Abstract
Background
This review is an update of a previously published review in the Cochrane Database of Systematic Reviews, 2005, Issue 4 (and last updated in the Cochrane Database of Systematic Reviews, 2013 issue 8), on local anaesthetic blockade (LASB) of the sympathetic chain to treat people with complex regional pain syndrome (CRPS).
Objectives
To assess the efficacy of LASB for the treatment of pain in CRPS and to evaluate the incidence of adverse effects of the procedure.
Search methods
For this update we searched the Cochrane Central Register of Controlled Trials (CENTRAL) (2015, Issue 9), MEDLINE (Ovid), EMBASE (Ovid), LILACS (Birme), conference abstracts of the World Congresses of the International Association for the Study of Pain, and various clinical trial registers up to September 2015. We also searched bibliographies from retrieved articles for additional studies.
Selection criteria
We considered randomised controlled trials (RCTs) that evaluated the effect of sympathetic blockade with local anaesthetics in children or adults with CRPS compared to placebo, no treatment, or alternative treatments.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. The outcomes of interest were reduction in pain intensity, the proportion who achieved moderate or substantial pain relief, the duration of pain relief, and the presence of adverse effects in each treatment arm. We assessed the evidence using GRADE (Grading of Recommendations Assessment, Development and Evaluation) and created a 'Summary of findings' table.
Main results
We included an additional four studies (N = 154) in this update. For this update, we excluded studies that did not follow up patients for more than 48 hours. As a result, we excluded four studies from the previous review in this update. Overall we included 12 studies (N = 461), all of which we judged to be at high or unclear risk of bias. Overall, the quality of evidence was low to very low, downgraded due to limitations, inconsistency, imprecision, indirectness, or a combination of these.
Two small studies compared LASB to placebo/sham (N = 32). They did not demonstrate significant short‐term benefit for LASB for pain intensity (moderate quality evidence).
One small study (N = 36) at high risk of bias compared thoracic sympathetic block with corticosteroid and local anaesthetic versus injection of the same agents into the subcutaneous space, reporting statistically significant and clinically important differences in pain intensity at one‐year follow‐up but not at short term follow‐up (very low quality evidence).
Of two studies that investigated LASB as an addition to rehabilitation treatment, the only study that reported pain outcomes demonstrated no additional benefit from LASB (very low quality evidence).
Eight small randomised studies compared sympathetic blockade to various other active interventions. Most studies found no difference in pain outcomes between sympathetic block versus other active treatments (low to very low quality evidence).
One small study compared ultrasound‐guided LASB with non‐guided LASB and found no clinically important difference in pain outcomes (very low quality evidence).
Six studies reported adverse events, all with minor effects reported.
Authors' conclusions
This update's results are similar to the previous versions of this systematic review, and the main conclusions are unchanged. There remains a scarcity of published evidence and a lack of high quality evidence to support or refute the use of local anaesthetic sympathetic blockade for CRPS. From the existing evidence, it is not possible to draw firm conclusions regarding the efficacy or safety of this intervention, but the limited data available do not suggest that LASB is effective for reducing pain in CRPS.
Plain language summary
Local anaesthetic sympathetic blockade for complex regional pain syndrome
Background
Local anaesthetic sympathetic blockade (LASB) is a common treatment for complex regional pain syndrome (CRPS). It involves blocking the activity of sympathetic nerves alongside the spine. The sympathetic nervous system mainly controls unconscious actions such as heart rate, blood flow, and perspiration. The injection of a local anaesthetic drug around the nerves temporarily blocks the function of the nerves. This updated review aimed to summarise the available evidence regarding whether LASB is effective at reducing pain in CRPS, how long any pain relief might last, and whether LASB is safe.
Key results and quality of the evidence
In September 2015, we found a limited number of small trials, all of which had design flaws. We did not find evidence that LASB was better than placebo in reducing pain, or that it provided additional pain relief when added to rehabilitation. While a number of small studies compared LASB to other treatments, most did not find that LASB was better. One small study found that injecting the thoracic (upper back) sympathetic nerves with local anaesthetic and steroid was better than injecting the same drugs just under the skin at one‐year follow‐up, but the study may have been prone to bias. Only six studies reported on the type and amount of side effects. These studies reported only minor side effects, but since some studies did not report this information we can draw no firm conclusions about the safety of LASB. The evidence was mostly of low or very low quality.
Overall, the evidence is limited, conflicting, and of low quality. While we cannot draw strong conclusions, the existing evidence is not encouraging.
Summary of findings
Summary of findings 1. LASB for pain intensity and duration of pain relief in adults with CRPS.
|
Patient or population: adults with CRPS Setting: secondary care Intervention/comparison: LASB vs various comparisons Outcome: pain intensity 0‐10 (VAS or NRS) | ||||
| Comparison | Studies | No of participants (studies) | Result (effect estimates reported where available from study report) | Quality of the evidence (GRADE) |
| LASB vs placebo | Aydemir 2006; Price 1998 | 23 (2) | No significant between‐group difference | ⊕⊕⊕⊝ Moderatea |
| Thoracic LASB + steroid vs subcutaneous local anaesthetic+ steroid | Rocha 2014 | 36 (1) | Favours LASB Mean difference (0‐10 scale) One month −1.25 (95% CI −3.2 to 0.7) One year −2.39 (95% CI −4.72 to −0.06) |
⊕⊝⊝⊝ Very lowb |
| LASB vs ultrasound block | Aydemir 2006 | 18 (1) | No significant between‐group difference | ⊕⊕⊝⊝ Lowc |
| LASB vs IVRB guanethidine | Bonelli 1983 | 19 (1) | No significant between‐group difference | ⊕⊝⊝⊝ Very lowb |
| LASB lumbar plexus vs pulsed radiofrequency lumbar plexus | Freitas 2013 | 40 (1) | No significant between‐group difference | ⊕⊝⊝⊝ Very lowb |
| LASB (lidocaine + clonidine) vs IVRB (lidocaine + clonidine) | Nascimento 2010 | 43 (1) | No significant between‐group difference | ⊕⊝⊝⊝ Very lowb |
| LASB + PT+ pharmacological vs PT + pharmacological | Rodriguez 2005 | 82 (1) | Favours SGB group | ⊕⊝⊝⊝ Very lowb |
| LASB + PT vs PT | Zeng 2003 | 60 (1) | No significant between‐group difference | ⊕⊝⊝⊝ Very lowb |
| Continuous LASB vs continuous brachial plexus block | Toshniwal 2012 | 33 (1) | Favours brachial plexus block | ⊕⊕⊝⊝ Lowc |
| Image‐guided LASB vs nonimage‐guided LASB | Yoo 2012 | 42 (1) | Mean difference 2 weeks postinjection −0.58 (95% CI −1.51 to 0.35) 4 weeks postinjection −0.74 (95% CI −1.36 to −0.12) |
⊝⊝⊝⊝ Very lowd |
| Outcome: hand pain 0‐3 scale | ||||
| LASB vs oral corticosteroids | Lim 2007 | 38 (1) | 15 day follow‐up, no significant between‐group difference at 30 day follow‐up 0.4 (95% CI −0.69 to −0.11), favours LASB with steroid |
⊝⊝⊝⊝ Very lowd |
| Outcome: duration of pain relief | ||||
| LASB bupivacaine + BTA vs LASB bupivacaine | Carroll 2009 | 9 (1) | Increased duration of relief with BTA Median time to analgesic failure (days): LASB bupivacaine + BTA 71 (95% CI 12 to 253) LASB bupivacaine 10 (95%CI 0 to 12) |
⊕⊕⊝⊝ Lowc |
a Downgraded once for imprecision. b Downgraded three times for limitations, inconsistency, and imprecision. cDowngraded twice for inconsistency and imprecision. dDowngraded four times for limitations, inconsistency, indirectness, and imprecision.
Background
This review is an update of a previously published review in the Cochrane Database of Systematic Reviews, 2005, Issue 4 (and last updated in the Cochrane Database of Systematic Reviews, 2013 Issue 8), on local anaesthetic sympathetic blockade for complex regional pain syndrome.
Description of the condition
Complex regional pain syndrome (CRPS) is an umbrella term for a variety of clinical presentations characterised by chronic persistent pain that is disproportionate to any preceding injury (if any) and that is not restricted anatomically to the distribution of a specific peripheral nerve (Bruehl 2010). The International Association for the Study of Pain (IASP) introduced the diagnostic label of CRPS in the 1990s (Merskey 1994), and since then, others have updated it in an attempt to improve its specificity (Harden 2006; Harden 2010). We present these modified diagnostic criteria (the 'Budapest criteria') in Table 2. The term CRPS encompasses a variety of earlier diagnostic terms, including reflex sympathetic dystrophy (RSD), reflex neurovascular dystrophy, Sudeck's atrophy, causalgia, and algodystrophy/algoneurodystrophy (Stanton‐Hicks 1995). CRPS can be classified into two subtypes: CRPS‐I, in which there is no identified peripheral nerve injury, and CRPS‐II, where symptoms are associated with a definable nerve lesion (Harden 2006). This distinction is not always easily made (Harden 2006). Both subtypes of CRPS are characterised by severe pain that is disproportionate to the inciting event, most commonly affecting the hand or foot but sometimes spreading to other body regions (Stanton‐Hicks 2002; Van Rijn 2011). Additionally CRPS presents with some or all of the following symptoms in the affected body parts: sensory disturbances; temperature changes; abnormal patterns of perspiration; swelling/oedema; reduced joint range of motion; movement abnormalities such as weakness, tremor, or dystonia; trophic changes such as skin atrophy, altered hair and nail growth, or localised osteoporotic changes (Bruehl 2010; De Mos 2009; Shipton 2009); and alterations in body perception (Lewis 2007; Lotze 2007; Moseley 2006). CRPS occurs most commonly following wrist fracture and subsequent immobilisation. However, cases can potentially occur after relatively minor trauma and may even occur spontaneously, albeit rarely (De Mos 2007; De Mos 2008; Sandroni 2003). The underlying pathophysiological mechanisms of CRPS are incompletely understood, although there is growing consensus that it is primarily a disorder of the nervous system. Research has identified abnormalities in the tissues of the affected area and the peripheral and central nervous systems (Jänig 2003; Marinus 2011). These include signs of increased neurogenic inflammation (Birklein 2001; Schinkel 2006; Schmelz 2001), an altered local immune response (Birklein 2014; Tan 2005), altered activity in the sympathetic nervous system (SNS) (Drummond 2004; Niehof 2006), increased sensitivity to normal SNS activity (Albrecht 2006; Ali 2000; Drummond 2001), and local tissue hypoxia (Birklein 2000; Koban 2003). Studies have also demonstrated changes in the brain in CRPS (Swart 2009), including alterations of the cortical (higher brain) representation of the affected body part (Maihöfner 2004; Pleger 2006), localised reductions in grey matter density and connectivity (Geha 2008), and altered inhibitory control (Schwenkreis 2003).
1. Budapest criteria: diagnostic criteria for complex regional pain syndrome.
|
To make the clinical diagnosis, the following criteria must be met: |
| 1. Continuing pain, which is disproportionate to any inciting event |
2. Must report at least one symptom in three of the four following categories.
|
3. Must display at least one sign at time of evaluation in two or more of the following categories:
|
| 4. There is no other diagnosis that better explains the signs and symptoms |
For research purposes, diagnostic decision rule should be at least one symptom in all four symptom categories and at least one sign (observed at evaluation) in two or more sign categories. A sign is counted only if it is observed at time of diagnosis.
Description of the intervention
Sympathetic blockade includes procedures that aim to temporarily impede the local function of the sympathetic nervous system. Usually an anaesthesiologist performs the procedure, injecting local anaesthetic directly into sympathetic neural structures that serve the affected limb(s) such as the stellate ganglion or the lumbar sympathetic chain (Nelson 2006). Radiologic guidance such as fluoroscopy or computerised tomography (CT) scan often ensures the accuracy of needle tip placement, and successful blockade is often monitored by direct (e.g., galvanic skin response) or indirect (increase in blood flow to the extremity or increase in temperature) assessment (Breivik 2009). This approach is distinct from the injection of neurolytic agents in an effort to destroy sympathetic nerves. LASBs are also commonly called stellate ganglion blockades (SGB) or, when performed in the lower body, lumbar sympathetic blockades (LSB).
How the intervention might work
People with persistent pain following nerve injury have long been observed to have abnormalities of autonomic nervous system function in the affected limb (temperature, blood flow, sweating) and abnormal skin texture or hair and nail growth attributed, at least in part, to local autonomic dysfunction (Bruehl 2010; De Mos 2009). Early uncontrolled observations of persistent improvement in signs and symptoms following local anaesthetic sympathetic blockade in people with what is now termed CRPS suggested that excessive sympathetic activity provoked or perpetuated this type of persistent pain (Campbell 1996). However, recent evidence regarding adrenaline content in venous effluents from affected limbs has not supported this hypothesis and suggests instead that any benefit of sympathetic blockade in CRPS may reflect transient reversal of a heightened local sensitivity to adrenaline (Binder 2009). These clinical impressions of persistent benefit from transient local anaesthetic sympathetic blockade in CRPS, reinforced by similar longstanding impressions of prolonged benefit after temporary local anaesthetics blockade in peripheral neuralgias, led to the incorporation of sympathetic block into current consensus treatment algorithms for CRPS (Carr 2011), although doubt remains over the contribution of the sympathetic nervous system to pain and the concept of sympathetically maintained pain in CRPS (Harden 2013).
Why it is important to do this review
Despite preclinical evidence that suggests the sympathetic nervous system is involved in the pathophysiology of CRPS, there is debate surrounding the contribution of the sympathetic nervous system to the clinical syndrome (Ochoa 1995; Schott 1995; Verdugo 1994a; Verdugo 1994b). The value of blocking the sympathetic nervous system is also disputed (Fine 1994; Hogan 1997; Jadad 1995; Verdugo 1994a). It is therefore important to evaluate the efficacy of sympathetic blockade with local anaesthetic in the treatment of CRPS. A meta‐analysis of the effect of sympathetic blockade with local anaesthetics in people with CRPS reported that up to 44% of those subjected to sympathetic blockade would be expected to have no pain relief. Due to the lack of randomised controlled trials, investigators obtained this estimate from pooling the results of observational studies (Cepeda 2002). Moreover, the review only evaluated English‐language studies, and it could have overlooked relevant RCTs. Hence, to overcome this limitation, we decided to perform a systematic review of the literature with no language restriction to determine both the efficacy and safety of sympathetic blockade with local anaesthetics to alleviate pain in people with CRPS.
Objectives
To assess the efficacy of LASB for the treatment of pain in CRPS and to evaluate the incidence of adverse effects of the procedure.
Methods
Criteria for considering studies for this review
Types of studies
We considered randomised controlled trials (RCTs). As blinding of sympathetic block is not always possible, we included trials that were either double‐blind, single‐blind, or open. We included studies that compared LASB with placebo interventions, no treatment, or alternative interventions. We also included studies that investigated the effect of adding LASB to other interventions.
Types of participants
We included studies that evaluated the effect of sympathetic blockade with local anaesthetics to treat CRPS in children or adults. We included studies even if the authors did not describe the constellation of symptoms necessary to diagnose CRPS and stated only that "patients with RSD/CRPS were included". We took this approach to avoid excluding any of the relatively few RCTs of this intervention. We placed no restrictions regarding the number of participants recruited to trials.
We excluded trials that evaluated sympathetic blockade for other pain syndromes such as radiculopathy, herpes zoster, postherpetic neuralgia, fibromyalgia, or phantom pain.
Types of interventions
We included studies that evaluated selective sympathetic blockade with local anaesthetics. We excluded studies that only evaluated somatic nerve blocks or studies that evaluated the effect of local anaesthetics or sympatholytic drugs administered orally, intravenously, or epidurally. We excluded studies that reported the results of combined sympatholytic therapies, such as surgical sympathectomy or guanethidine intravenous regional block plus local anaesthetic blockade of the sympathetic chain. We also excluded studies of ganglionide local opioid analgesia (GLOA), a technique in which clinicians locally inject opioids such as buprenorphine into the stellate ganglion, because this procedure does not block sympathetic activity.
Types of outcome measures
The outcomes of interest were pain intensity levels, duration of pain relief. and adverse events. For this update, we excluded studies that had only immediate follow‐up data (≤ 48 h), because this information provides little clinically relevant information about the effectiveness of this treatment. We applied this new criterion to studies that had been included in previous updates of this review.
Search methods for identification of studies
For this update, we used identical search strategies to that of our 2013 review update. For the search strategies, see Appendix 1 for the Cochrane Central Register of Controlled Trials (CENTRAL), Appendix 2 for MEDLINE, Appendix 3 for EMBASE, and Appendix 4 for LILACS.
We performed the search for the original review from November 2003 to January 2004 updated it on 17 November 2011 and 22 November 2012 (2013 update). The present update encompasses searches run from 22 November 2012 to 16 September 2015.
We evaluated non‐English language papers for inclusion.
For the 2016 update, we did not search the Cochrane Pain, Palliative and Supportive Care Group Specialised Register, as it is no longer updated.
Electronic searches
We searched the following databases for the update of this review.
CENTRAL (The Cochrane Library 2015, Issue 9).
MEDLINE (Ovid) (1966 to September 2015).
EMBASE (Ovid) (1974 to September 2015).
LILACS (Birme) (1982 to September 2015).
Searching other resources
Reference lists
We searched the bibliographies of retrieved articles for additional studies.
Unpublished studies
In order to minimise the impact of publication bias, we reviewed conference abstracts of the World Congresses of the International Association for the Study of Pain from 1995 up to 2014. For this update, we expanded the search of the original review by also searching relevant clinical trial registers (from inception) for upcoming trials. We searched the following clinical trial registers: the controlled trials register (15 October 2015; www.controlled-trials.com/), the United States National Institute of Health service ClinicalTrials.gov (15 October 2015; www.clinicaltrials.gov/); the Australian New Zealand Clinical trials register (15 October 2015; www.anzctr.org.au/), and the European Clinical Trials Register (7 December 2012; www.clinicaltrialsregister.eu/).
