Abstract
This study by Tóth et al. has defined that the N-terminal MHR1/2 domain is a conserved ADPR binding site in TRPM2 from ancient cnidarians to vertebrate, and that it is the key ligand binding site for invertebrate TRPM2 channel activation by ADPR, the same as observed in human and zebrafish TRPM2.
TRPM2, the most ancient TRPM family member, is a Ca2+-permeable nonselective cation channel that plays an important role in physiological functions such as insulin secretion, immune response, and core body temperature regulation (Perraud et al., 2001; Togashi et al., 2006; Yamamoto et al., 2008; Uchida et al., 2011; Song et al., 2016; Tan and McNaughton, 2016). It is uniquely activated in the presence of Ca2+ and PIP2 by ADP ribose (ADPR), a metabolite from NAD+ (Csanády and Törocsik 2009; Ernst et al., 2013; Guse 2015; Tóth and Csanády 2012). Recent structural studies on zebrafish and human TPPM2 (drTRPM2 and hsTRPM2; Huang et al., 2018, 2019, 2020) have revealed a novel ADPR binding site in the N-terminal MHR1/2 domain, and, at least in humans, a second ADPR binding site in the C-terminal NUDT9-H domain (Huang et al., 2018). However, it is unknown whether the MHR1/2 binding site also exists in invertebrates. Moreover, while both ADPR binding sites are indispensable for the channel activation of human TRPM2, their individual contribution and how they cooperatively open the channel remain unclear, because knocking out either binding site abolishes the channel response. The nucleotide binding preferences of these two sites are also unknown. In this issue of the Journal of General Physiology, Tóth et al. have addressed these key questions by ingeniously using a unique model system, an invertebrate TRPM2 from Nematostella vectensis (nvTRPM2), in which the MHR1/2 and the NUDT9-H domains operate independently (Tóth et al., 2020). This allowed them to knock out one binding site at a time and study the role of the other site. This study has defined that the N-terminal MHR1/2 domain is a conserved ADPR binding site across species, from ancient cnidarians to vertebrates, and that it is fundamental for TRPM2 channel activation by ADPR.
Broadly expressed in the body, TRPM2 is involved in many important physiological functions and has been considered as promising target for the treatment of Alzheimer’s disease or brain injury after stroke (Hara et al., 2002; Kaneko et al., 2006; Fonfria et al., 2005). Structurally, the TRPM2 channel is similar to other TRPM channels, having N-terminal TRPM homologue region domains (MHR1–4), a transmembrane domain (S1–S6), and a coiled-coil domain. What makes TRPM2 unique among the eight TRPM family members is the C-terminal NUDT9-H domain. This domain is so named because it shares 50% sequence similarity to the human NUDT9, which is a mitochondrial enzyme that binds to and hydrolyzes ADPR into AMP and ribose-5-phosphate (R5P; Perraud et al., 2001). Therefore, TRPM2 has been considered as one of the few “chanzymes”, that is, proteins with both channel function and enzymatic activity (Kühn et al., 2016; Iordanov et al., 2016; Tóth et al., 2014; Iordanov et al., 2019).
A consensus view in the field is that ADPR activates TRPM2 by binding to the NUDT9-H domain (Kühn and Lückhoff 2004; Yu et al., 2017; Fliegert et al., 2017), a view supported by the fact that human TRPM2 lacking the NUDT9-H domain does not respond to ADPR. However, this view is flawed, because Kühn et al. (2016) observed that nvTRPM2 retained ADPR-induced channel activation after deletion of the NUDT9-H domain, which leads to the idea that another ADPR binding site might exist. This has been structurally and functionally confirmed by two recent publications in which Huang et al. defined a novel ADPR binding site in the N-terminal MHR1/2 domain in both human and zebrafish TRPM2 (Huang et al., 2019, 2018). They were also able to visualize the ADPR binding in the NUDT9-H domain of human TRPM2 but not in the zebrafish TRPM2 because the domain is flexible in the latter structure.
