Abstract
Fusarium verticillioides, which causes ear, kernel and stem rots, has been reported as the most prevalent species on maize worldwide. Kernel infection by F. verticillioides results in reduced seed yield and quality as well as fumonisin contamination, and may affect seedling traits like germination rate, entire plant seedling length and weight. Maize resistance to Fusarium is a quantitative and complex trait controlled by numerous genes with small effects. In the present work, a Genome Wide Association Study (GWAS) of traits related to Fusarium seedling rot was carried out in 230 lines of a maize association population using 226,446 SNP markers. Phenotypes were scored on artificially infected kernels applying the rolled towel assay screening method and three traits related to disease response were measured in inoculated and not-inoculated seedlings: plant seedling length (PL), plant seedling weight (PW) and germination rate (GERM). Overall, GWAS resulted in 42 SNPs significantly associated with the examined traits. Two and eleven SNPs were associated with PL in inoculated and not-inoculated samples, respectively. Additionally, six and one SNPs were associated with PW and GERM traits in not-inoculated kernels, and further nine and thirteen SNPs were associated to the same traits in inoculated kernels. Five genes containing the significant SNPs or physically closed to them were proposed for Fusarium resistance, and 18 out of 25 genes containing or adjacent to significant SNPs identified by GWAS in the current research co-localized within QTL regions previously reported for resistance to Fusarium seed rot, Fusarium ear rot and fumonisin accumulation. Furthermore, linkage disequilibrium analysis revealed an additional gene not directly observed by GWAS analysis. These findings could aid to better understand the complex interaction between maize and F. verticillioides.
Keywords: GWAS, SNPs, Artificial inoculation, Fusarium verticillioides, Maize
Maize plants are attacked by several Fusarium species responsible for diseases such as root rot, stalk rot, seedling blight and ear rot, but Fusarium verticillioides (Sacc.) Nirenberg (synonyms Fusarium moniliforme, Gibberella fujikuroi MP-A or Gibberella moniliformis) (Bömke et al. 2008) has been reported as the most widespread fungal pathogen of maize worldwide (Leslie 1991; Danielsen et al. 1998; Chulze et al. 2000; Rahjoo et al. 2008). Fusarium infection can result in depleted seed yield and quality as well as fumonisin contamination. This fungus produces a wide range of mycotoxins that include fusaric acid, fusarins, and fumonisins (Desjardins and Proctor 2001). Fumonisins are the most prevalent (Munkvold 2003) and have been associated with esophageal cancer in humans, pulmonary edema in pigs, leukoencephalomalacia in horses and cancer-promoting activity in rats (Marasas 2001; Voss et al. 2002; Murillo-Williams and Munkvold 2008). Moreover, F. verticillioides can establish asymptomatically inside maize plants and kernels, impairing the possibility to easily detect affected grains (Munkvold and Desjardins 1997; Munkvold et al. 1997; Wilke et al. 2007).
F. verticillioides has available many infection pathways to reach plant tissues (Battilani et al. 2003), including the transmission from soil or the presence of seed borne F. verticillioides strains in all the plant tissues such as ears and kernels (Munkvold et al. 1997; Mesterházy et al. 2012). The frequency of fungal strain transmission from the seed depends on fungal characteristics, maize genotypes and environmental conditions (Wilke et al. 2007). Even though the complexity of maize-F. verticillioides interaction and the influence of seedling transmitted fungal strains on stalk, ear, and kernel rots as well as the fumonisin production was previously shown (Munkvold et al. 1997; Yates et al. 2003; Wilke et al. 2007; Williams et al. 2007), the potential genetic resistance to Fusarium seedling rot has not been widely examined yet.
Kernel infection by F. verticillioides can reduce seed germination of maize as well as vigor at different growth stages (Machado et al. 2013). The effect of F. verticillioides on the development of maize seedlings/plants under controlled conditions was previously evaluated (Machado et al. 2013). All traits measured (plant population stand, speed of seedling emergence, and height/weight of emerged plants) were negatively affected by the increasing fungal amounts present in the seeds (Machado et al. 2013). Recently, a genome-wide association study (GWAS) using artificially inoculated maize kernels allowed the identification of several SNPs and candidate genes significantly associated with Fusarium resistance at the seedling stage (Stagnati et al. 2019).
The cultivation of resistant germplasm is an efficient way to reduce yield loss and mycotoxin contamination, however, no maize genotypes immune to Fusarium infection have been identified and many commercial hybrids have less resistance than desired (Lanubile et al. 2010; Zila et al. 2013). Resistance to F. verticillioides in maize, which was mainly focused on Fusarium ear rot (FER), has been studied in different surveys based on artificial inoculation in field (Drepper and Renfro 1990; Reid and Zhu 2005; Reid et al., 2009; Lanubile et al. 2011, 2017; Zila et al. 2013; Maschietto et al. 2017), greenhouse (Danielsen et al. 1998; Lanubile et al. 2013, 2014a; Maschietto et al. 2016) and laboratory trials (Ju et al. 2017; Atabaki et al. 2018). Moreover, in order to develop genetically resistant germplasm to Fusarium researchers carried out Quantitative Trait Locus (QTL) mapping analysis and GWAS by testing several distinct population structures (McMullen et al. 2009; Yang et al. 2010; Zila et al. 2013, 2014; Warburton et al. 2013; Chen et al. 2016; Ju et al. 2017; Maschietto et al. 2017; Gaikpa and Miedaner 2019; Samayoa et al. 2019; Septiani et al. 2019).
In the present study, the Rolled Towel Assay (RTA) screening method was applied to a maize association panel (Flint-Garcia et al. 2005) for evaluating the effect of F. verticillioides infection on the vegetative response of maize seedlings, considering plant seedling length (PL), plant seedling weight (PW) and germination rate (GERM). The panel was previously analyzed for resistance to Fusarium infection of seedlings (FIS; Stagnati et al. 2019). In order to identify SNPs putatively associated with PL, PW and GERM, with and without kernel infection by F. verticillioides, GWAS and linkage disequilibrium (LD) analysis were performed.
Materials and Methods
Maize germplasm, kernel inoculation and phenotyping
The maize core diversity panel, sometimes referred as the “Goodman” association panel (Flint-Garcia et al. 2005; Zila et al. 2013) was used in this work. Seeds were retrieved from USDA-ARS-NCRPIS (Iowa State University, Regional Plant Introduction Station, Ames, Iowa, United States, 50011-1170). The panel was previously evaluated for response to F. verticillioides inoculation (FIS) by Stagnati and co-workers (2019). In that work, data about seedling germination were exclusively used in order to remove lines with <50% germination percentage (Stagnati et al., 2019). Accordingly, only 230 out of 302 inbred lines of the population having sufficient germination rate were screened and considered for further analysis in the present study (Table S1).
To artificially infect mature kernels and evaluate the effect of F. verticillioides on some vegetative traits of maize seedlings, the Rolled Towel Assay (RTA) phenotyping method was used (Ellis et al. 2011; Lanubile et al. 2015; Stagnati et al. 2019). For each inbred line, seeds with similar size and shape, and without visible damage, were selected. To reduce as much as possible the presence of contaminating fungi, seeds were surface-sterilized as previously described (Stagnati et al. 2019). RTA were prepared using 10 seeds for each inbred per towel of moistened geminating paper (Anchor Paper, Saint Paul, MN). Kernels were inoculated on the embryo side near the pedicel with 100 μl of 1x106 conidial suspension of F. verticillioides ITEM10027 (MPVP 294) and sterilized distilled water for treated and control towels, respectively. Incubation was performed for 7 days in the dark at 25° as previously reported (Bernardi et al. 2018; Stagnati et al. 2019).