Personal contact
We attempted to communicate with authors if we needed additional information that was not provided in the trial report. In addition, we provided the reference list of included studies to experts in the field to determine if any additional references were appropriate for the review.
Data collection and analysis
Selection of studies
Two review authors independently read each of the titles and abstracts of the reports identified by the search and discarded narrative reviews, case series, and case reports. If there was no abstract, we retrieved the full‐text report. If there was disagreement, the authors met to reach consensus, consulting an independent third review author if necessary. We retrieved in full all abstracts and reports that made reference to a trial of sympathetic blockade with local anaesthetics. Two review authors then independently assessed the full‐text articles. We did not anonymise the reports for the assessment.
Data extraction and management
Two review authors independently extracted the data. If there was disagreement, they met to reach consensus, consulting an independent third review author if necessary. We extracted the following data from each study.
Study details: study design (parallel or cross‐over), method of randomisation, presence or absence of blinding.
Demographic characteristics: age, sex, number of participants recruited, number of study withdrawals or drop‐outs, if any.
Participant clinical characteristics: duration of pain before sympathetic block, site of pain (arm, leg, mixed, or other such as facial).
Type of noxious initiating event (if known): surgery, fracture, crush injury, projectile, or stab injury.
Type of tissue injured: nerve, soft tissue, bone.
Presence of medico‐legal factors that may influence the experience of pain and the outcomes of therapeutic interventions.
Concomitant treatments that may affect outcome: antidepressants, physical therapy, etc.
Treatment characteristics: site of sympathetic block (cervical or lumbar), type of local anaesthetic used (including concentration and volume), evaluation of the technical adequacy of the block, duration of follow‐up, duration of the pain relief, number of blocks performed, method of pain assessment, and presence of complications or adverse effects.
Information on postprocedure analgesic requirements.
Information on conflicts of interest and statements of study support.
If authors reported pain intensity using a visual analogue scale or numeric rating scale, we extracted the mean and standard deviation of pain intensity in each study arm. If authors reported pain relief, we extracted the proportion of participants in each category of pain relief.
Assessment of risk of bias in included studies
We used a modified version of the Cochrane 'Risk of bias' tool with additional domains added in response to the recommendations of Moore 2010. On this basis we added two domains, 'size' and 'duration', using the thresholds for judgement suggested by Moore 2010. We have not added the 'outcome' domain as this is covered already by our choice of primary outcome measures. Thus in addition to the standard items in the 'Risk of bias' tool:
selection bias (random sequence generation, allocation concealment);
performance bias (blinding of participants and personnel);
detection bias (blinding of outcome assessment);
attrition bias (incomplete outcome data; consideration of analysis methods, e.g., imputation method);
reporting bias (selective reporting); and
other sources of bias;
We also assessed the following domains as recommended by Moore 2010.
Size (rating studies with fewer than 50 participants per arm as being at high risk of bias, those with between 50 and 199 participants per arm as being at unclear risk of bias, and 200 or more participants per arm as being at low risk of bias).
Duration (rating studies with follow‐up of two weeks as being at high risk of bias, two to seven weeks as being at unclear risk of bias and eight weeks or longer as being at low risk of bias).
Two review authors completed the 'Risk of bias' assessment for each included study independently. If there was disagreement, the authors met to reach consensus, consulting an independent third review author if necessary.
Measures of treatment effect
We compared the post‐treatment pain intensity scores between the trial arms. Where possible, we calculated the proportion of participants with a specific degree of pain relief and converted it into dichotomous information to yield the number of participants who obtained a moderately important benefit (30% pain relief) or a substantially important benefit (50% or more pain relief) as defined by the IMMPACT recommendations (Dworkin 2008). We planned to calculate the risk ratio (RR) as the measure of treatment effect and used this to calculate the number needed to treat for an additional beneficial outcome (NNTB) for 30% and 50% pain relief. We also collected data on the duration of pain relief postintervention where available.
For this update, we used the OMERACT 12 group's recommendations for minimally important difference for pain outcomes reported on a continuous scale (Busse 2015). They recommend 10 mm on a 0‐100 mm visual analogue scale (VAS) as the threshold for minimal importance for average between‐group change. They highlight that should be interpreted with caution as estimates that fall closely below this point may still reflect a treatment that benefits a considerable number of patients. We used this threshold but interpreted it cautiously.
Unit of analysis issues
No unit of analysis issues arose since we were unable to conduct a meta‐analysis due to insufficient data.
Dealing with missing data
Where insufficient data were presented to enter a study into the meta‐analysis, we contacted study authors to request access to the missing data.
Assessment of heterogeneity
We planned to assess heterogeneity and its impact using the Chi2 test and the I2 test (Higgins 2003; Higgins 2011). Where significant heterogeneity (P < 0.1) was present, we planned to conduct subgroup analyses. Preplanned comparisons included CRPS‐I versus CRPS‐II, children versus adults, and continuous versus single block. However, no meta‐analysis was possible.
Assessment of reporting biases
We considered the possible influence of publication/small study biases on review findings. For studies that utilised dichotomised outcomes, where possible, we planned to test for the possible influence of publication bias on each outcome by estimating the number of participants in studies with zero effect required to change the NNTB to an unacceptably high level (defined as an NNTB of 10) as outlined by Moore 2008.
Data synthesis
We pooled results where adequate data supported this, using Review Manager 5 software (RevMan 2012). Separate preplanned meta‐analyses included sympathetic blockade versus sham/placebo procedure and sympathetic blockade versus no treatment or usual care. We used a random‐effects model to combine the studies. We considered separate meta‐analyses for short‐term (up to two weeks postintervention), mid‐term (more than two to less than seven weeks postintervention) and long‐term (seven weeks or longer postintervention) outcomes where we identified adequate data.
Assessment of quality of available evidence
For this update we used the GRADE approach to assess the quality of evidence (Guyatt 2011a; Guyatt 2011b). Two reviewers independently applied the GRADE criteria to each key comparison. If there was disagreement, the authors met to reach consensus, consulting an independent third review if necessary. We present a summary of our judgements for each comparison in Appendix 5.
To ensure consistency of GRADE judgements, we applied the following criteria to each domain equally for all key comparisons of the primary outcome.
Limitations of studies: downgrade once if more than 25% of participants were from studies classified as being at a high risk of bias across any domain, excluding the 'study size' domain as this is accounted for in the assessment of imprecision.
Inconsistency: downgrade once if heterogeneity is statistically significant and the I2 value is more than 40%. When a meta‐analysis was not performed we downgraded once if trials did not show effects in the same direction.
Indirectness: downgrade once if more than 50% of the participants were outside the target group.
Imprecision: downgrade once if fewer than 400 participants for continuous data and fewer than 300 events for dichotomous data.
Publication bias: downgrade once where there is direct evidence of publication bias or if estimates of effect based on small scale, industry‐sponsored studies raising a high index of suspicion of publication bias.
Two review authors (NEO, BMW) judged whether these factors were present. We considered single studies to be inconsistent and imprecise, unless more than 400 participants were randomised for continuous outcomes or more than 300 for dichotomous outcomes. We applied the following definitions of the quality of the evidence (Balshem 2011).
High quality: We are very confident that the true effect lies close to that of the estimate of the effect.
Moderate quality: We are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low quality: Our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect.
Very low quality: We have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect.
'Summary of findings' table
We included a 'Summary of findings' table to present the main findings in a transparent and simple tabular format. In particular, we included key information concerning the quality of evidence, the magnitude of effect of the interventions examined, and the sum of available data on the outcome pain intensity, hand pain, and duration of pain relief.
Subgroup analysis and investigation of heterogeneity
We assessed heterogeneity and its impact using the Chi2 test and the I2 test (Higgins 2003; Higgins 2011). Where significant heterogeneity (P < 0.1) was present we planned to conduct subgroup analyses. Preplanned comparisons included CRPS‐I versus CRPS‐II, children versus adults, and repeated versus single blocks.
Where possible we used the proportion of people with adverse side effects in each treatment group to calculate the number needed to treat for an additional harmful outcome (NNTH).
Sensitivity analysis
When sufficient data were available, we conducted sensitivity analyses on the effect of including/excluding studies classified as being at unclear or high risk of bias.
Results
Description of studies
Results of the search
The previous update of this review included twelve studies (Aydemir 2006; Bonelli 1983; Carroll 2009; Meier 2009; Nascimento 2010; Price 1998, Raja 1991; Rodriguez 2005; Toshniwal 2012; Verdugo 1995, Wehnert 2002; Zeng 2003; combined N = 386). For this update, we included an additional four studies (Freitas 2013; Lim 2007; Rocha 2014; Yoo 2012; combined N = 154]). As our modified criteria excluded studies with follow‐up of 48 hours or less, we excluded four studies from this update that had been included in previous versions of this review (Meier 2009; Raja 1991; Verdugo 1995; Wehnert 2002; combined N = 79). Overall, we included 12 studies with 461 participants in this update.
One new study is awaiting classification, as it was published as a protocol for a trial and in abstract format only, and it is unclear whether the trial was completed (Kostadinova 2012).
Figure 1 presents a flow chart of the search screening process for the present update. We identified 461 studies through the database search strategy and none from searching other sources. After removing duplicates and screening titles and abstracts, we retrieved the full text for five studies. Of these, we included four new studies in the review.
1.
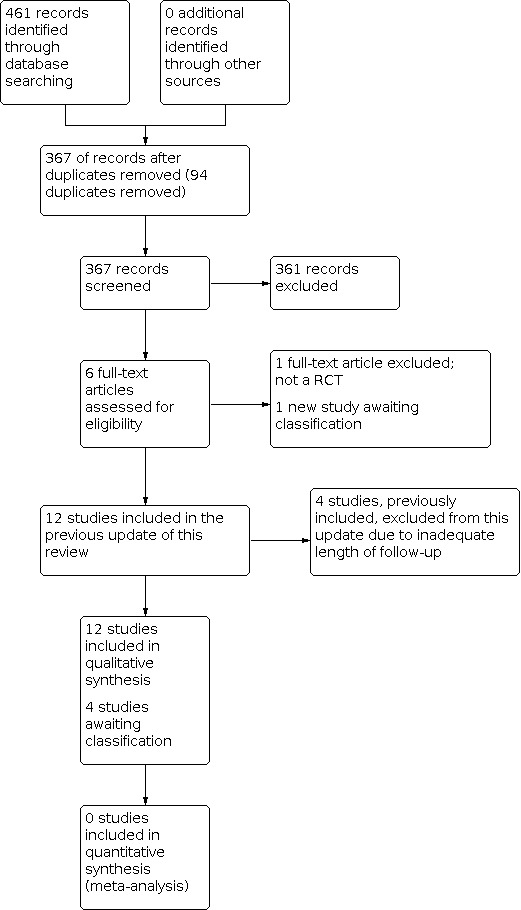
#Study flow diagram for updated searches
For this update we attempted to contact the authors of two studies: to retrieve essential data for Freitas 2013 and to check the status of the trial and request a report if available for Kostadinova 2012.
Included studies
We present full details of the studies in the Characteristics of included studies tables.
Study participants
All included studies evaluated only adult participants (Aydemir 2006; Bonelli 1983; Carroll 2009; Freitas 2013; Lim 2007; Nascimento 2010; Price 1998; Rocha 2014; Rodriguez 2005; Toshniwal 2012; Yoo 2012; Zeng 2003).
Nine studies included only people with upper limb CRPS treated with stellate ganglion blockade (Aydemir 2006; Bonelli 1983; Lim 2007; Nascimento 2010; Rocha 2014; Rodriguez 2005; Toshniwal 2012; Yoo 2012; Zeng 2003), and two studies included only people with lower limb CRPS treated with lumbar sympathetic blockade (Carroll 2009; Freitas 2013). The remaining study included a mix of upper and lower limb CRPS (Price 1998).
Study designs
Two studies used a cross‐over design (Carroll 2009; Price 1998), and 10 employed a parallel design (Aydemir 2006; Bonelli 1983; Freitas 2013; Lim 2007; Nascimento 2010; Rocha 2014; Rodriguez 2005; Toshniwal 2012; Yoo 2012; Zeng 2003). All included studies were small, with total numbers of participants ranging from 7 to 82.
LASB versus placebo
Two studies compared LASB versus placebo (Aydemir 2006; Price 1998).
Price 1998 (N = 7) compared stellate ganglion block (n = 4, 15 ml lidocaine 1%) versus lumbar sympathetic block (n = 3, 10 ml bupivacaine 0.125%) with normal saline injection in people with CRPS of the upper or lower extremities based on the IASP diagnostic criteria and investigated the proportion of participants who experienced 50% pain relief. Price 1998 also measured the duration of pain relief and the mean between‐group difference in pain relief on a visual analogue scale (VAS). Aydemir 2006 (N = 25) compared stellate ganglion lidocaine block (10 ml lidocaine 1%) plus sham stellate ganglion ultrasound block (n = 9) to a double‐sham condition (sham stellate ganglion lidocaine (10 ml saline) and ultrasound blocks). Both groups received rehabilitation treatment. Investigators measured spontaneous pain post‐treatment and at one‐month follow‐up.
LASB versus other interventions
In contrast to the original version of this review, we included studies, totaling nine, that compared LASB to other interventions (Aydemir 2006; Bonelli 1983; Carroll 2009; Freitas 2013; Lim 2007; Nascimento 2010; Rocha 2014; Toshniwal 2012; Yoo 2012).
Aydemir 2006 compared stellate ganglion lidocaine block (10 ml of 1%) plus sham stellate ganglion ultrasound block (n = 9) to stellate ganglion ultrasound 'block' (consisting of ultrasound delivered non‐invasively over the stellate ganglion) plus sham stellate ganglion lidocaine block (10 ml of saline; n = 9). Both groups received rehabilitation treatment. Investigators measured the primary outcome of spontaneous pain post‐treatment and at one‐month follow‐up.
Bonelli 1983 (N = 19) compared stellate ganglion block with bupivacaine (15 ml of 0.5%; n = 10) versus intravenous regional blockade (IVRB) with guanethidine (20 mg; n = 9) in patients with reflex sympathetic dystrophy. The primary outcome was the intensity of pain (measured using a 100 mm linear scale) measured post‐treatment at 15 minutes, 60 minutes, 24 hours and 48 hours as well as at one and three months.
Carroll 2009 (N = 9, of whom seven completed the study) compared sympathetic block with botulinum toxin A (75 units) plus bupivacaine (10 ml of 0.5%) versus bupivacaine alone (10 ml of 0.5%) in people with CRPS of the lower extremity. The primary outcome was the duration that pain (measured using a VAS) remained below baseline levels.
Freitas 2013 (N = 40) compared sympathetic block of the lumbar plexus with lidocaine and clonidine versus pulsed radiofrequency treatment of the same structure. Investigators measured pain intensity for up to six months follow‐up.
Lim 2007 (N = 36) compared a course of five stellate ganglion blocks with lidocaine versus a two‐week course of corticosteroids (prednisolone) in patients with CRPS following stroke. They used a self developed four‐point scale (0 to 3) of hand pain with passive movement and followed patients up to 30 days from the start of treatment.
Nascimento 2010 (N = 43) compared sympathetic block with lidocaine (70 mg 1% lidocaine) versus sympathetic block with lidocaine (70 mg 1% lidocaine) plus clonidine (30 μg) versus IVRB with lidocaine plus clonidine (7.0 ml solution, 1% lidocaine, 1 μg/kg clonidine). Investigators measured intensity of pain (VAS) and duration of pain relief post‐treatment and at one‐week follow‐up.
Rocha 2014 (N = 36) compared image‐guided thoracic sympathetic block with ropivacaine and triamcinolone versus injection of the same agents into the subcutaneous space. Authors described this comparison condition as an "active control" as it might be predicted to induce physiological effects. This allowed blinding of participants. Investigators followed up participants using the Brief Pain Inventory as an outcome measure at one month and one year. This study did not report a responder analysis.
Toshniwal 2012 compared continuous stellate ganglion block (SGB; n = 18; 280 ml, 0.125% bupivacaine at 2 ml/h for seven days) versus continuous infraclavicular brachial plexus block (n = 12; 400 ml, 0.125% bupivacaine at 5 ml/h for seven days) in people with CRPS‐I of the upper extremity. Both groups received concurrent physiotherapy sessions. The primary outcome was the subscale scores on the neuropathic pain scale measured over a four‐week period post‐treatment.
Yoo 2012 (N = 42) compared stellate ganglion block with image guidance to the same block versus no image guidance in participants with CRPS following stroke. Of note, the group with image guidance received a higher dose of lidocaine (10 ml) than the non‐guided group (5 ml). Investigators measured pain intensity with a VAS at two‐ and four‐week follow‐up.
LASB in addition to other therapies
Two studies evaluated the efficacy of LASB as an addition to other therapeutic management (Rodriguez 2005; Zeng 2003). Rodriguez 2005 evaluated physical therapy and pharmacological treatment with or without SGB (N = 41 per group, 10 cc, equal parts 2% lidocaine and 0.5% bupivacaine) in people with upper limb CRPS with a confirmed sympathetic component to their pain (50% pain reduction with screening, prerandomisation SGB). Investigators measured pain intensity, therapeutic efficacy (proportion with at least 50% pain reduction), and relapse rate at two months post‐treatment. Zeng 2003 compared SGB (dose not reported) plus rehabilitation versus rehabilitation alone in a group (N = 60) with shoulder‐hand syndrome following stroke. Pain (verbal rating scale) was measured at 10 and 20 days post‐treatment.
Excluded studies
In total, we excluded 26 studies. For this update, we excluded one study at the full‐text stage as it was not an RCT (Kastler 2013). In the last review we excluded two studies (Rodriguez 2006; Rodriguez 2008), as it was not clear whether they represented original trials in distinct cohorts or an expansion of the included trial by Rodriguez 2005, comprising many of the same participants' data. For this update we have reclassified these two studies to Studies awaiting classification and have again attempted to contact the study authors for clarification. See the table Characteristics of excluded studies for details of all studies excluded from all versions of this review.
