Abstract
Immunocompromised patients are highly susceptible to influenza virus infections. Although neuraminidase inhibitor (NAI) therapy has proved effective in these patients, the treatment regimens require optimization, which can be partly addressed via animal models. Here, we describe a pharmacologically immunosuppressed mouse model for studying the pathogenesis of influenza B viruses and evaluating the efficacy of antiviral treatment. We modeled clinical regimens for dexamethasone and cyclophosphamide to immunosuppress BALB/c mice that were then inoculated with B/Phuket/3073/2013 (Yamagata lineage) or B/Brisbane/60/2008 (BR/08, Victoria lineage) virus. Although both viruses caused morbidity and mortality in immunosuppressed mice, BR/08 was more virulent, consistently inducing greater morbidity and 100% lethality in mice inoculated with at least 103 TCID50/mouse. The replication of both viruses was prolonged in the lungs of immunosuppressed mice, but the extent of pulmonary inflammation in these mice was markedly less than that in immunocompetent animals. Most of the examined cytokines, including IFN-γ, IL-1β, and RANTES, were significantly decreased in the lungs of immunosuppressed mice, as compared to immunocompetent animals, until at least 10 days post-infection. Treatment with the NAI oseltamivir for 8 or 16 days increased the mean survival time and reduced virus spread in the lungs of immunosuppressed mice challenged with a lethal dose of BR/08 but did not completely provide protection or decrease the virus titers. Our data suggests that the synergy of the viral load and aberrant immune responses is a key contributor to the severity of infection, as well as the limited efficacy of oseltamivir, which in immunosuppressed mice curtails virus release without clearing infected cells.
Keywords: influenza B virus, immunosuppressed mice, pathogenesis, immune responses, antiviral treatment, oseltamivir
1. Introduction
Influenza B viruses cause significant disease burden during annual epidemics but remain less studied than influenza A viruses (Paul Glezen et al., 2013; Tafalla et al., 2016). Laboratory-confirmed influenza B viruses account for approximately 22% of influenza cases in the United States on average, but this proportion is highly variable depending on the season (Barr and Jelley, 2012; Blanton et al., 2017; Shang et al., 2016; WHO, 2017). Influenza B virus infections are clinically indistinguishable from influenza A virus infections, but the associated complications may differ (Koutsakos et al., 2016) and may include encephalitis, encephalopathy, gastrointestinal symptoms, or myositis (Burnham et al., 2013; Paddock et al., 2012). Influenza B viruses can be prevalent in children with cancer or other immunosuppressive conditions and can contribute to their deaths (Carr et al., 2011; CDC, 2017b; McCullers and Hayden, 2012; Peltola et al., 2003; Silvennoinen et al., 2011).
Influenza A and B viruses cause prolonged viral shedding and considerable morbidity and mortality in immunocompromised patients (Gooskens et al., 2009; Ljungman et al., 1993; Prasad and Spradbrow, 1977; Whimbey and Bodey, 1992), in whom progression of infection to the lower respiratory tract may induce severe disease. Influenza vaccines are often poorly immunogenic and unlikely to be fully protective in immunocompromised patients (Yousuf et al., 1997). Therefore, neuraminidase (NA) inhibitors (NAIs), the only class of drugs available for influenza prophylaxis and therapy, are particularly important in these patients, although there is a high risk of NAI resistance developing (Carr et al., 2011; Gubareva et al., 1998; van der Vries et al., 2013). Antiviral therapy reportedly improves clinical outcomes in immunocompromised patients (Boudreault et al., 2011; Lee and Ison, 2012), but current strategies require optimization (Ison, 2015; Whitley and Monto, 2006).
Preclinical studies in animal models can provide valuable information for optimizing treatment protocols for immunocompromised patients. There are multiple methods for modeling immunosuppression in mice (Bosma and Carroll, 1991; Mostafa et al., 2016; Wahlberg et al., 1992). One approach that mimics the treatment of hematologic malignant neoplasms involves using cyclophosphamide (CP) and dexamethasone (DEX), which are widely used clinically as monotherapies or in combination (Kaufman et al., 2009; Michallet et al., 2011). CP and DEX have different mechanisms of action and complementary immunosuppressive effects. CP is an alkylating agent, has myelosuppressive activity, and interferes with DNA replication, leading to apoptosis (Colvin, 2003). CP is used to treat aplastic anemia (Brodsky et al., 1996) and to prevent graft-versus-host disease in recipients of allogeneic hematopoietic stem cell transplants (Luznik et al., 2010) and rejection of organ transplants (Cooksley et al., 2005). DEX is a potent corticosteroid that causes apoptosis of lymphocytes (Estlin et al., 2000) and is given as pulse therapy for acute lymphoblastic leukemia, lymphoma, Hodgkin disease (Pui, 2006), and other inflammatory diseases (Li et al., 2017).
Data on influenza B viruses in immunosuppressed models is lacking. Here, we used pharmacologically immunosuppressed mice to characterize two influenza B viruses representing the Victoria and Yamagata lineages. We then evaluated the NAI oseltamivir in this model system, focusing on prolonged treatment.
Materials and methods
2.1. Viruses, cells, and compound
The human influenza B/Phuket/3073/2013 (PH/13) and B/Brisbane/60/2008 (BR/08) viruses (unadapted for replication in mice) were obtained from the Centers for Disease Control and Prevention, grown in 10-day-old embryonated chicken eggs for 72 h at 33°C, and the 50% tissue culture infectious dose (TCID50/mL) was determined in Madin-Darby canine kidney (MDCK) cells (American Type Culture Collection, Manassas, VA). Oseltamivir phosphate (oseltamivir) [ethyl(3R,4R,5S)-4-acetamido-5-amino-3-(1-ethylpropoxy)-1-cyclohexene-1-carboxylate] was dissolved in distilled water.
2.2. The immunosuppressive mouse model
Animal experiments were approved by the Animal Care and Use Committee of St. Jude Children’s Research Hospital (St. Jude) in compliance with National Institutes of Health guidelines and the Animal Welfare Act. Female 6-week-old BALB/c mice (The Jackson Laboratory, Bar Harbor, ME) were treated with DEX+CP (Sigma-Aldrich, St. Louis, MO). DEX (10 mg/kg/day in 0.2 mL) was administered to mice daily for 10 days intraperitoneally (ip), starting 1 day before inoculation with influenza B virus (−1 day post-infection [dpi]). CP (150 mg/kg/day in 0.3 mL) was administered ip as two doses at −1 and 5 dpi (Mostafa et al., 2016). The timing of virus infection in relation to immunosuppression was chosen to extend window of virus spread and clearance.
2.3. Pathogenicity of influenza B viruses in mice
Mice were anesthetized with isoflurane and inoculated intranasally with influenza B virus (102–105 TCID50/mouse in 30 L). Mice (n = 5/group/virus dose) were monitored daily for disease signs, weight loss, and survival (weight loss exceeding 25% was the endpoint for mortality). At 3, 6, and 10 dpi, lungs and nasal turbinates were collected from additional animals (n = 3/time point), and virus titers were determined by TCID50 assay in MDCK cells.
2.4. Differential leukocyte counts and flow cytometry
Mice (n = 5/group) were anesthetized, and blood samples were collected in 10% EDTA (disodium) by retro-orbital bleeding at −1, 3, 6, 10, 13, 17, and 24 dpi. Cells were enumerated and analyzed for differential leukocyte counts with a FORCYTE hematology analyzer (Oxford Science, Oxford, CT). For flow cytometry analysis of blood or bronchoalveolar lavage fluid (BALF) lymphocytes, cells were blocked with mouse BD Fc block (BD Biosciences, Franklin Lakes, NJ) in staining medium (0.5% FBS in PBS) for 10 min at 4°C, stained with CD3-APC, CD4-FITC, CD8-PE, CD19-PE-Cy7, and CD49b-APC-Cy7 antibodies (BD Biosciences) for 30 min at 4°C. Cellular populations were gated and quantified by FlowJo software (v. 7.6.1).
2.5. Cytokine and chemokine analysis
At 3, 6, and 10 dpi, the concentrations of each of 25 cytokines and chemokines were measured in lung homogenates (n = 3/group) with a MYCTOMAG-70K-PMX MILLIPLEX® MAP mouse cytokine/chemokine panel (Millipore). The plates were read on a Luminex 100/200 analyzer using the xPonent software.
2.6. Lung histopathology and immunohistochemistry
Lungs were collected at 6 or 16 dpi (n = 2/group), infused with 10% neutral-buffered formalin (Thermo Scientific), and stained with hematoxylin and eosin or subjected to immunohistochemical staining with anti-HA goat antiserum (B/Florida/04/2006, Yamagata lineage). The extent of virus infection and lung lesions was quantified as the percentage of the total alveolar lung field with active or inactive infection by using Aperio ImageScope software (Marathe et al., 2016).
2.7. Oseltamivir efficacy
Mice were anesthetized and inoculated intranasally with 5 MLD50/30 L/mouse of BR/08 (2.5 × 105 TCID50). Oseltamivir treatment (20 mg/kg/day by twice-daily oral gavage) was initiated 1 h after inoculation with BR/08 and continued for 8 (immunocompetent) or for 8 or 16 days (immunosuppressed). Control (inoculated, untreated) mice received sterile water. Mice (n = 5/group) were observed daily for disease signs, weight loss, and survival (weight loss of >25%). Additional groups of control and oseltamivir-treated mice (n = 3/group) were euthanized at 6 and 10 dpi, and virus titers were determined by TCID50 assay in MDCK cells.
2.8. Sequence analysis
Viral RNA was isolated from mouse lung homogenates at 6 and 9 dpi with an RNeasy Mini Kit (Qiagen). Samples were reverse transcribed and analyzed by PCR using primers specific for the NA gene (Tewawong et al., 2016). PCR products were purified with a QIAquick PCR Purification Kit (Qiagen). Sanger sequencing was performed by the Hartwell Center for Bioinformatics and Biotechnology at St. Jude. DNA sequences were completed and edited with the Lasergene sequence analysis software (DNASTAR, Madison, WI).