For each seedling, plant length (PL) from the tip to the end of the longest root, and fresh plant weight (PW) of maize genotypes in control (C) and inoculated (I) conditions were calculated. Germination percentage was evaluated as reported by Stagnati et al. (2019).
Association analysis, candidate gene discovery and linkage disequilibrium analysis
The ZeaGBSv2.7 set of SNP derived from Genotyping By Sequencing and available at Panzea (www.panzea.org) was used. Monomorphic and multiallelic SNPs and INDELs were discarded. Imputation was performed using the software Beagle 4.1 (Browning and Browning 2016). Heterozygous were set as missing data and removed the SNPs if >20% of missing data and minor allele frequency (MAF) <5%. After the final imputation, a set of 226,446 SNPs was used for association analysis.
Marker pairs with genotypic correlation higher than r = 0.5 were pruned according to a linkage disequilibrium based approach using the software Plink v1.07 (Purcell et al. 2007). A subset of around 100kb SNPs was obtained to compute the additive relationship matrix (K matrix) for the 230 inbred using TASSEL v5.2.25 (Bradbury et al. 2007).
Genome Wide Association Analysis (GWAS) was performed in Tassel (version 5.2.25). The mixed linear model (MLM) fitted by Tassel was:
Where y is the vector of phenotypes, β is a vector of the overall mean and the fixed effect estimate of an individual SNP, u is a vector of random line additive genetic effects, X and Z represent incidence matrices, and e is a vector of random residuals. Variance of random line effects was modeled as Var (u) = , where is the estimated additive polygenic variance. The optimum compression level option (compressed MLM) was used (Zhang et al. 2010).
To identify robust SNP associations, a resampling procedure was performed. In each of 100 data resamples, a random sample of 80% of inbred lines was selected from the population, GWAS was performed on the subset of lines. Only SNP markers determined as significant at P < 1x10−4 within at least 30% of data subsamples, i.e., a resample model inclusion probability (RMIP) threshold of 0.30, were considered as significant (Samayoa et al. 2015; Stagnati et al. 2019). Data manipulations and visualizations were performed using R software (R Core Team 2016).
Genes containing or close to associated SNPs were detected using Maize GDB genome browser using the B73 RefGen_V3 as the reference genome to place SNPs and genes avoiding low confidence genes and transposable elements. The function and conserved domains of proteins encoded by genes identified were retrieved submitting sequences to NCBI-protein BLAST (https://blast.ncbi.nlm.nih.gov) and restricting the search to Zea mays (taxid:4577). For SNPs positioned inside genes the possible effect on protein sequence was inquired. Introns and translated regions were identified using GeneWise online tool (http://www.ebi.ac.uk), coding sequence were translated using ExPASy (http://web.expasy.org/translate) and amino-acid variations were detected aligning the reference protein retrieved from MaizeGBD, and the SNP derived protein using MultAlin (http://multalin.toulouse.inra.fr).
Linkage disequilibrium (LD) measures (r2) were estimated using TASSEL version 5.0 between each significant SNP and other SNPs within a surrounding window of 60 adjacent SNPs (Samayoa et al. 2015; Stagnati et al. 2019). For clusters of tightly linked significant SNPs, LD analysis was computed for one of them; positions were considered linked to significant SNPs if r2 > 0.5 and the distance between the position and the SNP resulting from GWAS was greater than 2 Kb.
Data availability
All supporting data are included as supplemental files and are available at Figshare: https://figshare.com/s/54bb47a6abf418c859b9. Table S1. (Excel file) List of germplasm and phenotypic values. Table S2. (DOCX file) Table of LD analysis.
Results and Discussion
Phenotypic data
Seed germination was affected in different ways by artificial inoculation (Figure 1; Table S1). In 59 cases, germination rate were higher in the inoculated towels, whereas in 87 lines the germination was negatively affected by the fungus. Similar numbers were also observed in 84 lines without and with infection. Figure 1 illustrates the change in germination due to inoculation for 20 inbreds with the most extreme reactions after inoculation. The germination of inbreds I29 and CML238 was reduced by 70% resulting in 10 and 20%, respectively, after inoculation; germination of Il677a was reduced from 50 to 10% by the inoculation. I29 (a popcorn) and Il677a (a sweet corn) were the inbreds with the lowest germination rate after inoculation and both showed increased disease severity values following inoculation (Stagnati et al. 2019).
Figure 1.
Effect of inoculation with Fusarium verticillioides on germination percentage compared to control, in kernels of selected maize lines.
The ability of F. verticillioides to influence seed germination and vigor in maize was previously explored. Some studies reported that the fungus can condition host fitness promoting growth and inducing defense mechanisms such as lignin deposition in the cell wall (Yates et al. 1997). Seedlings grown from seeds inoculated with F. verticillioides showed reduced growth at early stages of infection, but by 21 days after planting the inoculated plants adjusted or overtook the mock plants in shoot diameter, root growth and dry weight (Yates et al. 1997). Additionally, previous reports in literature highlighted how maize seed lots with high incidence of this fungus (inoculum potential) underwent little or no reduction in germination or seedling growth, while others were seriously compromised by the fungus (Munkvold et al. 1997; Oren et al. 2003; Machado et al. 2013). Beside the fungal inoculum potential in a seed lot, further sources of variation in seedling germination should be taken in account, such as the genetic nature of the several inbred employed in this study that can have influenced the establishment of the maize-fungal association, determining even a beneficial effect to some hosting lines.
No significant correlations were observed in non-inoculated assays between either PL_C or PW_C and the FIS severity (Sev_C), previously evaluated in Stagnati et al. (2019), whereas a moderately negative correlation was found for the germination percentage (G_C; Figure 2). As expected, after F. verticillioides infection, PL_I, PW_I and G_I showed significant negative correlations with Sev_I (r =-0.75, -0.55 and -0.78 for PL_I, PW_I and G_I, respectively; Figure 2). In line with these achievements, previous studies reported that F. verticillioides infection diminished germination of maize kernels and hampered seedling growth and vigor (Van Wyk et al. 1988; Septiani et al. 2019).
Figure 2.
Correlation among disease severity (SEV; Stagnati et al. 2019), plant length (PL), plant weight (PW), germination percentage (G) traits measured in the control (C) or inoculated (I) plants. The distribution of each trait is shown on the diagonal. On the bottom of the diagonal the bivariate scatter plots with a fitted line are displayed. On the top of the diagonal the value of the correlation plus the significance level are reported as stars (*** p-value 0.001).
Furthermore, seedling traits (length, weight and germination) of both inoculated and control assays were significantly correlated each other (Figure 2). This finding could be explained by the inherent inbred line characteristics like seed size, weight and seedling growth vigor.
Association analysis and SNP discovery
GWAS analysis resulted in 42 SNPs, distributed across all chromosomes, significantly associated with the traits examined. 35 SNPs were located in 21 genes, while the remaining 7 were intergenic, but within 1.1 kb of known genes (Tables 1 and 2; Figure 3). A total of 25 genes were identified as containing or close to SNPs significantly associated with at least one of the measured traits. Six SNPs were in common between two traits each, i.e., four SNPs for PL_I and PW_C; one SNP for PL_I and GERM_I and one SNP for PL_C and PW_C.
