Abstract
Background
The presence of dilated intercellular spaces in the stratified squamous lining of the esophagus is the pathognomonic feature of reflux esophagitis secondary to gastroesophageal reflux disease (GERD). In addition to stomach acid, bile salts are major constituents of gastroesophageal refluxate. The aim of our study was to determine the effect of bile salts cocktail at different pHs on epithelial junctions in an in vitro transwell model of stratified esophageal squamous epithelium.
Discussion
Human telomerase reverse transcriptase (hTERT) immortalized primary esophageal EPC1 cells were grown on polyester transwell surfaces in calcium-enriched media. The cells exhibited gradual stratification into an 11-layered squamous epithelium over 7 days, together with epithelial barrier function as indicated by increased transepithelial electrical resistance (TEER). This stratified epithelium demonstrated well-formed tight junctions, adherens junctions, and desmosomes as visualized by immunofluorescence and electron microscopy. When exposed to short pulses of bile salts at pH 5, but not either condition alone, there was loss of stratification and decrease in TEER, concomitant with disruption of adherens junctions, tight junctions, and desmosomes, leading to the appearance of dilated intercellular spaces. At the cellular level, bile salts at pH 5 activated the Wnt pathway (indicated by increased β-catenin Ser552 phosphorylation).
Conclusion
In conclusion, in our in vitro transwell model bile salts at pH 5, but not bile salts or media at pH 5 alone, modulate Wnt signaling, disrupt different junctional complexes, and cause increased permeability of stratified squamous esophageal epithelium. These changes approximate the appearance of dilated intercellular space similar to that found in GERD patients.
Keywords: Gastroesophageal reflux disease, Dilated intercellular spaces, Bile salt
Introduction
Dilated intercellular space (DIS) is a histological feature of gastroesophageal reflux disease (GERD) especially in the early stages (Fig. 1). Almost 30 % of the US population experiences some reflux symptoms, the chief one being heartburn, with about half exhibiting daily symptoms.1 In reflux disease, the gastro-duodenal contents regurgitate into the lower end of the esophagus because of an incompetent lower esophageal sphincter (LES).2 Patients with protracted reflux are at increased risk of developing Barrett’s esophagus (BE), a precancerous lesion of esophageal adenocarcinoma (EAD).3
Fig. 1.

Dilated intercellular spaces in patient biopsies—biopsies taken from the lower esophagus of patients diagnosed with clinical GERD showed discernible dilatation of intercellular spaces with hematoxylin and eosin staining under both low and high magnification, whereas biopsies taken from non-GERD individuals showed tight intracellular connection with virtually no intercellular spaces (Fig. 1)
Gastric acid at pH 2–4, gastric pepsin, and bile salts from the duodenum are the chief constituents of gastro-duodeno-esophageal reflux.4 Gastric acid has been shown to induce DIS in rabbit esophageal epithelium by interfering with active sodium transport across the epithelium, with pepsin exacerbating the process.5 Bile salts, in combination with acid-pepsin, have also been implicated in inducing DIS in rabbit epithelium.6 Formation of DIS in the esophageal epithelium can be correlated with many GERD-related esophageal and extra-esophageal symptoms.7
Bile salts have been implicated in the pathogenesis of reflux disease and BE. Depending on the conjugation and ionization status bile salts might affect cell junctions extracellularly acting as a detergent, or intra-cellularly acting as signaling molecules.8–10 In our recent publication, we reported that bile salts at low pH promote loss of differentiation status of stratified squamous esophageal epithelium in vitro, at least in part, through epidermal growth factor activation, and downstream Akt and ERK1/2 pathways are active in post-injury mucosal repair.11 Wnt activation, happening downstream to EGFR activation, is another crucial event in the initiation of mucosal repair.12 In this publication, we aim to expand on those findings and determine the effect of bile salts cocktail at different pHs on epithelial junctions, permeability, and induction of DIS in our in vitro model of muscosal injury, and explore possible signaling mechanisms involved.
Materials and Methods
Human Biopsies
All human tissues were obtained from consenting patients according to protocols approved by the University of Rochester Research Subjects Review Board. Each biopsy was reviewed by the study pathologist (Z.Z.), and biopsies were used only if they contained 100 % of one tissue type. Biopsies were fixed in 4 % buffered formalin and embedded in paraffin. Once the paraffin blocks had solidified, 5-μm sections were cut with a microtome, mounted on positively charged glass slides, and allowed to dry. Sections were stained using standard hematoxylin and eosin (H&E) staining procedure.
Cell Culture
All experiments were performed using the human telomerase reverse transcriptase (hTERT)—immortalized primary esophageal cell line, EPC 1 (a generous gift from Dr. Anil Rustgi, University of Pennsylvania, Philadelphia, PA, USA).13 Cells were plated on Transwell® inserts with polyester membranes (0.4 μm) (Costar®, Corning, NY, USA) at 40 % cell density and grown to confluence in normal keratinocyte serum-free media (Gibco®, Carlsbad, CA, USA) supplemented with human recombinant EGF and bovine pituitary extract in both the apical and basal compartments. The basal compartment was replenished every alternate day. Once the cells reached confluence, the same media was supplemented to 0.6 mM calcium chloride (Invitrogen, Carlsbad, CA, USA).14 All treatments were done in Ca2+-supplemented media, unless otherwise specified (Fig. 2).
Fig. 2.
