Abstract
Background
The treatment of cancer is still unable to meet the needs of patients and remains a huge challenge. This study investigated the immune response and anti-cancer effect of silencing STAT3 combined with the use of anti-PD-L1 antibody.
Material/Methods
Transfected CT26.WT cells were used to subcutaneously inoculate C57B/L6 mice, which were subsequently injected with anti-PD-L1 antibody. Treated mice were examined for tumor formation and inflammation using HE staining. Tumors were investigated for apoptosis using the TUNEL assay. The expression of STAT3, PD-L1, and C-met was studied immunohistochemistrially and by using PCR and Western blot analysis.
Results
Four weeks after inoculation, tumors were observed in the inoculated mice. HE staining showed obvious inflammation in mice injected with cells that were silenced for STAT3 and injected with PD-L1 antibody. TUNEL assay showed low level of apoptosis in mice injected with cells silenced for STAT3 or injected with PD-L1 antibody, and higher level of apoptosis following combined treatment of STAT3 silencing and PD-L1 antibody injection. Immunohistochemistry, PCR, and Western blot analyses revealed that the expression of C-met, PD-L1, and STAT3 was significantly reduced in tumors following the combined treatment. Compared with treatment of STAT3 silencing or PD-L1 antibody injection, the combined treatment enhanced apoptosis.
Conclusions
Silencing STAT3 and PD-L1 antibody injection in combination increased apoptosis in tumor cells and thus offers better anti-cancer activity.
MeSH Keywords: Antibody-Dependent Cell Cytotoxicity; Immunity, Active; RNA, Small Interfering; Tumor Cells, Cultured
Background
Cancer results from uncontrolled clonal proliferation of cells due to various carcinogenic factors. With the development of industry and due to the unhealthy lifestyles, cancers are becoming widespread and occur in younger people. Cancer treatment is still unable to meet the needs of patients and remains a huge challenge. The tumor microenvironment is the internal and external environment where tumors occur, grow, and metastasize, and it affects the structure, function, and metabolism of tumor tissue [1]. The tumor microenvironment is one of the main factors that determine the development of tumors. The local pathological environment of tumor cells are determined by tumor cells, interstitial cells, and extracellular matrix [2]. Tumors and their microenvironment are mutually interdependent and antagonistic. Tumor cells alter and maintain their survival and development conditions through autocrine and paracrine functions, and affect the occurrence and development through changes in metabolism, secretion, immunity, structure, and function [3–6]. The immune response is a physiological process of the immune system to eliminate the antigen, which generates stimulation. This process is a comprehensive reflection of physiological functions of various parts of the immune system, including a series of physiological responses, such as antigen presentation, activation of lymphocyte, formation of immune molecules, and generation of immune effect [7]. Through an effective immune response, the internal environment is stabilized [8].
Immune response is affected by many genes and drugs [9]. Programmed cell death protein 1 (PD-1) is a receptor of the CD28 family, which is highly expressed in activated T cells and B cells. PD-1 has 2 ligands: PD-L1 and PD-L2. PD-L1 is less expressed in normal cells but is highly expressed in tumor cells [10]. When PD-1 binds to PD-L1, the PD-1 signaling pathway is activated, resulting in high expression of PD-1 and reduced T cell activity. Therefore, when the binding of PD-1 and PD-L1 is blocked by PD-L1 inhibitor, the negative regulation of the PD-1 signaling pathway is reduced, leading to the restoration of T cell activity and enhancement of immune response [11]. The signal transductor and activator of transcription 3 (STAT3) signaling pathway is a confluence where multiple carcinogenic pathways merge. STAT3 plays an important role in the occurrence of tumors [12]. Tumor cells affect immune cells through the STAT3 signaling pathway, and participate in the immune escape of tumor cells. Therefore, STAT3 has become an important protein in immunotherapy research [13]. Previous studies have shown that once activated by cytokines and growth factors, STAT3 promotes oncogenesis, inhibits apoptosis, and desensitizes tumor cells to chemotherapy drugs. Use of STAT3 inhibitor or inhibiting the STAT3 signaling pathway downregulates the expression of STAT3, leading to apoptosis and anti-tumor activity [14].
In this study, we investigated the effect of silencing STAT3 and anti-PD-L1 antibody, alone or in combination, on tumor cells, and the results provide new clues for cancer therapy.
Material and Methods
Cell line
The mouse colon cancer cell line CT26.WT was obtained from the Cell Bank of the Shanghai Academy of Science, and cultured in RPMI 1640 medium in 5% CO2 at 37°C. C57B/L6 mice were obtained from Slackking Experimental Animals, Hunan, China (permit no. SCXK 2016-0002). All animal experimental protocols were approved by the Ethics Committee for Animal Care and Study at Fujian Medical University.
