Summary
1. The European Water Framework Directive provides a framework for improving the ecological quality of stream ecosystems, with deviation from reference used as a measure of ecological status.
2. Here we examine the possibility of using less impacted stream sites from Latvia, Lithuania and Poland to establish a Danish reference network for macrophyte assemblages, and as a guiding image for identification of possible references sites within Denmark. Both approaches were evaluated using historical Danish records.
3. Four different macrophyte assemblages were identified for mid‐sized streams in the Central and Eastern Lowland ecoregions. Macrophyte assemblages could not be delineated using physical stream site characteristics; however a gradual change in assemblage composition was attributed to differences in alkalinity and human impact.
4. Assemblages of contemporary vegetation in Denmark were quite similar to those found in Polish, Latvian and Lithuanian streams (26–35%). However, more importantly, from species‐based predictions we noted higher similarity, particularly with Latvian and Lithuanian streams, before intensive land use commenced in Denmark (c. 1900). These results show that stream sites from these three countries can be considered in a Danish reference network.
5. Two of the four macrophyte assemblages comprised species such as Fontinalis antipyretica, Myriophyllum spicatum, Nuphar lutea, Potamogeton alpinus and P. perfoliatus that have a very scattered occurrence in the contemporary vegetation in Denmark. These groups were closely associated with the predictions from historic records, thereby lending support the conjecture that these assemblages could be part of the guiding image for the identification of potential reference sites within Denmark.
Keywords: historic data, lowland, macrophytes, natural condition, WFD
Introduction
The EU Water Framework Directive (WFD; European Commission, 2000) provides a legislative framework for a targeted effort to improve ecological quality of stream ecosystems based on monitoring and assessment using different stream organisms (e.g. fish, invertebrates, macrophytes and benthic diatoms). A central aspect in the directive is the reference concept which is used to assess the ecological impairment of sites (e.g. stream segments of a particular size) and to assess recovery of sites previously identified as impaired. According to the directive, reference conditions are described as ‘a state in the present or in the past corresponding to very low pressure without the effects of major industrialization, urbanization and intensification of agriculture, and with only very minor modification of physicochemistry, hydromorphology and biology’ (Wallin, Wiederholm & Johnson, 2003).
However, reference conditions as envisioned by the European Water Framework Directive are rare, and for many regions finding extant reference sites is particularly challenging due to the long‐term effects that land use has had on the integrity of streams. Several approaches have been used to establish reference values, such as the condition of systems in the absence of significant human disturbance, the best available sites within an area and the condition that sites might achieve if they were better managed (Stoddard et al., 2006). One of most common approaches to describe reference conditions is to quantify the biological condition at a set of sites that represent either minimally (MDC) or least (LDC) disturbed conditions (sensu Stoddard et al., 2006; Bailey, Norris & Reynoldson, 2004; Chaves et al., 2006).
The selection of MDC or LDC sites is often based on either the use of pre‐established inclusion/exclusion criteria or expert judgement. Although both theoretical and empirical work has focused on the characterization of pressure gradients to assess impacts on biological communities (e.g. Fore, Karr & Wisseman, 1996; Hering et al., 2006; Johnson et al., 2007), few studies have focused on understanding the variability of relatively unimpaired sites. This is unfortunate since according to the EU directive it is the biological communities at these sites which will be used to evaluate the degree of impairment. Furthermore, pressure‐impact relationships may not cover all types of single or combined pressures affecting biological communities. For example, stream macrophytes are well known for their response to nutrient pollution (see Birk, Korte & Hering, 2006), but the effect of other types of impairment are less well studied (Baattrup‐Pedersen & Riis, 1999; Wright et al., 2003; Daniel, Bernez & Haury, 2006; O’Hare et al., 2006; Pedersen, Baattrup‐Pedersen & Madsen, 2006). Consequently, the establishment of screening criteria and reliable thresholds for the selection of MDC or LDC sites for macrophyte assemblages is difficult.
The use of expert judgement for selecting MDC or LDC sites also has many weaknesses. First, bias may be introduced by focusing on species and assemblages that presently occur in best available sites. In lowland areas with intensive land use this bias may be significant, and species assemblages may differ substantially between the best sites and true reference sites. For example, several Potamogeton species such as P. alpinus Balbis, P. lucens L., P. polygonifolius Pourret and P. praelongus Wulf. were formerly widely distributed, but now many of these species are near or are extinct in lowland streams in several regions of Europe (Baggøe & Ravn, 1896; Raunkiær, 1895‐99; Mountford, 1994; work cited in Preston, 1995; Riis & Sand‐Jensen, 2001). Second, it is difficult to avoid subjectivity when using expert judgement. Thus limited knowledge on reference assemblages implies that the perception of what the reference condition represents differs among experts (Birk et al., 2006).
In this study, we describe reference assemblages for the mid‐sized lowland stream type of mixed geology (CIS WG2.A ECOSTAT, 2004) in the Central and Eastern Lowland ecoregions of Europe (e.g. Latvia, Lithuania, Poland, Germany, Sweden and Denmark). We used two approaches to establish a reference network for macrophyte assemblages in Danish streams. First, we examined the possibility of using stream sites from regions in Latvia, Lithuania and Poland with less intensive land use. These three countries are similar to Denmark in topography and climate, and all four countries belong to the Central Baltic River Geographical Intercalibration Group (GIG). Second, we examined the possibility of using macrophyte assemblages in these regions in the development of a guiding image for the identification of potential reference sites in Denmark (Stoddard et al., 2006). We see this guiding image as an extension to the expert judgement, building also on empirical data. Both approaches were validated using historical records for Danish macrophyte assemblages (see Riis & Sand‐Jensen, 2001 for the full reference list). These historic data provide a unique opportunity to gain insight into community structure and species occurrence before extensive human perturbation and therefore may be particularly valuable in the development of a guiding image for reference assemblages.
Methods
The reference dataset
Data were compiled from a number of different sources. First, we used a subset of the macrophyte data collected from mid‐sized lowland streams as part of the EU‐funded project STAR (Furse et al., 2006). This data set included stream sites classified as reference sites using a list of a priori exclusion criteria (Hering et al., 2003; Nijboer et al., 2004). Four sites were situated in Germany, seven in Poland, three in Sweden, four in Denmark and seven in Latvia. We chose to combine data from sites in Germany and Sweden as well as from the Baltic region and Poland to obtain a better measure of reference assemblages for the mid‐sized lowland stream type. Second, we used data from 15 stream sites in Latvia that had been classified as reference (Springe et al., 2006) and from 13 stream sites in Denmark that are considered as LDC sites (Baattrup‐Pedersen, Larsen & Riis, 2002; Pedersen et al., 2006). Third, new data were collected from 10 streams in Lithuania. These sites were selected to study the possibility of using data from an area which has experienced less intensive land use compared to Denmark. Lithuania and Denmark are topographically and climatically similar (average precipitation in Lithuania 661 mm compared to 712 mm in Denmark; average temperature 6 °C in Klaipeda compared to 7.7 °C in Denmark). The selected sites can be regarded as LDC sites, with intensive land use varying between 9–57% of total catchment area. The geographic location of the sampling sites is shown in Fig. 1.
