In December 2019, coronavirus disease 2019 (COVID-19), an infectious disease caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) caused an international outbreak. The World Health Organization designated this as a global pandemic on March 11, 2020, with over 200 countries affected worldwide. As of April 24, 2020, there were 2 790 986 patients with confirmed COVID-19 and 195 775 deaths worldwide, with the United States, Spain, Italy, France, Germany, United Kingdom, Turkey, and Iran surpassing China in the number of confirmed cases.1 In a consecutive series of 221 patients with confirmed COVID-19 admitted to a hospital in Wuhan, China, acute ischemic stroke occurred in 11(5%) of patients with a broad range of stroke subtypes.2 These patients with stroke were older, more likely to have cardiovascular risk factors, presenting with severe COVID-19 with multiple organ involvement. Of note, presence of COVID-19 in these patients does not imply that COVID-19 was the mechanism leading to the patient’s stroke.
Shortages of Personal Protective Equipment (PPE) such as N95 masks, facial shields, hand sanitizer, and cleansing wipes have presented a major challenge in the allocation of resources, as healthcare workers are frontline in the treatment of these patients.3 Redeployment of clinical staff, nursing, stroke and neurocritical care specialists to care for patients with COVID-19 may create staffing shortages for dedicated stroke care.
In an effort to mitigate the spread of COVID-19 to neuroscience healthcare workers, their patients, and their families, and to optimize allocation of healthcare resources, we present a modified algorithm to acute ischemic large vessel occlusion stroke workflow in the era of the COVID-19 pandemic. This guidance statement is based on shared best practices,4–6 consensus among academic and nonacademic practicing vascular and interventional neurologists, literature review, and would be adapted to the available resources of a local institution. The patients with acute stroke are a vulnerable group to address because these patients often come emergently from the community with little information. Radical changes are felt to be necessary to optimize the safety of the providing team and our patients, limit unnecessary tests, conserve PPE resources and mechanical ventilator usage. This document divides into the following: prehospital phase to the Emergency Department (ED), prethrombectomy procedure, thrombectomy intraprocedure, and postreperfusion therapy phases (Table).
Table 1.
Guidance Summary for Large Vessel Occlusion Stroke in the Era of COVID-19
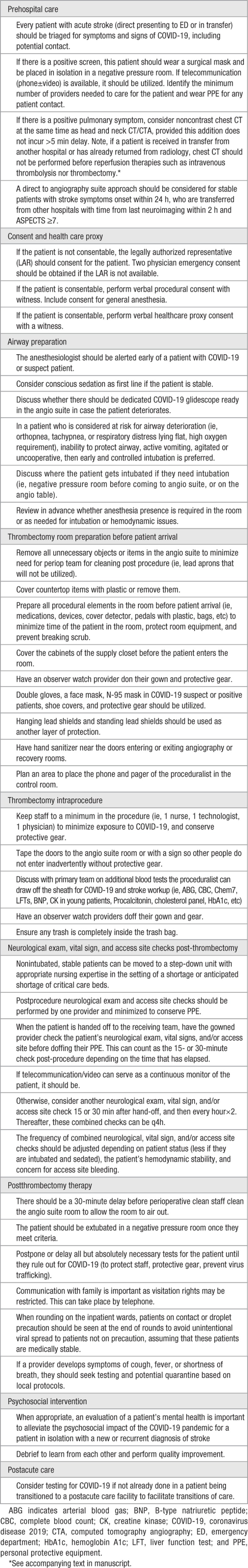
Prehospital and ED Care of Acute Large Vessel Occlusion
Adhering to existing local protocols, all patients (including stroke) presenting to the ED or as interhospital transfers, are screened for signs and symptoms of COVID-19. Any patient who is COVID-19 positive or screen positive should be managed under local protocols to ensure both patient and staff safety. Use of remote telestroke technology should be considered to obtain history and perform neurological examination, if available. During a Code Stroke, coordination between team members with predefined assigned roles (ie, one team member in PPE with patient, another member talks to family over telephone, looks at images, laboratories, and orders thrombolysis or medications) will help reduce staff exposure while maintaining quality care.