Reagents and equipment
Glucose RPMI 1640 medium (cat. no. KGM31800S-500) was obtained from Sijiqing Biologicals, Hangzhou, China. We obtained 0.25% trypsin (containing EDTA, cat. no. 20170101) from KGI Biotech, Beijing, China. The penicillin and streptomycin mixture (cat. no. 20160909) was from Leybold Tech, Beijing, China. Fetal bovine serum (FBS, cat. no. 1552680 2000) was from BI Biologicals, Beijing, China. Liposome 2000 reagents and anti-PDL-1 antibody (cat. no. ab10159R, 1: 200) were purchased from Abcam, USA. Hematoxylin (cat. no. AR11800-1) and eosin staining solution (cat. no. AR11800-2) were products of BOSTER, USA. Neutral resin (cat. no. CW0136), ultrapure RNA extraction kit (cat. no. CW0581M), and UltraSYBR mixture (cat. no. CW0957M) were purchased from CWBIO, Beijing, China. Scott blue staining (cat. no. G1865) and TUNEL kits (cat. no. C1088) were purchased from Beyotime Biotech, Beijing, China. Antibodies against GAPDH (cat. no. TA-08, 1: 3000), goat anti-mouse IgG (cat. no. ZB-2305, 1: 2000), and goat anti-rabbit IgG (cat. no. ZB-2301, 1: 2000) were purchased from ZSGB-Bio, Beijing, China. Rabbit antibodies against PDL-1 (cat. no. bs-10159R, 1: 1500) and C-met (cat. no. bs-0668R, 1: 600) were obtained from Bioss, Beijing, China. Polyclonal antibody against STAT3 (cat. no. A5511, 1: 1500) was from ABclonal, USA. Rabbit poly-HRP-labeled anti-rabbit IgG (cat. no. SV0002) was from Boster Biotech, Beijing, China. The micro-injection pump (AJ-5805) was a product of ANGEL, USA. Fluorescent PCR instrument (CFX Connect) and gel imaging system (Chemi DocTM XRS+) were from Bio-Rad laboratories (Shanghai) Co., Shanghai, China.
Vector construction
The sequence of the STAT3 gene was downloaded from Genebank (https://www.ncbi.nlm.nih.gov/) and the CDS sequence was used to design shRNA sequences (sense: GATCCGCACAATC TACGAAGAATCAACTTCCTGTCAGATTGATTCTTCGTAGATT GTGCTTTTTG, antisense: AATTCAAAAAAGCACAATCTA CGAAGAATCAAT CTGACAGGAAGTTGATTCTTCGTAGATTGTGCG) after adding BamHI site on the antisense strand and EcoRI site on the sense strand. Annealed double strands were ligated to pGreenpuro to generate a recombinant lentiviral vector sh-STAT3-pGreenpuro.
Transfection
Transfection of cells was performed using Lipofectamine 3000 Transfection Reagent according to the manufacturer’s instructions. Briefly, CT26.WT cells were seeded at a concentration of 1×105 in 6-well culture plates, grown to 80% confluence, and transfected with 50 nM sh-RNAs. Transfected cells were cultured in the 5% CO2 incubator at 37°C for 4 days and injected into mice.
Treatments
Five mice in each treatment group were injected subcutaneously with 200 μL/animal cell suspension containing 2×106 cells or/and injected intraperitoneally with anti-PD-L1 antibody (10 mg/kg) every 3 day for 14 days 2 weeks after the inoculation. For control, the mice were inoculated with un-transfected CT26.WT cells, and mice in STAT3 NC and sh-STAT3 groups were injected with CT26.WT cells transfected with STAT3 NC and sh-STAT3, respectively. For PD-L1 treatment, control mice inoculated with un-transfected CT26.WT cells were injected with anti-PD-L1 antibody (PD-L1), or transfected mice were injected with anti-PD-L1 antibody as above (PD-L1+STAT3 NC and PD-L1+STAT3, respectively). The treatment scheme and dose of anti-PD-L1 antibody were chosen based on a previous study [15].
HE staining
Tumor tissue was rinsed with PBS, fixed in 10% neutral formaldehyde, and embedded in paraffin. The sections were stained with HE and observed under a light microscope.
TUNEL assay
The assay was conducted as previously reported [16]. Briefly, tissue sections were baked at 65°C for 2 h and immersed in xylene for 2 h. They were rehydrated in a graded ethanol series, and proteinase K solution (50 μg/ml) was added dropwise. After incubation for 30 min at 37°C, the slices were washed 3 times with PBS. TUNEL assay was performed according to the manufacturer’s instructions. After adding TUNEL detection solution, the slides incubated at 45°C for 2 h and viewed under a fluorescence microscope.