We also identified one further study awaiting classification (Kostadinova 2012).
Risk of bias in included studies
We present the summary results of the 'Risk of bias' assessment in Figure 2 and Figure 3. We considered no studies to be at low risk of bias across all domains.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Only two studies clearly described an adequate randomisation process (Freitas 2013; Toshniwal 2012); we considered the other ten studies to be at unclear risk of bias for this domain. We judged four studies as being at a low risk of bias for allocation concealment (Aydemir 2006; Rocha 2014; Rodriguez 2005; Toshniwal 2012), and we assessed six studies as being at unclear risk of bias (Bonelli 1983; Freitas 2013; Lim 2007; Nascimento 2010; Yoo 2012; Zeng 2003). The remaining studies used a cross‐over study design (risk of bias for allocation concealment not applicable).
Blinding
We considered three studies to have blinded participants and personnel adequately (Aydemir 2006; Carroll 2009; Price 1998) (low risk of performance bias). We considered six studies to be at unclear risk of bias across this domain (Bonelli 1983; Freitas 2013; Nascimento 2010; Rocha 2014; Toshniwal 2012; Yoo 2012) as though the interventions were distinguishable, both were active invasive interventions. Three studies were at high risk of bias (Lim 2007; Rodriguez 2005; Zeng 2003;) as clinicians delivering the interventions were not blinded or the intervention conditions were clearly distinguishable. The outcome of interest for this review was self‐reported pain. In this situation, the patient acts as the assessor; therefore risk of detection bias is primarily dependent on participant blinding. For blinding of outcome assessment, we judged five studies to be at low risk of detection bias as they clearly reported blinding of the participants (Aydemir 2006; Carroll 2009; Freitas 2013; Price 1998; Rocha 2014), four studies at unclear risk of bias as it was unclear whether patients were adequately blinded (Bonelli 1983; Nascimento 2010; Toshniwal 2012; Yoo 2012), and three studies were judged to have high risk of detection bias because patients were not adequately blinded (Lim 2007; Rodriguez 2005; Zeng 2003).
Incomplete outcome data
We considered seven studies to be at unclear risk of bias due to incomplete outcome data (Aydemir 2006; Carroll 2009; Freitas 2013; Lim 2007; Rocha 2014; Rodriguez 2005; Yoo 2012) as a result of the levels of drop‐out reported or incomplete reporting of attrition.
Selective reporting
We judged three studies to be at high risk of bias for this domain due to incomplete reporting of pain scores (Freitas 2013; Price 1998; Rodriguez 2005). Carroll 2009 carried an unclear risk of bias for incomplete reporting of pain score at a secondary end point.
Adequate sample size?
We judged all studies to be at high risk of bias with regard to sample size as all had less than 50 participants per arm.
Adequate duration of follow‐up?
We considered all but four studies to be at high or unclear risk of bias based on inadequate duration of follow‐up (Bonelli 1983; Freitas 2013; Rocha 2014; Rodriguez 2005).
Other potential sources of bias
We judged two studies to be at high risk of bias for other reasons (Bonelli 1983; Rocha 2014). In Bonelli 1983, the LASB group had a significantly shorter duration of symptoms at baseline than the IVRB guanethidine group, and participants were significantly older. Rocha 2014 had average pain scores at baseline that differed by greater than one point between groups, but authors did not present tests for comparability at baseline. Three studies were at unclear risk of bias (Freitas 2013; Yoo 2012). Freitas 2013 and Rodriguez 2005 provided no baseline data, and neither Freitas 2013 nor Yoo 2012 gave details regarding concomitant treatments. We judged the two cross‐over studies to be at low risk of bias for carry‐over effects (Carroll 2009; Price 1998).
There were insufficient data to support a formal statistical analysis of reporting/small study biases for any comparison.
Sources of funding and conflicts of interest
While not formally included within the 'Risk of bias' assessment, we extracted information regarding study funding and potential conflicts of interest. Seven study reports offered no details regarding these issues (Aydemir 2006; Bonelli 1983; Freitas 2013; Nascimento 2010; Price 1998; Yoo 2012; Zeng 2003).
Carroll 2009 declared that the authors had filed a patent for the inclusion of botulinum toxin A in sympathetic blocks. Rodriguez 2005 declared financial support from governmental and non‐profit organisations. No study declared funding from industry sources. Toshniwal 2012 and Rocha 2014 declared no conflict of interest.
Effects of interventions
See: Table 1
For a summary of all core findings, see Table 1.
LASB versus placebo
For the comparison of LASB versus placebo, we rated all evidence as being of moderate quality.
Pain intensity
In Price 1998, there was no difference between lidocaine and normal saline; the same number of participants (6/7) achieved at least 50% pain relief at two weeks. In Aydemir 2006, spontaneous pain scores were no different from baseline to post‐treatment in either the group receiving lidocaine plus sham ultrasound SGB (Z = −0.18, P = 0.86) or in the group receiving sham lidocaine plus sham ultrasound (Z = −0.76, P = 0.45). Authors did not report between‐group comparisons.
Duration of pain relief
Price 1998 evaluated the duration of pain relief, finding that when local anaesthetic was administered, the mean duration of relief was longer (three days versus 19.9 hours in the saline group). However, short‐term relief was similar in both groups. In Aydemir 2006, spontaneous pain scores were no different from baseline to one‐month follow‐up in either the group receiving lidocaine (plus sham ultrasound SGB; Z = −1.05, P = 0.29) or in the group receiving sham lidocaine and sham ultrasound (Z = −0.68, P = 0.50). Authors reported no between‐group comparisons. None of the included studies reported postintervention analgesic requirements.
Adverse Events
Price 1998 and Aydemir 2006 did not report adverse events.
LASB versus other interventions
Pain relief
Most comparative studies reported no significant difference in pain between groups (Bonelli 1983; Freitas 2013; Nascimento 2010; low to very low quality evidence). Aydemir 2006 did not explicitly report between‐group differences, although they did not find any within‐group differences in spontaneous pain scores between baseline and post‐treatment nor at one‐month follow‐up in either the group receiving lidocaine SGB plus sham ultrasound SGB (Z‐scores listed above) or in the group receiving ultrasound SGB plus sham lidocaine (Z = −0.59, P = 0.55; Z = −0.63, P = 0.53, respectively; low quality evidence). Due to the variation in the interventions, there were not adequate data to allow pooling of the results.
Lim 2007 reported no significant difference in hand pain intensity (scale from 0 to 3) between LASB plus corticosteroid versus oral corticosteroids at 15‐day follow‐up (mean difference 0.00, 95% confidence interval (CI) −0.35 to 0.35; very low quality evidence) and a statistically significant difference in favour of LASB with steroid at 30 days (mean difference 0.40, 95% CI −0.69 to −0.11; very low quality evidence).
Rocha 2014 reported that thoracic LASB with ropivacaine and steroid did not result in a statistically significant difference in average pain scores compared to injection of the same agents into the subcutaneous space (described as an "active placebo" at one month (0 to 10 scale mean difference −1.25, 95% CI −3.20 to 0.70; very low quality evidence), but there was a statistically significant difference at one‐year follow‐up in favour of thoracic LASB (mean difference −2.39, 95% CI −4.72 to −0.06; very low quality evidence). While not significant at one‐month follow‐up, the point estimate at both time points exceeds our threshold for clinical importance. However, it is worth noting that at one‐year follow‐up, attrition in the active group was 16% and in the control group 26%, introducing a possible risk of bias.
Toshniwal 2012 reported significantly lower short‐term pain scores (on a 0 to 10 scale) in favour of the group receiving the continuous infraclavicular brachial plexus block versus the group receiving the continuous stellate ganglion block. Specifically, at 30 minutes, 2 hours and 12 hours, those receiving the continuous brachial plexus block had significantly lower intensity of pain (0.7, 0.5, and 0.7, respectively) and unpleasantness of pain (0.7, 0.7, and 0.8, respectively) compared with those receiving a continuous stellate ganglion block (intensity: 3.3, 2.7, and 1.9; unpleasantness: 3.0, 2.7, and 1.9). Dull pain intensity scores were significantly reduced for the brachial plexus block group versus the stellate ganglion block group at 2 hours (0.1 versus 2.4), 12 hours (0.6 versus 1.9), and 24 hours (1.3 versus 2.6) with deep pain also significantly reduced at these time points (2 hours −0.1 versus 2.3; 12 hours −0.7 versus 1.6; 24 hours −1.4 versus 2.4), as well as at 30 minutes postcannulation (0.1 versus 2.3). There were no statistically significant differences between groups for short‐term scores on any of the other Neuropathic Pain Scale components. Furthermore, there was no evidence of increased effectiveness for long‐term pain relief in one group over the other and no between‐group differences at any other time points. There was no statistical comparison of quality of pain differences between groups. We rated this evidence as being of low quality.
Yoo 2012 found no statistically significant or clinically important difference between image‐guided and non‐guided stellate ganglion block at two weeks postinjection (0 to 10 pain VAS mean difference −0.58, 95% CI −1.51 to 0.35; very low quality evidence); there was a statistically significant but clinically unimportant difference at four weeks postinjection (mean difference −0.74, 95% CI −1.36 to −0.12; very low quality evidence) in participants with CRPS following stroke.
Duration of pain relief
Carroll 2009 reported a significantly longer duration of analgesia in the botulinum toxin A group (median time to analgesic failure 71 days (95% CI 12 to 253; low quality evidence) compared with bupivacaine alone (< 10 days, 95% CI 0 to 12; P < 0.02; low quality evidence). However, while the authors reported that pain intensity declined significantly in the botulinum toxin A group, they did not provide numeric pain scores for either treatment group.
Adverse events
Only six studies provided specific data regarding adverse events, and the level of detail of this reporting was mixed.
Carroll 2009 reported moderate adverse events in one participant (14.2%) following the botulinum toxin type A LASB. This participant had significant nausea and emesis that began five5 hours after the injection and lasted two days, resolving spontaneously.
Freitas 2013 reported that paraesthesia during needle positioning in "1 out of 10" in the LASB group and "2 out of 10" in the pulsed radiofrequency group. This is likely to be an error as it suggests that there were 20 participants in total while the trial reports 40 participants. The study reports that all participants in both groups reported soreness at the injection site lasting five to seven days.
Nascimento 2010 also found mild adverse events for all three groups. The SGB group receiving lidocaine and clonidine (gGroup 2) reported the highest frequency of adverse events: 93.3% reported drowsiness (14/15), 13.3% dizziness (2/15), 13.3% hoarseness (2/15), 6.7% reported pain at the injection site (1/15), and 26.7% reported a feeling of dry mouth (4/15). The SGB group receiving only lidocaine (gGroup 1) reported the lowest frequency of adverse events, with nausea occurring in 6.5% (1/14), dizziness in 14.3% (2/14), hoarseness in 6.5% (1/14), and pain at the injection site in 6.5% (1/14). Lastly, the group receiving the IVintravenous (IV) regional block with lidocaine and clonidine (gGroup 3) reported drowsiness (46.1%; 6/13) and dizziness (7.7%; 1/13).
Rocha 2014 reported a similar overall rate of minor adverse events following thoracic blockade or subcutaneous injection with local anaesthetic and steroid and no major adverse events. Minor eventsThese included dizziness, blurred vision, puncture pain, increased pain, headache, nausea, vomiting, dysphagia, hoarseness, haematoma, dyspnoea, shivering, cold feeling, face swelling, and mouth numbness. Of note, 65% of participants in both groups reported "puncture pain";, 24% in the thoracic block group reported dyspnoea compared with 6% in the subcutaneous group. 35%Thirty‐five per cent in the thoracic block group reported dizziness compared to 12% in the subcutaneous group. Twenty‐four per cent in the thoracic block group reported dizziness compared to no participants in the subcutaneous group.
Toshniwal 2012 found adverse events in both groups. In the continuous stellate ganglion block group, Horner's syndrome was most common (94.7%) while initial motor weakness was the most common adverse event in the continuous infraclavicular brachial plexus group (100%). Positive catheter tip culture occurred in 61.1% (11/18) of the stellate ganglion block group and in 8.3% (1/12) in the brachial plexus group; investigators observed no signs of infection at the catheter site were observed in either group. Catheter migration was found in 5.2% (1/19) of the stellate ganglion block group (versus 7.1% (1/14) of the brachial plexus group). Lastly, hoarseness of voice (for initial 12 hours) was found in 16.7% (3/18) of participants in the stellate ganglion block group reported hoarseness of voice (for initial 12 hours).
Yoo 2012 reported no adverse events in the group who received ultrasound guided blocks and two haematomas at the injection site for those who received unguided blocks.
LASB in addition to other interventions
Pain relief
Zeng 2003 and Rodriguez 2005 investigated the effectiveness of adding LASB to rehabilitation versus rehabilitation or medication alone. Zeng 2003 found no benefit of adding LASB at 10 days (0‐10 Verbal Rating scale mean difference 0.2, 95% CI −1.3 to 1.7; very low quality evidence) or 20 days (mean difference 0.1, 95% CI −0.97 to 1.17; very low quality evidence). Rodriguez 2005 reported treatment efficacy (proportion with at least 50% pain reduction) at the two‐month follow‐up to be 46% in favour of the SGB group, an absolute risk reduction of 17% in favour of the SGB group with a number needed to treat for an additional beneficial outcome (NNTB) of 6. The NNTB suggests that six people with CRPS would need to be treated with SGB (in addition to physical and pharmacological therapy) to prevent one relapse. There was a higher relapse rate in the control group (37%) versus the SGB group (20%) (hazard ratio (HR) 2.7, 95% CI 1.1 to 6.7; very low quality evidence). The Kaplan‐Meier estimates of the cumulative probability of not having a relapse at two months was 80% in the SGB group and 63% in the control group. However, this study did not report data for pain intensity or the proportion who achieved meaningful pain relief.
Duration of pain relief
No studies specifically presented data on the duration of pain relief for this comparison.
Adverse events
Zeng 2003 and Rodriguez 2005 did not report adverse events.
Discussion
Summary of main results
The objective of this review was to assess the efficacy of LASB for the treatment of pain in CRPS and to evaluate the incidence of adverse effects of the procedure.
Previous versions of this review revealed the scarcity of published evidence to support the use of LASB for CRPS and raised questions about its efficacy.
LASB versus placebo or no treatment
This update reveals little progress in developing high quality evidence to support the use of LASB for CRPS since the last update in 2013. There are only two placebo‐controlled randomised studies that met our modified inclusion criteria for this update (Aydemir 2006; Price 1998), both of which have very small sample sizes. We can draw no firm conclusions from this evidence. It is notable that the results to date are not suggestive of a significant effect of LASB over placebo even in the very short term (30 minutes to two hours), the time frame that theory would suggest local anaesthetic is likely to have its maximum benefit. We could not estimate the duration of pain relief, if any.
LASB versus other interventions
In a change from the original version of this review, we took the decision to include trials that compared LASB with alternative interventions or that evaluated the effect of adding LASB to other treatments. We identified a number of such studies investigating a range of comparisons, and the majority of these demonstrated no significant difference between the intervention and control groups. It is notable that in one small study (Bonelli 1983), LASB did not demonstrate superior effectiveness when compared to intravenous regional blockade (IVRB) with guanethidine, an intervention for which there is consistent evidence of no effect (Jadad 1995; McQuay 1997; O'Connell 2013).
One small study (N = 36) at high risk of bias suggests a potentially clinically important effect for thoracic sympathetic blockade with bupivacaine and triamcinolone on average daily pain at one month and one year when compared to injection of the same agents into the subcutaneous space, though this difference was not statistically significant at one‐ month follow‐up (Rocha 2014). The subcutaneous injection in this study was used as an active control condition, and might be expected to have systemic effects.
Carroll 2009 provided limited evidence that, compared with LASB alone, sympathetic blockade with botulinum toxin A added to local anaesthetic may prolong analgesia. Another single study, Rodriguez 2005, provided limited evidence to suggest that when added to usual physical therapy and pharmacological treatment, LASB may reduce the risk of relapse, but we found this study to be at high risk of bias across multiple domains; it did not report data for pain relief, and the lack of a sham condition raises the possibility that the observed improvement may have resulted from non‐specific effects. In contrast, Zeng 2003 found no benefit of adding cervical sympathetic blockade to usual comprehensive rehabilitative treatment for pain outcomes.
There is limited evidence that, compared with continuous infraclavicular brachial plexus blocks, continuous stellate ganglion LASB results in less relief in short‐term pain intensity, pain unpleasantness, deep pain and dull pain (Toshniwal 2012). The same study also provides limited evidence of no difference in longer‐term pain relief (up to four weeks) between groups (Toshniwal 2012).
Given the limited evidence available and the various sources of potential bias and uncertainty, we conclude that there is little credible evidence to support the use of LASB for CRPS and that the majority of the limited evidence available suggests that LASB may be ineffective.
Adverse events
The reporting of adverse events in the identified studies was inconsistent and limited. Given this lack of reporting and the small size of all of the included studies, we cannot confidently draw conclusions regarding the safety of LASB. While those adverse events that have been reported appear to be minor, it is not currently possible to rule out the potential for rare but serious adverse events. To obtain a better estimate of the incidence and nature of adverse events, it might be necessary to review evidence from non‐randomised observational study designs, but that was beyond the scope of this review.
Overall completeness and applicability of evidence
By undertaking a systematic search of unpublished and grey literature and consulting experts in the field, we have limited the risk of excluding important and relevant evidence. We judged all of the included studies as being at unclear risk of bias in at least one domain, reflecting a common lack of clarity in many study reports. We deemed three as being at high risk of bias specifically for the selective reporting of outcomes. This represents a significant challenge to a confident interpretation of an already limited evidence base.
We attempted to contact the authors of seven studies, with mixed success. Two responded and provided available data (Nascimento 2010; Toshniwal 2012). However, as we were unable to source two studies (Kostadinova 2012; Salinas Cerda 1997), did not receive a response from one to provide full data (Freitas 2013), and were unable to include two studies due to lack of clarity over whether the participant population overlapped with another included study (Rodriguez 2006; Rodriguez 2008), it is possible that we are missing relevant data.
