Abstract
As a result of the 2019 novel human coronavirus (COVID-19) global spread, medical examiner/coroner offices will inevitably encounter increased numbers of COVID-19–infected decedents at autopsy. While in some cases a history of fever and/or respiratory distress (eg, cough or shortness of breath) may suggest the diagnosis, epidemiologic studies indicate that the majority of individuals infected with COVID-19 develop mild to no symptoms. Those dying with—but not of—COVID-19 may still be infectious, however. While multiple guidelines have been issued regarding autopsy protocol in cases of suspected COVID-19 deaths, there is some variability in the recommendations. Additionally, limited recommendations to date have been issued regarding scene investigative protocol, and there is a paucity of publications characterizing COVID-19 postmortem gross and histologic findings. A case of sudden unexpected death due to COVID-19 is presented as a means of illustrating common autopsy findings, as well as diagnostic and biosafety considerations. We also review and summarize the current COVID-19 literature in an effort to provide practical evidence-based biosafety guidance for medical examiner-coroner offices encountering COVID-19 at autopsy.
Key Words: autopsy biosafety, coronavirus, COVID-19, SARS-CoV-2
SARS-CoV-2 is a novel human coronavirus that was detected as the causative agent of human respiratory infection (COVID-19) in Wuhan, China, in December 2019. It is related to SARS-CoV and MERS-CoV, other human coronaviruses responsible for outbreaks of severe acute respiratory syndrome first detected in China in 2002–2003 and in Saudi Arabia in 2012, respectively.1 Based on currently available global data, COVID-19 is thought to cause mild, limited, or no symptoms in at least 80% of those infected.2–4 A substantial portion of COVID-infected individuals are suspected to be asymptomatic carriers; estimates derived from select populations of quarantined individuals who underwent widespread testing (eg, cruise ship passengers and Japanese citizens evacuated from China) range from 18% to 33%.5,6 Of those known to be COVID-19 positive, approximately 20% to 30% will develop more severe disease necessitating hospitalization. Such disease may progress to acute respiratory distress syndrome (ARDS), sepsis, multiorgan failure, and death.7–9 Risk factors for severe disease have been reported to include advanced age, hypertension, and diabetes, although recent data suggest hospitalization may be more common among younger COVID-19 patients than previously suspected.7,8
Case fatality ratios range widely from 0.2% to 7.7%, depending on the population age, prevalence of comorbidities, testing criteria and availability, health care capacity, and how cases are defined.10–13 A variety of electronic databases provide ongoing daily monitoring of COVID-19 cases tabulated by country.14–16 Inevitably, there will be an increased number of suspected or confirmed COVID-19 deaths encountered at autopsy. While multiple guidelines have been issued regarding autopsy protocol in cases of suspected or confirmed COVID-19 deaths, there is some variability in the recommendations.17–21 Additionally, limited recommendations to date have been issued regarding scene investigative protocol. We present a case of sudden unexpected death due to COVID-19 as a means of illustrating suggestive autopsy findings, diagnostic considerations, and recommended scene and morgue biosafety practices in novel coronavirus deaths.
CASE REPORT
History
A 58-year-old woman reported a week of fevers and respiratory difficulty. Her medical history was significant for insulin-dependent type 2 diabetes mellitus (hemoglobin A1c; range, 7%–11%), obesity, hyperlipidemia, mild intermittent asthma, and chronic lower extremity swelling with ulceration. She was instructed to stay home and self-quarantine by her retail store employer. She was found dead in her bedroom after last being known alive the night before. Emergency medical services responded to the scene and attempted resuscitation before pronouncing death and notifying the medical examiner's office.
Scene Investigation
Two medicolegal death investigators performed a scene investigation. Family members were interviewed outside the residence, eliciting the information that several of the decedent's coworkers and an adult member of her household had also developed respiratory illness symptoms. At least 6 ft (ie, 2 m) of separation was maintained between investigators and family members during interview. Prior to entering the residence, investigators donned personal protective equipment (PPE) sufficient for contact and droplet precautions including gloves, fluid-resistant gown, a barrier face mask, and goggles.17,18,20 A camera was used to document the scene photographically; otherwise, minimal equipment was brought into the residence. Following documentation, the body was examined and placed in a plastic shroud and then sealed in a body bag and loaded into the transport vehicle. After the body was secured, investigators doffed their PPE and performed hand hygiene with an alcohol-based gel. The body was then transported to the morgue and logged in without opening the outer body bag. The transport vehicle was disinfected with a dilute bleach solution.
Autopsy Protocol
An autopsy was performed in a separate negative-pressure isolation suite adjacent to the main morgue room but on its own heating, ventilation, and air-conditioning circuit with 16 air changes per hour. Only 2 operators (ie, a pathologist and a technician) were permitted in the isolation suite during the case. A second technician acted as a circulator and monitored the case via an observation window; communication was facilitated via inexpensive low-power walkie-talkies. A small isolation cooler attached to the isolation room acted as an airlock to facilitate transfer of materials into and out of the isolation suite during the prosection (Fig. 1).
FIGURE 1.

A and B, Isolation cooler “airlock” entrance from body receiving area (A) and access to the isolation suite (B); note that full PPE required for entry into the airlock.
In accordance with guidelines, PPE utilized by autopsy prosectors included scrubs, dedicated autopsy footwear with shoe covers, a long-sleeve water resistant gown or Tyvek suit, a long-sleeved splash apron, double gloves and cut gloves, hair covers, and a powered air-purifying respirator (PAPR) with full-face shield (Fig. 2).17–21 Grossly soiled PPE was frequently wiped down with disinfectant wipes or dilute bleach solution during the procedure. Doffing of PPE at the end of the procedure occurred in the isolation cooler airlock and was monitored by an inspector.
FIGURE 2.

Autopsy PPE on left with a full face-shield PAPR and liquid impervious gown; scene investigation PPE on right with disposable facemask respirator (shown with N95, but current requirements are for surgical mask only), fluid resistant gown, and eye protection.