Immunohistochemistry
Immunohistochemistry detection assay was performed as previously described [17]. The 2-μm tissue sections were placed onto coated slides, deparaffinized with xylene, and rehydrated as in the TUNEL assay. After washing in water, the slides were autoclaved for 3 min in sodium citrate buffer for antigen retrieval. Endogenous peroxidase activity was suppressed with hydrogen peroxidase for 5 min at room temperature. After rinsing with tris-buffered saline (TBS), the tissue sections were incubated with primary antibodies against STAT3, PD-L1, and C-met for 30 min. The sections were then rinsed with TBS and incubated with secondary antibody for 30 min. Diaminobenzidine (DAB) and hematoxylin chromogen (Dako, Glostrup, Denmark) method was used for color development. The sections were observed under a light microscope and the intensity of staining was qualified using Imaging Pro-plus (v6) software (Media Cybernetics Corporation, USA).
Real-time quantitative PCR for gene expression
Gene expression using real-time quantitative PCR was performed as previously reported [18]. Briefly, 200 ng of RNA isolated from tumor tissues was reverse-transcribed in a total volume of 10 μl using the High-Capacity cDNA Transcriptase Reverse kit (Applied Biosystems by Life Technologies, Carlsbad, CA, USA) according to manufacturer’s recommendations. Reactions were performed on a 96-thermal cycler for 10 min at 25ºC, 2 h at 37ºC, and 5 min at 85ºC. A total of 2.5 μl of the resulting cDNA was used to pre-amplify the cDNA using the UltraSYBR mixture in a total volume of 12 μl. Non-fluorescent probes were used at 1X. Pre-amplification cycling conditions were 10 min at 95ºC followed by 14 cycles, each consisting of 15 s at 95ºC and 4 min at 60ºC. RT-qPCR was performed on a Fluorescence PCR instrument (CFX Connect). Human glyceraldehyde-3-phosphate dehydrogenase, GADPH (Hs03929097_g1) was used as an internal reference. The PCR analysis was carried out in a total volume of 10 μl reaction containing 1.5 μl diluted and pre-amplified cDNA and 10 μl UltraSYBR mixture. The cycling conditions were 50ºC for 2 min, 95ºC for 10 min, followed by 40 cycles, each consisting of 15 s at 95ºC and 1 min at 60ºC. Primer sequences are listed in Table 1.
Table 1.
Primers for PCR.
| Gene | Primer sequence (5′-3′) | |
|---|---|---|
| PD-L1 | Forward | CCATCTTATTATGCCTTGGTGTAG |
| Reverse | TTTGCTTCTTTGAGTTTGTATCTTG | |
| STAT3 | Forward | ACCAACAATCCCAAGAATGTAAACT |
| Reverse | CATGTGATCTGACACCCTGAATAAT | |
| C-met | Forward | CACATTTTCCTTGGTGCCACTAAC |
| Reverse | CCTGATAAATTGGCTTTGCTGCT | |
| GAPDH | Forward | GAAGGTCGGAGTCAACGGAT |
| Reverse | CCTGGAAGATGGTGATGGG | |
The data were analyzed using the Applied Biosystems software RQ Manager v1.2.1. Relative expression was calculated using the comparative Ct method to obtain fold change value (2−ΔΔCt) according to a previously described protocol [19].
Western blot analysis
Tissues were ground and lysed with RIPA buffer containing protease and phosphatase inhibitors (Roche, UK). After centrifugation at 6950 g for 20 min, 50 μg protein in the supernatants was used in polyacrylamide gel electrophoresis (SDS-PAGE), transferred to a PVDF membrane, and reacted to appropriate primary and secondary antibodies before visualization with a chemiluminescence kit. The intensity of blot signals was quantified using ImageQuant TL analysis software (General Electric, UK).
Statistical analysis
All data are presented as means±standard error of the mean (SEM) generated from at least 3 independent experiments. Analysis was performed using SPSS v19.0 (SPSS, Chicago, IL, USA). Statistical comparisons between experimental and control groups were assessed by using the t test. P<0.05 was considered statistically significant.
Results
Tumor formation
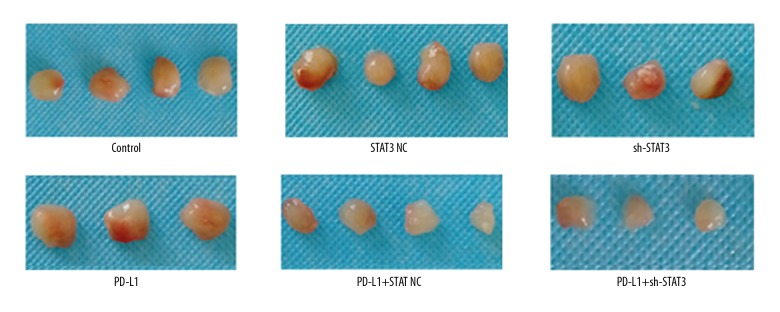
After subcutaneous inoculation, all mice were healthy and survived. Four weeks later, tumors (Figure 1) were observed in 21 mice and were similar in size within the groups (Figure 1). However, tumors derived from sh-STAT3-transfected cells or injected with anti-PD-L1 antibody were relatively smaller, and the tumors in the STAT3+PD-L1 group grew slowly and were the smallest (Figure 1).
Figure 1.