Quality of the evidence
We did not judge any of the included studies to be at low risk of bias across all domains. Indeed, all but two studies carried an unclear risk of bias for random sequence generation and all but four for allocation concealment. These factors have been demonstrated to exaggerate the effects of studies, particularly those with subjective outcomes, such as pain (Schulz 1995; Wood 2008). We assessed all studies to be at high risk of bias for inadequate sample size and only four studies to be at low risk of bias for adequate duration of follow‐up. Small studies may well be underpowered to detect a clinical effect, but conversely there is empirical evidence that small published clinical trials in pain have a tendency to exaggerate treatment effects (Moore 2010; Nüesch 2010). These numerous sources of potential bias might alone explain any observed positive effects in the included studies. Thus, all of the evidence identified should be interpreted with caution.
Applying the GRADE approach, ratings across all comparisons were either low or very low quality except for the comparison of LASB versus placebo, which was of moderate quality. This rating is the result of the criteria we decided upon a priori when updating the searches. However, this moderate rating still merits some caution, since it is based on so few data. It is our view that for future updates, the rating of imprecision might be downgraded twice in the event that a comparison consists of fewer than 100 participants. Since each comparison consists of only one or two very small studies, and since all studies are at unclear or high risk of bias across various domains, it would be reasonable to characterise the entire body of included evidence as of low or very low quality.
Potential biases in the review process
While we have attempted to identify all eligible trials using a comprehensive search strategy, we may have still missed some key literature. Only three included studies used a positive response to a prior LASB to attempt to establish sympathetically maintained pain as part of their inclusion criteria (Carroll 2009; Price 1998; Rodriguez 2005). This speaks to a wider issue concerning the use of LASB in CRPS. It is possible that LASB might only be effective in a subgroup of people with CRPS with sympathetic dysfunction, or perhaps in people with other characteristics. However, to date evidence of predictors of a positive response to LASB are elusive (Sethna 2012).
The decision to exclude studies with only very short term follow‐up (≤ 48 hours) has led to the exclusion of studies that had been included in previous updates of this review. We took this decision on the basis that such studies are of more value in terms of investigating the diagnostic potential of LASB, which was not the purpose of our review. These studies do not provide clinically useful information in terms of treatment effectiveness over a reasonable period of time. This review focused on pain as a primary outcome and did not consider outcomes such as function or other clinical signs. However, LASB is commonly conducted with the primary goal of pain relief.
Agreements and disagreements with other studies or reviews
Our results do not change the overall conclusions of earlier versions of this review. Similarly a number of earlier systematic reviews have included evaluations of the evidence for LASB, and all have similarly agreed that the evidence is limited and that there is no clear evidence for the efficacy of LASB (Forouzanfar 2002; Perez 2010; Tran 2010). Van Eijs 2011 recommended that LASB be considered for the treatment of CRPS if conservative multidisciplinary management has failed. However, they rated the evidence for the effectiveness of CRPS as level 2B+, characterised as "multiple RCTs, with methodologic weaknesses, yield contradictory results better or worse than the control treatment. Benefits closely balanced with risk and burdens, or uncertainty in the estimates of benefits, risk and burdens." This classification of the evidence seems consistent with our own conclusions, though we feel this level of evidence precludes clinical recommendations.
Authors' conclusions
Implications for practice.
For people with CRPS
LASB is a treatment that may be offered for CRPS to help reduce pain and other symptoms. There is a scarcity of published evidence and a lack of high quality evidence to support or refute its effectiveness, though the available evidence is not encouraging. Due to the scarcity of evidence it is not possible to draw confident conclusions about the safety of LASB. People should consider this information when deciding whether to agree to receive the treatment.
For clinicians
There is a scarcity of published evidence and a lack of high quality evidence to support or refute its effectiveness, though the available evidence is not encouraging. One study, judged to be at high risk of bias, provides very low quality evidence that LASB may reduce the risk of recurrence of pain when added to rehabilitation and standard pharmacological care, and one study, also judged to be at high risk of bias, suggests that thoracic sympathetic block with local anaesthetic and corticosteroid may be effective. However, on the basis of such evidence it is not possible to make any clinical recommendations. Due to the scarcity of evidence it is not possible to draw confident conclusions about the safety of LASB.There is currently little credible evidence to support the use of LASB for CRPS.
For policy makers and funders
The available evidence relating to the effectiveness of LASB for CRPS is not compelling. While there is substantial uncertainty regarding the effectiveness of alternative therapeutic options (O'Connell 2013), it is not clear that investment in this procedure provides clinical value.
Implications for research.
General
If LASB is to continue to be offered to people with CRPS, there is a clear need for further, better quality research into its efficacy. It seems likely that the best chance of delivering high quality trials is through multicentre, collaborative research projects that can recruit from larger clinical populations. While many studies investigate the effect of adding therapeutic agents to LASB, there remains substantial uncertainty regarding the efficacy of simple local anaesthetic blockade for CRPS.
Design
Reducing this uncertainty requires adequately powered trials that utilise placebo controls, ensure adequate blinding and confirm the technical adequacy of the block. Future trials should use established diagnostic criteria and clearly report the type of CRPS under investigation. Trials should also consider the IMMPACT recommendations for the design of trials in pain to ensure that outcomes, thresholds for clinical importance and study designs are optimal (Dworkin 2008; Dworkin 2009; Dworkin 2010; Turk 2008a; Turk 2008b).
Measurement (endpoints)
Future trials should measure both immediate pain relief and long‐term (≥ 6 month) outcomes from LASB. Furthermore, future trials should adhere to the CONSORT guidance on standards of reporting and should clearly report all adverse events (Altman 2012).
What's new
| Date | Event | Description |
|---|---|---|
| 22 March 2021 | Review declared as stable | See updated Published notes. |
History
Protocol first published: Issue 1, 2004 Review first published: Issue 4, 2005
| Date | Event | Description |
|---|---|---|
| 28 July 2016 | Review declared as stable | See Published notes. |
| 7 March 2016 | New citation required but conclusions have not changed | The conclusions of the review remain unchanged. |
| 16 October 2015 | New search has been performed | This updated review used refined exclusion criteria (exclude studies with follow‐up of <48hrs) (see Differences between protocol and review). This resulted in the exclusion of 4 studies from this update that had been included in previous versions of this review (Meier 2009; Raja 1991; Verdugo 1995; Wehnert 2002). We also updated the data analysis that included consideration of the minimally important difference (as per OMERACT 12 group recommendations) and evaluation of the quality of evidence using the GRADE approach. |
| 26 June 2013 | New search has been performed | This updated review used an expanded search strategy, updated Risk of Bias assessment, and updated inclusion criteria. These changes resulted in inclusion of 10 additional studies compared with the initial review (n = 363 additional participants); two studies compared LASB to a placebo/inert treatment (Aydemir 2006; Price 1998), the remaining nine studies compared LASB with an active treatment [Bonelli 1983; Carroll 2009; Meier 2009; Nascimento 2010; Raja 1991; Toshniwal 2012; Wehnert 2002; Zeng 2003) or investigated the effect of adding LASB to an active treatment (Rodriguez 2005). Despite these methodological updates and inclusion of new studies, the conclusions of the review remain unchanged; there is a dearth of published evidence for LASB and the available evidence suggests lack of efficacy. Readers of the original review would benefit from reading this update as new evidence is provided for treatment comparisons between LASB and other active interventions (for example, intravenous regional anesthesia). |
| 26 June 2013 | New citation required but conclusions have not changed | Despite methodological updates and inclusion of new studies, the conclusions of the review remain unchanged; there is a dearth of published evidence for LASB and the available evidence suggests lack of efficacy. |
| 3 October 2011 | Amended | The following changes have been made to the methodology of the protocol. We have made them all to bring the protocol up to date with the current PaPaS author guidelines: We have chosen to adopt a modified version of the Cochrane ROB tool with additional criteria added in response to the recommendations of Moore et al. (2010). As such we have added 2 additional criteria “Size” and “Duration” using the thresholds for judgement suggested by Moore 2010. We have not added the “Outcome” criteria as this is covered already by our choice of primary outcome measures. We have rewritten the data synthesis/ analysis sections to fit the current RevMan headings. We now specify that we will calculate Risk Ratio for achieving a moderately important benefit (30% or more) or a substantially important benefit (50% or more) and have specified time windows for short, medium and long term follow up. We suggest the following preplanned subgroup analyses where adequate data allow: CRPS I vs II, Adults vs children and single vs continuous blockade. We have added a planned sensitivity analyses, where data are sufficient, to allow testing of the effect of including/ excluding studies whose risk of bias is unclear or high. |
| 3 October 2011 | Amended | The Background section has been substantively rewritten to fit the headings now suggested in RevMan. |
| 22 September 2011 | Amended | Searching other resources ‐ unpublished studies: We have expanded this search strategy to also include clinical and controlled trial registers, such as http://www.controlledtrials.com/, the Australian New Zealand Clinical Trials Register (http://www.anzctr.org.au/), and a European Clinical Trials Register (https://www.clinicaltrialsregister.eu/). |
| 21 September 2011 | Amended | We have inserted a new Table (under other Tables) that provides the new Budapest criteria for diagnosing CRPS. |
| 21 September 2011 | Amended | We have added new search terms to the search strategy that will make it more sensitive and conforms to updates in treatment (for example, Botox now being used for sympathetic chain blockades). Also attached is an updated search strategy for Medline, created in collaboration with Jane Hayes from PaPaS. |
| 21 September 2011 | Amended | Methods: selection of studies. Two independent reviewers will screen the titles and abstracts of the search results in order to determine which full text articles to retrieve. This is changed from one reviewer. |
| 21 September 2011 | Amended | Addition of new criteria for considering studies for this review (Types of interventions). |
| 21 September 2011 | Amended | Addition of new criteria for considering studies for this review (Types of participants). |
| 9 November 2009 | Amended | Contact details updated. |
| 30 October 2008 | Amended | Converted to new review format. |
Notes
Assessed for updating in 2016
A new search within two years is not likely to identify any potentially relevant studies likely to change the conclusions. Therefore, this review has now been stabilised following discussion with the authors and editors. The review will be re‐assessed for updating in five years. If appropriate, we will update the review before this date if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Assessed for updating in 2021
In March 2021 we did not identify any potentially relevant studies likely to change the conclusions. Therefore, this review has now been stabilised following discussion with the authors and editors. The review will be reassessed for updating in two years. If appropriate, we will update the review before this date if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Please note that Neil O'Connell is the PaPaS Co‐ordinating Editor and he was not involved in the editorial assessment or decisions when considering this review for updating; we thank the Editors Dr Peter Cole and Professor Christopher Eccleston for their input.
Acknowledgements
We thank the Pain, Palliative and Supportive Care Review Group for running the searches and supporting the review process.
We would also like to thank Arturo Lawson, Murat Dalkilinc, Luciana Macedo, Ann Meulders, Eric Parent, Andrea Wand, Eva Bosch and Ein‐Soon Shin for their assistance in interpreting non‐English language trials.
Cochrane Review Group funding acknowledgement: The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane PaPaS Group. Disclaimer: The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, National Health Service (NHS) or the Department of Health.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Complex Regional Pain Syndromes] explode all trees
#2 complex regional pain syndrome
#3 reflex sympathetic dystrophy
#4 reflex neurovascular dystrophy
#5 (RSD or RND)
#6 shoulder hand syndrome
#7 algoneurodystrophy
#8 algodystrophy
#9 sudeck*
#10 causalgia
#11 (sympathetic* near/3 pain*)
#12 SMP
#13 ((posttraumatic or post‐traumatic) next dystrophy)
#14 neuralgia
#15 MeSH descriptor: [Neuralgia] explode all trees
#16 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15
#17 MeSH descriptor: [Sympatholytics] explode all trees
#18 MeSH descriptor: [Nerve Block] explode all trees
#19 MeSH descriptor: [Anesthetics, Local] explode all trees
#20 bupivacaine
#21 lidocaine
#22 guanethidine
#23 (nerve* near/5 block*)
#24 (stellate near/5 block*)
#25 (sympathetic* near/5 block*)
#26 sympatholytic*
#27 (local near/5 (anaesthetic* or anesthetic*))
#28 #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27
#29 #16 and #28
Appendix 2. MEDLINE search strategy
1 exp Complex Regional Pain Syndromes/
2 complex regional pain syndrome.mp.
3 CRPS.mp.
4 reflex sympathetic dystrophy.mp.
5 reflex neurovascular dystrophy.mp.
6 (RSD or RND).mp.
7 shoulder hand syndrome.mp.
8 algoneurodystrophy.mp.
9 algodystrophy.mp.
10 sudeck*.mp.
11 causalgia.mp.
12 (sympathetic* adj3 pain*).mp.
13 SMP.mp.
14 ((posttraumatic or post‐traumatic) adj dystrophy).mp.
15 neuralgia.mp. or exp Neuralgia/
16 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15
17 exp Sympatholytics/
18 exp Nerve Block/
19 exp Anesthetics, Local/
20 bupivacaine.mp.
21 lidocaine.mp.
22 guanethidine.mp.
23 (nerve* adj5 block*).mp.
24 (stellate adj5 block*).mp.
25 (sympathetic* adj5 block*).mp.
26 sympatholytic*.mp.
27 (local adj5 (anaesthetic* or anesthetic*)).mp.
28 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27
29 16 and 28
30 randomized controlled trial.pt.
31 controlled clinical trial.pt.
32 randomized.ab.
33 placebo.ab.
34 drug therapy.fs.
35 randomly.ab.
36 trial.ab.
37 groups.ab.
38 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37
39 29 and 38
Appendix 3. EMBASE search strategy
1 exp Complex Regional Pain Syndromes/
2 complex regional pain syndrome.mp.
3 CRPS.mp.
4 reflex sympathetic dystrophy.mp.
5 reflex neurovascular dystrophy.mp.
6 (RSD or RND).mp.
7 shoulder hand syndrome.mp.
8 algoneurodystrophy.mp.
9 algodystrophy.mp.
10 sudeck*.mp.
11 causalgia.mp.
12 (sympathetic* adj3 pain*).mp.
13 SMP.mp.
14 ((posttraumatic or post‐traumatic) adj dystrophy).mp.
15 neuralgia.mp. or exp Neuralgia/
16 or/1‐15
17 exp Sympatholytics/
18 exp Nerve Block/
19 exp Anesthetics, Local/
20 bupivacaine.mp.
21 lidocaine.mp.
22 guanethidine.mp.
23 (nerve* adj5 block*).mp.
24 (stellate adj5 block*).mp.
25 (sympathetic* adj5 block*).mp.
26 sympatholytic*.mp.
27 (local adj5 (anaesthetic* or anesthetic*)).mp.
28 or/17‐27
29 16 and 28
30 random$.tw.
31 factorial$.tw.
32 crossover$.tw.
33 cross over$.tw.
34 cross‐over$.tw.
35 placebo$.tw.
36 (doubl$ adj blind$).tw.
37 (singl$ adj blind$).tw.
38 assign$.tw.
39 allocat$.tw.
40 volunteer$.tw.
41 Crossover Procedure/
42 double‐blind procedure.tw.
43 Randomized Controlled Trial/
44 Single Blind Procedure/
45 or/30‐44
46 (animal/ or nonhuman/) not human/
47 45 not 46
48 29 and 47
Appendix 4. LILACS search strategy
"complex regional pain syndrome" or CRPS or "reflex sympathetic dystrophy" or "reflex neurovascular dystrophy" or RSD or RND or "shoulder hand syndrome" or algoneurodystrophy or algodystrophy or sudeck$ or causalgia or sympathetic pain$ or SMP [Words] and bupivacaine or lidocaine or guanethidine or (nerve$ block$) or (stellate block$) or (sympathetic$ block$) or sympatholytic$ or (local anaesthetic$) or (local anesthetic$) [Words]
Appendix 5. GRADE judgements by comparison
| Comparison | Studies | N for comparison | Result | GRADE CRITERIA | RATING | ||||
| LIMITATIONS | INCONSISTENCY | INDIRECTNESS | IMPRECISION | PUBLICATION BIAS | |||||
| LASB vs placebo |
Price 1998 Aydemir 2006 |
23 | No difference | — | — | — | X | — | Moderate |
| Thoracic LASB + steroid vs subcutaneous | Rocha 2014 | 36 | 1 month –clinically important difference (not sig). | X | X | — | X | — | Very low |
| 1 year clinically important difference (sig) | X | X | — | X | — | ||||
| LASB vs ultrasound block | Aydemir 2006 | 18 | No difference | — | X | — | X | — | Low |
| LASB vs IVRB guanethidine | Bonelli 1983 | 19 | No difference | X | X | — | X | — | Very low |
| LASB vs BTA | Carroll 2009 | 9 | Increased duration of relief with BTA | X | — | X | — | Low | |
| LASB vs pulsed radiofrequency | Freitas 2013 | 40 | No difference | X | X | — | X | — | Very low |
| LASB vs oral corticosteroids | Lim 2007 | 38 | No difference | X | X | X | X | — | Very low |
| LASB vs IVRB (both lidocaine + clonidine) | Nascimento 2010 | 43 | No difference | X | X | — | X | — | Low |
| LASB + PT + pharma vs PT + pharma | Rodriguez 2005 | 82 | Favours SGB | X | X | — | X | — | Very low |
| LASB+ vs PT | Zeng 2003 | 60 | No difference | X | X | — | X | — | Very low |
| Continuous LASB vs continuous brachial plexus block | Toshniwal 2012 | 33 | Favours brachial plexus block | — | X | — | X | — | Low |
| Image‐guided LASB vs nonimage‐guided | Yoo 2012 | 42 | No difference | X | X | X | X | — | Very low |
X = downgrade on this criteria; BTA: botulinum toxin A; IVRB: intravenous regional blockade; LASB: local anaesthetic sympathetic blockade; PT: physical therapy; SGB: stellate ganglion blockades.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aydemir 2006.
