Abstract
The aim of this study was to evaluate the results of single-session sclerotherapy with mixture of alcohol and polidocanol and a subsequent injection of albendazole for devisceration of hydatid cysts in the spleen. Eight patients (four women and four men, average age 22.9±11.4 with hydatid cyst in the spleen were treated with 10 minutes time of exposure to mixture of ethanol 95% and polidocanol 1%. After that, 2 to 5 ml of albendazole was injected into the cyst cavity. Two patients had 2 cysts. At follow-up the patients were examined with clinical and biochemical examinations, ultrasonography, and serologic test for echinococcal antibody titres. The mean hospital stay was 2.5±0.93 days. During the follow-up period, mean cyst diameter decreased from 46±16.4 mm to 13.6±16.26 mm. In all ten cysts, a reduction of post procedural recolection of fluid over 40% was observed. Five cysts (50%) disappeared during the follow-up period. All cysts (5) smaller then 50 mm in diameter disappeared during follow-up period. After an initial rise, the echinococcal-an-tibody titres fell progressively and at the last follow-up were negative (< 1: 160) in 7 (88%) patients. No complications were observed, except for pain, fever and urticaria during the first 24-hours after the procedure. Sclerotherapy using only one session and 10 min time of exposure to the mixture of ethanol and polidocanol, and a subsequent injection of al-bendasole solution represents an effective treatment of hydatid cysts in the spleen. This procedure is even more efficacious for hydatid cyst with diametar smaller then 50mm.
Keywords: Spleen hydatid cyst, PAIR, Interventional ultrasound
INTRODUCTION
Hydatid disease is a worldwide zoonosis produced by the larval stage of the Echinococcus tapeworm. Echinococcosis is a zoonosis transmitted by dogs in livestock-raising areas and accidentally affects humans. Human infection is acquired from ingestion of the parasite eggs from infected animals. Echinococcus granulosus causes cystic echinococcosis in humans, a condition that is found throughout the world, particularly in the great grazing regions of the world as Africa, South America, the Mediterranean region, the Middle East, Australia and New Zealand (1, 2). The most frequent site of hydatid cysts is in the liver (50% to 70% of cases), followed by the lungs (20% to 30%), and less frequently, the spleen, kydneys, heart, bones, central nervous system, and elsewhere. The ultimate goal of treatment is elimination of the germinal layer of the hydatid cyst. Currently, three treatment options are available : surgery, medical treatment and percutaneous drainage. Surgery was the only treatment option available until the mid 1980’s (2, 3). However, drug therapy with mebendasol and albendasol (4, 5, 6, 7) and percutaneous drainage have been introduced as alternative treatments. Percutaneoous treatment was called PAIR, Puncture-Aspiration-Injection-Reaspira-tion. Percutaneous drainage is minimally invasive and very effective in the treatment of hydatidosis (8, 9, 10, 11). Later, PAIR-derived technique was introduced for treatment of complicated hydatid cysts, cysts containing non-drainable material and cysts with difficult approach for intervention (12, 13, 14). We now report on a prospective study of modified PAIR technique for percutaneous treatment of hydatid cysts in the spleen.
FIGURE 2.