Tumor formation following sh-STAT3 and anti-PD-L1 antibody treatments.
Growth of tumors
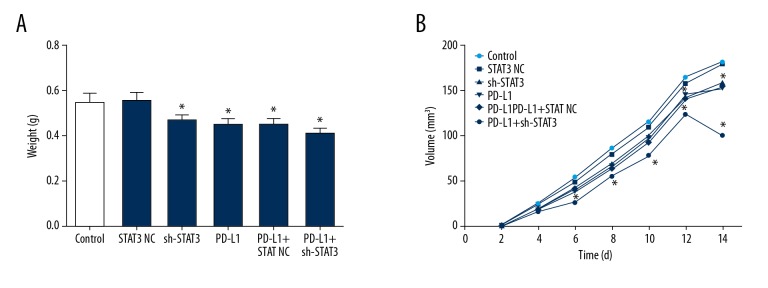
Compared with mice in the control and NC vector groups, the weights of tumor-derived cells transfected with sh-STAT3, injected with anti-PD-L1 antibody, and after combined treatments were significantly smaller (Figure 2A, P<0.05). The volume of tumors in the STAT3+PD-L1 group even began to decline 12 days after tumor formation (Figure 2B) (P<0.05).
Figure 2.
Tumor mass (A) and volume (B) following sh-STAT3 and anti-PD-L1 antibody treatments. * Denotes P<0.05 vs. control
HE staining
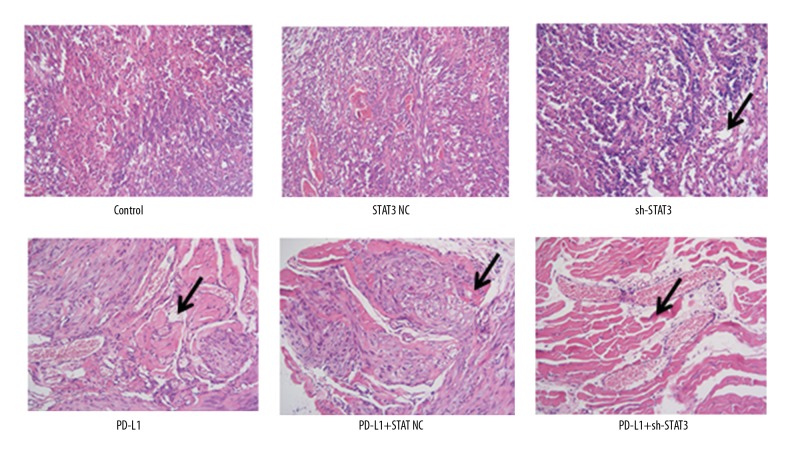
Compared with mice in the control group, tumor cells in the NC group were arranged more tightly, while they were relatively loosely arranged in the mice treated with anti-PD-L1 antibody and sh-STAT3 (Figure 3), where small vacuoles were visible and evident and cells were arranged irregularly, particularly in the mice that received combined treatments, where cell morphology was extremely irregular and inflammation was strong.
Figure 3.
HE staining of tumor tissues following sh-STAT3 and anti-PD-L1 antibody treatment alone or in combination. Arrows indicate inflamed and vacuolated tissues.
Apoptosis
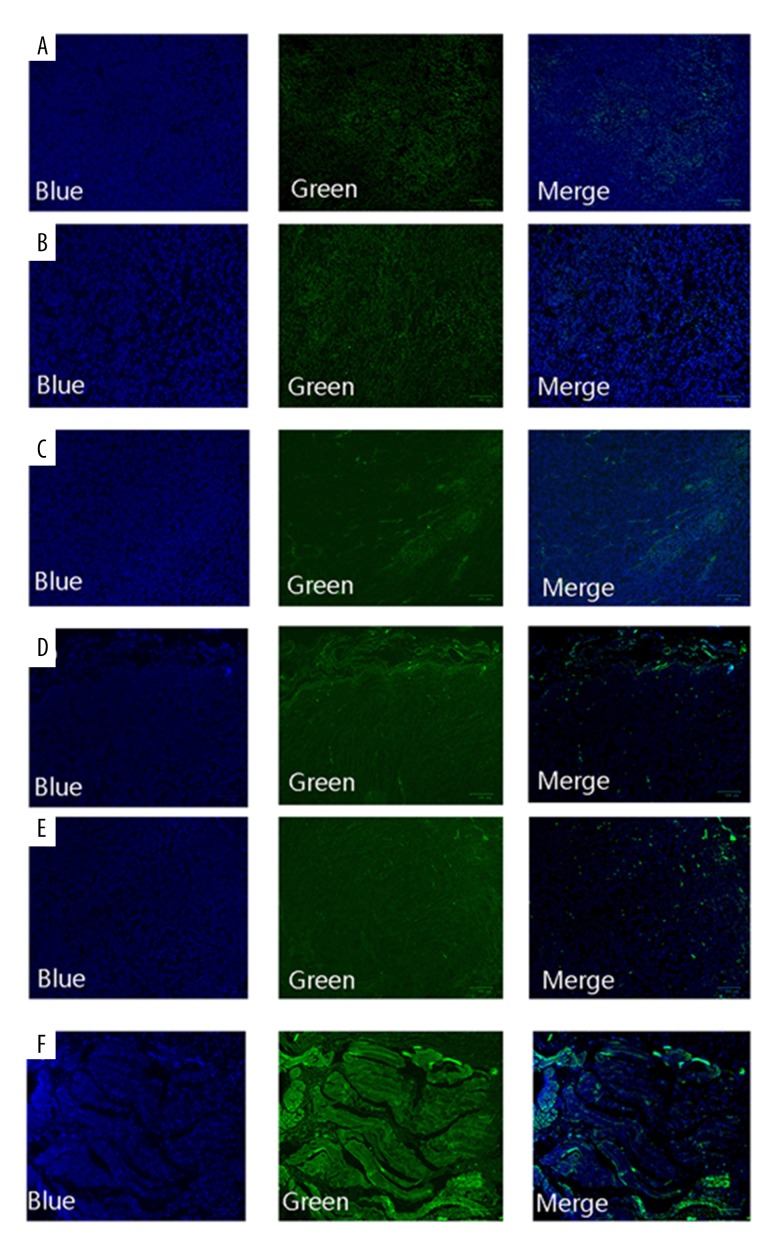
As shown in Figure 4, normal cells were regularly-shaped and only the nuclei were stained blue by DAPI. In contrast, apoptotic cells were chaotically arranged and were green-colored. Compared with the control group, few apoptotic cells were observed in the NC group. Green fluorescence was enhanced in the sh-STAT3 and PD-L1 groups, and was the strongest in the sh-STAT3+PD-L1 group, suggesting that apoptosis was significantly increased in that group.
Figure 4.
TUNEL assay results of apoptosis in cancer cells following sh-STAT3 and anti-PD-L1 antibody treatment alone or in combination. (A) Control; (B) NC; (C) sh-STAT3; (D) anti-PD-L1 antibody; (E) NC+PD-L1; (F) sh-STAT3+PD-L1.
Expression of C-met, PD-L1, and STAT3
