Abstract
The opioid crisis continues to affect pregnant and postpartum women the United States, with the number of pregnant women diagnosed with opioid use disorder (OUD) quadrupling over the last decade. The associated increase in morbidity and mortality among mother and baby warrants prompt, targeted intervention efforts that improve engagement, linkage of care, and treatment retention. Patient navigation (PN) is a chronic care intervention that can directly address this need by helping women identify medical, behavioral, and psychosocial care goals. Moreover, PN can assist women in preparing for, engaging in, and maintaining patient participation in necessary services. Specifically, PN includes strengths-based case management, 1–1 clinical support, motivational interviewing, and addiction-relapse prevention programming. The objective of this article is to present the study protocol of a pilot multisite randomized clinical trial, entitled: Optimizing Pregnancy and Treatment Interventions for Moms 2.0 (OPTI-Mom 2.0; NCT03833245). In this study, we build upon a proof-of-concept study, employing evidence-informed frameworks for protocol and intervention expansion in order to construct a PN intervention tailored for pregnant women with OUD in central Utah and southwestern Pennsylvania. Our protocol provides an initial framework of a potentially impactful intervention and may guide development of future programs. Importantly, this study further establishes the evidence-base—with potential to ameliorate serious adverse opioid-related outcomes and improve health for women and their children.
Keywords: Opioid use disorder, pregnancy, multi-site, clinical trial
Introduction
The opioid epidemic in the United States continues to result in serious health consequences for pregnant and postpartum women. From 1999–2014, the prevalence of pregnant women diagnosed with opioid use disorder (OUD) at delivery quadrupled [1]. OUD during pregnancy is associated with adverse maternal and neonatal health outcomes, such as preterm birth, low birthweight, and neonatal opioid withdrawal syndrome (NOWS), which are associated with substantial expenditures of health care resources [2–7]. This escalation in morbidity and mortality due to opioid use during pregnancy and postpartum period [8] calls for rapid and targeted intervention efforts designed to engage, link, and retain pregnant women in OUD treatment.
OUD, like any addiction, is a chronic disease that can be managed and treated successfully with appropriate, evidence-based care [9]. Thus, OUD among pregnant women requires interventions that last beyond the pregnancy episode and take into account the full milieu of potential needs. Standard care for pregnant women with OUD involves medication assisted treatment, with methadone or buprenorphine, combined with additional behavioral health services [10, 11]. Recent data has demonstrated that 44% of pregnant women do not receive medication-assisted treatment with either methadone or buprenorphine and less than one-third receive behavioral health services [12]. Owing to the manifold challenges faced by pregnant women with OUD that result in low retention in treatment [13], patient navigation (PN) works to reduce barriers to healthcare engagement by guiding patients through the complex and often fragmented healthcare and social service systems [14, 15]. PN is a chronic care intervention [16] that can be used to link and retain pregnant women with OUD in treatment. Specifically, PN has the potential to aid pregnant women with OUD to identify needs; determine behavioral health, medical, psychosocial care goals; and collaboratively prepare for, engage in, and maintain activity in these necessary services and thus ameliorate threats to maternal and neonatal health and wellbeing. Previous research that has employed motivational incentives (i.e., contingency management) among pregnant women has shown up to a 3-fold increase in study retention [17], 4–6 weeks longer substance treatment retention [18, 19], and a 6 fold increase in retention for substance treatment-related activities [19] compared to controls. However, PN and behavioral and physical health care linkage/retention for pregnant women with OUD has not been documented in the literature previously.
The objective of this article is to report the study protocol of a pilot multisite randomized clinical trial, entitled: Optimizing Pregnancy and Treatment Interventions for Moms 2.0 (OPTI-Mom 2.0; NCT03833245). This study is testing a PN intervention for pregnant women with OUD and assessing linkage/retention in care. Importantly, this study builds upon a proof-of-concept project among 21 pregnant women with who were provided the PN intervention that utilized a one-group repeated measures design [20].
Materials and Methods
Study Design
To plan the current study, our team’s first step was to transform our previous protocol into a two-group, single-blind, multi-site, randomized clinical trial using the guide provided by Chung et al. [52] for planning multisite trials. Specifically, we utilized face-to-face exercises for trial planning, which employed the Nominal Group Method [52] and included an unstructured/critical topical discussion, question/answer session, and consensus building [52, 53]. Due to differences in the geographic location of the investigative team members (southwestern Pennsylvania and central Utah), planning sessions were held via web conference. [21, 22]Decisions made by the group during these web planning meetings have been applied to the study protocol and procedures manuals for this project. The following design reflects many of the decisions made during this process.
Participant Recruitment and Assessment
Pregnant women with OUD who present to one of two academic health centers located in Southwestern Pennsylvania or Central Utah are approached for potential participation. Project investigators are faculty within these two health systems and have track records of clinical investigation and service provision within these settings. The two health care systems within this project are large, tertiary care, academic medical centers that serve urban, suburban, and rural patients in their respective regions. Both systems provide a full complement of general and specialty maternal and neonatal healthcare services, including perinatal addiction care. Pregnant women from each system are identified through clinic-based outreach, electronic health records (automated electronic data warehouse identification and advertisement as well manual searches), and outreach and advertisements to local organizations that serve this population. Women identified are contacted to provide a study overview and a screening invitation by study coordinators via face-to-face communication if present within the clinical settings or by mailed letter if not present. Following identification, screening is carried out by study research staff. Women not interested in screening are asked to provide their reason for not screening.
Study screening identifies if women are: pregnant, ≥18 years, English speaking, plan to carry their babies to delivery, and meet Diagnostic Statistical Manual-V [23] criteria for OUD. Women excluded are those with a psychotic or a manic episode in the last 30 days documented in their medical record or through self-report. Self-report psychosis assessment is performed using the subscale from the Behavior and Symptom Identification Scale [24], and self-reported mania is assessed using Altman Self-Rating Mania Scale [25]. Women beyond the 32nd week of gestation are also excluded to allow up to 8 weeks for prenatal intervention delivery. Those who cannot provide contact information for themselves, collateral contact information of 2 persons, or who plan to move from the area within 2 months after their delivery are also excluded from the study. Finally, women who have been enrolled in a medication-assisted treatment program for > 6 weeks are excluded to eliminate potential participants who are already actively engaged in OUD treatment. Participants with symptoms of intoxication (e.g., slurred speech, dozing off) are only consented after these are resolved; the consent document also includes a consent quiz to ensure comprehension of key elements of study participation.
Eligible women who express interest in the study are asked to provide signed informed consent for study participation. The informed consent process is carried out by study research staff and complies with all ethical and procedural requirements approved by a single Institutional Review Board (IRB) approved at both institutions, with the University of Utah acting as the single IRB. Participants also sign a release of medical information that authorizes research team members to review prenatal care, treatment program records, and neonates’ medical records.