The autopsy was performed with minor modifications to standard procedures. In order to minimize potential organism aerosolization, the sink-mounted vacuum aspirator, sink drain tissue grinder, and station hose were not used. All fluid collections were ladled from body cavities. Although the head was opened with a powered oscillating saw, the upper half of the body, head of the table, and floor surrounding these areas were misted with disinfectant immediately upon cessation of powered sawing and before brain removal. Only one prosector remained in the room during the sawing; for at least 10 minutes afterward (ie, 2 full air changes), no additional personnel entered the room. Cleaning of the body and autopsy table was accomplished with towels that were subsequently discarded. Limited body and specimen photography was accomplished either in situ or on a small photography board near the body.
Postautopsy Procedures
Following autopsy, the body was closed in the standard fashion and cleaned with damp towels and disinfectant. A clean body table with a clean body bag and plastic shroud spread out on it had been placed in the isolation cooler airlock by the second technician. This was moved into the isolation suite, and the body was transferred onto the clean shroud, wrapped tightly, sealed with tape, and then zipped into the clean body bag. This procedure allowed the outer surfaces of the shroud and body bag to remain essentially clean. Once sealed, the outer bag was labeled and signed by the pathologist and technician, each verifying the identity of the decedent. The outer surface of the body bag was then sprayed with disinfectant, and the body table was removed to the isolation cooler where residual liquid disinfectant was removed, and the bag labeled as infectious.
Release procedures were modified to prevent opening the body bag or shroud for visual identification confirmation by funeral home staff while the body was still in the facility. The dual signatures of the pathologist and technician acted as the confirmation that the body bag contained the correct decedent, and release to the funeral home was performed without opening the outer bag. The infectious nature of the case was disclosed to the funeral home personnel at the time of release. Notably, none of the personnel involved in this index case (ie, pathologist, technicians, scene investigators) suffered any symptoms consistent with COVID infection in the 2 weeks following their involvement in the case nor since.
Gross Findings
External examination revealed an obese (body mass index 38 kg/m2) middle-aged adult woman with fully developed rigor mortis and blanching livor mortis. Changes of lower extremity venous stasis and scarring consistent with the history of lower leg ulcers were present. On internal examination, there were no pleural effusions or other abnormal fluid collections. Thick mucus was noted in the airways, however. The lungs were moderately heavy and edematous (right: 818 g, left: 705 g) and had a relatively firm texture throughout. Areas of hemorrhage were evident in the right upper and middle lobes and to a lesser extent in the left lower lobe (Fig. 3.) Hilar and mediastinal lymph nodes appeared enlarged.
FIGURE 3.
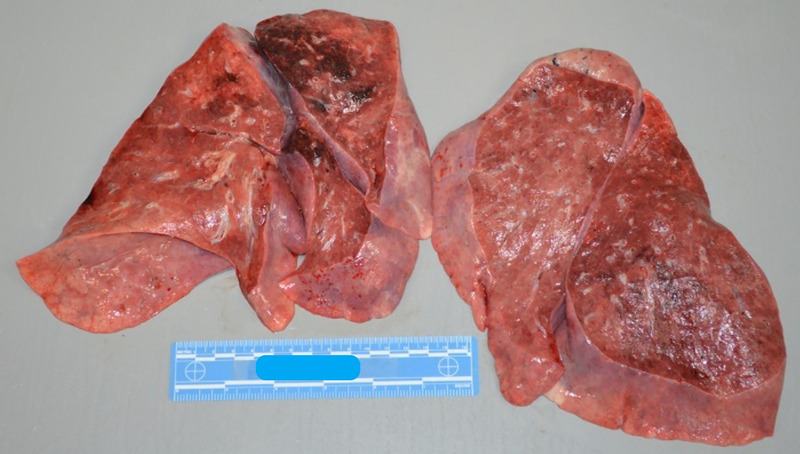
Unfixed moderately heavy, edematous, and relatively firm lungs with areas of hemorrhage in the right upper/middle lobes and left lower lobe.
The heart weighed 438 g and exhibited moderate coronary atherosclerosis in each of the main coronary distributions, but there were no occlusions or critical stenoses. The myocardium was free of obvious infarcts and had the expected firm texture and red-brown color. The left ventricle was 1.2 to 1.4 cm thick. The cardiac valves were normal. There was moderate infrarenal aortic atherosclerosis. The kidneys were finely granular and had focal cortical scars (right 170 g, left 183 g). The spleen had a normal-appearing capsule and parenchyma (148 g). The liver (1990 g) was grossly unremarkable. The brain (1221 g) exhibited hydrocephalus ex vacuo; the frontal horns measured 2.8 cm in greatest dimension at the level of the temporal poles. Tissue sections were placed in 10% neutral-buffered formalin and allowed to fix for 72 hours prior to histologic sampling. Hematoxylin-eosin staining was performed by an outside laboratory.
Microscopic Findings
Histologic examination of the lungs revealed diffuse proteinaceous edema and dense amphophilic concretions along alveolar septae consistent with hyaline membranes. The lung architecture was preserved, and the septae were of normal thickness, but mild mononuclear infiltrates were present throughout. There was prominent desquamating pneumocyte hyperplasia with focal multinucleated cells and bizarre forms. Acute alveolar hemorrhage and collections of reactive foamy alveolar macrophages were focally present, as were collections of alveolar fibrin (Fig. 4.) No viral inclusions or specific cytopathic changes were identified. Suppurative inflammation was absent. No fibroblastic foci, fibrosis, granulomas, or foreign bodies were appreciated.
FIGURE 4.