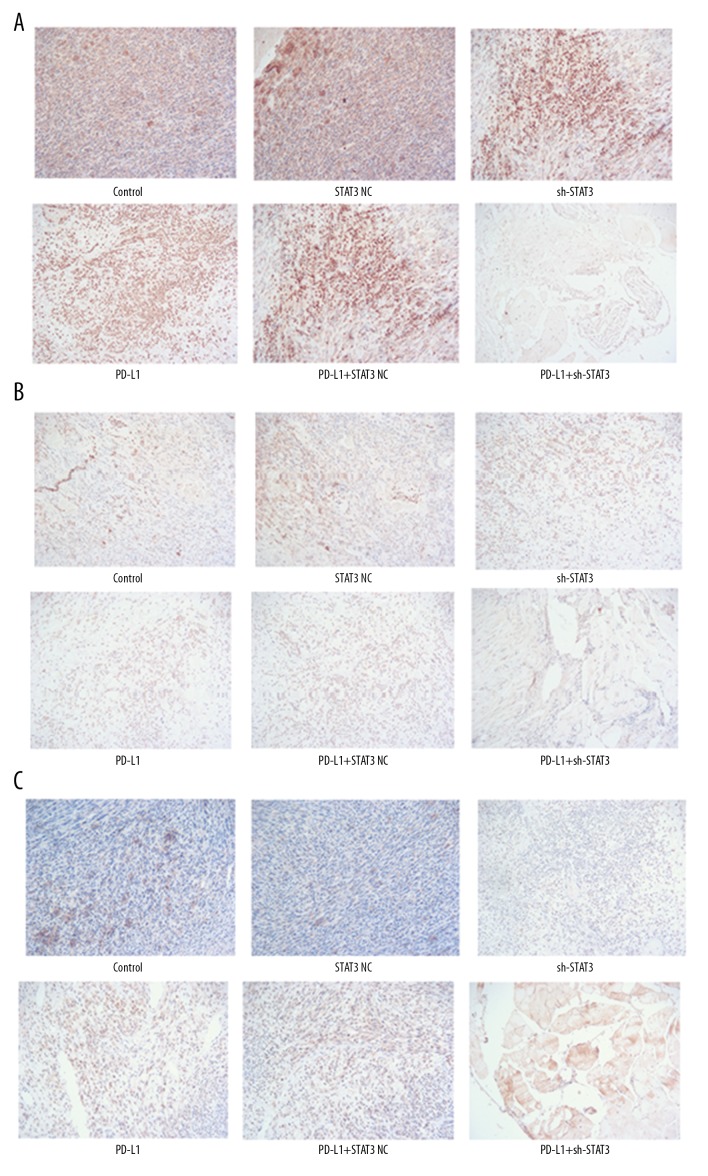
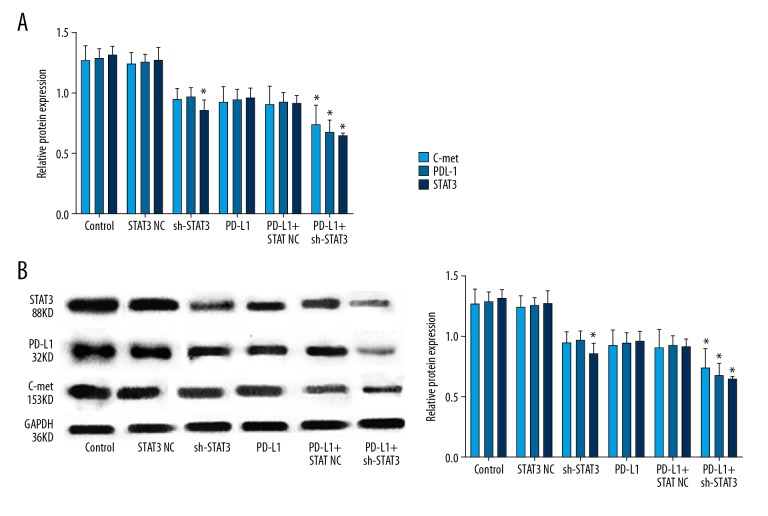
Immunohistochemistry showed that cells in the NC and control groups had similar and more brown-yellow labeling for the 3 proteins, while there were obviously fewer brown-yellow colored granules in the other 3 groups, indicating that the expression of C-met, PD-L1, and STAT3 decreased following sh-STAT3 and anti-PD-L1 treatments (Figure 5). The reduction was more remarkable following combined sh-STAT3 and anti-PD-L1 treatment. qPCR data and Western blot analysis showed similar results for mRNA and protein levels of these genes (Figure 6).
Figure 5.
Immunohistochemistry assays of C-met, PD-L1, and STAT3 following sh-STAT3 and anti-PD-L1 antibody treatment alone or in combination. (A) C-met; (B) PD-L1; (C) STAT3.
Figure 6.
Relative mRNA and protein levels of C-met, PD-L1, and STAT3 following sh-STAT3 and anti-PD-L1 antibody treatment alone or in combination. (A) Relative mRNA level; (B) Left panel: representative Western blot, right panel: relative protein level. * Denotes P<0.05 vs. control.
Discussion
For tumors, particularly malignancy, treatment is still challenging. Radiotherapy or drug therapy often cause a number of adverse effects and complications, such as nausea, hair loss, and reduced health. Anti-PD-LI antibody has been demonstrated to be safe, with few adverse effects [20]. We therefore investigated its effect on immune response in the tumor microenvironment in combination with sh-STAT3 and anti-tumor activity.
The results of the present study showed that treatment with PD-L1 antibody or silencing STAT3 expression inhibited the growth of tumors, and it was found that the combined use of PD-L1 antibody and sh-STAT3 had better anti-tumor activity; specifically, the weight and volume of the tumors decreased significantly after the combined treatment. We also found that treatment with PD-L1 antibody or sh-STAT3 promoted apoptosis of tumor cells, and the effect of combined treatment was more obvious. Studies