Following consent and recruitment, all participants are provided with a baseline assessment. Participants are also assessed at 14-weeks following the baseline survey (after completion of the prenatal navigation sessions for PN participants) and at 2 and 6 months postpartum. Assessors are blinded to participant intervention condition. In both study arms, women receive $30 for completing the baseline assessment, $40 for the second assessment, $50 for the third assessment, and $75 for the final assessment. Patients who complete all 4 assessments are given a completion bonus of $50. Women in both arms also may be reimbursed $10 for transportation/parking costs for each study-related visit. Following the baseline assessment, participants are randomized to standard care or the PN intervention. Randomization is stratified by hospital site and performed in blocks of 6 to ensure an even distribution of participants at the medical centers in PN and standard care groups. All screening and outcome data are captured on encrypted tablet devices via REDCap surveying software [26, 27] and are stored centrally. The study randomization list and subsequent condition assignment for both sites are also housed and operated within the REDCap platform [26, 27].
Intervention Conditions
Standard care.
The standard care condition within the two health systems includes brief case management, referral, and limited follow up. Brief case management involves the participant speaking to the clinic or hospital social worker who conducts an assessment for behavioral health and social service needs. All women are referred to OUD pharmacotherapy and any identified behavioral health or social service needs. The southwestern Pennsylvania site offers resources for an onsite inpatient initiation to methadone with continued methadone maintenance provided by federally licensed community methadone treatment programs. The southwestern Pennsylvania site also offers outpatient initiation to buprenorphine (mono-product), with maintenance provided by an onsite outpatient buprenorphine treatment program or through community partners. Patients in central Utah are offered in-patient initiation to buprenorphine (buprenorphine-naloxone product) onsite or from community partners. Methadone services are offered by referral, which are provided by federally licensed methadone treatment programs within the community. To avoid influence of the compensatory equalization of treatments bias (i.e., increased service provision by social workers delivering standard care that would compete with PN), we have not exposed social work staff to the PN intervention protocol/training, and these staff are regularly encouraged by project investigators to continue to provide services as usual. Our team also conducted interviews with social work staff before study initiation to capture what standard care involves at each medical center in order to characterize details of these services.
Patient navigation.
The PN intervention is delivered by the study navigator, who, in this study, is a master’s level research clinician. Figure 2 contains an overview of the PN intervention. The prenatal portion of the PN intervention includes delivery of up to 10 sessions. The postnatal portion of the intervention is delivered as 4 sessions over 8 weeks. Women who complete the prenatal portion of the intervention before delivery receive regular calls/texts until delivery when the navigator encourages and reinforces abstinence and treatment retention.
Figure 2. PN intervention outlining session objectivesa,b.
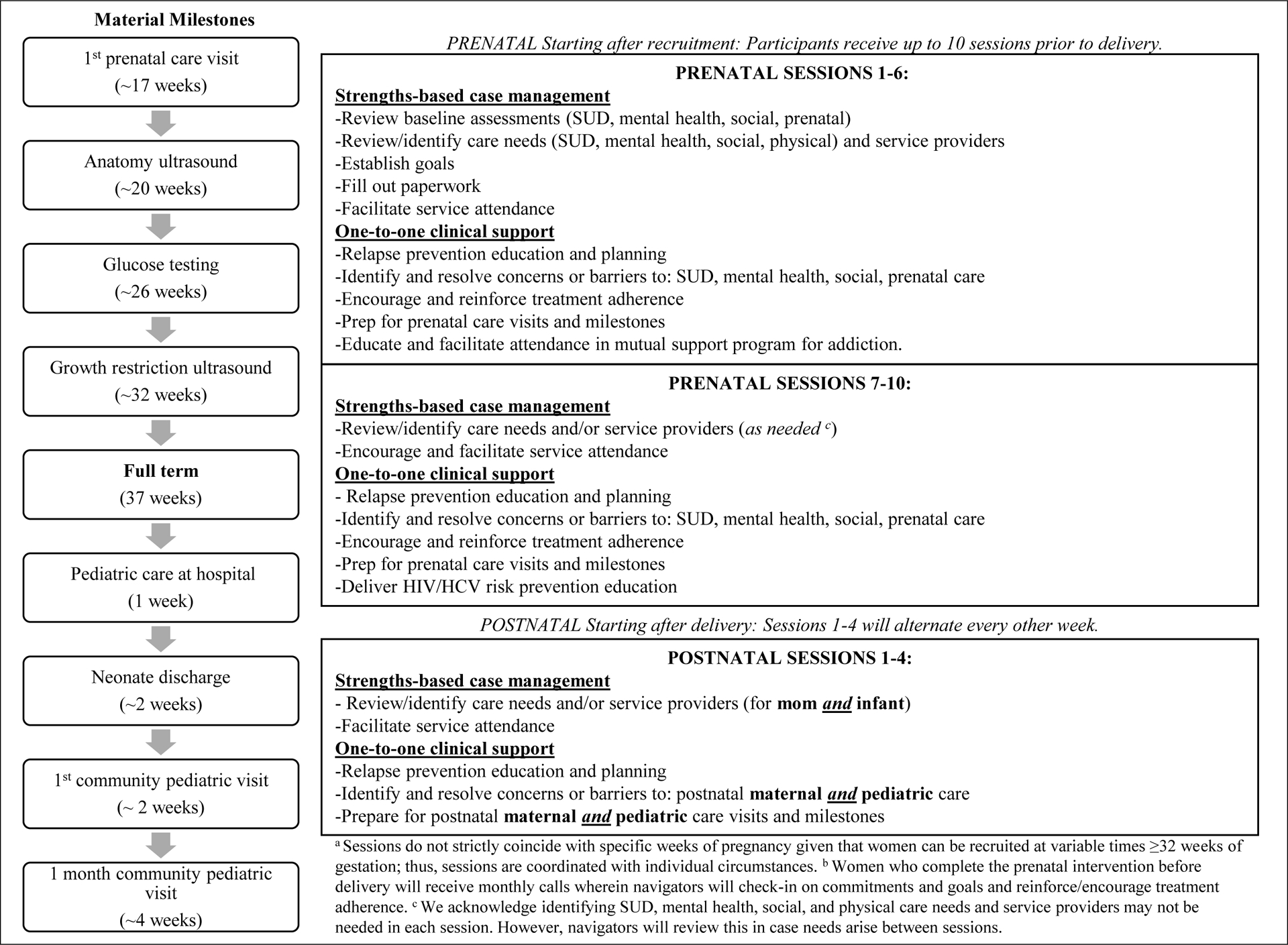
Our PN model is based on the work of Parker [28] and the recently completed Project HOPE study (Hospital Visit as Opportunity for Prevention and Engagement for HIV-Infected Drug Users [29, 30]). The study navigators underwent a two-day training in motivational interviewing tailored to the study intervention manual. The navigators also received a half-day training in the intervention protocol by study investigators. Intervention fidelity checking occurs throughout the study by audio recording all PN sessions, which are selected at random for fidelity assessments and feedback. Fidelity assessments cover intervention protocols, case management, and motivational interviewing.