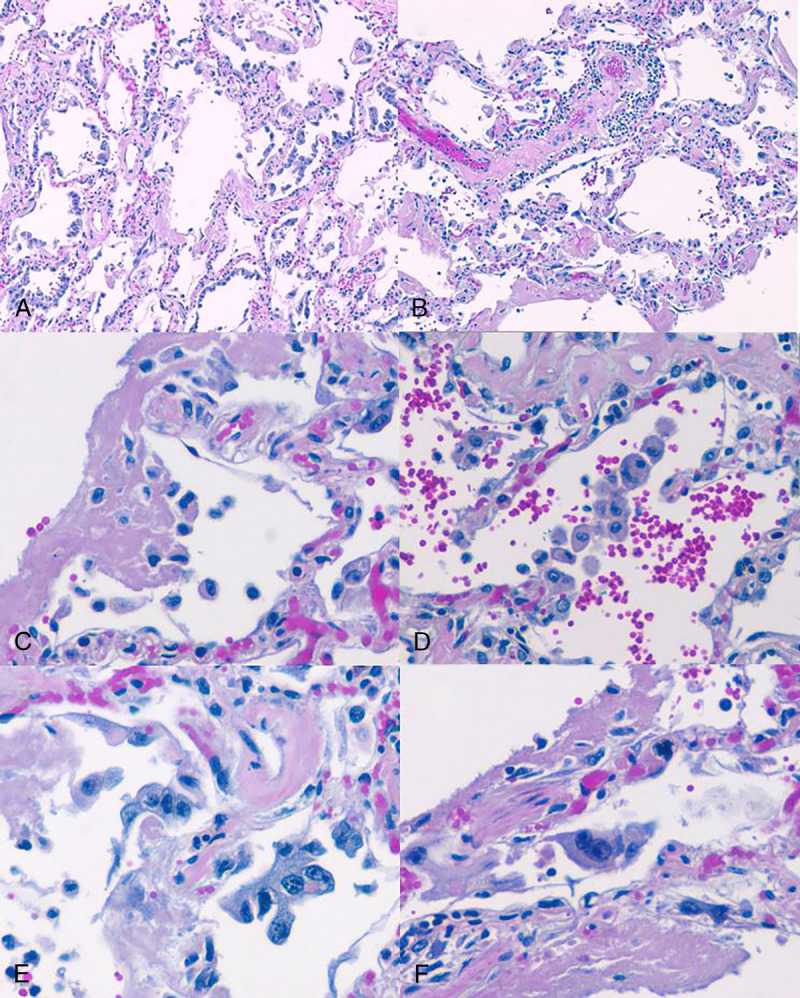
A–F, Hematoxylin-eosin–stained sections at original magnification of 100× (A, B) and 400× (C–F). Lung parenchyma exhibits inflamed septae, hyaline membranes, and pneumocyte hyperplasia (A); septal and perivascular mononuclear inflammation (B); hyaline membranes and alveolar fibrin (C); intra-alveolar macrophages and reactive pneumocytes (D); marked pneumocyte hyperplasia (E); with sporadic multinucleated cells (F).
Sections of myocardium demonstrated myocyte hypertrophy with interstitial and perivascular fibrous tissue but no acute ischemic changes or inflammatory infiltrates. The liver exhibited mild steatosis and central lobular pallor and congestion, but no significant portal or lobular inflammation. Renal changes included arteriolosclerosis, mesangial sclerosis and hypercellularity, and focal global glomerulosclerosis. A section of medulla had no inflammatory or ischemic changes. An incidental adrenal cortical nodule and a focus of papillary adenocarcinoma of the thyroid were also detected.
Ancillary Testing
Dacron-tipped swabs of the right and left main bronchi were procured, placed into viral transport medium, and submitted to the Washington State Public Health Laboratory for both SARS-CoV-2 and influenza testing. Bacterial swabs of areas of potential consolidation were placed in Amies transport media tubes and submitted to the University of Washington microbiology laboratory for culture. The reverse transcriptase–polymerase chain reaction (RT-PCR) results for SARS-CoV-2 returned as positive 11.5 hours after submission. Negative influenza results returned several days later. Bacterial cultures were positive for methicillin-sensitive Staphylococcus aureus and Streptococcus viridans. Given the lack of acute histologic inflammation in the lungs, these bacterial culture results were interpreted as being most likely contaminants or postmortem artifact.
Death Certification
Based on the above autopsy and investigative findings, and in accordance with US National Vital Statistics certification guidelines, the cause of death was determined to be ARDS due to viral pneumonia due to COVID-19.22 Other significant contributory factors included type 2 diabetes mellitus, hypertension, and obesity. The state department of health was notified.
DISCUSSION
Currently every state in the United States has reported COVID-19 cases, resulting in a total of more than 46,000 deaths to date.23 Inevitably, medical examiner/coroner (ME/C) offices will encounter increased numbers of SARS-CoV-2–infected decedents at autopsy as a result of COVID-19 spread. While in some cases a history of fever and/or respiratory distress (eg, cough or shortness of breath) may suggest the diagnosis, epidemiologic studies indicate that the majority of individuals infected with COVID-19 develop mild to no symptoms.2 Even those dying with—but not of—COVID-19 may still be infectious. Transmission of SARS-CoV-2 from presymptomatic/asymptomatic individuals has been documented, although the frequency remains to be established.24–27 Medical examiner/coroners must use their judgment to determine whether postmortem COVID-19 testing and/or autopsy should be pursued. In addition to suggestive antemortem signs/symptoms, epidemiologic factors may also help guide decisions such as history of contact with a known COVID-19–positive case, or being a part of a cluster of respiratory illness cases in a closed setting (eg, a nursing care facility).17 The presented autopsy case and literature review are intended to help familiarize ME/C offices with COVID-19 disease features, diagnostic strategies, and key biosafety principles.