have shown that PD-L1 antibodies can inhibit the proliferation of tumor cells and promote their apoptosis [21], and continuous and excessive activation of the STAT3 gene accelerates the lymph node metastasis of tumors by activating transcription of downstream genes [22,23]. In addition, this study found that PD-L1 antibody or sh-STAT3 induced an inflammatory reaction in tumor tissues, producing vacuoles in tumor cells. Cellular immunity is the most important and universal immune system against cancer cells. In the immune response against tumor cells, T lymphocytes play the most significant role, recognizing and killing tumor cells [24].
PD-1 and its ligand, PD-L1, play an important role in the immune escape process of tumors. They are necessary immunosuppressive molecules in the human body. Therefore, they have been targeted to regulate to generate anti-tumor activity. Persistent and high expression of PD-L1 results in loss of immune surveillance and escape of tumor cells. PD-L1 antibody enhances the biological function of T cells, leading to the inhibition of growth of tumors and increased apoptosis [25]. PD-L1 is an induced cell-surface protein in malignant tumors and it inhibits T cell-mediated tumor response by acting on the T cell receptor PD-1 [26]. Immune response is a complex process depending on the balance of activators and inhibitors in various pathways. Tumor cells may maintain an immunosuppressive microenvironment that is conducive to the development of the tumor. Interaction between PD-1 receptor and ligand is a main inhibitory pathway for cancer cells [27]. On the surface of cancers and immune cells, such as antigen-presenting cells, PD-L1 is expressed and binds to PD-1 to inhibit the migration of T cells, leading to the accumulation and secretion of cytotoxic mediators, and blocking the enhancement of anti-cancer immunity [28]. Combined anti-cancer immunotherapy has gained increased attention. In combined therapy, the efficacy of PD-L1 inhibitors is more significant [11].