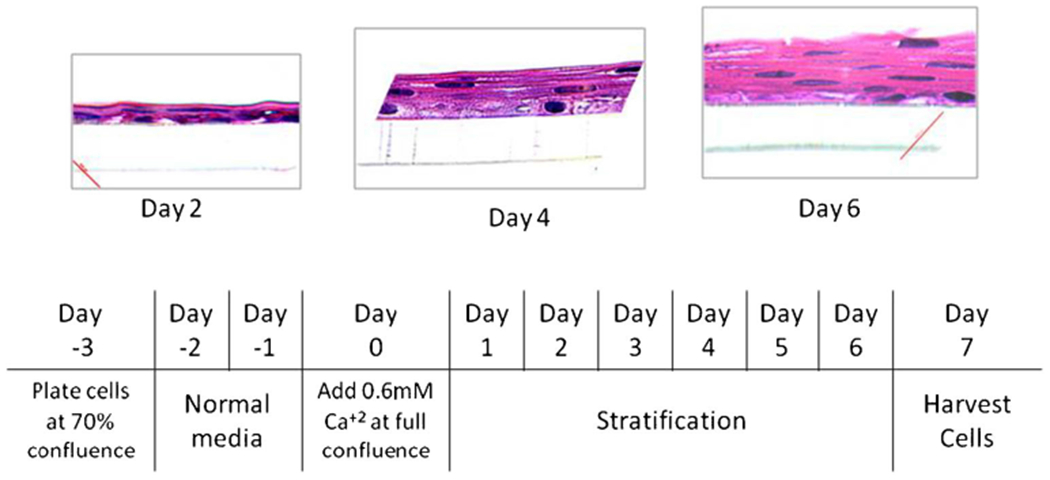
EPC1 cells stratify in presence of calcium on polyester transwells—hematoxylin and eosin staining of EPC1 cells on transwell gradually stratifies in 6 days of culture in media enriched with calcium. The culture protocol is shown in the schema below
Bile Salts Treatment
Bile salts and acid treatments were performed using a protocol modified from Liu et al,15 only in the apical media of the transwell. Confluent cells were treated with control media, bile salts cocktail at pH 7.4, pH 5 media, and bile salts cocktail at pH 5 for 15-min pulses at three times a day for up to 6 days. Cells were cultured in the control media between treatments. Cells were assessed for transepithelial electrical resistance (TEER) every morning before the first treatment. For assessment of tissue morphology, cells were harvested the morning after the last treatment. For signaling experiments, cells were grown to full confluence in the presence of calcium. They were then deprived of human recombinant EGF and bovine pituitary extract and left overnight before further treatment. The following day, cells were treated with bile salts cocktail at pH 5 for 5 min, 15 min, 30 min, 1 h, 2 h, 4 h, 8 h, and 16 h. In parallel, cells were treated with the control media for 16 h. Replicate transwells were harvested immediately after the last treatment for isolation of protein and Western blot analysis.
Bile Salts Cocktail Components
The bile salts cocktail consisted of 170 μM glycocholic acid (TCI America, Portland, OR, USA), 25 μM taurocholic acid, 125 μM glycochenodeoxycholic acid, 25 μM taurochenodeoxycholic acid, 50 μM glycodeoxycholic acid, 8 μM taurodeoxycholic acid, and 100 μM deoxycholic acid (all from Sigma Chemical Co., St. Louis, MO, USA).
Transepithelial Electrical Resistance
The cells in transwell culture were daily monitored by measuring the TEER using the Epithelial Voltohmmeter (EVOM2, World Precision Instruments, Inc., Sarasota, FL). The chopstick electrodes were placed in both the apical and basal compartments with the tip fully immersed in the media for the measurement. Each transwell was measured thrice at three different positions, and the mean value was taken and then multiplied with the total surface area of the transwell. For each condition, an average of three replicate transwell readings was used to plot the TEER graph. The principles of TEER measurement are comprehensively illustrated in a review by Benson et al.16
Formalin Fixation and Paraffin Embedding
Cells grown on transwell inserts were rinsed with Hank’s balanced salt solution (HBSS; Gibco® Carlsbad, CA, USA) at 37 °C and fixed in 4 % buffered formalin. Cells were then rinsed with HBSS and dehydrated using a graded ethanol wash (35–100 %, 10-min washes). After the last wash, cells on transwells were treated with clearing agent Histo-Clear (National Diagnostics, Atlanta, GA, USA) for 10 min and then infiltrated with liquid paraffin in an oven set at 58 °C for 1 h. The transwell plates with inserts immersed in paraffin were removed from the oven to allow the paraffin to solidify. Special care was taken when removing the membranes from the transwell inserts by using Harris Uni-Core™ 8.0-mm punch biopsy razors (Ted Pella Inc., Redding, CA) and by embedding them longitudinally in paraffin boats with cassettes. Once the paraffin blocks had solidified, 5-μm sections were cut with a microtome, then mounted on positively charged glass slides, and allowed to dry. Sections were stained with hematoxylin and eosin (H&E).
Protein Isolation and Western Blot Analysis
Protein was isolated from cultured EPC1 cells after harvesting them with ice-cold radioimmunoprecipitation assay buffer. Western blot analysis was performed with rabbit anti-phospho serine 552 β-catenin antibody (1:1000; secondary 1:5000) and rabbit anti-β-catenin (1:1000; secondary 1:5000 antibody) from Cell Signaling Technology, Danvers, MA, USA. Rabbit anti β-tubulin antibody (1:1000; secondary1:5000), from Cell Signaling Technology, Danvers, MA, USA, was used as the loading control. Secondary antibodies were all from Cell Signaling Technology, Danvers, MA, USA.