Transmission
It is suspected that SARS-CoV-2—similar to SARS-CoV and MERS-CoV—began as a zoonotic coronavirus that subsequently spread to humans.1 Community- and health care–associated person-to-person transmission were documented early in the pandemic, and direct or close contact with infectious persons is believed to be the major mode of transmission.28,29 Transmission occurs through exposure to infectious droplets originating from the respiratory tract; infectious droplets may be released from an infected individual via sneezing, coughing, talking, or undergoing an aerosolizing procedure such as intubation or autopsy.30,31 Reportedly droplets neither typically spread beyond 6 ft (2 m) nor linger in air, although some evidence has suggested a longer range of spread may be possible.31,32 Less commonly infection may arise as a result of indirect transmission through fomites, especially if the eyes, face, or mouth are contacted after touching an infected surface.31,33 SARS-CoV-2 has also been detected in blood and anal swabs; increasing evidence suggests that fecal-oral transmission may be another potential route of spread.34–36
Scene Investigation
As in the current presented case, investigators are advised to mitigate risk of potential SARS-CoV-2 exposure at death scenes by standing at a distance of 6 ft or greater when conducting interviews and requesting interviewees to remain outside the residence while investigators enter. As the Centers for Disease Control and Prevention (CDC) has recently issued recommendations for the public to wear cloth facial coverings when at risk of social-based transmission, scene investigators may consider encouraging interviewees to don cloth facial masks.37 Scene investigators should don contact and droplet precaution PPE when entering residences. Decontamination of all potentially contaminated equipment, careful body bagging procedures, and investigator hand hygiene are also encouraged. Anecdotally, this has been the scene investigative policy of the Snohomish County Medical Examiner's Office, which despite being the country with the second highest number of positive/confirmed COVID cases in the state of Washington has had no scene investigators who tested positive for SARS-CoV-2 to date. In order to more efficiently triage cases, ME/C offices may elect to have scene investigators procure nasopharyngeal (NP) viral testing swabs at the scene. Scene investigative recommendations are summarized in Table 1.
TABLE 1.
Scene Investigative Recommendations in Suspected/Confirmed COVID-19 Cases

Morgue and PPE
Each office is advised to carefully assess its own infrastructure, supplies, and staffing to determine whether suspected or confirmed COVID-19 deaths can be safely prosected on site. Current CDC and World Health Organization (WHO) recommendations are that suspected/confirmed COVID-19 autopsies be prosected in an airborne infection isolation rooms at negative pressure relative to surrounding areas and ideally with at least 12 air changes per hour (although 6 will suffice in older structures.)17,20 Air from isolation rooms should be exhausted directly outside or else pass through a high-efficiency particulate air (HEPA) filter if recirculated.17,18 Recommended PPE includes standard autopsy protection (ie, double surgical gloves interposed with a layer of cut-proof synthetic mesh gloves, scrub suit worn under an impermeable long-sleeve gown/apron, goggles or face shield, shoe covers, and surgical cap).17–20 As autopsy prosection may generate aerosols, respiratory protection consisting of a disposable N95 respirator or PAPR with HEPA filter is also indicated.17–21
Given national PPE supply limitations, ME/C offices are encouraged to restrict autopsy attendance to key personnel only (ie, prosector and technician).38 In order to preserve eyewear, preferential use of powered air purifying respirators or goggles, which can be reused following appropriate disinfection, should be considered.39 In general, PAPRs are preferable to N95 respirator masks as they provide even higher protection to prosectors and—with appropriate disinfection of reusable elements after use—can be repeatedly used, thus assisting with PPE conservation efforts.40 Powered air-purifying respirator filters should be replaced in accordance with manufacturer recommendations. Some studies have suggested the possibility of decontaminating and reusing N95 masks via UV germicidal irradiation, hydrogen peroxide vapor sterilization, and simply allowing respirators to hang to dry for 3 days.38,41–45 Proposals for respirator mask construction have also been offered.38 Further details regarding PPE conservation strategies are beyond the bounds of the current review but are available through the CDC.39
Autopsy Protocol
Aerosol generation at autopsy may occur through inadvertent splashing of fluids, puncturing of fluid pockets under pressure, and using vacuum-assisted suction devices and sink-mounted tissue grinders.38–42 However, the most likely aerosol-generating autopsy procedure is use of powered oscillating bone saws, particularly during brain removal.46–50 High densities of aerosolized particles are generated in the region of the saw placing the user at risk. While N95 respirator masks physically filter 95% of particles at least 1 μm in size from the inspired air, CDC and OSHA guidelines nonetheless suggest avoiding use of an oscillating bone saw at autopsy in cases of suspected/confirmed COVID-19. If a saw is used, it is recommended that a vacuum shroud be attached to assist in capturing aerosols.17,18 Other oscillating saw aerosol reduction techniques to consider include moistening the saw blade before cutting, using autopsy tables with built-in ventilation, tenting plastic around the decedent's neck and head to entrap aerosols, and wearing a HEPA-filtered PAPR rather than N95 mask.46–49 As in the current presented case, other general splash and aerosol reduction techniques (eg, eschewing use of vacuum assisted aspirator, sink tissue grinder, or hose) are also advised. Autopsy protocol recommendations are summarized in Table 2.
TABLE 2.
