Abstract
Numerous studies have evaluated the effects of exercise training on obese children and adolescents. However, the impact of aerobic and/or resistance exercise alone, without any other interventions, on vascular markers and C-reactive protein (CRP) in obese children and adolescents is still not clear. We performed a literature search in Ovid Medline, PubMed, and SCOPUS databases to identify articles on the effects of exercise on vascular markers and CRP among obese children and adolescents, published between January 2009 and May 2019. Only full-text articles in English that reported on the effect of aerobic and/or resistance exercise on the vascular markers pulse wave velocity (PWV), carotid intima-media thickness (CIMT), flow-mediated dilatation (FMD), augmentation index (AIx), or CRP in obese children and adolescents (5–19 years old) were included. The literature search identified 36 relevant articles; 9 articles that fulfilled all the inclusion criteria were selected by two independent reviewers. Aerobic exercise or a combination of aerobic and resistance exercise training significantly improved CIMT and PWV in obese children and adolescents in all studies in which they were measured (2 studies for PWV and 4 studies for CIMT). However, the effects of exercise on FMD and CRP levels were inconclusive, as only half of the studies demonstrated significant improvements (1/2 studies for FMD and 4/8 studies for CRP). The results of our review support the ability of exercise to improve vascular markers such as PWV and CIMT in obese children and adolescents. This finding is important as obesity is a modifiable risk factor of cardiovascular disease (CVD), and exercise may help in reducing the future occurrence of CVD in this population.
Keywords: Exercise, vascular marker, inflammation, C-reactive protein, obese children and adolescents
INTRODUCTION
Obesity is a complex condition that involves extra deposition of adipose tissue and visceral adiposity, as well as adipocyte dysfunction [1]. Obesity is considered as a worldwide emergency in public health, affecting both adults and children. In the United States (USA), obesity is considered as a distinct disease and the third leading cause of preventable death, accounting for 216,000 deaths in 2005 alone [2,3]. The economic burden of lifetime medical cost linked to obesity is summed up to nearly $14 billion [4].
The prevalence of obesity especially in children has been dramatically increasing since 1975. The rising incidence of overweight/obese children globally is worrying, and it has tripled in the past four decades. In 2016, more than 18% of children and adolescents aged 5–19 years were overweight/obese worldwide, which is equivalent to 340 million individuals, compared to only 4% in 1975 [5].
Since 2012, cardiovascular disease (CVD) is the leading cause of death in the world [5]. One of the contributing risk factors is obesity. Farpour-Lambert et al. indicated that the first sign of atherosclerosis may develop even before puberty in obese children [6]. Therefore, obese children possess a higher risk of metabolic syndrome and CVD [7].
Exercise is one form of physical activity that has been proven in the prevention and treatment of CVD by improving CVD risk profile, reducing the number of cardiovascular events and hospitalization in patients with CVD [8-11]. A review of 20 studies on overweight and obese children and adolescents showed the positive effect of exercise in reducing body mass index (BMI) [12]. To achieve significant health effect, children and adolescents should perform at least moderate-intensity activities [13], 60 minutes daily as recommended by the WHO [5]. However, obese children spend more time in low-intensity physical activities as they have lower cardiorespiratory fitness (CRF) compared to their normal-weight peers [14].
There are several vascular markers that can be utilized to predict and assess the development of atherosclerosis. Atherosclerosis can be reflected upon the local changes of the affected artery caused by the atherosclerotic plaque. Atherosclerosis may affect the elasticity, endothelial function, and inflammatory state of the affected artery, which can be assessed by several parameters including pulse wave velocity (PWV), carotid intima-media thickness (CIMT), flow-mediated dilatation (FMD), augmentation index (AIx), and C-reactive protein (CRP) [15,16].
PWV measures arterial stiffness, whereby higher PWV indicates less arterial elasticity. A compromised elasticity of the vessels causes arterial stiffness and may jeopardize the functional properties of large arteries, therefore, signaling the early stage of atherosclerosis [17]. AIx also measures arterial stiffness through the ascending aorta pressure waveform [18]. Early and late stage of subclinical atherosclerosis can be detected using CIMT [19]. Meanwhile, FMD assesses endothelial function by calculating artery relaxation in response to increased shear stress [20]. CRP or high-sensitivity (hs)-CRP is a marker of inflammation and measuring its plasma level is a predictive tool to signify the inflammatory state of the affected artery. hs-CRP is more sensitive than CRP and it can be detected as low as 0.15 mg/dL [21].
The benefits of exercise on reducing the CVD-related risk among obese children are unparalleled. Vascular markers such as PMV, CIMT, and FMD are good assessment tools in evaluating atherosclerosis. However, to the best of our knowledge, no studies have systematically correlated exercise with vascular markers and inflammation in CVD risk assessment. As childhood obesity increases the risk of CVD [22], studies on vascular markers and inflammation among obese children and adolescents are strongly needed. Therefore, this review aimed to elucidate the influence of exercise on vascular markers with CRP among obese children.
