Abstract
Experimental studies of burns require the use of different animal models. The aim of this work was to establish experimental model of thermal injuries and to evaluate the effects of topical agents on healing of the burn wounds. Forty female Wistar rats were randomly classified in 4 groups and isolated for 2 weeks before the onset of experiment. Animals were primarily anaesthetized with pentobarbital-sodium and then shaved (skin area of their back with diameters 5 cm x 5 cm). A round metal stamp with contact area of 5 cm2 and total weight of 100 g was heated up to 80°C and then applied without additional pressure on the depilated skin of the back for 14 seconds. This procedure produced a standardized burn wound. Induced burn wounds were immediately drowned in the 4°C-water for 3 s in order to maintain microcirculation. After the inducement of thermal injures, all rats were treated with 1% silver sulfadiazine cream, herbal topical preparations or were not treated at all. Burn wounds were treated twice a day until the healing completion. The result of treatment application was a significant reduction of burn wound diameters. Herbal topical preparations expressed positive therapeutic effects on the parameters of burn wounds. The efficiency of silver sulfadiazine cream in burn wound healing was significantly more expressed in comparison to healing process in control group of animals (p<0,001). We conclude that herbal topical preparations efficiently caused the completion of burn wound healing process without scar formation.
Keywords: burns, animal model, rats, herbal remedies
INTRODUCTION
Pharmacological-toxicological studies present an important contribution in the evaluation of protective effects of the new drugs in treatment of burns. These researches consist of preliminary (in vitro), preclinical (in vivo), and clinical researches (1,2). Animal models are successfully used in investigation of the different diseases and diverse pathophysiologic processes. The utilization of animal models provides careful control of alterations, because all animals are modified in similar manner. At the same time, it should be kept in mind that such alterations might affect the course of investigated diseases. Beside the limited utilization of laboratory animals, animal models have some advantages in comparison to similar studies in humans. Little mammals have accelerated life cycle. That fact has been successfully used in shortening of the duration and costs of complex researches. The advantage of animal models is possibility to observe a great number of subjects allowing the generation of adequate data and confirmation of the complete importance of statistical analysis. In addition, using animal models make possible the realisation of more precise control of experiment conduction. The best option is to use the most investigated and most available animals. However, the most available animals are not necessarily the most appropriate ones for required animal model (3). An increased interest in development and utilization of in vitro and in vivo experimental models appeared in the beginning of nineties of the past century, particularly interest in investigation of the pathophysiologic skin disorders. For the majority of dermatological problems there are no models that completely reflect all symptoms and mechanisms normally present in clinical praxis (4). A unique problem in dermatological pharmacology is skin burn. Experimental variables might be better controlled in animal models reflecting, in significant extent, the situation in humans (5). Burns induce complex skin damages.
MATERIAL AND METHODS
STUDY PLAN AND EXPERIMENTAL ANIMALS
Prospective, cross-sectional, intervention animal study was designed to evaluate the influence of topical herbal preparations in burn wound healing process. Animals were raised and cared according to European Convention for the protection of vertebrate animals used for experimental and other scientific purposes (6). Healthy laboratory Rattus rats of Wistar type and albino sort were used in the study (rodents, mammals). These were all female animals with body weight of approximately 232 g. Animals originated from their own brood. During experiment performed during summertime, all animals were situated in appropriate cages, in rooms with natural light regime and humidity level, on controlled temperature (22 ± 2°C). Experimental animals were given food and water ad libitum.
STUDY PROTOCOL AND EXPERIMENTAL MODEL
Forty Wistar rat females were randomly sorted in four groups and put in quarantine for two weeks before the initiation of experiment. Each group consisted of 10 animals treated according to following schedule:
Group I -topical treatment with sulphadiazine silver cream;
Group I -topical treatment with Jomelop (extract of herbs);
Group III -topical treatment with HD ointment (contains an aqueous extract of following plants (Sambucus nigra L. Caprifoliaceae, Sanguisorba minor Bertol. Rosaceae, Teucrium chamaedrys L. Labiateae, Polypodium vulgaraeL. Polypodiaceae);
Group IV -control group.