Autopsy Recommendations in Suspected/Confirmed COVID-19 Cases

Gross and Histologic Features
While much literature has been published regarding COVID-19 signs/symptoms and clinical course, there is currently a paucity of data regarding expected postmortem gross and histologic findings. The case presented herein adds to those few previously published studies. Lungs are often heavy and histologically reveal edema with focal/diffuse hyaline membrane formation, pneumocyte hyperplasia, patchy mononuclear inflammatory infiltrates, some multinucleated cells, and a lack of definitive intranuclear or intracytoplasmic viral inclusions.51,52 Overall, pulmonary pathologic features generally appear consistent with early/organizing diffuse alveolar damage and resemble those seen in SARS autopsies51–53 While myocarditis has been reported in association with COVID-19, it appears to be a less common complication.54,55 Most typically (as in the presented case), minimal mononuclear myocardial inflammatory involvement is found. Rarely, COVID-19–associated encephalitis has been reported.56
Decontamination
In an experimental study, viable SARS-CoV-2 was detected up to 72 hours after being placed onto plastic and stainless-steel surfaces; it also remained viable in an experimentally created aerosol for at least 3 hours, although infectious titers per liter of air decreased over time.57 A recent review and analysis of literature found that generally human coronaviruses can remain infectious for up to 9 days on inanimate surfaces, depending on surface type and temperature; at temperatures ≥30°C (ie, 86°F), viral persistence shortened.58 Given that SARS-CoV-2 may remain viable on a variety of inanimate surfaces for a matter of days, careful morgue decontamination procedures are key. Surface disinfection with diluted household bleach solutions (ie, of 0.1% sodium hypochlorite), 0.5% hydrogen peroxide solutions, or alcohol solutions (ie, with at least 70% ethanol) has been shown to inactivate human coronaviruses within 1 minute.58,59 For hospital-grade disinfectants, staff should verify their suitability for use against SARS-CoV-2 and ensure disinfectants remain on surfaces in accordance with US Environmental Protection Agency recommendations.60,61 While conclusive data regarding how long infectious human remains may harbor infectious SARS-CoV-2 are lacking, autopsies in cases of SARS found that SARS-CoV could be recovered from lung tissue and grown in cell culture up to 7 days after death.53,62 Considering that there is reportedly 75% to 80% similarity between the viral genomes of SARS-CoV-2 (causative agent of COVID-19) and SARS-CoV (causative agent of SARS), these results suggest that protective precautions are called for even in cases of delayed suspected COVID-19 death investigations or autopsies.1,63 While limited data exist regarding formalin inactivation of SARS-CoV-2–infected tissue, formalin fixation at room temperature has been shown to inactivate most of SARS-CoV (ie, close to assay detection limits) within a day.63,64
Diagnosis
In cases of suspected COVID-19 in which full autopsies are performed, submission of SARS-CoV-2 swabs from both upper and bilateral lower respiratory tracts is recommended by the CDC.17 If NP and bilateral tracheobronchial/lung swabs are procured, it is recommended they be submitted in separate vials each containing 2 to 3 mL of viral transport medium.17 Medical examiner/coroner offices, however, must be cognizant of their current state health department testing capacity and proceed accordingly.17,19 A rational strategy for minimizing the number of swab submissions per autopsy is to submit swabs only from the lower respiratory tract. Additionally, sampling both lungs with a single swab may increase recovery of viral RNA. Concurrent testing for other causes of respiratory illness such as influenza is also strongly encouraged.17,19 In order to preserve limited viral testing kit supply, ME/C offices are encouraged to communicate with their testing laboratories to verify whether COVID-19, as well as other respiratory viral pathogen testing, can be performed on a single swab. Prosectors should submit separate samples for bacterial/fungal organisms as indicated. In cases of suspected COVID-19 in which autopsy is not performed, NP swab testing for COVID-19, as well as other respiratory pathogens, is recommended.17
Only synthetic fiber swabs with plastic shafts should be submitted for SARS-CoV-2 RT-PCR testing, as others may inactivate virus or otherwise inhibit the testing. In the event that swabs were not procured at autopsy, fixed autopsy tissue specimens may also be submitted to CDC in suspected COVID-19 deaths for immunohistochemical, molecular, or other assay characterization.17 Recent diagnostic advancements include the Food and Drug Administration emergency use authorization enabling US laboratories already certified for high-complexity testing to develop and validate their own SARS-CoV-2 testing; this markedly augmented diagnostic capability as previously all testing was performed mainly by US public health laboratories.65 Additionally, there has been emergency use authorization approval of rapid COVID-19 PCR diagnostic tests that some manufacturers report can deliver results in less than an hour.66 Recently, a serologic enzyme-linked immunosorbent assay testing to assess for COVID-19 antibodies has received Food and Drug Administration approval, and more are in development; these are expected to prove of utility in more accurately calculating disease transmission and mortality rates.66–68
Case Triage
Previous surveys have indicated that morgues are heterogeneous in their preparedness to handle highly infectious disease cases.69,70 Facility environmental controls, availability of PPE, and staffing are all factors that ME/C must carefully assess in determining whether suspected/confirmed COVID-19 deaths will be autopsied.17,19 The majority of known COVID-19 deaths technically should not fall under ME/C office jurisdiction as they are natural deaths predominantly occurring in hospitalized patients.71 While autopsy is unlikely to be necessary in such cases—and is, in fact, discouraged by OSHA—ME/C may nonetheless be called upon to facilitate death certification ensuring accurate public health disease tracking, or in cases of medicolegal significance (homicides, accidents or suicides) where a decedent dies in the hospital and is COVID-19 positive due to community spread.18,71
In deaths occurring outside the hospital setting, ME/C offices are tasked with determining whether deaths are attributable to COVID-19 and performing autopsies as needed.71 Medical examiner/coroner offices may elect to triage cases by taking viral NP swabs in suspected COVID-19 deaths and reserve autopsy for those decedents with negative testing results or else cases in which SARS-CoV-2 infection is thought to be incidental rather than the cause of death. Performing an NP swab for SARS-CoV-2 requires neither a negative-pressure room nor N95 respirator; basic contact and droplet precaution PPE are sufficient.17 In cases in which autopsy is merited, the above described precautions should be followed to prevent morgue staff–acquired COVID-19 infection. Some offices have elected to perform limited autopsies (eg, precluding brain/spinal cord removal) in cases of suspected/known COVID-19 positivity to reduce morgue staff aerosol exposure. Others have incorporated antemortem or postmortem CT radiology as a means of helping triage decedents meriting testing and/or autopsy.19,21 Establishing collaborative relations with larger ME/C offices capable of safely autopsying suspected highly infectious disease cases should be encouraged. Globally, mobile biosafety autopsy facilities/laboratories with sophisticated infrastructure have been deployed during disease epidemics such as SARS and Ebola virus; advantages include the ability to mobilize rapidly to areas of need with minimal footprint.72–74 Such mobile facilities may be advisable in the United States as well.