Figure 1.
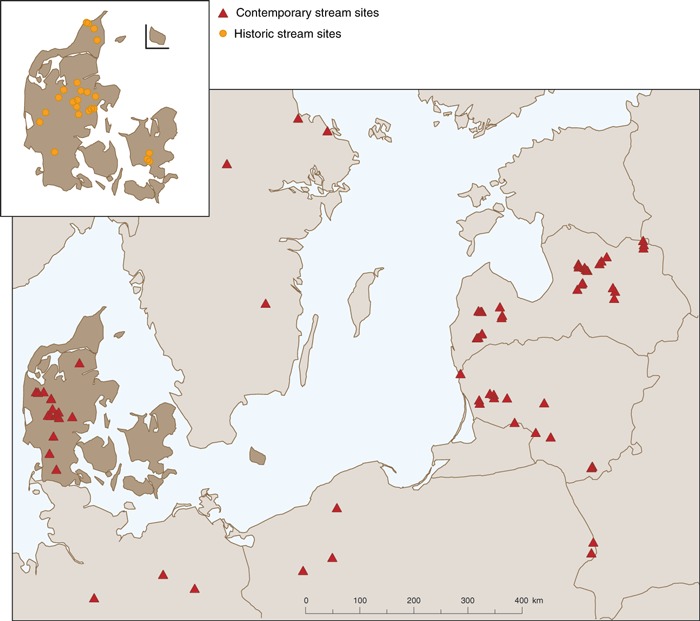
Map showing the location of the lowland mid‐sized stream sites used in the present study.
The Lithuanian stream sites were surveyed in summer 2006 according to the Danish sampling protocol (Pedersen & Baattrup‐Pedersen, 2003). A 100‐m long stream reach was surveyed and macrophyte recordings were made in approximately 250 plots (25 × 25 cm) adjacently placed along 10 cross‐sectional transects in each reach. A cover score was allocated to each species present in the plots using an abundance scale: 1 = 1–5%, 2 = 6–25%, 3 = 26–50%, 4 = 51–75%, 5 = 76–100%. Species abundance was then calculated as the sum of cover scores to the maximum score sum (i.e. the number of plots multiplied by the maximal score of five). Water depth was measured to the nearest centimetre at a fixed point in each of the plots and the mean depth was calculated from these recordings. The dominant substratum type was categorized as stone (>60 mm diameter), gravel (3–60 mm), coarse sand (1–3 mm), fine sand (0.25–1 mm), silt (<0.25 mm), hard clay or peat in each plot. The frequency of the various substratum types was calculated from these recordings. Stream width was measured at each transect and averaged to give the mean width of the stream reach. Stream water alkalinity was determined by potentiometric end‐point titration with HCL on 100 ml samples of stream water (Hutchinson, 1957). The macrophyte, depth and substratum data were further processed into broader categories to enable comparison with the STAR dataset that were sampled using the AQEM/STAR site protocol and the MTR protocol (available at http://www.eu-star.at under ‘Protocols’). This approach was also used previously with reliable results (Baattrup‐Pedersen et al., 2004).
Finally, historic data were integrated in the analysis for 27 Danish stream localities distributed throughout Denmark (Fig. 1; Riis & Sand‐Jensen, 2001). These data were based on published literature, excursion reports and herbarium specimens kept in the Botanical Museum, Copenhagen and cover the period 1876–1920.
Data analysis
A total of 184 macrophyte species were recorded (160 vascular species and 24 mosses, liverworts and macroalgae), of which 73 species were submerged and amphibious species according to previous classifications (Riis, Sand‐Jensen & Vestergaard, 2000; Szoszkiewicz et al., 2006). The taxonomic resolution of macroalgae, liverworts and mosses varied among regions except for the common water moss, Fontinalis antipyretica Hedw., and were excluded from the analyses.
Detrended correspondence analysis (DCA) on both species abundance and presence/absence data were used to determine multivariate patterns in macrophyte community structure. Plots with less than three species and species occurring in less than three plots were excluded leaving 63 plots and 69 species for further analyses. DCA was run with nonlinear rescaling, detrending by 26 segments and without down‐weighting of rare species. In the final ordination, we used presence/absence data because no additional information could be retrieved using abundance data. Analysis of similarity (anosim) was used to test the significance for among‐country differences in macrophyte assemblages.
The species scores for the first three DCA axes were used in an agglomerative hierarchical clustering of the species to obtain groups of species occurring under similar environmental conditions. We used the Sørensen distance measure with the flexible beta linkage method at beta = −0.25. The frequency of species allocated to the various clusters was calculated for each site as the proportion of species belonging to each of the clusters to the total number of species belonging to the clusters.
Detrended correspondence analysis species scores for the first three axes were used to predict site scores for 27 historic stream records (c. 1900) (Riis & Sand‐Jensen, 2001). These scores were only calculated for sites with at least three species co‐occurring in the historic and contemporary dataset (23 sites). In total we used species scores from 15 of the 36 recorded species in the historic dataset (see Table 1). Species which were not present in the contemporary dataset included several Potamogeton species (e.g. P. acutifolius Link, P. densus L., P. filiformis Pers., P. frisii Rupr., P. lucens, P. nodosus Poiret, P. obtusifolius Mert. & Koch, P. praelongus, P. polygonifolius, P. pusillus L., P. zosterifolius Schum.) as well as Ceratophyllum demersum L., Luronium natans (L.) Rafin., Oenanthe fluviatilis (Bab.) Coleman, Myriophyllum verticellatum L., Stratiotes aloides L.and Zannichellia sp. Furthermore, a total of 11 Potamogeton hybrids were not included in contemporary data.
Table 1.