Immunolabeling Cells on Transwell Membrane
The cells on transwells were fixed with 4 % paraformaldehyde at 37 °C for 20 min at room temperature. Cells were rinsed again and permeated with 0.5 % Triton X-100 solution for 10 min at room temperature. After rinsing again, the membranes with the fixed cells were cut out of the transwell very carefully using a needle tip and placed on paraffin films. Membranes were then blocked with 1 % bovine serum albumin (BSA) solution overnight and then incubated with primary antibodies diluted 1:100 in 1 % BSA for an hour. Thereafter, membranes were rinsed and incubated with fluorochrome-conjugated secondary antibodies diluted 1:1000 in 1 % BSA for an hour. Finally, after rinsing the excess antibodies with PBS, the membranes were mounted on plus slides with cover slips. The stratified cell layers were visualized using confocal microscopy.
Electron Microscopy
Cells on transwell membranes were fixed with 4 % formaldehyde and 1 % glutaraldehyde solution, and sent to University of Rochester Medical Center Electron Microscope Research Core (Rochester, NY) for sample preparation, visualization, and analysis using a transmission electron microscope (Hitachi TEM 7650).
Results
EPC1 Cells on Polyester Transwells Stratify in the Presence of Calcium
By restricting transepithelial transport through cell junctions, every epithelial layer exerts an electrical resistance.16 This is called epithelial barrier function. Greater the amount of stratification and/or stronger the cell-cell junctions, higher will be the electrical resistance and lower will be the transepithelial permeability. When EPC1 cells were grown in vitro on polyester transwells in media enriched with calcium, they gradually became increasingly stratified over 6 days of culture, as visualized by hematoxylin and eosinstained cross-sections (Fig. 2). As the epithelium stratified, the transepithelial electrical resistance (TEER) increased in parallel (Fig. 3a, b—blue line) indicative of increased epithelial barrier function and functional cell junction formation.
Fig. 3.

Treatment with bile acid at pH 5 decreases epithelial resistance—transepithelial electrical resistance increased through day 1–6 as EPC1 cells stratified on transwells (a, b—blue line). TEER reduced overnight after the third pulse treatment with cocktail of bile salts at pH 5 (a, b—yellow line), but with neither alone (a—red and green lines)
Treatment with Bile Salts at pH 5 Decreases Epithelial Barrier Function
In order to investigate the effects of extracellular pH and bile salts on formation of esophageal epithelial barrier, we compared the dynamics of TEER in four different experimental conditions: (i) EPC1 cells growing in normal culture medium with neutral pH 7.4, (ii) cells growing under neutral pH in the presence of bile salts cocktail (BA, pH 7.4), (iii) cells growing in the acidified medium with pH 5.0, and (iv) cells growing in the acidic medium with bile salts (BA, pH 5.0). Remarkably, EPC1 cells growing in the presence of bile salts at pH 5 developed significantly lower TEER (Fig. 3a: yellow line), compared to cells growing in neutral pH medium (Fig 3a: blue line), thereby indicating attenuated assembly of the epithelial barrier. By contrast, TEER was not significantly affected by cell exposure to either pH 5 media alone or bile salts at the neutral pH (Fig. 3a, b). Next, we asked if exposure to bile salts at acidic pH can only prevent assembly of the epithelial barrier or it also disrupts preexisting barrier. To do that, we allowed EPC1 cells to form a barrier by culturing them for the first 3 days in neutral medium and then exposing the cells to pulses of acidic medium with bile salts. Figure 3b shows that cell exposure to bile salts in acidic conditions efficiently disrupted already formed epithelial barrier.
Bile Salts at pH 5 Causes Loss of Stratification
Therefore, we next determined if exposure to bile salts at low pH affects epithelial stratification and assembly of intercellular junctions in EPC1 cells. Electron micrograph suggested that apical treatment with bile salts at pH 5, but not bile salts at pH 7.4, reduced stratification in EPC1 cells on transwells. Treatment with bile salts at pH 5 resulted in a seven-layered stratification which is significantly lower comparing to 11-layered stratification of the control EPC1 cells. By contrast, bile salts at pH 7.4 did not affect stratification, whereas cell exposure to acidic medium alone only modestly decreased stratification (to nine layers) (Fig. 4).
Fig. 4.

Bile salts at pH 5 cause loss of stratification—electron microscopy of EPC1 cells grown on transwells after 7 days of treatment. Apical side is on the top, and basal side along with the polyester filter can be seen at the bottom. Nucleated basal cells can be seen at the bottom, and non-nucleated, flattened, terminally differentiated keratinocytes can be seen on the top. Cells treated with the control media, bile salts at pH 7.4, show similar levels of stratification. Cells treated with bile salts at pH 5 show considerable loss of stratification. pH 5 also shows some loss of stratification
Bile Salts at pH 5 Disrupts Major Epithelial Junctions
In order to examine assembly of epithelial junctions under our experimental conditions, we analyzed the morphology of three major types of junctional complexes such as desmosomes, adherens junctions (AJ), and tight junctions (TJ). Desmosomes were analyzed by transmission electron microscopy, whereas AJ and TJ were visualized by immunofluorescence labeling and confocal microscopy. Electron micrographs of cells growing in the neutral medium show a close alignment of cell membranes of contacting cells and abundant desmosomes visualized as prominent electron-dense areas at cell-cell contacts. Cell exposure to bile salts at pH 5 caused a marked loss of desmosomes that was accompanied by the diminished intercellular contacts and dilatation of intracellular spaces (Fig. 5). Treatment with bile acid at neutral pH also showed some degree of desmosomal disruption especially in the suprabasal layer (Figs. 4 and 5), indicating that pH may be modulating the effect of bile salts on esophageal epithelium. By contrast, desmosomes were essentially unchanged following treatment with media at pH 5 alone.
Fig. 5.