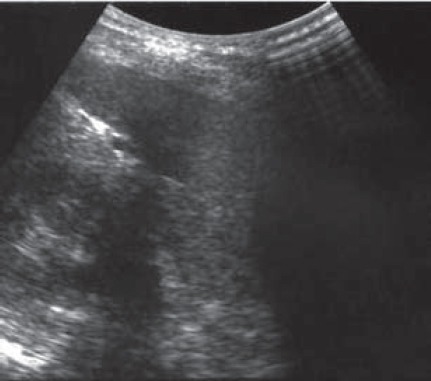
Spleen of the same patient after procedure
PATIENTS AND METHODS
Patients that were admitted to our hospital with hydatid cyst in the spleen between May 2000 and February 2003, were included. Patients that were enrolled had symptoms, such as pain in left hypochodrium, or were asimp-tomatic at the time the cyst in the spleen was diagnosed. Spleen infestation with Echinococcus granulosus was confirmed serologicly (IgG >1:160) in all cases. Hydatid cyst was diagnosed in four female and four male patients, with a mean age of 22,9±11,4. Patients signed written informed consent. On ultrasonografic examination, six patients had univesicular cysts, which were rounded with well-defined borders and contained pure fluid, and two had multivesicular cysts, with pure fluid in each vesicle. All patients undergoing procedure were treated with albendazole, administrated orally in a dose of 10 mg per kilogram of body weight per day. Albendazole prophylaxis was started one week before the procedure and continued for three weeks thereafter. The procedure was performed in three steps. In step one, the cyst was punctured under ultrasound guidance, using 18-gauge needle, and the fluid content of the cyst was subtotally aspirated. In step two, the cyst cavity was nearly filled with a mixture of 95% alcohol and 1% polidocanol (ethoxysclerol), which was left in the cavity for 10 minutes. In step three, the cyst was aspirated completely, and 2 to 5 mililiter of albendasole was injected into the cavity depending on the cyst size. The cyst fluid was subjected to cytologic and micro-biologic examination. After the procedure, the patient was observed for 24 hours and then discharged from the hospital. Examinations were performed at the time of enrollment, at one and four months, and subsequently every three months during the follow-up period. The ultimate goal of treatment was the disappearance of the cyst. (Figure 1 and 2). Other important parameters of efficacy were the size of the cyst over time, the length of the hospital stay, and any complications related to the procedure. A secondary parameter of efficacy was the serum echinococcal-an-tibody titer over time. Differences in cystic diameter before and after therapy were examined using the Wil-coxon signed-rank test. All p values were two-tailed.
FIGURE 1.
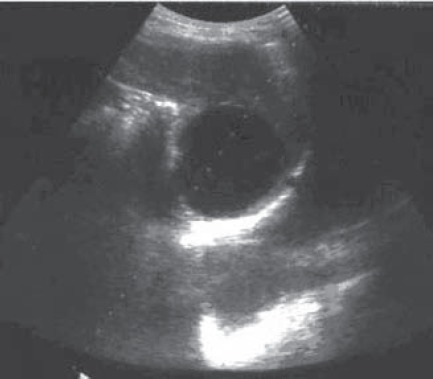
Hydatid cyst in the spleen before devisceration
RESULTS
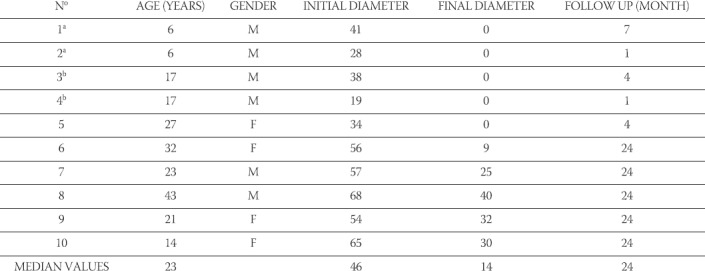
Ten spleen hydatid cysts in eight patients were treated by single-session sclerotherapy performed with a 10 minute time of exposure to alcohol 95% and polidoca-nol 1% mixture. Consequently, 2 to 5 ml (deepending of cyst volume) of albendasole solution was injected into the cyst cavity. All the patients recieved the assigned treatment. The mean hospital stay was 2,5±0,93 days, and it was significantly shorter in relation to others procedures. The procedure was successful in all eight patients in relation to devisceration of the cysts. The mean diametar of the cyst was decreased from 46±16,4mm before treatment to 13,6±16,26mm at the last examination. This represents a reduction of diametar of 50%-100% (median 92%, p < 0,001). The time of observation was 7-24 months (Figure 3). After the procedure, the maximal diametar was reduced in all cysts. In the two, the maximal diameter was reduced over 40%, and in all others over 50% compared with the one before the procedure. Five of ten cysts disappeared between 4 and 24 months after the treatment (Table 1). All of them had initial diameter smaller then 50 mm. Two patients (25%) had a fourfold rise in antibody ti-tres in the first four months after the procedure. At the last follow-up examination, all patients had smaller levels of antibody titres than before treatment, and seven of them (88%) had negative antibody titres(< 1:160). There were no complications during procedures, except for the pain. Two patients (25%) had fever within 24 hours after the procedure, one patient had transiet hypotension, and one had urticaria. Cultures of cyst fluid obtained from the cysts during puncture failed to document a microbial cause of fever. All complications disappeared within 48 hours after the procedure.