Co‐occurring species in the contemporary and historic datasets used for the calculation of site scores for 27 historic stream records from Danish stream sites around 1900 (Riis & Sand‐Jensen, 2001)
| Co‐occurring species in the historic and contemporary dataset |
|---|
| Alisma plantago‐aquatica L. |
| Ranunculus ssp. |
| Berula erecta (Hudson) |
| Butomus umbellatus L. |
| Elodea canadensis L. C. Rich. |
| Myriophyllum alterniflorum DC. |
| Myriophyllum spicatum L. |
| Nuphar lutea (L.) Sm. |
| Potamogeton alpinus Balbis |
| Potamogeton crispus L. |
| Potamogeton natans L. |
| Potamogeton pectinatus L. |
| Potamogeton perfoliatus L. |
| Saggittaria saggitifolia L. |
| Sparganium emersum Rehman |
Site scores were calculated for sites with at least three co‐occurring species.
Tree‐based classification models (CART) were performed to explore relations between macrophyte assemblage patterns and physical stream characteristics. The CART models gave classifications rules that separated groups based on stream site characteristics. The analyses were performed for the individual groups separately using cluster occurrence (>30%) as response and physical stream site characteristics as predictor variables. In addition, we performed Spearman rank correlation analyses on all sites where environmental variables were available.
Results
Stream site typology and characteristics
The stream sites were, except for those in Lithuania, previously classified as mid‐sized lowland streams based on altitude and size typology (Hering et al., 2004; Baattrup‐Pedersen et al., 2006). Eight of the Lithuanian stream sites could also be classified as mid‐sized lowland streams, but two sites were situated in catchments slightly below (Skroblus catchment size, 77.8 km2) or above (Dubysa catchment size, 1532 km2) the boundaries for mid‐sized streams (e.g. 100–1000 km2).
Stream characteristics varied within and among regions, but overlapped regarding both reach (altitude, distance to source and slope) and site (width, depth and substratum) characteristics (Table 2). In particular, altitude (4.4–215 m a.s.l.) and distance of stream sites to source (2.0–238 m), varied among the Swedish streams, whereas stream slope varied among the Danish streams (0.24–34 m km−1). All width, depth and substratum categories were represented in all stream groups. The Danish stream sites were deeper than stream sites in Latvia, Lithuania, Poland and Sweden (WA depth; t‐test with Bonferroni correction, P < 0.05), but they were not wider (WA width; t‐test with Bonferroni correction, P > 0.05). Coverage of the different substratum types did not differ significantly among the Danish stream sites and stream sites in other regions (WA substratum; t‐test with Bonferroni correction, P > 0.05).
Table 2.
Site characteristics (minimum and maximum) and mean (± SD) values for width, depth and substratum for streams in Denmark (D), Germany (G), Poland (P), Latvia (La), Lithuania (Li) and Sweden (S)
| D (n = 17) | G (n = 4) | P (n = 7) | La (n = 22) | Li (n = 10) | S (n = 3) | |
|---|---|---|---|---|---|---|
| Site characteristics | ||||||
| Latitude (°N) | 55.1–56.8 | 52.9–60.6 | 52.7–57.0 | 56.6–57.5 | 54.1–56.0 | 57.6–60.3 |
| Longitude (°E) | 8.5–9.8 | 10.1–17.3 | 15.9–26.0 | 21.8–27.3 | 21.1–24.3 | 14.8–18.2 |
| Altitude (m a.s.l.) | 5.3–60.0 | 45.0–250.0 | 80.0–165.0 | 10.0–183.0 | 3.1–113.7 | 4.4–215.3 |
| Distance to source (km) | 9.0–75 | 10–33 | 16–62 | 3.0–48 | 15–144 | 2.0–238 |
| Slope (m km−1) | 0.24–34 | 0.7–9.0 | 0.3–5.0 | 0.2–8.2 | 0.1–10 | 1.6–5.5 |
| Stream width | ||||||
| <1 m | 0 | 0 | 41 ± 42.6 | 20 ± 33.6 | 0 | 1.0 ± 1.7 |
| 1–5 m | 41 ± 45.8 | 25 ± 50 | 21 ± 20.4 | 34 ± 38.2 | 6.0 ± 19.0 | 81 ± 30.9 |
| 5–10 m | 47 ± 44.9 | 75 ± 49.8 | 23 ± 29.8 | 34 ± 42.8 | 54 ± 40.9 | 18 ± 31.8 |
| 10–20 m | 12 ± 31.5 | 0.25 ± 0.5 | 13 ± 26.3 | 12 ± 31.2 | 36 ± 38.7 | 0 |
| >20 m | 0 | 0 | 1.4 ± 3.8 | 0 | 3.3 ± 10.4 | 0 |
| Weighted average* | 2.9 ± 0.64 | 2.8 ± 0.50 | 2.1 ± 1.06 | 2.3 ± 0.90 | 3.3 ± 0.52 | 2.2 ± 0.33 |
| Stream depth | ||||||
| <0.25 m | 12.4 ± 22.9 | 19.8 ± 33.6 | 55.0 ± 29.9 | 38.0 ± 23.4 | 31.2 ± 16.7 | 61.7 ± 44.8 |
| 0.25–0.5 m | 18.9 ± 15.3 | 20.3 ± 17.6 | 40.0 ± 26.3 | 43.9 ± 20.5 | 34.7 ± 9.9 | 28.3 ± 27.5 |
| 0.5–1.0 m | 51.3 ± 21.8 | 41.8 ± 33.0 | 5.0 ± 11.2 | 17.5 ± 20.6 | 30.5 ± 17.1 | 10.0 ± 17.3 |
| >1.0 m | 17.4 ± 22.9 | 18.25 ± 28.4 | 0 | 0.7 ± 2.3 | 3.6 ± 4.7 | 0 |
| Weighted average* | 2.74 ± 0.59 | 2.59 ± 0.96 | 1.5 ± 0.37 | 1.81 ± 0.41 | 2.07 ± 0.41 | 1.48 ± 0.62 |
| Stream substratum | ||||||
| Bedrock | 0 | 0 | 9.3 ± 14.6 | 0 | 0 | 15 ± 25.7 |
| Boulders/cobbles | 5.6 ± 15.8 | 0 | 23 ± 16.8 | 16 ± 21.2 | 19 ± 15.5 | 25 ± 21.8 |
| Pebbles/gravel | 12 ± 12.9 | 13 ± 25.0 | 34 ± 28.2 | 28 ± 29.6 | 20 ± 12.2 | 36 ± 19.3 |
| Sand | 57 ± 27.5 | 66 ± .4.3 | 11 ± 9.4 | 44 ± 38.9 | 43 ± 21.4 | 13 ± 14.4 |
| Silt | 17 ± 21.3 | 21 ± 21.7 | 7.1 ± 9.5 | 6.2 ± 19.1 | 2.3 ± 3.9 | 7.0 ± 11.3 |
| Clay | 0.5 ± 1.1 | 0.8 ± 1.0 | 16 ± 29.4 | 0.23 ± 1.1 | 0.8 ± 1.4 | 3.3 ± 5.8 |
| Silt over other substrata | 0.2 ± 0.7 | 0 | 0 | 0 | 2.6 ± 2.6 | 0 |
| Weighted average | 4.0 ± 0.6 | 4.1 ± 0.3 | 3.3 ± 1.2 | 3.3 ± 0.8 | 3.5 ± 0.5 | 2.7 ± 1.1 |
Numbers in parentheses show the number of study streams.