Bile salts at pH 5 cause desmosomal disruption—disruption of desmosomes (solid arrow) as well as DIS (patterned arrow) is markedly visible at higher magnification after treatment with bile salts at pH 5 and at pH 7.4 to lesser extent. The desmosomal architecture remains intact after treatment with pH 5 media, similar to the control
Immunofluorescence labeling and confocal microscopy was used to examine the effects of pH and bile salts on integrity of AJ and TJ. In EPC1 cells growing at neutral pH, assembly of mature AJ and TJ in the apical epithelial layer was manifested by the appearance of characteristic “chicken wire” labeling pattern for E-cadherin and ZO-1, respectively (Fig. 6). Treatment with bile salts at pH 5 disrupted this characteristic labeling leading to predominantly cytoplasmic accumulation of E-cadherin and ZO-1. By contrast, exposure to either pH 5 alone or bile salts at neutral pH did not affect junctional labeling of E-cadherin or ZO-1 (Fig. 6a). The observed effects of bile salts and acidic pH were not limited to selective junctional proteins, and similar mislocalization was observed for other molecular components of AJ (p120 catenin, α-catenin, and β-catenin) and TJ (occludin and AF6) (Fig. 6b, c). Overall, this data strongly suggests that bile salts at pH 5 disrupt esophageal epithelial barriers by causing disassembly of AJ, TJ, and desmosomes.
Fig. 6.

Bile salts at pH 5 cause desmosomal disruption—in a, adherens junction protein E-cadherin is visualized in red, and tight junction protein zonula occludin-1 is visualized in green under confocal microscopy. Treatment of bile salts at pH 5 on stratified EPC1 cells on transwells caused disruption of cell-cell junctions, whereas pH 5 only or bile salts at neutral pH has no effect on the cell-cell junctions. In b, adherens junction proteins p120 catenin is visualized in red, and tight junction protein occludin is visualized in green under confocal microscopy. In c, adherens junction proteins AF6 is visualized in green, and adherens junction-associated proteins α-catenin and β-catenin are visualized in red under confocal microscopy. Both figures indicate treatment with bile salts at pH 5 on stratified EPC1 cells on transwells caused disruption of cell-cell junctions
Bile Salts at Low pH Activate Wnt Signaling Pathway
To elucidate the possible mechanism by which bile salts at pH 5 might induce disruption of tight junctions and/or dilation of intracellular space, the status of Wnt was determined by assessing the level of phosphorylation of β-catenin at serine 552 residue. Bile salts at pH 5 caused Wnt activation within as early as 30 min of treatment (Fig. 7a) and were also consistent with 6 days of pulse treatment (Fig. 7b).
Fig. 7.
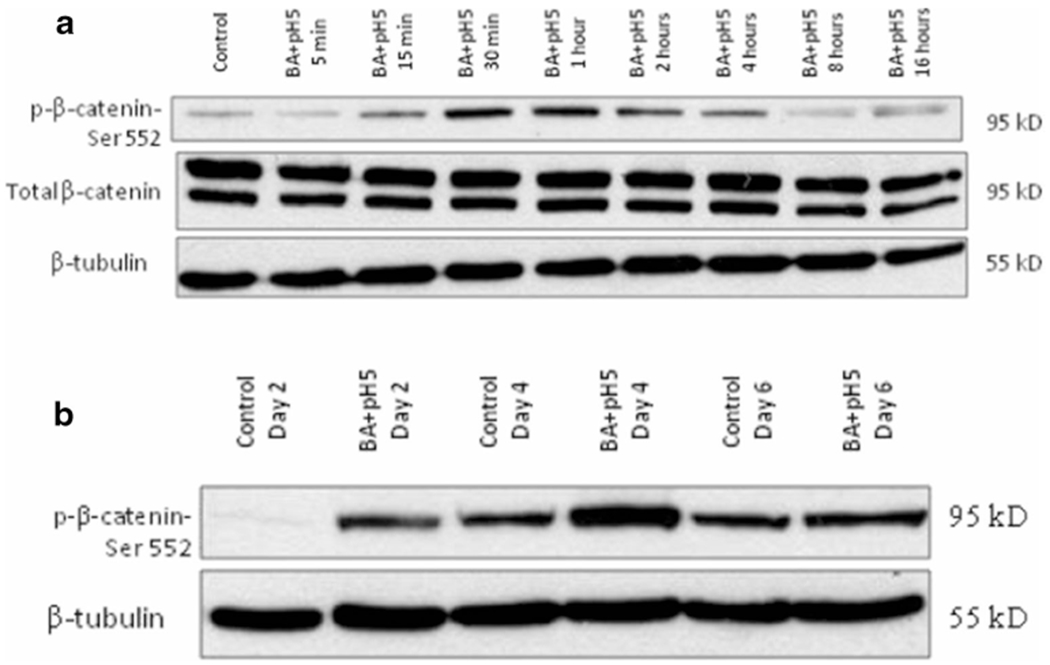
Bile salts at low pH activate WNT pathway—bile salts at pH 5 caused β-catenin serine residue-552 phosphorylation in EPC1 cells on transwells, following 30 min of treatment, indicating WNT activation, with maximum phosphorylation seen at 1 h (a). Activation persisted with longterm treatment on days 2, 4, and 6 of treatment (b). Total β-catenin level remained unchanged. β-tubulin served as the loading control
Discussion
The most important innate defense for the esophageal epithelium against noxious luminal environment is the lower esophageal sphincter (LES). In health, the esophageal and the gastric luminal contents are separated by a functional LES, which is a high pressure zone at the gastroesophageal junction (GEJ). The LES normally remains closed all the time, except when food is being passaged from esophagus to stomach, when it opens as a part of receptive relaxation of the stomach or transient lower esophageal sphincter relaxation (TLERS).17 However, in reflux disease, due to anatomic damage to the LES, the high pressure zone is lost and gastro-duodenal contents are regurgitated freely into the lower esophagus. Through a protracted course of the disease, the epithelial defenses may fail resulting in epithelial injury manifest by the appearance of dilated intercellular space (DIS), followed by epithelial inflammation and erosions.18 Even before the inflammation sets in, the epithelium actively battles the luminal assault by initiating epithelial restitution, a process in which anti-apoptotic, anti-oxidant, and cell migration promoting genes are activated to restore the epithelial integrity and barrier function.19 Formation of DIS can arguably be viewed as a histological feature of a pro-migratory epithelium, where intercellular junctions are deregulated to facilitate the process of epithelial restitution.