| Study characteristics | ||
| Methods | RCT, parallel design | |
| Participants | N = 25; divided into 3 groups (group 1: n = 9; group 2: n = 9; group 3: n = 7) Mean age (SD): Group 1: 21.9 years (1.05) Group 2: 21.4 years (0.73) Group 3: 21.1 years (0.38) Sex: not reported. Upper limb CRPS‐I (dominant arm: group 1, n = 6; group 2, n = 9; group 3, n = 2); excluded if had SGB block in last month. Diagnostic criteria: IASP (Bruehl 1999) Duration of symptoms: Group 1 (0‐3 months, n = 5; 3‐6 months, n = 2; > 6 months, n = 2) Group 2 (0‐3 months, n = 6; 3‐6 months, n = 2; > 6 months, n = 1) Group 3 (0‐3 months, n = 5; 3‐6 months, n = 0; > 6 months, n = 2) Type of initiating injury: Group 1 (trauma, n = 7; fracture, n = 2; idiopathic, n = 0) Group 2 (trauma, n = 2; fracture, n = 5; idiopathic, n = 2) Group 3 (trauma, n = 3; fracture, n = 4; idiopathic, n = 0) Concomitant treatments: all groups received 21 sessions of physiotherapy, which involved exercises, contrast baths, transcutaneous electrical nerve stimulation (TENS), and pneumatic compression. If necessary, all groups had access to medical treatment, which involved 500 mg oral paracetamol pill, maximum of six tablets (3 g) per day. Medicolegal factors: not reported Previous treatment: not reported |
|
| Interventions | Group 1: stellate ganglion lidocaine block (real) plus sham stellate ganglion ultrasound block Group 2: stellate ganglion ultrasound block (real) plus sham stellate ganglion lidocaine block Group 3: sham stellate ganglion lidocaine block and sham stellate ganglion ultrasound block For the purpose of this review, we included comparisons between group 1 and group 3 as placebo‐controlled and comparisons of group 1 and group 2 as comparison with another active intervention. SGB lidocaine (real): Location: C6 level; 1.5 cm lateral to central line, 4‐5 cm deep Dosage: 10 ml of 1% lidocaine Number of blocks performed: 10 Eval. of technical adequacy of block: No. SGB (sham): Identical site, but injected 10 ml of saline. Number of blocks not reported. SGB ultrasound (real): Probe size of 1 cm2; 5 min of intermittent ultrasound at 3 watt/cm2 over the affected site (over stellate ganglion) Number of treatments not reported SGB ultrasound (sham): 5 min, no energy delivered Number of treatments not reported |
|
| Outcomes | Spontaneous pain: 0‐10cm visual analogue scale Outcomes measured pretreatment, post‐treatment, and at one‐month follow‐up Adverse events/side effects not reported |
|
| Country of origin | Turkey | |
| Study aim | To investigate the efficacies of stellate ganglion blockage (SGB) with lidocaine and ultrasound in CRPS | |
| Notes | This study was translated and interpreted by a researcher fluent in Turkish. The study author, TS, worked with the researcher to fully interpret and score. Conflict of interests not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized" Comment: Method of randomisation not reported |
| Allocation concealment (selection bias) | Low risk | Envelope method used to conceal allocation; group assignment generated by an independent person |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double blind" Comment: reported that participants and personnel blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Reported that outcome assessors blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Drop‐outs/withdrawals not reported |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were adequately reported on |
| Adequate sample size? | High risk | Group 1, n = 9; group 2, n = 9; group 3, n = 7 |
| Adequate duration of follow‐up? | Unclear risk | One‐month follow‐up |
| Free of other bias? | Low risk | Pain scores were not significantly different between groups at baseline; identical timing of outcome assessment between groups |
Bonelli 1983.
| Study characteristics | ||
| Methods | RCT, parallel design | |
| Participants | N = 19 Mean age (SD): 52.33 years (5.04) Sex: not reported Diagnosis of RSD following peripheral nerve injury At least 3 of the following clinical signs: hyperpathia, allodynia, vasomotor disturbance, trophic signs, oedema, limited motion Mean (SD) duration of pain: Stellate ganglion block group: 6.55 (3.94) months IVRB guanethidine group: 17.55(14.9) months Previous treatment not reported; concomitant treatment not reported 2 lost to follow‐up at 3 months in SGB group Baseline pain (0‐100 scale) mean (SD): Stellate ganglion block group: 70.5 (27.36) IVRB guanethidine group: 65 (25.46) Medico‐legal factors: not reported |
|
| Interventions | SGB (n = 10) versus IVRB guanethidine block (n = 9); treatment period of 16 days SGB: bupivacaine (0.5%) 15 ml No. of blocks: 8 (1 every other day for 16 days) Evaluation of adequacy of block? Skin temperature, plethysmographic wave IVRB guanethidine (20 ml), heparin (500 μl), isotonic saline (25 ml) No. of blocks: 4 (every 4 days) |
|
| Outcomes | Pain: 100 mm linear scale (specific details not reported) Pain measured at baseline and post‐treatment at 15 min, 60 min, 24 h, 48 h, 1 month and 3 months Adverse events only mentioned in discussion (alludes to none in either group) |
|
| Country of origin | Italy | |
| Study aim | To compare the effects of regional IVRB with guanethidine with stellate ganglion blocks in people with severe RSD following peripheral nerve injury of the upper limb | |
| Notes | Conflicts of interest not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomly allocated to two groups" Comment: Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participant blinding not reported. The interventions are likely to be distinguishable. Unsure if participants aware of study hypothesis. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data post‐treatment and one‐month follow‐up. 3 months: 2/10 missing from SGB group, no missing data for IVRB group |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in methods were reported in the results |
| Adequate sample size? | High risk | N = 19; n = 10 stellate ganglion block group, n = 9 regional IV guanethidine |
| Adequate duration of follow‐up? | Low risk | Follow‐up of 3 months |
| Free of other bias? | High risk | At baseline, SGB group had a significantly shorter duration of symptoms than the IVRB guanethidine group (mean (SD): 6.55 (3.94) months versus 17.55 (14.9) months; P < 0.05) and were significantly older (mean (SD): 52.33 (5.04) years versus 42.77 (4.65) years; P < 0.01). |
Carroll 2009.
| Study characteristics | ||
| Methods | RCT cross‐over | |
| Participants | N = 9 Age mean (range) 49.4 (38‐67) Sex: 1 male Lower limb CRPS‐I, with duration of pain of at least 6 months, spontaneous pain rating > 6/10, unsuccessful therapy with at least 2 non‐opioid medications (for neuropathic pain), at least a 50% reduction in pain for > 5 h but < 2 weeks from a previous lumbar sympathetic injection Inciting events: tarsal tunnel surgery n = 1, bunionectomy/cast n = 1, crush injury n = 1, plantar fasciectomy n = 1, foreign body removal n = 1, ankle arthroscopy n = 1, ankle fracture/cast n = 1, metatarsal fracture n = 1, back surgery n = 1 Diagnostic criteria: IASP (Merskey 1994) Medico‐legal factors: not reported Mean duration of pain (range): 3.8 years (2‐14) Baseline mean pain levels, 10 cm VAS (range): 7.2 cm (4.7‐8.9) Concomitant treatments: not reported but participants asked not to cease existing therapies, but not to start new therapies during study period |
|
| Interventions | Lumbar sympathetic blocks: Anterolateral border of L2 vertebral body, fluoroscopy guided Active: 10 ml of 0.5% bupivacaine with an added 75 units botulinum toxin A Control: 10 ml of 0.5% bupivacaine |
|
| Outcomes | Primary outcome: time to analgesic failure (time for pain to return to baseline level) Daily pain intensity ‐ 10 cm VAS, measured for 7 days prior to first injection and recorded daily until participants reported their pain returned to baseline or 1 month (whichever was longer) Adverse events reported |
|
| Country of origin | USA | |
| Study aim | To determine whether adding BTA to lumbar sympathetic blockade increases the duration of analgesia | |
| Notes | Authors declared that they had filed a patent for BTA in sympathetic blocks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned to which injection they received first" Comment: method of randomisation not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "All physicians and patients involved in the study were blinded to which injection contained botulinum toxin A. Data were not unblinded for any patient until the study was completed, and no interim analyses were performed"; "in the crossover injection, the patient received an identical injection" Comment: injections were identical and participants blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: as above Comment: self reported outcomes and participants were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2/9 participants did not complete the study (one due to technical issues related to the block ‐ malpositioning of the needle ‐ and one because outcome forms were not returned). Only 1 participant received BTA first, and this participant dropped out. Due to complete blinding and use of a cross‐over study design, the effect that this drop‐out has on the results is unclear. |
| Selective reporting (reporting bias) | Unclear risk | Full data not presented for the secondary end point (VAS pain scores over time); comparison of within‐injection group change over time provided, but comparison of between‐injection group differences not provided. |
| Adequate sample size? | High risk | N = 9 |
| Adequate duration of follow‐up? | Unclear risk | Quote: "Patients continued to record daily VAS until they reported their pain had returned to baseline or 1 month, whichever was longer" Comment: Follow‐up was observed until pain returned to baseline levels ‐ for some this was only 4 weeks |
| Free of other bias? | Low risk | Cross‐over study design ensured similarity between groups; participants allowed to continue current medications but could not start new medications. Quote: "patients were eligible for their crossover injection 1 month after they reported their pain had returned to baseline" Comment: 1 month washout period observed, after pain had returned to baseline levels |
Freitas 2013.
| Study characteristics | ||
| Methods | RCT parallel | |
| Participants | N = 40 (though adverse event reporting implies 20 and no CONSORT flowchart presented). Presume 20 per group. Age: not reported Sex: not reported Lower limb CRPS‐I. Diagnostic criteria: IASP Bruehl 1999 Duration of pain prior to block: > 6 months Type of initiating injury: not reported Concomitant treatments: not reported Medico‐legal factors: excluded if pending litigation Previous treatment: unresponsive to medication and physiotherapy (such as oral gabapentin 2400 mg/d, oral amitriptyline 100 mg/d, and oral carbamazepine 1000 mg/d and physiotherapy for more than 6 months |
|
| Interventions | Group 1: LASB lumbar plexus sympathetic block L2‐3 and L3‐4. 15 ml lidocaine and 100 mcg clonidine at each level Group 2. Pulsed radiofrequency (PRF) lumbar plexus L2‐3 and L3‐4, x3 120sec cycles at each level at 42º C. 1 ml of 2% lidocaine injected at each level Evaluation of technical adequacy of blocks: no Number of blocks: 1 per site, on 1 occasion Concomitant treatments: not reported |
|
| Outcomes | Pain intensity VAS, neuropathic pain scale. No numerical data provided. SF‐36. Means reported but no measures of variance Statistically significant difference seen in "hot pain" at "final score" but this time point is not clearly defined ‐ likely 6 months. Adverse events: "2 out of 10" had paraesthesia following PRF. "1 out of 10" had paraesthesia following LASB. Note; this implies 20 rather than 40 participants. |
|
| Country of origin | Brazil | |
| Study aim | To determine whether percutaneous PRF applied directly to the sympathetic lumbar plexus was more effective than lumbar sympathetic blocks, and, if so, whether this could be achieved without the risks associated with traditional ablative procedures | |
| Notes | Conflicts of interest not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomized to either PRF or sympathetic lumbar block according to computer generated random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information given |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: Personnel not blinded however, comparison is between two active invasive interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: Participants blinded to group assignment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 1 participant in pulsed radiofrequency group dropped out ‐ reasons unclear and unclear whether data missing or excluded |
| Selective reporting (reporting bias) | High risk | Pain outcome data are not reported in numerical format |
| Adequate sample size? | High risk | N = 40 entered the study but reporting of adverse events suggests 20 participants |
| Adequate duration of follow‐up? | Low risk | 6‐month follow‐up |
| Free of other bias? | Unclear risk | No baseline data presented; no detail given regarding concomitant treatments |
Lim 2007.
| Study characteristics | ||
| Methods | Parallel RCT | |
| Participants | N = 38 Mean age (SD): Group 1: 61.4 years (10.1) Group 2. 58.7 years (12.1) Sex: 21 female, 17 male Upper Limb CRPS poststroke Diagnostic criteria: unclear though described a "three phase bone scan test" Duration of symptoms: not reported Type of initiating injury: stroke. Concomitant treatment: conservative physical therapy including passive joint movements Medico‐legal factors: not reported Previous treatment: not reported |
|
| Interventions | Group 1: LASB stellate ganglion, lidocaine Dosage: 10 ml 0.5% lidocaine Number of blocks: 5 blocks: 1 every 3 days for 15 days Evaluation of technical adequacy of block: not reported Group 2: oral corticosteroids for 14 days. 60 mg of oral prednisolone was administered in two 30mg doses depending on the hormone cortisol rhythm on days 1‐3, and applied 40 mg on days 4‐6, 30 mg on days 7‐9, and 20 mg on days 10‐12. Single dose of 10 mg was administered on days 13‐14. Oral administration was stopped after 14 days. |
|
| Outcomes | 0‐3 scale hand pain with passive movement or palpation. Measured at 15 and 30 days from start of treatment | |
| Country of origin | Korea | |
| Study aim | To compare the therapeutic effects between stellate ganglion block and steroid therapy in poststroke complex regional pain syndrome | |
| Notes | Adverse events not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no details reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Personnel not blinded. Non‐invasive control qualitatively different to active intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: Inadequate blinding of participants |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no information reported on attrition |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes adequately reported |
| Adequate sample size? | High risk | Comment: N = 38 |
| Adequate duration of follow‐up? | Unclear risk | Comment: 30 days follow‐up |
| Free of other bias? | Low risk | Comment: no other bias detected |
Nascimento 2010.
| Study characteristics | ||
| Methods | RCT, parallel | |
| Participants | N = 43 Mean age (range): Group 1: 37.7 years (27‐54) Group 2: 38.6 years (25‐50) Group 3: 39.0 years (27‐50) Sex: Group 1 (n = 14): 1 male Group 2 (n = 15): 1 male Group 3 (n = 14): 1 male Upper extremity CRPS‐I Diagnostic criteria: IASP (Merskey 1994) Mean duration of pain (range): Group 1: 24.2 months (3‐72) Group 2: 24.2 months (8‐72) Group 3: 22.3 months (2‐48) Mean baseline pain intensity (SE), 10 cm VAS scale: Group 1: 8.7 cm (0.3) Group 2: 8.7 cm (0.3) Group 3: 8.3 cm (0.3) Inciting event: Repetitive strain injury n = 18, carpal tunnel syndrome n = 11, late postsurgical pain n = 8, fracture and long‐lasting immobilisation n = 3, stab wound n = 2, unknown n = 1 Previous treatment for CRPS: unsuccessful use of tricyclic antidepressants, gabapentin, opioids or anti‐convulsants At admission all free of drugs. Medico‐legal factors: not reported |
|
| Interventions | Group 1: SGB, anterior paratracheal approach, fluoroscopy‐guided, 70 mg 1% lidocaine Group 2: SGB, identical approach,70 mg 1% lidocaine + 30 μg clonidine Group 3: IVRB 7.0 ml solution 1% lidocaine with 1 μg/kg clonidine. Tourniquet pressure released 30 min later. Evaluation of adequacy of block? yes, temperature checked No. of blocks: 5, x 1 weekly |
|
| Outcomes | Pain intensity 0‐10 cm VAS (anchors "no pain" to "worst pain imaginable") Pain was measured immediately before and soon after the end of each procedure. Pain intensity scored daily. Pain measured one week after the last procedure. Duration of analgesia calculated as the interval between the end of the procedure and the time at which VAS ≥ 3 was recorded. Adverse events reported |
|
| Country of origin | Brazil | |
| Study aim | To compare the efficacy of IVRB produced by combining lidocaine with clonidine, to that of SGB produced by the injection of lidocaine, alone or combined with clonidine, into the stellate ganglion, for the management of pain in people with upper extremity CRPS‐I | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned to one of three experimental groups" Comment: method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated that study was blinded nor whether participants were blind to the study hypotheses |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Side effects and effectiveness of treatment were recorded by another author who was unaware of the procedure" Comment: while side effects and effectiveness were recorded by a blinded assessor, the use of self reported outcome in participants who may not have been blind to the study hypothesis is a possible risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Excluded 2/45 with clear reasons and clear n reported for all graphs |
| Selective reporting (reporting bias) | Low risk | Adequately reported results for all prespecified outcomes (from methods section) |
| Adequate sample size? | High risk | n = 45; 15 participants per treatment group |
| Adequate duration of follow‐up? | High risk | 1 week follow‐up |
| Free of other bias? | Low risk | No differences between groups for important prognostic factors, participants not taking any medication at inclusion, outcome assessment timing identical between groups |
Price 1998.