The primary goal of the current PN intervention is linking participants before and after delivery to treatment/psychosocial care and clinical support for participants’ retention in those services. All PN participants are referred to a medication-assisted treatment program by the study navigator. A major challenge for pregnant women with OUD within these two health systems is linking to services for ongoing medication treatment for OUD and subsequently being retained in care before and after delivery. Women are encouraged to work with their providers to choose the opioid pharmacotherapy, buprenorphine or methadone, which will best meet their needs, and continue with this treatment throughout pregnancy and the postpartum period. The PN intervention also encourages engagement with prenatal and postpartum healthcare and effective transition of newborns into pediatric care.
The PN intervention specifically encompasses two complementary and necessary services: strengths-based case management (SBCM) and 1-to-1 clinical support (see Table 2). PN sessions last 45–60 minutes. Due to challenges keeping pregnant women with OUD engaged in care, the intervention emphasizes community outreach. For example, the navigator can visit participants’ neighborhoods to meet social and family networks to empathically encourage and support engagement with health, social services systems, and recovery support (i.e., narcotics anonymous, alcoholics anonymous) if she becomes disengaged in care or follow up.
Table 2.
Study Measures
| Source | Screen | Baseline | 14 weeks | 2 months | 6 months |
|---|---|---|---|---|---|
| Self-Report | X | X | X | X | |
| Self-Report | X | X | X | X | |
| Medical record | X | X | X | X | |
| Medical record | X | X | X | X | |
| Medical record/ patient | X | X | X | X | X |
| Agency records | X | X | X | X | |
| Self-Report | X | X | X | X | |
| Self-Report | X | X | X | X | |
| Self-Report | X | X | |||
| Self-Report | X | X | |||
| Medical record | X | ||||
| Medical record | X | X | X | X | |
| Medical record | X | X | X | X | |
| Medical record | X | ||||
| Medical record | X | X | X | X | |
| Medical record | X | X | |||
| Self-Report | X | X | X | X | |
| Self-Report | X | X | X | X | |
| Self-Report | X | X | X | X | |
| Self-Report | X | X | X | X | |
| Self-Report | X | X | X | X | |
| Self-Report | X | X | X | X | X |
Tx=Treatment
SBCM is an evidenced-based component of the PN model that has been demonstrated to help individuals with substance use disorders and chronic health conditions engage in needed care [31, 32]. Navigators apply the specialized skills of SBCM to link individuals to ongoing medication treatment for OUD and guide participants to active engagement in health and social services. SBCM gives patients responsibility for, and ownership of, their recovery [32] and has been shown to have a variety of positive effects, particularly treatment retention [33] and linkage with community services [34]. SBCM utilizes patients’ strengths for goal setting and developing a working alliance [32] between providers and patients. Specific elements the navigators focus on include: helping patients obtain and complete paperwork; accessing and engaging in drug/mental health counseling/treatment/mutual support, social services uptake, medication treatment for OUD linkage/retention; and engagement in pre/post-natal care and pediatric/developmental care for newborns.
One-to-one clinical support is an essential PN component for identifying, establishing, and retaining health behavior improvement goals. One-to-one clinical support is designed to motivate and assist participants in recognizing and overcoming internal/external barriers to care, including: emotional support, decision support, lifestyle change support, monitoring outcomes of screening/diagnostics, health behavior, and HIV and Hepatitis C virus (HCV) prevention education, which addresses needle sharing and unsafe sex [28]. Participants receive support and verbal reinforcement for completion of paperwork, engaging in drug/mental health treatment, social services uptake, agonist adherence, and engaging in pediatric/developmental care for their infants. One-to-one clinical support is delivered using motivational interviewing skills [35]. Motivational interviewing is an evidence-based approach for promoting health behavior change in healthcare settings [35, 36]. Navigators collaborate with participants to resolve ambivalence toward change by guiding them to establish their own goals and strategies, which enables women to take increased ownership in outcomes while also building self-efficacy and increasing the likelihood of achieving abstinence and pharmacotherapy for OUD retention goals. Motivational Interviewing is particularly valuable in aiding and empowering women to resolve barriers to continued care that may come as a result of having discouraging experiences within health care, treatment, or social service systems.
Intervention Augmentation
To expand our intervention approach from our proof-of-concept study to include greater relapse prevention capabilities, we utilized Marlatt and Gordon’s relapse prevention model [37, 38]. This model posits that both immediate determinants and covert antecedents predict and precede substance use relapse [37]. To systematically infuse relapse prevention into the postnatal PN sessions, we employed the ADAPT-ITT framework (Assessment, Decision, Administration, Production, Topical Experts, Integration, Training, and Testing, Table 1; [39]). ADAPT-ITT was originally designed as a framework for adapting evidence-based HIV interventions.
Table 1.
ADAPT-ITT framework (Assessment, Decision, Administration, Production, Topical Experts, Integration, Training, and Testing
| Assessment | Provided relapse prevention written materials to the project coinvestigators with the assignment to read and mark up the materials. Completing this task, investigators attended a 2-hour web conference where they: (1) received a presentation on relapse prevention among substance using populations, and (2) provided brief verbal summaries of their assigned reading materials to one another in the group. |
| Decision | Following the presentation and summaries, the web conference involved roundtable discussion, eliciting suggestions regarding needed intervention components. |
| Administration | Meeting participants provided specific recommendations on how to incorporate the identified components into the PN manual. |
| Production | Concluding the web conference, handwritten notes and comments recorded during the Decision phase were requested by the project leader. Once received, this information and the identified relapse prevention content was infused into the PN intervention. |
| Topical Experts | Adapted intervention sessions materials were circulated to the project coinvestigators for review and comment. |
| Integration | Comments and edits from the review of the sessions were returned for finalization and incorporation into the intervention manual. |
| Training | The project leader authored instructions for the adapted sessions on how navigators are to become familiar and proficient in the relapse prevention content, and how navigators are trained. |
| Testing | Navigators trianed in the relpase prevention infused study materials deliver this materials througout the study. |
Our application of the ADAPT-ITT framework resulted in relapse prevention content delivery as the initial task of sessions 2–13 and is called the “Relapse Prevention Check-Up.” Within the Check-Up, the participant is shown a card that contains 6 topics: (1) managing cravings, (2) recognizing and challenging thinking errors, (3) coping with emotions, (4) structuring time and avoiding boredom, (5) engaging with positive social support, and (6) developing healthy habits and self-care practices. Should PN sessions occur over the phone, the navigator verbally relates these topics to the participant. The navigator, following principles of motivational interviewing, asks the participant if there are any of the topics she knows about and/or would like to talk about. After the participant relates what she knows or would like to know more about, the navigator asks permission to share more specific information on topics from the card. The patient navigator then provides education on the topic based on the Marlatt et al. model [37, 38]. The navigator employs a Relapse Prevention Plan Worksheet, developed in this phase, to summarize the discussions and the information shared during sessions where relapse prevention is discussed. The navigator provides the summary sheet to the participant during the final prenatal and final postnatal intervention sessions as a record and for reference (or mails the sheet to the participant if the session occurs telephonically).