MATERIALS AND METHODS
Literature review
A review of previous literature was performed to identify relevant studies focusing on the effects of exercise on vascular markers and inflammation among obese children/adolescents. The literature search was conducted using Ovid Medline, PubMed, and Scopus databases (published between January 2009 and May 2019). The search strategy involved a combination of three keywords: 1) “exercise” OR “physical activity” AND 2) (“pulse wave velocity” OR “arterial stiffness”) OR “augmentation index” OR (“flow-mediated dilatation” OR “endothelial function”) OR “carotid intima media thickness” OR (“inflammation” OR “C-reactive protein”) AND 3) “obese” OR “overweight” AND “child” OR “adolescent”.
Selection of research articles
Non-English articles were excluded from this review. Review articles, proceedings, supplementary issues, poster presentations, books, bibliographies, letter to the editor, case reports, and consensus/statement/guideline were also excluded from the review. For this review, only studies reporting the effects of exercise on 1) vascular markers or 2) inflammation in 3) obese children/adolescents were included.
Inclusion and exclusion criteria
Only studies that stated the direct effects of exercise on at least one of the vascular markers or CRP among obese children/adolescents were included. Studies were taken into account if at least one of these cardiovascular risk markers was measured: 1) AIx; 2) CIMT; 3) FMD; 4) PWV; or 5) CRP. The type of activity only involved aerobic and/or resistance exercises. The study population was limited to children and adolescents within the age range of 5–19 years.
Data extraction and management
Articles were screened in three phases before finally included in this review. Initially, the titles and keywords that did not fulfill the inclusion criteria were excluded. The remaining articles that met the inclusion criteria were further screened by abstracts. In the final phase, the remaining articles were judged by two independent reviewers thoroughly to exclude any articles that did not meet the inclusion criteria. The articles selected for this review were finalized only when both reviewers agreed to the inclusion of the selected articles. Any differences in opinion were resolved by proper discussion. To standardize data extraction from the selected articles, a data extraction form was designed that collects data comprising of: 1) population and sample size; 2) mean age; 3) age range; 4) male percentage; 5) BMI; 6) types of exercise; 7) methodology of exercise intervention; and 8) effect of exercise on selected vascular markers or inflammation.
Search results
The literature searches identified 600 potentially relevant articles. Based on the titles, keywords, and abstract, the two independent reviewers excluded 140 items as they did not meet the inclusion criteria. Another 283 articles were further excluded as the studies did not involve exercise as an intervention step, no direct measurement on the effects of exercise on the vascular markers of interest or inflammation, or the study populations were not obese children/adolescents. Next, 27 out of 177 remaining articles were excluded due to duplication. Out of the remaining 36 articles, only 9 articles were included in this review for further assessment and data extraction. To remove any confounding factors, 27 articles were excluded due to the studies involving combination of other interventions such as diet restriction/counseling and psychological counseling during the intervention period, which was in contrast with our inclusion criteria. Figure 1 shows the flowchart of the selection process, including the reasons for the exclusion.
FIGURE 1.
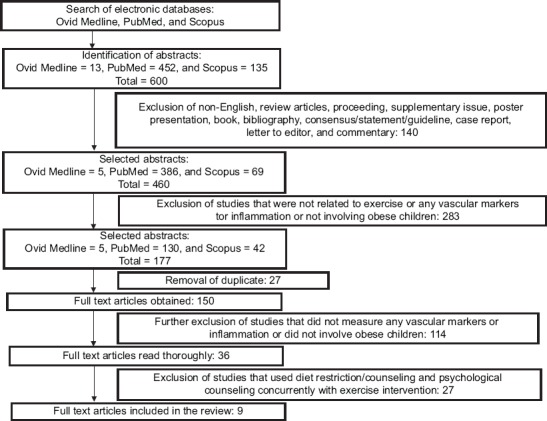
Flowchart of article selection and exclusion process.
Study characteristics
Tables 1 and 2 show the characteristics of the selected studies. All studies were published between January 2009 and May 2019. The types of exercise included aerobic and resistance exercises. The age range of the individuals involved was between 5 and 19 years and it followed the WHO age classification and the physical requirement for obesity in children and adolescents [1]. All studies, except Chuensiri et al. [23], measured CRP or hs-CRP as an inflammatory marker, while none of the studies measured AIx. Two studies used brachial-ankle PWV (baPWV) to measure arterial stiffness. CIMT and FMD were determined in 4 and 2 studies, respectively. All studies involved obese children/adolescents without existing comorbidities, except for 1 study that involved obese children/adolescents with hyperinsulinemia and abdominal obesity.
TABLE 1.
Demographic data of the subjects from the included studies

TABLE 2.