Electrophysiological experiments support the idea that the MHR1/2 binding site is crucial for the channel gating of human and zebrafish TRPM2 (Kühn et al., 2019; Huang et al., 2018, 2019). The key residues involved in ADPR binding in the MHR1/2 domain are conserved across species. However, whether the same binding site exists in invertebrate TRPM2 remains unexplored. Interestingly, the two ADPR molecules in human TRPM2 show different molecular geometries, the one in the MHR1/2 domain being U-shaped and the one in the NUDT9-H domain having an extended shape (Huang et al., 2019). This implies that the two domains may contribute differently to channel gating, but this is challenging to study because, at least in humans and zebrafish, the two binding sites are functionally cooperative, and knocking out either one abolishes the channel response to ADPR.
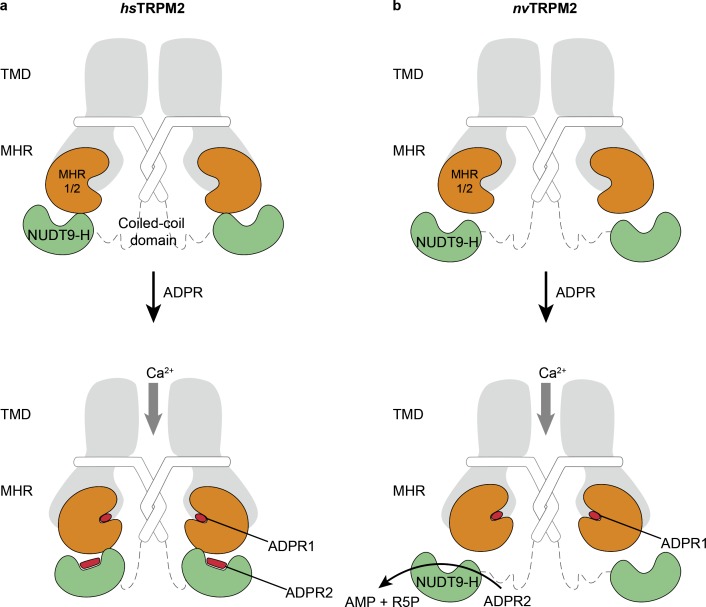
To address these key questions, Csanady and colleagues have taken advantage of the invertebrate TRPM2 from N. vectensis (nvTRPM2) because its ADPR-dependent channel activation is apparently independent of its NUDT9-H domain. This is also indicated by a structural study showing that the nvNUDT9-H domain showed no interaction with the rest of the protein and is thus invisible in the high-resolution structure (Zhang et al., 2018). They have previously reported that the NUDT9-H domain of nvTRPM2 is indeed capable of hydrolyzing ADPR to AMP and R5P (Fig. 1 a). That enzymatic activity has been lost in the vertebrate TRPM2, which is in agreement with the fact that the key residues for enzymatic activity are conserved only in invertebrate TRPM2 (Iordanov et al., 2019, 2016).
Figure 1.
ADPR binding sites in human TRPM2 (hsTRPM2) and an invertebrate TRPM2 from N. vectensis (nvTRPM2). (a) In hsTRPM2, binding of ADPR in both the N-terminal MHR1/2 domain and the C-terminal NUDT9-H domain is indispensable for channel activation. The ADPR1 is in a U-shape, while ADPR2 is in an extended shape. (b) In nvTRPM2, the channel activation requires only binding of ADPR into the MHR1/2 domain, while the NUDT9-H domain has only enzymatic activity to hydrolyze ADPR into AMP and R5P.
