Antonio Bertoletti and Anthony Tanoto Tan discuss the opportunities and challenges of CAR-T cell therapy in chronic infections.
Abstract
While therapy with T cells engineered with a chimeric antigen receptor (CAR) or a classical T cell receptor (TCR) is revolutionizing cancer treatment, its adoption in infectious diseases has been met with considerable resistance. Can we find its value for the cure of infections?
Adoptive therapy with T cells engineered with a chimeric antigen receptor (CAR) or TCR (indicated herein as CAR/TCR-T cells) has transitioned from an experimental approach to a main strategy of care in cancer treatment. While it has triggered an astonishing proliferation of clinical trials in the cancer space (>500 in the last 3 yr; MacKay et al., 2020), clinical applications in the context of infections have met considerable inertia, and only two clinical trials in HIV infection are currently ongoing. Here, we want to discuss what we consider to be the obstacles that prevent the acceptance of CAR/TCR-T cells as a potential treatment in different infectious diseases. We also contend that specific chronic infections like hepatitis B virus (HBV) might benefit from the adoption of this approach and discuss how technological advances in lymphocyte engineering might boost this process.
The production of CAR/TCR-T cells requires specialized personnel and infrastructure and is very expensive. Thus, the hypothesis to treat infectious diseases with high incidence in developing countries, like tuberculosis and malaria, with this approach is nowadays cost-prohibitive and unrealistic. It is logical that research efforts targeting these pathogens are diverted mainly toward vaccine development or therapeutic small molecules that target their replication. However, in some infectious diseases, CAR/TCR-T cells might offer a rational and practical approach despite the inherent drawbacks.
Infections in immunosuppressed patients
Viral infections or relapses developing in immunosuppressed patients (i.e., patients after hematopoietic stem cell or organ transplantation with human cytomegalovirus or EBV reactivation) have been shown to be responsive to adoptive immunotherapy with autologous in vitro expanded virus-specific T cells derived from memory or naive T cell populations (Houghtelin and Bollard, 2017). These expanded virus-specific T cells can be substituted by autologous T cells engineered in vitro through the introduction of pathogen-specific CAR or TCRs. In vitro, the antiviral function of the latter is identical to T cells of the same specificity isolated from the peripheral blood (Gehring et al., 2011). Coupled with the availability of new TCRs specific for rare viruses, like hepatitis E virus (Soon et al., 2019), that affect immunosuppressed patients, CAR/TCR-T cells can find a place in the treatment of patients affected by these pathogens. One caveat could be that while antiviral T cells generated naturally are multispecific and polyclonal (Houghtelin and Bollard, 2017), engineered CAR/TCR-T cells are monospecific and monoclonal. Whether this lack of diversity would lead to selection of CAR/TCR escape variants will have to be taken into consideration.
CAR/TCR-T cells in HIV infection
Another area of interest for CAR/TCR-T cells is in the control or eradication of persistent infections. This has been explored mainly in the context of HIV infection, in which a considerable amount of work has been invested to engineer T cells with different CAR constructs comprising of either the extracellular domain of CD4 or HIV-specific broadly neutralizing antibodies (reviewed in Kuhlmann et al., 2018) with the intention of eliminating the majority of circulating virus-producing T cells or latent HIV reservoirs. Despite promising results in preclinical works, only two trials are now testing CAR-T cells in HIV-infected patients. There are several reasons for such low “penetrance” in the clinical arena. First, patients under antiviral treatment live a normal life. To compete with antiretroviral therapy, CAR/TCR therapy should therefore achieve extremely demanding clinical end points like the eradication of HIV through lysis of all cells with latent HIV reservoirs. In addition, the ability of HIV to mutate and escape CTL pressure, the possible infection of the transferred CAR-T cells, the low expression of HIV antigens in latently infected cells that is difficult to safely reactivate, and the decrease in HIV antigen load during antiretroviral therapy all contribute to the increased difficulty to apply CAR/TCR-T cell therapy in HIV (Kuhlmann et al., 2018).
Peculiarity of HBV infection
We believe that chronic infections with HBV, alone or in conjunction with hepatitis D virus, have characteristics that make it uniquely suited for CAR/TCR-T cell therapy.