Table 1. Chromosome (CHR) location, number of genes and SNPs localized inside (IN) or outside (OUT) predicted coding regions of genes significantly associated with the traits PL_C, PL_I, PW_C, PW_I, GERM_C and GERM_I.
| TRAIT | CHR | GENES | SNPs | IN | OUT |
|---|---|---|---|---|---|
| PL_C | 4 | 2 | 2 | 2 | 0 |
| PL_I | 1 | 1 | 4 | 3 | 1 |
| PL_I | 3 | 1 | 1 | 1 | 0 |
| PL_I | 5 | 1 | 3 | 3 | 0 |
| PL_I | 9 | 3 | 3 | 2 | 1 |
| PW_C | 1 | 2 | 5 | 3 | 2 |
| PW_C | 4 | 1 | 1 | 1 | 0 |
| PW_I | 1 | 1 | 1 | 1 | 0 |
| PW_I | 3 | 1 | 7 | 7 | 0 |
| PW_I | 5 | 1 | 1 | 1 | 0 |
| GERM_C | 8 | 1 | 1 | 1 | 0 |
| GERM_I | 1 | 1 | 1 | 1 | 0 |
| GERM_I | 2 | 2 | 2 | 1 | 1 |
| GERM_I | 3 | 3 | 3 | 2 | 1 |
| GERM_I | 5 | 4 | 4 | 4 | 0 |
| GERM_I | 6 | 1 | 1 | 1 | 0 |
| GERM_I | 8 | 2 | 2 | 1 | 1 |
Table 2. Marker name, allelic variants (SNP), number of lines carrying each allelic variant (OBS), additive effect, p-values for the association between the SNP and the phenotype, resample model inclusion probability (RMIP) and proportion of the phenotypic variance associated with the SNP (R2) referred to the traits PL_C, PL_I, PW_C, PW_I, GERM_C and GERM_I.
| N° | Trait | Markera | SNPb | OBSc | Additive effectd | P-value | R2 | RMIP |
|---|---|---|---|---|---|---|---|---|
| 1 | PL_C | S4_237392707 | A/G | 216/14 | −5.50 | 3.85*10−06 | 0.08 | 0.4 |
| 2 – 19 | PL_C | S4_768454 | G/A | 150/76 | −2.89 | 2.52*10−06 | 0.09 | 0.3 |
| 3 – 15 | PL_I | S1_45515811 | A/G | 105/23 | −2.63 | 5.19*10−07 | 0.08 | 0.4 |
| 4 – 16 | PL_I | S1_45515933 | G/T | 122/106 | 2.64 | 5.86*10−07 | 0.08 | 0.4 |
| 5 – 17 | PL_I | S1_45515951 | T/C | 122/106 | 2.64 | 5.86*10−07 | 0.08 | 0.4 |
| 6 – 18 | PL_I | S1_45516086 | G/C | 106/122 | 2.64 | 5.86*10−07 | 0.08 | 0.4 |
| 7 – 33 | PL_I | S3_166796369 | G/A | 217/12 | −5.65 | 5.56*10−06 | 0.07 | 0.4 |
| 8 | PL_I | S5_30755308 | G/T | 155/74 | −2.84 | 1.01*10−06 | 0.08 | 0.5 |
| 9 | PL_I | S5_30755309 | C/A | 154/75 | −3.08 | 1.31*10−07 | 0.10 | 0.8 |
| 10 | PL_I | S5_30755313 | G/T | 154/75 | −3.08 | 1.31*10−07 | 0.10 | 0.8 |
| 11 | PL_I | S9_105426127 | G/A | 192/37 | −4.43 | 1.60*10−07 | 0.10 | 0.9 |
| 12 | PL_I | S9_105564038 | C/A | 183/47 | −3.20 | 2.00*10−06 | 0.07 | 0.4 |
| 13 | PL_I | S9_108462691 | C/T | 209/20 | −4.76 | 3.79*10−07 | 0.08 | 0.4 |
| 14 | PW_C | S1_290520672 | G/T | 188/38 | −0.09 | 2.18*10−06 | 0.08 | 0.5 |
| 15 – 3 | PW_C | S1_45515811 | G/A | 123/105 | 0.07 | 2.29*10−07 | 0.08 | 0.6 |
| 16 – 4 | PW_C | S1_45515933 | G/T | 122/106 | 0.07 | 3.30*10−07 | 0.08 | 0.6 |
| 17 – 5 | PW_C | S1_45515951 | T/C | 106/122 | 0.07 | 3.30*10−07 | 0.08 | 0.6 |
| 18 – 6 | PW_C | S1_45516086 | G/C | 106/122 | 0.07 | 3.30*10−07 | 0.08 | 0.6 |
| 19 - 2 | PW_C | S4_768454 | G/A | 150/76 | −0.07 | 2.72*10−06 | 0.08 | 0.5 |
| 20 | PW_I | S1_3366706 | T/C | 198/30 | 0.10 | 1.34*10−06 | 0.07 | 0.4 |
| 21 | PW_I | S3_200433261 | C/T | 162/65 | −0.07 | 3.39*10−06 | 0.08 | 0.3 |
| 22 | PW_I | S3_200433799 | C/T | 152/77 | −0.08 | 1.78*10−06 | 0.08 | 0.5 |
| 23 | PW_I | S3_200433801 | G/C | 152/77 | −0.08 | 1.78*10−06 | 0.08 | 0.5 |
| 24 | PW_I | S3_200433802 | A/C | 152/77 | −0.08 | 1.78*10−06 | 0.08 | 0.5 |
| 25 | PW_I | S3_200433803 | T/C | 152/77 | −0.08 | 1.78*10−06 | 0.08 | 0.5 |
| 26 | PW_I | S3_200433807 | T/C | 152/77 | −0.08 | 1.78*10−06 | 0.08 | 0.5 |
| 27 | PW_I | S3_200433834 | G/A | 152/77 | −0.08 | 1.78*10−06 | 0.08 | 0.5 |
| 28 | PW_I | S5_31445260 | T/C | 131/98 | 0.07 | 7.31*10−06 | 0.08 | 0.4 |
| 29 | GERM_C | S8_164380034 | G/T | 158/68 | −5.54 | 2.53*10−08 | 0.10 | 0.7 |
| 30 | GERM_I | S1_174846151 | C/T | 214/16 | 10.77 | 6.61*10−08 | 0.08 | 0.5 |
| 31 | GERM_I | S2_2069896 | G/A | 215/14 | −12.08 | 2.71*10−08 | 0.09 | 0.6 |
| 32 | GERM_I | S2_219731457 | A/G | 182/44 | −6.82 | 6.16*10−06 | 0.07 | 0.4 |
| 33-7 | GERM_I | S3_166796369 | G/A | 217/12 | −14.21 | 5.72*10−09 | 0.11 | 0.8 |
| 34 | GERM_I | S3_206944106 | A/G | 217/13 | 12.03 | 1.53*10−07 | 0.09 | 0.5 |
| 35 | GERM_I | S3_206950032 | G/T | 217/13 | 12.03 | 1.53*10−07 | 0.09 | 0.5 |
| 36 | GERM_I | S5_201596318 | C/G | 212/16 | −10.14 | 9.04*10−07 | 0.08 | 0.5 |
| 37 | GERM_I | S5_216440094 | C/T | 216/13 | −12.78 | 9.66*10−08 | 0.09 | 0.7 |
| 38 | GERM_I | S5_24609625 | G/C | 119/108 | −5.63 | 9.80*10−07 | 0.08 | 0.3 |
| 39 | GERM_I | S5_6906047 | T/C | 179/46 | −7.19 | 4.97*10−06 | 0.08 | 0.3 |
| 40 | GERM_I | S6_150075477 | G/A | 163/66 | −6.07 | 4.87*10−06 | 0.07 | 0.4 |
| 41 | GERM_I | S8_100397575 | T/C | 214/16 | −10.40 | 4.88*10−07 | 0.08 | 0.5 |
| 42 | GERM_I | S8_171745673 | A/G | 203/24 | −11.29 | 2.52*10−07 | 0.09 | 0.6 |
Figure 3.
Manhattan plots of the traits PL_C (A), PL_I (B), PW_C (C), PW_I (D), GERM_C (E) and GERM_I (F). SNPs significantly associated to the traits are indicated by green dots.
