Abstract
A survey of persistence of anti-HBs after hepatitis B vaccination has shown that five years after vaccination on a sample of 152 persons, or 82,53%, stands at >10 IU/I. Long term immunogenicity of vaccinated children remained at 88,89%, health workers 79,41% and drug addicts 64,28%. The results of these studies in Bosnia and Herzegovina show the high level of protection hepatitis B vaccine agains HBV infection. Vaccination against viral hepatitis B results in immunologic memory response among the vaccinated, and even after a decrease of anti-HB level following the third vaccine dose inoculation, a booster dose is not needed. Immunity remains steady and a booster dose is not recommended.
Keywords: Immune memory, hepatitis B vaccination, long term efficacy
INTRODUCTION
Hepatitis B infection is found worldwide but the prevalence varies greatly among different countries. It is estimated that a half of the world population has experienced infection and there are 350 million chronically infected individuals. Hepatitis B is responsible for 1,5 million deaths per year. Around 40% of chronically infected individuals will die as a result of their infection. Chronic HBV infection may take the form of a Chronic Active Hepatitis (CAH) or Chronic Persistent Hepatitis (CPH) or minimal hepatitis. The distinction can only be made on a histological examination of the liver. CAH is far more common in active carriers as it is indicative of active viral replication Cirrhosis and hepatocellular carcinoma (HCC) is thought to be more common in active carriers. In European countries where immunisation against viral hepatitis B is conducted, the level of incidence of viral hepatitis disease has dropped by 80% compared to the period when immunisation process was not administered (1). Data from Italy shows that in the period between 1985 and 1990 when immunisation against viral hepatitis B was introduced, the incidence of this disease was reduced by more than 80%. Research in Spain has shown that an introduction of hepatitis B immunisation reduced the hepatitis B positive markers prevalence by 46% since the rate of HBV infection reduced from 16,9% to 9,1% with the introduction of the above mentioned (2). Nine years after the inclusion of immunisation against viral hepatitis B in the regular immunisation programme for children in Saudi Arabia, the prevalence of HbsAg was significantly reduced (3,4).
SUBJECTS AND METHODS
The primary study was to compare the referring results of research on persistence of anti-HBs after hepatitis vaccination in the world, with those in Bosnia and Herzegovina. The kinetics of anti-HBs after hep-B vaccination is very similar in every vaccinated individual, irrespective of the peak antibody level after third vaccine. An anti-HBs se-rological test result of 10ml IU/ml indicates immunity.
RESULTS
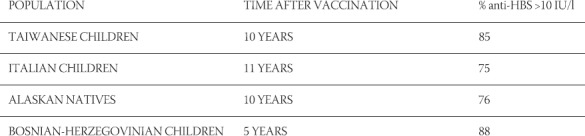
This research encompassed 186 recipients of a vaccine against viral hepatitis B, who received three doses of the vaccine five to six years ago. Results have shown that the persistence of anti-HBs after hepatitis B vaccination >10 IU/l stands at 82,53% on average (Table 1). The largest incidence of anti-HBs >10IU/l level was registered among the population of children vaccinated against viral hepatitis B at a rate of 88,89%, then follow health workers at a rate of 79,41%, whilst intravenous drug users show the lowest rate at 64,28%. When compared with similar findings in other countries, results in Bosnia and Herzegovina show persistence of anti-HBs after immunisation rate to reflect in similar percentages (Table 2) These results show that 5 to 11 years after immunisation, those vaccinated against viral hepatitis B display a reduction in the titer of antibodies. A present-day question arises as to whether the fall in anti-HB <10IU/l among those immunised against viral hepatitis B represents a loss of immunity, and whether a hepatitis B booster vaccine is necessary to boost immunity.
TABLE 1.
Persistence of anti-HBs after hepatitis Β vaccination
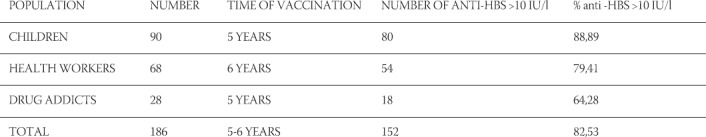
TABLE 2.