In this issue of the Journal of General Physiology, Tóth et al. took a step further in studying the role of MHR1/2 domain in nvTRPM2. They first explored the functional properties of the nvTRPM2 by deleting its NUDT9-H domain (Tóth et al., 2020). Despite significantly reduced expression (as also observed in human and zebrafish TRPM2; Huang et al., 2018, 2019), the NUDT9-H deletion construct remained responsive to ADPR, in agreement with the previous report by Kühn et al. (2016). They further determined the half-maximal effective concentration (EC50) of this truncation construct for channel activation by ADPR, which is in the same range as that of wild type nvTRPM2, supporting the idea that the MHR1/2 binding site is directly in charge of channel activation in nvTRPM2. In contrast, the NUDT9-H domain does not contribute to channel gating, but solely to enzymatic activity. This result beautifully illustrated that the two ADPR binding sites in invertebrate TRPM2 such as nvTRPM2 have completely independent functions. This differs from vertebrate TRPM2, in which both MHR1/2 and NUDT9-H domains participate cooperatively in channel gating (Fig. 1).
Next, Tóth et al. (2020) generated mutants of key residues involved in ADPR binding in the MHR1/2 domain, as identified from the human and zebrafish TRPM2 structures (Huang et al., 2018, 2019), and performed electrophysiological experiments. In these mutants, ADPR produced little or no current, confirming that the N-terminal MHR1/2 binding site is the only one responsible for ADPR-induced channel activation in nvTRPM2.
Lastly, to establish whether the two ADPR binding sites in nvTRPM2 have different affinities for ADPR and its analogues, Tóth et al. (2020) again took advantage of the functional independence of the two binding sites. They reported the EC50 for the MHR1/2 binding site (responsible for channel activation) and the Km for the hydrolysis of ADPR and its analogues by the isolated nvNUDT9-H domain. While nvTRPM2 responded to the tested ligands with variable efficacies and potencies, the isolated nvNUDT9-H domain showed little selectivity among the ligands. These results provide the first glimpse of nucleotide selectivity and specificity at these two ADPR binding sites and provide useful information for further development of TRPM2-specific drugs.
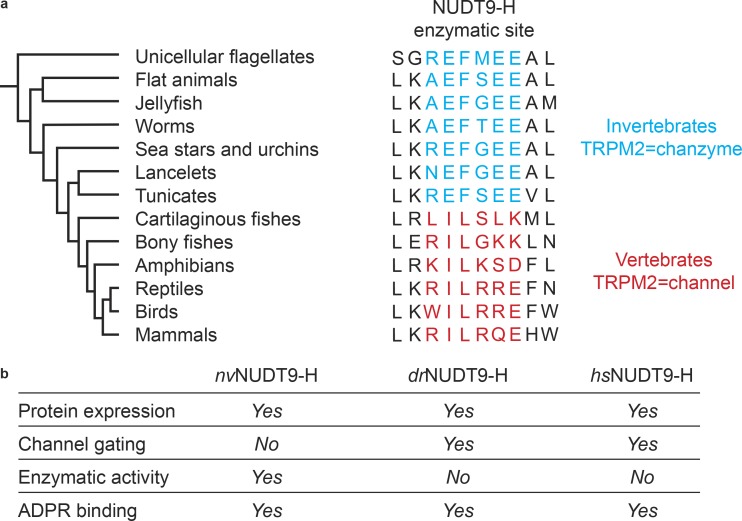
Taken together, the work by Tóth et al. and by other groups (Kühn et al., 2016, 2019; Huang et al., 2018, 2019) has established that the N-terminal MHR1/2 domain in TRPM2 is a conserved ADPR binding site in charge of channel gating from invertebrate to vertebrate TRPM2, while the NUDT9-H domain has changed its function along the evolutionary path. In Fig. 2, we summarize the current knowledge of the structural and functional differences of the NUDT9-H domain among invertebrate and vertebrate TRPM2. Briefly, in nvTRPM2, the NUDT9-H domain is solely in charge of enzymatic activity, hydrolyzing ADPR into AMP and R5P to inactivate the channel, but it does not directly affect channel gating. In contrast, the enzymatic activity of the vertebrate NUDT9-H domain has been lost, but it has gained function to be involved in channel gating. It is yet to be determined how the two ADPRs binding cooperatively activate human TRPM2 and what their individual contributions are.
Figure 2.
The function of NUDT9-H domain. (a) Sequence alignment of the key residues in the NUDT9-H domain that are involved in enzymatic activity (figure adapted from Iordanov et al., 2019). (b) Comparison of the functions of NUDT9-H in different species.