The astonishing efficacy of antiviral treatments in patients with chronic hepatitis C (hepatitis C virus clearance in 95% of treated patients) has exposed the inadequacy of current HBV treatments in which antiviral agents achieve suppression of viral replication but cure less than 5% of patients. As such, HBV treatment needs to be administered indefinitely. However, unlike in HIV, current HBV antiviral therapy with nucleoside analogues blocks HBV-DNA synthesis without affecting antigen production. Thus, HBV-infected hepatocytes under nucleoside analogue treatment express HBV antigens and can be targeted by CAR/TCR-T cells. In addition, the compact nature of the HBV genome in which a single DNA sequence is translated into different HBV proteins reduces the likelihood of the emergence of mutated viruses with conserved fitness, which in turn makes it less likely for HBV to escape T cell recognition. The peculiar characteristics of the defective antiviral immunity present in chronic hepatitis B (CHB) patients make it also more amenable to a reconstitution through CAR/TCR therapy. HBV-specific T cell frequency in CHB patients is extremely low in comparison to other persistent viral infection (like HIV, EBV, and human cytomegalovirus), as a consequence of the persistent presence of large quantity of viral antigens (HBsAg is present in patients’ serum at concentrations of 20–50 µg/ml) and of antigen presentation by hepatocytes that severely impairs the CD8 T lymphocytes expansion (Bénéchet et al., 2019). Such impairment of the HBV-specific T cell compartment in CHB patients makes it unlikely that their autologous HBV-specific T cells can be expanded and recovered through check-point inhibitors or vaccine therapy. Indeed, recovery of HBV-specific T cells in vitro using anti-PD1/anti–PD-L1 antibodies is minimal and clinical trials with anti-PD1 antibodies resulted in only 1 out of 12 patients achieving HBV cure (Gane et al., 2019). Lastly, the hepatotrophic nature of HBV and the generally intact T cell compartment (except for HBV-specific T cells) will not interfere with a CAR/TCR-T cell therapy strategy like that observed in HIV.
Hence, engineering HBV-specific T cells using TCRs specific for HLA class I–restricted HBV epitopes or with a CAR specific for HBsAg (the HBV envelope antigen secreted in large quantity by infected hepatocytes) can provide a well-characterized, sizeable, and functionally intact population of HBV-specific T cells. In HBV animal models, treatment with these human engineered HBV-specific CAR/TCR-T cells demonstrated anti-HBV activity (Krebs et al., 2013; Kah et al., 2017) and eradicated HBV infection when used in combination with agents blocking HBV infection (Wisskirchen et al., 2019). Such efficacy in animal models, as seen in the work on HIV, does not translate immediately to clinical success, but evidence obtained in patients with CHB infection receiving transplantation of vaccine or HBV-primed bone demonstrated that adoptive reconstitution of HBV-specific immunity has the potential to cure chronic HBV infection (Lau et al., 1997).
Safety concerns in treating infections affecting vital organs
The evidence that supports the potential of CAR/TCR-T cells in the treatment of HBV need to be balanced by other critical arguments.
CAR/TCR-T cells are primarily engineered with the intention to directly lyse the targeted cells. Their application in cancer patients is relatively straightforward, with the main purpose to eliminate or reduce the tumor burden. In an infectious disease setting where organs essential for life are infected (like the liver in HBV) and where the T cells mediate protection but also organ pathology, the use of CAR/TCR-T cells has to be evaluated with caution. Fatal clinical events occur in some patients treated with CAR/TCR-T cells when an excessive number of tumor (cytokine storm) or nontumor cells (off-tumor toxicity) were lysed (MacKay et al., 2020). This shows the danger that is lurking when large numbers of activated T cells are infused in patients.
Thus, while the establishment of a memory T cell response able to provide long-term protection represents the “holy grail” of immunotherapy, the infusion of T cells stably expressing pathogen-specific CAR/TCR poses the risk that these T cells might proliferate and wipe out all the infected cells that might be the majority of the infected organ.
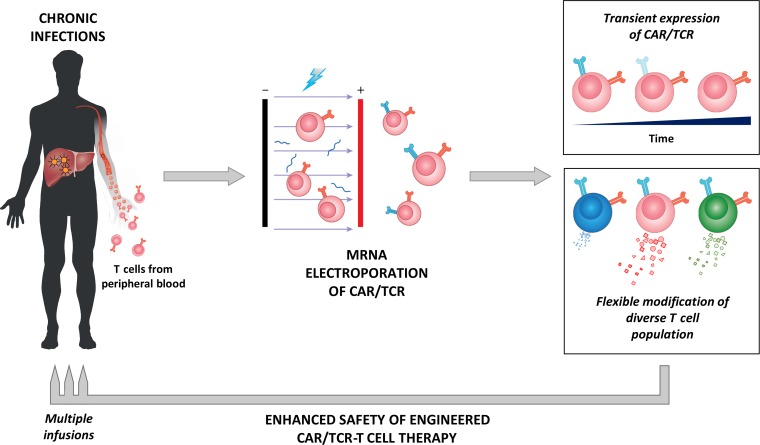
The introduction of a suicidal gene into the CAR/TCR-T cells can be a solution to this problem. Another option that we are presently pursuing is to engineer T cells that express CAR/TCR only transiently through CAR/TCR mRNA electroporation (Fig. 1). mRNA-electroporated T cells express TCR for 48–72 h, and this allows for adoptive transfer of escalating numbers of TCR T cells in patients to evaluate their clinical and toxic effects. While mRNA-electroporated TCR-T cells showed a reduced potency against cancer and infections in comparison to stably transfected T cells in animal models (Koh et al., 2013), its deployment in immunocompetent patients can result in the triggering of new secondary antitumor responses. Recent data in immunocompetent cancer models have also shown that the efficacy of CAR-T cell therapy was dependent on their ability to initiate a secondary immune response and not solely on the quantity of adoptively transferred CAR-T cells (Etxeberria et al., 2019). This seems to occur in a compassionate trial performed in liver-transplanted patients with hepatocellular carcinoma relapses. The objective reduction of lung metastasis observed after mRNA TCR-T cell infusion in one patient could not be explained by a direct effect of the infused TCR-T cells, but rather by their ability to trigger a secondary immune response (Tan et al., 2019). Thus, even though the adoptive transfer of mRNA-electroporated T cells requires multiple infusions, the possibility that such treatment will trigger an endogenous anti-pathogen response might increase their efficacy. Another opportunity offered by CAR/TCR expression through mRNA electroporation is the ability to directly engineer nonactivated and noncycling T cells. This results in the production of TCR-T cells expressing low quantities of perforin and granzyme, which have lower inflammatory/lytic capability yet intact ability to activate antiviral mechanisms in target cells (Koh et al., 2018).