2.9. Serologic assay
Sera were collected by retro-orbital bleeding at 22 dpi, treated with receptor-destroying enzyme (Denko-Seiken, San Jose, CA), heat-inactivated at 56°C for 1 h, and tested by hemagglutination inhibition (HI) assay with 0.5% turkey red blood cells (Rockland Immunochemicals).
2.10. Statistical analysis
Virus titers, cell counts, and cytokine/chemokine levels were compared by one-way ANOVA with Bonferroni’s post-test (GraphPad Prism 5.0 software). The Kaplan-Meier method was used to estimate the probability of survival, and the log-rank test was used to compare survival rates.
3. Results
3.1. Morbidity and mortality
In immunocompetent mice, inoculation with 102 to104 TCID50 of PH/13 caused 5% weight loss without mortality (Fig. 1A, B). Inoculation with 105 TCID50 of PH/13 caused 10% weight loss and 20% mortality. BR/08 virus was more virulent; inoculation with 104 or 105 TCID50 caused pronounced weight loss and killed 40% and 100% of animals, respectively (Fig. 1C, D). In uninfected mice, DEX+CP treatment caused 5% to 10% weight loss at 8 dpi, and the animals quickly regained the weight after treatment ended (Fig. 1E, G). In immunosuppressed mice, inoculation with 103 TCID50 of PH/13 promoted no additional weight loss until 10 dpi, followed by drastic weight loss to 14 dpi, resulting in 40% mortality (Fig. 1E, F). Animals inoculated with 104 or 105 TCID50 of PH/13 gradually lost weight, and all mice died by 16 and 14 dpi, respectively. Inoculating immunosuppressed mice with 103 to 105 TCID50 of BR/08 caused rapid weight loss and death by 6–7 dpi (Fig. 1G, H). Thus, immunosuppressed mice were more susceptible than immunocompetent animals to infection with PH/13 and BR/08 viruses. The pathogenicity increased with the virus dose, and BR/08 was more virulent than PH/13 in both immunocompetent and immunosuppressed mice.
Figure 1. Morbidity and mortality of immunocompetent and immunosuppressed mice inoculated with influenza B viruses.
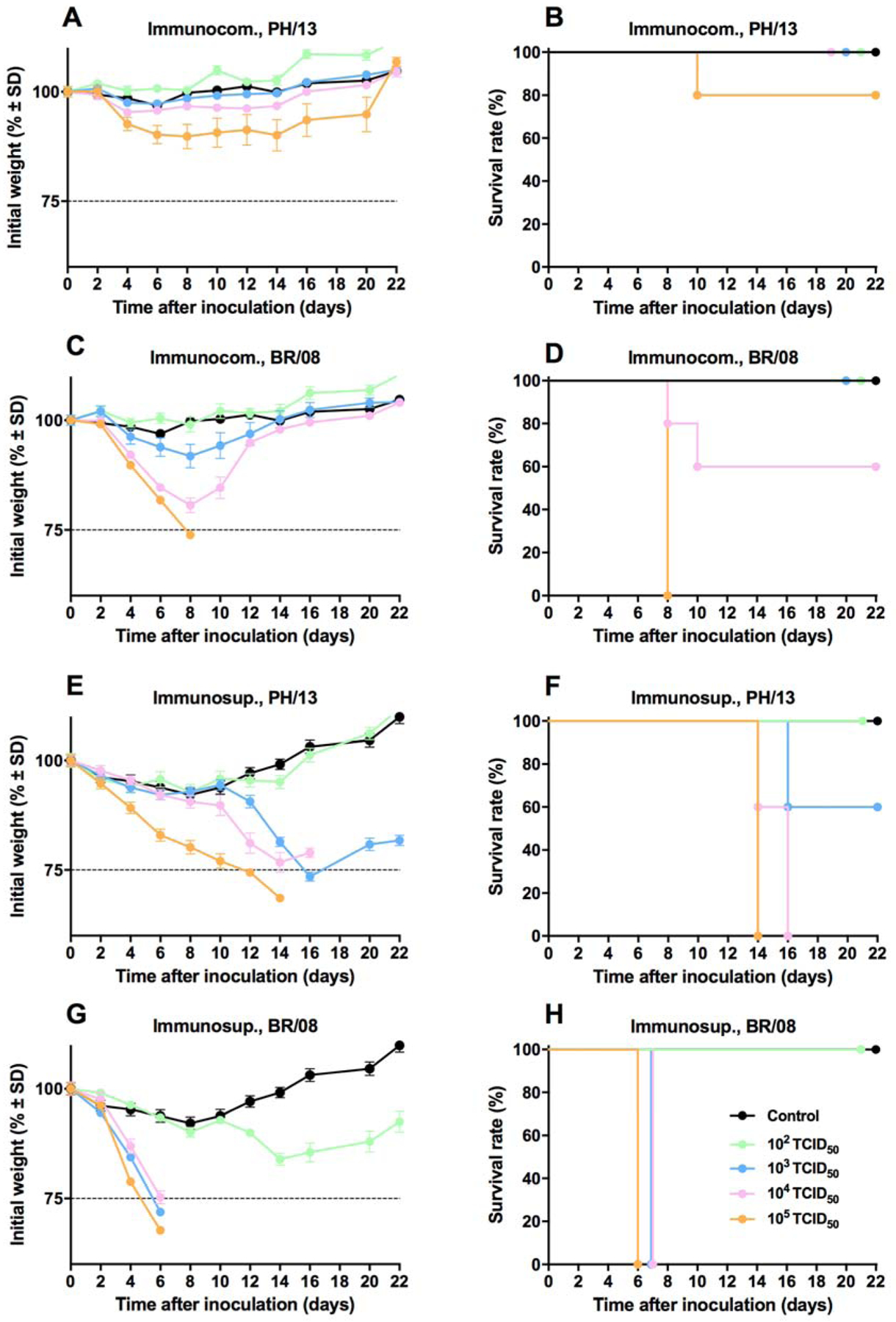
Female 6- to 8-week-old immunocompetent and immunosuppressed (DEX+CP-treated) BALB/c mice (n = 5/group) were anesthetized with isoflurane and inoculated intranasally with influenza B viruses at a range of doses (102–105 TCID50/mouse in 30 μL). The graphs show the weight loss (A, C, E, and G) and survival (B, D, F, and H) of immunocompetent mice inoculated with PH/13 (A, B) or BR/08 (C, D) viruses and of immunosuppressed mice inoculated with PH/13 (E, F) or BR/08 (G, H) viruses. Control uninfected animals (black line) received sterile PBS (A-D) or DEX+CP (E-H). The horizontal dotted line in panels A, C, E, and G indicates the endpoint for mortality (25% loss of initial weight). The probabilities of survival were determined by Kaplan-Meier and log-rank tests. Abbreviations: DEX+CP, dexamethasone and cyclophosphamide; PH/13, B/Phuket/3073/2013; BR/08, B/Brisbane/60/2008.
3.2. Viral load
In immunocompetent mice, PH/13 and BR/08 replicated in the nasal turbinates and lungs, with generally higher pulmonary titers (Fig. 2A, B). At 10 dpi, both viruses persisted in the nasal turbinates, but only BR/08 was detected in the lungs. In immunosuppressed mice inoculated with 102 TCID50, PH/13 and BR/08 viruses persisted in the nasal turbinates up to 16 dpi and in the lungs up to 10 dpi (PH/13) or 16 dpi (BR/08). The highest titers were found in nasal turbinates at 10 dpi (range, 5–5.2 log10TCID50/mL) and in lungs at 3 dpi (range, 4.5–5.5 log10TCID50/mL) (Fig. 2A, B). Replication of PH/13 and BR/08 in lungs was efficient at a dose of 105 TCID50. Overall, lung titers in the two models were comparable early in infection, but higher titers (P < 0.05) and persistence of both viruses in the lungs of the immunosuppressed mice was detected at later time points.
Figure 2. Viral load in respiratory tract of immunocompetent and immunosuppressed mice inoculated with influenza B viruses.
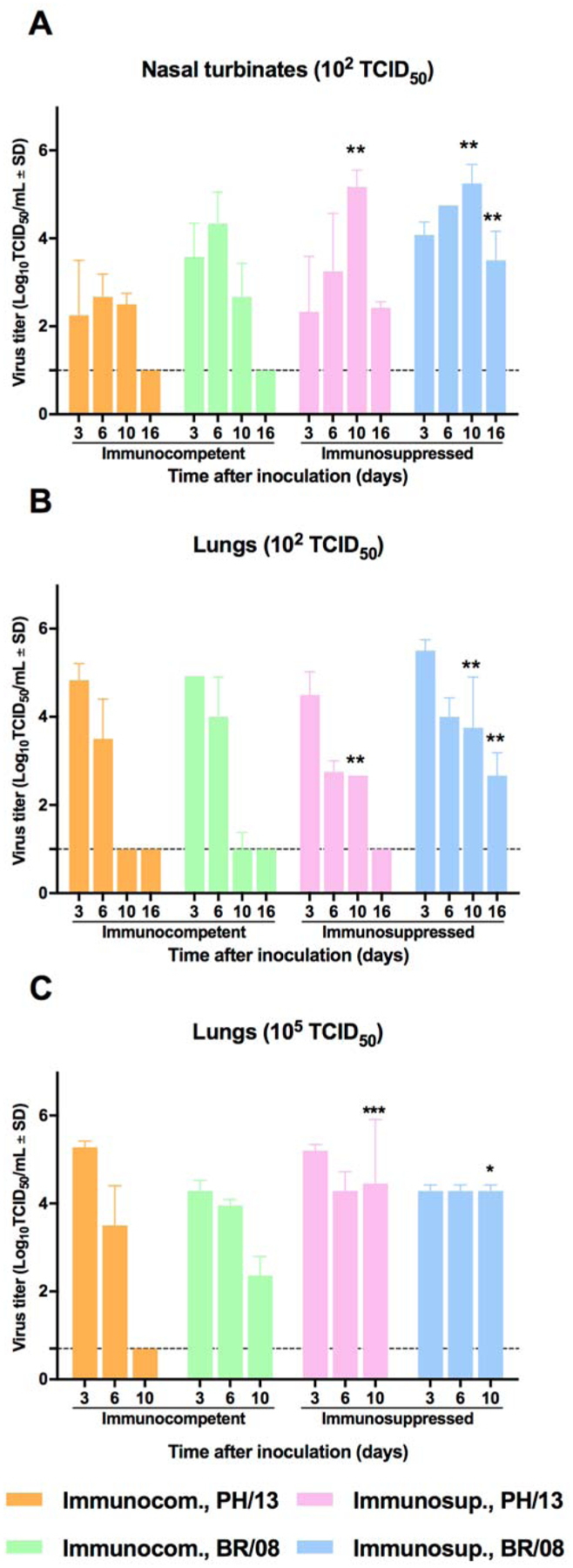
Immunocompetent and immunosuppressed (DEX+CP-treated) BALB/c mice were anesthetized with isoflurane and inoculated intranasally with 102 TCID50/mouse (A, B) or 105 50 TCID50/mouse (C) of influenza PH/13 or BR/08 virus. Virus titers were determined in the lungs and nasal turbinates of mice (n = 3/group) at 3, 6, 10, and 16 dpi by TCID50 assays in MDCK cells. The bars represent the mean virus titers ± SDs in mouse nasal turbinates (A) and lungs (B, C). *P < 0.05, **P < 0.01, and ***P< 0.001 for comparisons of immunocompetent and immunosuppressed mice by one-way ANOVA with Bonferroni’s multiple comparison post-test.