Epithelial injury and disruption of intercellular tight junctions leading to DIS formation as well as loss of barrier function has been well characterized in rabbit as well as human esophagus using ex vivo culture systems.6,20 Recently, Kalabis et al. have shown that mouse esophageal keratinocytes can be co-cultured with esophageal fibroblasts on transwells coated with collagen matrix to give rise to stratified squamous epithelium.21 Previously, we have reported the development of a simplified transwell culture system where EPC1 cells can be grown into stratified squamous epithelium without the need for co-culture or coated matrix.11 In order to study epithelial injury in response to simulated gastroesophageal reflux in an in vitro culture system, our EPC1 transwell culture was assessed for stratification and epithelial barrier function. When grown on transwells in the presence of the calcium-enriched medium EPC1 cells gradually formed a stratified epithelium with a corresponding increase in the TEER with increasing stratification. At day 7 of culture, EPC1 epithelium reached 11 layers of stratification (Fig. 4). Both increase in stratification as well as TEER can be attributed to calcium-induced formation of cell junctions, which we assessed thereby.
Cells in a stratified epithelium need an abundance of cell junctions in the form of tight junctions, adherens junctions, and desmosomes in order to remain attached to the piling layers of cells in three dimension, and hemi-desmosomes to attach to the basement membrane.22,23 Stratified EPC1 epithelium on transwells had well-formed desmosomal junctions when visualized by transmission electron microscopy, in addition to well-delineated tight junctions when visualized for ZO-1 and occludin by immunofluorescence staining. The stratified epithelium also expressed well-formed adherens junctions when visualized for adherens junction protein E-cadherin, AF-6, and adherens junction-associated proteins α, β, and p120 catenins. β-catenin not only functions in maintaining adherens junctions by remaining tethered to E-cadherin at the cell surface, it is also a transcription factor that when translocated to the nucleus activates the Wnt pathway, thus promoting cell proliferation, migration, and epithelial repair.24 Akt-mediated β-catenin serine-552 phosphorylation can release it from the junctional complex thus enabling its nuclear translocation and Wnt activation.25
In GERD patients with acid related symptoms, acid suppression is the mainstay of therapy. DIS in non-erosive reflux disease does not respond very well to acid suppression therapy, thus raising the possibility of the bile salts as reflux components being chiefly responsible for the histologic phenotype.26,27 The esophageal lumen is protected from transient acid exposure by the buffering action of bicarbonate coming from saliva and esophageal submucosal glands, as well as the clearing action of gravity and esophageal peristalsis.28 Generally, the intact epithelium is protected from pepsin-mediated damage if the refluxing pH is greater than 3, which is the gastric pH range in the majority of patients on long-term acid suppression therapy. On the other hand, acid suppression does not stop regurgitation of bile salts through a damaged LES, and the natural ability of gastric juice to precipitate bile salts is lost as pH rises beyond 3.29 Thus, in the refractory GERD patients, it is likely that bile salts might be responsible for inducing DIS in esophageal epithelium.
In our study, we chose to test media at pH 5, alone or in combination with bile salts, as it represents the reflux pH of patients under long-term acid suppression therapy.30,31 Also, pH 5 is very close to the pKa (acid dissociation constant) of the bile salts used in our treatment cocktail, thus facilitating their membrane permeability and hindering detergent action.32 The bile salts cocktail used here is physiologically relevant to that found in patient reflux.4 Thus, the treatment combination of bile salts at pH 5 very closely mimics the composition and pH found in most reflux patients in clinical settings. Bile salts at pH 5 disrupted tight junctions, adherens junctions, and desmosomes. As a result, there was significant reduction of epithelial stratification as well as decreased TEER. The effect on TEER highly corresponded with the commencement of treatment with bile salts and pH 5 cocktail, indicating a quite rapid effect on the junctions. Of note, bile salts at neutral pH caused some degree of desmosomal disruption but had no effect on the tight and adherens junctions. pH 5 alone did not cause any disruption of desmosomes, adherens, and tight junctions, nor did it affect the barrier function as indicated by TEER measurement but caused some degree of loss of stratification. Therefore, only treatment condition that had consistent effects on TEER, desmosomes, AJs, and TJs was the cocktail bile salts at pH 5.