For the trait PL_C only two SNP associations were discovered, both on chromosome 4, but inside two different genes (Figure 3A). Eleven SNPs were found associated with the trait PL_I (Figure 3B): including a cluster of four SNPs in or near the same gene on chromosome 1, one SNP on chromosome 3, three SNPs inside the same gene on chromosome 5 and further three SNPs on chromosome 9. Five SNPs in or near two different genes on chromosome 1 and a SNP on chromosome 4 were associated with PW_C (Figure 3C). For PW_I an associated SNP was found in a gene on chromosome 1, seven SNPs were in a gene on chromosome 3 and one SNP was in a gene on chromosome 5 (Figure 3D).
For the trait GERM_C only one significant SNP was identified and it was inside a gene on chromosome 8 (Figure 3E). For GERM_I thirteen SNPs were observed (Figure 3F). One is located in a gene on chromosome 1, two SNPs highlighted two genes on chromosome 2 and three SNPs were found on chromosome 3 associated with 3 genes. On chromosome 4, 4 SNPs were identified inside the same number of genes; on chromosome 6 a SNP highlighted one gene and on chromosome 8 two SNPs, one inside and one close by two different genes.
The highest number of genes was identified on chromosome 5, while chromosome 6 had only one gene associated to disease traits. Genes highlighted were generally at the end of the chromosome than in centromere or pericentromeric regions. No SNPs on chromosomes 7 and 10 were associated to traits.
Genes associated with PL, PW and GERM traits
Table 3 reports all genes containing or close to SNP markers deriving from GWAS and significantly associated to the traits length, weight and germination in control and inoculated seedlings. A SNP (S4_768454) was found inside the gene GRMZM2G058394 and associated with traits PL_C and PW_C. This gene encodes a putative thaumatin like-protein (TLP). TLPs are implicated in host defense and in various developmental processes. These genes are expressed in response to environmental perturbations, drought, cold and pathogen attacks. Therefore, TLPs are classified as pathogenesis related protein (PR) (Marchler-Bauer et al. 2015). TLPs are universal in plants. The antifungal activity of TLPs is well reported (Liu et al. 2010; Sharma et al. 2020) and for some of them the binding activity to β-1,3-D-glucanase is described. β-1,3-D-glucan is a key component of fungal cell wall and the activity of this enzyme is important in the breakdown of fungal cell (Liu et al. 2010; Sharma et al. 2020). The TLP family also includes a xylanase inhibitor (Liu et al. 2010; Fierens et al. 2007); considering that xylan is an important component of plant cell wall, the xylanase activity of TLPs is one of the mechanisms by which these proteins are involved in plant-defense mechanism (Liu et al. 2010). In maize ears and silks infected by F. verticillioides, thaumatin gene had lower expression ratios in resistant lines compared to susceptible ones. In contrast, kernels of the resistant lines showed higher gene expression before fungal infection, highlighting the key role of constitutive resistance in limiting pathogen attack (Lanubile et al. 2010, 2012a,b; Maschietto et al. 2016). The SNP found in this gene did not cause an amino-acid change in the protein.
Table 3. Genes containing or near SNPs significantly associated with the traits PL, PW and GERM in control and Fusarium inoculated seedlings. For each marker, the genomic position (pos_AGP3), the surrounding or adjacent gene, the type and the conserved domain of the encoded protein (where available) and the amino-acid variation caused by the SNP are reported.
| N° | Gene_id | Strand1 | pos_AGP3 | Description | Conserved domains | Genic2 | Amino-acid3 |
|---|---|---|---|---|---|---|---|
| 1 | GRMZM5G851807 | + | 237936341 | Putative DNA-directed RNA polymerase family protein | PRK13042, superantigen-like protein, DUF3223, SPT5 elongation factor | TRUE | E_UTR |
| 2–19 | GRMZM2G058394 | + | 768454 | Putative thaumatin domain family protein | Glycoside hydrolase family 64 | TRUE | No change |
| 3–15 | GRMZM2G087186 | — | 45520333 | Pyruvate decarboxylase isozyme 3 | Pyruvate decarboxylase domains | TRUE | No change |
| 4–16 | 45520455 | TRUE | E_UTR | ||||
| 5–17 | 45520473 | TRUE | E_UTR | ||||
| 6–18 | 45520608 | 107 | NA | ||||
| 7–33 | GRMZM2G141638 | + | 166839392 | Putative AP2/EREBP transcription factor superfamily protein | AP2 domain | TRUE | No change (T1), E_UTR(T2) |
| 8 | GRMZM2G027232 | + | 30775149 | 50S ribosomal protein L11; | Ribosomal protein | TRUE | E_UTR |
| 9 | 30775150 | TRUE | E_UTR | ||||
| 10 | 30775154 | TRUE | E_UTR | ||||
| 11 | GRMZM2G031370 | + | 106433186 | Uncharacterized protein | RABGAP_TBC domain GTPase activator protein in yeast | TRUE | E_UTR |
| 12 | GRMZM2G058162 | — | 106571097 | putative ubiquitin-conjugating enzyme family | UBCc superfamily | 400 | NA |
| 13 | GRMZM2G338376 | — | 108482377 | Uncharacterized protein | Serine threonine protein kinase | TRUE | Intron |
| 14 | GRMZM2G012224 | + | 290593516 | Putative uncharacterized protein | DUF642/ and CBM_4_9_Carbohydrate binding domain | 1114 | NA |
| 20 | GRMZM2G426802 | — | 3359556 | Signal transducer | WD40 signal transducer, | TRUE | Intron |
| 21 | GRMZM5G800586 | — | 200505296 | Uncharacterized protein | BURP domain of unknown function | TRUE | Intron |
| 22 | 200505834 | TRUE | E_UTR | ||||
| 23 | 200505836 | TRUE | E_UTR | ||||
| 24 | 200505837 | TRUE | E_UTR | ||||
| 25 | 200505838 | TRUE | E_UTR | ||||
| 26 | 200505842 | TRUE | E_UTR | ||||
| 27 | 200505869 | TRUE | E_UTR | ||||
| 28 | GRMZM2G154954 | + | 31465101 | Glycine rich protein 3 | No conserved domains | TRUE | No change |
| 29 | GRMZM2G024799 | — | 163929482 | SF16 protein | No conserved domains | TRUE | E_UTR |
| 30 | GRMZM2G008250 | + | 174872171 | Uncharacterized protein | No conserved domains | TRUE | No change |
| 31 | GRMZM2G040627 | + | 2072269 | Uncharacterized protein | Uracil-DNA glycosylase /DNA repair | TRUE | No change |
| 32 | GRMZM2G025783 | — | 220400327 | Protein kinase Kelch repeat:Kelch | Kelch and Fbox associated with LRR | 484 | NA |
| 34 | GRMZM2G497710 | + | 207023192 | Uncharacterized protein | Wall-associated receptor kinase galacturonan-binding, GUB_WAK_bind domain. | TRUE | E_UTR |
| 35 | GRMZM2G028568 | + | 207029118 | Uncharacterized protein | Serine threonine protein kinase(stress response/ GUB_WAK domain | 45 | NA |
| 36 | GRMZM2G071970 | + | 6914037 | Brittle stalk-2-like protein 4 | COBRA-like protein | TRUE | E_UTR |
| 37 | GRMZM2G032648 | — | 24629650 | Uncharacterized protein | CBS domain | TRUE | No change (T1) E_UTR (T2) |
| 38 | GRMZM2G125432 | + | 201652485 | NA | Putative SET-domain protein | TRUE | E_UTR (T1-2) |
| 39 | GRMZM2G171801 | — | 216492681 | NA | DEAD-box ATP-dependent RNA helicase 48-like | TRUE | R317K |
| 40 | GRMZM2G129288 | + | 150269434 | Uncharacterized protein | TUB and F-box domain | TRUE | Intron (T1-2-3), E_UTR (T4-5) |
| 41 | GRMZM2G126010 | — | 99908826 | Actin-1 | Nucleotide-Binding Domain of the sugar kinase/HSP70/actin superfamily | TRUE | E_UTR |
| 42 | GRMZM2G139460 | + | 171298650 | Uncharacterized protein | DUF4228 | 229 | NA |
1: indicates if the gene is on the forward (+) or reverse (-) strand. 2: indicates if the marker is inside (TRUE) a gene, otherwise the distance from the closest gene is reported. 3: indicate the amino acid variation (reference amino acid/position of variation/new amino acid), SNPs located in un-translated regions are reported (E_UTR); if the SNP had different consequences on different transcripts, of the same gene, that of interest is reported, NA means that the marker is not inside the gene.