*Indicates significant differences among means.
Macrophyte assemblage structure
Eigen values of the first three DCA axes were 0.376, 0.218 and 0.172, respectively (Fig. 2). Gradient length of the first DCA axis was 3.57, indicating that a unimodal model would best fit the data. The first and second DCA axes represented a geographical separation of stream sites, with sites in Denmark (DCA1 axis scores ranged from 203 to 298), clearly separated from sites in Sweden (DCA1: 0–106), Poland (DCA1: 160–203), Latvia (DCA1: 49–168) and Lithuania (DCA1: 100–198), whereas overlap existed with sites in Germany (DCA1: 177–219). Particularly DCA1 correlated significantly with several stream characteristics (P < 0.05). The most important correlations (r > 0.4) were found between DCA1 and depth (WA depth, r = 0.58), DCA1 and substratum (WA substratum, r = 0.48) and DCA1 and altitude (r = −0.43). DCA 2 correlated significantly with latitude (r = 0.25) and DCA 3 correlated significantly with width (WA width r = 0.36) and depth (WA depth r = 0.25).
Figure 2.
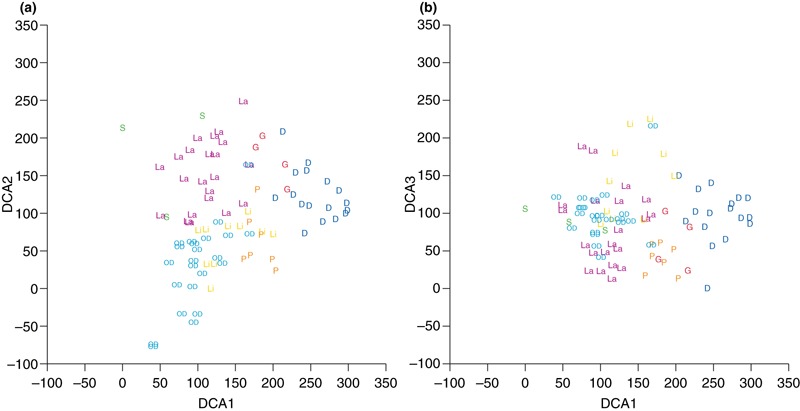
Scatter plots showing DCA1 versus DCA2 (a) and DCA1 versus DCA3 (b) based on presence‐absence data of macrophytes species from 63 mid‐sized lowland streams in the Central and Eastern Lowland ecoregions of Europe and projection of predicted DCA site scores for 23 historic mid‐sized stream sites distributed throughout Denmark based on the co‐occurrence of species in the historic and contemporary dataset (see Fig. 1). The historic records cover the period 1876‐1920, i.e. before the time period of significant human impact in Danish streams. Abbreviation as follows: D, Denmark; G, Germany; P, Poland; La, Latvia; Li, Lithuania; S, Sweden; OD, old Danish records.
Bray‐Curtis pair‐wise similarity tests also revealed geographical patterns in assemblage composition (Table 3; anosim, P < 0.05). Overall, similarity varied from 12.2% (Germany – Sweden) to 50.8% (Denmark – Denmark). Within country similarity was generally higher than among countries similarities (Table 3). Sites in Poland and Lithuania exhibited high similarities with the Danish sites (average 35.0% and 28. 9%, respectively), whereas sites in Sweden exhibited very low similarity (13.0%).
Table 3.
Average (±SD) of Bray‐Curtis similarity within and between macrophyte assemblages in the different regions
| D (n = 17) | G (n = 4) | P (n = 7) | La (n = 22) | Li (n = 10) | S (n = 3) | |
|---|---|---|---|---|---|---|
| D | 50.8 (16.7) | |||||
| G | 27.7 (12.0) | 43.6 (14.2) | ||||
| P | 35.0 (11.4) | 29.0 (13.6) | 44.9 (14.6) | |||
| La | 25.6 (11.3) | 29.1 (13.2) | 27.7 (12.1) | 38.1 (14.9) | ||
| Li | 28.9 (10.8) | 20.5 (12.9) | 32.9 (14.2) | 29.5 (14.2) | 39.4 (13.6) | |
| S | 13.0 (10.6) | 12.2 (10.9) | 16.6 (15.1) | 24.2 (15.0) | 15.2 (14.7) | 33.6 (14.6) |
See Table 2 for country abbreviations.
The historic records from 23 Danish stream sites were classified as mid‐sized lowland streams according to altitude and size typology (Riis & Sand‐Jensen, 2001). The predicted DCA1 (average 99, range 41–168), DCA2 (average 30, range −77–164) and DCA3 (average 98, range 4–215) site scores overlapped, in particular with site scores from streams in Latvia and Lithuania (Fig. 2) and to a lesser degree with site scores from Poland, whereas no overlap occurred with the contemporary Danish streams sites (Fig. 2).
Species groups and regional occurrence
Cluster analysis revealed seven clusters. However, three groups were represented by only two to four species, namely, group 2 (Poa sp., Polygonum amphidium L.), group 6 (Potamogeton praelongus, Carex hirta L., Juncus bulbosus L., Poa pratensis L.) and group 7 (Alisma plantago‐aquatica L., Veronica beccabunga L., Lysimachia thyrsiflora L.) (Table 4). These three groups were excluded from further analysis. Group 1 was species rich (25 species), consisting primarily of amphibious (n = 5) and terrestrial (n = 18) species which are able to grow emergent within streams (Table 4). The terrestrial species included both tall monocots and smaller dicots. The most common species in group 1 were associated with the land–water ecotone, namely, Phalaris arundinacea L. (found in 57% of the streams sites), Myosotis spp. (51%), Berula erecta (Hudson) (40%) and Glyceria maxima (Hartman) (29%). Group 3 was predominated by primarily submerged species such as Sparganium emersum Rehman (83%), Elodea canadensis L. C. Rich (54%), Callitriche sp. (44%) and Ranunculus sp. (43%). Two amphibious species (S. erectum L., 59% and Veronica anagallis‐aquatica L., 49%) were also important species within this group, whereas terrestrial species had a more scattered occurrence. Group 4 was predominated by amphibious and terrestrial species, but submerged species, such as P. perfoliatus L. and Myriophyllum spicatum L., were also present. Similar to group 1, the most common species in group 4 (i.e. Mentha aquatica L., 35%, Equisetum fluviatile L., 25% and Iris pseudocaris L., 19%) were associated with the land–water ecotone. Group 5 consisted of floating‐leaved, submerged as well as terrestrial species, with Fontinalis antipyretica (41%), Nuphear lutea (L.) Sm. (30%), Scirpus sylvaticus L. (17%) and P. alpinus (16%) being the most abundant species (Table 4).