Measures
Table 2 lists the measurement domain, the instrument itself, the source, and timing during the study when the measurement is captured. Primary outcomes for this study will capture information on: OUD and other substance use disorder (SUD) treatment linkage/retention, opioid abstinence, adherence to MAT, and linkage/retention in psychosocial services for participants before randomization compared through 6-months post-delivery. Secondary outcomes involve capturing prenatal care, HIV/HCV risk behaviors, and depression and anxiety for participants before randomization compared through 6-months post-delivery; and child/mother indicators following delivery through 6-months. In addition to examining the effect of treatment condition on the above outcomes, covariates that will be collected in this study will assess behavioral, physical, and psychosocial domains (Table 2). Demographic domains are also assessed and include age, race, education level, employment status, marital status, number of other children, and health insurance status.
Sample Size
Given that this project is designed as a pilot study to test an expanded intervention and associated protocols and procedures, our sample size is not based on a power estimate. Rather, our sample size is based on estimates of how many patients can be screened and consented within the study timeframe, an appropriate method for pilot studies [40]. Based on medical record examination, we anticipate an average of 199 potential recruits each year between both medical centers during timeline of recruitment across 15 months. If approximately 70% are eligible and interested, and of those, 70% provide informed consent; we will recruit 122 participants in this study who will be randomized to the PN (n=61) or standard care conditions (n=61, Figure 1).
Figure 1: Anticipated Study Recruitment.
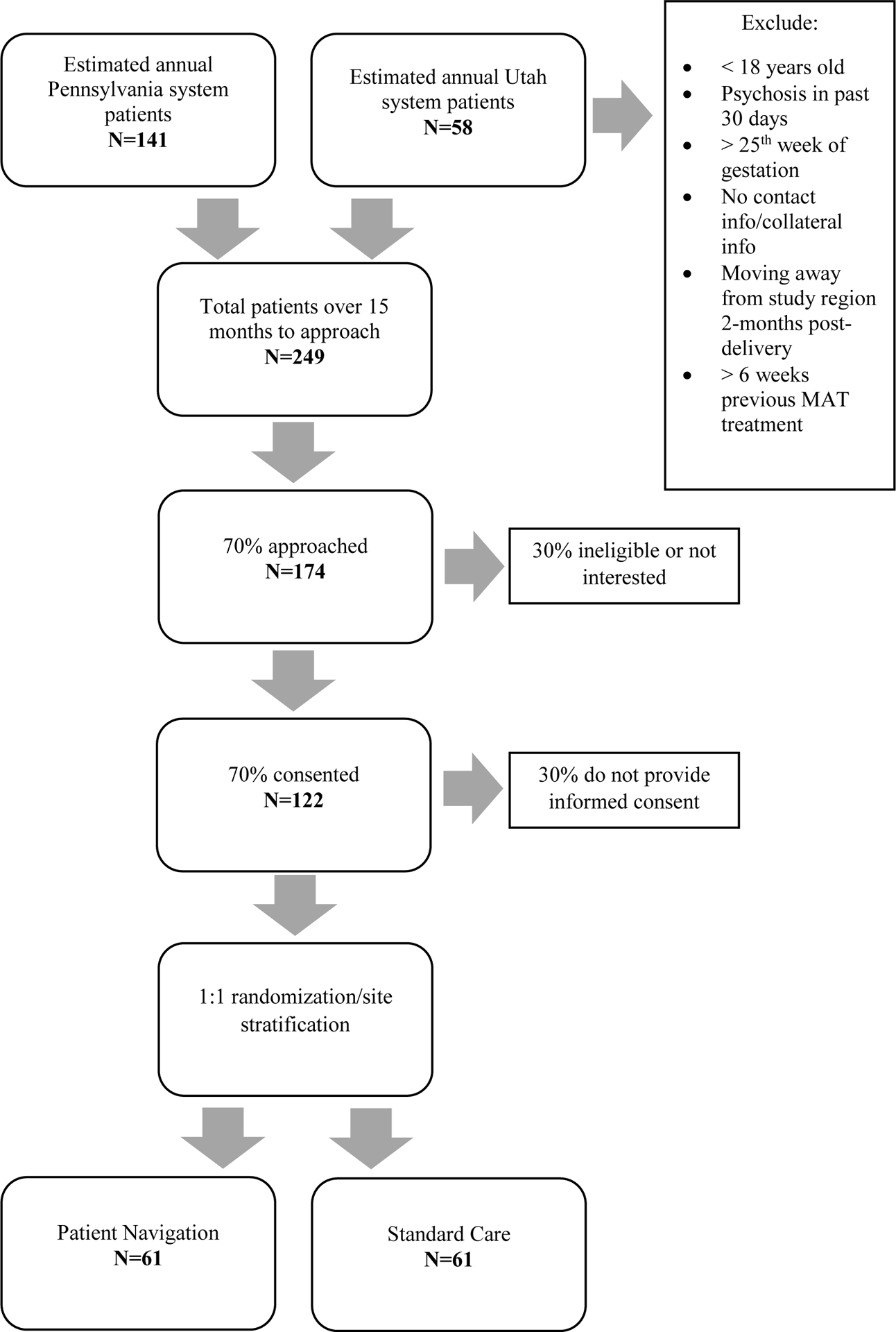
Definitive estimation and hypothesis testing are not the aim of this pilot study. However, the target sample size will allow estimation of odds ratios (for comparisons of linking participants before and after delivery to treatment/psychosocial care and clinical support and for participants’ retention in those services) along with 95% confidence intervals comparing outcome rates in the treatment and control arms, while accounting for within site clustering. The lower bounds of these confidence intervals will differ from the odds ratio estimates by a factor of 1/3 to 1/2 and the upper bounds will differ by a factor of 2 to 3 for a broad range of overall rates and true odds ratios for each site. In particular, this estimation accuracy requires that outcome rates be in the range 12–88% and true odds ratios be in the range 1/50 to 50 for each site. The pilot study will allow detection of strong signals of preliminary benefit and provide suggestions of benefit for subtler signals.
