Volatile, Thermally Stable, Reactive Transition Metal Amidinates. Inorg. Chem 2003, 42, 7951–7958. [DOI] [PubMed] [Google Scholar]
- (94).Hagadorn JR; Arnold J Titanium(II), -(III), and -(IV) Complexes Supported by Benzamidinate Ligands. Organometallics 1998, 17, 1355–1368. [Google Scholar]
- (95).Bai G; Roesky HW; Noltemeyer M; Hao H; Schmidt H-G Synthesis of the First Compound with a Rhombohedral Ti6(μ3-NH)6(μ3-N)2 Core Structure by Ammonolysis of a Titanium Chelate in a Two-Phase System. Organometallics 2000, 19, 2823–2825. [Google Scholar]
- (96).Hagadorn JR; Arnold J Low-Valent Chemistry of Titanium Benzamidinates Leading to New Ti μ-N2, μ-O, Alkyl Derivatives, and the Cyclometalation of TMEDA. J. Am. Chem. Soc 1996, 118, 893–894. [Google Scholar]
- (97).Benac BL; Burgess EM; Arduengo AJ 1,3Dimethylimidazole-2-Thione. Org. Synth 1986, 64, 92. [Google Scholar]
- (98).Doddi A; Peters M; Tamm M N-Heterocyclic Carbene Adducts of Main Group Elements and Their Use as Ligands in Transition Metal Chemistry. Chem. Rev 2019, 119, 6994–7112. [DOI] [PubMed] [Google Scholar]
- (99). Attempts to coordinate the carbene moiety of 3 have been performed with the following: CuBr, CuI, AgOTf, AuCl(SMe2), PdCl2(MeCN)2, Pd(PtBu3)2, [RhCl(COD)]2, AlCl3, GaCl3, and B(C6F5)3.
- (100).Kilpatrick AFR; Green JC; Cloke FGN Reactivity of a Dititanium Bis(Pentalene) Complex toward Heteroallenes and Main-Group Element−Element Bonds. Organometallics 2017, 36, 352–362. [Google Scholar]
- (101).Carbó JJ; García-López D; González-del Moral O; Martín A; Mena M; Santamaría C Carbon−Nitrogen Bond Construction and Carbon−Oxygen Double Bond Cleavage on a Molecular Titanium Oxonitride: A Combined Experimental and Computational Study. Inorg. Chem 2015, 54, 9401–9412. [DOI] [PubMed] [Google Scholar]
- (102).Varga V; Mach K; Polášek M; Sedmera P; Hiller J; Thewalt U; Troyanov SI TitanoceneBis(Trimethylsilyl) Acetylene Complexes: Effects of Methyl Substituents at the Cyclopentadienyl Ligands on the Structure of Thermolytic Products. J. Organomet. Chem 1996, 506, 241–251. [Google Scholar]
- (103).Odom AL; Cummins CC A Chromium(VI) Nitrido-Silylmethyl Complex and a Chromium(V) μ-Nitrido Dimer: Synthetic and Structural Details. Organometallics 1996, 15, 898–900. [Google Scholar]
- (104).Marek R; Lyčka A; Kolehmainen E; Sievänen E; Toušek J 15N NMR Spectroscopy in Structural Analysis: An Update (2001 – 2005). Curr. Org. Chem 2007, 11, 1154–1205. [Google Scholar]
- (105).Girolami GS; Rauchfuss TB; Angelici RJ Synthesis and Technique in Inorganic Chemistry, 3rd ed.; University Science Books: Sausalito, CA, 1999. [Google Scholar]
- (106).Blessing RH An Empirical Correction for Absorption Anisotropy. Acta Crystallogr., Sect. A 1995, 51, 33–38. [DOI] [PubMed] [Google Scholar]
- (107).Sheldrick GM SHELXT - Integrated Space- Group and Crystal-Structure Determination. Acta Crystallogr., Sect. A 2015, 71, 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (108).Albrecht C; Krüger T; Wagner C; Rüffer T; Lang H; Steinborn D Zur Reaktivität von Titanocenkomplexen [Ti(Cp′)2(η2-Me3SiC≡CSiMe3)] (Cp′ = Cp, Cp*) Gegenüber Benzoldicarbonsäuren. Z. Anorg. Allg. Chem 2008, 634, 2495–2503. [Google Scholar]
- (109).Bruce AE; Bruce MRM; Tyler DR Photochemistry and Electronic Structure of the (η5-C5H5)2TiS5 Complex. J. Am. Chem. Soc 1984, 106, 6660–6664. [Google Scholar]
- (110).Fochi G; Floriani C; Bart JCJ; Giunchi G Azo-Complexes of Bis(Cyclopentadienyl)-Titanium and -Vanadium; Model Systems for N-N Multiple Bond Activation. J. Chem. Soc., Dalt. Trans 1983, 1515–1521. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.