If there is positive screening for COVID-19, this patient should wear a surgical mask and immediately be placed in a negative pressure room in the ED if one is available.7 A test for COVID-19 should be considered if the patient meets local criteria for investigation. If a patient or their family is unable to provide corroborative history, then a surgical mask should be placed on this patient, the patient tested for COVID-19 and precautions in place.
Team members evaluating acute stroke code patients in person should be kept to a minimum (ie, one physician, one nurse) and wear appropriate PPE including full sleeved gown, surgical mask, eye protection (face shield or equivalent), and gloves.8 Personal protection should be escalated to N95 mask, hair cap, double gloves, shoe covers in the setting of contact with patient with confirmed COVID-19 and/or aerosolizing events such as coughing, sneezing, nebulizer treatment, suctioning, nasogastric tube placement, bag mask ventilation, CPR, and intubation.
A dedicated computed tomography (CT) scan room for patients with COVID-19 should be established if multiple CT rooms are available. If there are positive pulmonary symptoms, consider low-dose chest CT at the same time as the performance of CT head with CT angiography head and neck, as long as the addition of the chest CT does not incur >5-minute delay to treatment. CT chest may facilitate COVID-19 diagnosis.9,10 However, decision-making to test a patient for COVID-19 or about need for quarantine should be based on local protocols. Of note, if a patient had a head CT/CT angiography at an outside hospital or has returned to the ER from head CT/CT angiography, repeat CT to evaluate for chest pathology should not be performed before reperfusion therapy or thrombectomy.
If CT perfusion is part of an institution’s protocol for selection of thrombectomy patients in the late window, it should be performed at the same time as CT head and CT angiography. Recent data suggest that in the 6- to 24-hour time window, clinical core mismatch by Alberta Stroke Program Early CT Score (ASPECTS) scores (6–10) on noncontrast CT overlaps with clinical core mismatch by CT perfusion or MRI using DAWN criteria11 in nearly 80% of cases.12 Given the overwhelming benefit of thrombectomy noted in DAWN, it is reasonable to assume that meaningful benefit from thrombectomy exists when imaging criteria defining the clinical core mismatch in DAWN are substituted by ASPECTS scores on noncontrast CT. Lack of CT perfusion or MRI capabilities in a resource constrained environment should not be a deterrent from thrombectomy in the 6- to 24-hour time window.
As it would minimize exposure to emergency department and CT suite personnel, a direct to the angiography suite approach should be considered for stable transferred patients with stroke symptoms onset within 24 hours, particularly if the time from the outside hospital imaging to arrival is <2 hours and CT ASPECTS is ≥7. This approach has been shown to safely reduce times to reperfusion and may lead to better functional outcomes.12,13
Airway Preparation
The anesthesiologist should be alerted early of a COVID-19 or suspect patient. Local policies for intubation and general anesthesia versus conscious sedation differ at different centers. If appropriate, consider conscious sedation as first-line to protect anesthesiologists from exposure and to protect our patients from unnecessary intubation as well as conserving mechanical ventilator resources.14,15 Converting a patient from conscious sedation to general anesthesia in the middle of the procedure in the angiography suite should be avoided due to high risk of aerosolization in a positive pressure room. In a patient who is considered at risk for airway deterioration (ie, orthopnea, tachypnea, or respiratory distress lying flat, high oxygen requirement), unable to protect their airway, agitation, uncooperative, or actively vomiting, then early and controlled intubation is preferred. An aerosol box can be used as a cover as an additional measure of PPE protection during intubation.16 High-flow oxygen, bag-valve mask, and noninvasive positive pressure ventilation are not recommended due to the concern for aerosolization of virus. Rapid sequence intubation may be the preferred course.
There should be a discussion regarding where the patient gets intubated if it is seen necessary (ie, in a negative pressure room in the ED, operating theater, ICU versus angiography suite). If no negative pressure room is immediately available, the treatment plans should continue forward. Any breach in the ventilator tubing should be avoided, which can be a source for aerosolization and exposure to health care workers.