3.3. Immunologic changes
To characterize the immunosuppression caused by DEX+CP treatment, we determined the total and differential lymphocyte and neutrophil counts in blood samples from mice inoculated with 102 TCID50 of PH/13 or BR/08. Neither virus had a noticeable effect on the total lymphocyte or neutrophil counts (Fig. 3A, B), except that BR/08 caused a significantly reduced lymphocyte count at 3 dpi (P < 0.05) (Fig. 3A). After DEX+CP treatment, the lymphocyte counts started to drop markedly, being particularly low between 3 and 24 dpi (Fig. 3A). DEX+CP treatment decreased the peripheral neutrophil counts at 3 and 10 dpi; this was followed by significant increases at 13 and 17 dpi (P < 0.05) (Fig. 3B), with no apparent differences between control and virus-infected animals. Percentages of CD4+ and CD8+ T lymphocytes markedly increased at 13 dpi (Fig. 3C, D), whereas percentages of B cells were reduced (Fig. 3E). Peripheral lymphocyte counts increased slightly between 13 and 17 dpi, during which time the lymphocyte populations consisted mainly of natural killer (NK) cells, with almost no detectable B lymphocytes (Fig. 3E, F). The rebound increase in the peripheral neutrophils is probably due to an increase in colony-stimulating activity due to reduction of feedback inhibition after the discontinuation of chemotherapy (Broxmeyer, 1978).
Figure 3. Lymphocyte and neutrophil counts in immunocompetent and immunosuppressed mice inoculated with influenza B viruses.
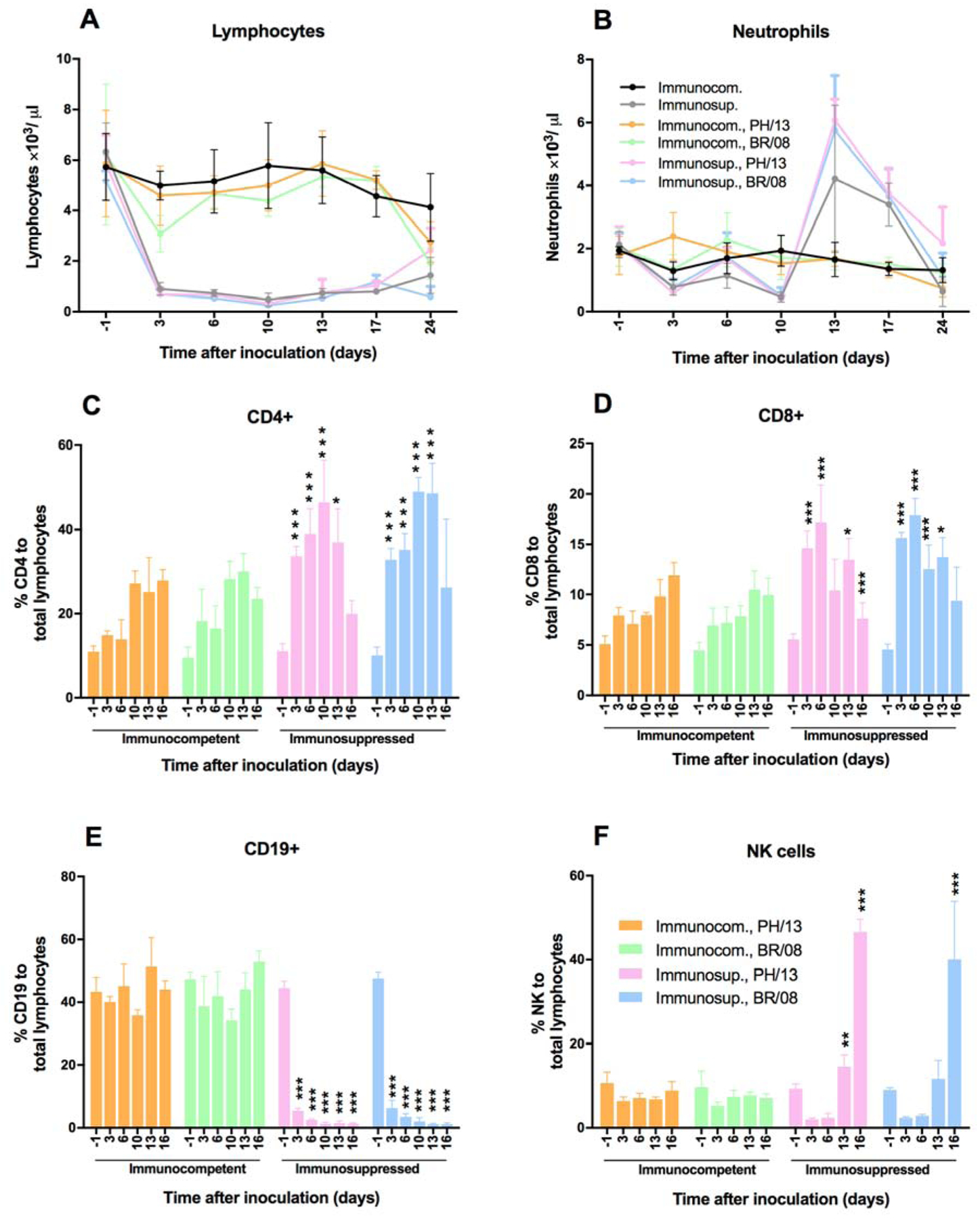
Immunocompetent and immunosuppressed (DEX+CP-treated) BALB/c mice were anesthetized with isoflurane and inoculated intranasally with 102 TCID50/mouse of influenza PH/13 or BR/08 virus. Blood was collected from uninfected control (PBS-treated) mice and from DEX+CP-treated uninfected and virus-inoculated mice (n = 5/group) by the retro-orbital route at −1, 3, 6, 10, 13, 17, and 24 dpi. Total lymphocyte (A), neutrophil (B), CD4+ cell (C), CD8+ cell (D), CD19+ cell (E), and NK cell (F) counts were determined with an automatic cell counter, and lymphocyte populations were differentiated by flow cytometry. Bars represent the mean values ± SDs. *P < 0.05, **P < 0.01, and ***P < 0.001 for comparisons of immunocompetent and immunosuppressed mice one-way ANOVA with Bonferroni’s multiple comparison post-test.
In immunocompetent mice, induction of pulmonary cytokines peaked at 6 dpi, being significantly reduced at later time points. BR/08 induced significantly higher levels of IFN-γ, IL-1β, IL-10, IL-12(P40), and IL-12(P70) (P < 0.05) than did PH/13 (Fig. 4). DEX+CP treatment reduced most of the examined inflammatory mediators [including IFN-γ, IL-1α, IL-1β, IL-10, IL-12(P40), and IL-12(P70)] to near the control baseline levels and reduced the levels of the others (including IP-10 and TNF-α) relative to those in immunocompetent mice (P < 0.05). Immunocompetent mice developed anti-HA antibodies against influenza B virus (HI titer 160–320), whereas immunosuppressed mice had no detectable antibodies (data not shown). Thus, DEX+CP treatment caused significant lymphopenia that persisted until the end of the study and correlated with reduced or delayed release of inflammatory mediators and an inability to elicit a specific humoral immune response.
Figure 4. Induction of pulmonary cytokine/chemokine responses in immunocompetent and immunosuppressed mice inoculated with influenza B viruses.