Table 4.
Species list for each species group delineated using agglomerative hierarchical clustering of DCA1‐3 species scores
| Group | Species |
|---|---|
| 1 | Phalaris arundinacea L., Myosotis spp., Berulaerecta (Hudson),Glyceriafluitans (L.) R. Br.,Glyceriamaxima (Hartman),Agrostisstolonifera L.,Potamogetonnatans L.,Equisetumpalustre L.,Ranunculusrepens L.,Epilobiumhirsutum L.,Juncuseffuses L.,Poatrivialis L.,Galiumpalustre L.,Carexacuta L.,Filipendulaulmaria (L.) Maxim., Epilobiumparviflorum Schreber,Myriophyllumalterniflorum DC,Nasturtium sp., Stellariapalustris Retz., Lotus pedunculatus Cav., Deschampsia caespitose (L.) Beauv., Lysimachia vulgaris L., Mentha verticillata L., Juncus articulatus L., Montia sp. |
| 2 | Poa sp., Polygonum amphibium L. |
| 3 | Sparganium emersum Rehman,Lemnaminor L.,Sparganiumerectum L.,Elodeacanadensis L. C. Rich.,Veronicaanagallis‐aquatica L.,Callitriche spp., Ranunculus spp., Carex rostrata Stokes, Potentilla palustris (L.), Menyanthes trifoliata L. |
| 4 | Menthaaquatica L.,Equisetumfluviatile L.,Lemnatrisulca L.,Irispseudocaris L.,Solanumdulcamara L.,Rorippaamphibian (L.) Besser,Potamogetonperfoliatus L.,Rumexhydrolapathum Hudson,Sagittariasagittifolia L., Lycopus europaeus L., Sium latifolium L., Stachys palustris L., Hydrocharis morsus‐ranae L., Myriophyllum spicatum L., Caltha palustris L., Potamogeton pectinatus L. |
| 5 | Fontinalis antipyretica Hedw., Nupharlutea (L.) Sm., Scirpuslacustris L., Potamogetonalpinus Balbis,Scirpussylvaticus L., Carexacutiformis Ehrh., Butomusumbellates L., Potamogeton crispus L. |
| 6 | Potamogeton praelongus Wulf., Carex hirta L., Juncus bulbosus L., Poa pratensis L. |
| 7 | Alismaplantago‐aquatica L.,Veronicabeccabunga L., Lysimachia thyrsiflora L. |
Species in bold were found in at least 10% of the stream sites.
Species are presented in order of frequency from high to low per species group.
Species belonging to all groups were present in streams in all regions (Table 5). In particular, species from group 3 were widespread and occurred in all of the stream sites (Table 5). Group 1 and group 3 species often co‐occurred (species frequency; r = 0.61, P < 0.05). Marked differences were noted in species abundances in groups among regions (Table 6). For example, group 1 species were abundant in Danish stream sites, group 3 species in the Danish, Latvian and Lithuanian sites, group 4 species in the Polish sites and group 5 species in the Polish, Latvian and Lithuanian sites. Mainly groups 1 and 3 had high (>60%) and groups 4 and 5 had low (up to 40–60%) species abundances (Table 6). The predicted site scores for the historic records were closely associated with groups 4 and 5 (Fig. 3).
Table 5.
Number of sites from different regions with one or more species present from the different groups
| Group | D (n = 17) | G (n = 4) | P (n = 7) | La (n = 22) | Li (n = 10) | S (n = 3) |
|---|---|---|---|---|---|---|
| 1 | 17 | 3 | 7 | 15 | 10 | 2 |
| 3 | 17 | 4 | 7 | 22 | 10 | 3 |
| 4 | 14 | 2 | 7 | 15 | 6 | 1 |
| 5 | 7 | 2 | 7 | 17 | 10 | 2 |
The total number of sites included in the analysis within each region are given in parentheses.
See Table 2 for country abbreviations.
Table 6.
Frequency class calculated as the number of species belonging to a given group to the total number of species within the group for stream sites in each region
| Group | Frequency class (%) | D (n = 17) | G (n = 4) | P (n = 7) | La (n = 22) | Li (n = 10) | S (n = 3) |
|---|---|---|---|---|---|---|---|
| C1 | 0 | 0 | 1 | 0 | 7 | 0 | 1 |
| >0–20 | 4 | 3 | 4 | 15 | 8 | 2 | |
| 20–40 | 3 | 0 | 3 | 0 | 2 | 0 | |
| 40–60 | 3 | 0 | 0 | 0 | 0 | 0 | |
| >60 | 7 | 0 | 0 | 0 | 0 | 0 | |
| C3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| >0–20 | 1 | 0 | 1 | 2 | 1 | 1 | |
| 20–40 | 1 | 2 | 3 | 9 | 4 | 1 | |
| 40–60 | 1 | 2 | 2 | 8 | 4 | 1 | |
| >60 | 14 | 0 | 1 | 3 | 1 | 0 | |
| C4 | 0 | 3 | 2 | 0 | 7 | 4 | 2 |
| >0–20 | 11 | 2 | 2 | 14 | 4 | 1 | |
| 20–40 | 3 | 0 | 1 | 1 | 1 | 0 | |
| 40–60 | 0 | 0 | 3 | 0 | 1 | 0 | |
| >60 | 0 | 0 | 1 | 0 | 0 | 0 | |
| C5 | 0 | 10 | 2 | 0 | 5 | 0 | 1 |
| >0–20 | 2 | 2 | 3 | 5 | 3 | 1 | |
| 20–40 | 5 | 0 | 4 | 11 | 3 | 1 | |
| 40–60 | 0 | 0 | 0 | 0 | 3 | 0 | |
| >60 | 0 | 0 | 0 | 1 | 1 | 0 |
The most frequent class is marked in bold. The number of sites is given in parenthesis.
See Table 2 for country abbreviations.