Body Disposition
Neither CDC nor WHO guidelines require double-bagging of known COVID-19–positive decedents; however, ME/C offices should ensure that any body fluids leaking from orifices are contained.17,20 Utilizing cotton packing of orifices, a plastic shroud, or a second body bag is a potential method of preventing fluid leakage. Following appropriate decontamination of the body bag, a method of clearly designating the infectious nature of contents to funeral home personnel should be used (eg, body bag notification sticker); ME/C offices may want to consider modifying body release procedures to preclude contamination risks of opening body bag in morgue for funeral personnel identification.75 There is currently no CDC nor WHO requirement for cremation of COVID-19–positive decedents.17,20 While decedents can be buried or cremated as per current state requirements, burial may result in storage difficulties as social distancing–related funeral service delays. Further details of recommended funeral home/mortuary protocol in cases of COVID-19–positive decedents can be accessed on both the CDC and the National Funeral Directors Association websites.76,77 Medical examiner/coroner offices are encouraged to closely collaborate with funeral homes/mortuaries to ensure body transportation and storage needs are met. In jurisdictions with a required waiting period prior to cremation (eg, 48 hours), investigating the possibility of a waiver in order to alleviate decedent storage issues may be advisable.
CONCLUSIONS
Although much remains to be learned about SARS-CoV-2, recommendations for handling decedents with known or suspected COVID-19 are available from multiple national and international organizations.17–21 Additionally, much can be inferred from experiences with prior coronavirus outbreaks such as SARS-CoV and MERS-CoV. In order to ensure safety of scene investigators, ME/Cs, and morgue staff, developing an understanding of SARS-CoV-2 transmission, presentation, and prevention strategies is key. The current case presentation with literature review is intended to better inform and provide practical evidence-based biosafety guidance for ME/C offices encountering COVID-19 at autopsy. As community spread of COVID-19 increases, ME/C offices are encouraged to employ these biosafety principles broadly, as COVID carriers may be presymptomatic or asymptomatic, and yet still present infectious risk at autopsy. Medical examiner/coroner offices are cautioned that suspicious scene investigative history (eg, nursing home resident or individual from homeless encampment) cannot necessarily be relied upon to identify COVID carriers. It is hoped that by documenting and sharing postmortem findings ME/C offices can provide new insights into the pathogenesis of SARS-CoV-2 and potentially assist in formulating therapeutic strategies to reduce disease mortality.
Footnotes
The authors report no conflict of interest.
REFERENCES
- 1.Lu R Zhao X Li J, et al. Genomic characterization and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention [published online February 24, 2020]. JAMA. 2020. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization (WHO) Report of the WHO-China joint mission on Coronavirus Disease 2019 (COVID-19). February 28, 2020. Available at: https://www.who.int/publications-detail/report-of-the-who-china-joint-mission-on-coronavirus-disease-2019-(covid-19). Accessed April 4, 2020.
- 4.Li R Pei S Chen B, et al. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV2) [published online March 16, 2020]. Science. 2020. pii: eabb3221. doi: 10.1126/science.abb3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nishiura H Kobayashi T Suzuki A, et al. Estimation of the asymptomatic ratio of novel coronavirus infections (COVID-19) [published online March 13, 2020]. Int J Infect Dis. 2020. pii: S1201–9712(20)30139–9. doi: 10.1016/j.ijid.2020.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mizumoto K Kagaya K Zarebski A, et al. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the diamond princess cruise ship, Yokohama, Japan, 2020. Euro Surveill. 2020;25(10):2000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou F Yu T Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study [published online March 11, 2020]. Lancet. 2020. pii: S0140–6736(20)30566–3. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention (CDC) Severe outcomes among patients with Coronavirus Disease 2019 (COVID-19)—United States, February 12–March 16, 2020 [punlished online March 18, 2020]. Morb Mortal Wkly Rep. 10.15585/mmwr.mm6912e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.European Centre for Disease Control and Prevention (ECDC) Coronavirus disease 2019 (COVID-19) pandemic: increased transmission in the EU/EEA and the UK—seventh update. 2020. Available at: https://www.ecdc.europa.eu/en/publications-data/rapid-risk-assessment-coronavirus-disease-2019-covid-19-pandemic. Accessed April 4, 2020.
- 10.Lazzerini M, Putoto G. COVID-19 in Italy: momentous decisions and many uncertainties [published online March 18, 2020]. Lancet Glob Health. 2020. pii: S2214-109X(20)30110–8. doi: 10.1016/S2214-109X(20)30110-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verity R Okell LC Dorigatti I, et al. Estimates of the severity of coronavirus disease 2019: a model-based analysis [published online March 30, 2020]. Lancet Infect Dis. 2020; pii: S1473–3099(20)30243–7. doi: 10.1016/S1473-3099(20)30243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization (WHO) WHO director-general's opening remarks at the media briefing on COVID-19. 2020. Available at: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---3-march-2020. Accessed April 4, 2020.
- 13.Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy [published online March 23, 2020]. JAMA. 2020; doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 14.European Centre for Disease Prevention and Control (ECDC) COVID-19 situation update—worldwide. Available at: https://www.ecdc.europa.eu/en/geographical-distribution-2019-ncov-cases. Accessed April 4, 2020.
- 15.World Health Organization (WHO) Coronavirus disease (COVID-2019) situation reports. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports. Accessed April 4, 2020.
- 16.Johns Hopkins University (JHU) Coronavirus COVID-19 global cases by the Center for Systems Science and Engineering (CSSE) at JHU. Available at: https://coronavirus.jhu.edu/map.html. Accessed April 4, 2020.
- 17.Centers for Disease Control and Prevention (CDC) Collection and submission of postmortem specimens from deceased persons with known or suspected COVID-19, March 2020 (interim guidance). 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-postmortem-specimens.html. Accessed March 26, 2020.
- 18.Occupational Safety and Health Administration (OSHA) COVID-19 control and prevention. Available at: https://www.osha.gov/SLTC/covid-19/controlprevention.html. Accessed March 26, 2020.