| Study characteristics | ||
| Methods | Quasi‐randomised controlled trial (cross‐over) | |
| Participants | N = 7 (3 lower extremity, 4 upper extremity pain) Mean age: 42 years (SD 11; range: 32‐52) Sex: 3 male CRPS‐I or ‐II of upper or lower extremities (excluded if CRPS in multiple areas) Diagnostic criteria: IASP (Merskey 1994) Mean duration of symptoms: 3 years (SD 2 years; range 18 months to 7 years) Inciting event: trauma (n = 6), surgical (n = 1) Medico‐legal factors: not reported Previous treatment: not reported Concomitant treatment: all participants continued concomitant physical therapy and medications |
|
| Interventions | Active condition: SGB with lidocaine (15 ml of lidocaine 1%) Lumbar sympathetic blockade with 15 ml 1% lidocaine (test solution) followed by 10 ml bupivacaine 0.125% Evaluation of technical adequacy of block? yes ‐ evaluated Horner's syndrome and surface skin temperature for stellate ganglion blocks. Nothing reported for lumbar blocks. Control condition: Stellate ganglion: 15 ml saline Lumbar: 15 ml saline + 10 ml saline The blocks were separated by a period of 7‐10 days Number of blocks: 1 for each condition |
|
| Outcomes | Pain intensity and pain unpleasantness (0‐10 VAS) Pain outcomes measured every 15 min for 1.5 h prior to injection and every 15 min for 1 h following injection. Pain outcomes then rated 4 times a day (morning, midday, afternoon, evening) for 7 days postinjection Time to peak analgesia measured as the VAS unit difference between pre‐injection baseline pain rating and the lowest VAS rating in the first hour Duration of pain relief measured as the time it took for pain intensity to return to 50% of the difference between baseline and peak analgesic effect |
|
| Country of origin | USA | |
| Study aim | To evaluate the diagnostic and therapeutic value of local anaesthetic sympathetic blocks | |
| Notes | Author confirmed the quasi‐random allocation in correspondence; adverse events not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Four patients received S first with LA block second, and the order was reversed for the remaining 3 patients" Comment: quasi‐random process used, not truly random. Due to cross‐over study design and successful blinding, we feel this presents an unclear risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Both the patient and the physician administering the sympathetic ganglia injections were blind with regard to the contents of the injecting syringe (S or LA) and with regard to whether skin surface temperature changes or Horner's syndrome occurred"; "The syringe was filled. . . by a third person who maintained the code for the contents of the syringes and the double‐blind nature of the study"; "None of the 7 patients reported subjective differences between effects of S and LA blocks within the first hour after block. However, 2 patients correctly determined that they had received S injection because of the shorter duration of relief received." Comment: blinding completed and blinding success was formally assessed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were self rated and participants were blinded to treatment group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2/7 participants missed all pain unpleasantness data but pain intensity data complete |
| Selective reporting (reporting bias) | High risk | Group results for pain unpleasantness scores not reported |
| Adequate sample size? | High risk | N = 7 |
| Adequate duration of follow‐up? | High risk | 7 days follow‐up |
| Free of other bias? | Low risk | Quote: "Medication use and physical therapy were as similar as possible for the time periods following both saline and lidocaine blocks, and medications were not, as a rule, significantly adjusted during the study period." Comment: also, cross‐over study design ensured similarity between groups for important outcomes and outcome assessment at same time periods Procedures separated by 7‐ ‐ 10 days. Figures illustrate pain returned to baseline levels prior to next block. |
Rocha 2014.
| Study characteristics | ||
| Methods | Parallel RCT | |
| Participants | N = 36 Mean age (SD): Group 1: 42 years (13.5) Group 2: 44.4 years (8.9) Sex: 19 female, 17 male Upper limb CRPS Diagnostic criteria: IASP (Merskey 1994) then switched to IASP (Harden 2010). Participants screened under old criteria were then excluded. Duration of symptoms (months): Group 1: 22.7 (26.3) Group 2: 21.0 (2.16) Type of initiating injury: mixed: fractures, contusions, surgery, work‐related Concomitant treatments: unclear Previous treatment: 4 week standardised multimodal protocol including physical therapy, oral analgesic polytherapy: antidepressants, analgesics, opioids, gabapentin and psychological input. Medico‐legal factors: not reported |
|
| Interventions | Group 1: LASB T2 sympathetic ganglion (fluoroscopically guided) Group 2: subcutaneous space injection of same agents (fluoroscopy also used) Agents: 10 ml anaesthetic + steroid. 5 ml 75% ropivacaine, 5 ml triamcinolone Evaluation of technical adequacy of block: yes ‐ measurement of arm temperature Number of blocks: 1 |
|
| Outcomes | Average pain score from Brief Pain Inventory McGill Pain Questionnaire Adverse events 1 month and 1 year follow‐up |
|
| Country of origin | Brazil | |
| Study aim | To evaluate the efficacy of TSB for upper limb CRPS‐I | |
| Notes | Authors declare no conflict of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no details reported |
| Allocation concealment (selection bias) | Low risk | Comment: participants asked to pick an unmarked opaque envelope containing allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: Personnel not blinded however, comparison is between two active invasive interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: assessor blinded to intervention |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: 21% attrition at long‐term follow‐up and imbalanced across groups. No details provided regarding reasons for loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes reporting adequate |
| Adequate sample size? | High risk | Comment: N = 36 |
| Adequate duration of follow‐up? | Low risk | Comment: 1‐year follow‐up |
| Free of other bias? | High risk | Comment: average pain scores at baseline differ by more than 1 point |
Rodriguez 2005.
| Study characteristics | ||
| Methods | RCT, parallel design | |
| Participants | N= 82 Mean age (no SDs provided): Group 1: 44.1 years Group 2: 46.1 years, Sex: Group 1: 36.6% male Group 2: 46.3% male Upper limb CRPS (type I or type II) with presence of pain mediated by the sympathetic nervous system (defined as a decrease in resting pain by 50% with a stellate ganglion block) Diagnostic criteria: IASP (Merskey 1994; Reinders 2002) Inciting event: 71.4% of CRPS cases were secondary to accidental or violent trauma and 18% occurred following surgical procedures. Mean duration of symptoms (no SDs provided): Group 1: 253.7 days Group 2: 213.4 days Medico‐legal factors: Group 1: 14.6% had a compensation claim Group 2: 24.4% had a compensation claim Previous treatment: participants were excluded if they had previous stellate ganglion blocks; no other previous treatment reported Concomitant treatment: Group 1 also received physical therapy and pharmacological treatment |
|
| Interventions | Group 1: SGB, physical therapy and pharmacological treatment Site of block: paratracheal at the height of the cricoid cartilage Number of blocks: 5 Type of substance injected: 10 cc of volume with equal parts of 2% lidocaine and 0.5% bupivacaine Evaluation of technical adequacy: increase in temperature of at least 1° C of the hand and face (affected side) and the presence of Horner's syndrome (ptosis of the upper eye lid and conjunctivitis) Group 2: Physical therapy and pharmacological treatment only(Control group): Received physical therapy and pharmacological treatment |
|
| Outcomes | Pain intensity (VAS). Measured at baseline, 1 month and 2 months. Exact follow‐up time appears to be variable among participants (i.e. followed for more than 2 months in some). Therapeuctic efficacy: number of participants with at least 50% reduction in the pain. Measured at 2 months postintervention. Efficacy: (incidence of pain in control group − incidence of pain in the SGB group)/incidence of pain in the control group * 100 Absolute risk reduction (incidence of pain in control group − incidence of pain in the intervention group). Measured at 2 months postintervention. NNTB = 1/ARR (calculated at 2 months postintervention) Relapse (return of pain to less than 50% reduction or return of pain to baseline levels or above); determined at 2 months postintervention |
|
| Country of origin | Colombia | |
| Study aim | To determine the analgesic efficacy of the stellate ganglion blockade (SGB) in the alleviation of pain mediated by the sympathetic nervous system in patients with Complex Regional Pain Syndrome | |
| Notes | This is the first published study by Rodriguez. There are 2 other published studies and one IASP abstract that use an identical study design. It is unclear if all the studies represent the same cohort. We attempted to contact the authors 3 times with no success. This study was translated and interpreted by a researcher fluent in Spanish. The study author, TS, worked with the researcher to fully interpret and score. Funded by Colciencias and the Universidad Libre Seccional Cali. No conflict of interest stated. No adverse events reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized" Comment: method of randomisation unclear |
| Allocation concealment (selection bias) | Low risk | Opaque envelopes used; randomly given to each participant |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "double blind" Comment: Probably not. Index and control groups are not indistinguishable, and success of blinding was not tested |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The investigator was reported to be blinded; however, outcomes were self reported and participants were likely not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Participants that dropped out or underwent surgery were excluded from the analysis. The number of excluded participants per group is not reported. |
| Selective reporting (reporting bias) | High risk | No pain scores were given and time to relapse was unclear |
| Adequate sample size? | High risk | N = 82 (41 in each group) |
| Adequate duration of follow‐up? | Low risk | Follow‐up of 2 months |
| Free of other bias? | Unclear risk | No baseline data given on pain intensity; unsure if groups were similar at baseline |
Toshniwal 2012.
| Study characteristics | ||
| Methods | RCT, parallel design | |
| Participants | N= 33 Mean age (SD): Group 1: 44.33 years (13.6) Group 2: mean age 42 years (16.6) Sex: Group 1 (n=19): 6 male Group 2 (n=14): 7 male CRPS‐I of the upper extremity which had lasted at least 3 months and was refractory to medical management. People receiving any interventional procedure for the condition were excluded. Diagnostic criteria: Bruehl 1999 ), Mean duration of pain (SD): Group 1: 8.8 months (4.4). Group 2: 9.3 months (SD 2.8) Inciting event: not reported Medico‐legal factors: not reported Previous treatment: not reported Concomitant treatment: physiotherapy (4 weeks), no change in medication |
|
| Interventions | Continuous stellate ganglion block (CSG) versus continuous infraclavicular brachial plexus block (CIBP). Both groups received physiotherapy (as per recommendations from same physiotherapist) for 4 weeks. No change in regular medications in either group. Group 1: CSG block Site: stellate ganglion ‐ 20 gauge IV cannula was inserted anterolaterally into the neck, lateral to the cricoid cartilage. Cannula inserted until the C6 tubercle was hit at which time the stylet was removed and the cannula vertically sutured to the skin. Cannula position confirmed via injection of 2 ml of radio‐opaque dye under fluoroscopy Number of blocks: continuous block for 7 days Type/amount of anaesthetic: bolus of 10 ml (5 + 5 ml) 0.25% bupivacaine was injected. An elastomeric pump (solution of 0.125% bupivacaine 280 ml, delivering at 2 ml/h) was attached to the catheter. Pump was changed on day 5. Evaluation of technical adequacy: measured temperature difference between arms (> 1.5° C temperature increase in the affected arm considered adequate sympatholysis) and degree of vasodilatation using plethysmography scores (where an increase in the waveform reading score by 2 was considered improved circulation secondary to sympatholysis/vasodilatation). Group 2: CIBP block Site: brachial plexus ‐ identified using nerve stimulation by vertical approach and inserting a Contiplex D needle with catheter Position was confirmed via injection of 3 cc of radio‐opaque dye under fluoroscopy Number of block: continuous block for 7 days Type/amount of anaesthetic: bolus of 30 ml 0.25% bupivacaine was injected through the catheter. An elastomeric pump containing 0.125% bupivacaine 400 ml delivering at 5 ml/h was connected to the catheter. Pump was changed on days 3 and 6. Evaluation of technical adequacy: As above. Both groups had an increase in temperature of the blocked arm (vs contralateral hand) and improvement in circulation (at 30 min); no difference between groups. |
|
| Outcomes | Neuropathic pain scale ‐ components analysed separately (intensity, sharp, hot, dull, cold, sensitive, itchy, unpleasant, deep pain, surface pain, and quality of pain). Scale was 0 (i.e., intensity, 0 = no pain) to 10 (i.e., intensity, 10 = most intense pain sensation imaginable). Measured at 6 min, 30 min, 2 h, 12 h, and 24 h, day 2, day 3, day 4, day 5, day 6, day 7, week 2, week 3, week 4 Adverse events reported |
|
| Country of origin | USA | |
| Study aim | To compare the efficacy of continuous stellate ganglion (CSG) block with that of continuous infraclavicular brachial plexus (CIBP) block in management of CRPS type I of upper extremity | |
| Notes | The authors acknowledged editorial support from 2 doctors from Wayne State University, Detroit. The authors declared that they have nothing to disclose and no conflict of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients. . . were randomly assigned to receive CSG block or CIBP block using a computer‐generated table of random numbers (50 numbers in two columns)" Comment: likely done |
| Allocation concealment (selection bias) | Low risk | Quote: "Group allocation was concealed in sealed opaque envelopes that were not opened until patient consent had been obtained" Comment: likely done |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Both active interventions but does not mention blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcomes are self rated, thus unclear risk due to uncertainty whether participants were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Three patients were dropped from the study. One patient from each group was excluded from the study as their catheters became dislodged during the follow‐up period, and one patient in the CIBP group was excluded because he failed to follow up after 2 weeks" Comment: drop‐out rates < 20% (1 group had 1/19 drop out (5.3%) and 1 had 2/14 drop out (14.3%)). Similar reasons for drop‐out |
| Selective reporting (reporting bias) | Low risk | Reports all outcomes and all between‐group differences |
| Adequate sample size? | High risk | n = 18 (CSG), n = 12 (CIBP) |
| Adequate duration of follow‐up? | Unclear risk | 4 weeks of follow‐up |
| Free of other bias? | Low risk | Groups were similar on important prognostic factors |
Yoo 2012.
| Study characteristics | ||
| Methods | Parallel RCT | |
| Participants | N = 42 Mean age (SD): Group 1: 61.3 years (5.6) Group 2: 59.1 years (4.5) Sex: 25 males, 20 females (note: likely error as not consistent with overall N) CRPS poststroke, upper limb Type of initiating injury: stroke Diagnostic criteria: IASP Harden 2010 Duration of symptoms ‐ "duration since stroke". Group 1: 2.8 months (1.1) Group 2: 2.3 months (0.9) Previous treatment: not reported Concomitant treatments: not reported Medico‐legal factors: not reported |
|
| Interventions | Group 1: stellate ganglion block without ultrasound guidance. 10 ml lidocaine injection Group 2: stellate ganglion block with ultrasound guidance. 5 ml lidocaine injection Number of blocks: 2, 1 week apart Evaluation of technical adequacy of block: none |
|
| Outcomes | Pain intensity VAS, 0‐10, anchors "no pain" and "the highest pain" 2‐ and 4‐week follow‐up Adverse events |
|
| Country of origin | Korea | |
| Study aim | To evaluate the effectiveness of ultrasound‐guided SGB by comparing with the blind SGB in poststroke CRPS patients in reducing pain and swelling of the affected limb | |
| Notes | Conflict of interest not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: Participant blinding not reported. The interventions are likely to be distinguishable, however both were active invasive interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: blinding of participants is unclear |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: there are anomalies between group numbers in the text and table 1 |
| Selective reporting (reporting bias) | Low risk | Comment: outcome reporting adequate |
| Adequate sample size? | High risk | Comment: N = 42 |
| Adequate duration of follow‐up? | Unclear risk | Comment: follow‐up stated at 4 weeks |
| Free of other bias? | Unclear risk | Comment: no information given on concomitant treatment |
Zeng 2003.
| Study characteristics | ||
| Methods | RCT, parallel | |
| Participants | N = 60 Age range: 38‐71 Sex: 42 males Shoulder‐hand syndrome following stroke Diagnostic criteria not reported Duration of symptoms; described as "in the early stages of SHS complicated with paralysis" Previous treatment not specified Mean baseline pain, 0‐10 VRS (SD): SGB + rehab group: 6.95 (3.24) Rehab‐only group: 6.85 (3.24) Medico‐legal factors: not reported Concomitant treatments: not reported |
|
| Interventions | Stellate ganglion block + rehabilitation versus rehabilitation only SGB: anterior entry, transverse process of C7, agent, dose not reported Rehabiliation details: reports "comprehensive treatment" eliminating causes of oedema, avoid weight loading of limb, avoid limb trauma, remove factors causing shoulder pain, movement exercises, joint mobilisations, ice therapy, physical therapy |
|
| Outcomes | Pain VAS (0 = no pain, 2 = little pain, 4 = frequent mild pain or occasional severe pain, 6 = severe pain but tolerable, 8 = continuous pain and intolerable, 10 = severe pain that couldn't be touched). Pain was measured before treatment, at 10 days and 20 days post‐treatment |
|
| Country of origin | China | |
| Study aim | Effect of stellate ganglion is observed on base of comprehensive rehabilitation treatment (sic) | |
| Notes | No statement of financial support or conflict of interest; adverse events not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly divided into two groups" Comment: method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Index and control groups are not indistinguishable and success of blinding was not tested |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participant‐rated outcomes; participants not blinded (as above) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop‐outs |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes adequately reported on |
| Adequate sample size? | High risk | N = 60 (30 in each group) |
| Adequate duration of follow‐up? | Unclear risk | 20 days follow‐up |
| Free of other bias? | Low risk | Quote: "All patients were in early stage of SHS complicated with paralysis"; "there weren't statistical differences at age, sex, rehabilitation kind" Comments: no difference between groups in age, sex, rehabilitation and duration of symptoms; outcome assessment timing identical |
ARR: absolute risk reduction; BTA: botulinum toxin A; CIBP: continuous infraclavicular brachial plexus block; CRPS: complex regional pain syndrome; CSG: continuous stellate ganglion block; IASP: International Association for the Study of Pain;IV: intravenous; IVRB: intravenous regional blockade; LASB: local anaesthetic sympathetic blockade; NNTB: number needed to treat for an additional beneficial outcome; PRF: pulsed radiofrequency; RCT: randomised controlled trial; RSD: reflex sympathetic dystrophy; SD: standard deviation; SE: standard error; SF‐36: 36‐item short‐form health survey; SGB: stellate ganglion blockade; SMP: sympathetically maintained pain; VAS: visual analogue scale; VRS: verbal report scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ackerman 2006 | Not randomised |
| Arias 1989 | Not randomised |
| Catala 1994 | Sympathetic blockade versus intravenous lidocaine for postherpetic neuralgia |
| Dellemijn 1994 | Sympathetic blockade versus phentolamine infusion |
| Erickson 1993 | Not randomised |
| Farcot 1990 | Not randomised |
| Fukusaki 1995 | Nerve blocks (including sympathetic blocks) for cervical radiculopathy |
| Garrido 2005 | Not randomised |
| Glynn 1993 | Not randomised |
| Hartrick 2004 | Not randomised |
| ISRCTN71968956 | Not randomised |
| Kastler 2013 | Not randomised |
| Linson 1983 | Not randomised |
| Malmqvist 1992 | Not randomised |
| Meier 2009 | < 48 h follow‐up postblock |
| Perrigot 1982 | Not a local sympathetic block |
| Quevedo 2005 | Not randomised |
| Raja 1991 | < 48 h follow‐up postblock |
| Schurmann 2001 | Not randomised |
| Steinbrocker 1953 | Not randomised |
| Tran 2000 | Sympathetic blockade plus iohexol versus sympathetic blockade plus saline; evaluating the effect of the contrast agent iohexol |
| Verdugo 1995 | <48 h follow‐up postblock |
| Wang 1985 | Not randomised |
| Wehnert 2002 | <48 h follow‐up postblock |
| Yucel 2009 | Not randomised |
Characteristics of studies awaiting classification [ordered by study ID]
Kostadinova 2012.
| Methods | Parallel RCT |
| Participants | N = 32 |
| Interventions | Stellate ganglion block with bupivacaine vs bupivocaine plus neostigmine |
| Outcomes | Not reported |
| Notes | Abstract of protocol only available ‐ authors contacted for further details |
Rodriguez 2006.
| Methods | Parellel RCT |
| Participants | N = 71 |
| Interventions | SGB, physical therapy and pharmacological treatment vs physical therapy and pharmacological treatment |
| Outcomes | Pain intensity VAS 10 cm. Proportion with 50% pain relief 1 month postblock |
| Notes | Not clear whether a distinct trial or an extension of Rodriguez 2005. Authors contacted for clarification. |
Rodriguez 2008.
| Methods | Parellel RCT |
| Participants | N = 114 |
| Interventions | SGB, physical therapy and pharmacological treatment vs physical therapy and pharmacological treatment |
| Outcomes | Pain intensity VAS 10 cm. Time to relapsing. 6‐month follow‐up |
| Notes | Conference abstract. Not clear whether a distinct trial or an extension of Rodriguez 2005. Authors contacted for clarification. |
Salinas Cerda 1997.
| Methods | Study report not retrievable |
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Notes | — |
RCT: randomised controlled trial; SGB: stellate ganglion blockade; VAS: visual analogue scale.