Analyses and Hypotheses
We will employ descriptive statistics to summarize central tendency, frequencies, and proportions for patient demographics and substance use, health, social, and maternal/neonatal indicators. T-tests and χ2 tests will be used to assess mean and proportion differences between baseline and outcome variables by study group at each time point. We will also develop a series of multilevel models to examine treatment, time, and covariate effects associated with our primary and secondary study outcomes [41, 42]. In particular, outcomes will be compared between the study arms in the context of logistic mixed effects models with random effects over time within subject and fixed effects for time trends within each study arm, as well as site and covariates including demographics, substance use, health, social, and maternal/neonatal indicators. Importantly, this multilevel perspective accommodates both overall time-trends and patient-specific time-trends. The multilevel framework allows for flexible treatment of time where change as a putative outcome may be nonlinear, accelerate, or decelerate at different rates across time. The framework also accommodates unequal numbers of observations and unequal spacing of observations across participants (i.e., missing data patterns). All multilevel models will be adjusted for site, demographics, and participant session completion.
Employing the above described multilevel modeling framework, we hypothesize (H), H1: PN recipients will have superior linkage and retention in: (a) OUD and other substance use disorder treatment, (b) psychosocial services, and (c) pre/postnatal care compared to standard care, and H2: a larger portion of PN patients will be (a) adherent to medication treatment for OUD and (b) drug abstinent compared to standard care.
Discussion
OUD is a treatable chronic condition and pregnancy is an optimal time for behavior change among women [43–50]. Medication for OUD treatment (i.e., methadone or buprenorphine) is key in the care of pregnant women with OUD, but it must also be combined with additional behavioral health services that target psychosocial aspects of addiction [10, 11].
Operating within a chronic care paradigm, PN attempts to break down barriers that prevent individuals with OUD from not only accessing medication-assisted treatment (i.e., methadone or buprenorphine), but also from engaging with health, psychiatric, social, and family services that are often not provided through medication-assisted treatment programs [14, 15, 28, 51–53]. To address barriers such as fear/anxiety, communication, transportation, finances, the medical system, and lack of information [14, 51, 53], navigators develop 1-to-1 relationships to provide personalized support focusing on individual needs [14, 15, 51, 53]. However, PN has only recently begun to be researched in relation to behavioral health problems [29, 54–56], such as smoking cessation improvements [57], HIV management [58] and our team’s proof-of-concept study that demonstrated intervention feasibility among pregnant women with OUD.
Specifically, our proof-of-concept study findings demonstrated promising initial results for women in a number of behavioral and physical health domains, including improvements in opioid/substance use, mental health, and prenatal care outcomes [20]. This multisite pilot trial study expands upon this previous PN intervention to further establish the internal and external validity of these findings and the potential value of the PN intervention for pregnant women with OUD. Methods for intervention and protocol expansion followed evidence-informed frameworks that facilitated systematic and objective steps. The study intervention also includes added content to prevent drug use relapse among participants, and the protocol has established methods/procedures necessary for conducting a multisite randomized clinical trial to pilot test the PN intervention across two health systems.
Strengths and Limitations
This study includes several strengths. PN is an evidence-based practice that has demonstrated efficacy for a number of other chronic health conditions. Further, unlike most studies that provide medication and/or behavioral/psychosocial interventions during pregnancy, our current study collects outcome information after delivery, for approximately 2 months postpartum. This study likewise advances the field by building upon our preliminary findings by expanding its design to a multisite randomized trial within two regional health systems.
This study also possesses limitations. Recruitment for this study is limited to a convenience sample within the catchment areas of the two tertiary care health systems in urban settings, and thus findings are likely limited in terms of their external validity beyond these regions. We look forward to utilizing what is learned in this study to extend this model of care to larger geographic areas and broader population in future research to strengthen generalizability. We also recognize there may be a broad spectrum of severity of OUD among recruited participants, resulting in varying levels of needs. This variability, combined with limitations of availability of health/social services for participants living outside of urban areas with greater resources, may results in some participants having greater needs, access to treatment, and support than others. We anticipate adjusting outcome analyses for level of engagement in services as well as urbanicity of dwelling. Notwithstanding these limitations, PN represents a significant potential for aiding pregnant women with OUD to locate, engage, and remain engaged in OUD treatment during pregnancy and the postpartum period.
Conclusion
Pregnant women with OUD face a number of significant health and social challenges during pregnancy and following delivery. Interventions designed to collaboratively address and support recovery from this chronic health problem are paramount. Thus, the results of this study testing PN compared to standard care will produce and evaluate necessary protocols/procedures and pilot data preparatory to a large scale, fully-powered, multisite randomized trial. Importantly, this study further establishes the evidence-base—with the potential to ameliorate serious adverse opioid-related outcomes and improve health for women and their children.
Support:
This project was supported by a grant from the Centers for Disease Control and Prevention (R01CE002996).
Footnotes
Competing Interests: None
References
- [1].Haight SC, Ko JY, Tong VT, Bohm MK, Callaghan WM, Opioid Use Disorder Documented at Delivery Hospitalization - United States, 1999–2014, MMWR. Morbidity and Mortality Weekly Report 67(31) (2018) 845–849. doi: 10.15585/mmwr.mm6731a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Shainker SA, Saia K, Lee-Parritz A, Opioid addiction in pregnancy, Obstetrical & Gynecological Survey 67(12) (2012) 817–25. doi: 10.1097/OGX.0b013e3182788e8c. [DOI] [PubMed] [Google Scholar]