Physical exercise parameters and significant effects of exercise on vascular markers and inflammation in the included studies

RESULTS AND DISCUSSION
Exercise is a form of physical activity that is widely known for its health-improving benefits. Exercise is considered as lifestyle intervention that helps to reduce excess fat deposit and improves cardiovascular function [24,25]. The two types of exercise that are commonly practiced are aerobic and resistance exercises. Aerobic exercise helps to improve cardiovascular adaptations and increase CRF without significantly changing muscle strength. This kind of activity utilizes energy from aerobic metabolism in the form of adenosine triphosphate (ATP) [26]. In contrast, resistance exercise increases muscle strength without significantly increasing CRF, and this involves neuromuscular adaptations [27]. Both types of exercise training improve systolic blood pressure, low-density lipoprotein (LDL), triglycerides (TGs), fasting blood glucose, body composition, and FMD in obese children [28-32]. However, the combination of aerobic and resistance exercises results in a greater improvement of CRF and the quality of life among CVD patients with obesity [33]. Furthermore, the American College of Sports Medicine recommended a combination of aerobic and resistance exercise for significant weight loss [34]. A recent study also suggested this combination of exercise to achieve a substantial reduction in fat deposits among overweight and obese youths [35].
PWV quantifies arterial stiffness non-invasively. An increase in PWV is directly proportional to increased stiffness of the artery. This signifies lower vascular compliance that increases the risk of future cardiovascular events [36]. In this review, only two studies measured PWV, specifically baPWV, and both showed significant reductions after 12 weeks of exercise intervention [23,37]. The exercise-induced reduction of arterial stiffness may be mediated by a decrease of α-adrenergic receptor tone in arterial smooth muscle cells; thus, increasing arterial elasticity [38]. Maeda et al. suggested that a decrease in endothelin-1 improves arterial stiffness. Low level of endothelin-1 reduces the shear stress on the arterial wall hence decreasing vascular tone. Consequently, decreased vascular tone leads to better arterial compliance [39]. Previous studies have reported that better arterial compliance was achieved through the combination of aerobic and resistance exercises rather than aerobic exercise alone [23,37]. In older adults, combined exercise programs showed more significant effects in reducing baPWV compared to aerobic exercise alone [24]. Recent studies further supported that resistance exercise leads to better cardiovascular outcomes [40-42].
CIMT is a measure of the thickness of the tunica media and intima layer in the carotid arterial wall and is used to assess the extent of carotid atherosclerotic diseases [43,44]. The early stage of atherosclerosis is marked by the thickening of the intima-media layer [45]. Surprisingly, obese children have CIMT similar to a 45-year-old, which reflects on the build-up of plaque in the arteries. In addition, these children have a risk of developing CVD as early as the age of 30 [46]. Exercise intervention affects CIMT the most, and all four studies measuring CIMT showed a significant reduction in CIMT after completion of 12 weeks of exercise training [6,23,47,48]. The significant CIMT reduction in all studies may be due to substantial CRF improvement. A moderate- to high-intensity exercise training increases CRF, and CRF is considered as the primary determinant of CIMT value [49]. On the other hand, increased shear stress during exercise modulates arterial wall structure by reducing the level of monocyte chemoattractant protein-1 (MCP-1) and vascular cell adhesion molecule (VCAM). This decreases vascular wall permeability to LDL cholesterol and eventually reduces the thickness of the arterial wall [50].
FMD measures the dilation of an artery when blood flow increases, by high-resolution ultrasound [51]. FMD can also be interpreted as a tool to assess endothelial function whereby the dilation of artery depends on the release of nitric oxide (NO) by the endothelial cells. FMD showed a significant correlation with cardiovascular events in patients with chest pain but without previous coronary artery disease (CAD) who had undergone coronary angiography [52]. Moreover, a multi-ethnic study on atherosclerosis that involved more than 3000 subjects supported the fact that FMD is a viable tool to predict future cardiovascular events [53]. In this review, two studies measured FMD, but only one showed a significant increment after aerobic exercise training [6,23]. Aerobic training increases cardiac output and puts an impact (shear stress) on vascular walls, which in turn stimulates the release of NO into bloodstream [54]. Endothelial NO is a known vasodilator produced mainly by endothelial nitric oxide synthase (eNOS). An increase in NO will trigger vasodilatation thus reflecting upon increased FMD. Chuensiri et al. found that blood NO level increases following aerobic exercise training [23]. This finding is in accordance with the study by Mantione et al., where the level of NO in the exhaled air in moving subjects was much lower compared to fully resting subjects [55]. In addition, the eNOS level doubled in CAD patients who exercised compared to sedentary CAD patients [56].
CRP or hs-CRP is an inflammatory marker strongly associated with an increased risk of CVD [57]. Physically active individuals have 19–35% lower level of CRP compared to inactive fellows [58]. Hence, the level of CRP fluctuates, depending on the physical activity of individuals [59]. From our analysis, only half of the studies that measured CRP demonstrated a significant reduction in CRP after exercise intervention among obese children and adolescents [37,47,60,61]. A previous review also reported the similar trend, where less than half of exercise intervention contributed to significant improvements in CRP [62]. Another study highlighted the importance of body weight and fat percentage reduction in decreasing CRP level in exercised subjects [57]. All studies with significant CRP improvement showed a decrease in body weight and fat percentage post-exercise. These findings could indicate that improved CRP is not directly correlated with exercise intervention but is dependent on the level of weight reduction through exercise [63].