Persistence of anti-HB after hepatitis Β vaccination
Recent opinions, as well as numerous epidemiologic research, show the presence of immunity among those vaccinated with three vaccines against viral hepatitis besides the reduction in the titter rate to >10 IO/l. WHO does not recommend hepatitis B vaccine booster doses based on the following:
Many studies have shown that infants, children and adults who have responded to a complete hepatitis B immunisation. Series are protected from disease for as long as 15 years, even if they lose protective antibodies over time
Long-term protection relies on immunologic memory, which allows a protective anamnestic antibody response after exposure to the hepatitis B virus.
Immunologic memory is made-up of a complex interplay among memory B cells, memory T helper cells, memory cytotoxic lymphocytes (CTI), and antigen/antibody complexes. In vitro studies have shown that if the hepatitis B vaccine administered in a primary series initially induces and anti-HBs titre greater than or equal to 10 IU/ml, then memory B and T-helper cells retain the capacity to generate antibodies following re-exposure to HbsAg even if the anti-HBs titre falls to less than 10ml IU/ml later on (4,5). Some of the difficulties in determining the ength of protection after hepatitis B immunisation are summarised below:
Follow-up studies with an observation time much longer than 10 years are still rare
The number of vaccines available for follow-up decreases with time and the data become less significant.
In countries with low endemicity, the risk of hepatitis B infection is very lowso that challenge will be rare
Imunological memory, thus far, has been emonstrated mainly byanamnestic response to re-vaccination whie reliable and sensitive cellular tests are seldom used.
DISCUSSION
Immunisation against viral hepatitis B today represents one of the most valuable preventative programmes which significantly reduces the rate if incidence of this disease. The best proof of the efficiency of this vaccine is the reduction in the incidence of the viral hepatitis B among the citizens as claimed by the USA Health Service. Taiwan was the first country in the World to introduce immunisation against viral hepatitis B in 1984. Since 1986, all children are vaccinated against it. The programme also significantly reduced the incidence of viral hepatitis B among the non-vaccinated population HbsAg positive rate, from 8,1% to 2,3% in 2000 (5). Almost all individuals adequately vaccinated against hepatitis B have shown evidence of immunity in the form of persisting anti-HBs (protective body that develops following recovery from hepatitis B virus infection or after vaccination) and/or in vitro B-cell stimulation or an anamnestic response to a vaccine challenge(6,7) Protection against HBV infection if bound to anti-HB concentration of >10 IL/l (measured 1 to 3 months after administration of the last dose of the primary schedule). Antibody persistence depends on the initial (peak) anti-HBs concentration. The kinetics of anti-HBs after hep-B vaccination is very similar in every vaccinated individual, irrespective of the peak antibody level after third vaccine. An anti-HBs se-rological test result of 10ml IU/ml indicates immunity. Active immunisation against HBV is indicated for groups with an increased risk of acquiring this infection. These groups include medical personnel involved in care of patients who are potential carriers of the virus, laboratory staff, people working in high risk institutions such as those for mentally handicapped, individuals requiring repeated blood transfusions and/or blood products and those who may require haemodialysis in future. The vaccine takes up to 6 months to produce adequate protection and should not be given to people who are naturally immune to HbsAg positive, or suffering from acute hepatitis B. The vaccine may be given to HIV positive individuals.
CONCLUSION
Research findings have shown that the persistence of anti-HB s after hepatitis B vaccination >10IU/l shows on 152 (82,52%) vaccinated persons five years after the vaccination has been administered. These research findings have also shown a high level of anti-HBs displayed among the persons vaccinated against viral hepatitis B. The lowest percentage of anti-HBs >10 IU/l was found among the group of sampled drug addicts 64,28%, with the largest among children at a rate of 88,89%. It is important to emphasise that among those immunised with three doses, an immunologic memory is manifested after the administration on the hepatitis B vaccine. This immunologic memory is long-term regardless of the level of anti-HBs persistence, therefore a booster dose vaccine is not recommended regardless of the reduction in the level of anti-HBs.