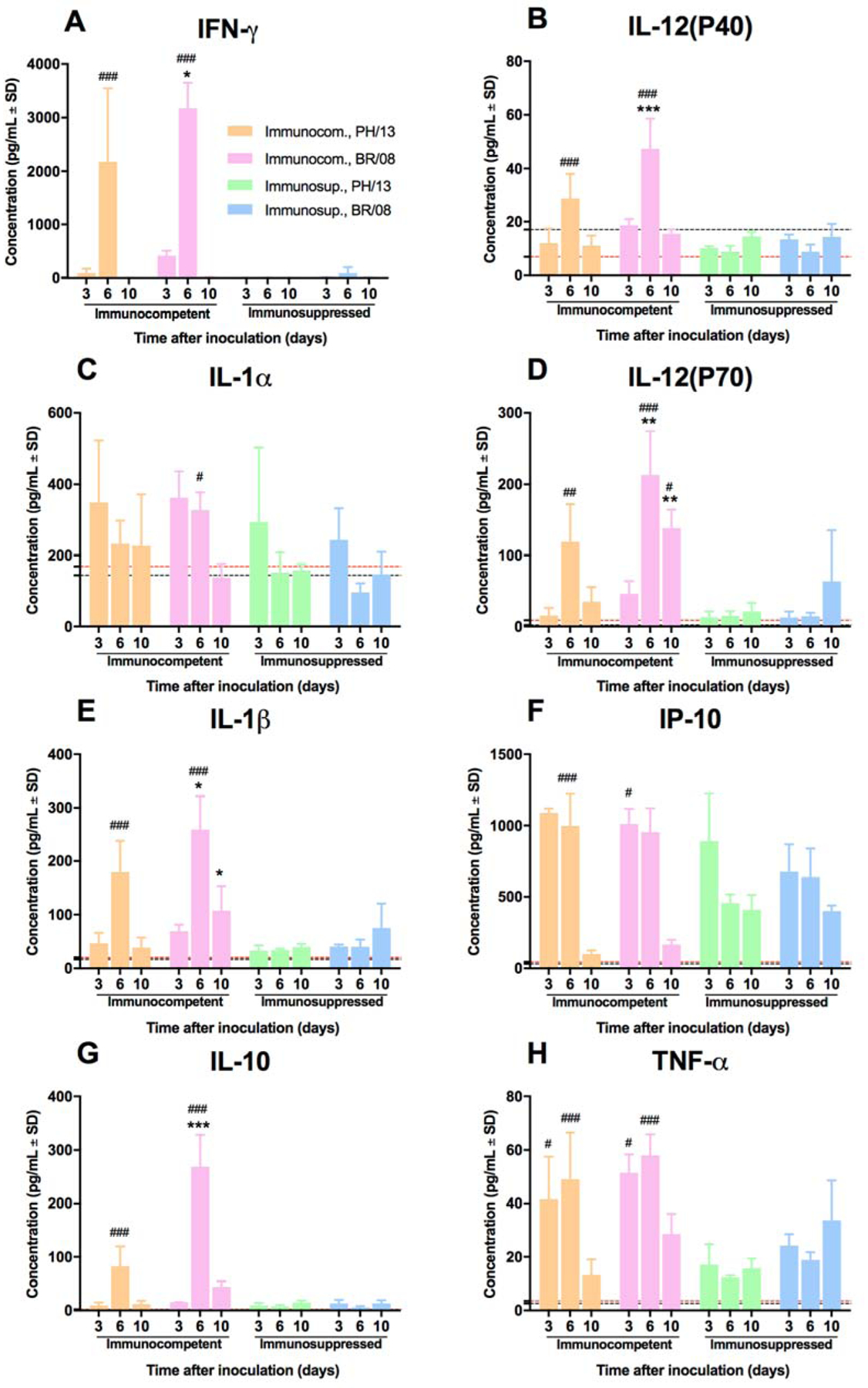
Immunocompetent and immunosuppressed (DEX+CP-treated) BALB/c mice were anesthetized with isoflurane and inoculated intranasally with 102 TCID50/mouse of influenza PH/13 or BR/08 viruses. The expression levels of IFN-γ (A), IL-12(P40) (B), IL-1α (C), IL-12(P70) (D), IL-1β (E), IP-10 (F), IL-10 (G), and TNF-α (H) were assayed in lung homogenates at 3, 6, and 10 dpi. Bars represent mean values ± SDs (n = 3/group/time-point). *P < 0.05, and ***P < 0.001 for comparisons of PH/13 and BR/08 virus–infected immunocompetent mice; and #P < 0.05 ##, P < 0.01, and ###P < 0.001 for comparisons of PH/13 and BR/08 virus–infected immunocompetent and immunosuppressed mice by one-way ANOVA with Bonferroni’s multiple comparison post-test. The level of cytokine/chemokine expression in control uninfected mice is indicated by a black dotted line for immunocompetent mice and by a red dotted line for immunosuppressed mice.
3.4. Histologic changes
In immunocompetent mice, PH/13 caused less extensive and less severe virus infection and lung alveolar damage than did BR/08. Inflammatory cell infiltrates were relatively mild and were limited to perivascular connective tissues (Fig. 5A), and viral antigen staining was restricted to type II pneumocytes and intra-alveolar cell debris (Fig. 5B). In contrast, BR/08 infection was associated with prominent perivascular and intra-alveolar inflammatory cell infiltrates (Fig. 5E) and with viral antigen staining covering the alveolar surfaces (Fig. 5F). Histomorphometry revealed that PH/13 infection in lungs (Fig. 5C, D) was less extensive than BR/08 infection (Fig. 5G, H) at 6 and 16 dpi. In immunosuppressed mice, perivascular and intra-alveolar inflammation was markedly reduced in both PH/13- and BR/08-infected lungs (Fig. 5I, M). Where present, intra-alveolar inflammation consisted primarily of alveolar macrophages, which were also positive for viral antigen (Fig. 5J). Compared with the pattern observed in immunocompetent mice, there was a marked increase in the extent and intensity of staining of alveolar and bronchiolar epithelial cells following infection with either virus (Fig. 5J, N). The overall extent of PH/13 virus infection in immunosuppressed mice at 6 dpi was much less extensive than BR/08 infection (Fig. 5K, O). Pulmonary lesions were essentially absent in PH/13-infected mice at 16 dpi but were still quite extensive in immunosuppressed BR/08-infected mice (Fig. 5L, P).
Figure 5. Histopathologic changes in the lungs of immunocompetent and immunosuppressed mice inoculated with influenza B viruses.
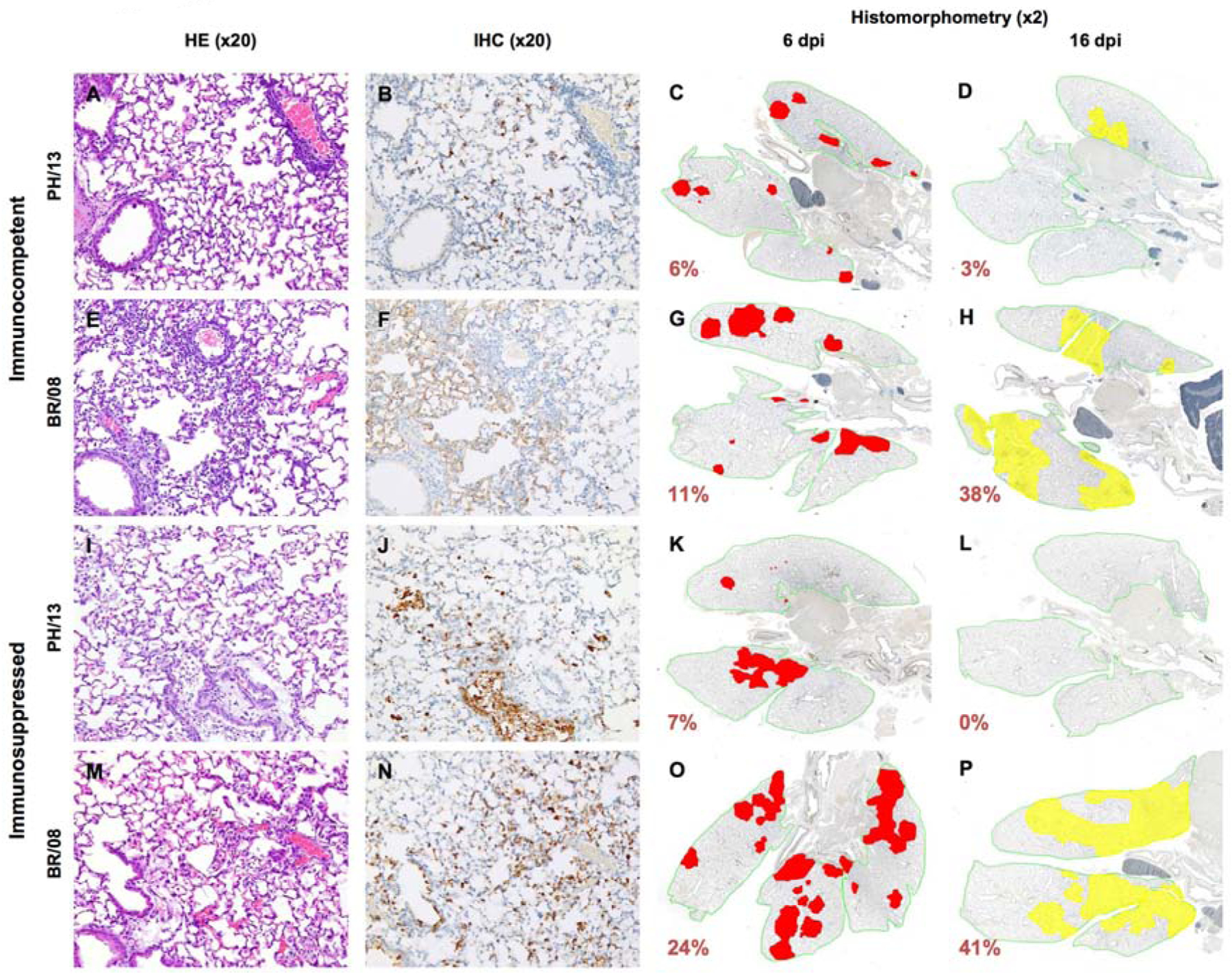
Immunocompetent and immunosuppressed (DEX+CP-treated) BALB/c mice were anesthetized with isoflurane and inoculated intranasally with 102 TCID50/mouse of influenza PH/13 or BR/08 virus. Pulmonary lesions were evaluated at 6 or 16 dpi (n = 2/group). Mouse lungs were fixed in 10% neutral buffered formalin and stained with hematoxylin-eosin (HE) (A, E, I, and M), subjected to immunohistochemical (IHC) staining with anti–influenza B antiserum (B, F, J, and N), or analyzed by histomorphometry at 6 dpi (C, G, K, and O) or 16 dpi (D, H, L, and P). Representative images for each treatment group are shown (magnification: ×20 [HE and IHC] or ×2 [histomorphometry]). In the histomorphometry images, the total lung areas examined are outlined in green; areas of active infection with antigen-positive cells are shown in red; and areas of inactive infection with lesions but negligible antigen are shown in yellow. The percentage of the total lung area represented by the lesions is indicated for each image.