Differences between protocol and review
This 2016 updated review used an expanded search strategy. In particular, we used additional search terms and also searched clinical trial registers for potentially relevant studies. For this update, we excluded studies that had only immediate follow‐up data (≤ 48 h), because this information provides little clinically relevant information about the clinical effectiveness of this treatment.
Further, we used an updated version of the 'Risk of bias' assessment – specifically, we included 'size of treatment groups' and 'duration of follow‐up' in the 'Risk of bias' evaluation. This updated review also included studies comparing local anaesthetic blockade (LASB) versus other active treatments (original review compared LASB with placebo/inert treatments only). Lastly, we updated the data analysis that included consideration of the minimally important difference (as per OMERACT 12 group recommendations) and evaluation of the level of evidence using the GRADE approach.
Contributions of authors
NEO: informed the modification of the protocol; acted as the arbiter reviewer; led the data synthesis and the writing of the manuscript.
BMW: informed the modification of the protocol; screened, identified and evaluated studies; extracted data; and contributed to the writing of the manuscript.
WG: informed the modification of the protocol; screened, identified and evaluated studies; extracted data; and contributed to the writing of the manuscript
DBC: designed the original protocol and consulted on the modifications; contributed to the writing of the manuscript.
FB: informed the modification of the protocol; assisted in the clinical trial register searches; and contributed to the writing of the manuscript.
TRS: led the modification and writing of the protocol; performed the literature search; screened, identified and evaluated studies; extracted data; informed the data synthesis; and informed the writing of the manuscript.
Sources of support
Internal sources
No sources of support supplied
External sources
Saltonstall Foundation, USA
Javeriana University School of Medicine, Colombia
Declarations of interest
NEO: none known.
BMW: none known.
WG: none known.
DBC: none known; DBC practiced anesthesiology and pain medicine in busy academic medical centers from 1986‐2005, directly treating patients with CRPS, but has not treated people with CRPS since 2005.
FB: none known; FB is a practicing neurologist and pain treatment specialist who treats patients with CRPS.
TRS: none known.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Aydemir 2006 {published data only}
- Aydemir K, Taskaynatan MA, Yazicloglu K, Ozgul A. The effects of stellate ganglion block with lidocaine and ultrasound in complex regional pain syndrome: A randomized, double blind, placebo controlled study. Journal of Rheumatology and Medical Rehabilitation 2006;17(3):193-200. [Google Scholar]
Bonelli 1983 {published data only}
- Bonelli S, Conoscente F, Movilia PG, Restelli L, Francucci B, Grossi E. Regional intravenous guanethidine vs. stellate ganglion block in reflex sympathetic dystrophies: a randomized trial. Pain 1983;16(3):297-307. [DOI] [PubMed] [Google Scholar]
Carroll 2009 {published data only}
- Carroll I, Clark JD, Mackey S. Sympathetic block with botulinum toxin to treat complex regional pain syndrome. Annals of Neurology 2009;65(3):348-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Freitas 2013 {published data only}
- Freitas TS, Deusdara R, Kessler I. Pulsed radiofrequency of sympathetic lumbar plexus versus sympathetic block in the management of lower limb complex regional pain syndrome type. Stereotactic and functional neurosurgery 2013;91:107. [Google Scholar]
Lim 2007 {published data only}
- Lim K B, Lee H J, Joo S J, Kim J Y, Lim S S. The Comparision of Effects between Stellate Ganglion Block and Oral Corticosteroid Therapy in Post-stroke Complex Regional Pain Syndrome. Journal of Korean Academy of Rehabilitation Medicine 2007;31(4):417-22. [Google Scholar]
Nascimento 2010 {published data only}
- Nascimento MSA, Klamt JG, Prado WA. Intravenous regional block is similar to sympathetic ganglion block for pain management in patients with complex regional pain syndrome type I. Brazilian Journal of Medical and Biological Research 2010;43(12):1239-44. [DOI] [PubMed] [Google Scholar]
Price 1998 {published data only}
- Price DD, Long S, Wilsey B, Rafii A. Analysis of peak magnitude and duration of analgesia produced by local anesthetics injected into sympathetic ganglia of complex regional pain syndrome patients. Clinical Journal of Pain 1998;14(3):216-26. [DOI] [PubMed] [Google Scholar]
Rocha 2014 {published data only}
- Rocha RDO, Teixeira MJ, Yeng LT, Cantara MG, Faria VG, Liggieri V et al. Thoracic sympathetic block for the treatment of complex regional pain syndrome type I: a double-blind randomized controlled study. Pain 2014;155(11):2274-81. [DOI] [PubMed] [Google Scholar]
Rodriguez 2005 {published data only}
- Rodriguez RF, Bravo LE, Tovar MA, Castro F, Ramos GE, Mendez F. Determination of the analgesic efficacy of the stellate ganglion blockade in the alleviation of pain mediated by the sympathetic nervous system in patients with Complex Regional Pain Syndrome [Determinacion de la eficacia analgesica de los bloqueos del ganglio estrellado en el alivio del dolor mediado por el sistema nervioso simpatico, en pacientes con sindrome doloroso regional complejo del miembro superior]. Revista Colombiana de Anestesiología 2005;33(3):153-9. [Google Scholar]
Toshniwal 2012 {published data only}
- Toshniwal G, Sunder R, Thomas R, Dureja GP. Management of complex regional pain syndrome type I in upper extremity - Evaluation of continuous stellate ganglion block and continuous infraclavicular brachial plexus block: a pilot study. Pain Medicine 2012;13(1):96-106. [DOI] [PubMed] [Google Scholar]
Yoo 2012 {published data only}
- Yoo SD, Jung SS, Kim HS, Yun DH, Kim DH, Chon J. Efficacy of ultrasonography guided stellate ganglion blockade in the stroke patients with complex regional pain syndrome. Annals of Rehabilitation Medicine 2012;36(5):633-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zeng 2003 {published data only}
- Zeng X, Chen S, Guan C, Jiang L, Wang L. Block of ganglion stellatum on improving edema and range of movement in shoulder-hand syndrome. Chinese Journal of Clinical Rehabilitation 2003;7(7):1194-5. [Google Scholar]
References to studies excluded from this review
Ackerman 2006 {published data only}
- Ackerman WE, Zhang JM. Efficacy of stellate ganglion blockade for the management of type 1 complex regional pain syndrome. Southern Medical Journal 2006;99(10):1084-8. [DOI] [PubMed] [Google Scholar]
Arias 1989 {published data only}
- Arias LM, Bartkowski R, Grossman KL, Schwartzman RJ, Tom CM. Sufentanil stellate ganglion injection in the treatment of refractory reflex sympathetic dystrophy. Regional Anesthesia 1989;14(2):90-2. [PubMed] [Google Scholar]
Catala 1994 {published data only}
- Catala E, Ferrandiz M, Aliaga L, Serra R, Castro MA, Villar JM. Intravenous lidocaine compared with sympathetic blocks as treatment for post-herpetic neuralgia. A 1-year survey. The Pain Clinic 1994;7(3):205-10. [Google Scholar]
Dellemijn 1994 {published data only}
- Dellemijn PL, Fields HL, Allen RR, McKay WR, Rowbotham MC. The interpretation of pain relief and sensory changes following sympathetic blockade. Brain 1994;117(6):1475-87. [DOI] [PubMed] [Google Scholar]
Erickson 1993 {published data only}
- Erickson SJ, Hogan QH. CT-guided injection of the stellate ganglion: description of technique and efficacy of sympathetic blockade. Radiology 1993;188(3):707-9. [DOI] [PubMed] [Google Scholar]
Farcot 1990 {published data only}
- Farcot JM, Grasser C, Foucher G, Marin Braun F, Ehrler S, Demangeat JL, et al. Local intravenous treatment of algodystrophy of the hand: buflomedil versus guanethidine, long term follow-up [Traitements locaux intra-veineux des algodystrophies de la main: buflomédil versus guanéthidine, suivi à long terme]. Annales de Chirurgie de la Main et du Membre Supérieur: Organe Officiel des Sociétés de Chirurgie de la Main 1990;9(4):296-304. [DOI] [PubMed] [Google Scholar]
Fukusaki 1995 {published data only}
- Fukusaki M, Matsumoto M, Yamaguchi K, Nakamura H, Sumiwaka K. The role of nerve blocks to deal with pain associated with cervical radiculopathy. The Pain Clinic 1995;8(3):219-25. [Google Scholar]
Garrido 2005 {published data only}
- Garrido B, Fernandez-Suarez L, Bosch F, Rabi MC, Hernandez-Arteaga M. Complex regional pain syndrome type I. Management with sympathetic blockade and other therapies [Síndrome doloroso regional complejo tipo 1.Tratamiento mediante bloqueos simpáticos y más]. Revista de la Sociedad Española del Dolor 2005;12(7):417-24. [Google Scholar]
Glynn 1993 {published data only}
- Glynn C, Casale R. Morphine injected around the stellate ganglion does not modulate the sympathetic nervous system nor does it provide pain relief. Pain 1993;53(33):37. [DOI] [PubMed] [Google Scholar]
Hartrick 2004 {published data only}
- Hartrick CT, Kovan JP, Naismith P. Outcome prediction following sympathetic block for complex regional pain syndrome. Pain Practice 2004;4(3):222-8. [DOI] [PubMed] [Google Scholar]
ISRCTN71968956 {published data only}
- ISRCTN71968956. Percutaneous sympathetic blockade in complex regional pain syndrome type 1: a prospective clinical investigation on predictors of sympatheticaly (sic) maintained pain ID - 7. http://www.isrctn.com/ISRCTN71968956 (accessed 15 October 2015).
Kastler 2013 {published data only}
- Kastler A, Aubry S, Sailley N, Michalakis D, Siliman G, Gory G, et al. CT-guided stellate ganglion blockade vs. radiofrequency neurolysis in the management of refractory type I complex regional pain syndrome of the upper limb. European Radiology 2013;25(5):1316-22. [DOI] [PubMed] [Google Scholar]
Linson 1983 {published data only}
- Linson MA, Leffert R, Todd DP. The treatment of upper extremity reflex sympathetic dystrophy with prolonged continuous stellate ganglion blockade. Journal of Hand Surgery 1983;8(2):153-9. [DOI] [PubMed] [Google Scholar]
Malmqvist 1992 {published data only}
- Malmqvist ELA, Bengtsson M, Sorensen J. Efficacy of stellate ganglion block: a clinical study with bupivacaine. Regional Anesthesia 1992;17(6):340-7. [PubMed] [Google Scholar]
Meier 2009 {published data only}
- Meier PM, Zurakowski D, Berde CB, Sethna NF. Lumbar sympathetic blockade in children with complex regional pain syndromes: a double blind placebo-controlled crossover trial. Anesthesiology 2009;111(2):372-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Perrigot 1982 {published data only}
- Perrigot M, Bergego C, Hocini A, Pierrot Deseilligny E. Algodystrophic syndrome in hemiplegia. Clinical and therapeutic study [Le syndrome algodystrophique chez l'hémiplégique. Etude clinique et thérapeutique]. Annales de Médecine Interne 1982;133(8):544-8. [PubMed] [Google Scholar]
Quevedo 2005 {published data only}
- Quevedo JP, Purgavie K, Platt H, Strax TE. Complex regional pain syndrome involving the lower extremity: a report of 2 cases of sphenopalatine block as a treatment option. Archives of Physical Medicine and Rehabilitation 2005;86(2):335-7. [DOI] [PubMed] [Google Scholar]
Raja 1991 {published data only}
- Raja SN, Treede RD, Davis KD, Campbell JN. Systemic alpha-adrenergic blockade with phentolamine: a diagnostic test for sympathetically maintained pain. Anesthesiology 1991;74(4):691-8. [DOI] [PubMed] [Google Scholar]
Schurmann 2001 {published data only}
- Schurmann M, Gradl G, Wizgal I, Tutic M, Moser C, Azad S, et al. Clinical and physiologic evaluation of stellate ganglion blockade for complex regional pain syndrome type I. Clinical Journal of Pain 2001;17(1):94-100. [DOI] [PubMed] [Google Scholar]
Steinbrocker 1953 {published data only}
- Steinbrocker O, Neustadt D, Lapin L. Shoulder-hand syndrome:sSympathetic block compared with corticotropin and cortisone therapy. JAMA 1953;153(9):788-91. [DOI] [PubMed] [Google Scholar]
Tran 2000 {published data only}
- Tran KM, Frank SM, Raja SN, El Rahmany HK, Kim LJ, Vu B. Lumbar sympathetic block for sympathetically maintained pain: changes in cutaneous temperatures and pain perception. Anesthesia and Analgesia 2000;90(6):1396-401. [DOI] [PubMed] [Google Scholar]
Verdugo 1995 {published data only}
- Verdugo RJ, Moya MF, Cea JG, Salinas HA, Bilbeny CJ. Stellate ganglion block in reflex sympathetic dystrophy: a double-blind crossover study. In: Program & Abstracts of the 1st Scientific Meeting of the European Federation of IASP Chapters; 1995 May 18-21; Verona (Italy). Diegum (Belgium): European Federation of IASP Chapters (EFIC), 1995:52.
Wang 1985 {published data only}
- Wang JK, Erickson RP, Ilstrup DM. Repeated stellate ganglion blocks for upper-extremity reflex sympathetic dystrophy. Regional Anesthesia 1985;10(3):125-8. [Google Scholar]
Wehnert 2002 {published data only}
- Wehnert Y, Müller B, Larsen B, Kohn D. Sympathetically maintained pain (SMP): phentolamine test vs sympathetic nerve blockade. Comparison of two diagnostic methods [Sympathisch unterhaltener Schmerz (SMP) – Phentolamintest vs. Sympathikusblockade Vergleich zweier diagnostischer Methoden]. Der Orthopäde 2002;31(11):1076-83. [DOI] [PubMed] [Google Scholar]
Yucel 2009 {published data only}
- Yucel I, Demiraran Y, Ozturan K, Degirmenci E. Complex regional pain syndrome type I: efficacy of stellate ganglion blockade. Journal of Orthopaedic Traumatology 2009;10(4):179-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Kostadinova 2012 {published data only}
- Kostadinova M, Blaise G, Homsy A, De Gagne N, Del Pino S F. The effect of stellar ganglion block with bupivacaine combined or not with neostigmine on the pain relief in patients with complex regional pain syndrome (1343376). In: Canadian Journal of Anesthesia. Proceedings of the Canadian Anesthesiologists' Society Annual Meeting; 2012 June 15-18; Quebec City (Canada). Toronto (Canada): Canadian Anesthesiologists' Society, 2012; Supplement 1:1-90.
Rodriguez 2006 {published data only}
- Rodriguez RF, Bravo LE, Tovar MA, Castro F, Ramos GE, Daza P. Study of the analgesic efficacy of stellate ganglion blockade in the management of the complex regional pain syndrome in patients with pain mediated by sympathetic nervous system: preliminary study [Determinación de la eficacia analgésica de los bloqueosdel ganglio estrellado en el síndrome doloroso regionalcomplejo con dolor mediado por el sistema nerviososimpático: estudio preliminar]. Revista de la Sociedad Española del Dolor 2006;13(4):230-7. [Google Scholar]
Rodriguez 2008 {published data only}
- Rodriguez RF, Bravo LE, Tovar MA, Ramos GE. Sympathetic blockades in sympathetic mediated pain. In: The 12th World Congress of PainAbstract viewer; 2008 Aug 17-22; Glasgow (Scotland). Washington (DC): International Association for the Study of Pain (IASP), 2008:PM 349.