If the decision is for conscious sedation, consideration for a dedicated COVID-19 glidescope or video laryngoscopy can be prepared in the angiography suite in case the patient deteriorates. Advance discussion on whether the anesthesiologist is required in the angiography suite during the case should be reviewed or as needed for intubation or hemodynamic support. If the patient requires intubation in the angiography suite, all nonessential persons should leave the room. Following intubation, any person entering the room should be in full PPE because of concern for residual aerosolization of virus post intubation.
Procedural Consent and Health Care Proxy
If the patient is unable to consent, a legally authorized representative should consent for the patient. If no contact can be reached, 2-physician emergency consent may be obtained or the proper documentation for the treatment risks and benefits and the failed attempts to contact family can be made in the medical record as per local institutional protocols.
If the patient is consentable, it is preferable to have a patient verbally authorize staff to sign the consent form for them. Inanimate objects such as pens and tablets can become a vehicle of spread for COVID-19. This may be considered appropriate in the setting of the COVID-19 pandemic; however, local standards should be adhered to. The staff assistant would sign the patient’s name and document themselves as witness in the presence of the patient. If the patient declines directed signature, they should be provided a new pen and sign the form.
At the same time, as the procedural consent, consent for general anesthesia should be obtained.
In a consentable patient, designation of a healthcare proxy consent should be conducted with a staff witness in the event the patient loses the ability to provide informed consent. This step may be important because rehabilitation or long-term care facilities may require this document to accept a candidate patient.
Preprocedure Room Preparation
The charge nurse and technologist should be alerted as soon as there is a suspected or patient with confirmed COVID-19 patient and room preparation dependent on the institution. If there are multiple angiography rooms available, a COVID-19 room can be designated. The procedure room should be cleared of any unnecessary items (ie, lead aprons that will not be utilized) to minimize the need of perioperative staff cleaning post-procedure. Countertop items should be covered with plastic or removed. The detectors on the angiography suite, foot pedal, and lead shields should be covered in plastic or an equivalent. The hanging lead shields and standing lead shields can be used as another layer of protection for the proceduralist.
The table, medications, and procedural preparation should be made in advance as much as possible to improve speed to reperfusion, limit the time the patient is in the suite, limit the need to break in and out of the room to retrieve materials or break scrub. The cabinets of the supply materials should be covered before the patient comes in the room.
There should be a designated space for the proceduralist phone and/or pager in the control room and communication maintained with the proceduralist via intercom or walkie-talkie if there is an urgent call.
If circumstances allow, it is optimal to have an observer ensure proper donning of gown and protective gear by each member in the procedure room per institutional protocol. This should include double gloving, wearing a face mask that covers the eyes, N95 mask in COVID-19 suspected patients, and wearing shoe covers.
There should be hand sanitizer stations near the doors entering or exiting the angio suite room as well as in patient recovery areas.
Thrombectomy Intraprocedure
In the procedure, staff should be kept to a minimum (ie, 1 nurse, 1 technologist, 1 physician) to limit provider exposure and limit use of protective gear. Door entry to the angiography suite should be taped with a sign to prevent people from entering inadvertently without protective gear. Most angiography suites are positive pressure rooms. Opening any doors to the angiography suite should be minimized once the patient is in the room to prevent movement of the virus to adjacent spaces.
In the control room, consider limiting the number of people to maintain a 6-foot distance between team members. These persons should wear a mask if the door between the angio suite and control room is opened because the angiography suite is likely to be a positive pressure room and can contaminate the adjacent rooms.
Negative or even fluid balance should be maintained given the risk for pulmonary edema in patients with COVID-19. Heparinized bag flushes should be monitored closely to ensure inadvertent excess fluid administration. Blood loss should be minimized given concurrent national shortages of blood.