FIGURE 3.
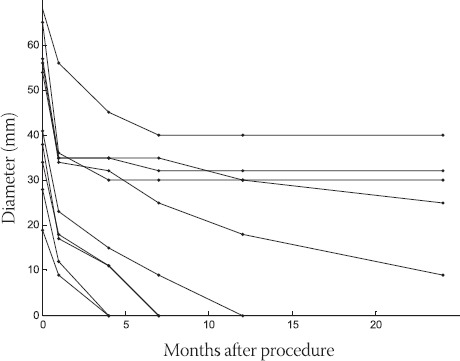
Cyst diameter in ten cysts following procedureA pattern of post-procedural recollection of fluid later followed by diameter reduction (median diameter reduction 92%) was observed in all ten cysts.
TABLE 1.
Ten cysts in eight patients treated with single-session sclerotherapy with mixture of alcochol and polidocanol and injection of albendazole solution thereafter.
DISCUSSION
Percutaneous therapeutic treatment of hydatid cysts has long been discouraged because of the risk of anaphylaxis and intraperitoneal seeding. Nevertheless, accidental and intended diagnostic puncture of what appeared to be hydatid cysts happened to occur uneventful (15). In early 1990’s, a systematic percutaneous technique was introduced, at the first as treatment for liver hydatid cysts (8). To prevent intraabdominal seeding, the hyda-tid cyst is punctured through normal tissue. The tissue collapses when needle or catheter is removed and thus serves as protection against leakage of the cyst fluid (12, 16). This technique was based on the one introduced for percutaneous treatment of non-parasitic cysts with 95% alcohol (17) and called PAIR. Usually albendazole prophylaxis is started one week before PAIR and continued for three to eight weeks thereafter (10, 11, 18, 19). Many studies demonstrated that PAIR was safe, successful in 90%-100% of cases, and had few relapses (04%) (9, 10, 11, 16, 19, 20, 21, 22). The WHO Informal Working Group on Echinococcosis reported the results of 765 hydatid cysts treated in multiple centres. Success rate was 99,7%, failure rate 0,26%, relaps rate 1,57%, and complication rate 14,8%. Anaphylactic shock (one patient died) and spillage occurred in 0,52% each. Minor complications were observed in 13,7 % (10, 16, 23) Hydatid cysts in the spleen have a specificity to be found in smaller organ than the liver and lung cysts, hidden by the rib arch and therefore difficult to approach for introducing of catheter. Hydatid cysts of the spleen are usualy smaller then 50 mm in diameter. Some authors suggest that small (< 50-60 mm) and large (> 50-60 mm) cysts should be treated differently (24, 25, 26). It has been suggested that cysts smaller then 50-60 mm in diameter should not undergo catheterization after the initial drainage. For these, after the initial PAIR procedure, only single-session sclerotherapy with a 10 minute time of exposure to the mixture of alcohol and polidocanol, and injection of albendazole solution thereafter is enough for devisceration. As a result of our study, hydatid cysts smaller then 50 mm had regression faster and completely in relation to the ones greater then 50mm in diameter. Some patients in our study showed an increase of antibody titres soon after the intervention. After follow-up period, all patients had antibody titres less than before the procedure, and most of them had negative antibody titres. Our results of level of antibody titres are similar to the results of other studies (19, 27). Routine culture of the aspirated fluid to detect possible bacterial infection or contamination is also done in more investigations (19, 24, 25, 26, 27). In our study, microbiologic examinations were negative. There were no serious complications from the interventions. The reason is probably that modified PAIR is less agressive in realation to classic PAIR procedure and surgery treatment.
CONCLUSION
Modified PAIR (with injection polidocanol and albendazole) can be performed safely and results in the disappearance of the hydatid cysts in the spleen. The efficacy of this procedure is similar to that of standard PAIR procedure (especially in cysts smaller then 50 mm), and treatment with cystectomy, in terms of reducing the size of the cyst and causing its disappearance over follow-up period of two years. The advantages of modified PAIR include a shorter hospital stay and a lower complication rate.