A SNP in the gene GRMZM2G338376, which encodes a protein kinase with domains common to the receptor like kinase (RLK), was associated with PL_I. RLKs are surface transmembrane receptors able to perceive different signals and the presence of pathogens. The RLK family includes the BAK1 that is involved in brassinosteroid-mediated plant development. Interestingly, in previous studies this gene was highly expressed in maize coleoptile at V1 stage (Sekhon et al. 2011) and presented a strong modulation in resistant ears at 72 hr after F. verticillioides infection (Lanubile et al. 2014a; Wang et al. 2016). Furthermore, previous association mapping analysis identified BAK1 in two QTL for Fusarium seedling rot resistance (qFSR4.2) and seedling weight (qSWT3.1) in inoculated kernels of a Multi-parent Advance Generation Intercross (MAGIC) maize population (Septiani et al. 2019).
GRMZM2G154954, including the SNP S5_31445260 associated with PW_I, encodes the glycine rich protein 3. These proteins are key component of plant cell wall, and their expression was described in response to lesions caused by biotic and abiotic factors, in defense against Pseudomonas syringae, and two glycine rich peptides isolates from Capsella bursa-pastoris were active against several bacteria and fungi (Mangeon et al. 2010).
The SNP S2_219731457 associated with GERM_I was near the gene GRMZM2G025783. The protein encoded by this gene contains the F-box domain that mediates protein-protein interactions in several contests and in polyubiquitination. The F-box associated to the Tub superfamily domain was found also in the gene GRMZM2G129288, which was highlighted by the SNP 40, located in the UTR of all the 5′ transcripts for this gene (Marchler-Bauer et al. 2015).
Additional SNPs associated with GERM_I were identified in the GRMZM2G028568 and GRMZM2G125432 genes. GRMZM2G028568 encodes an uncharacterized protein with the Serine/threonine kinase domain, involved in plant resistance, and the wall-associated receptor kinase galacturonan-binding, which is an extracellular part of the serine/threonine kinase binding cell-wall pectins. This domain is responsible of the phosphorylation of Serine-Threonine residues and implicated in plant resistance (Afzal et al. 2008). GRMZM2G125432 encodes a protein with Pre-SET and SET domains. Pre-SET domain stabilizes SET domain, which is a lysine methyltransferase involved in protein-protein interactions (Thorstensen et al. 2011). A group of SET domains have been classified as PR proteins.
Linkage disequilibrium analysis
Linkage disequilibrium (LD) analysis was performed in a window of 60 adjacent SNPs on the same chromosome to identify correlations between markers. Genome-wide, 4 SNPs or groups of SNPs were detected in LD of r2 greater than or equal to 0.5 to significantly associated markers (Table S2). Generally, for each trait-associated SNP examined, from 1 to 3 SNPs were detected to be in LD r2 > 0.5.
Regarding the trait PW_I, only the gene GRMZM5G800586 was found both by GWAS and LD analysis; this gene encodes a protein of unknown function.
For the trait GERM_I, three positions were found in LD in the gene GRMZM2G497710 that was also identified by GWAS; also GRMZM2G125432 already reported as containing a SNP was the closest to a position found by LD analysis.
Genome-wide, three genes were identified by both analyses (GWAS and LD); while LD analysis exclusively revealed only one new gene.
Comparison between genes detected by GWAS and QTL for resistance to Fusarium
Since QTL often refer to chromosomal regions that may span several Mb of the genome, in which several genes may be located, genes overlapping previously reported QTL involved in resistance to FER, fumonisin accumulation, Fusarium seed rot (FSR) and seedling traits, including seedling length, weight and FIS, were investigated (Figures 4-6). Genes containing or adjacent to significant SNPs identified by GWAS analysis were located on chromosomes according to their physical position. In parallel, known QTL for the traits mentioned above were located on chromosomes according to ‘bin’ positions (Zila et al. 2013; Lanubile et al. 2014b; Ju et al. 2017; Maschietto et al. 2017; Septiani et al. 2019).
Figure 4.
Localization of QTL and genes containing or adjacent to SNPs identified by GWAS on chromosomes 1, 2 and 3. Genes associated with different traits (PL_C; PL_I; PW_C; PW_I; GERM_C; GERM_I) from this study are indicated by the black arrow. Candidate genes from Zila et al. (2013) are indicated by the orange arrow. Horizontal lines of different colors represent different QTL intervals for resistance to Fusarium and fumonisin accumulation identified by previous reports: pink and gray (Fusarium ear rot and fumonisins from Lanubile et al. 2014b and Maschietto et al. 2017, respectively); green (Fusarium seed rot from Ju et al. 2017); blue (Fusarium seedling rot, plant length and weight from Septiani et al. 2019). The centromere is indicated by a red label on the chromosome.
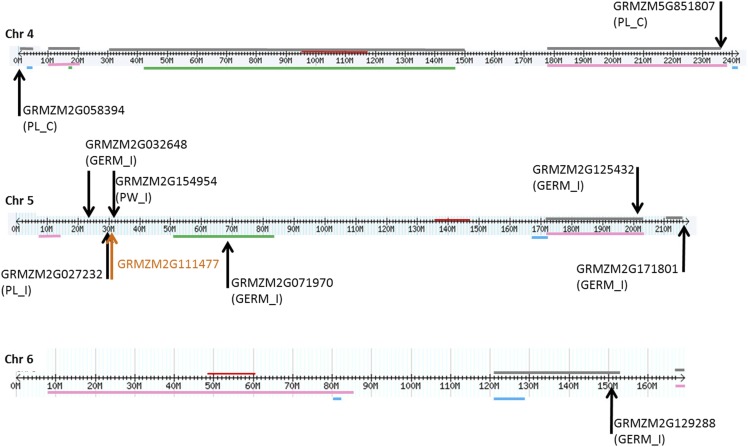
Figure 6.
Localization of QTL and genes containing or adjacent to SNPs identified by GWAS on chromosomes 8 and 9. Genes associated with different traits (PL_C; PL_I; PW_C; PW_I; GERM_C; GERM_I) from this study are indicated by the black arrow. Candidate genes from Zila et al. (2013) are indicated by the orange arrow. Horizontal lines of different colors represent different QTL intervals for resistance to Fusarium and fumonisin accumulation identified by previous reports: pink and gray (Fusarium ear rot and fumonisins from Lanubile et al. 2014b and Maschietto et al. 2017, respectively); green (Fusarium seed rot from Ju et al. 2017); blue (Fusarium seedling rot, plant length and weight from Septiani et al. 2019). The centromere is indicated by a red label on the chromosome.