After the procedure is completed, discuss with the admitting team and draw blood tests from the sheath that may be necessary for COVID-19 and stroke workup if not already done (ie, arterial blood gas, complete blood count, Chem 7, creatine kinase, and hypercoagulable panel in younger patients, B-type natriuretic peptide, troponin, hemoglobin A1c, cholesterol panel, etc) to limit the need for additional blood draws and exposures.
Cone-beam head CT (Xper or Dyna) should be considered while the patient is on the angiography suite table post-procedure to obviate the need for travel to CT, with the caveat of limited quality.
Ensure any trash is completely inside the trash bag.
If the room is big enough, place red tape on the floor of the angio suite 6 feet from the patient’s bed. This would be the area outside of which a provider would doff their gown. Again, an observer to watch team members doff off their gown and gear can be helpful to identify potential contamination or technique mistakes, if available.
Neurological, Vital Signs, and/or Access Site Checks Postreperfusion Therapy
Nonintubated, stable patients can be moved to a step-down unit with appropriate nursing expertise in the setting of a shortage or anticipated shortage of critical care beds.6 Repatriation or transfer of a patient post-thrombectomy from a comprehensive stroke center to a primary stroke center with appropriate physician and nursing expertise can be considered in the setting of hospitals overwhelmed by a shortage of ventilators or critical care beds while maintaining thrombectomy access. Communication between transferring and receiving teams, advance notification to patient families of repatriation is important to maintain optimal patient care.
Postprocedure or postthrombolytic neurological exam and/or access site checks should be combined and performed by one person and the frequency minimized to conserve PPE. When the patient is handed off to the receiving team, have the provider check the neurological exam, vitals, and/or access site before doffing their gown. This can qualify as the 15- or 30-minute check post-procedure or post thrombolytic, depending on the time that has elapsed.
Video can be utilized as a continuing monitor of the patient’s neurological exam and/or access site. Otherwise, consider another combined exam, vital sign, and/or access site check 15 or 30 minutes after hand-off, and then every hour×2. Thereafter, if the patient has remained stable, the intervals for the combined checks can be spread to q4h. The frequency of checks should be adjusted depending on the patient’s status (less if they are intubated and sedated), hemodynamic stability, perceived risk of hemorrhagic transformation, and concern for bleeding at the access site.
Post-Procedure
There should be a minimum 30 minutes delay before perioperative cleaning staff cleans the angiography suite to allow the room to air out.7
In-room providers should wash their hands, sanitize, and change out their scrubs or follow local protocol. Telephone communication with the patient’s family should then be pursued as with any reperfusion therapy or procedure, but even more so with the COVID-19 pandemic and restriction of family/visitors.
Intubated patients should be extubated in a negative pressure room.
A definitive diagnosis of COVID-19 should be made as soon as possible as patients who rule out will decrease the use of protective equipment.7 Any tests that do not change management should be delayed or deferred (to protect staff, virus trafficking, and conserve protective gear).7
Imaging of COVID-19 or suspected patients should be limited to imaging that will impact management.17
When rounding, the usual sequence is by acuity of patient illness or geographic convenience. In the era of the COVID-19 pandemic, assuming that all patients are equally stable, patients on contact or droplet precaution should be rounded on at the end of rounds to avoid unintentional viral spread to patients not on precautions as clinical circumstances allow.
If a provider develops any symptoms of cough, fever, or shortness of breath, they should seek testing and potential quarantine based on local protocols.
Psychosocial Intervention
When appropriate, an evaluation of a patient’s mental health is important to alleviate the psychosocial impact of the COVID-19 epidemic for a patient in isolation with a new or recurrent diagnosis of stroke.18
It is helpful to debrief with the team to learn, improve best practices and workflow. Healthcare workers, particularly nurses and frontline healthcare workers directly engaged in the care of patients with COVID-19 are vulnerable to the psychological burden of depression, anxiety, insomnia, and distress.19
Postacute Care
In preparation for the patient’s postacute care, testing for COVID-19 may be required for a patient being discharged to a postacute care facility, regardless of whether the patient was being treated for COVID-19 at the hospital.20 Patients who are asymptomatic or with minor signs of infection have been shown capable of shedding potentially infectious virus.21 Long- or short-term care facilities are vulnerable to respiratory disease outbreaks, including the spread of COVID-19.22 Early coordinated communication between the primary team, case management, and postacute care facilities is important to reduce bottlenecks in patient transitions once the patient is medically ready.