Acknowledgments
Merritt C. Maduke served as editor.
We thank David Nadziejka for technical editing.
W. Lü is supported by the National Institutes of Health (NIH; grant R56HL144929). J. Du is supported by a McKnight Scholar Award, a Klingenstein-Simon Scholar Award, a Sloan Research Fellowship and the NIH (grant R01NS111031).
The authors declare no competing financial interests.
References
- Csanády L., and Törocsik B.. 2009. Four Ca2+ ions activate TRPM2 channels by binding in deep crevices near the pore but intracellularly of the gate. J. Gen. Physiol. 133:189–203. 10.1085/jgp.200810109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst I.M., Fliegert R., and Guse A.H.. 2013. Adenine Dinucleotide Second Messengers and T-lymphocyte Calcium Signaling. Front. Immunol. 4:259 10.3389/fimmu.2013.00259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliegert R., Watt J.M., Schöbel A., Rozewitz M.D., Moreau C., Kirchberger T., Thomas M.P., Sick W., Araujo A.C., Harneit A., et al. 2017. Ligand-induced activation of human TRPM2 requires the terminal ribose of ADPR and involves Arg1433 and Tyr1349. Biochem. J. 474:2159–2175. 10.1042/BCJ20170091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonfria E., Marshall I.C., Boyfield I., Skaper S.D., Hughes J.P., Owen D.E., Zhang W., Miller B.A., Benham C.D., and McNulty S.. 2005. Amyloid beta-peptide(1-42) and hydrogen peroxide-induced toxicity are mediated by TRPM2 in rat primary striatal cultures. J. Neurochem. 95:715–723. 10.1111/j.1471-4159.2005.03396.x [DOI] [PubMed] [Google Scholar]
- Guse A.H. 2015. Calcium mobilizing second messengers derived from NAD. Biochim. Biophys. Acta. 1854:1132–1137. 10.1016/j.bbapap.2014.12.015 [DOI] [PubMed] [Google Scholar]
- Hara Y., Wakamori M., Ishii M., Maeno E., Nishida M., Yoshida T., Yamada H., Shimizu S., Mori E., Kudoh J., et al. 2002. LTRPC2 Ca2+-permeable channel activated by changes in redox status confers susceptibility to cell death. Mol. Cell. 9:163–173. 10.1016/S1097-2765(01)00438-5 [DOI] [PubMed] [Google Scholar]
- Huang Y., Winkler P.A., Sun W., Lü W., and Du J.. 2018. Architecture of the TRPM2 channel and its activation mechanism by ADP-ribose and calcium. Nature. 562:145–149. 10.1038/s41586-018-0558-4 [DOI] [PubMed] [Google Scholar]
- Huang Y., Roth B., Lü W., and Du J.. 2019. Ligand recognition and gating mechanism through three ligand-binding sites of human TRPM2 channel. eLife. 8:e50175 10.7554/eLife.50175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Fliegert R., Guse A.H., Lü W., and Du J.. 2020. A Structural Overview of the Ion Channels of the TRPM Family. Cell Calcium. 85. In press. 102111 10.1016/j.ceca.2019.102111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iordanov I., Mihályi C., Tóth B., and Csanády L.. 2016. The proposed channel-enzyme transient receptor potential melastatin 2 does not possess ADP ribose hydrolase activity. eLife. 5:e17600 10.7554/eLife.17600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iordanov I., Tóth B., Szollosi A., and Csanády L.. 2019. Enzyme activity and selectivity filter stability of ancient TRPM2 channels were simultaneously lost in early vertebrates. eLife. 8:e44556 10.7554/eLife.44556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko S., Kawakami S., Hara Y., Wakamori M., Itoh E., Minami T., Takada Y., Kume T., Katsuki H., Mori Y., and Akaike A.. 2006. A critical role of TRPM2 in neuronal cell death by hydrogen peroxide. J. Pharmacol. Sci. 101:66–76. 10.1254/jphs.FP0060128 [DOI] [PubMed] [Google Scholar]