Figure 3.
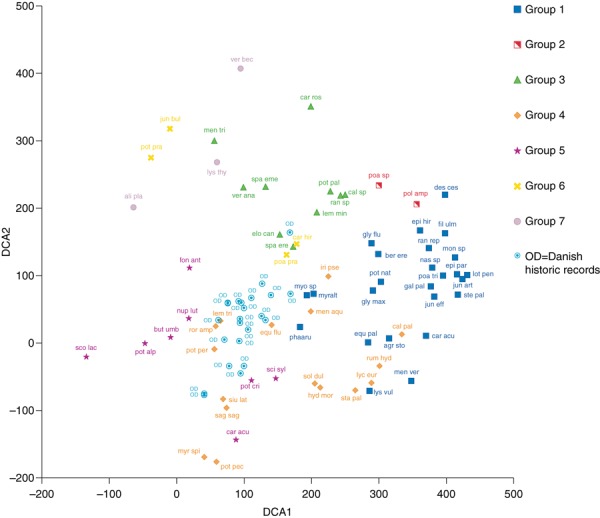
Scatter plot of DCA axes 1 and 2 of species scores based on presence‐absence data of 69 macrophyte species from 63 mid‐sized lowland streams in the Central and Eastern Lowland ecoregions of Europe and predicted site scores for the historic data (see legend to Fig. 2). Species groups, delineated using agglomerative cluster analysis, are shown with different symbols. Abbreviation as follows: Group 1: pha_aru: Phalaris arundinacea, myo_sp.: Myosotis spp., ber_ere: Berula erecta, gly_flu: Glyceria fluitans, gly_max: Glyceria maxima, agr_sto: Agrostis stolonifera, pot_nat: Potamogeton natans, equ_pal: Equisetum palustre, ran_rep: Ranunculus repens, epi_hir: Epilobium hirsutum, jun_eff: Juncus effuses, poa_tri: Poa trivialis, gal_pal: Galium palustre, car_acu: Carex acuta, fil_ulm: Filipendula ulmaria, epi_par: Epilobium parviflorum, myr_alt: Myriophyllum alterniflorum, nas_sp.: Nasturtium sp., ste_pal: Stellaria palustris., lot_ped: Lotus pedunculatus, des_cae: Deschampsia caespitose, lys_vul: Lysimachia vulgaris, men_ver: Mentha verticillata, jun_art: Juncus articulatus, mon_sp.: Montia sp. Group 2: Poa_sp.: Poa sp., pol_amp: Polygonum amphibium. Group 3: Spa_eme: Sparganium emersum, lem_min: Lemna minor, spa_ere: Sparganium erectum., elo_can: Elodea canadensis., ver_ana: Veronica anagallis‐aquatica, call_sp.: Callitriche spp., ran_sp.: Ranunculus spp., car_ros: Carex rostrata, pot_pal: Potentilla palustris, men_tri: Menyanthes trifoliata. Group 4: Men_aqu: Mentha aquatica, equ_flu: Equisetum fluviatile, lem_tri: Lemna trisulca, iri_pse: Iris pseudocaris, sol_cul: Solanum dulcamara, ror_amp: Rorippa amphibian, pot_per: Potamogeton perfoliatus, rum_hyd: Rumex hydrolapathum, sag_sag: Sagittaria sagittifolia, lyc_eur: Lycopus europaeus, siu_lat: Sium latifolium, sta_pal: Stachys palustris, hyd_mor: Hydrocharis morsus‐ranae, myr_spi: Myriophyllum spicatum, cal_pal: Caltha palustris, pot_pec: Potamogeton pectinatus. Group 5: Fon_ant: Fontinalis antipyretica, nup_lut: Nuphar lutea, sci_lac: Scirpus lacustris, pot_alp: Potamogeton alpinus, sci_syl: Scirpus sylvaticus, car_acu: Carex acutiformis, but_umb: Butomus umbellates, pot_cri: Potamogeton crispus. Group 6: Pot_pra: Potamogeton praelongus, car_hir: Carex hirta, jun_bul: Juncus bulbosus, poa_pra: Poa pratensis. Group 7: Ali_pla: Alisma plantago‐aquatica, ver_bec: Veronica beccabunga, lys_thy: Lysimachia thyrsiflora.
Clusters and stream site characteristics
Among stream variability in assemblage composition was not related to variability in longitude, latitude, altitude, slope, distance to source or weighted averages of depth, substratum and width (i.e. no significant solutions were achieved in the tree constructions using these variables, P > 0.05). Correlation confirmed these analyses; few strong correlations (r > 0.4) between cluster abundance and the eight environmental variables were significant. Species abundances of group 1 and 3 were negatively correlated with longitude (r = −0.63 and r = −0.44, respectively) and positively correlated with stream depth (WA depth, r = 0.46 and r = 0.49, respectively). Groups 3 abundance was also correlated with substratum fineness (WA substratum, r = 0.47).
Discussion
Reference sites in the Baltic region and Poland
Contemporary stream vegetation in mid‐sized streams in Denmark shared similarities with similar‐sized streams in Poland, Latvia and Lithuania (26–35%). However, more importantly we found from species‐based predictions even higher similarity before intense human land use commenced in Denmark around 1900. The historic stream sites resembled the contemporary sites from principally Latvia and Lithuania. Therefore stream sites within these countries can be considered in a Danish reference network.
The species‐based prediction approach used here likely provides the best estimation of the similarity of the contemporary stream vegetation in the Baltic region and Poland with the historic vegetation in Denmark. First, predictions were based on the co‐occurrence of several species as opposed to comparisons based on presence/absence of individual species. Interpretation of the historic data is thus less affected by low and variable sampling effort and different sampling methodologies compared to those used today. Both differences in sample effort and methodology are often considered to limit the use of historic data. Second, species used in predictions comprised both disturbance‐insensitive species that are currently widely distributed (e.g. E. canadensis, S. emersum and B. erecta) and disturbance‐sensitive species that have undergone marked declines (e.g. P. alpinus, Butomus umbellatus L. and A. plantago‐aquatica: Riis & Sand‐Jensen, 2001; Baattrup‐Pedersen et al., 2004). Representation of species covering different sensitivities to disturbance means that the predictions are less prone to distortion compared to predictions based solely on the presence of disturbance‐sensitive species.
