Abstract
Currently, statins are the first-line therapies for dyslipidemia and atherosclerotic cardiovascular disease, however, their hypolipidemic effects have not been satisfactory. We performed a meta-analysis to compare lipid-lowering efficacy and safety of ezetimibe and statin combination therapy with double-dose statin monotherapy in patients with high cardiovascular risk. Fourteen studies involving 3105 participants were included in the final analysis; 1558 (50.18%) participants received ezetimibe and statin combination therapy and 1547 (49.82%) received double-dose statin monotherapy. Eight studies reported the percentages of changes in several lipid parameters from baseline to endpoint in both groups. Lipid parameters changed more significantly in patients coadministered with ezetimibe and statin (low-density lipoprotein cholesterol [LDL-C]: MD = -9.39, 95% CI -13.36 to -5.42; non-high-density lipoprotein cholesterol [non-HDL-C]: MD = -10.36, 95% CI -14.23 to -6.50; total cholesterol [TC]: MD = -8.11, 95% CI -10.95 to -5.26; and triglyceride [TG]: MD = -5.96, 95% CI -9.12 to -2.80), with moderate to high heterogeneity among the studies. Two out of fourteen studies investigated several different statins. Our subgroup analysis showed that, compared with double-dose atorvastatin monotherapy, ezetimibe and atorvastatin combination therapy significantly decreased LDL-C, non-HDL-C, TC, and TG levels by 14.16%, 14.01%, 11.06%, and 5.96%, respectively (p < 0.001). No significant difference was found in the incidence of laboratory-related adverse events (AEs) between statin combination therapy and monotherapy. Overall, ezetimibe and statin combination therapy significantly decreased LDL-C, non-HDL-C, and TC levels in patients with high cardiovascular risk, among which ezetimibe combined with atorvastatin had the best therapeutic effect. Compared with ezetimibe and statin combination therapy, double-dose statin monotherapy did not increase the risk of AEs.
Keywords: Efficacy, safety, statin, ezetimibe, combination therapy
INTRODUCTION
Cardiovascular disease is one of the main causes of mortality around the world [1]. Among diverse etiological factors, dyslipidemia is the most important reversible risk factor for cardiovascular diseases [2]. Previous studies showed that serum cholesterol levels were directly correlated with coronary heart disease (CHD)-related mortality [3-5]. A meta-analysis of individual data obtained from 174,000 participants in 27 randomized trials showed that every 1.0 mmol/L reduction of low-density lipoprotein cholesterol (LDL-C) level resulted in a 12% reduction in vascular mortality [6]. Thus, developing effective and safe therapies for treating dyslipidemia is crucial for the prevention and treatment of cardiovascular diseases.
In general, hypolipidemic drugs are classified into the following categories: hydroxymethylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors (statins), niacin, cholic acid chelating agents, ezetimibe, and PCSK9 monoclonal antibody. Notably, statins are the first-line therapeutic approach for dyslipidemia and atherosclerotic cardiovascular disease (ASCVD) [7]. Unfortunately, the recommended LDL-C levels cannot be reached in partial patients receiving statin monotherapy. Thus, the current clinical hypolipidemic guidelines recommend powerful lipid-lowering drugs, such as statins at high doses, for treating patients with ultrahigh risk of ASCVD [7-9]. However, high doses of statins may cause side effects in patients, especially in the elderly. Some clinical trials showed that the risks of adverse events (AEs), such as myopathy and liver injury, generally increased with increasing doses of statins [10]. It remains controversial whether high doses of statins are good for treating patients with high cardiovascular risk.
In the current study, the primary efficacy endpoint was the percentage of change in lipid parameters. Moreover, the safety of two treatment strategies was assessed by reviewing adverse experiences and laboratory values. According to the 2018 Cholesterol Clinical Practice Guidelines, a high cardiovascular risk is defined as a history of multiple major ASCVD events or one major ASCVD event and multiple other high-risk conditions [11].
A meta-analysis is an effective statistical tool for overcoming the limitations of individual studies with different sample sizes. Thus, we performed a meta-analysis to compare lipid-lowering efficacy and safety between ezetimibe and statin combination therapy and double-dose statin monotherapy in patients with high cardiovascular risk.
MATERIALS AND METHODS
Search strategy
The meta-analysis was conducted in accordance with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement [12]. In December 2018, two of the authors searched six databases independently, including PubMed, Cochrane Central, EMBASE, Medline, CNKI, and Wanfang. The search terms, deliberately generic, included (“Ezetimibe” OR “Ezetrol” OR “SCH-58235” OR “SCH58235” OR “Zetia”) AND (“Hydroxymethylglutaryl-CoA Reductase Inhibitors” OR “HMG-CoA Statins” OR “HMG-CoA Reductase Inhibitors” OR “Hydroxymethylglutaryl-CoA Inhibitors” OR “Statins” OR “Hydroxymethylglutaryl-Coenzyme A Inhibitors”).