- Kühn F.J., and Lückhoff A.. 2004. Sites of the NUDT9-H domain critical for ADP-ribose activation of the cation channel TRPM2. J. Biol. Chem. 279:46431–46437. 10.1074/jbc.M407263200 [DOI] [PubMed] [Google Scholar]
- Kühn F.J., Kühn C., Winking M., Hoffmann D.C., and Lückhoff A.. 2016. ADP-Ribose Activates the TRPM2 Channel from the Sea Anemone Nematostella vectensis Independently of the NUDT9H Domain. PLoS One. 11:e0158060 10.1371/journal.pone.0158060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn F.J.P., Ehrlich W., Barth D., Kühn C., and Lückhoff A.. 2019. Functional importance of NUDT9H domain and N-terminal ADPR-binding pocket in two species variants of vertebrate TRPM2 channels. Sci. Rep. 9:19224 10.1038/s41598-019-55232-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perraud A.L., Fleig A., Dunn C.A., Bagley L.A., Launay P., Schmitz C., Stokes A.J., Zhu Q., Bessman M.J., Penner R., et al. 2001. ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature. 411:595–599. 10.1038/35079100 [DOI] [PubMed] [Google Scholar]
- Song K., Wang H., Kamm G.B., Pohle J., Reis F.C., Heppenstall P., Wende H., and Siemens J.. 2016. The TRPM2 channel is a hypothalamic heat sensor that limits fever and can drive hypothermia. Science. 353:1393–1398. 10.1126/science.aaf7537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan C.H., and McNaughton P.A.. 2016. The TRPM2 ion channel is required for sensitivity to warmth. Nature. 536:460–463. 10.1038/nature19074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togashi K., Hara Y., Tominaga T., Higashi T., Konishi Y., Mori Y., and Tominaga M.. 2006. TRPM2 activation by cyclic ADP-ribose at body temperature is involved in insulin secretion. EMBO J. 25:1804–1815. 10.1038/sj.emboj.7601083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tóth B., and Csanády L.. 2012. Pore collapse underlies irreversible inactivation of TRPM2 cation channel currents. Proc. Natl. Acad. Sci. USA. 109:13440–13445. 10.1073/pnas.1204702109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tóth B., Iordanov I., and Csanády L.. 2014. Putative chanzyme activity of TRPM2 cation channel is unrelated to pore gating. Proc. Natl. Acad. Sci. USA. 111:16949–16954. 10.1073/pnas.1412449111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tóth B., Iordanov I., and Csanády L.. 2020. Selective profiling of N- and C-terminal nucleotide-binding sites in a TRPM2 channel. J. Gen. Physiol. 152:e201912533 10.1085/jgp.201912533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida K., Dezaki K., Damdindorj B., Inada H., Shiuchi T., Mori Y., Yada T., Minokoshi Y., and Tominaga M.. 2011. Lack of TRPM2 impaired insulin secretion and glucose metabolisms in mice. Diabetes. 60:119–126. 10.2337/db10-0276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S., Shimizu S., Kiyonaka S., Takahashi N., Wajima T., Hara Y., Negoro T., Hiroi T., Kiuchi Y., Okada T., et al. 2008. TRPM2-mediated Ca2+influx induces chemokine production in monocytes that aggravates inflammatory neutrophil infiltration. Nat. Med. 14:738–747. 10.1038/nm1758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu P., Xue X., Zhang J., Hu X., Wu Y., Jiang L.H., Jin H., Luo J., Zhang L., Liu Z., and Yang W.. 2017. Identification of the ADPR binding pocket in the NUDT9 homology domain of TRPM2. J. Gen. Physiol. 149:219–235. 10.1085/jgp.201611675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Tóth B., Szollosi A., Chen J., and Csanády L.. 2018. Structure of a TRPM2 channel in complex with Ca2+ explains unique gating regulation. eLife. 7:e36409 10.7554/eLife.36409 [DOI] [PMC free article] [PubMed] [Google Scholar]