- 19.Hanley B Lucas SB Youd E, et al. Autopsy in suspected COVID-19 cases [published online March 20, 2020]. J Clin Pathol. 2020; pii: jclinpath-2020-206522. doi: 10.1136/jclinpath-2020-206522. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization (WHO) Infection prevention and control for the safe management of a dead body in the context of COVID-19. 2020. Available at: https://apps.who.int/iris/bitstream/handle/10665/331538/WHO-COVID-19-lPC_DBMgmt-2020.1-eng.pdf. Accessed March 26, 2020.
- 21.Fineschi V Aprile A Aquila I, et al. Management of the corpse with suspect, probable or confirmed COVID-19 respiratory infection—Italian interim recommendations for personnel potentially exposed to material from corpses, including body fluids, in morgue structures and during autopsy practice. Pathologica Epub 2020; 10.32074/1591-951X-13-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Vital Statistics System (NVSS) Guidance for certifying deaths due to coronavirus disease 2019 (COVID-19). 2020. Available at: https://www.cdc.gov/nchs/nvss/covid-19.htm. Accessed April 4, 2020.
- 23.Centers for Disease Control and Prevention Coronavirus disease 2019 COVID-19: cases in U.S. 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed March 28, 2020.
- 24.Rothe C Schunk M Sothmann P, et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382(10):970–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu Z Song C Xu C, et al. Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing [published online March 4, 2020], China. Sci China Life Sci. 2020. doi: 10.1007/s11427-020-1661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei WE Li Z Chiew CJ, et al. Presymptomatic transmission of SARS-CoV-2—Singapore, January 23–March 16, 2020 [published online April 1, 2020]. Morb Mortal Wkly. doi: http://dx.doi.org/10.15585/mmwr.mm6914e1external icon. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoehl S Rabenau H Berger A, et al. Evidence of SARS-CoV-2 infection in returning travelers from Wuhan, China. N Engl J Med. 2020;382(13):1278–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan JF-W Yuan S Kok K-H To KK-W, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghinai I McPherson TD Hunter JC, et al. First known person-to-person transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the USA. Lancet. 2020;395:1137–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zou L Ruan F Huang M, et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382(12):1177–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention (CDC) How coronavirus spreads. 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/prepare/transmission.html. Accessed March 26, 2020.
- 32.Bourouiba L. Turbulent gas clouds and respiratory pathogen emissions: potential implications for reducing transmission of COVID-19 [published online March 26, 2020]. JAMA. 2020. doi: 10.1001/jama.2020.4756. [DOI] [PubMed] [Google Scholar]
- 33.Cai J Sun W Huang J, et al. Early release—indirect virus transmission in cluster of COVID-19 cases, Wenzhou, China, 2020—volume 26, number Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens [published online March 11, 2020]. JAMA. doi: 10.1001/jama.2020.3786. [Google Scholar]
- 34.Zhang W Du RH Li B, et al. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect. 2020;9(1):386–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ong SWX Tan YK Chia PY, et al. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA. 2020; doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gu J, Han B, Wang J. COVID-19: Gastrointestinal manifestations and potential fecal-oral transmission [published online March 3, 2020]. Gastroenterology. 2020; pii: S0016–5085(20)30281-X. doi: 10.1053/j.gastro.2020.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention (CDC) Recommendation regarding the use of cloth face coverings, especially in areas of significant community-based transmission. 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/cloth-face-cover.html. Accessed April 5, 2020.
- 38.Livingston E, Desai A, Berkwits M. Sourcing personal protective equipment during the COVID-19 pandemic [published online March 28, 2020]. JAMA. 2020; doi: 10.1001/jama.2020.5317. [DOI] [PubMed] [Google Scholar]
- 39.Centers for Disease Control and Prevention (CDC) Coronavirus disease 2019 (COVID-19): strategies to optimize the supply of PPE and equipment. 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/index.html. Accessed March 31, 2020.
- 40.Centers for Disease Control and Prevention (CDC) The National Personal Protective Technology Laboratory (NPPTL): respirator fact sheet. 2012. Available at: https://www.cdc.gov/niosh/npptl/topics/respirators/factsheets/respsars.html. Accessed March 31, 2020.
- 41.Lindsley WG Martin SB Jr. Thewlis RE, et al. Effects of ultraviolet germicidal irradiation (UVGI) on N95 respirator filtration performance and structural integrity. J Occup Environ Hyg. 2015;12(8):509–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mills D Harnish DA Lawrence C, et al. Ultraviolet germicidal irradiation of influenza-contaminated N95 filtering facepiece respirators. Am J Infect Control. 2018;46(7):e49–e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lowe JL Paladino KD Farke JD, et al. N95 filtering facepiece respirator ultraviolet germicidal irradiation (UVGI) process for decontamination and reuse. Available at: https://www.nebraskamed.com/sites/default/files/documents/covid-19/n-95-decon-process.pdf. Accessed March 31, 2020. [DOI] [PMC free article] [PubMed]
- 44.Schwartz A Stiegel M Greeson N, et al. Decontamination and reuse of N95 respirators with hydrogen peroxide vapor to address worldwide personal protective equipment shortages during the SARS-CoV-2 (COVID-19) Pandemic. Available at: https://absa.org/decontamination-and-reuse-of-n95-respirators-with-hydrogen-peroxide-vapor-to-address-worldwide-personal-protective-equipment-shortages-during-the-sars-cov-2-covid-19-pandemic/. Accessed March 31, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsai PP. Information and FAQs on performance, protection, and sterilization of masks against COVID-19. 2020. Available at: https://utrf.tennessee.edu/information-faqs-performance-protection-sterilization-of-masks-against-covid-19/. Accessed March 31, 2020.