3.5. Efficacy of oseltamivir against lethal BR/08 infection
To determine the efficacy of oseltamivir against BR/08 virus, mice received oseltamivir treatment for 8 or 16 days (Table 1). Untreated immunocompetent control mice progressively lost weight, and all animals died between 8 and 10 dpi; their mean survival was 8.6 days. With oseltamivir treatment, 80% of the immunocompetent mice survived infection, and virus was cleared from their lungs by 10 dpi. Untreated immunosuppressed control mice rapidly lost weight and did not survive beyond 8 dpi; their mean survival was 7.4 days. In contrast, both 8-and 16-day treatment with oseltamivir significantly increased the mean survival of immunosuppressed mice challenged with a lethal dose of BR/08 to 9.5 and 13.8 days, respectively (P < 0.05). However, only the 16-day treatment rescued 20% animals from death and promoted virus clearance from lungs at 14 dpi (Table 1).
Table 1.
Efficacy of oseltamivir treatment of immunocompetent and immunosuppressed BALB/c mice inoculated with a lethal dose of influenza BR/08 virus.
| Oseltamivir dose (mg/kg/d)a | No. of treatment days | No. of surviving mice/total no. of mice (%) | Mean day to death ± SD | Mean weight loss (% ± SD) on dpi:b | Lung virus titer (mean ± SD) (log10QTCID50/ml) on dpi:c | ||||
|---|---|---|---|---|---|---|---|---|---|
| 6 | 10 | 14 | 6 | 10 | 14 | ||||
| Immunocompetent mice | |||||||||
| 0 | 8 | 0/5 (0) | 8.6 ± 0.9 | 25.7 ± 1.3 | NA | NA | 3.5 ± 0.0 | NA | NA |
| 20 | 8 | 4/5 (80) | 18.6 ± 5.4* | 17.4 ± 0.9 | 14.6 ± 2.0 | 3.1 ± 0.9 | 2.5 ± 0.7 | < | < |
| Immunosuppressed mice | |||||||||
| 0 | 8 | 0/5 (0) | 7.4 ± 0.9 | 21.2 ± 2.6 | NA | NA | 2.6 ± 1.2 | NA | NA |
| 20 | 8 | 0/5 (0) | 9.5 ± 1.6* | 13.6 ± 1.9 | 17.9 ± 1.2 | NA | 3.5 ± 0.0 | 4.1 ± 0.4 | 4.5 ± 0.1 |
| 20 | 16 | 1/5 (20) | 13.8 ± 4.4* | 11.9 ± 1.5 | 19.8 ± 2.2 | 24.8 ± 1.5 | 3.2 ± 0.4 | 3.5 ± 0.0 | < |
Oseltamivir was administered by oral gavage at a dose of 20 mg/kg/day (0.1 mL/mouse) twice daily for 8 (immunocompetent mice) or for 8 or16 (immunosuppressed mice) days. Control (virus-inoculated, untreated) mice received sterile water.
Loss of weight was calculated for each mouse as a percentage of its weight on day 0.
Virus titers were determined in the lungs of mice (n = 3/group/time-point) at 6, 10, and 14 dpi by TCID50 assays in MDCK cells.
, virus not detected by the TCID50 assay (detection limit was 1.0 log10TCID50/mL).
P < 0.01 for comparisons of untreated and oseltamivir-treated mice inoculated with BR/08 virus by one-way ANOVA with Bonferroni’s multiple comparison post-test. NA, not applicable (all mice in the group died).
Untreated virus-infected immunocompetent mice showed prominent perivascular and intra-alveolar inflammation (Fig. 6A), with widespread infection of alveolar epithelium and macrophages (Fig. 6B). Extensive areas of the lungs were actively infected or contained thickened septa and inflammatory cells in the absence of viral antigen (Fig. 6C). Although lungs of oseltamivir-treated mice showed similar levels of perivascular inflammation, they had less intra-alveolar inflammation (Fig. 6D) and fewer infected alveolar pneumocytes (Fig. 6E, F). The proportion of the lungs showing active infection was also markedly reduced in oseltamivir-treated groups (Fig. 6F). Histopathologic examination of immunosuppressed mice revealed minimal perivascular and intra-alveolar inflammation in both untreated and oseltamivir-treated animals (Fig. 6G, J). Numerous infected type II pneumocytes were evident in all immunosuppressed lungs (Fig. 6H, K), but oseltamivir treatment markedly reduced the extent of bronchiolar epithelium involvement (Fig. 6K). Immunosuppressed mice had no areas of inactive pulmonary lesions, but the active infection was much more extensive in the untreated controls than in the oseltamivir-treated mice (Fig. 6I, L). Oseltamivir treatment had no apparent effect on the release of pulmonary inflammatory mediators in immunosuppressed mice, except for a slight increase in IFN-γ at 6 dpi (Fig. 7A).
Figure 6. Histopathologic changes in the lungs of immunocompetent and immunosuppressed mice inoculated with influenza BR/08 virus and treated with oseltamivir.
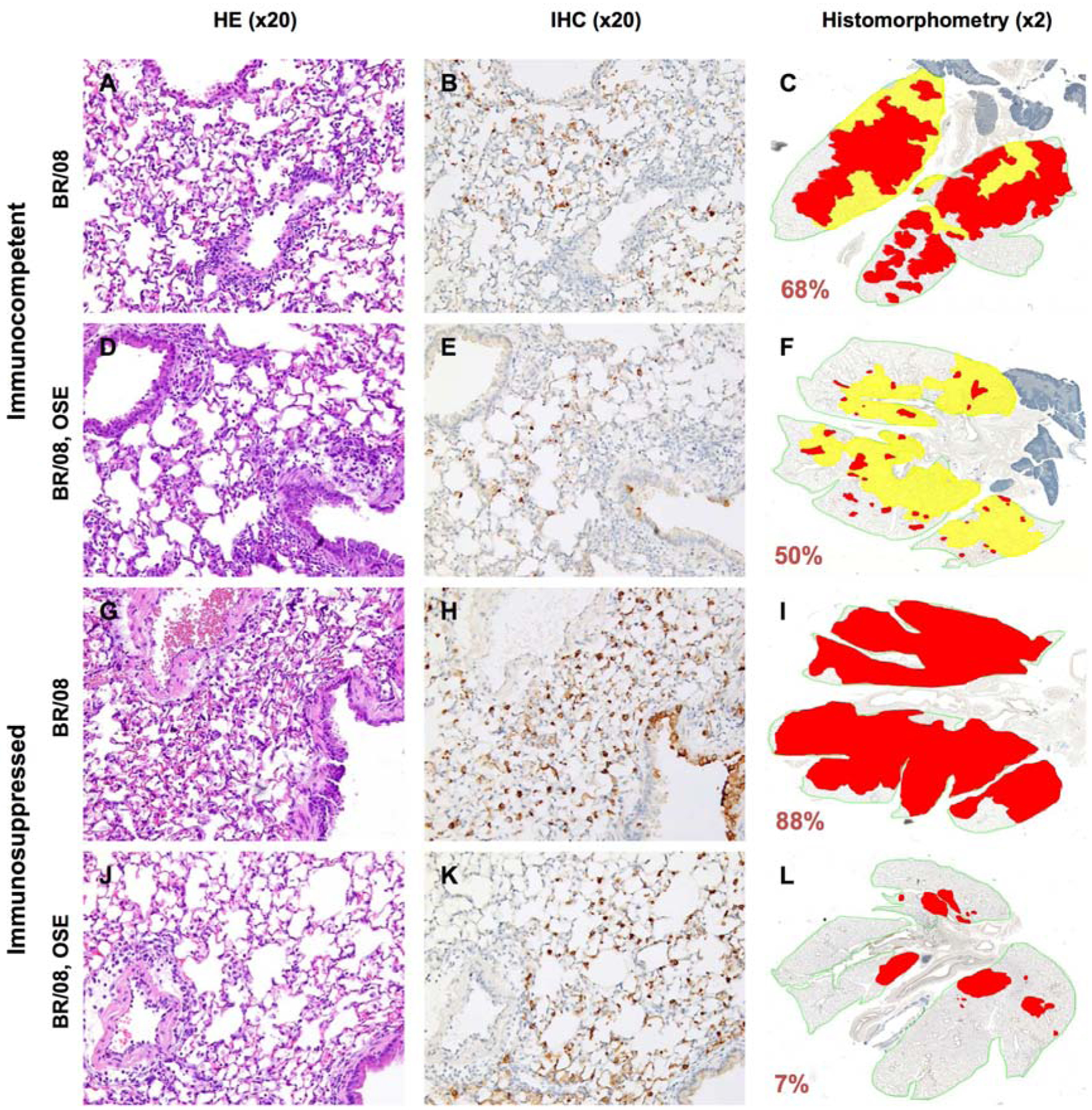
Immunocompetent and immunosuppressed (DEX+CP-treated) BALB/c mice were anesthetized with isoflurane and inoculated intranasally with a 5 MLD50 dose of BR/08 virus. The NAI oseltamivir was administered by oral gavage at a dose of 20 mg/kg/day (0.1 mL/mouse) twice daily for 8 (immunocompetent mice) or 16 (immunosuppressed mice) days. Control animals received sterile water for 8 days. Pulmonary lesions were evaluated at 6 dpi (n = 2/group). Mouse lungs were fixed in 10% neutral-buffered formalin and stained with HE (A, D, G, and J), subjected to IHC staining with anti–influenza B antiserum (B, E, H, and K), or analyzed by histomorphometry (C, F, I, and L). Representative images for each treatment group are shown (magnification: ×20 [A, B, D, E, G, H, J, and K] or ×2 [C, F, I, and L]). The total lung areas examined are outlined in green; areas of active infection with antigen-positive cells are shown in red, and areas of inactive infection with lesions but negligible antigen are shown in yellow. The percentage of the total lung area represented by the lesions is indicated for each image.
Figure 7. Pulmonary cytokine/chemokine responses in immunocompetent and immunosuppressed mice inoculated with influenza BR/08 virus and treated with oseltamivir.