On chromosome 1, genes were well distributed and associated for all traits (Figure 4). The genes GRMZM2G012224 and GRMZM2G087186 associated with PW_C, the later one also associated with PL_C, and GRMZM2G008250 with GERM_I were located inside known QTL found for both FER and fumonisin accumulation resistance. Additionally, the gene GRMZM2G426802 related to PW_I overlapped the QTL for FSR. In a previous work, Zila et al. (2013) reported the gene GRMZM2G703598 associated with FER resistance on chromosome 1 detected in the same association population employed in this study and assayed in field conditions.This gene did not co-localize within any QTL in the map (Figure 4).
Two genes (GRMZM2G040627 and GRMZM2G025783) on chromosome 2 and one (GRMZM2G141638) on chromosome 3 related to GERM_I overlapped two QTL regions reported for FER and fumonisin accumulation (Figure 4).
The genes associated with PL_C, GRMZM2G058394 and GRMZM5G851807, were located at the ends of QTL reported for FER and fumonisin resistance on chromosome 4 (Figure 5).
Figure 5.
Localization of QTL and genes containing or adjacent to SNPs identified by GWAS on chromosomes 4, 5 and 6. Genes associated with different traits (PL_C; PL_I; PW_C; PW_I; GERM_C; GERM_I) from this study are indicated by the black arrow. Candidate genes from Zila et al. (2013) are indicated by the orange arrow. Horizontal lines of different colors represent different QTL intervals for resistance to Fusarium and fumonisin accumulation identified by previous reports: pink and gray (Fusarium ear rot and fumonisins from Lanubile et al. 2014b and Maschietto et al. 2017, respectively); green (Fusarium seed rot from Ju et al. 2017); blue (Fusarium seedling rot, plant length and weight from Septiani et al. 2019). The centromere is indicated by a red label on the chromosome.
On chromosome 5, two genes (GRMZM2G125432 and GRMZM2G171801) related to GERM_I were located inside QTL reported for FER and fumonisin resistance. One more gene (GRMZM2G071970) associated with the same trait was located inside a known QTL involved in FSR resistance (Figure 5). Moreover, the gene GRMZM2G11147 identified by Zila et al. (2013) was very close to the genes GRMZM2G027232 and GRMZM2G154954 for PL_I and PW_I, respectively.
One gene related to GERM_I (GRMZM2G129288) was located inside a QTL reported for fumonisin accumulation on chromosome 6 (Figure 5).
Two genes associated with GERM_I traits (GRMZM2G126010 and GRMZM2G139460) overlapped with QTL intervals for resistance to fumonisin accumulation and FSR on chromosome 8, respectively, and one additional gene for GERM_C (GRMZM2G024799) was in the QTL for FER (Figure 6).
On chromosome 9, three genes (GRMZM2G031370, GRMZM2G058162 and GRMZM2G338376) were found associated with PL_I inside a QTL reported for fumonisin accumulation (Figure 6).
No gene associations were observed on chromosomes 7 and 10.
In the recent work by Ju et al. (2017), three QTL for FSR were found associated to three genes identified in this study, two for GERM_I and one for PW_I (Figures 4-6). This finding once again confirms how F. verticillioides infection limits germination of maize kernels and reduces seedling weight and vigor. Conversely, no correspondence was observed with QTL previously described by Septiani et al. (2019), where the seedling weight and length as well as Fusarium seedling rot traits were evaluated by the same phenotyping method. This could be explained by the different genetic materials employed for mapping in the two researches (association mapping panel vs. multi-parent population-MAGIC) and the use of diverse genome assemblies.
Conclusions
In this work, a bioassay was applied to the maize association panel tested for seedling related traits to F. verticillioides resistance. GWAS analysis revealed 42 SNPs associated with three phenotypic traits: plant seedling length (PL), plant seedling weight (PW) and germination rate. In total, 25 genes were found associated with the SNPs and many of these genes had a role in disease response.
Comparison between genes found in our GWAS analysis and previous QTL mapping studies reported for resistance to FER, fumonisin accumulation and seedling traits revealed that the majority of the genes, associated with the SNPs, localized inside QTL for these traits.
These findings will help us to better understand the existing complex interaction between maize and F. verticillioides in order to improve genomic selection for Fusarium resistance at the seedling stage. Functional validation of the most recurring candidate genes will be required to verify their role in the pathways to hamper fungal infection in maize.
Acknowledgments
This research was funded by the European Union’s Horizon 2020 research and innovation program under Grant Agreement No. 678781 (MycoKey). The authors thank Paola Battilani and Paola Giorni for kindly providing F. verticillioides strain.
Footnotes
Supplemental material available at figshare: https://figshare.com/s/54bb47a6abf418c859b9
Communicating editor: E. Akhunov
Literature Cited
- Afzal A. J., Wood A. J., and Lightfoot D. A., 2008. Plant receptor-like serine threonine kinases: roles in signaling and plant defense. Mol. Plant Microbe Interact. 21: 507–517. 10.1094/MPMI-21-5-0507 [DOI] [PubMed] [Google Scholar]
- Atabaki A., Rahjoo V., Hanafi M. M., Abiri R., Zamanizadeh H. R. et al. , 2018. Morpho-molecular identification, pathogenicity variation, mating biology and fumonisin production of Fusarium species in Zea Mays L. Int. J. Plant Pathol. 7: 31–50. [Google Scholar]
- Battilani P., Rossi V., and Pietri A., 2003. Modelling Fusarium verticillioides infection and fumonisin synthesis in maize ears. Asp. Appl. Biol. 68: 91–100. [Google Scholar]
- Bernardi J., Stagnati L., Lucini L., Rocchetti G., Lanubile A. et al. , 2018. Phenolic profile and susceptibility to Fusarium infection of pigmented maize cultivars. Front. Plant Sci. 9: 1189 10.3389/fpls.2018.01189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bömke C., Rojas M. C., Hedden P., and Tudzynski B., 2008. Loss of gibberellin production in Fusarium verticillioides (Gibberella fujikuroi MP-A) is due to a deletion in the gibberellic acid gene cluster. Appl. Environ. Microbiol. 74: 7790–7801. 10.1128/AEM.01819-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury P. J., Zhang Z., Kroon D. E., Casstevens T. M., Ramdoss Y. et al. , 2007. TASSEL: Software for association mapping of complex traits in diverse samples. Bioinformatics 23: 2633–2635. 10.1093/bioinformatics/btm308 [DOI] [PubMed] [Google Scholar]
- Browning B. L., and Browning S. R., 2016. Genotype imputation with millions of reference samples. Am. J. Hum. Genet. 98: 116–126. 10.1016/j.ajhg.2015.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Zhang L., Liu S., Li Z., Huang R. et al. , 2016. The genetic basis of natural variation in kernel size and related traits using a four-way cross population in maize. PLoS One 11: e0153428 10.1371/journal.pone.0153428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chulze S. N., Ramirez M. L., Torres A., and Leslie J. F., 2000. Genetic variation in Fusarium section Liseola from no-till maize in Argentina. Appl. Environ. Microbiol. 66: 5312–5315. 10.1128/AEM.66.12.5312-5315.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsen S., Meyer U. M., and Funck Jensen D., 1998. Genetic characteristics of Fusarium verticillioides isolates from maize in Costa Rica. Plant Pathol. 47: 615–622. 10.1046/j.1365-3059.1998.00277.x [DOI] [Google Scholar]
- Desjardins, A. E., and R. H. Proctor, 2001 Biochemistry and genetics of Fusarium toxins. In: Fusarium: Paul E. Nelson Memorial Symposium (Ed. Summerell, B.A. Leslie, J.F., Backhouse, D., Bryden, W.L. and Burgess, L.W.). American Phytopathological Society, St. Paul, MN, 50‒69. [Google Scholar]
- Drepper W. J., and Renfro B. L., 1990. Comparison of methods for inoculation of ears and stalks of maize with Fusarium moniliforme. Plant Dis. 74: 952–956. 10.1094/PD-74-0952 [DOI] [Google Scholar]