- [3].ACOG, ACOG Committee Opinion No. 524: Opioid abuse, dependence, and addiction in pregnancy, Obstetrics and Gynecology 119(5) (2012) 1070–6. doi: 10.1097/AOG.0b013e318256496e. [DOI] [PubMed] [Google Scholar]
- [4].Jones HE, Heil SH, Baewert A, Arria AM, Kaltenbach K, Martin PR, Coyle MG, Selby P, Stine SM, Fischer G, Buprenorphine treatment of opioid-dependent pregnant women: a comprehensive review, Addiction 107 Suppl 1 (2012) 5–27. doi: 10.1111/j.1360-0443.2012.04035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].McQueen KA, Murphy-Oikonen J, Gerlach K, Montelpare W, The impact of infant feeding method on neonatal abstinence scores of methadone-exposed infants, Advances in neonatal care : official Journal of the National Association of Neonatal Nurses 11(4) (2011) 282–90. doi: 10.1097/ANC.0b013e318225a30c. [DOI] [PubMed] [Google Scholar]
- [6].Whiteman VE, Salemi JL, Mogos MF, Cain MA, Aliyu MH, Salihu HM, Maternal Opioid Drug Use during Pregnancy and Its Impact on Perinatal Morbidity, Mortality, and the Costs of Medical Care in the United States, Journal of Pregnancy 2014 (2014) 906723. doi: 10.1155/2014/906723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Roussos-Ross K, Reisfield G, Elliot I, Dalton S, Gold M, Opioid use in pregnant women and the increase in neonatal abstinence syndrome: what is the cost?, Journal of Addiction Medicine 9(3) (2015) 222–5. doi: 10.1097/ADM.0000000000000122. [DOI] [PubMed] [Google Scholar]
- [8].Smid MC, Stone NM, Baksh L, Debbink MP, Einerson BD, Varner MW, Gordon AJ, Clark EAS, Pregnancy-Associated Death in Utah: Contribution of Drug-Induced Deaths, 133(6) (2019) 1131–1140. doi: 10.1097/AOG.0000000000003279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].NIDA, Drugs, Brains, and Behavior: The Science of Addiction, National Institutes of Health, National Institute on Drug Abuse, Bethesda, MD, 2010. [Google Scholar]
- [10].Amato L, Minozzi S, Davoli M, Vecchi S, Psychosocial and pharmacological treatments versus pharmacological treatments for opioid detoxification, The Cochrane Database of Systematic Reviews (9) (2011) Cd005031. [DOI] [PubMed] [Google Scholar]
- [11].Amato L, Minozzi S, Davoli M, Vecchi S, Psychosocial combined with agonist maintenance treatments versus agonist maintenance treatments alone for treatment of opioid dependence, The Cochrane Database of Systematic Reviews (10) (2011) Cd004147. [DOI] [PubMed] [Google Scholar]
- [12].Krans EE, Kim JY, James AE 3rd, Kelley D, Jarlenski MP, Medication-Assisted Treatment Use Among Pregnant Women With Opioid Use Disorder, Obstetrics and Gynecology 133(5) (2019) 943–951. doi: 10.1097/AOG.0000000000003231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Martin CE, Longinaker N, Terplan M, Recent trends in treatment admissions for prescription opioid abuse during pregnancy, Journal of Substance Abuse Treatment 48(1) (2015) 37–42. doi: 10.1016/j.jsat.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Freeman HP, Rodriguez RL, History and principles of patient navigation, Cancer 117(S15) (2011) 3537–3540. doi: 10.1002/cncr.26262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].McDonald KM, Sundaram V, Bravata DM, Lewis R, Lin N, Kraft S, McKinnon M, Paguntalan H, Owens DK, Closing the quality gap: a critical analysis of quality improvement strategies, in: Shojania KG, McDonald KM, Wachter RM, Owens DK (Eds.) Care Coordination, Agency for Healthcare Research and Quality, Rockville, MD, 2007. [PubMed] [Google Scholar]
- [16].ImprovingChronicCare, The Chronic Care Model, 2014. http://www.improvingchroniccare.org/. (Accessed June 3 2014).
- [17].Brigham G, Winhusen T, Lewis D, Kropp F, Incentives for retention of pregnant substance users: a secondary analysis, Journal Of Substance Abuse Treatment 38(1) (2010) 90–95. doi: 10.1016/j.jsat.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Svikis DS, Lee JH, Haug NA, Stitzer ML, Attendance incentives for outpatient treatment: effects in methadone- and nonmethadone-maintained pregnant drug dependent women, Drug and Alcohol Dependence 48 (1997) 33–41. doi: 10.1016/s0376-8716(97)00101-4. [DOI] [PubMed] [Google Scholar]
- [19].Jones HE, O’Grady KE, Tuten M, Reinforcement-based treatment improves the maternal treatment and neonatal outcomes of pregnant patients enrolled in comprehensive care treatment, The American Journal on Addictions / American Academy of Psychiatrists in Alcoholism and Addictions 20(3) (2011) 196–204. doi: 10.1111/j.1521-0391.2011.00119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Cochran GT, Hruschak V, Abdullah W, Krans E, Douaihy AB, Bobby S, Fusco R, Tarter R, Optimizing Pregnancy Treatment Interventions for Moms (OPTI-Mom): A Pilot Study, Journal of Addiction Medicine 12(1) (2018) 72–79. doi: 10.1097/ADM.0000000000000370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Guydish J, Tajima B, Manser ST, Jessup M, Strategies to encourage adoption in multisite clinical trials, Journal of Substance Abuse Treatment 32(2) (2007) 177–88. doi: 10.1016/j.jsat.2006.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Walker R, Morris DW, Greer TL, Trivedi MH, Research staff training in a multisite randomized clinical trial: Methods and recommendations from the Stimulant Reduction Intervention using Dosed Exercise (STRIDE) trial, Addiction Research & Theory 22(5) (2014) 407–415. doi: 10.3109/16066359.2013.868446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].A. American Psychiatric, D.S.M.T.F. American Psychiatric Association, Diagnostic and statistical manual of mental disorders : DSM-5, 5th ed. ed., Arlington, VA: : American Psychiatric Association; 2013. [Google Scholar]
- [24].Eisen SV, Normand SL, Belanger AJ, Spiro A 3rd, Esch D, The Revised Behavior and Symptom Identification Scale (BASIS-R): reliability and validity, Medical Care 42(12) (2004) 1230–41. doi: 10.1097/00005650-200412000-00010. [DOI] [PubMed] [Google Scholar]
- [25].Altman EG, Hedeker D, Peterson JL, Davis JM, The Altman Self-Rating Mania Scale, Biological Psychiatry 42(10) (1997) 948–55. doi: 10.1016/S0006-3223(96)00548-3. [DOI] [PubMed] [Google Scholar]
- [26].Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, McLeod L, Delacqua G, Delacqua F, Kirby J, Duda SN, The REDCap consortium: Building an international community of software platform partners, Journal of Biomedical Information 95 (2019) 103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG, Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support, Journal of Biomedical Information 42(2) (2009) 377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Parker VA, Lemak CH, Navigating patient navigation: crossing health services research and clinical boundaries, Advances In Health Care Management 11 (2011) 149–183. 10.1108/S1474-8231(2011)0000011010. [DOI] [PubMed] [Google Scholar]
- [29].Metsch L, Project HOPE: Hospital Visit as Opportunity for Prevention and Engagement for HIV-Infected Drug Users, 2014. http://ctndisseminationlibrary.org/protocols/ctn0049.htm. (Accessed May 6 2014).