Inclusion criteria
Comparisons of the efficacy or safety of double-dose statin monotherapy vs. statin and ezetimibe combination therapy;
Reports on the percentages of changes in lipid parameters from baseline to endpoint, including LDL-C, high-density lipoprotein cholesterol (HDL-C), non-HDL-C, total cholesterol (TC), and triglyceride (TG);
Information on clinical AEs;
Randomized control trials.
Exclusion criteria
Abstracts, letters, case reports, reviews, or nonclinical studies;
Studies with insufficient data to estimate standard deviation (SD) and 95% confidence interval (CI);
Studies that contained duplicate data or repeated analyses;
Studies that excluded original data to calculate the percentages of changes in lipid parameters.
Data extraction and quality assessment
All candidate articles were independently evaluated and extracted by two authors (Yunyun Zhu and Shaoyi Lin). Extracted data were further verified by a senior author, and any discrepancies were resolved by a consensus agreement. Data items to be collected were determined before the literature search. The risk of bias in each individual trial was assessed using the Cochrane Collaboration’s tool (Figure S1 and S2).
FIGURE S1.
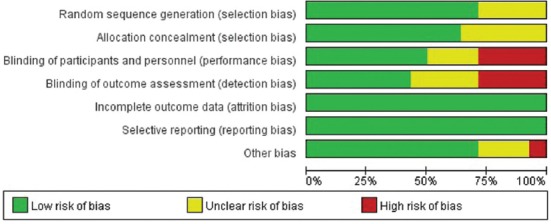
Risk of bias graph. Review of authors’ judgments about each item presented as percentages across all included studies.
FIGURE S2.
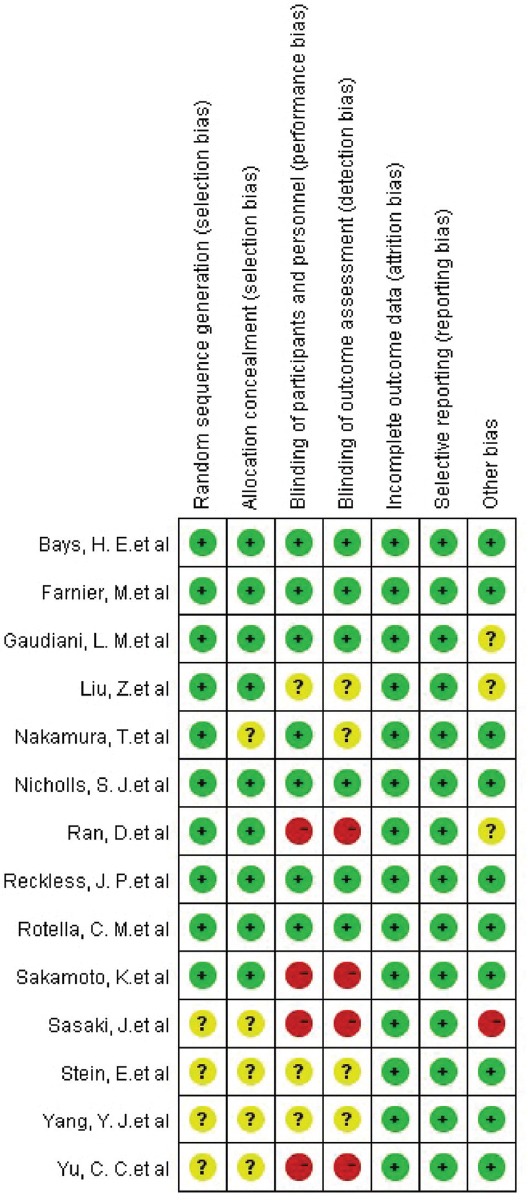
Risk of bias summary. Review of authors’ judgments about each risk of bias item for each included study.
Statistical analysis
The meta-analysis was performed using Review Manager Version 5.3. (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen) and Stata SE12.0 Software (StataCorp LLC, Texas, USA). Summary estimates were represented as mean difference (MD) and 95% CI. Continuous variables were reported as mean and SD, with nonparametric data transformed to mean (SD) as previously described [13]. Cochran’s Q test and Higgins I2 statistic were performed to assess the heterogeneity of included trials. Heterogeneity was considered significant when p < 0.10 or I2 value > 50%, where a random-effect model was applied. Otherwise, a fixed-effect model was applied. Subgroup analysis and sensitivity analysis were performed to explore the sources of heterogeneity. For all analyses, two-sided p < 0.05 was considered statistically significant. The quality of outcome was assessed using the Grading of Recommendations, Assessment, Development, and Evaluation system. Publication bias was assessed visually by funnel plots and statistically by Egger’s test.