The level of CRP is influenced by the level of pro-inflammatory cytokines, such as interleukin-6 (IL-6), that are involved in atherosclerosis progression. IL-6 has been suggested as an essential link to the rising level of CRP in CVD. IL-6 is secreted in adipose tissue, lymphocytes, and activated macrophages, and it controls the synthesis of CRP in the liver. Therefore, a decrease of body weight in exercised subjects will reduce IL-6 and, in turn, CRP, as less fat deposit is available [62]. IL-6 may also be induced by the pro-inflammatory cytokines IL-1β and tumor necrosis factor-α (TNF-α), which are overexpressed in obesity. These are, however, not investigated herein. Overexpression of IL-1β and TNF-α amplifies the inflammatory cascade and activates reactive oxygen species (ROS) production eventually promoting CVD risk [64,65]. Exercise may help to reduce the overexpression of IL-1β and TNF-α, which consecutively decreases IL-6 and CRP [66]. A low level of CRP results in low permeability of the vascular wall to LDL, thus, reducing the risk of CVD [49].
A non-significant decrease in CRP may be due to low exercise intensity or duration. Longer exercise duration causes a constant elevation of basal metabolic rate that leads to a significant loss in body weight and fat deposit [23]. Another factor that may affect the results is the time of the CRP measurement. The time of CRP measurements was not stated in the included studies, except in Vasconcellos et al. [47] and Park et al. [48], and this may be one of the confounding factors. Plaisance and Grandjean suggested that CRP level should be observed days after the final exercise session for a better reflection on CVD risk [59].
Other confounding factors that could affect the results of our review are hormonal and sex differences between the included studies. These two factors are known to affect vascular function and inflammatory level [67,68]. It was observed that aortic stiffness and CRP level changed during menstrual cycle whereby their level was increased during follicular phase compared to midluteal phase [67,68].
Limitations of our study are: 1) few studies were examined; 2) only CRP or hs-CRP was measured as the marker of inflammation; and 3) other CVD risk factors such as oxidative stress markers, lipid profiles, and coagulation factors were not included.
CONCLUSION
Aerobic and/or resistance exercise exert positive effects on vascular markers, namely PWV, CIMT, and FMD. However, the impact of exercise on the inflammatory marker CRP is still controversial, as only half of the study included in this review showed a significant reduction. The effect of exercise on AIx was not discussed in this review, because the included studies did not measure AIx. In addition, our conclusion is limited to the 9 studies that focused on exercise only, without the interference of other types of intervention, and on 5–19 years old obese children and adolescents. More studies should be conducted to address these issues.
ACKNOWLEDGMENTS
This research was funded by The National University of Malaysia, GUP-2017-096.
Footnotes
Conflict of interest statement: The authors declare no conflict of interests
REFERENCES
- 1.Khan M, Joseph F. Adipose tissue and adipokines:The association with and application of adipokines in obesity. Scientifica. 2014;2014:1–7. doi: 10.1155/2014/328592. https://doi.org/10.1155/2014/32←. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pollack A. A.M.A. Recognizes Obesity as a Disease. [Last accessed on 2018 May 20]. Available from: http://www.nytimes.com/2013/06/19/business/ama-recognizes-obesity-as-a-disease.html.
- 3.Danaei G, Ding EL, Mozaffarian D, Taylor B, Rehm J, Murray CJ, et al. The preventable causes of death in the United States:Comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med. 2009;6:e1000058. doi: 10.1371/journal.pmed.1000058. https://doi.org/10.1371/journal.pmed.1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finkelstein EA, Graham WC, Malhotra R. Lifetime direct medical costs of childhood obesity. Pediatrics. 2014;133:854–62. doi: 10.1542/peds.2014-0063. https://doi.org/10.1542/peds.2014-0063. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Obesity and Overweight. [Last accessed on 2018 May 10]. Available from: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight .
- 6.Farpour-Lambert NJ, Aggoun Y, Marchand LM, Martin XE, Herrmann FR, Beghetti M, et al. Physical activity reduces systemic blood pressure and improves early markers of atherosclerosis in pre-pubertal obese children. J Am Coll Cardiol. 2009;54:2396–406. doi: 10.1016/j.jacc.2009.08.030. https://doi.org/10.1016/j.jacc.2009.08.030. [DOI] [PubMed] [Google Scholar]