- [30].Metsch LR, Feaster DJ, Gooden L, Matheson T, Stitzer M, Das M, Jain MK, Rodriguez AE, Armstrong WS, Lucas GM, Nijhawan AE, Drainoni ML, Herrera P, Vergara-Rodriguez P, Jacobson JM, Mugavero MJ, Sullivan M, Daar ES, McMahon DK, Ferris DC, Lindblad R, VanVeldhuisen P, Oden N, Castellon PC, Tross S, Haynes LF, Douaihy A, Sorensen JL, Metzger DS, Mandler RN, Colfax GN, del Rio C, Effect of Patient Navigation With or Without Financial Incentives on Viral Suppression Among Hospitalized Patients With HIV Infection and Substance Use: A Randomized Clinical Trial, JAMA 316(2) (2016) 156–70. doi: 10.1001/jama.2016.8914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Brun C, Rapp RC, Strengths-based case management: individuals’ perspectives on strengths and the case manager relationship, Social Work 46(3) (2001) 278–88. doi: 10.1093/sw/46.3.278. [DOI] [PubMed] [Google Scholar]
- [32].Saleebey DE, The strengths perspective in social work practice, Longman, New York, 2009. [PubMed] [Google Scholar]
- [33].Jansson LM, Svikis DS, Breon D, Cieslak R, Intensity of Case Management Services Does More Better for Drug-Dependent Women and Their Children?, Social Work in Mental Health 3(4) (2005) 63–78. 10.1300/J200v03n04_04. [DOI] [Google Scholar]
- [34].Rapp RC, Otto AL, Lane DT, Redko C, McGatha S, Carlson RG, Improving linkage with substance abuse treatment using brief case management and motivational interviewing, Drug & Alcohol Dependence 94(1–3) (2008) 172–182. doi: 10.1016/j.drugalcdep.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Miller W, Rollnick S, Motivational Interviewing: Helping People to Change, The Guilford Press, New York, 2013. [Google Scholar]
- [36].Rollnick S, Miller WR, Butler C, Motivational interviewing in health care: helping patients change behavior, Guilford Press, New York, 2008. [Google Scholar]
- [37].Larimer ME, Palmer RS, Marlatt GA, Relapse prevention. An overview of Marlatt’s cognitive-behavioral model, Alcohol research & health : the journal of the National Institute on Alcohol Abuse and Alcoholism 23(2) (1999) 151–60. [PMC free article] [PubMed] [Google Scholar]
- [38].Haug N, Sorensen JL, Gruber V, Song Y, Relapse Prevention for Opioid Dependence, in: Marlatt AG, Donovan D (Eds.), Relapse prevention: maintenance strategies in the treatment of addictive behaviors, Guilford Press, New York, 2005. [Google Scholar]
- [39].Wingood GM, DiClemente RJ, The ADAPT-ITT Model: A Novel Method of Adapting Evidence-Based HIV Interventions, JAIDS Journal of Acquired Immune Deficiency Syndromes 47 Suppl 1, The First National Scientific Meeting of the Social and Behavioral Science Research Network(Supplement 1) (2008) S40–S46. doi: 10.1097/QAI.0b013e3181605df. [DOI] [PubMed] [Google Scholar]
- [40].Leon AC, Davis LL, Kraemer HC, The Role and Interpretation of Pilot Studies in Clinical Research, Journal of Psychiatric Research 45(5) (2011) 626–629. 10.1016/j.jpsychires.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Rabe-Hesketh S, Anders S, Multilevel and Longitudinal Modeling Using Stata, Third Edition Volume II: Categorical Responses, Counts, and Survival, StataCorp LP, College Station, TX, 2012. [Google Scholar]
- [42].Li B, Lingsma H, Steyerberg E, Lesaffre E, Logistic random effects regression models: a comparison of statistical packages for binary and ordinal outcomes, BMC Medical Research Methodology 11(1) (2011) 77. doi: 10.1186/1471-2288-11-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Waller CS, Zollinger TW, Saywell RW Jr., Kubisty KD, The Indiana Prenatal Substance Use Prevention Program: its impact on smoking cessation among high-risk pregnant women, Indiana medicine : the journal of the Indiana State Medical Association 89(2) (1996) 184–7. [PubMed] [Google Scholar]
- [44].Ostbye T, McBride C, Demark-Wahnefried W, Bastian L, Morey M, Krause KM, Brouwer R, Turner B, Interest in healthy diet and physical activity interventions peripartum among female partners of active duty military, Military Medicine 168(4) (2003) 320–5. 10.1093/milmed/168.4.320. [DOI] [PubMed] [Google Scholar]
- [45].Joy EA, Van Hala S, Marshall E, Pregnancy as an opportunity for behavior change, Current Sports Medicine Reports 3(6) (2004) 327–9. [DOI] [PubMed] [Google Scholar]
- [46].Crawford JT, Tolosa JE, Goldenberg RL, Smoking cessation in pregnancy: why, how, and what next, Clinical Obstetrics and Gynecology 51(2) (2008) 419–35. doi: 10.1097/GRF.0b013e31816fe9e9. [DOI] [PubMed] [Google Scholar]
- [47].Phelan S, Pregnancy: a “teachable moment” for weight control and obesity prevention, American Journal of Obstetrics and Gynecology 202(2) (2010) 135.e1–8. doi: 10.1016/j.ajog.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Bohrer J, Ehrenthal DB, Other adverse pregnancy outcomes and future chronic disease, Seminars in Perinatology 39(4) (2015) 259–63. doi: 10.1053/j.semperi.2015.05.003. [DOI] [PubMed] [Google Scholar]
- [49].Atkinson L, Shaw RL, French DP, Is pregnancy a teachable moment for diet and physical activity behaviour change? An interpretative phenomenological analysis of the experiences of women during their first pregnancy, British Journal of Health Psychology 21(4) (2016) 842–858. doi: 10.1111/bjhp.12200. [DOI] [PubMed] [Google Scholar]
- [50].O’Brien CM, Cramp C, Dodd JM, Delivery of Dietary and Lifestyle Interventions in Pregnancy: is it Time to Promote the Use of Electronic and Mobile Health Technologies?, Seminars in Reproductive Medicine 34(2) (2016) e22–7. doi: 10.1055/s-0036-1583533. [DOI] [PubMed] [Google Scholar]
- [51].Farrisi D, Dietz N, Patient navigation is a client-centered approach that helps to engage people in HIV care, HIV Clinician 25(1) (2013) 1–3 http://www.deltaaetc.org/hcarticles/articles%20as%20pdf/winter%202013%20articles%20as%20pdf/patientnavigation.pdf. [PubMed] [Google Scholar]
- [52].Parker VA, Clark JA, Leyson J, Calhoun E, Carroll JK, Freund KM, Battaglia TA, Patient navigation: development of a protocol for describing what navigators do, Health Services Research 45(2) (2010) 514–531. doi: 10.1111/j.1475-6773.2009.01079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Piper LE, Patient service navigator: improving quality and services and reducing cost under the Affordable Care Act, The Health Care Manager 33(1) (2014) 47–52. doi: 10.1097/01.HCM.0000440619.54761.f9. [DOI] [PubMed] [Google Scholar]