Delineation of reference assemblages
We identified four macrophyte groups (e.g. groups 1, 3, 4 and 5) in the Central and Eastern Lowland ecoregions (ecoregions 14 and 15, respectively, according to Illies, 1978) that were continuously distributed within the region. These groups can be regarded as subgroupings to the lowland stream reference community previously identified (C6 – see Baattrup‐Pedersen et al., 2006). We were unable to discriminate among‐group variability in macrophyte assemblages using physical stream site and size‐related variables. But we found a clear geographical pattern in species composition, in particular for groups 1 and 5. Group 1 sites were more abundant in the most western parts of the region, which included western Jutland, an area that remained ice‐free during the last glaciation, whereas group 5 sites were most abundant in the eastern lowlands of the Baltic region and Poland.
Many species found in group 1 were small amphibious and terrestrial, i.e. species typically found occurring in streams with well developed and gradual transitional zones between water and land (Mountford & Chapman, 1993; Kemp, Harper & & Crosa, 1999; Pedersen et al., 2006). This zone is particularly well developed in highly mobile streams located in regions with glacio‐fluvial outwash plains and sandy soils which are typical of western Denmark. We found that the group 1 assemblage was similar to the Myriophyllum‐community described by Riis et al. (2000), with the concurrent presence of Glyceria fluitans (L.) R. Br., P. natans L. and Myriophyllum alterniflorum DC. The Myriophyllum‐community has been found to be closely associated with low alkalinity in Danish streams (Riis et al., 2000). Earlier studies have shown how differences in physiology and use of bicarbonate and/or carbon dioxide in photosynthesis affect the distribution of macrophytes in streams (Riis et al., 2000; Dodkins, Rippey & Hale, 2005; Feijoo & Lombardo, 2007). We suggest that the decline in the abundance of group 1 species from the western part of Denmark to the eastern parts in Latvia and Lithuania is associated with changes in stream water alkalinity. Alkalinity of streams situated in western Jutland has previously been found to be lower (mean 0.7 mmol L−1) than streams in the eastern part of Jutland (1.7 mmol L−1) and on the Isles of Funen and Seeland (4.9 mmol L−1 and 3.6 mmol L−1, respectively) (Riis, unpubl. data). By comparison, the mean alkalinity of the Latvian (3.9 mmol L−1, Springe, unpubl. data) and Lithuanian (3.3 mmol L−1) streams were similar to streams on the Isles of Funen and Seeland.
The most abundant species in group 5, F. antipyretica, is particularly abundant in stream sites that are unimpacted by hydromorphological degradation (O’Hare et al., 2006). Most of the species that belong to this group can also be found in contemporary Danish stream vegetation. However, many of these species have declined, i.e. M. spicatum, P. alpinus and B. umbellatus (Riis & Sand‐Jensen, 2002; Baattrup‐Pedersen et al., 2004) and are now often only abundant locally but not regionally. These distribution patterns have been attributed to habitat degradation (eutrophication and hydromorphological impairment) leading to spatial restrictions, and future projections indicate further declines if habitats continue to be become impaired (Riis & Sand‐Jensen, 2002). The decline in the abundance of group 5 species from Poland and the Baltic region to Denmark is therefore likely associated with differences in the intensity of human impacts on the stream integrity, a finding that is also supported by the close association of this group with the historic data (discussed below).
Guiding image for reference assemblages in Denmark
Our study showed that analysing macrophyte assemblages in relatively undisturbed sites with those described historically in Denmark was very useful for obtaining a more complete understanding of reference assemblages in Danish streams. In particular, we were able to identify groups 4 and 5 that were closely associated with the species‐predicted site scores of the historic records. These groups could therefore contribute to the guiding image for the identification of potential reference sites within Denmark. Furthermore, we propose that this assemblage‐based approach can be a valuable supplement to other approaches, such as the use screening criteria for pressures or impacts (CIS 2.3 REFCOND) or the use of the best available sites as surrogates for the true reference condition. Thus by integrating macrophyte assemblages in the screening for potential reference sites, the weaknesses associated with the use of thresholds for pressures or impacts that are poorly documented is reduced. In addition, a combined approach probably also gives the most reliable description of the reference assemblages. Many species that were formerly widespread are now found in marginal habitats (Riis & Sand‐Jensen, 2002; Baattrup‐Pedersen et al., 2004) and their dispersal within and among stream systems is most likely restricted. Therefore, these species may not be present locally in reaches that have suitable environmental conditions and fulfil screening thresholds simply because of regional declines. Neglecting these species would result in a distorted description of reference assemblages, thereby affecting stream assessments.
Acknowledgments
This work was in part supported by the Danish Forest and Nature Agency. We greatly appreciate the efforts of the researchers in the framework of the EU project STAR in collecting and providing a subset of the data used. We also thank Klaus B. Fries and Nicholas John Bell for field assistance.
References
- Baattrup‐Pedersen A. & Riis T. (1999) Macrophyte diversity and composition in relation to substratum characteristics in regulated and unregulated Danish streams. Freshwater Biology, 42, 375–385. [Google Scholar]
- Baattrup‐Pedersen A., Larsen S.E. & Riis T. (2002) Long‐term effects of stream management on plant communities in two Danish lowland streams. Hydrobiologia, 481, 33–45. [Google Scholar]
- Baattrup‐Pedersen A., Szoszkiewicz K., Nijboer R., O’Hare M. & Ferreira T. (2006) Macrophyte communities in unimpacted European streams: variability in assemblage patterns, abundance and diversity. Hydrobiologia, 566, 179–196. [Google Scholar]
- Baattrup‐Pedersen A., Friberg N., Pedersen M.L., Skriver J., Kronvang B. & Larsen S.E. (2004) Anvendelse af Vandrammedirektivet i danske vandløb (in Danish). Danmarks Miljøundersøgelser. Faglig rapport fra DMU 499. 145 s. Available at: http://www2.dmu.dk/1_viden/2_Publikationer/3_fagrapporter/rapporter/FR499.PDF. (Last accessed on 25 March 2008) [Google Scholar]