All animals were anaesthetised by intraperitoneally applied pentobarbital sodium, in a dose of 35 mg/kg. Subsequent to that, animal hair was removed from the scapula zone of right side of the beck (5 cm x 5 cm). Burns were caused by adherence of the round metal seal heated on 80°C in water bath and through contact thermometer control for 14 seconds. Metal seal was just reclined on the rat skin without additional pressure. The dimensions of the utilized metal seal were: radius 2.5 cm, weight 100.0 g. In order to maintain the microcirculation of the burn wounds and to stimulate the conversion from burns of partial thickness to burns of full thickness, burns were immediately sunk in 4°C-water for 3 s. Following the inducement of burns, each animal was put individually in proper cage and left to rest for four hours. Though that period, careful monitoring of the burn expansion and animal general state were performed. Test preparations were applied twice a day until the complete re-epithelization of burn wound was achieved. Burn wound radius was measured daily by ruler throughout determination of its widest diameter. For the period of monitoring of the parameters of burn wound healing, two animals from each group were excluded in order to monitor changes of the burn wound surface. Animals were photographed each day using Olimpus digital camera, while burn wound surface was detected throughout software program Aut°CAD. Macroscopic and microscopic analyses were performed by classic morphofunctional method of examination.
STATISTICAL ANALYSIS
The following parameters were monitored in each group of animals: change in body weight, burn healing time, burn healing speed, morbidity parameters and mortality rate, level of achieved skin reparation that is histological status of burns. The initial animal body weights were measured just prior the inducement of burns. Subsequent to that, body weight determination was performed daily throughout first 10 days, and then in 5 day-intervals until the complete burn wound healing was achieved. All obtained results were presented and documented through digital Figures taken by a high-resolution Olympus camera. All differences pointed as p<0,05 were considered as significant.
RESULTS
CHANGES IN BODY WEIGHT
The percentage of body weight changes was analysed throughout t-test in order to determine the significance of differences. Obtained values are presented on Graph 1, with additional note, that every point is representing the percentage of average change in body weight of all animals from each animal group.
GRAPH 1.
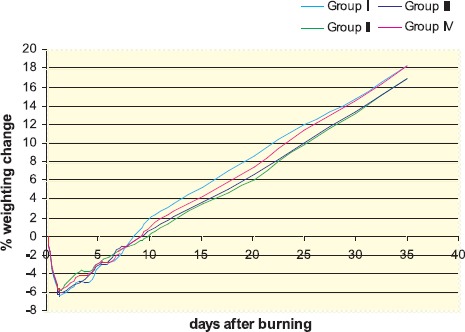
Changes in body weight of rats
BURN HEALING TIME
The study results of cumulative sizes of burn radiuses indicate the presence of statistically significant difference between treated and control animals. Bimodal grouping might be observed, as well. Animal groups treated with tested preparations are on one side while control group of animals is on the other side.
BURN SURFACE
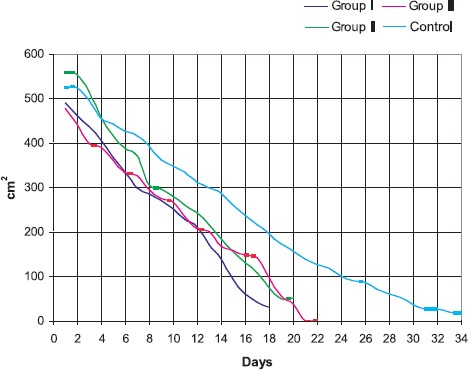
Exponential reduction in sizes of surfaces of burn wounds alongside with time distances from the moment of burn inducement is evident on Graph 4.
GRAPH 2.
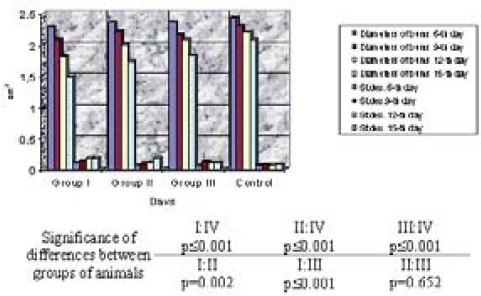
A cumulative overview of burn radiuses
GRAPH 3.