The STAT3 signaling pathway has a profound impact on tumor formation and the tumor microenvironment. STAT3 is upregulated in cancer cells, and is considered to be a key mediator of tumor-related immunosuppressor. The promoter region upstream the PD-L1 gene contains 4 potential STAT3 binding sites [29], which are approximate to the transcriptional starting site, suggesting that STAT3 has a regulatory role in PD-L1 transcription. PD-L1 is an antibody that is dependent on the expression of STAT3 [29]. C-met is a receptor tyrosine kinase. After ligand binding, it activates the downstream pathways to play a role in organ development and cancer progression [30]. C-met is overexpressed in cancer cells and plays a major role in the growth and survival of tumors [31]. Although C-met-induced cancer proliferation is independent of PD-L1, C-met participates in immune escape through interaction with PD-1 expressed in T cells and other immune cells, resulting in overexpression of PD-L1 in cancer cells [32]. In the present study, anti-PD-L1 antibody was injected into mice after inoculation of tumor cells. Immunohistochemistry, PCR, and Western blot results showed that, compared with the control group, there were no significant changes in STAT3, PD-L1, and C-met expression in cells transfected with NC vector. However, when anti-PD-L1 antibody was injected into mice inoculated with STAT3-silenced cells, the expression of these genes was downregulated. HE staining revealed that after the combined treatment, cells were loosely arranged, with many vacuoles, and were obviously inflamed. Apoptosis was also evident as large green-stained areas following the combined treatment. These results suggest that silencing STAT3 enhances STAT3-induced immune response if it is combined with anti-PD-L1anibody, resulting in the formation of immune cells and increased activity of immune cells. As a result, apoptosis of cancer cells is promoted, leading to inhibition of cancer cells and better anti-cancer effect. This evidence that PD-L1 antibody and downregulation of STAT3 expression inhibit the growth of tumors provides a potential clinical option for tumor treatment, although more work is needed to further validate the anti-tumor activity and rule out adverse effects.
Conclusions
We demonstrated that combined use of anti-PD-L1 antibody and silencing of STAT3 can enhance immune response and generate better anti-tumor activity.
Footnotes
Source of support: This study was supported by the National Nature and Science Foundation, Fujian Province (2016Y0034)
References
- 1.Mlecnik B, Bindea G, Kirilovsky A, et al. The tumor microenvironment and Immunoscore are critical determinants of dissemination to distant metastasis. Sci Transl Med. 2016;8:327ra26. doi: 10.1126/scitranslmed.aad6352. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y, Yu J. Advance of tumor microenvironment in endometrial cancer. J Mod Oncol. 2016;16:152–56. [Google Scholar]
- 3.Spill F, Reynolds DS, Kamm RD, Zaman MH. Impact of the physical microenvironment on tumor progression and metastasis. Curr Opin Biotechnol. 2016;40:41–48. doi: 10.1016/j.copbio.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ren H, Zhao Z, Zhang J, et al. Effect of heat shock treated cell culture supernatant on the activity of umbilical cord blood lymphocytes. Chinese J Lab Diagn. 2013;16:998–1001. [Google Scholar]
- 5.Singh SR, Rameshwar P, Siegel P. Targeting tumor microenvironment in cancer therapy. Cancer Lett. 2016;380:203–4. doi: 10.1016/j.canlet.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 6.Sounni NE, Noel A. Targeting the tumor microenvironment for cancer therapy. Clin Chem. 2013;59:85–93. doi: 10.1373/clinchem.2012.185363. [DOI] [PubMed] [Google Scholar]
- 7.Joyce JA, Fearon DT. T cell exclusion, immune privilege, and the tumor microenvironment. Science. 2015;348:74–80. doi: 10.1126/science.aaa6204. [DOI] [PubMed] [Google Scholar]
- 8.Hansson GK, Libby P. The immune response in atherosclerosis: A double-edged sword. Nat Rev Immunol. 2006;6:508–19. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 9.Marriott AS, Vasieva O, Fang Y, et al. NUDT2 disruption elevates diadenosine tetraphosphate (Ap4A) and down-regulates immune response and cancer promotion genes. PLoS One. 2016;11:e0154674. doi: 10.1371/journal.pone.0154674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mellati M, Eaton KD, Brooks-Worrell BM, et al. Anti-PD-1 and anti-PDL-1 monoclonal antibodies causing type 1 diabetes. Diabetes Care. 2015;38:e137–38. doi: 10.2337/dc15-0889. [DOI] [PubMed] [Google Scholar]
- 11.Zhen Y, Yi J. [Research progress of PD 1/PD-L1 inhibitor in tumor immunotherapy]. Guangdong Medical Journal. 2016;37:3301–3. [in Chinese] [Google Scholar]