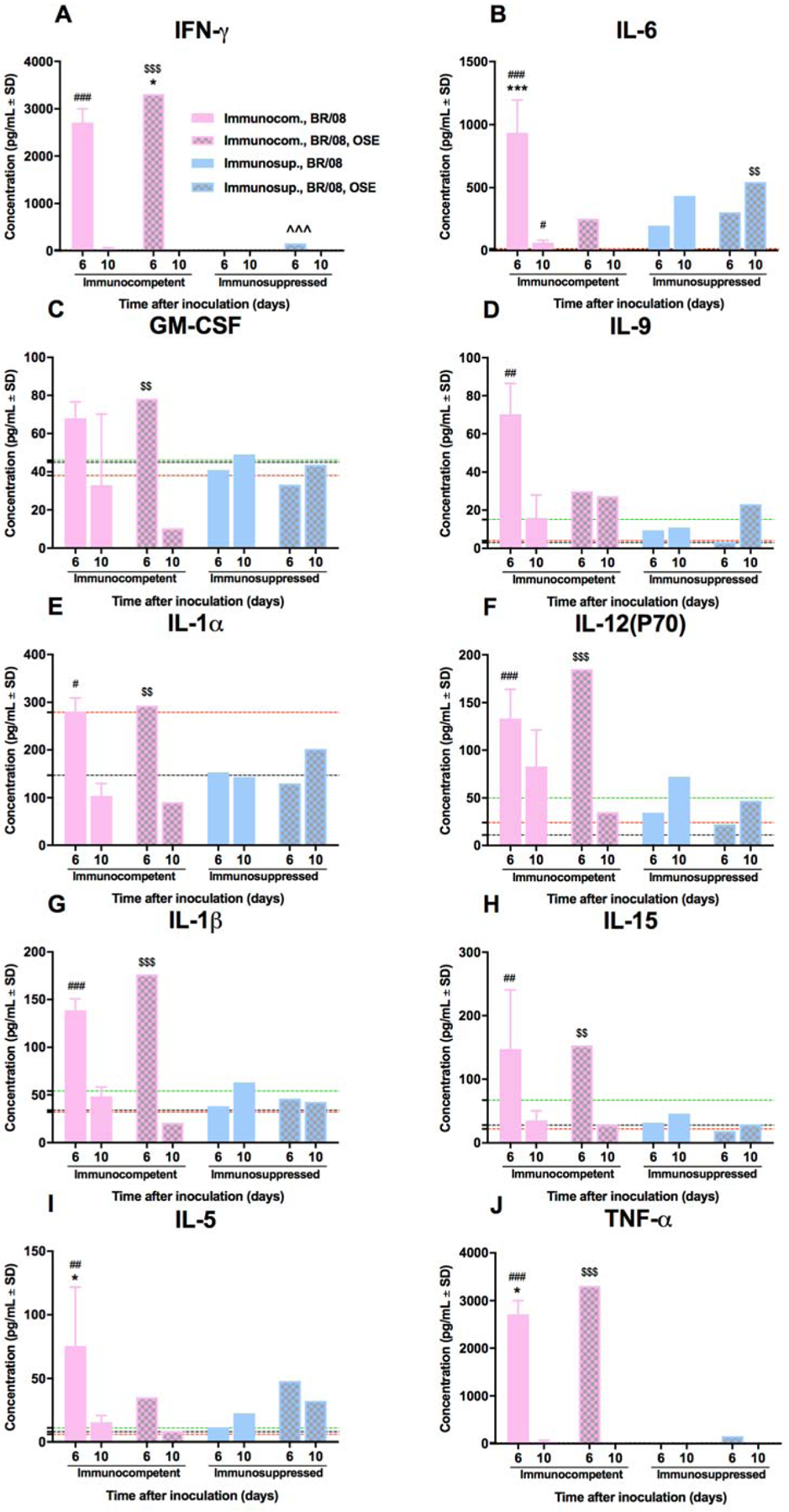
Immunocompetent and immunosuppressed (DEX+CP-treated) BALB/c mice were anesthetized with isoflurane and inoculated intranasally with influenza BR/08 virus and treated with oseltamivir as described in the legend for Figure 6. The expression levels of IFN-γ (A), IL-6 (B), GM-CSF (C), IL-9 (D), IL-1α (E), IL-12(P70) (F), IL-1β (G), IL-15 (H), IL-5 (I), and TNF-α (J) were assayed in lung homogenates at 6 and 10 dpi. Bars represent mean values ± SDs (n = 3/group/time-point). *P < 0.05, **P < 0.01, and ***P < 0.001 for comparisons of untreated and oseltamivir-treated immunocompetent mice; ^^^P < 0.001 for comparison of untreated and oseltamivir-treated immunosuppressed mice; #P < 0.05, ##P < 0.01, and ###P < 0.001 for comparisons of untreated and DEX+CP–treated mice; and $$P < 0.01, and $$$P < 0.001 for comparison of oseltamivir-treated immunocompetent and immunosuppressed mice by one-way ANOVA with Bonferroni’s multiple comparison post-test. The level of cytokine/chemokine expression in control uninfected mice is indicated by a black dotted line for immunocompetent mice, by a red dotted line for immunosuppressed mice, and by a green dotted line for immunosuppressed, oseltamivir-treated mice.
To determine whether oseltamivir-resistant variants emerged during treatment, we extracted viral RNA directly from the lung homogenates of mice and sequenced the NA genes. Sequencing of the samples obtained at 6 and 10 dpi did not reveal any NA substitutions associated with NAI resistance.
4. Discussion
The animal models available for studying influenza B viruses are limited (Bouvier and Lowen, 2010; Thangavel and Bouvier, 2014). Here, we established a pharmacologically immunosuppressed mouse model, and compared the pathogenesis of two antigenically distinct influenza B viruses, as well as the efficacy of prolonged oseltamivir treatment. We demonstrated that BR/08 and PH/13 viruses differ in their pathogenicity and that immunosuppressed mice were more susceptible to infection than were immunocompetent animals. Although BR/08 and PH/13 viral titers were similar in immunosuppressed mice, BR/08 virus persisted longer than PH/13 and mortality was higher with BR/08, supporting the conclusion that BR/08 was more virulent than PH/13. The more severe alveolar damage and more extensive pulmonary spread with BR/08 were consistent with the higher morbidity and mortality caused by that virus. The different degrees of immunopathology caused by influenza B viruses might be important contributors to their overall pathogenicity. Oseltamivir was effective in controlling the spread of BR/08 virus in the lungs of both immunocompetent and immunosuppressed mice but failed to improve viral clearance or prevent mortality in immunosuppressed mice.
The immunosuppressive treatment regimen used in this study mimicked clinical drug schedules in order to transiently suppress the normal immune functions. Neutropenia and lymphopenia are usually associated with severe lower respiratory tract infections in immunocompromised patients (El Saleeby et al., 2008); hence, we used the total lymphocyte and neutrophil counts as readouts of the immune status of the mice. DEX+CP treatment caused severe lymphopenia, which was associated with the absence of anti-HA antibodies. In virus-infected immunosuppressed mice, virus lung clearance (at 16 dpi) correlated with a slight rebound in the total lymphocytes count and a marked increase in NK cells. That slight increase in lymphocytes correlated with virus clearance from the lung and reductions in nasal titers in PH/13-infected mice, reductions in nasal and lung titers in BR/08-infected mice, and their peak weight loss. Based on the earlier rebound of NK cells, we hypothesize that they play a role in viral clearance, especially in mild infection. NK cells were recently shown to be important in Sendai virus clearance after the discontinuation DEX+CP treatment (Mostafa et al., 2016). The current study perceived that the increase in NK cells may be insufficient for virus clearance in the more severe infection model. NK cell recovery may contribute to the virus-induced immunopathology, as NK cells not only confer protection during influenza infection but also prompt pathology (Schultz-Cherry, 2015). Immunosuppression initially dampened the expression of several inflammatory mediators [IFN-γ, IL-1α, IL-1β, IL-10, IL-12(P40), and IL-12(P70)], resulting in minimal inflammation in lungs early in infection. This finding correlated with the significant initial lymphopenia and neutropenia caused by DEX+CP treatment. Recovery from immunosuppression, with an increase in NK cells and neutrophils, correlated with delayed weight loss in immunosuppressed mice and suggested the development of an immune restoration syndrome (IRS)-like phenomenon (Bosamiya, 2011; Sharma and Soneja, 2011). This is further supported by the clinical deterioration observed during immune system recovery in the immunosuppressed mice. Similarly, prolonged influenza B virus replication, attenuated cellular immune responses, and variable pathogenicity was observed in the mice with varying permanent immunocompromised status (Pascua et al., 2017). Moreover, sustained NK cell response contributed to the observed pathogenicity of influenza B virus in these genetically-modified mice, and reconstitution of lymphocyte responses was not noted.
Oral oseltamivir is the most commonly prescribed antiviral for prophylaxis and treatment of high-risk patients, including immunocompromised individuals (CDC, 2017a; Dutkowski, 2010; Ison, 2013; Ison et al., 2012; Shinjoh et al., 2012; Whitley and Monto, 2006)). Only limited preclinical data is available on antiviral treatment of influenza A virus infection in immunocompromised hosts (Kitano et al., 2013; Pham et al., 2013; Sidwell et al., 2003; van der Vries et al., 2013) and none is available for influenza B virus infection. Oseltamivir and the investigational NAI A-322278 were effective in reducing viral replication and weight loss in immunocompetent and immunocompromised mice infected with lethal and sublethal doses of A(H2N2) virus (Ison et al., 2006). Interestingly, oseltamivir and peramivir (Wester and Shetty, 2016) had different efficacies against influenza A(H1N1)pdm09 virus in CP-treated mice (Kitano et al., 2013). Although peramivir significantly reduced mortality in immunosuppressed mice, oseltamivir afforded no survival benefit (Kitano et al., 2013). Moreover, clinical studies in Japan have shown that oseltamivir is less efficacious than peramivir against influenza B virus infections in immunocompetent patients (Kawai et al., 2005; 2006; Sugaya et al., 2007). Prolonged influenza B virus shedding was noted among immunocompromised patients during NAI treatments (Carr et al., 2011; Gubareva et al., 1998). Our data suggests limited efficacy of oseltamivir against influenza B virus infections in immunosuppressed mice. An 8-day course of oseltamivir protected 80% of immunocompetent mice from lethal BR/08 infection, and at 6 dpi, the immune responses, aided by oseltamivir, initiated regulation of virus replication in the lungs. In contrast, immunosuppressed mice failed to establish immune responses early in infection, leaving the burden of virus control to oseltamivir alone. Although a 16-day course of oseltamivir provided some protection (20%), the absence of efficient immune responses late in the infection probably contributed to the inferior outcomes with that drug. Compounding the problem, if an IRS-like phenomenon develops upon the cessation of DEX+CP treatment, it might lead to a rebound of inflammatory immune responses and their associated immunopathology. Discontinuing antiviral drug treatment might also allow viral replication to rebound, as previously observed in immunocompromised mice (Ison et al., 2006). Our animal models emphasize the need for antiviral treatment optimization in this category of patients.