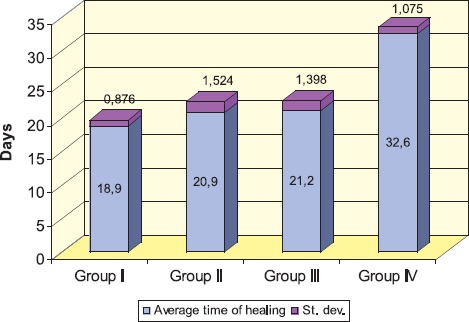
Time to achieve the complete burn wound healing
GRAPH 3.
Time to achieve the complete burn wound healing
MACROSCOPIC MANIFESTATION
Macroscopic expression of burn wounds was observed daily while the improvement in healing process was recorded. Burn wounds were defined as completely healed when eshar was entirely separated and wound surface re-epithelized.
Day 6: An improvement in wound healing process is observed (Figure 1A, D, G). Control burn is dry and shrivelled together with the presence of necrotic tissue. Its size approximately corresponds to size of three day-old treated burn (Figure 1J). Also, a difference in inflammatory reaction between treated and control animals is evident. Day 12: A significant improvement in healing process is evident. Formed incrustation is already dropped down or is in process of dropping down. Additionally, a difference in healing process between burns treated with herbal preparations and those treated with sulphadiazine silver is evident (Figure 1B, E,H). Healing of the control burns is characterised by irregular contraction of the newly-formed tissue (Figure 1K). Burn wounds treated with tested preparations are visibly less expressed in comparison to control ones, while being characterised by noticeable epithelization of the margins and uniform contraction of the newly-formed tissue. Day 22: Treated burn wounds are completely healed with minor and cosmetically acceptable scars. Cheloids, formed in rats treated with herbal preparations, are cos-metically much more acceptable if compared to those observed in rats treated with sulphadiazine silver or in control animals (Figure 1C, F,I). Rough healing process, accompanied with the formation of uneven and bumpy scars, is evident in control animals (Figure 1L).
FIGURE 1.
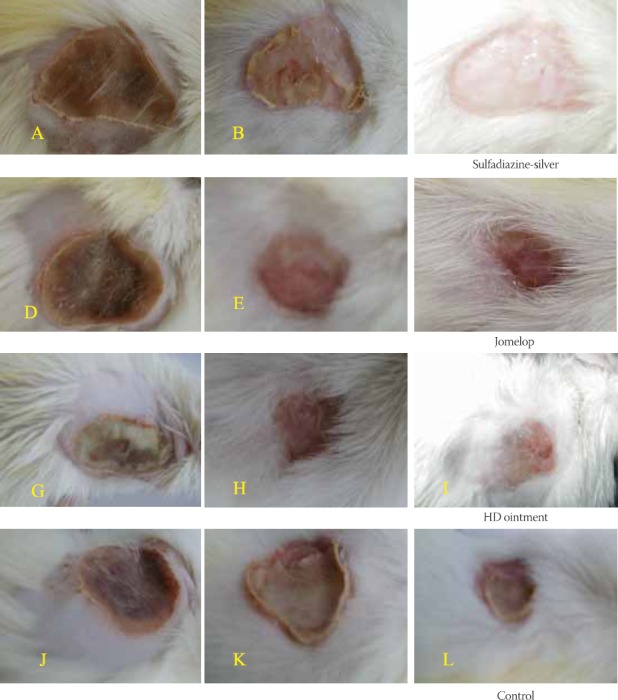
Macroscopic expression of the surface of burns treated with different preparation after 6,12 and 22 days
MORBIDITY PARAMETERS AND MORTALITY RATE
There were no signs of morbidity in our study of experimental burns. Additionally, no mortality was recorded during the study period. Achieved results are congruent with literature reports (mortality rate ranging from 0% to 5% in burns of partial thickness). Day 6: A wide zone of coagulated necrotic mass with visible keratinous superficial layer is evident in animals treated with sulphadiazine silver. It is separated from healthy skin by demarcation line formed of inflammatory-cell infiltration with prevailing presence of the leukocytes. Stripes of immature granulation tissue full of cells spread from the burn margins bellow the burn zone. Connective fibres in skin corium are swelled while vessels were mostly hyperaemic. Normal skin with numerous hair follicles and sweat glands are visible near the burn zone (Figure 2A). Day 6: Evidently distracted epidermis partially homogenous and with acidophilic properties is evident in animals treated with Jomelop. It is surrounded with massive cell detritus. Occasionally, in the deeper zone of cellular-inflammatory infiltration, numerous erythrocytes are present (Figure 2D). Some parts of demarcation zone, in the form of a slightly wider area, are characterised by coagulation exudate with accumulation of cellular-inflammatory infiltration and residues of necrotic tissue. In deep wound zone no structure alteration is evident. Day 6: A coagulation necrotic mass with abundant cellular detritus is present in animals treated with HD ointment (Figure 2C). Demarcation zone is formed of numerous inflammatory cells, mostly leukocytes. Connective tissue components are not present below the centre of burn wound. Scab material is directly reclined on granulation tissue with some cellular-inflammatory