Conclusions
We live in uncharted times amidst the COVID-19 pandemic. The word crisis in Chinese is composed of 2 characters, one representing danger, the other opportunity. We cannot see this dangerous enemy, the coronavirus. Every opportunity and detail to recalibrate our acute neurological workflow to protect our frontline healthcare workers, our families, our colleagues, and our patients should be sought, implemented, and adapted to a resource-constrained environment. It is incumbent upon us to protect each other so that we are not unknowingly exposed or spread to our most vulnerable patients, while at the same time, providing optimal care, patient safety, and access for our patients with stroke. Optimizing protection of the healthcare worker should not compromise emergency stroke patient care. This guidance statement pertains to current practice and can change as new evidence arises.
Acknowledgments
We thank review and helpful comments on our manuscript by Dr Mitchell Elkind.
Disclosures
Dr Nguyen is Principal Investigator of the CLEAR study (CT for Late Endoascular Reperfusion) funded by Medtronic; serves on the Data Safety Monitoring Board for TESLA (Thrombectomy for Emergent Salvage of Large Anterior Circulation Ischemic Stroke), ENDOLOW (Endovascular Therapy for Low NIHSS Ishemic Strokes), SELECT 2 (A Randomized Controlled Trial to Optimize Patient’s Selection for Endovascular Treatment in Acute Ischemic Stroke) trials. Dr Jovin is advisor/investor for Anaconda, Route92, VizAi, FreeOx, and Blockade Medical; received personal fees, Data Safety Monitoring Board and steering committee fees from Cerenovus; Medtronic grants, and advisor/stockholder for Corindus. He serves as Principal Investigator for the DAWN (DWI or CTP Assessment With Clinical Mismatch in the Triage of Wake Up and Late Presenting Strokes Undergoing Neurointervention) and AURORA (Analysis of Pooled Data From Randomized Studies of Thrombectomy More Than 6 Hours After Last Known Well) trials (Stryker Neurovascular). Dr Nogueira disclosures are Stryker Neurovascular (DAWN Trial Principal Investigator—no compensation, TREVO [Trevo Registry Post Marketing Surveillance] Registry Steering Committee—no compensation; significant consultant); Cerenovus/ Neuravi (ENDOLOW Trial Principal Investigator—no compensation, EXCELLENT [Embotrap Extraction & Clot Evaluation & Lesion Evaluation for Neurothrombectomy] Registry Principal Investigator—no compensation, ARISE-2 trial [Analysis of Revascularization in Ischemic Stroke With EmboTrap] Steering Committee—no compensation, Physician Advisory Board, modest); Phenox (PROST Trial [Preset for Occlusive Stroke Treatment] Principal Investigator, Physician Advisory Board, modest); Anaconda (Physician Advisory Board, modest); Genentech (physician advisory board, modest); Biogen (CHARM Trial [BII093 (glibenclamide) for Severe Cerebral Edema Following Large Hemispheric Infarction] Steering Committee; physician advisory board, modest); Prolong Pharmaceuticals (physician advisory board, modest); Brainomix (physician advisory board, stock options); Viz-AI (physician advisory board, stock options); Corindus Vascular Robotics (physician advisory board, stock options); Vesalio (physician advisory board, stock options); Ceretrieve (physician advisory board, stock options); Astrocyte (physician advisory board, stock options); Cerebrotech (physician advisory board, stock options); Imperative Care (Imperative Trial Principal Investigator, modest). Diogo Haussen is consultant for Stryker, Vesalio and Cerenovus; has stock options with VizAi. A.E. Hassan is consultant and speaker for Medtronic, Stryker, Microvention, Penumbra, Balt, Viz Ai, Scientia, Genentec, and GE Healthcare; received personal fees with Cerenovus outside of submitted work. Dr Ortega-Gutierrez is consultant for Medtronic and Stryker Neurovascular. Dr Hsiang-Yi Chou receives research support from the National Institutes of Health 1 R21 NS113037-01. Dr Janardhan reports grants from National Science Foundation (Principal Investigator), other from Insera Therapeutics, Inc (Board Member); other from the Society of Vascular and Interventional Neurology outside submitted work; has notice of allowances or awarded >65 patents in the United States and worldwide and over 20 patents pending in the United States and worldwide related to the broader field but not part of this article. Dr Yavagal reports personal fees from Medtronic, personal fees from Cerenovus, other from Rapid Medical, personal fees from Vascular Dynamics, other from Poseydon, other from Neurosave, and other from Neuralanalytics outside the submitted work. Dr Liebeskind is consultant to Cerenovus, Genentech, Stryker, Medtronic as Imaging Core Lab.