Figure 1.
CAR/TCR-T cells engineered through mRNA electroporation confer enhanced safety features suited for their deployment in patients with chronic viral diseases. The labile nature of mRNA results in a transient expression of transgenic CAR/TCR that limits the functional lifespan of the engineered T cells to ~3–5 days. This allows for a conservative dose escalation clinical trial design to closely monitor for potential toxicities. The flexibility of the technique to modify even non-activated T cells resulting in CAR/TCR-T cells with reduced cytolytic capacity, while maintaining antiviral effects, can further enhance the safety of the approach in viral infected patients.
In conclusion, we cannot deny the existence of practical difficulties and safety concerns that still prevent the widespread clinical translation of CAR/TCR-T cell therapy for infectious diseases. However, we believe that similar to other innovations that have changed the practice of medicine, acceptance of new treatments requires dedicated work and solid knowledge that can only be derived from the accumulation of experimental and clinical data. The practical difficulties of implementation and the criticisms about safety of CAR/TCR-T cells should therefore enhance our efforts to design better products that can be more safely applied to the treatment of infectious diseases. We also need to carefully evaluate clinical and immunological data generated from patients in whom T cells specific for viral antigens are used to primarily target not infected but viral-related cancer cells. The data gathered from these patients, who are the unfortunate individuals suffering from the most awful consequences of chronic infections, might hopefully provide the knowledge that will allow the implementation of CAR/TCR-T cell treatment in early stages of infection and thus prevent the development of such cancers.
References
- Bénéchet A.P., et al. Nature. 2019 doi: 10.1038/s41586-019-1620-6. [DOI] [Google Scholar]
- Etxeberria I., et al. Cancer Cell. 2019 doi: 10.1016/j.ccell.2019.10.006. [DOI] [Google Scholar]
- Gane E., et al. J. Hepatol. 2019 doi: 10.1016/j.jhep.2019.06.028. [DOI] [Google Scholar]
- Gehring A.J., et al. J. Hepatol. 2011 doi: 10.1016/j.jhep.2010.10.025. [DOI] [Google Scholar]
- Houghtelin A., and Bollard C.M. Front. Immunol. 2017 doi: 10.3389/fimmu.2017.01272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kah J., et al. J. Clin. Invest. 2017 doi: 10.1172/JCI93024. [DOI] [Google Scholar]
- Koh S., et al. Mol. Ther. Nucleic Acids. 2013 doi: 10.1038/mtna.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh S., et al. Gastroenterology. 2018 doi: 10.1053/j.gastro.2018.03.027. [DOI] [Google Scholar]
- Krebs K., et al. Gastroenterology. 2013 doi: 10.1053/j.gastro.2013.04.047. [DOI] [Google Scholar]
- Kuhlmann A.-S., et al. Curr. Opin. HIV AIDS. 2018 doi: 10.1097/COH.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau G.K., et al. Hepatology. 1997 doi: 10.1002/hep.510250631. [DOI] [Google Scholar]
- MacKay M., et al. Nat. Biotechnol. 2020 doi: 10.1038/s41587-019-0329-2. [DOI] [Google Scholar]
- Soon C.F., et al. J. Hepatol. 2019 doi: 10.1016/j.jhep.2019.06.005. [DOI] [Google Scholar]
- Tan A.T., et al. Gastroenterology. 2019 doi: 10.1053/j.gastro.2019.01.251. [DOI] [Google Scholar]
- Wisskirchen K., et al. J. Clin. Invest. 2019 doi: 10.1172/JCI120228. [DOI] [Google Scholar]