infiltration and blood vessels. Relatively rich, multiplied granulation tissue is present beside and below the burn zone. Day 6: There are numerous hyperkeratotic cells in fattened stratum corneum of control burns. Massive granulation inflammatory infiltration in the form of demarcation line is bond to corneal layer, beneath which, necrotic coagulated masses containing profuse cellular detritus are evident. Bellow the burn zone, some corium blood vessels are thrombosed while granulation tissue fills almost entire corium (Figure 2J). Day 12: A crust material, in the form of cellular detritus islets accompanied with deep-below demarcation zone, is observed in histological slices of burn wound treated with sulphadiazine silver (Figure 2B). In profound layers of connective tissue there are numerous of blood vessels surrounded by moderate number of fixed and mobile cells. Epidermis is thin and made of just few cell rows. Hair follicles are activated in the process of support in reconstruction of epidermis. Day 12: In histological tissue slices of burned skin treated with Jomelop, intensive fibroblast activation is evident particularly in the transition zone between burned and healthy skin (Figure 2E). A crust material, exceeding structurally preserved epidermis, can be observed. In maintained epidermis, especially alongside the margins of burn, there is a hypertrophic stratum spinosum. A granulation tissue with numerous blood vessels is below the burn zone. In healthy skin, stratum spinosum cells are multiplied and large while containing circular and huge nuclei. Stratum granulo-sum is a bit wider while stratum lucidum is practically invisible. Stratum granulosum is directly followed by hyperkeratotic stratum corneum, particularly in the zone of margin with superficial desquamation part. Day 12: In histological tissue slices made of burned skin treated with HD ointment, masses of destructed cellular debris are separated from multiplied granulation tissue (Figure 2H). Connective tissue components of corium are destructed in the form of areoles. Day 12: A crust formation, in the form of unstructured line beneath the skin tissue made of fibroblasts and inflammation cellular infiltration, is evident in control burn tissue slices (Figure 2K). Neutrophilic granulocytes, cellular detritus and mass of coccoid bacteria are recognisable in necrotic mass. Signs of oedema are visible in deeper layers of dermis. Connective fibres are swelled and separated while rich cellular infiltrations, mostly made of leukocytes, are evident. Day 22: Burns treated with sulphadiazine silver are covered with thin epidermis including keratin layer. In granulation tissue, somewhere below surface and somewhere a bit deeper, there is a disposal of calcium salts in the form of long continuous or irregular stripes parallel with epithelium (Figure 2C). Day 22: There is cellular infiltration forming a wide zone in superficial parts of dermis beneath the preserved epidermis of burns treated with Jomelop. Deep dermis is oede-matous with moderately dilated blood vessels. In central wound zone, masses of necrotising tissue and significant amounts of cellular detritus are present (Figure 2F). Day 22: Thin epidermis with unevenly wide layer of corneal cells is present in burns treated with HD ointment. Margins are formed of closely serried fibres allocated in unequal directions and containing numerous fibroblasts. Thin layer of inflammatory cellular infiltration reaches the formed epidermis, but only between the zones newly-formed and previous epithelium (Figure 2I). Day 22: Tightly serried fibres in asymmetrical directions with many fibroblasts and mobile cells are evident in tissue slices made of control burns (Figure 2L).