References
- 1.Johns Hopkins. Coronavirus Resource Center. https://coronavirus.jhu.edu. Accessed April 24, 2020.
- 2.Li Y, Wang M, Zhou Y, Chang J, Xian Y, Mao L, et al. Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. [published online March 13, 2020]. Lancet. doi: 10.1136/svn-2020-000431. https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3550025. Accessed April 10, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bauchner H, Fontanarosa PB, Livingston EH. Conserving supply of personal protective equipment-A call for ideas. [published online March 20, 2020]. JAMA. doi: 10.1001/jama.2020.4770. https://jamanetwork.com/journals/jama/fullarticle/2763590. Accessed April 10, 2020. [DOI] [PubMed] [Google Scholar]
- 4.Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019;50:e344–e418. doi: 10.1161/STR.0000000000000211. doi: 10.1161/STR.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 5.Eskey CJ, Meyers PM, Nguyen TN, Ansari SA, Jayaraman M, McDougall CG, et al. American Heart Association Council on Cardiovascular Radiology and Intervention and Stroke Council. Indications for the performance of intracranial endovascular neurointerventional procedures: a Scientific Statement from the American Heart Association. Circulation. 2018;137:e661–e689. doi: 10.1161/CIR.0000000000000567. doi: 10.1161/CIR.0000000000000567. [DOI] [PubMed] [Google Scholar]
- 6.Lyden P. Temporary emergency guidance to US stroke centers during the COVID-19 pandemic on behalf of the AHA/ASA Stroke Council Leadership. [published online April 1, 2020]. Stroke. https://www.ahajournals.org/doi/10.1161/Strokeaha.120.030023. Accessed April 10, 2020. [Google Scholar]
- 7.Han Y, Zeng H, Jiang H, Yang Y, Yuan Z, Cheng X, et al. CSC Expert consensus on principles of clinical management of patients with severe emergent cardiovascular disease during the COVID-19 epidemic. Circulation. 2020;48:189–194. doi: 10.1161/CIRCULATIONAHA.120.047011. [DOI] [PubMed] [Google Scholar]
- 8.Khosravani H, Rajendram P, Notario L, Chapman MG, Menon BK. Protected code stroke. Hyperacute stroke management during the coronavirus disease 2019 (COVID-19) pandemic. [published online April 1, 2020]. Stroke. doi: 10.1161/STROKEAHA.120.029838. . Accessed April 10, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi H, Han X, Jiang N, Cao Y, Alwalid O, Gu J, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425–434. doi: 10.1016/S1473-3099(20)30086-4. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fang Y, Zhang H, Xie J, Lin M, Ying L, Pang P, et al. Sensitivity of Chest CT for COVID-19: comparison to RT-PCR. [published online February 19, 2020]. Radiology. doi: 10.1148/radiol.2020200432. https://pubs.rsna.org/doi/10.1148/radiol.2020200432. Accessed April 10, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. DAWN Trial Investigators. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378:11–21. doi: 10.1056/NEJMoa1706442. doi: 10.1056/NEJMoa1706442. [DOI] [PubMed] [Google Scholar]
- 12.Desai S, Tonetti DA, Molyneaux BJ, Atchaneeyasakul K, Rocha M, Jovin TG, et al. Interaction between time, ASPECTS, and clinical mismatch. [published online April 3, 2020]. J Neurointerv Surg. doi: 10.1136/neurintsurg-2020-015921. https://jnis.bmj.com/content/early/2020/04/03/neurintsurg-2020-015921. Accessed April 10, 2020. [DOI] [PubMed] [Google Scholar]