- 7.Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. 2004;350:2362–74. doi: 10.1056/NEJMoa031049. https://doi.org/10.1056/nejmoa031049. [DOI] [PubMed] [Google Scholar]
- 8.Lewinter C, Doherty P, Gale CP, Crouch S, Stirk L, Lewin RJ, et al. Exercise-based cardiac rehabilitation in patients with heart failure:A meta-analysis of randomised controlled trials between 1999 and 2013. Eur J Prev Cardiol. 2015;22:1504–12. doi: 10.1177/2047487314559853. https://doi.org/10.1177/2047487314559853. [DOI] [PubMed] [Google Scholar]
- 9.Sibilitz KL, Berg SK, Tang LH, Risom SS, Gluud C, Lindschou J, et al. Exercise-based cardiac rehabilitation for adults after heart valve surgery. Cochrane Database Syst Rev. 2016;3:CD010876. doi: 10.1002/14651858.CD010876.pub2. https://doi.org/10.1002/14651858.cd010876.pub2. [DOI] [PubMed] [Google Scholar]
- 10.Lane R, Ellis B, Watson L, Leng GC. Exercise for intermittent claudication. Cochrane Database Syst Rev. 2014;7:CD000990. doi: 10.1002/14651858.CD000990.pub3. https://doi.org/10.1002/14651858.cd000990.pub3. [DOI] [PubMed] [Google Scholar]
- 11.Kelley GA, Kelley KS, Pate RR. Exercise and BMI in overweight and obese children and adolescents:A systematic review and trial sequential meta-analysis. Biomed Res Int. 2015;2015:704539. doi: 10.1155/2015/704539. https://doi.org/10.1249/01.mss.0000466061.73312.fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janssen I, Leblanc AG. Systematic review of the health benefits of physical activity and fitness in school-aged children and youth. Int J Behav Nutr Phys Act. 2010;7:40. doi: 10.1186/1479-5868-7-40. https://doi.org/10.1186/1479-5868-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trost SG, Kerr LM, Ward DS, Pate RR. Physical activity and determinants of physical activity in obese and non-obese children. Int J Obes Relat Metab Disord. 2001;25:822–9. doi: 10.1038/sj.ijo.0801621. https://doi.org/10.1038/sj.ijo.0801621. [DOI] [PubMed] [Google Scholar]
- 14.Roberfroid D, San Miguel L, Thiry N. Novel Serum Biomarkers for the Prediction of Cardiovascular Risk:A Technology Assessment (HTA) Brussels: Belgian Health Care Knowledge Center (KCE); 2013. Belgian:Creative Common Lisence. [Google Scholar]
- 15.Polak JF, Pencina MJ, Pencina KM, O'Donnell CJ, Wolf PA, D'Agostino RB, Sr, et al. Carotid-wall intima-media thickness and cardiovascular events. N Engl J Med. 2011;365:213–21. doi: 10.1056/NEJMoa1012592. https://doi.org/10.1056/nejmoa1012592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults:A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2010;122:e584–636. doi: 10.1161/CIR.0b013e3182051b4c. https://doi.org/10.1161/cir.0b013e3182051b4c. [DOI] [PubMed] [Google Scholar]
- 17.Wilmer CV, Nichols W, O'Rourke MF. McDonald's Blood Flow in Arteries Theoretical, Experimental and Clinical Principles. London: CRC Press; 2005. [Google Scholar]
- 18.Pignoli P, Tremoli E, Poli A, Oreste P, Paoletti R. Intimal plus medial thickness of the arterial wall:A direct measurement with ultrasound imaging. Circulation. 1986;74:1399–406. doi: 10.1161/01.cir.74.6.1399. https://doi.org/10.1161/01.cir.74.6.1399. [DOI] [PubMed] [Google Scholar]
- 19.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, et al. Abridged version of the expert consensus document on arterial stiffness. Artery Res. 2007;1:2–12. doi: 10.1093/eurheartj/ehl254. https://doi.org/10.1016/j.artres.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Moens AL, Goovaerts I, Claeys MJ, Vrints CJ. Flow-mediated vasodilation:A diagnostic instrument, or an experimental tool? Chest. 2005;127:2254–63. doi: 10.1378/chest.127.6.2254. https://doi.org/10.1378/chest.127.6.2254. [DOI] [PubMed] [Google Scholar]
- 21.Futterman LG, Lemberg L. High-sensitivity C-reactive protein is the most effective prognostic measurement of acute coronary events. Am J Crit Care. 2002;11:482–6. https://doi.org/10.4037/ajcc2002.11.5.482. [PubMed] [Google Scholar]
- 22.Daniels SR. The consequences of childhood overweight and obesity. Future Child. 2006;16:47–67. doi: 10.1353/foc.2006.0004. https://doi.org/10.1353/foc.2006.0004. [DOI] [PubMed] [Google Scholar]
- 23.Chuensiri N, Suksom D, Tanaka H. Effects of high-intensity intermittent training on vascular function in obese preadolescent boys. Child Obes. 2018;14:41–9. doi: 10.1089/chi.2017.0024. https://doi.org/10.1089/chi.2017.0024. [DOI] [PubMed] [Google Scholar]
- 24.Figueroa A, Park SY, Seo DY, Sanchez-Gonzalez MA, Baek YH. Combined resistance and endurance exercise training improves arterial stiffness, blood pressure, and muscle strength in postmenopausal women. Menopause. 2011;18:980–4. doi: 10.1097/gme.0b013e3182135442. https://doi.org/10.1097/gme.0b013e3182135442. [DOI] [PubMed] [Google Scholar]