- [54].Marr AL, Pillow T, Brown S, Southside medical homes network: linking emergency department patients to community care, Prehospital and Disaster Medicine 23(3) (2008) 282–4. 10.1017/S1049023X00065018. [DOI] [PubMed] [Google Scholar]
- [55].Koester KA, Morewitz M, Pearson C, Weeks J, Packard R, Estes M, Tulsky J, Kang-Dufour MS, Myers JJ, Patient navigation facilitates medical and social services engagement among HIV-infected individuals leaving jail and returning to the community, AIDS Patient Care And STDs 28(2) (2014) 82–90. 10.1089/apc.2013.0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Goff SL, Pekow PS, White KO, Lagu T, Mazor KM, Lindenauer PK, IDEAS for a healthy baby--reducing disparities in use of publicly reported quality data: study protocol for a randomized controlled trial, Trials 14 (2013) 244. doi: 10.1186/1745-6215-14-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Lasser KE, Kenst KS, Quintiliani LM, Wiener RS, Murillo J, Pbert L, Xuan Z, Bowen DJ, Patient navigation to promote smoking cessation among low-income primary care patients: a pilot randomized controlled trial, Journal Of Ethnicity in Substance Abuse 12(4) (2013) 374–390. 10.1080/15332640.2013.81931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Bradford JB, Coleman S, Cunningham W, HIV System Navigation: an emerging model to improve HIV care access, AIDS Patient Care And STDs 21 Suppl 1 (2007) S49–S58. 10.1089/apc.2007.9987. [DOI] [PubMed] [Google Scholar]
- [59].Cacciola JS, Alterman AI, Lynch KG, Martin JM, Beauchamp ML, McLellan AT, Initial reliability and validity studies of the revised Treatment Services Review (TSR-6), Drug and Alcohol Dependence 92(1–3) (2008) 37–47. 10.1016/j.drugalcdep.2007.06.004. [DOI] [PubMed] [Google Scholar]
- [60].Sobell M, Sobel L, Bogardis J, Leo G, Skinner W, Problem drinkers’ perceptions of whether treatment goals should be self-selected or therapist-selected, Behavioral Therapy 23 (1992) 43–52. 10.1016/S0005-7894(05)80307-7. [DOI] [Google Scholar]
- [61].Sobell L, Sobell M, Timeline follow-back: a technique for assessing self-reported alcohol consumption, in Measuring Alcohol Consumption: Psychosocial and Biological Methods, in: J A, R L (Eds.) Humana Press, Totowa, New Jersey, 1992. [Google Scholar]
- [62].Napper LE, Fisher DG, Reynolds GL, Johnson ME, HIV Risk Behavior Self-Report Reliability at Different Recall Periods, AIDS and Behavior 14(1) (2010) 152–61. 10.1007/s10461-009-9575-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Pascoe JM, Ialongo NS, Horn WF, Reinhart MA, Perradatto D, The reliability and validity of the maternal social support index, Family Medicine 20(4) (1988) 271–276. [PubMed] [Google Scholar]
- [64].Barroso NE, Hungerford GM, Garcia D, Graziano PA, Bagner DM, Psychometric Properties of the Parenting Stress Index-Short Form (PSI-SF) in a High-Risk Sample of Mothers and their Infants, Psychological Assessment 28(10) (2016) 1331–1335. 10.1037/pas0000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Hawley NL, Brown C, Nu’usolia O, Ah-Ching J, Muasau-Howard B, McGarvey ST, Barriers to Adequate Prenatal Care Utilization in American Samoa, Maternal and Child Health Journal (2013). 10.1007/s10995-013-1368-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Tandon SD, Cluxton-Keller F, Colon L, Vega P, Alonso A, Improved adequacy of prenatal care and healthcare utilization among low-income Latinas receiving group prenatal care, Journal of women’s health (2002) 22(12) (2013) 1056–61. 10.1089/jwh.2013.4352. [DOI] [PubMed] [Google Scholar]
- [67].Tayebi T, Zahrani ST, Mohammadpour R, Relationship between adequacy of prenatal care utilization index and pregnancy outcomes, Iranian Journal of Nursing and Midwifery Research 18(5) (2013) 360–6. [PMC free article] [PubMed] [Google Scholar]
- [68].Kurtzman JH, Wasserman EB, Suter BJ, Glantz JC, Dozier AM, Measuring Adequacy of Prenatal Care: Does Missing Visit Information Matter?, Birth (Berkeley, Calif.) (2014). [DOI] [PubMed] [Google Scholar]
- [69].Skinner HA, The drug abuse screening test, Addictive Behaviors 7(4) (1982) 363–371. [DOI] [PubMed] [Google Scholar]
- [70].Yudko E, Lozhkina O, Fouts A, A comprehensive review of the psychometric properties of the Drug Abuse Screening Test, Journal of Substance Abuse Treatment 32(2) (2007) 189–198. doi: 10.1016/j.jsat.2006.08.002. [DOI] [PubMed] [Google Scholar]
- [71].Gavin DR, Ross HE, Skinner HA, Diagnostic validity of the drug abuse screening test in the assessment of DSM-III drug disorders, British Journal of Addiction 84(3) (1989) 301–307. DOI: 10.1111/j.1360-0443.1989.tb03463.x. [DOI] [PubMed] [Google Scholar]
- [72].Meneses-Gaya I.C.d., Zuardi AW, Loureiro SR, Crippa J.A.d.S., Psychometric properties of the Fagerström Test for Nicotine Dependence, Jornal Brasileiro de Pneumologia 35 (2009) 73–82. 10.1590/S1806-37132009000100011. [DOI] [PubMed] [Google Scholar]
- [73].McHorney CA, Ware JE, Raczek AE, The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs, Medical Care 31(3) (1993) 247–263. [DOI] [PubMed] [Google Scholar]
- [74].Spitzer RL, Williams JB, Kroenke K, Hornyak R, McMurray J, Validity and utility of the PRIME-MD patient health questionnaire in assessment of 3000 obstetric-gynecologic patients: the PRIME-MD Patient Health Questionnaire Obstetrics-Gynecology Study, American Journal of Obstetrics and Gynecology 183(3) (2000) 759–69. 10.1067/mob.2000.106580. [DOI] [PubMed] [Google Scholar]
- [75].Spitzer RL, Kroenke K, Williams JB, Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire, JAMA 282(18) (1999) 1737–44. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- [76].Smith TC, Smith B, Jacobson IG, Corbeil TE, Ryan MAK, Reliability of Standard Health Assessment Instruments in a Large, Population-Based Cohort Study, Annals of Epidemiology 17(7) (2007) 525–532. 10.1016/j.annepidem.2006.12.002. [DOI] [PubMed] [Google Scholar]
- [77].Hides L, Lubman DI, Devlin H, Cotton S, Aitken C, Gibbie T, Hellard M, Reliability and validity of the Kessler 10 and Patient Health Questionnaire among injecting drug users, Australian & New Zealand Journal of Psychiatry 41(2) (2007) 166–168. 10.1080/00048670601109949. [DOI] [PubMed] [Google Scholar]