- Ellis M. L., Broders K. D., Paul P. A., and Dorrance A. E., 2011. Infection of soybean seed by Fusarium graminearum and effect of seed treatments on disease under controlled conditions. Plant Dis. 95: 401–407. 10.1094/PDIS-05-10-0317 [DOI] [PubMed] [Google Scholar]
- Fierens E., Rombouts S., Gebruers K., Goesaert H., Brijs K. et al. , 2007. TLXI, a novel type of xylanase inhibitor from wheat (Triticum aestivum) belonging to the thaumatin family. Biochem. J. 403: 583–591. 10.1042/BJ20061291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint-Garcia S. A., Thuillet A. C., Yu J., Pressoir G., Romero S. M. et al. , 2005. Maize association population: a high-resolution platform for quantitative trait locus dissection. Plant J. 44: 1054–1064. 10.1111/j.1365-313X.2005.02591.x [DOI] [PubMed] [Google Scholar]
- Gaikpa D. S., and Miedaner T., 2019. Genomics-assisted breeding for ear rot resistances and reduced mycotoxin contamination in maize: methods, advances and prospects. Theor. Appl. Genet. 132: 2721–2739. 10.1007/s00122-019-03412-2 [DOI] [PubMed] [Google Scholar]
- Ju M., Zhou Z., Mu C., Zhang X., Gao J. et al. , 2017. Dissecting the genetic architecture of Fusarium verticillioides seed rot resistance in maize by combining QTL mapping and genome-wide association analysis. Sci. Rep. 7: 46446 10.1038/srep46446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanubile A., Pasini L., and Marocco A., 2010. Differential gene expression in kernels and silks of maize lines with contrasting levels of ear rot resistance after Fusarium verticillioides infection. J. Plant Physiol. 167: 1398–1406. 10.1016/j.jplph.2010.05.015 [DOI] [PubMed] [Google Scholar]
- Lanubile A., Pasini L., Lo Pinto M., Battilani P., Prandini A. et al. , 2011. Evaluation of broad spectrum sources of resistance to Fusarium verticillioides and advanced maize breeding lines. World Mycotoxin J. 4: 43–51. 10.3920/WMJ2010.1206 [DOI] [Google Scholar]
- Lanubile A., Bernardi J., Battilani P., Logrieco A., and Marocco A., 2012a Resistant and susceptible maize genotypes activate different transcriptional responses against Fusarium verticillioides. Physiol. Mol. Plant Pathol. 77: 52–59. 10.1016/j.pmpp.2011.12.002 [DOI] [Google Scholar]
- Lanubile A., Bernardi J., Marocco A., Logrieco A., and Paciolla C., 2012b Differential activation of defense genes and enzymes in maize genotypes with contrasting levels of resistance to Fusarium verticillioides. Environ. Exp. Bot. 78: 39–46. 10.1016/j.envexpbot.2011.12.006 [DOI] [Google Scholar]
- Lanubile A., Logrieco A., Battilani P., Proctor R. H., and Marocco A., 2013. Transcriptional changes in developing maize kernels in response to fumonisin-producing and nonproducing strains of Fusarium verticillioides. Plant Sci. 210: 183–192. 10.1016/j.plantsci.2013.05.020 [DOI] [PubMed] [Google Scholar]
- Lanubile A., Ferrarini A., Maschietto V., Delledonne M., Marocco A. et al. , 2014a Functional genomic analysis of constitutive and inducible defense responses to Fusarium verticillioides infection in maize genotypes with contrasting ear rot resistance. BMC Genomics 15: 710 10.1186/1471-2164-15-710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanubile A., Maschietto V., and Marocco A., 2014b Breeding maize for resistance to mycotoxins, Mycotoxin Reduction in Grain Chains, edited by Leslie J. F., and Logrieco A. F.. John Wiley & Sons, Ltd, Chichester, UK: 10.1002/9781118832790.ch4 [DOI] [Google Scholar]
- Lanubile A., Muppirala U. K., Severin A. J., Marocco A., and Munkvold G. P., 2015. Transcriptome profiling of soybean (Glycine max) roots challenged with pathogenic and non-pathogenic isolates of Fusarium oxysporum. BMC Genomics 16: 1089 10.1186/s12864-015-2318-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanubile A., Maschietto V., Borrelli V. M., Stagnati L., Logrieco A. F. et al. , 2017. Molecular basis of resistance to Fusarium ear rot in maize. Front. Plant Sci. 8: 1774 10.3389/fpls.2017.01774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie J. F., 1991. Mating populations in Gibberella fujikuroi (Fusarium section Liseola). Phytopathology 81: 1058–1060. [Google Scholar]
- Liu J. J., Sturrock R., and Ekramoddoullah A. K., 2010. The superfamily of thaumatin-like proteins: its origin, evolution, and expression towards biological function. Plant Cell Rep. 29: 419–436. 10.1007/s00299-010-0826-8 [DOI] [PubMed] [Google Scholar]
- Machado J. C., Machado A. Q., Pozza E. A., Machado C. F., and Zancan W. L. A., 2013. Inoculum potential of Fusarium verticillioides and performance of maize seeds. Trop. Plant Pathol. 38: 213–217. 10.1590/S1982-56762013000300005 [DOI] [Google Scholar]
- Mangeon A., Junqueira R. M., and Sachetto-Martins G., 2010. Functional diversity of the plant glycine-rich proteins superfamily. Plant Signal. Behav. 5: 99–104. 10.4161/psb.5.2.10336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marasas W. F. O., 2001. Discovery and occurrence of the fumonisins: A historical perspective. Environ. Health Perspect. 109: 239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchler-Bauer A., Derbyshire M. K., Gonzales N. R., Lu S., Chitsaz F. et al. , 2015. CDD: NCBI’s conserved domain database. Nucleic Acids Res. 43: 222–226. 10.1093/nar/gku1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maschietto V., Lanubile A., De Leonardis S., Marocco A., and Paciolla C., 2016. Constitutive expression of pathogenesis-related proteins and antioxydant enzyme activities triggers maize resistance towards Fusarium verticillioides. J. Plant Physiol. 200: 53–61. 10.1016/j.jplph.2016.06.006 [DOI] [PubMed] [Google Scholar]
- Maschietto V., Colombi C., Pirona R., Pea G., Strozzi F. et al. , 2017. QTL mapping and candidate genes for resistance to Fusarium ear rot and fumonisin contamination in maize. BMC Plant Biol. 17: 20 10.1186/s12870-017-0970-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMullen M. D., Kresovich S., Villeda H. S., Bradbury P., Li H. et al. , 2009. Genetic properties of the maize nested association mapping population. Science 325: 737–740. 10.1126/science.1174320 [DOI] [PubMed] [Google Scholar]
- Mesterházy Á., Lemmens M., and Reid L. M., 2012. Breeding for resistance to ear rots caused by Fusarium spp. in maize – a review. Plant Breed. 131: 1–19. 10.1111/j.1439-0523.2011.01936.x [DOI] [Google Scholar]
- Munkvold G. P., McGee D. C., and Carlton W. M., 1997. Importance of different pathways for maize kernel infection by Fusarium moniliforme. Phytopathology 87: 209–217. 10.1094/PHYTO.1997.87.2.209 [DOI] [PubMed] [Google Scholar]
- Munkvold G. P., and Desjardins A. E., 1997. Fumonisins in maize can we reduce their occurrence? Plant Dis. 81: 556–565. 10.1094/PDIS.1997.81.6.556 [DOI] [PubMed] [Google Scholar]
- Munkvold G. P., 2003. Cultural and genetic approaches to managing mycotoxins in maize. Annu. Rev. Phytopathol. 41: 99–116. 10.1146/annurev.phyto.41.052002.095510 [DOI] [PubMed] [Google Scholar]
- Murillo-Williams A., and Munkvold G. P., 2008. Systemic infection by Fusarium verticillioides in maize plants grown under three temperature regimes. Plant Dis. 92: 1695–1700. 10.1094/PDIS-92-12-1695 [DOI] [PubMed] [Google Scholar]
- Oren L., Ezrati S., Cohen D., and Sharon A., 2003. Early events in the Fusarium verticillioides-maize interaction characterized by using a green fluorescent protein-expressing transgenic isolate. Appl. Environ. Microbiol. 69: 1695–1701. 10.1128/AEM.69.3.1695-1701.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S., Neale B., Todd-Brown K., Thomas L., Ferreira M. A. et al. , 2007. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81: 559–575. 10.1086/519795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahjoo V., Zad J., Javan-Nikkhah M., Mirzadi Gohari A., Okhorvat S. M. et al. , 2008. Morphological and molecular identification of Fusarium isolated from maize ears in Iran. J. Plant Pathol. 90: 463–468. [Google Scholar]
- R Core Team, 2016 R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/.