- 25.Weinstein Y, Kamerman T, Berry E, Falk B. Mechanical efficiency of normal-weight prepubertal boys predisposed to obesity. Med Sci Sports Exerc. 2004;36:567–73. doi: 10.1249/01.mss.0000121958.99985.a5. https://doi.org/10.1249/01.mss.0000121958.99985.a5. [DOI] [PubMed] [Google Scholar]
- 26.Patel H, Alkhawam H, Madanieh R, Shah N, Kosmas CE, Vittorio TJ, et al. Aerobic vs anaerobic exercise training effects on the cardiovascular system. World J Cardiol. 2017;9:134–8. doi: 10.4330/wjc.v9.i2.134. https://doi.org/10.4330/wjc.v9.i2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lambert CP, Evans WJ. Adaptations to aerobic and resistance exercise in the elderly. Rev Endocr Metab Disord. 2005;6:137–43. doi: 10.1007/s11154-005-6726-5. https://doi.org/10.1007/s11154-005-6726-5. [DOI] [PubMed] [Google Scholar]
- 28.Escalante Y, Saavedra JM, García-Hermoso A, Domínguez AM. Improvement of the lipid profile with exercise in obese children:A systematic review. Prev Med. 2012;54:293–301. doi: 10.1016/j.ypmed.2012.02.006. https://doi.org/10.1016/j.ypmed.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 29.García-Hermoso A, Saavedra JM, Escalante Y. Effects of exercise on resting blood pressure in obese children:A meta-analysis of randomized controlled trials. Obes Rev. 2013;14:919–28. doi: 10.1111/obr.12054. https://doi.org/10.1111/obr.12054. [DOI] [PubMed] [Google Scholar]
- 30.Dias KA, Green DJ, Ingul CB, Pavey TG, Coombes JS. Exercise and vascular function in child obesity:A meta-analysis. Pediatrics. 2015;136:e648–59. doi: 10.1542/peds.2015-0616. https://doi.org/10.1542/peds.2015-0616. [DOI] [PubMed] [Google Scholar]
- 31.García-Hermoso A, Saavedra JM, Escalante Y, Sánchez-López M, Martínez-Vizcaíno V. Endocrinology and adolescence:Aerobic exercise reduces insulin resistance markers in obese youth:A meta-analysis of randomized controlled trials. Eur J Endocrinol. 2014;171:R163–71. doi: 10.1530/EJE-14-0291. https://doi.org/10.1530/eje-14-0291. [DOI] [PubMed] [Google Scholar]
- 32.Schranz N, Tomkinson G, Olds T. What is the effect of resistance training on the strength, body composition and psychosocial status of overweight and obese children and adolescents?A systematic review and meta-analysis. Sports Med. 2013;43:893–907. doi: 10.1007/s40279-013-0062-9. https://doi.org/10.1007/s40279-013-0062-9. [DOI] [PubMed] [Google Scholar]
- 33.Marzolini S, Oh PI, Brooks D. Effect of combined aerobic and resistance training versus aerobic training alone in individuals with coronary artery disease:A meta-analysis. Eur J Prev Cardiol. 2012;19:81–94. doi: 10.1177/1741826710393197. https://doi.org/10.1177/1741826710393197. [DOI] [PubMed] [Google Scholar]
- 34.American College of Sports Medicine, editor. ACSM's Guidelines for Exercise Testing and Prescription. Philadelphia, USA: Lippincott Williams and Wilkins; 2013. [DOI] [PubMed] [Google Scholar]
- 35.García-Hermoso A, Ramírez-Vélez R, Ramírez-Campillo R, Peterson MD, Martínez-Vizcaíno V. Concurrent aerobic plus resistance exercise versus aerobic exercise alone to improve health outcomes in paediatric obesity:A systematic review and meta-analysis. Br J Sports Med. 2018;52:161–6. doi: 10.1136/bjsports-2016-096605. https://doi.org/10.1136/bjsports-2016-096605. [DOI] [PubMed] [Google Scholar]
- 36.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness:A systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1318–27. doi: 10.1016/j.jacc.2009.10.061. https://doi.org/10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 37.Wong A, Sanchez-Gonzalez MA, Son WM, Kwak YS, Park SY. The effects of a 12-week combined exercise training program on arterial stiffness, vasoactive substances, inflammatory markers, metabolic profile, and body composition in obese adolescent girls. Pediatr Exerc Sci. 2018;30:480–6. doi: 10.1123/pes.2017-0198. https://doi.org/10.1123/pes.2017-0198. [DOI] [PubMed] [Google Scholar]
- 38.Sugawara J, Komine H, Hayashi K, Yoshizawa M, Otsuki T, Shimojo N, et al. Reduction in alpha-adrenergic receptor-mediated vascular tone contributes to improved arterial compliance with endurance training. Int J Cardiol. 2009;135:346–52. doi: 10.1016/j.ijcard.2008.04.007. https://doi.org/10.1016/j.ijcard.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maeda S, Sugawara J, Yoshizawa M, Otsuki T, Shimojo N, Jesmin S, et al. Involvement of endothelin-1 in habitual exercise-induced increase in arterial compliance. Acta Physiol (Oxf) 2009;196:223–9. doi: 10.1111/j.1748-1716.2008.01909.x. https://doi.org/10.1111/j.1748-1716.2008.01909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tikkanen E, Gustafsson S, Ingelsson E. Associations of fitness, physical activity, strength, and genetic risk with cardiovascular disease:Longitudinal analyses in the UK biobank study. Circulation. 