- Baggøe J. & Ravn F.K. (1896) Excursioner til jyske søer og vandløb i sommeren 1895 (in Danish). Botanisk Tidskrift, 20, 288–326. [Google Scholar]
- Bailey R.C., Norris R.H. & Reynoldson T.B. (2004) Bioassessment of Freshwater Ecosystems Using the Reference Condition Approach. Kluwer Academic Publishers, Boston. [Google Scholar]
- Birk S., Korte T. & Hering D. (2006) Intercalibration of assessment methods for macrophytes in lowland streams: direct comparison and analysis of common metrics. Hydrobiologia, 566, 417–430. [Google Scholar]
- Chaves M.L., Costa J.L., Chainho P., Costa M.J. & Prat N. (2006) Selection and validation of reference sites in small river basins. Hydrobiologia, 573, 133–154. [Google Scholar]
- Daniel H., Bernez I. & Haury J. (2006) Relationships between macrophytic vegetation and physical features of river habitats: the need for a morphological approach. Hydrobiologia, 570, 11–17. [Google Scholar]
- Dodkins I., Rippey B. & Hale P. (2005) An application of canonical correspondence analysis for developing ecological quality assessment metrics for river macrophytes. Freshwater Biology, 50, 891–904. [Google Scholar]
- European Commission (2000) Directive 2000/60/EC of the European Parliament and of the Council – Establishing a Framework for Community action in the Field of Water Policy. Brussels, Belgium. [Google Scholar]
- Feijoo C. & Lombardo R.J. (2007) Baseline water quality and macrophyte assemblages in Pampean streams: A regional approach. Water Research, 41, 1399–1410. [DOI] [PubMed] [Google Scholar]
- Fore L.S., Karr J.R. & Wisseman R.W. (1996) Assessing invertebrate responses to human activities: evaluating alternative approaches. Journal of the North American Benthological Society, 15, 212–231. [Google Scholar]
- Furse M., Hering D., Moog O. et al. (2006) The STAR project: context, objectives and approaches. Hydrobiologia, 566, 3–29. [Google Scholar]
- Hering D., Moog O., Sandin L. & Verdonschot P.F.M. (2004) Overview and application of the AQEM assessment system. Hydrobiologia, 516, 1–20. [Google Scholar]
- Hering D., Johnson R.K., Kramm S., Schmutz S., Szoszkiewicz K. & Verdonschot P.F.M. (2006) Assessment of European streams with diatoms, macrophytes, macroinvertebrates and fish: a comparative metric‐based analysis of organism response to stress. Freshwater Biology, 51, 1757–1785. [Google Scholar]
- Hering D., Buffagni A., Moog O. et al. (2003) The development of a system to assess the ecological quality of streams based on macroinvertebrates – design of the sampling programme within the AQEM project. International Review of Hydrobiology, 88, 345–361. [Google Scholar]
- Hutchinson G.E. (1957) A Treatise on Limnology, Vol. 1 Wiley and Sons, New York. [Google Scholar]
- Illies J. (1978) Limnofauna Europaea. Gustav Fischer Verlag, Stuttgart. [Google Scholar]
- Johnson R.K., Furse M.T., Hering D. & Sandin L. (2007) Ecological relationships between stream communities and spatial scale: implications for designing catchment‐level monitoring programmes. Freshwater Biology, 52, 939–958. [Google Scholar]
- Kemp J.L., Harper D.M. & & Crosa G. (1999) Use of functional habitats to link ecology with morphology and hydrology in river rehabilitation. Aquatic Conservation: Marine and Freshwater Ecosystems, 9, 159–178. [Google Scholar]
- Mountford J.O. (1994) Floristic change in English grazing marshes: the impact of 150 years of drainage and land‐use change. Watsonia, 20, 3–24. [Google Scholar]
- Mountford J.O. & Chapman J.M. (1993) Water regime requirements of British wetland vegetation: using the moisture classifications of Ellenberg and Londo. Journal of Environmental Management, 38, 275–288. [Google Scholar]
- Nijboer R.C., Johnson R.K., Verdonschot P.F.M., Sommerhauser M. & Buffagni A. (2004) Establishing reference conditions for European streams. Hydrobiologia, 516, 91–105. [Google Scholar]
- O’Hare M.T., Baattrup‐Pedersen A., Nijboer R., Szoszkiewicz K. & Ferreira T. (2006) Macrophyte communities of European streams with altered physical habitat. Hydrobiologia, 566, 197–210. [Google Scholar]
- Pedersen M.L. & Baattrup‐Pedersen A. (2003) Økologisk overvågning i vandløb og på vandløbsnære arealer under NOVANA 2004‐2009 (in Danish). Danmarks Miljøundersøgelser. Teknisk anvisning fra DMU 21: 128 s. Available at: http://www2.dmu.dk/1_viden/2_Publikationer/3_tekanvisning/rapporter/TA21.pdf. (Last accessed on 25 March 2008) [Google Scholar]
- Pedersen T.C.M., Baattrup‐Pedersen A. & Madsen T.V. (2006) Effects of stream restoration and management on plant communities in lowland streams. Freshwater Biology, 51, 161–179. [Google Scholar]
- Preston C.D. (1995) Pondweeds of Great Britain and Ireland. Botanical Society of the British Isles, Handbook No. 8. London. [Google Scholar]
- Raunkiær C. (1895-99) De danske blomsterplanters naturhistorie Enkimbladede (in Danish). Gyldendal, Copenhagen. [Google Scholar]
- Riis T. & Sand‐Jensen K. (2001) Historical changes in species composition and richness accompanying perturbation and eutrophication of Danish lowland streams over 100 years. Freshwater Biology, 46, 269–280. [Google Scholar]
- Riis T. & Sand‐Jensen K. (2002) Abundance‐range size relationships in stream vegetation in Denmark. Plant Ecology, 161, 175–183. [Google Scholar]
- Riis T., Sand‐Jensen K. & Vestergaard O. (2000) Plant communities in lowland Danish streams: species composition and environmental factors. Aquatic Botany, 66, 255–272. [Google Scholar]
- Springe G., Sandin L., Briede A. & Skuja A. (2006) Biological quality metrics: their variability and appropriate scale for assessing streams. Hydrobiologia, 566, 153–172. [Google Scholar]
- Stoddard J.L., Larsen D.P., Hawkins C.P., Johnson R.K. & Norris R.H. (2006) Setting expectations for the ecological condition of streams: the concept of reference condition. Ecological Applications, 16, 1267–1276. [DOI] [PubMed] [Google Scholar]
- Szoszkiewicz K., Ferreira T., Korte T., Baattrup‐Pedersen A., Davy‐Bowker J. & O’Hare M. (2006) European river plant communities: the importance of organic pollution and the usefulness of existing macrophyte metrics. Hydrobiologia, 566, 211–234. [Google Scholar]
- Wallin M., Wiederholm T. & Johnson R. (2003) Guidance on establishing reference condition and ecological status class boundaries for inland surface waters. Final Report to the European Commission from CIS Working Group 2.3. REFCOND.
- Wright J.F., Clarke R.T., Gunn R.J.M., Winer J.M., Kneebone N.T. & Davy‐Bowker J. (2003) Response of the flora and macroinvertebrate fauna of a chalk stream site to changes in management. Freshwater Biology, 48, 894–911. [Google Scholar]