RESULTS
Search results
A total of 2493 initial citations were retrieved through electronic searches, among which 45 studies were identified as potential candidates after screening. Further, 107 studies that involved non-ezetimibe intervention (n = 17), non-double-dose statin intervention (n = 33), or different primary endpoints (n = 57) were excluded from our study. The details of the study selection process are shown in Figure 1.
FIGURE 1.
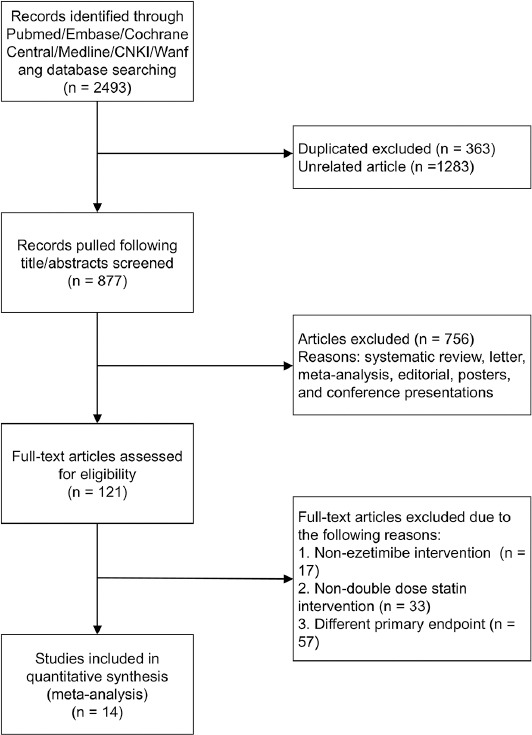
Flow chart of the selection process.
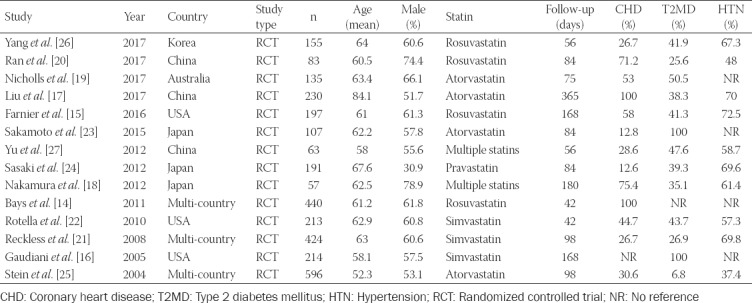
Fourteen studies [14-27] involving 3105 participants were included for final quantitative analysis, among which 1558 (50.18%) participants received ezetimibe and statin combination therapy and 1547 (49.82%) received double-dose statin monotherapy. The average age of participants was 68.2 years with 30.9%–78.9% males. The follow-up duration in these studies ranged from 42 days to 365 days. All research subjects were high-risk population for cardiovascular diseases based on their medical histories of cardiovascular diseases (i.e., CHD) or related diseases (i.e., diabetes, hypertension, etc.). Among the fourteen studies, two studies investigated multiple statins of different types. The statins used in the study by Nakamura et al. [18] included atorvastatin, pravastatin, rosuvastatin, and pitavastatin, and the statins used in the study by Yu et al. [27] included simvastatin, atorvastatin, and pravastatin. Additional baseline and patient characteristics are listed in Table 1.
TABLE 1.
The main characteristics of studies included in the meta‑analysis
The Primary efficacy variable – the percentages of changes in lipid parameters from baseline to endpoint
A total of eight studies reported data about the percentages of changes in lipid parameters from baseline to endpoint, including LDL-C, HDL-C, non-HDL-C, TC, and TG, in both 10-mg ezetimibe plus statin group and double-dose statin group.