Our mouse model can be used to study the effects of other NAIs and investigational antivirals via two modified strategies. First, increasing the dosage might better control virus spread in the absence of adaptive immunity but may confer little clinical benefit (Ariano et al., 2010; Kumar et al., 2010; Lee et al., 2013; Writing Committee, 2008) and cause other complications, such as decreased enteric absorption of the drug in certain high-risk individuals (Jullien et al., 2011). A second strategy would be to prolong treatment, but this could lead to the emergence of drug-resistant viruses. In our study, increased treatment duration in immunosuppressed mice did not lead to virus resistance, but this is not consistent with the high incidence of NAI resistance (up to 33%) reported in immunocompromised patients (Carr et al., 2011; van der Vries et al., 2013). In future studies with this model, both increased dosage and prolonged treatment may be examined individually and in combination. As immunosuppression in individuals can be acute (due to drug treatment) or persistent (due to genetics), a “one-size-fits-all” strategy is unlikely to emerge. Mouse models like ours are important preclinical tools to test antiviral options for respiratory viruses such as influenza B viruses, with the goal to improve the treatment outcomes in affected patients.
Highlights.
B/Brisbane/60/2008 virus was more pathogenic than B/Phuket/3073/2013 virus in immunosuppressed mice.
Differences in the immunopathology caused by influenza B viruses might influence their respective overall pathogenicity.
Oseltamivir therapy was less effective in immunosuppressed mice than in immunocompetent B/Brisbane/60/2008-infected mice.
Our immunosuppressed mouse model could facilitate evaluations of established and investigational anti-influenza drugs.
Acknowledgments
This work was supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Centers of Excellence for Influenza Research and Surveillance (CEIRS), contract no. HHSN272201400006C, and by ALSAC. We thank Keith A. Laycock for excellent editing of the manuscript and the staff of the Hartwell Center for Bioinformatics and Biotechnology for their help with the Sanger sequencing. The NAI oseltamivir phosphate was provided by Roche Innovation Centre (Basel, Switzerland).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors have no personal or financial affiliation with a commercial entity that might pose a conflict of interest.
References
- Ariano RE, Sitar DS, Zelenitsky SA, Zarychanski R, Pisipati A, Ahern S, Kanji S, Rello J, Kumar A, 2010. Enteric absorption and pharmacokinetics of oseltamivir in critically ill patients with pandemic (H1N1) influenza. CMAJ : Canadian Medical Association journal = journal de l’Association medicale canadienne 182, 357–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr IG, Jelley LL, 2012. The coming era of quadrivalent human influenza vaccines: who will benefit? Drugs 72, 2177–2185. [DOI] [PubMed] [Google Scholar]
- Blanton L, Mustaquim D, Alabi N, Kniss K, Kramer N, Budd A, Garg S, Cummings CN, Fry AM, Bresee J, Sessions W, Garten R, Xu X, Elal AI, Gubareva L, Barnes J, Wentworth DE, Burns E, Katz J, Jernigan D, Brammer L, 2017. Update: Influenza Activity - United States, October 2, 2016-February 4, 2017. MMWR. Morbidity and mortality weekly report 66, 159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosamiya SS, 2011. The immune reconstitution inflammatory syndrome. Indian journal of dermatology 56, 476–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosma MJ, Carroll AM, 1991. The SCID mouse mutant: definition, characterization, and potential uses. Annual review of immunology 9, 323–350. [DOI] [PubMed] [Google Scholar]
- Boudreault AA, Xie H, Leisenring W, Englund J, Corey L, Boeckh M, 2011. Impact of corticosteroid treatment and antiviral therapy on clinical outcomes in hematopoietic cell transplant patients infected with influenza virus. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 17, 979–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier NM, Lowen AC, 2010. Animal Models for Influenza Virus Pathogenesis and Transmission. Viruses 2, 1530–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky RA, Sensenbrenner LL, Jones RJ, 1996. Complete remission in severe aplastic anemia after high-dose cyclophosphamide without bone marrow transplantation. Blood 87, 491–494. [PubMed] [Google Scholar]
- Broxmeyer HE, 1978. Inhibition in vivo of mouse granulopoiesis by cell-free activity derived from human polymorphonuclear neutrophils. Blood 51, 889–901. [PubMed] [Google Scholar]
- Burnham AJ, Baranovich T, Govorkova EA, 2013. Neuraminidase inhibitors for influenza B virus infection: efficacy and resistance. Antiviral research 100, 520–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr S, Ilyushina NA, Franks J, Adderson EE, Caniza M, Govorkova EA, Webster RG, 2011. Oseltamivir-resistant influenza A and B viruses pre- and postantiviral therapy in children and young adults with cancer. The Pediatric infectious disease journal 30, 284–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC, 2017a. https://http://www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm.
- CDC, 2017b. https://http://www.cdc.gov/flu/weekly/.
- Colvin OM, 2003. Adventures with an enigmatic anticancer drug. Cancer biology & therapy 2, 301–303. [DOI] [PubMed] [Google Scholar]
- Cooksley CD, Avritscher EB, Bekele BN, Rolston KV, Geraci JM, Elting LS, 2005. Epidemiology and outcomes of serious influenza-related infections in the cancer population. Cancer 104, 618–628. [DOI] [PubMed] [Google Scholar]
- Dutkowski R, 2010. Oseltamivir in seasonal influenza: cumulative experience in low- and high-risk patients. The Journal of antimicrobial chemotherapy 65 Suppl 2, ii11–ii24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Saleeby CM, Somes GW, DeVincenzo JP, Gaur AH, 2008. Risk factors for severe respiratory syncytial virus disease in children with cancer: the importance of lymphopenia and young age. Pediatrics 121, 235–243. [DOI] [PubMed] [Google Scholar]
- Estlin EJ, Ronghe M, Burke GA, Yule SM, 2000. The clinical and cellular pharmacology of vincristine, corticosteroids, L-asparaginase, anthracyclines and cyclophosphamide in relation to childhood acute lymphoblastic leukaemia. British journal of haematology 110, 780–790. [DOI] [PubMed] [Google Scholar]
- Gooskens J, Jonges M, Claas EC, Meijer A, Kroes AC, 2009. Prolonged influenza virus infection during lymphocytopenia and frequent detection of drug-resistant viruses. The Journal of infectious diseases 199, 1435–1441. [DOI] [PubMed] [Google Scholar]
- Gubareva LV, Matrosovich MN, Brenner MK, Bethell RC, Webster RG, 1998. Evidence for zanamivir resistance in an immunocompromised child infected with influenza B virus. The Journal of infectious diseases 178, 1257–1262. [DOI] [PubMed] [Google Scholar]
- Ison MG, 2013. Influenza prevention and treatment in transplant recipients and immunocompromised hosts. Influenza and other respiratory viruses 7 Suppl 3, 60–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ison MG, 2015. Optimizing antiviral therapy for influenza: understanding the evidence. Expert review of anti-infective therapy 13, 417–425. [DOI] [PubMed] [Google Scholar]
- Ison MG, Mishin VP, Braciale TJ, Hayden FG, Gubareva LV, 2006. Comparative activities of oseltamivir and A-322278 in immunocompetent and immunocompromised murine models of influenza virus infection. The Journal of infectious diseases 193, 765–772. [DOI] [PubMed] [Google Scholar]
- Ison MG, Szakaly P, Shapira MY, Krivan G, Nist A, Dutkowski R, 2012. Efficacy and safety of oral oseltamivir for influenza prophylaxis in transplant recipients. Antiviral therapy 17, 955–964. [DOI] [PubMed] [Google Scholar]
- Jullien V, Hubert D, Launay O, Babany G, Lortholary O, Sermet I, 2011. Pharmacokinetics and diffusion into sputum of oseltamivir and oseltamivir carboxylate in adults with cystic fibrosis. Antimicrobial agents and chemotherapy 55, 4183–4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman M, Limaye SA, Driscoll N, Johnson C, Caramanica A, Lebowicz Y, Patel D, Kohn N, Rai K, 2009. A combination of rituximab, cyclophosphamide and dexamethasone effectively treats immune cytopenias of chronic lymphocytic leukemia. Leukemia & lymphoma 50, 892–899. [DOI] [PubMed] [Google Scholar]
- Kawai N, Ikematsu H, Iwaki N, Maeda T, Satoh I, Hirotsu N, Kashiwagi S, 2006. A comparison of the effectiveness of oseltamivir for the treatment of influenza A and influenza B: a Japanese multicenter study of the 2003–2004 and 2004–2005 influenza seasons. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 43, 439–444. [DOI] [PubMed] [Google Scholar]
- Kawai N, Ikematsu H, Iwaki N, Satoh I, Kawashima T, Maeda T, Miyachi K, Hirotsu N, Shigematsu T, Kashiwagi S, 2005. Factors influencing the effectiveness of oseltamivir and amantadine for the treatment of influenza: a multicenter study from Japan of the 2002–2003 influenza season. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 40, 1309–1316. [DOI] [PubMed] [Google Scholar]
- Kitano M, Kodama M, Itoh Y, Kanazu T, Kobayashi M, Yoshida R, Sato A, 2013. Efficacy of repeated intravenous injection of peramivir against influenza A (H1N1) 2009 virus infection in immunosuppressed mice. Antimicrobial agents and chemotherapy 57, 2286–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsakos M, Nguyen TH, Barclay WS, Kedzierska K, 2016. Knowns and unknowns of influenza B viruses. Future microbiology 11, 119–135. [DOI] [PubMed] [Google Scholar]