REFERENCES
- 1.Mele A, Stroffolini T. Hepatitis B in Italy where we areten years after the introduction of mas vaccination. J. Med. Virol. 2002;67:440–43. doi: 10.1002/jmv.10092. [DOI] [PubMed] [Google Scholar]
- 2.Dominguez A, Vidal J. Changesin the seroepidemiology of hepatitis B infection in Catalonia 1989-1996. Vaccine. 2000;18:23452350. doi: 10.1016/s0264-410x(00)00028-1. [DOI] [PubMed] [Google Scholar]
- 3.Faeh FZ, Ayoola EA. Seroepidemiologyof hepatitis B virus infection in Saudi Arabian children a baseline survey for mass vaccination against hepatitis. B.J. Infect. 1992;24:197–206. doi: 10.1016/0163-4453(92)93006-c. [DOI] [PubMed] [Google Scholar]
- 4.Al-Faleh F.Z, Ramia S. Seroepidemiologyof hepatitis B virus infection in Saudi children 8 years after a mass hepatitis B vaccination programme. J Infect. 1999;38:167–170. doi: 10.1016/s0163-4453(99)90245-1. [DOI] [PubMed] [Google Scholar]
- 5.Lin Y.C, Chang M.H. Long-term immunogenicity and efficacy of universal hepatitis Bvirusvaccination in Taiwan. J. Infect. Dis. 2003;187:134–138. doi: 10.1086/345871. [DOI] [PubMed] [Google Scholar]
- 6.European Concensus Group on Hepatitis B. Immunity. Are booster immunization needed for lifelong hepatitis B immunity? Lancet. 2000;335:561–565. [PubMed] [Google Scholar]
- 7.Jilg W, Smidth M. Perzistence of specific antibodies after hepatitis B vaccination. J Hepatol. 1988;6:201–207. doi: 10.1016/s0168-8278(88)80032-1. [DOI] [PubMed] [Google Scholar]
- 8.Geseman M. Quantificationof hepatitis B vaccine-induced antibodies as a predictor of anti-HBs persistence. Vaccine. 1995;13:443–447. doi: 10.1016/0264-410x(94)00010-k. [DOI] [PubMed] [Google Scholar]
- 9.Velinga A, Van Damme P. Modellingong-term persistence OK of hepatitis B antibodies after vaccination. J Med Virol. 1999;57:100–103. doi: 10.1002/(sici)1096-9071(199902)57:2<100::aid-jmv2>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 10.Wainwright RB. Protection provided by hepatitis B vaccine in a Yupik Eskimo population-results of a 10 year study. J Infect. Dis. 1997;175:674–677. doi: 10.1093/infdis/175.3.674. [DOI] [PubMed] [Google Scholar]
- 11.West D.J, Calandra G.B. Vaccine induced immunologic memory for hepatitis B surface antigen. implications for policy on booster vaccination. Vaccine. 1996;14:1019–1027. doi: 10.1016/0264-410x(96)00062-x. [DOI] [PubMed] [Google Scholar]
- 12.Carman W, Thomas H. Viralgenetic variation hepatitis B virus as a clinical example. Lancet. 1993;341:349–353. doi: 10.1016/0140-6736(93)90146-8. [DOI] [PubMed] [Google Scholar]
- 13.Feitelson M.A. Biology of hepatitis B virus variants. Lab. Invest. 1994;71:324–349. [PubMed] [Google Scholar]
- 14.Tiollais P, Charnay P. Biology of hepatitis B virus. Science. 1981;213:406–411. doi: 10.1126/science.6264599. [DOI] [PubMed] [Google Scholar]
- 15.Shizuma T, Hasegawa K. Molecularanalysis of antigenicity and immunogenicity of a vaccine-induced escape mutants of hepatitis B virus. J Gastroenterol. 2003;28:244–253. doi: 10.1007/s005350300043. [DOI] [PubMed] [Google Scholar]
- 16.Francois G, Kew M. Mutanthepatitis viruses:a matter of academic interest only or a problem with far-reaching implications? Vaccine. 2001;19:3799–3815. doi: 10.1016/s0264-410x(01)00108-6. [DOI] [PubMed] [Google Scholar]
- 17.Oon C.J. Current aspects of hepatitis B surface antigen mutants in Singapore. J. Viral. Hepat. 1998;5:17–23. doi: 10.1046/j.1365-2893.1998.0050s2017.x. [DOI] [PubMed] [Google Scholar]