REFERENCES
- 1.Pedrosa I, Saiz A, Arrazola J, Ferreirós J, Pedrosa C.S. Hydatid Disease. Radiologic and Pathologic Features and Complications. Radiographics. 2000;20:795–817. doi: 10.1148/radiographics.20.3.g00ma06795. [DOI] [PubMed] [Google Scholar]
- 2.Ammann R.W, Eckert J. Cestodes Echinococcus Gastroenterol. Clin. North. Am. 1996;25:655–689. doi: 10.1016/s0889-8553(05)70268-5. [DOI] [PubMed] [Google Scholar]
- 3.Milićevic M. Hydatiddisease. In: Blumgart L.H, editor. Surgery of the liver and biliary tract. 2nd ed. Vol. 2. Edinburgh, Scotland: Churchill Livingstone; 1994. pp. 1121–1150. [Google Scholar]
- 4.Horton R.J. Albendazole in treatment of human cystic echinococcosis 12 years of experience. Acta. Trop. 1997;64:7993. doi: 10.1016/s0001-706x(96)00640-7. [DOI] [PubMed] [Google Scholar]
- 5.Teggi A, Lastilla M.G, De Rosa F. Therapy of human hydatid disease with mebendazole and albendazole. Antimicrob. Agents. Chemother. 1993;37:1679–1684. doi: 10.1128/aac.37.8.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franchi C, Di Vico B, Teggi A. Long-term evaluation of patients with hydatidosis treated with benzimidazole carbamates. Clin. Infect. Dis. 1999;29:304–309. doi: 10.1086/520205. [DOI] [PubMed] [Google Scholar]
- 7.Shantz P.M. Editorial response Treatment of cystic echinococcosis-improving but still limited. Clin. Infect. Dis. 1999;29:310311. doi: 10.1086/520206. [DOI] [PubMed] [Google Scholar]
- 8.Mueller P.R, Dawson S.L, Ferrucci J.T, Jr, Nardi G.L. Hepatic echinococcal cyst successful percutaneous drainage. Radiology. 1985;155:627–628. doi: 10.1148/radiology.155.3.3890001. [DOI] [PubMed] [Google Scholar]
- 9.Garlaschelli A, Marangio A, Gulizia R, Filice C, Brunetti E. PAIRfor abdominal cystic echinococcosis results of a 15 year experience as a contribution to an evidence-based management of the disease. Acta. Tropica. 2002:S91–92. [Google Scholar]
- 10.Zerem E, Šabanović Z, Smajić M. Percutaneoustreatment of abdominal and retroperitoneal echinococcal cysts using ultrasonog-raphy. Med. Arh. 2002;57(1 Suppl 2):71–73. [PubMed] [Google Scholar]
- 11.Brigić E, Zerem E, Terzić S. Ultrasonographicguidance of percutaneous drainage as a new method of treatment of echinococcal cysts. Med. Arh. 2003;57(1):13–15. [PubMed] [Google Scholar]
- 12.Schipper H.G, Lameris J.S, van Delden O.M, Rauws E.A, Kager P.A. Percutaneous evacuation (PEVAC) of multivesicular echi-nococcal cysts with or without cystobiliary fistulas which contain non-drainable material first results of a modified PAIR method. Gut. 2002;50:718–723. doi: 10.1136/gut.50.5.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haddad M.C, Sammak B.M, Al Karawi M. Percutaneoustreatment of heterogenous predominantly solid echopattern echino-coccal cysts of the liver. Cardiovasc Intervent. Radiol. 2000;23:121–125. doi: 10.1007/s002709910024. [DOI] [PubMed] [Google Scholar]
- 14.Brunetti E, Morangio A, Gulizia R, Filice C. Ultrasound-guided radiofrequency thermal ablation of prevalently solid echinococcal liver cysts. Acta. Tropica. 2002:S90–91. [Google Scholar]