FIGURE 2.
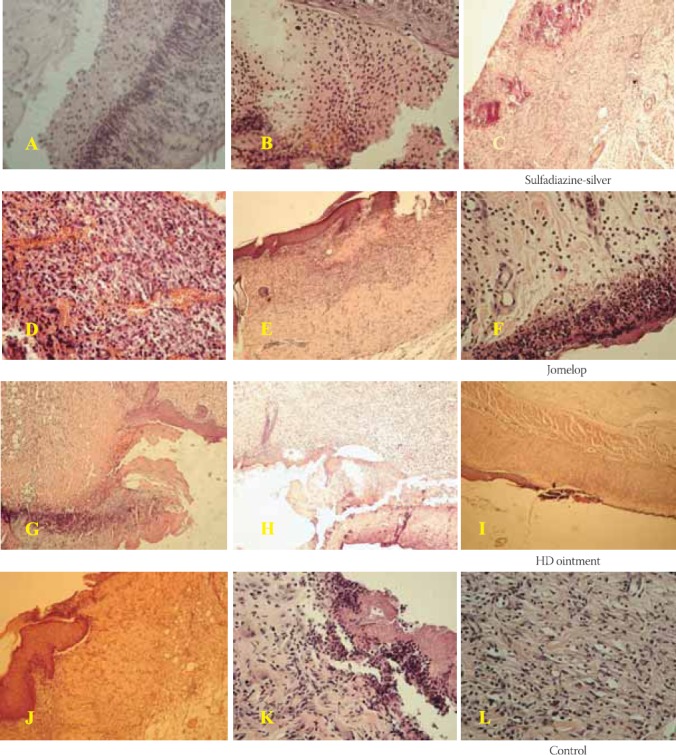
Histological appearance of the burns treated with different topical preparations after 6,12 and 22 days
DISCUSSION
There are many in vitro and in vivo models of wound healing described in literature. The choice of animal model depends on numerous factors including animal availability, costs, handling, as well as their anatomical and functional similarity with human body. Small animals are more frequently used in wound healing studies. Nevertheless, there are a lot of anatomical and physiological differences between frequently utilized small mammalians and humans. Pig skin is the most resembled to human skin from the anatomical and physiological point of view. But, the size of pigs is limiting factor for their larger utilization as animal models (7). In our model, a very available animal sort accomplishing all requirements is used (8,9,10). Body weight loss is a crucial characteristic of post-burning period. Hypermetabolic response of burned animals is frequently up to two times increased comparing to the state of normal metabolism. Body weight loss in post-burning period is in a direct proportion with depth and size of burn, physical state of burns, as well as the choice of treatment. In initial post-burning period, body weight loss is a result of metabolism and excretion of separated fluid inside the burns. Comparison between body masses of animals with identical burns is in direct relation with energy differences or metabolic balance (11). In our study, a statistically significant difference in the change of body mass between differently treated experimental animals is not established. Two probable curing phases in thermal wound healing processes are noticed in our histologically analysed material. The first one would be the removal of ne-crotic material, while the second one would be wound cicatrisation, that is, the appearance of newly-formed tissue. Control animals, not treated with protective medical preparations, have prolonged both first and second phase. From the reparation point of view, the most efficient healing is observed in wounds treated with sulphadiazine silver. Our finding is entirely in accordance with results noted by Isler et al., Snelling and Roberts, as well as by Fox et al. (5,10,12). In addition, subsequent healing phases are in accordance with the intensity of initial catabolism, wound purification and resorption of disintegration products. In the same time, these are basic determinants of anabolism extent and reparation status, as well (5). It is necessary to emphasise that minor skin injuries provide a greater possibility for inner immune system to accomplish more successful infection inhibition. Denatured eschar products, in II degree burns, primarily make a solid cover representing temporary blockage of the wound. Burn wound healing represent a dynamic relation between necrosis and pus against and macrophage and fibroblast activation. Burn wound healing can be divided to primary, secondary and tertiary healing. The significance