Desmosomal disruption by bile salts both at pHs 5 and 7.4 created a striking resemblance to DIS in EPC1 epithelium when visualized under high magnification in electron microscopy. There seemed to be a larger dosage effect with bile salts at pH 5 when compared to bile salts at pH 7.4, indicating the unionized fraction as the effector molecules. At pH 5, a pH closer to the pKa of bile salts, there is a greater amount of unionized bile salts (about 50% of total bile salt concentration) in solution which are responsible for increased damage to the cell junctions than at pH 7.4.32 At neutral pH, the damage was restricted to desmosomes only, as there were reduced amounts of unionized bile salts (less than 1 % of total concentration) present to damage the other types of cell junctions. Perhaps with longer treatment time-course, damage to other cell junctions might have been observed even with bile salts at neutral pH. Percentage ionization of bile salts at a specific pH can be calculated by the derivative of Henderson-Hasselbalch equation33:-
Moreover, the cocktail of bile salts at pH 5 caused Wnt activation by β-catenin phosphorylation at serine 552. We have previously reported that bile salts at pH 5 activate EGFR, Akt, and ERK1/2 pathways in EPC1 cell transwell culture.11 Wnt activation might as well be downstream event following EGFR activation.25
Bile salts are known to disrupt cell junctions and reduce TEER in Caco-2 cell lines.9,34 Bile salts are also known to serine phosphorylate β-catenin and release it from membrane-bound E-cadherin (adherens junction) complex, and thus activate Wnt signaling following its nuclear translocation in human colon cancer cell lines.12 We have shown in our previous publication that bile salts activate EGFR, downstream Akt and ERK1/2 pathways.11 Akt-mediated serine 552 phosphorylation of β-catenin is known to release it from membrane-bound E-cadherin junction complex, leading to β-catenin-mediated cell proliferation and migration.25 Thus, taking our previous study, literature evidences and our current data into consideration, we propose the following mechanism by which bile salts may disrupt cell junctions to induce DIS formation (Fig. 8). At basal condition in a terminally differentiated stratified squamous cell, Wnt signaling is inactive, and β-catenin binds to E-cadherin and is tethered to the adherens junction. Bile acid, possible by activating EGFR pathway and downstream Akt phosphorylation, might cause β-catenin phosphorylation at Ser552 residue triggering its release and subsequent disruption of the adherens junction complex. β-catenin then translocates into the nucleus and activates Wnt pathway. Thus, bile salts treatment of terminally differentiated squamous cells might induce a partial and temporary undifferentiated state in them through activation of Wnt, thereby facilitating mucosal repair. As the effects are more enhanced with bile salts at pH 5 than at pH 7.4, this points to an intracellular signaling action by unionized fraction of bile salts, rather than a detergent action. Bile salts can generate intracellular reactive oxygen species, which in turn can activate EGFR pathway by releasing pro-ligands.9 Further study is needed to elucidate the exact mechanism of action.
Fig. 8.
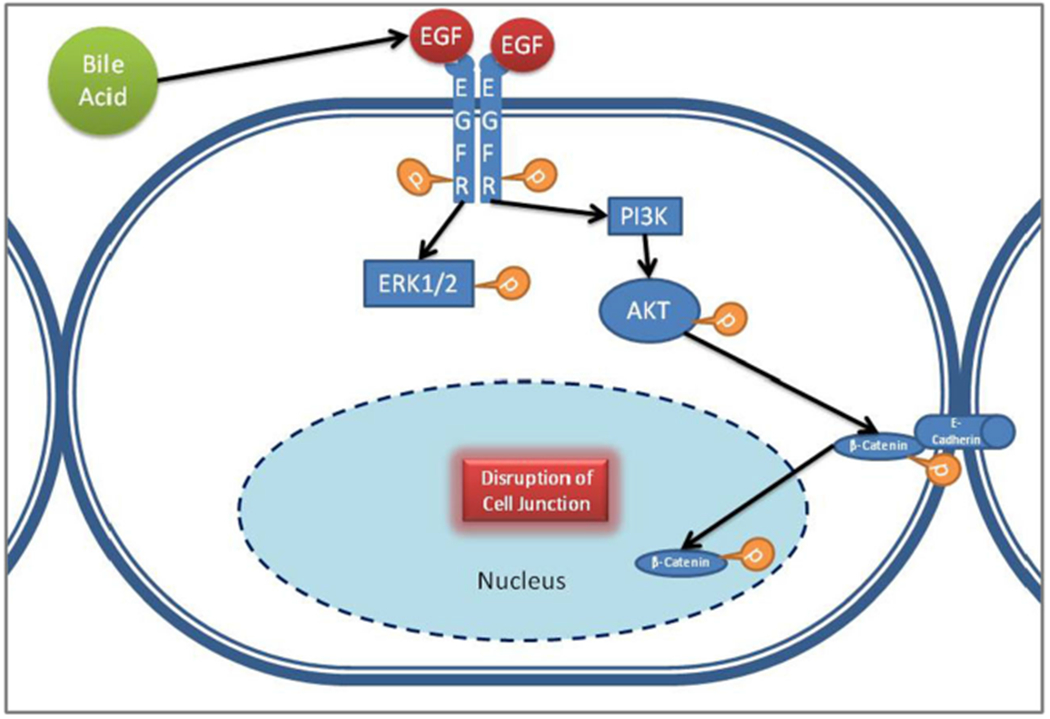
Hypothetical sequence of events leading to junction disruption—at basal condition in a terminally differentiated stratified squamous cell, WNT signaling is inactive, and β-catenin binds to E-cadherin and is tethered to the adherens junction. Bile salts, possible by activating EGFR pathway and downstream Akt phosphorylation, might cause β-catenin phosphorylation at Ser552 residue triggering its release and subsequent disruption of the adherens junction complex
In conclusion, we have demonstrated the utility of our simple EPC1 transwell culture model to explore morphological and molecular effects of simulated reflux on stratified epithelium. Our data demonstrate that bile salts at pH 5, but not either alone, disrupts tight junction complexes and causes increased permeability of stratified squamous esophageal epithelium. These changes approximate the appearance of dilated intercellular space similar to that found in the GERD patients.