- 13.Mendez B, Requena M, Aires A, Martins N, Boned S, Rubiera M, et al. Direct transfer to angio-suite to reduce workflow times and increase favorable clinical outcome. Stroke. 2018;49:2723–2727. doi: 10.1161/STROKEAHA.118.021989. doi: 10.1161/STROKEAHA.118.021989. [DOI] [PubMed] [Google Scholar]
- 14.Abou-Chebl A, Lin R, Hussain MS, Jovin TG, Levy EI, Liebeskind DS, et al. Conscious sedation versus general anesthesia during endovascular therapy for acute anterior circulation stroke: preliminary results from a retrospective, multicenter study. Stroke. 2010;41:1175–1179. doi: 10.1161/STROKEAHA.109.574129. doi: 10.1161/STROKEAHA.109.574129. [DOI] [PubMed] [Google Scholar]
- 15.Emanuel EJ, Persad G, Upshur R, Thome B, Parker M, Glickman A, et al. Fair allocation of scarce medical resources in the time of Covid-19. [published online March 23, 2020]. N Engl J Med. doi: 10.1056/NEJMsb2005114. https://www.nejm.org/doi/full/10.1056/NEJMsb2005114. Accessed April 10, 2020. [DOI] [PubMed] [Google Scholar]
- 16.Canelli R, Connor CW, Gonzalez M, Nozari A, Ortega R. Barrier enclosure during endotracheal intubation. [published online April 3, 2020]. N Engl J Med. doi: 10.1056/NEJMc2007589. https://www.nejm.org/doi/pdf/10.1056/NEJMc2007589. Accessed April 10, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mossa-Basha M, Meltzer CC, Kim DC, Tuite MJ, Kolli KP, Tan BS. Radiology department preparedness for COVID-19; radiology scientific expert panel. [published online March 16, 2020]. Radiology. doi: 10.1148/radiol.2020200988. https://pubs.rsna.org/doi/10.1148/radiol.2020200988. Accessed April 10, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duan L, Zhu G. Psychological interventions for people affected by the COVID-19 epidemic. Lancet Psychiatry. 2020;7:300–302. doi: 10.1016/S2215-0366(20)30073-0. doi: 10.1016/S2215-0366(20)30073-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lai J, Ma S, Wang Y, Cai Z, Hu J, Wei N, et al. Factors associated with mental health outcomes among health care workers exposed to coronavirus disease 2019. JAMA Netw Open. 2020;3:e203976. doi: 10.1001/jamanetworkopen.2020.3976. doi: 10.1001/jamanetworkopen.2020.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grabowski DC, Joynt Maddox KE. Postacute care preparedness for COVID-19. Thinking ahead. [published online March 25, 2020]. JAMA. doi: 10.1001/jama.2020.4686. https://jamanetwork.com/journals/jama/fullarticle/2763818. Accessed April 10, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoehl S, Rabenau H, Berger A, Kortenbusch M, Cinatl J, Bojkova D, et al. Evidence of SARS-CoV-2 infection in returning travelers from wuhan, China. N Engl J Med. 2020;382:1278–1280. doi: 10.1056/NEJMc2001899. doi: 10.1056/NEJMc2001899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McMichael TM, Currie DW, Clark S, Pogosjans S, Kay M, Schwarz NG, et al. Epidemiology of Covid-19 in a long-term care facility in King County, Washington. [published online March 27, 2020]. N Engl J Med. doi: 10.1056/NEJMoa2005412. https://www.nejm.org/doi/full/10.1056/NEJMoa2005412. Accessed April 10, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]