2018;137:2583–91. doi: 10.1161/CIRCULATIONAHA.117.032432. https://doi.org/10.1161/circulationaha.117.032432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shiroma EJ, Cook NR, Manson JE, Moorthy MV, Buring JE, Rimm EB, et al. Strength training and the risk of Type 2 diabetes and cardiovascular disease. Med Sci Sports Exerc. 2017;49:40–6. doi: 10.1249/MSS.0000000000001063. https://doi.org/10.1249/mss.0000000000001063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Åberg ND, Kuhn HG, Nyberg J, Waern M, Friberg P, Svensson J, et al. Influence of cardiovascular fitness and muscle strength in early adulthood on long-term risk of stroke in Swedish men. Stroke. 2015;46:1769–76. doi: 10.1161/STROKEAHA.115.009008. https://doi.org/10.1161/strokeaha.115.009008. [DOI] [PubMed] [Google Scholar]
- 43.Raghuveer G. Lifetime cardiovascular risk of childhood obesity. Am J Clin Nutr. 2010;91:1514S–1519S. doi: 10.3945/ajcn.2010.28701D. https://doi.org/10.3945/ajcn.2010.2⇽d. [DOI] [PubMed] [Google Scholar]
- 44.Głowińska-Olszewska B, Tołwińska J, Urban M. Relationship between endothelial dysfunction, carotid artery intima media thickness and circulating markers of vascular inflammation in obese hypertensive children and adolescents. J Pediatr Endocrinol Metab. 2007;20:1125–36. https://doi.org/10.1515/jpem.2007.20.10.1125. [PubMed] [Google Scholar]
- 45.Nafikudin M, Nawawi H, Muid S, Annuar R, Yusoff K, Khalid BA, et al. Measurement of intima-media thickness of common carotid arteries using ultrasound in patients with familial and non-familial hypercholesterolaemia and correlation of intima-media thickness to obesity. Med J Malaysia. 2003;58:647–52. https://doi.org/10.1016/s1567-5688(03)91239-6. [PubMed] [Google Scholar]
- 46.Le J, Zhang D, Menees S, Chen J, Raghuveer G. “Vascular age”is advanced in children with atherosclerosis-promoting risk factors. Circ Cardiovasc Imaging. 2010;3:8–14. doi: 10.1161/CIRCIMAGING.109.880070. https://doi.org/10.1161/circimaging.109.880070. [DOI] [PubMed] [Google Scholar]
- 47.Vasconcellos F, Seabra A, Cunha F, Montenegro R, Penha J, Bouskela E, et al. Health markers in obese adolescents improved by a 12-week recreational soccer program:A randomised controlled trial. J Sports Sci. 2016;34:564–75. doi: 10.1080/02640414.2015.1064150. https://doi.org/10.1080/02640414.2015.1064150. [DOI] [PubMed] [Google Scholar]
- 48.Park JH, Miyashita M, Kwon YC, Park HT, Kim EH, Park JK, et al. A 12-week after-school physical activity programme improves endothelial cell function in overweight and obese children:A randomised controlled study. BMC Pediatr. 2012;12:111. doi: 10.1186/1471-2431-12-111. https://doi.org/10.1186/1471-2431-12-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scholl J, Bots ML, Peters SA. Contribution of cardiorespiratory fitness, relative to traditional cardiovascular disease risk factors, to common carotid intima-media thickness. J Intern Med. 2015;277:439–46. doi: 10.1111/joim.12271. https://doi.org/10.1111/joim.12271. [DOI] [PubMed] [Google Scholar]
- 50.Cunningham KS, Gotlieb AI. The role of shear stress in the pathogenesis of atherosclerosis. Lab Invest. 2005;85:9–23. doi: 10.1038/labinvest.3700215. https://doi.org/10.1038/labinvest.3700215. [DOI] [PubMed] [Google Scholar]
- 51.Flammer AJ, Anderson T, Celermajer DS, Creager MA, Deanfield J, Ganz P, et al. The assessment of endothelial function:From research into clinical practice. Circulation. 2012;126:753–67. doi: 10.1161/CIRCULATIONAHA.112.093245. https://doi.org/10.1161/circulationaha.112.093245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Neunteufl T, Heher S, Katzenschlager R, Wölfl G, Kostner K, Maurer G, et al. Late prognostic value of flow-mediated dilation in the brachial artery of patients with chest pain. Am J Cardiol. 2000;86:207–10. doi: 10.1016/s0002-9149(00)00857-2. https://doi.org/10.1016/s0002-9149(00)00857-2. [DOI] [PubMed] [Google Scholar]
- 53.Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, et al. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study:The multi-ethnic study of atherosclerosis. Circulation. 2009;120:502–9. doi: 10.1161/CIRCULATIONAHA.109.864801. https://doi.org/10.1161/circulationaha.109.⇈01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Whyte JJ, Laughlin MH. The effects of acute and chronic exercise on the vasculature. Acta Physiol (Oxf) 2010;199:441–50. doi: 10.1111/j.1748-1716.2010.02127.x. https://doi.org/10.1111/j.1748-1716.2010.02127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mantione KJ, Esch T, Stefano GB. Detection of nitric oxide in exhaled human breath:Exercise and resting determinations. Med Sci Monit. 2007;13:MT1–5. [PubMed] [Google Scholar]