To extend the relevance of this study in the clinical setting, acid suppression therapy likely will not prevent DIS formation and subsequent initiation of inflammatory response in esophageal epithelium as long as the patient is refluxing bile salts. Our experiments showed that bile salts at neutral pH had less damaging effects on the epithelium, but proton-pump inhibitor (PPI) monotherapy rarely raises the gastric pH beyond 6.30,31,35 Recent studies have shown promise for use of sodium alginate in decreasing bile salt refluxate.36 For the refractory GERD patients who demonstrate mixed reflux pattern in 24-h distal pH-impedance monitoring, the role of sodium alginate along with PPI as the anti-reflux therapy might be a potential area for future exploration.
Acknowledgments
The authors would like to thank Dr. Roy C. Orlando (UNC School of Medicine, NC) for helpful discussions. The study was performed in the Department of Surgery, University of Rochester Medical Center, Rochester, NY. The data in the paper was presented in parts as a poster in Digestive Disease Week, 2013 (Orlando, FL). Parts of the paper also feature in the doctoral thesis of Dr. Sayak Ghatak, Biology, University of Rochester (URL: http://hdl.handle.net/1802/28858).
References
- 1.El-Serag HB, Sweet S, Winchester CC, and Dent J, Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeVault KR, Castell DO, and American College of G, Updated guidelines for the diagnosis and treatment of gastroesophageal reflux disease. Am J Gastroenterol, 2005. 100(1): p. 190–200. [DOI] [PubMed] [Google Scholar]
- 3.Souza RF, Krishnan K, and Spechler SJ, Acid, bile, and CDX: the ABCs of making Barrett’s metaplasia. Am J Physiol Gastrointest Liver Physiol, 2008. 295(2): p. G211–8. [DOI] [PubMed] [Google Scholar]
- 4.Kauer WK, Peters JH, DeMeester TR, Feussner H, Ireland AP, Stein HJ, and Siewert RJ, Composition and concentration of bile acid reflux into the esophagus of patients with gastroesophageal reflux disease. Surgery, 1997. 122(5): p. 874–81. [DOI] [PubMed] [Google Scholar]
- 5.Orlando RC, Powell DW, and Carney CN, Pathophysiology of acute acid injury in rabbit esophageal epithelium. J Clin Invest, 198168(1): p. 286–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farre R, van Malenstein H, De Vos R, Geboes K, Depoortere I, Vanden Berghe P, Fornari F, Blondeau K, Mertens V, Tack J, and Sifrim D, Short exposure of oesophageal mucosa to bile acids, both in acidic and weakly acidic conditions, can impair mucosal integrity and provoke dilated intercellular spaces. Gut, 2008. 57(10): p. 1366–74. [DOI] [PubMed] [Google Scholar]
- 7.Orlando RC, Dilated intercellular spaces and chronic cough as an extra-oesophageal manifestation of gastrooesophageal reflux disease. Pulm Pharmacol Ther, 2011. 24(3): p. 272–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hofmann AF and Mysels KJ, Bile acid solubility and precipitation in vitro and in vivo: the role of conjugation, pH, and Ca2+ ions. J Lipid Res, 1992. 33(5): p. 617–26. [PubMed] [Google Scholar]
- 9.Araki Y, Katoh T, Ogawa A, Bamba S, Andoh A, Koyama S, Fujiyama Y, and Bamba T, Bile acid modulates transepithelial permeability via the generation of reactive oxygen species in the Caco-2 cell line. Free Radic Biol Med, 2005. 39(6): p. 769–80. [DOI] [PubMed] [Google Scholar]
- 10.Yasuda H, Hirata S, tnoue K, Mashima H, Ohnishi H, and Yoshiba M, Involvement of membrane-type bile acid receptor M-BAR/TGR5 in bile acid-induced activation of epidermal growth factor receptor and mitogen-activated protein kinases in gastric carcinoma cells. Biochem Biophys Res Commun, 2007. 354(1): p. 154–9. [DOI] [PubMed] [Google Scholar]
- 11.Ghatak S, Reveiller M, Toia L, Ivanov A, Godfrey TE, and Peters JH, Bile Acid at Low pH Reduces Squamous Differentiation and Activates EGFR Signaling in Esophageal Squamous Cells in 3-D Culture. Journal of Gastrointestinal Surgery, 2013. 17(10): p. 1723–1731. [DOI] [PubMed] [Google Scholar]
- 12.Pai R, Tarnawski AS, and Tran T, Deoxycholic acid activates beta-catenin signaling pathway and increases colon cell cancer growth and invasiveness. Mol Biol Cell, 2004. 15(5): p. 2156–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harada H, Nakagawa H, Oyama K, Takaoka M, Andl CD, Jacobmeier B, von Werder A, Enders GH, Opitz OG, and Rustgi AK, Telomerase induces immortalization of human esophageal keratinocytes without p16INK4a inactivation. Mol Cancer Res, 2003. 1(10): p. 729–38. [PubMed] [Google Scholar]
- 14.Deyrieux AF and Wilson VG., Invitro culture conditions to study keratinocyte differentiation using the HaCaT cell line. Cytotechnology, 2007. 54(2): p. 77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu T, Zhang X, So CK, Wang S, Wang P, Yan L, Myers R, Chen Z, Patterson AP, Yang CS, and Chen X, Regulation of Cdx2 expression by promoter methylation, and effects of Cdx2 transfection on morphology and gene expression of human esophageal epithelial cells. Carcinogenesis, 2007. 28(2): p. 488–96. [DOI] [PubMed] [Google Scholar]