Salinas Cerda 1997 {published data only}
- Salinas Cerda, H. Reflex sympathetic dystrophy: long-term control of sympatic block with guanethidine [Distrofia simpática refleja: control a largo plazo del bloqueo simpático con guanetidina]. Dolor 1997;5:17-22. [Google Scholar]
Additional references
Albrecht 2006
- Albrecht PJ, Hines S, Eisenberg E, Pud D, Finlay DR, Davar G et al. Pathologic alterations of cutaneous innervation and vasculature in affected limbs from patients with complex regional pain syndrome. Pain 2006;120(3):244-66. [DOI] [PubMed] [Google Scholar]
Ali 2000
- Ali Z, Raja SN, Wesselmann U, Fuchs PN, Meyer RA, Campbell JN. Intradermal injection of norepinephrine evokes pain in patients with sympathetically maintained pain. Pain 2000;88(2):161-8. [DOI] [PubMed] [Google Scholar]
Altman 2012
- Altman DG, Moher D, Schultz KF. Improving the reporting of randomised trials: The CONSORT statement and beyond. Statistics in Medicine 2012;31(25):2985-97. [DOI] [PubMed] [Google Scholar]
Balshem 2011
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401-6. [DOI] [PubMed] [Google Scholar]
Binder 2009
- Binder A, Baron R. Complex regional pain syndrome, including applications of neural blockade. In: Cousins MJ, Carr DB, Horlocker TT, Bridenbaugh PO, editors(s). Cousins & Bridenbaugh's Neural Blockade in Clinical Anesthesia and Management of Pain. 4th edition. Philadelphia: Lippincott Williams & Wilkins, 2009:1154-68. [Google Scholar]
Birklein 2000
- Birklein F, Weber M, Neundörfer B. Increased skin lactate in complex regional pain syndrome: evidence for tissue hypoxia? Neurology 2000;55(8):1213-5. [DOI] [PubMed] [Google Scholar]
Birklein 2001
- Birklein F, Schmelz M, Schifter S, Weber M. The important role of neuropeptides in complex regional pain syndrome. Neurology 2001;57(12):2179-84. [DOI] [PubMed] [Google Scholar]
Birklein 2014
- Birklein F, Drummond PD, Li W, Schlereth T, Albrecht N, Finch PM et al. Activation of cutaneous immune responses in complex regional pain syndrome. Journal of Pain 2014;15(5):485-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Breivik 2009
- Breivik H, Cousins MJ. Sympathetic neural blockade of upper and lower extremity. In: Cousins MJ, Carr DB, Horlocker TT, Bridenbaugh PO, editors(s). Cousins & Bridenbaugh's Neural Blockade in Clinical Anesthesia and Management of Pain. 4th edition. Philadelphia: Lippincott Williams & Wilkins, 2009:848-85. [Google Scholar]
Bruehl 1999
- Bruehl S, Harden RN, Galer BS, Saltz S, Bertram M, Backonja M et al. External validation of IASP diagnostic criteria for Complex Regional Pain Syndrome and proposed research diagnostic criteria. Pain 1999;81(1-2):147-54. [DOI] [PubMed] [Google Scholar]
Bruehl 2010
- Bruehl S. An update on the pathophysiology of complex regional pain syndrome. Anesthesiology 2010;113(3):713-25. [DOI] [PubMed] [Google Scholar]
Busse 2015
- Busse JW, Bartlett SJ, Dougados M, Johnston BC, Guyatt GH, Kirwan JR et al. Optimal strategies for reporting pain in clinical trials and systematic reviews: recommendations from an OMERACT 12 workshop. Journal of Rheumatology 2015;42(10):1962-1970. [DOI] [PubMed] [Google Scholar]
Campbell 1996
- Campbell JN. Complex regional pain syndrome and the sympathetic nervous system. Pain 1996;68:89-96. [Google Scholar]
Carr 2011
- Carr DB. Local anesthetic blockade for neuralgias: “Why is the sky blue, daddy?”. Anesthesia and Analgesia 2011;112(6):1285. [DOI] [PubMed] [Google Scholar]
De Mos 2007
- De Mos M, De Bruijn AGJ, Huygen FJPM, Dieleman JP, Stricker BHC, Sturkenboom MCJM. The incidence of complex regional pain syndrome: a population-based study. Pain 2007;129(1-2):12-20. [DOI] [PubMed] [Google Scholar]
De Mos 2008
- De Mos M, Huygen FJPM, Dieleman JP, Koopman JSHA, Stricker BHC, Sturkenboom MCJM. Medical history and the onset of complex regional pain syndrome (CRPS). Pain 2008;139(2):458-66. [DOI] [PubMed] [Google Scholar]
De Mos 2009
- De Mos, Sturkenboom MCJM, Huygen FJPM. Current understandings on complex regional pain syndrome. Pain Practice 2009;9(2):86-99. [DOI] [PubMed] [Google Scholar]
Drummond 2001
- Drummond PD, Finch PM, Skipworth S, Blockey P. Pain increases during sympathetic arousal in patients with complex regional pain syndrome. Neurology 2001;57(7):1296-303. [DOI] [PubMed] [Google Scholar]
Drummond 2004
- Drummond PD, Finch PM. Persistence of pain induced by startle and forehead cooling after sympathetic blockade in patients with complex regional pain syndrome. Journal of Neurology, Neurosurgery and Psychiatry 2004;75(1):98-102. [PMC free article] [PubMed] [Google Scholar]
Dworkin 2008
- Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. Journal of Pain 2008;9(2):105-21. [DOI] [PubMed] [Google Scholar]
Dworkin 2009
- Dworkin RH, Turk DC, McDermott MP, Peirce-Sandner S, Burke LB, Cowan P et al. Interpreting the clinical importance of group differences in chronic pain clinical trials: IMMPACT recommendations. Pain 2009;146(3):238-44. [DOI] [PubMed] [Google Scholar]
Dworkin 2010
- Dworkin RH, Turk DC, Peirce-Sandner S, Baron R, Bellamy N, Burke LB et al. Research design considerations for confirmatory chronic pain clinical trials: IMMPACT recommendations. Pain 2010;149(2):177-93. [DOI] [PubMed] [Google Scholar]
Fine 1994
- Fine PG, Roberts WJ, Gillette RG, Child TR. Slowly developing placebo responses confound tests of intravenous phentolamine to determine mechanisms underlying idiopathic chronic low back pain. Pain 1994;56(2):235-42. [DOI] [PubMed] [Google Scholar]
Forouzanfar 2002
Geha 2008
- Geha PY, Baliki MN, Harden RN, Bauer WR, Parrish TB, Apkarian AV. The brain in chronic CRPS pain: abnormal gray-white matter interactions in emotional and autonomic regions. Neuron 2008;60(4):570-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011a
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso-Coello P et al. GRADE guidelines: 4. Rating the quality of evidence - study limitations (risk of bias). Journal of Clinical Epidemiology 2011;64(4):407-15. [DOI] [PubMed] [Google Scholar]
Guyatt 2011b
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J et al. GRADE guidelines: 1. Introduction - GRADE evidence profiles and summary of findings table. Clinical Journal of Clinical Epidemiology 2011a;64(4):383-94. [DOI] [PubMed] [Google Scholar]
Harden 2006
- Harden RN, Bruehl S. Introduction and diagnostic considerations. In: Harden RN, editors(s). Complex Regional Pain Syndrome: Treatment Guidelines. Reflex Sympathetic Dystrophy Syndrome Association, 2006. [Google Scholar]
Harden 2010
- Harden RN, Bruehl S, Perez RS, Birklein F, Marinus J, Maihofner C et al. Validation of proposed diagnostic criteria for complex regional pain syndrome. Pain 2010;150(2):268-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harden 2013
- Harden RN, Oaklander AL, Burton AW, Perez RS, Richardson K, Swan M et al. Complex regional pain syndrome: practical diagnostic and treatment guidelines, 4th edition. Pain Medicine 2013;14(2):180-229. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook of Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from: www.cochrane-handbook.org.
Hogan 1997
- Hogan QH, Abram SE. Neural blockade for diagnosis and prognosis. A review. Anesthesiology 1997;86(1):216-41. [DOI] [PubMed] [Google Scholar]
Jadad 1995
- Jadad AR, Carroll D, Glynn CJ, McQuay HJ. Intravenous regional sympathetic blockade for pain relief in reflex sympathetic dystrophy: a systematic review and a randomized, double blind crossover study. Journal of Pain and Symptom Management 1995;10(1):13-20. [DOI] [PubMed] [Google Scholar]
Jänig 2003
- Jänig W, Baron R. Complex regional pain syndrome: mystery explained? The Lancet Neurology 2003;2(11):687-97. [DOI] [PubMed] [Google Scholar]
Koban 2003
- Koban M, Leis S, Schultze-Mosgau S, Birklein F. Tissue hypoxia in complex regional pain syndrome. Plastic and Reconstructive Surgery 2003;104(1-2):149-57. [DOI] [PubMed] [Google Scholar]
Lewis 2007
- Lewis JS, Kersten P, Mccabe CS, Mcpherson KM, Blake DR. Body perception disturbance: a contribution to pain in complex regional pain syndrome (CRPS). Pain 2007;133(1-3):111-9. [DOI] [PubMed] [Google Scholar]
Lotze 2007
- Lotze M, Moseley GL. Role of distorted body image in pain. Current Rheumatology Reports 2007;9(6):488-96. [DOI] [PubMed] [Google Scholar]
Maihöfner 2004
- Maihöfner C, Handwerker HO, Neundörfer B, Birklein F. Cortical reorganization during recovery from complex regional pain syndrome. Neurology 2004;63(4):693-701. [DOI] [PubMed] [Google Scholar]
Marinus 2011
- Marinus J, Moseley GL, Birklein F, Baron R, Maihofner C, Kingery WS et al. Clinical features and pathophysiology of complex regional pain syndrome. The Lancet Neurology 2011;10(7):637-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
McQuay 1997
- McQuay HJ, Moore RA, Eccleston C, Morley S, Williams AC. Systematic review of outpatient services for chronic pain control. Health Technology Assessment 1997;1(6):1-135. [PubMed] [Google Scholar]
Merskey 1994
- Merskey H, Bogduk N. Classification of Chronic Pain: Descriptions of Chronic Pain Syndromes and Definitions of Pain Terms. 2nd edition. Seattle: IASP Press, 1994. [Google Scholar]
Moore 2008
- Moore RA, Barden J, Derry S, McQuay HJ. Managing potential publication bias. In: McQuay HJ, Kalso E, Moore RA, editors(s). Systematic Reviews in Pain research: Methodology Refined. Seattle: IASP Press, 2008:15-23. [Google Scholar]
Moore 2010
- Moore RA, Eccleston C, Derry S, Wiffen P, Bell RF, Straube S et al. "Evidence" in chronic pain--establishing best practice in the reporting of systematic reviews. Pain 2010;150(3):386-9. [DOI] [PubMed] [Google Scholar]
Moseley 2006
- Moseley GL. Why do people with complex regional pain syndrome take longer to recognize their affected hand? Neurology 2006;62(12):2182-6. [DOI] [PubMed] [Google Scholar]
Nelson 2006
- Nelson DV, Stacey BR. Interventional therapies in the management of complex regional pain syndrome. Clinical Journal of Pain 2006;22(5):438-42. [DOI] [PubMed] [Google Scholar]
Niehof 2006
- Niehof SP, Huygen FJPM, Van der Weerd RWP, Westra M, Zijlstra FJ. Thermography imaging during static and controlled thermoregulation in complex regional pain syndrome type 1: diagnostic value and involvement of the central sympathetic system. BioMedical Engineering Online 2006;5:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nüesch 2010
- Nüesch E, Trelle S, Reichenbach S, Rutjes AWS, Tschannen B, Altman DG et al. Small study effects in meta-analyses of osteoarthritis trials: meta-epidemiological study. BMJ 2010;341(7766):241. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Connell 2013
- O'Connell NE, Wand BM, McAuley J, Marston L, Moseley GL. Interventions for treating pain and disability in adults with complex regional pain syndrome- an overview of systematic reviews. Cochrane Database of Systematic Reviews 2013, Issue 4. Art. No: CD009416. [DOI: 10.1002/14651858.CD009416.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ochoa 1995
- Ochoa JL, Verdugo RJ. Reflex sympathetic dystrophy. A common clinical avenue for somatoform expression. Neurologic Clinics 1995;13(2):351-63. [PubMed] [Google Scholar]
Perez 2010
- Perez RS, Zollinger PE, Dijkstra PU, Thomassen-Hilgersom IL, Zuurmond WW, Rosenbrand KCJ et al. Evidence-based guidelines for complex regional pain syndrome type 1. BMC Neurology 2010;10:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pleger 2006
- Pleger B, Ragert P, Schwenkreis P, Forster AF, Wilimzig C, Dinse H et al. Patterns of cortical reorganization parallel impaired tactile discrimination and pain intensity in complex regional pain syndrome. Neuroimage 2006;32(2):503-10. [DOI] [PubMed] [Google Scholar]
Reinders 2002
- Reinders MF, Geertzen JHB, Dijkstra PU. Complex Regional Pain Syndrome: Use of the International Association for the Study of Pain Diagnostic Criteria Defined in 1994. Clinical Journal of Pain 2002;18(4):207-15. [DOI] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Sandroni 2003
- Sandroni P, Benrud-Larson LM, McClelland RL, Low PA. Complex regional pain syndrome type I: incidence and prevalence in Olmsted county, a population-based study. Pain 2003;103(1-2):199-207. [DOI] [PubMed] [Google Scholar]
Schinkel 2006
- Schinkel C, Gaertner A, Zaspel J, Zedler S, Faist E, Schuermann M. Inflammatory mediators are altered in the acute phase of posttraumatic complex regional pain syndrome. Clinical Journal of Pain 2006;22(3):235-9. [DOI] [PubMed] [Google Scholar]
Schmelz 2001
- Schmelz M, Weber M, Birklein F, Neundo B. Facilitated neurogenic inflammation in complex regional pain syndrome. Pain 2001;91(3):251-7. [DOI] [PubMed] [Google Scholar]
Schott 1995
- Schott GD. An unsympathetic view of pain. The Lancet 1995;345(8950):634-6. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408-12. [DOI] [PubMed] [Google Scholar]
Schwenkreis 2003
- Schwenkreis P, Janssen F, Rommel O, Pleger B, Volker B, Hosbach I, et al. Bilateral motor cortex disinhibition in complex regional pain syndrome (CRPS) type I of the hand. Neurology 2003;61(4):515-9. [DOI] [PubMed] [Google Scholar]
Sethna 2012
- Sethna NF. Sympathetic nerve blocks, pragmatic trials and responder analysis. Anesthesiology 2012;116(1):12-4. [DOI] [PubMed] [Google Scholar]
Shipton 2009
- Shipton EA. Complex regional pain syndrome: mechanisms, diagnosis, and management. Current Anaesthesia and Critical Care 2009;20(5-6):209-14. [Google Scholar]
Stanton‐Hicks 1995
- Stanton-Hicks 1995. Reflex sympathetic dystrophy: changing concepts and taxonomy. Pain 1995;63(1):127-33. [DOI] [PubMed] [Google Scholar]
Stanton‐Hicks 2002
- Stanton-Hicks MD, Burton AW, Bruehl SP, Carr DB, Harden RN, Hassenbusch SJ et al. An updated interdisciplinary clinical pathway for CRPS: report of an expert panel. Pain Practice 2002;2(1):1-16. [DOI] [PubMed] [Google Scholar]
Swart 2009
- Swart CMA, Stins JF, Beek PJ. Cortical changes in complex regional pain syndrome (CRPS). European Journal of Pain 2009;13(9):902-7. [DOI] [PubMed] [Google Scholar]
Tan 2005
- Tan ECTH, Oyen WJG, Goris RJA. Leukocytes in complex regional pain syndrome type I. Inflammation 2005;29(4-6):182-6. [DOI] [PubMed] [Google Scholar]
Tran 2010
Turk 2008a
- Turk DC, Dworkin RH, Revicki D, Harding G, Burke LB, Cella D et al. Identifying important outcome domains for chronic pain clinical trials: An IMMPACT survey of people with pain. Pain 2008;137(2):276-85. [DOI] [PubMed] [Google Scholar]
Turk 2008b
- Turk DC, Dworkin RH, McDermott MP, Bellamy N, Burke LB, Chandler JM et al. Analyzing multiple endpoints in clinical trials of pain treatments: IMMPACT recommendations. Pain 2008;139(3):485-93. [DOI] [PubMed] [Google Scholar]
Van Eijs 2011
- Van Eijs F, Stanton-Hicks M, Van Zundert J, Faber CG, Lubenow TR, Mekhail N et al. Evidence-based interventional pain medicine according to clinical diagnoses. 16. Complex regional pain syndrome. Pain Practice 2011;11(5):70-87. [DOI] [PubMed] [Google Scholar]
Van Rijn 2011
- Van Rihn MA, Marinus J, Putter H, Bosselaar SRJ, Moseley GL, Van Hilten JJ. Spreading of complex regional pain syndrome: not a random process. Journal of Neural Transmission 2011;118(9):1301-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Verdugo 1994a
- Verdugo RJ, Ochoa JL. Sympathetically maintained pain I. Phentolamine block questions the concept. Neurology 1994;44(6):1003-10. [DOI] [PubMed] [Google Scholar]
Verdugo 1994b
- Verdugo RJ, Campero M, Ochoa JL. Phentolamine sympathetic block in painful polyneuropathies. II. Further questioning of the concept of "sympathetically maintained pain". Neurology 1994;44(6):1010-14. [DOI] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Juni P, Altman DG et al. Empirical evidence of bias in treatment effect estimates with different interventions and outcomes: meta-epidemiological study. BMJ 2008;336:601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Cepeda 2002
- Cepeda MS, Lau J, Carr DB. Defining the therapeutic role of local anesthetic sympathetic blockade in complex regional pain syndrome: a narrative and systematic review. Clinical Journal of Pain 2002;18(4):216-33. [DOI] [PubMed] [Google Scholar]
Cepeda 2005
- Cepeda S, Carr D. Local anesthetic sympathetic blockade for complex regional pain syndrome. Cochrane Database of Systematic Reviews 2005, Issue 4. Art. No: CD004598. [DOI: 10.1002/14651858.CD004598] [DOI] [PubMed] [Google Scholar]
Cepeda 2010
- Cepeda MS, Carr DB, Lau J. Local anaesthetic sympathetic blockade for complex regional pain syndrome. Cochrane Database of Systematic Reviews 2010, Issue 1. Art. No: CD004598. [DOI: 10.1002/14651858.CD004598.pub2] [DOI] [PubMed] [Google Scholar]
Stanton 2013
- Stanton TR, Wand BM, Carr DB, Birklein F, Wasner GL, O'Connell NE. Local anaesthetic sympathetic blockade for complex regional pain syndrome. Cochrane Database of Systematic Reviews 2013, Issue 8. Art. No: CD004598. [DOI: 10.1002/14651858.CD004598.pub3] [DOI] [PubMed] [Google Scholar]