- 56.Hambrecht R, Adams V, Erbs S, Linke A, Kränkel N, Shu Y, et al. Regular physical activity improves endothelial function in patients with coronary artery disease by increasing phosphorylation of endothelial nitric oxide synthase. Circulation. 2003;107:3152–8. doi: 10.1161/01.CIR.0000074229.93804.5C. https://doi.org/10.1161/01.cir.0000074229.93804.5c. [DOI] [PubMed] [Google Scholar]
- 57.Fedewa MV, Hathaway ED, Ward-Ritacco CL. Effect of exercise training on C reactive protein:A systematic review and meta-analysis of randomised and non-randomised controlled trials. Br J Sports Med. 2017;51:670–6. doi: 10.1136/bjsports-2016-095999. https://doi.org/10.1136/bjsports-2016-095999. [DOI] [PubMed] [Google Scholar]
- 58.Ford ES. Does exercise reduce inflammation?Physical activity and C-reactive protein among U.S. Adults. Epidemiology. 2002;13:561–8. doi: 10.1097/00001648-200209000-00012. https://doi.org/10.1097/00001648-200209000-00012. [DOI] [PubMed] [Google Scholar]
- 59.Plaisance EP, Grandjean PW. Physical activity and high-sensitivity C-reactive protein. Sports Med. 2006;36:443–58. doi: 10.2165/00007256-200636050-00006. https://doi.org/10.2165/00007256-200636050-00006. [DOI] [PubMed] [Google Scholar]
- 60.Seo YG, Lim H, Kim Y, Ju YS, Lee HJ, Jang HB, et al. The effect of a multidisciplinary lifestyle intervention on obesity status, body composition, physical fitness, and cardiometabolic risk markers in children and adolescents with obesity. Nutrients. 2019;11:e137. doi: 10.3390/nu11010137. https://doi.org/10.3390/nu11010137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.da Silva PL, de Mello MT, Cheik NC, Sanches PL, Correia FA, de Piano A, et al. Interdisciplinary therapy improves biomarkers profile and lung function in asthmatic obese adolescents. Pediatr Pulmonol. 2012;47:8–17. doi: 10.1002/ppul.21502. https://doi.org/10.1002/ppul.21502. [DOI] [PubMed] [Google Scholar]
- 62.Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation, obesity, stress and coronary heart disease:Is interleukin-6 the link? Atherosclerosis. 2000;148:209–14. doi: 10.1016/s0021-9150(99)00463-3. https://doi.org/10.1016/s0021-9150(99)00463-3. [DOI] [PubMed] [Google Scholar]
- 63.Michigan A, Johnson TV, Master VA. Review of the relationship between C-reactive protein and exercise. Mol Diagn Ther. 2011;15:265–75. doi: 10.1007/BF03256418. https://doi.org/10.2165/11593400-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 64.Dinarello CA. Historical insights into cytokines. Eur J Immunol. 2007;37(Suppl 1):S34–45. doi: 10.1002/eji.200737772. https://doi.org/10.1002/eji.200737772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Accattato F, Greco M, Pullano SA, Carè I, Fiorillo AS, Pujia A, et al. Effects of acute physical exercise on oxidative stress and inflammatory status in young, sedentary obese subjects. PLoS One. 2017;12:e0178900. doi: 10.1371/journal.pone.0178900. https://doi.org/10.1371/journal.pone.0178900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dimitrov S, Hulteng E, Hong S. Inflammation and exercise:Inhibition of monocytic intracellular TNF production by acute exercise via β2-adrenergic activation. Brain Behav Immun. 2017;61:60–8. doi: 10.1016/j.bbi.2016.12.017. https://doi.org/10.1016/j.bbi.2016.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aminuddin A, Hakim LA, Chan SY, Elyatulnadia S, Akma HH, Afifa NS, et al. The changes of aortic stiffness during normal menstrual cycle. Med Health. 2018;13:117–29. https://doi.org/10.17576/MH.2018.1301.12. [Google Scholar]
- 68.Gursoy AY, Caglar GS, Kiseli M, Pabuccu E, Candar T, Demirtas S, et al. CRP at early follicular phase of menstrual cycle can cause misinterpretation for cardiovascular risk assessment. Interv Med Appl Sci. 2015;7:143–6. doi: 10.1556/1646.7.2015.4.2. https://doi.org/10.1556/1646.7.2015.4.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mendelson M, Michallet AS, Monneret D, Perrin C, Estève F, Lombard PR, et al. Impact of exercise training without caloric restriction on inflammation, insulin resistance and visceral fat mass in obese adolescents. Pediatr Obes. 2015;10:311–9. doi: 10.1111/ijpo.255. https://doi.org/10.1111/ijpo.255. [DOI] [PubMed] [Google Scholar]
- 70.Nascimento H, Costa E, Rocha S, Lucena C, Rocha-Pereira P, Rêgo C, et al. Adiponectin and markers of metabolic syndrome in obese children and adolescents:Impact of 8-month regular physical exercise program. Pediatr Res. 2014;76:159–65. doi: 10.1038/pr.2014.73. https://doi.org/10.1038/pr.2014.73. [DOI] [PubMed] [Google Scholar]