- 16.Benson K, Cramer S, and Galla HJ, Impedance-based cell monitoring: barrier properties and beyond. Fluids Barriers CNS, 2013. 10(1): p. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Epstein FH, Mittal RK, and Balaban DH, The esophagogastric junction. New England Journal of Medicine, 1997. 336(13): p. 924–932. [DOI] [PubMed] [Google Scholar]
- 18.Castell DO, Murray JA, Tutuian R, Orlando RC, and Arnold R, Review article: the pathophysiology of gastro-oesophageal reflux disease—oesophageal manifestations. Aliment Pharmacol Ther, 2004. 20 Suppl 9: p. 14–25. [DOI] [PubMed] [Google Scholar]
- 19.de Vries DR, Ter Linde JJ, van Herwaarden MA, Schwartz MP, Shephard P, Geng MM, Smout AJ, and Samsom M, In GERD patients, mucosal repair associated genes are upregulated in non-inflamed oesophageal epithelium. J Cell Mol Med, 2009. 13(5): p. 936–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bjorkman EV, Edebo A, Oltean M, and Casselbrant A, Esophageal barrier function and tight junction expression in healthy subjects and patients with gastroesophageal reflux disease: functionality of esophageal mucosa exposed to bile salt and trypsin in vitro. Scand J Gastroenterol, 2013. 48(10): p. 1118–26. [DOI] [PubMed] [Google Scholar]
- 21.Kalabis J, Wong GS, Vega ME, Natsuizaka M, Robertson ES, Herlyn M, Nakagawa H, and Rustgi AK, Isolation and characterization of mouse and human esophageal epithelial cells in 3D organotypic culture. Nat Protoc, 2012. 7(2): p. 235–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cavey M and Lecuit T, Molecular bases of cell-cell junctions stability and dynamics. Cold Spring Harb Perspect Biol, 2009. 1(5): p. a002998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orlando RC, Esophageal mucosal defense mechanisms. GI Motility online, 2006. [Google Scholar]
- 24.Orsulic S, Huber O, Aberle H, Arnold S, and Kemler R, E-cadherin binding prevents beta-catenin nuclear localization and beta-catenin/LEF-1-mediated transactivation. Journal of cell science, 1999. 112(8): p. 1237–1245. [DOI] [PubMed] [Google Scholar]
- 25.Fang D, Hawke D, Zheng Y, Xia Y, Meisenhelder J, Nika H, Mills GB, Kobayashi R, Hunter T, and Lu Z, Phosphorylation of beta-catenin by AKT promotes beta-catenin transcriptional activity. J Biol Chem, 2007. 282(15): p. 11221–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orlando LA and Orlando RC, Dilated intercellular spaces as a marker of GERD. Current gastroenterology reports, 2009. 11(3): p. 190–194. [DOI] [PubMed] [Google Scholar]
- 27.Vela MF, Craft BM, Sharma N, Freeman J, and Hazen-Martin D, Refractory heartburn: comparison of intercellular space diameter in documented GERD vs. functional heartburn. The American journal of gastroenterology, 2010. 106(5): p. 844–850. [DOI] [PubMed] [Google Scholar]
- 28.Orlando RC, Mechanisms of reflux-induced epithelial injuries in the esophagus. Am J Med, 2000. 108 Suppl 4a: p. 104S–108S. [DOI] [PubMed] [Google Scholar]
- 29.DeMeester TR, Clinical biology of the Barrett’s metaplasia, dysplasia to carcinoma sequence. Surg Oncol, 2001. 10(3): p. 91–102. [DOI] [PubMed] [Google Scholar]
- 30.Atanassoff PG, Brull SJ, Weiss BM, Landefeld K, Alon E, and Rohling R, The time course of gastric pH changes induced by omeprazole and ranitidine: a 24-hour dose-response study. Anesth Analg, 1995. 80(5): p. 975–9. [DOI] [PubMed] [Google Scholar]
- 31.Dehn TC, Shepherd HA, Colin-Jones D, Kettlewell MG, and Carroll NJ, Double blind comparison of omeprazole (40 mg od) versus cimetidine (400 mg qd) in the treatment of symptomatic erosive reflux oesophagitis, assessed endoscopically histologically and by 24 h pH monitoring. Gut, 1990. 31(5): p. 509–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hofmann A and Hagey L, Bile acids: chemistry, pathochemistry, biology, pathobiology, and therapeutics. Cellular and Molecular Life Sciences, 2008. 65(16): p. 2461–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Po HN and Senozan NM, The Henderson-Hasselbalch equation: Its history and limitations. Journal of Chemical Education, 200178(11): p. 1499–1503. [Google Scholar]
- 34.Raimondi F, Santoro P, Barone MV, Pappacoda S, Barretta ML, Nanayakkara M, Apicella C, Capasso L, and Paludetto R, Bile acids modulate tight junction structure and barrierfunction of Caco-2 monolayers via EGFR activation. Am J Physiol Gastrointest Liver Physiol, 2008. 294(4): p. G906–13. [DOI] [PubMed] [Google Scholar]
- 35.Hunt RH, Armstrong D, James C, Chowdhury SK, Yuan Y, Fiorentini P, Taccoen A, and Cohen P, Effect on intragastric pH of a PPI with a prolonged plasma half-life: comparison between tenatoprazole and esomeprazole on the duration of acid suppression in healthy male volunteers. Am J Gastroenterol, 2005. 100(9): p. 1949–56. [DOI] [PubMed] [Google Scholar]
- 36.Strugala V, Avis J, Jolliffe IG, Johnstone LM, and Dettmar PW, The role of an alginate suspension on pepsin and bile acids—key aggressors in the gastric refluxate. Does this have implications for the treatment of gastrooesophageal reflux disease? J Pharm Pharmacol, 200961(8): p. 1021–8. [DOI] [PubMed] [Google Scholar]