- Reid L. M., and Zhu X., 2005. Screening corn for resistance to common diseases in Canada, Agriculture and Agri-Food Canada, Ottawa. [Google Scholar]
- Reid L. M., Zhu X., Parker A., and Yan W., 2009. Increased resistance to Ustilago zeae and Fusarium verticillioides in maize inbred lines bred for Fusarium graminearum resistance. Euphytica 165: 567–578. 10.1007/s10681-008-9782-6 [DOI] [Google Scholar]
- Samayoa L. F., Malvar R. A., Olukolu B. A., Holland J. B., and Butrón A., 2015. Genome-wide association study reveals a set of genes associated with resistance to the Mediterranean corn borer (Sesamia nonagrioides L.) in a maize diversity panel. BMC Plant Biol. 15: 35 10.1186/s12870-014-0403-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samayoa L. F., Cao A., Santiago R., Malvar R. A., and Butrón A., 2019. Genome-wide association analysis for fumonisin content in maize kernels. BMC Plant Biol. 19: 166 10.1186/s12870-019-1759-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekhon R. S., Lin H., Childs K. L., Hansey C. N., Buell C. R. et al. , 2011. Genome-wide atlas of transcription during maize development. Plant J. 66: 553–563. 10.1111/j.1365-313X.2011.04527.x [DOI] [PubMed] [Google Scholar]
- Septiani P., Lanubile A., Stagnati L., Busconi M., Nelissen H. et al. , 2019. Unravelling the genetic basis of Fusarium seedling rot resistance in the MAGIC maize population: novel targets for breeding. Sci. Rep. 9: 5665 10.1038/s41598-019-42248-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A., Shumayla S., Tyagi S., Alok A., Singh K. et al. , 2020. Thaumatin-like protein kinases: Molecular characterization and transcriptional profiling in five cereal crops. Plant Sci. 290: 110317 10.1016/j.plantsci.2019.110317 [DOI] [PubMed] [Google Scholar]
- Stagnati L., Lanubile A., Samayoa L. F., Bragalanti B., Giorni P. et al. , 2019. A genome wide association study reveals markers and genes associated with resistance to Fusarium verticillioides infection of seedlings in a maize diversity panel. G3 (Bethesda) 9: 571–579. 10.1534/g3.118.200916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorstensen T., Grini P. E., and Aalen R. B., 2011. domain proteins in plant development. Biochim. Biophys. Acta 1809: 407–420. 10.1016/j.bbagrm.2011.05.008 [DOI] [PubMed] [Google Scholar]
- Van Wyk P., Scholtz D., and Marasas W., 1988. Protection of maize seedlings by Fusarium moniliforme against infection by Fusarium graminearum in the soil. Plant Soil 107: 251–257. 10.1007/BF02370554 [DOI] [Google Scholar]
- Voss K. A., Howard P. C., Riley R. T., Sharma R. P., Bucci T. J. et al. , 2002. Carcinogenicity and mechanism of action of fumonisin B1: A mycotoxin produced by Fusarium moniliforme (=F. verticillioides). Cancer Detect. Prev. 26: 1–9. 10.1016/S0361-090X(02)00011-9 [DOI] [PubMed] [Google Scholar]
- Wang Y., Zhou Z., Gao J., Wu Y., Xia Z. et al. , 2016. The mechanisms of maize resistance to Fusarium verticillioides by comprehensive analysis of RNA-seq data. Front. Plant Sci. 7: 1654 10.3389/fpls.2016.01654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton M. L., Williams W. P., Windham G. L., Murray S. C., Xu W. et al. , 2013. Phenotypic and genetic characterization of a maize association mapping panel developed for the identification of new sources of resistance to Aspergillus flavus and aflatoxin accumulation. Crop Sci. 53: 2374–2383. 10.2135/cropsci2012.10.0616 [DOI] [Google Scholar]
- Williams L. D., Glenn A. E., Zimeri A. M., Bacon C. W., Smith M. A. et al. , 2007. Fumonisin disruption of ceramide biosynthesis in maize roots and the effects on plant development and Fusarium verticillioides-induced seedling disease. J. Agric. Food Chem. 55: 2937–2946. 10.1021/jf0635614 [DOI] [PubMed] [Google Scholar]
- Wilke A. L., Bronson C. R., Tomas A., and Munkvold G. P., 2007. Seed transmission of Fusarium verticillioides in maize plants grown under three different temperature regimes. Plant Dis. 91: 1109–1115. 10.1094/PDIS-91-9-1109 [DOI] [PubMed] [Google Scholar]
- Yang X., Yan J., Shah T., Warburton M. L., Li Q. et al. , 2010. Genetic analysis and characterization of a new maize association mapping panel for quantitative trait loci dissection. Theor. Appl. Genet. 121: 417–431. 10.1007/s00122-010-1320-y [DOI] [PubMed] [Google Scholar]
- Yates I. E., Bacon C. W., and Hinton D. M., 1997. Effects of endophytic infection by Fusarium moniliforme on corn growth and cellular morphology. Plant Dis. 81: 723–728. 10.1094/PDIS.1997.81.7.723 [DOI] [PubMed] [Google Scholar]
- Yates I. E., Arnold J. W., Hinton D. M., Basinger W., and Walcott R. R., 2003. Fusarium verticillioides induction of maize seed rot and its control. Can. J. Bot. 81: 422–428. 10.1139/b03-034 [DOI] [Google Scholar]
- Zhang Z., Ersoz E., Lai C. Q., Todhunter R. J., Tiwari H. K. et al. , 2010. Mixed linear model approach adapted for genome-wide association studies. Nat. Genet. 42: 355–360. 10.1038/ng.546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zila C. T., Samayoa L. F., Santiago R., Butrón A., and Holland J. B., 2013. A genome-wide association study reveals genes associated with fusarium ear rot resistance in a maize core diversity panel. G3 (Bethesda) 3: 2095–2104. 10.1534/g3.113.007328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zila C. T., Ogut F., Romay M. C., Gardner C. A., Buckler E. S. et al. , 2014. Genome wide association study of Fusarium ear rot disease in the U.S.A. maize inbred line collection. BMC Plant Biol. 14: 372 10.1186/s12870-014-0372-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All supporting data are included as supplemental files and are available at Figshare: https://figshare.com/s/54bb47a6abf418c859b9. Table S1. (Excel file) List of germplasm and phenotypic values. Table S2. (DOCX file) Table of LD analysis.