- Kumar D, Michaels MG, Morris MI, Green M, Avery RK, Liu C, Danziger-Isakov L, Stosor V, Estabrook M, Gantt S, Marr KA, Martin S, Silveira FP, Razonable RR, Allen UD, Levi ME, Lyon GM, Bell LE, Huprikar S, Patel G, Gregg KS, Pursell K, Helmersen D, Julian KG, Shiley K, Bono B, Dharnidharka VR, Alavi G, Kalpoe JS, Shoham S, Reid GE, Humar A, American Society of Transplantation, H.N.C.S.G., 2010. Outcomes from pandemic influenza A H1N1 infection in recipients of solid-organ transplants: a multicentre cohort study. The Lancet. Infectious diseases 10, 521–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N, Hui DS, Zuo Z, Ngai KL, Lui GC, Wo SK, Tam WW, Chan MC, Wong BC, Wong RY, Choi KW, Sin WW, Lee EL, Tomlinson B, Hayden FG, Chan PK, 2013. A prospective intervention study on higher-dose oseltamivir treatment in adults hospitalized with influenza a and B infections. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 57, 1511–1519. [DOI] [PubMed] [Google Scholar]
- Lee N, Ison MG, 2012. Diagnosis, management and outcomes of adults hospitalized with influenza. Antiviral therapy 17, 143–157. [DOI] [PubMed] [Google Scholar]
- Li X, DuBois DC, Song D, Almon RR, Jusko WJ, Chen X, 2017. Modeling Combined Immunosuppressive and Anti-inflammatory Effects of Dexamethasone and Naproxen in Rats Predicts the Steroid-Sparing Potential of Naproxen. Drug metabolism and disposition: the biological fate of chemicals. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungman P, Andersson J, Aschan J, Barkholt L, Ehrnst A, Johansson M, Weiland O, 1993. Influenza A in immunocompromised patients. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 17, 244–247. [DOI] [PubMed] [Google Scholar]
- Luznik L, Jones RJ, Fuchs EJ, 2010. High-dose cyclophosphamide for graft-versus-host disease prevention. Current opinion in hematology 17, 493–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marathe BM, Wong SS, Vogel P, Garcia-Alcalde F, Webster RG, Webby RJ, Najera I, Govorkova EA, 2016. Combinations of Oseltamivir and T-705 Extend the Treatment Window for Highly Pathogenic Influenza A(H5N1) Virus Infection in Mice. Scientific reports 6, 26742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullers JA, Hayden FG, 2012. Fatal influenza B infections: time to reexamine influenza research priorities. The Journal of infectious diseases 205, 870–872. [DOI] [PubMed] [Google Scholar]
- Michallet AS, Rossignol J, Cazin B, Ysebaert L, 2011. Rituximab-cyclophosphamide-dexamethasone combination in management of autoimmune cytopenias associated with chronic lymphocytic leukemia. Leukemia & lymphoma 52, 1401–1403. [DOI] [PubMed] [Google Scholar]
- Mostafa HH, Vogel P, Srinivasan A, Russell CJ, 2016. Non-invasive Imaging of Sendai Virus Infection in Pharmacologically Immunocompromised Mice: NK and T Cells, but not Neutrophils, Promote Viral Clearance after Therapy with Cyclophosphamide and Dexamethasone. PLoS pathogens 12, e1005875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paddock CD, Liu L, Denison AM, Bartlett JH, Holman RC, Deleon-Carnes M, Emery SL, Drew CP, Shieh WJ, Uyeki TM, Zaki SR, 2012. Myocardial injury and bacterial pneumonia contribute to the pathogenesis of fatal influenza B virus infection. The Journal of infectious diseases 205, 895–905. [DOI] [PubMed] [Google Scholar]
- Pascua PNQ, Mostafa HH, Marathe BM, Vogel P, Russell CJ, Webby RJ, Govorkova EA, 2017. Pathogenicity and peramivir efficacy in immunocompromised murine models of influenza B virus infection. Scientific reports 7, 7345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul Glezen W, Schmier JK, Kuehn CM, Ryan KJ, Oxford J, 2013. The burden of influenza B: a structured literature review. American journal of public health 103, e43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltola V, Ziegler T, Ruuskanen O, 2003. Influenza A and B virus infections in children. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 36, 299–305. [DOI] [PubMed] [Google Scholar]
- Pham VL, Nakayama M, Itoh Y, Ishigaki H, Kitano M, Arikata M, Ishida H, Kitagawa N, Shichinohe S, Okamatsu M, Sakoda Y, Tsuchiya H, Nakamura S, Kida H, Ogasawara K, 2013. Pathogenicity of pandemic H1N1 influenza A virus in immunocompromised cynomolgus macaques. PloS one 8, e75910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad LB, Spradbrow PB, 1977. Multiplication of turkey herpes virus and Marek’s disease virus in chick embryo skin cell cultures. Journal of comparative pathology 87, 515–520. [DOI] [PubMed] [Google Scholar]
- Pui CH, 2006. Central nervous system disease in acute lymphoblastic leukemia: prophylaxis and treatment. Hematology. American Society of Hematology. Education Program, 142–146. [DOI] [PubMed] [Google Scholar]
- Schultz-Cherry S, 2015. Role of NK cells in influenza infection. Current topics in microbiology and immunology 386, 109–120. [DOI] [PubMed] [Google Scholar]
- Shang M, Blanton L, Kniss K, Mustaquim D, Alabi N, Barnes S, Budd A, Davlin SL, Kramer N, Garg S, Cummings CN, Flannery B, Fry AM, Grohskopf LA, Olsen SJ, Bresee J, Sessions W, Garten R, Xu X, Elal AI, Gubareva L, Barnes J, Wentworth DE, Burns E, Katz J, Jernigan D, Brammer L, 2016. Update: Influenza Activity - United States, October 2-December 17, 2016. MMWR. Morbidity and mortality weekly report 65, 1439–1444. [DOI] [PubMed] [Google Scholar]
- Sharma SK, Soneja M, 2011. HIV & immune reconstitution inflammatory syndrome (IRIS). The Indian journal of medical research 134, 866–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinjoh M, Takano Y, Takahashi T, Hasegawa N, Iwata S, Sugaya N, 2012. Postexposure prophylaxis for influenza in pediatric wards oseltamivir or zanamivir after rapid antigen detection. The Pediatric infectious disease journal 31, 1119–1123. [DOI] [PubMed] [Google Scholar]
- Sidwell RW, Bailey KW, Morrey JD, Wong MH, Baldwin TJ, Smee DF, 2003. Inhibition of influenza virus infections in immunosuppressed mice with orally administered peramivir (BCX-1812). Antiviral research 60, 17–25. [DOI] [PubMed] [Google Scholar]
- Silvennoinen H, Peltola V, Vainionpaa R, Ruuskanen O, Heikkinen T, 2011. Incidence of influenza-related hospitalizations in different age groups of children in Finland: a 16-year study. The Pediatric infectious disease journal 30, e24–28. [DOI] [PubMed] [Google Scholar]
- Sugaya N, Mitamura K, Yamazaki M, Tamura D, Ichikawa M, Kimura K, Kawakami C, Kiso M, Ito M, Hatakeyama S, Kawaoka Y, 2007. Lower clinical effectiveness of oseltamivir against influenza B contrasted with influenza A infection in children. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 44, 197–202. [DOI] [PubMed] [Google Scholar]
- Tafalla M, Buijssen M, Geets R, Vonk Noordegraaf-Schouten M, 2016. A comprehensive review of the epidemiology and disease burden of Influenza B in 9 European countries. Human vaccines & immunotherapeutics 12, 993–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewawong N, Chansaenroj J, Klinfueng S, Vichiwattana P, Korkong S, Thongmee T, Theamboonlers A, Payungporn S, Vongpunsawad S, Poovorawan Y, 2016. Lineage-specific detection of influenza B virus using real-time polymerase chain reaction with melting curve analysis. Archives of virology 161, 1425–1435. [DOI] [PubMed] [Google Scholar]
- Thangavel RR, Bouvier NM, 2014. Animal models for influenza virus pathogenesis, transmission, and immunology. Journal of immunological methods 410, 60–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Vries E, Stittelaar KJ, van Amerongen G, Veldhuis Kroeze EJ, de Waal L, Fraaij PL, Meesters RJ, Luider TM, van der Nagel B, Koch B, Vulto AG, Schutten M, Osterhaus AD, 2013. Prolonged influenza virus shedding and emergence of antiviral resistance in immunocompromised patients and ferrets. PLoS pathogens 9, e1003343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlberg J, Holmberg A, Bergh S, Hultman T, Uhlen M, 1992. Automated magnetic preparation of DNA templates for solid phase sequencing. Electrophoresis 13, 547–551. [DOI] [PubMed] [Google Scholar]
- Wester A, Shetty AK, 2016. Peramivir injection in the treatment of acute influenza: a review of the literature. Infection and drug resistance 9, 201–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whimbey E, Bodey GP, 1992. Viral pneumonia in the immunocompromised adult with neoplastic disease: the role of common community respiratory viruses. Seminars in respiratory infections 7, 122–131. [PubMed] [Google Scholar]
- Whitley RJ, Monto AS, 2006. Prevention and treatment of influenza in high-risk groups: children, pregnant women, immunocompromised hosts, and nursing home residents. The Journal of infectious diseases 194 Suppl 2, S133–138. [DOI] [PubMed] [Google Scholar]
- WHO, 2017. http://www.who.int/influenza/surveillance_monitoring/updates/2017_05_15_surveillance_update_289.pdf?ua=1.
- Writing Committee of the Second World Health Organization Consultation on Clinical Aspects of Human Infection with Avian Influenza, A.V., Abdel-Ghafar AN, Chotpitayasunondh T, Gao Z, Hayden FG, Nguyen DH, de Jong MD, Naghdaliyev A, Peiris JS, Shindo N, Soeroso S, Uyeki TM, 2008. Update on avian influenza A (H5N1) virus infection in humans. The New England journal of medicine 358, 261–273. [DOI] [PubMed] [Google Scholar]
- Yousuf HM, Englund J, Couch R, Rolston K, Luna M, Goodrich J, Lewis V, Mirza NQ, Andreeff M, Koller C, Elting L, Bodey GP, Whimbey E, 1997. Influenza among hospitalized adults with leukemia. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 24, 1095–1099. [DOI] [PubMed] [Google Scholar]