of secondary burn wound healing has the most important role in investigation of the new topical preparations (13). The results of our study show that tested preparations reduce the duration of exudative and inflammatory phases of the wound healing in rats with standardised burns. Obtained results are consistent with those found in literature, representing the favourable effects of application of different herbal extracts on the processes of wound healing (14,15,16). The improvement in burn healing is monitored throughout the wound radius measuring method. Our study results demonstrate that burns treated with sulphadiazine silver heal more rapidly than control burns. Additionally, burns treated with herbal preparations heal more rapidly than control burns. The intensification of general protective response observed in rats after the application of tested preparations (day 9 vs. day 6) may explain the reduction of the burn radius. Applied herbal preparations probably improve anabolic phase of the burn wound healing by enabling a simpler resorption of the disintegration products and better tissue nourishment. The results of our study show that inception of the epithelisation of wound margins is more rapid in the group of animals treated with herbal preparations than in the control group of animals. In addition, wound healing is accompanied with more uniformed contraction and slighter cicatricial tissue than in control group. Scars formed in animals treated with herbal preparations are less visible and have, from the cosmetic point of view, better appearance. Neuromuscular deficit, hypermetabolism and immu-nosuppression are processes causing the weakening of organism while possessing a crucial role in morbidity and mortality of the patients suffering from burns. The prominent metabolic response in thermal wounds is manifested by the increased oxygen consumption (17). Almost identical results are achieved in human and animal studies (18). Neither mortality nor mortality in treated and control animals are noted in our study of experimental burns. Our study results are consistent with already published ones obtained from studies elaborating the same parameters (19). Histo-logical findings confirm results achieved throughout monitoring of the healing parameters of treated and control burns. Approximately one third of traditional “medicines” are purposed to treat wounds in relation to only 1-3% of all contemporary medicines (20). Our results clearly point the efficacy of topical herbal preparations in inducement of the wound healing process.
CONCLUSION
A simple, reproductive, low-cost and recommendable animal model for the investigation of thermal wound healing is adopted allowing insignificantly low morbidity and mortality in tested animals. The efficacy if sulphadiazine silver versus tested topical herbal preparations in the treatment of burn wounds is confirmed, as well. Additionally, tested herbal preparations appear to be efficacious in the healing of experimental burns comparing to control burn wounds.