- 15.Lewall D.B, Mc Crokell S.J. Rupture of echinococcal cysts Diagnosis, Classification and clinical implications. AJR. 1986;146(2):458–470. doi: 10.2214/ajr.146.2.391. [DOI] [PubMed] [Google Scholar]
- 16.Schipper H.G, Kager P.A. Diagnosis and treatment of hepatic echinococcosis an overview. Scand. J. Gastroenterol. Suppl. 2004;(241):50–55. doi: 10.1080/00855920410011004. [DOI] [PubMed] [Google Scholar]
- 17.Larssen T.B, Rosendahl K, Horn A, Jensen D.K, Rórvik J. Singlesessionalcohol sclerotherapy in symptomatic benign hepatic cysts performed with a time of exposure to alcohol of 10 min initial results. Eur. Radiol. 2003;13:2627–2632. doi: 10.1007/s00330-003-1923-7. [DOI] [PubMed] [Google Scholar]
- 18.Vankastesan P. Albendazole. Journal of antimicrobial chemotherapy. 1998;41:145–147. doi: 10.1093/jac/41.2.145. [DOI] [PubMed] [Google Scholar]
- 19.Khuroo M.S, Dar M.Y, Yattoo G.N, Zarger S.A, Javid G, Khan B.A, Iqbalboda M. Percutaneousdrainage versus albedasol therapy in hepatic hydatidosis a prospective, randomized study. Gastroenterology. 1993;104:1452–1459. doi: 10.1016/0016-5085(93)90355-g. [DOI] [PubMed] [Google Scholar]
- 20.Bastid C, Azar C, Doyer M, Sahel J. Percutaneoustreatment of hydatid cysts under sonographic guidance. Dig. Dis. Sci. 1994;39:1576–1580. doi: 10.1007/BF02088067. [DOI] [PubMed] [Google Scholar]
- 21.Gargouri M, Ben Amor N, Ben Chehida F, Hammou A, Gharbi H.A, Ben Cheikh M, Kchouk H, Ayachi K, Golvan J.Y. Percutaneous treatment of hydatid cysts (Echinococcus granulosus) Cardiovasc. Intervent. Radiol. 1990;13(3):169–173. doi: 10.1007/BF02575469. [DOI] [PubMed] [Google Scholar]
- 22.Etlik O, Arslan H, Bay A, Sakarya M.E, Harman M, Temizoz O, Kayan M, Bakan V, Unal O. Abdominalhydatid disease long-term results of percutaneous treatment. Acta. Radiol. 2004;45(4):383–389. doi: 10.1080/02841850410005651. [DOI] [PubMed] [Google Scholar]
- 23.Filice C, Brunetti E, Bruno R, Crippa F.G. WHO-Informal Working Group on Echinococcosis-PAIR Network. Percutaneous drainage of echinococcal cysts (PAIR-puncture, aspiration, injection, reaspiration) results of a worldwide survey for assessment of its safety and efficacy. Gut. 2000;47:156–157. doi: 10.1136/gut.47.1.156b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akhan O, Ozmen M.N, Dincer A, Sayek I.S, Gocman A. Liverhydatid disease long-term results of percutaneous treatment. Radiology. 1996;198:259–264. doi: 10.1148/radiology.198.1.8539390. [DOI] [PubMed] [Google Scholar]
- 25.Men S, Hekimoglu B, Yucesoy C, Arda I.S, Baran I. Percutaneoustreatment of hepatic hydatid cysts an alternative to surgery. AJR. 1999;172:83–89. doi: 10.2214/ajr.172.1.9888745. [DOI] [PubMed] [Google Scholar]
- 26.Ustunsoz B, Akhan O, Kamiloglu M.A, Somuncu I, Ugurel M.S, Cetiner S. Percutaneoustreatment of hydatid cysts of the liver long term results. AJR. 1999;172(1):91–96. doi: 10.2214/ajr.172.1.9888746. [DOI] [PubMed] [Google Scholar]
- 27.Khuroo M.S, Wani N.A, Javid G, Khan B.A, Yattoo G.N, Shah A.H, Jeelani S.G. Percutaneous drainage compared with surgery for hepatic hydatid cysts. New. Eng. J. Med. 1997;337(13):881887. doi: 10.1056/NEJM199709253371303. [DOI] [PubMed] [Google Scholar]