- 12.Ma X, Zhang C, Wang J. [Effects of sinal transducer and activator of transcription 3 on the thmorogenesis]. Letters in Biotechnology. 2009;20:224–26. [in Chinese] [Google Scholar]
- 13.Zou B, Yang W. [Progress in the study of relationship between STAT3 and tumor immues escape]. Chinese J Clinicians. 2013;7:159–62. [in Chinese] [Google Scholar]
- 14.Kortylewski M, Xin H, Kujawski M, et al. Regulation of the IL-23 and IL-12 balance by Stat3 signaling in the tumor microenvironment. Cancer Cell. 2009;15:114–23. doi: 10.1016/j.ccr.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Longoria TC, Eskander RN. Immune checkpoint inhibition: Therapeutic implications in epithelial ovarian cancer. Recent Pat Anticancer Drug Discov. 2015;10:133–44. doi: 10.2174/1574892810666150504121000. [DOI] [PubMed] [Google Scholar]
- 16.Kyrylkova K, Kyryachenko S, Leid M, Kioussi C. Detection of apoptosis by TUNEL assay. Methods Mol Biol. 2012;887:41–47. doi: 10.1007/978-1-61779-860-3_5. [DOI] [PubMed] [Google Scholar]
- 17.Bachman J. Immunohistochemistry on freely floating fixed tissue sections. Methods Enzymol. 2013;533:207–15. doi: 10.1016/B978-0-12-420067-8.00013-1. [DOI] [PubMed] [Google Scholar]
- 18.Jozefczuk J, Adjaye J. Quantitative real-time PCR-based analysis of gene expression. Methods Enzymol. 2011;500:99–109. doi: 10.1016/B978-0-12-385118-5.00006-2. [DOI] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Swaika A, Hammond WA, Joseph RW. Current state of anti-PD-L1 and anti-PD-1 agents in cancer therapy. Mol Immunol. 2015;67:4–17. doi: 10.1016/j.molimm.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 21.Blank C, Mackensen A. Contribution of the PD-L1/PD-1 pathway to T-cell exhaustion: an update on implications for chronic infections and tumor evasion. Cancer Immunol Immunother. 2007;56:739–45. doi: 10.1007/s00262-006-0272-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bui QT, Im JH, Jeong SB, et al. Essential role of Notch4/STAT3 signaling in epithelial-mesenchymal transition of tamoxifen-resistant human breast cancer. Cancer Lett. 2017;390:115–25. doi: 10.1016/j.canlet.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 23.Ren D, Lin B, Zhang X, et al. Maintenance of cancer stemness by miR-196b-5p contributes to chemoresistance of colorectal cancer cells via activating STAT3 signaling pathway. Oncotarget. 2017;8:49807–23. doi: 10.18632/oncotarget.17971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strickland KC, Howitt BE, Shukla SA, et al. Association and prognostic significance of BRCA1/2-mutation status with neoantigen load, number of tumor-infiltrating lymphocytes and expression of PD-1/PD-L1 in high grade serous ovarian cancer. Oncotarget. 2016;7:13587–98. doi: 10.18632/oncotarget.7277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15:486–99. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nowicki TS, Anderson JL, Federman N. Prospective immunotherapies in childhood sarcomas: PD1/PDL1 blockade in combination with tumor vaccines. Pediatr Res. 2016;79:371–77. doi: 10.1038/pr.2015.246. [DOI] [PubMed] [Google Scholar]
- 27.Ishii H, Azuma K, Kawahara A, et al. Programmed cell death-ligand 1 expression and immunoscore in stage II and III non-small cell lung cancer patients receiving adjuvant chemotherapy. Oncotarget. 2017;8:61618–25. doi: 10.18632/oncotarget.18651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bertucci F, Finetti P, Colpaert C, et al. PDL1 expression in inflammatory breast cancer is frequent and predicts for the pathological response to chemotherapy. Oncotarget. 2015;6:13506–19. doi: 10.18632/oncotarget.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rachel B, Mitchell T, Gary P, et al. STAT3 is necessary for liver myeloid-derived suppressor cell PDL-1-dependent inhibition of genetically modified T cell function. J Immunol. 2014;125:123–38. [Google Scholar]
- 30.Modali SD, Parekh VI, Kebebew E, Agarwal SK. Epigenetic regulation of the lncRNA MEG3 and its target c-MET in pancreatic neuroendocrine tumors. Mol Endocrinol. 2015;29:224–37. doi: 10.1210/me.2014-1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Z, Lin SX, Liang HZ, He JH. [Expression of heptocyte growth factor/c-Met system in nasopharyngeal carcinoma and its biological significance]. Chin J Pathol. 2005;34:75–79. [in Chinese] [PubMed] [Google Scholar]
- 32.Balan M, Mier y Teran E, Waaga-Gasser AM, et al. Novel roles of c-Met in the survival of renal cancer cells through the regulation of HO-1 and PD-L1 expression. J Biol Chem. 2015;290:8110–20. doi: 10.1074/jbc.M114.612689. [DOI] [PMC free article] [PubMed] [Google Scholar]