REFERENCES
- 1.Webb M.L. Research. In: Alfonso R Gennaro, et al., editors. Remington:The Science and Practice of Pharmacy. 20th ed. Philadelphia: College of Pharmacy and Science; 2000. pp. 81–88. [Google Scholar]
- 2.Luellmann H, Mohr K. Pharmakologie und Toxikologie, 14. komplett ueberarbeitete und neu gestaltete Auflage. Georg Thieme Verlag Stuttgart-New York. 1999:40–41. [Google Scholar]
- 3.Rouse M.S, Wilson W.R. General Methodologies for Animal Models. In: Oto Zak., editor. Handbook of animal models of infection Experimental Models in Antimicrobial Chemotherapy. San Diego, London, Boston, New York, Sydney, Tokyo, Toronto: Academic Press; 1999. pp. 9–12. [Google Scholar]
- 4.Bouclier M, Cavey D, Kail N, Hensby C. Experimentalmodels in skin pharmacology:Pharmacological reviews. 1990;42(2):127–151. [PubMed] [Google Scholar]
- 5.Isler H, Bauen A, Baschong W. Topicaltreatment of standardized burns with a protein-free haemodialysate. Burns. 1991;17(2):93–98. doi: 10.1016/0305-4179(91)90130-9. [DOI] [PubMed] [Google Scholar]
- 6.European Convention for the protection of vertebrate animals used for experimental and other scientific purposes. Official Journal L 222. (Document 299A0824-01) 1999:0031–0037. [Google Scholar]
- 7.Sullivan T.P, Eaglstein W.H, Davis S.C, Mertz P. Thepig as a model for human wound healing. Wound. Repair. Regen. 2001;9(2):6676. doi: 10.1046/j.1524-475x.2001.00066.x. [DOI] [PubMed] [Google Scholar]
- 8.Walker H.L, Mason A.D. A standard animal burn. J. Trauma. 1968;8:1049–1051. doi: 10.1097/00005373-196811000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Mikuš D, Sikirić P, Seiwerth S, et al. Pentadecapeptide BPC 157 cream improves burn-wound healing and attenuates burn-gastric lesions in mice. Burns. 2001;27(8):817–27. doi: 10.1016/s0305-4179(01)00055-9. [DOI] [PubMed] [Google Scholar]
- 10.Snelling C.F, Roberts F.J. Comparisonof 1% sulfadiazine with and without 1°% chlorhexidine digluconate for topical antibacterial effect in the burnt infected rat. J Burn. Care. Rehabil. 1988;9(1):35–40. doi: 10.1097/00004630-198801000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Thorton J.W, Hess C.A, Cassingham V, Bartlett R. Epidermalgrowth factor in the healing of second degree burns a controlled animal study. Burns. 1981;8:156–160. doi: 10.1016/0305-4179(82)90079-1. [DOI] [PubMed] [Google Scholar]
- 12.Fox C.L, Rao T.N, Azmeth R, Gandhi S.S, Modak S. Comparativeevaluation of zinc sulfadiazine and silver sulfadiazine in burn wound infection. J Burn. Care. Rehabil. 1990;11(2):112–117. doi: 10.1097/00004630-199003000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Brown R, Kinsty D. Strategiesfor cell engineering in tissue repair. Wound. Rep. Reg. 1997;5:212–221. doi: 10.1046/j.1524-475X.1997.50304.x. [DOI] [PubMed] [Google Scholar]
- 14.Mensah A.Y, Sampson J, Houghton P.J, et al. Effects of Buddleja globosa leaf and its constituents relevant to wound healing. J. Ethno-pharmacol. 2001;77(2-3):219–226. doi: 10.1016/s0378-8741(01)00297-5. [DOI] [PubMed] [Google Scholar]
- 15.Parck E.H, Chun M.J. Wound healing activity of Opuntia ficus-in-dica. Fitoterapia. 2001;72(2):165–167. doi: 10.1016/s0367-326x(00)00265-3. [DOI] [PubMed] [Google Scholar]
- 16.Choi S.W, Son B.W, Son Y.S, Park Y.I, Lee S.K, Chung M.H. Thewound-healing effect of glycoprotein fraction isolated from aloe vera. Br J Dermatol. 2001;145(4):535–545. doi: 10.1046/j.1365-2133.2001.04410.x. [DOI] [PubMed] [Google Scholar]
- 17.Herndon D.N, Wilmore D.W, Mson A.D. Development an analysis of a small animal model simulating the human post burn hypo metabolic response. J. Surg. Res. 1978;25:394. doi: 10.1016/s0022-4804(78)80003-1. [DOI] [PubMed] [Google Scholar]
- 18.Barrow R.E, Meyer N.A, Jeschke M.G. Effect of varying burn size and ambient temperature on the hyper metabolic rate in thermally injured rats. J. Surg. Res. 2001;99(2):253–257. doi: 10.1006/jsre.2001.6183. [DOI] [PubMed] [Google Scholar]
- 19.Mantle D, Gok M.A, Lennard T.W. Adverse and beneficial effects of plant extracts an skin and skin disorders. Adverse Drug React. Toxicol. Rev. 2001;20(2):89–103. [PubMed] [Google Scholar]