- 46.Nolte KB, Taylor DG, Richmond JY. Biosafety considerations for autopsy. Am J Forensic Med Pathol. 2002;23(2):107–122. [DOI] [PubMed] [Google Scholar]
- 47.Pluim JME Jimenez-Bou L Gerretsen RRR, et al. Aerosol production during autopsies: the risk of sawing in bone. Forensic Sci Int. 2018;289:260–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pluim JME, Loeve AJ, Gerretsen RRR. Minimizing aerosol bone dust during autopsies. Forensic Sci Med Pathol. 2019;15(3):404–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wenner L Pauli U Summermatter K, et al. Aerosol generation during bone-sawing procedures in veterinary autopsies. Vet Pathol. 2017;54(3):425–436. [DOI] [PubMed] [Google Scholar]
- 50.Brooks EG, Utley-Bobak SR. Autopsy biosafety: recommendations for prevention of meningococcal disease. Acad Forensic Pathol. 2018;8(2):328–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tian S Hu W Niu L, et al. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer [published online February 28, 2020]. J Thorac Oncol. 2020; pii: S1556–0864(20)30132–5. doi: 10.1016/j.jtho.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu Z Shi L Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome [published online February 18, 2020]. Lancet Respir Med. 2020. pii: S2213–2600(20)30076-X. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tse GM To KF Chan PK, et al. Pulmonary pathological features in coronavirus associated severe acute respiratory syndrome (SARS). J Clin Pathol. 2004;57(3):260–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ruan Q Yang K Wang W, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China [published online March 3, 2020]. Intensive Care Med. 2020. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Inciardi RM Lupi L Zaccone G, et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) [published online March 27, 2020]. JAMA Cardiol. doi:10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xinhuanet Beijing hospital confirms nervous system infections by novel coronavirus. 2020. Available at: http://www.xinhuanet.com/english/2020-03/05/c_138846529.htm. Accessed March 31, 2020.
- 57.van Doremalen N Bushmaker T Morris DH, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1 [published online March 17, 2020]. N Engl J Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kampf G, Todt D, Pfaender S. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect. 2020;104(3):246–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Centers for Disease Control and Prevention (CDC) Detailed disinfection guidance: interim recommendations for US households with suspected/confirmed coronavirus disease 2019. 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/prepare/cleaning-disinfection.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fcommunity%2Fhome%2Fcleaning-disinfection.html. Accessed March 26, 2020.
- 60.American Chemistry Council Center for Biocide Chemistries Novel coronavirus (COVID-19)–fighting products. 2020. Available at: https://www.americanchemistry.com/Novel-Coronavirus-Fighting-Products-List.pdf. Accessed March 27, 2020.
- 61.US Environmental Protection Agency (EPA) List N: disinfectants for use against SARS-CoV-2. 2020. Available at: https://www.epa.gov/pesticide-registration/list-n-disinfectants-use-against-sars-cov-2. Accessed March 27, 2020.
- 62.Tang JW To KF Lo AW, et al. Quantitative temporal-spatial distribution of severe acute respiratory syndrome-associated coronavirus (SARS-CoV) in post-mortem tissues. J Med Virol. 2007;79(9):1245–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Henwood AF. Coronavirus disinfection in histopathology [published online March 1, 2020]. J Histotechnol. 2020;1–3. doi: 10.1080/01478885.2020.1734718. [DOI] [PubMed] [Google Scholar]
- 64.Darnell ME Subbarao K Feinstone SM, et al. Inactivation of the coronavirus that induces severe acute respiratory syndrome, SARS-CoV. J Virol Methods. 2004;121(1):85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.US Food and Drug Administration (FDA) Coronavirus (COVID-19) update: FDA issues new policy to help expedite availability of diagnostics. 2020. Available at: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-new-policy-help-expedite-availability-diagnostics. Accessed March 28, 2020.
- 66.Daley J. Here's how coronavirus tests work—and who offers them. Sci Am. 2020; Available at: https://www.scientificamerican.com/article/heres-how-coronavirus-tests-work-and-who-offers-them/. Accessed March 27, 2020. [Google Scholar]
- 67.US Food and Drug Administration (FDA) Cellex SARS-CoV-2 IgG/IgM rapid test—letter of authorization. Available at: https://www.fda.gov/media/136622/download. Accessed April 4, 2020.
- 68.Johns Hopkins Center for Health Security Serology-based tests for COVID-19. 2020. Available at: http://www.centerforhealthsecurity.org/resources/COVID-19/Serology-based-tests-for-COVID-19.html#sec5. Accessed April 5, 2020.
- 69.Le AB Brooks EG McNulty LA, et al. U.S. medical examiner/coroner capability to handle highly infectious decedents. Forensic Sci Med Pathol. 2019;15(1):31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fusco FM Scappaticci L Schilling S, et al. A 2009 cross-sectional survey of procedures for post-mortem management of highly infectious disease patients in 48 isolation facilities in 16 countries: data from EuroNHID. Infection. 2016;44(1):57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aiken SS Gill JR, National Association of Medical Examiners (NAME) . COVID-19 press release. Available at: https://www.thename.org/assets/docs/COVID-19%20PRESS%20RELEASE.pdf. Accessed March 31, 2020.
- 72.Chui P Chong P Chong B, et al. Mobile biosafety level-4 autopsy facility—an innovative solution. Appl Biosaf. 2007;12(4):238–244. [Google Scholar]
- 73.Zhang Y, Gong Y, Wang C. Rapid deployment of a mobile biosafety level-3 laboratory in Sierra Leone during the 2014 Ebola virus epidemic. PLoS Negl Trop Dis. 2017;11(5):e0005622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rao V, Bordelon E. Mobile high-containment biological laboratories deployment: opportunities and challenges in expeditionary deployments to outbreak response. Appl Biosaf. 2019;24(1):20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Snohomish County Medical Examiner Office Medical examiner's COVID-19 release procedure & handling guidelines. Available at: https://www.snohomishcountywa.gov/5585/COVID-19. Accessed March 31, 2020.
- 76.Centers for Disease Control and Prevention (CDC) Coronavirus disease 2019 (COVID-19): frequently asked questions. Available at: https://www.cdc.gov/coronavirus/2019-ncov/faq.html#funerals. Accessed March 31, 2020.
- 77.National Funeral Directors Association (NFDA) Situation update: novel Coronavirus (COVID-19). Available at: https://www.nfda.org/covid-19. Accessed March 31, 2020.