Combination of ezetimibe and statin was correlated with a greater percentage of LDL-C change from baseline (MD = -9.39, 95% CI -13.36 to -5.42). However, there was greater heterogeneity among the studies (I2 = 75%, p < 0.001) (Figure 2A). Then, statins were classified into subgroups for a subgroup analysis (Figure 2B). In rosuvastatin subgroup, the point estimation of MD (95% CI) was -3.30 (-7.45, 0.86) (p = 0.12), suggesting that there was no statistical significance between rosuvastatin in combination with ezetimibe and double-dose rosuvastatin. The results obtained with Q-test and I2-test showed that there was no heterogeneity among the studies in rosuvastatin subgroup (I2 = 0%, p = 0.59). Compared with double-dose atorvastatin treatment, LDL-C levels after ezetimibe plus atorvastatin treatment decreased by 14.16%, with a statistically significant difference (MD = -14.16, 95% CI -16.01 to -12.31; p < 0.001), and no heterogeneity was observed between the studies (I2 = 0.00%, p = 0.90). While no significant differences in the lipid-lowering efficacies were observed between the two subgroups (rosuvastatin vs. atorvastatin; p = 0.12), high heterogeneity (I2 = 55%) was observed, suggesting multiple statin subgroups were the main source of heterogeneity.
FIGURE 2.
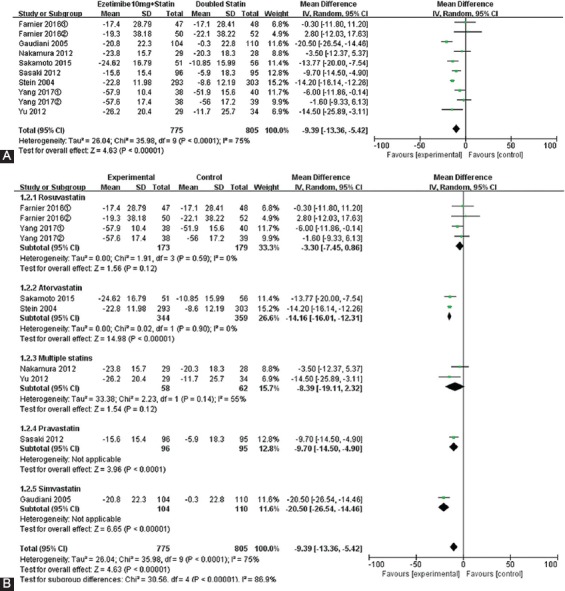
Forest plot of the percentages of changes in low-density lipoprotein cholesterol (LDL-C) (A). Forest plot of subgroup meta-analysis by the type of statin for LDL-C (B).
The results for non-HDL-C and TC were similar to the results for LDL-C. The changes in non-HDL-C and TC from baseline were more significant in patients receiving ezetimibe and statin combination therapy vs. double-dose statin monotherapy (non-HDL-C: MD = -10.36, 95% CI -14.23 to -6.50, p < 0.001; TC: MD = -8.11, 95% CI -10.95 to -5.26, p < 0.001) (Figures 3A and 4A). However, there was greater heterogeneity among the studies (non-HDL-C: I2 = 75%, TC: I2 = 73%). Compared with double-dose atorvastatin subgroup, non-HDL-C and TC levels declined by 14.01% (p < 0.001) and 11.06% (p < 0.001), respectively, in ezetimibe plus atorvastatin group, with statistically significant differences (non-HDL-C: 95% CI -15.85 to -12.18; TC: 95% CI -12.61 to -9.50) (Figures 3B and 4B), and there was no heterogeneity between the studies (I2 = 0%). There was significant heterogeneity in rosuvastatin subgroup (I2 = 61%), suggesting that rosuvastatin subgroup was the main source of heterogeneity for TC.
FIGURE 3.
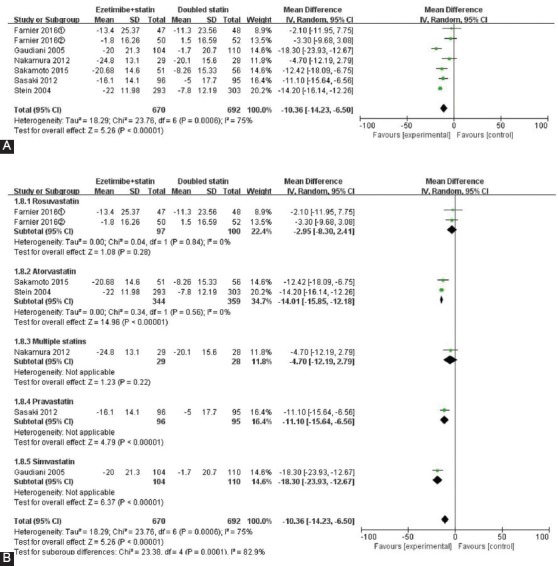
Forest plot of the percentages of changes in non-high-density lipoprotein cholesterol (non-HDL-C) (A). Forest plot of subgroup meta-analysis by the type of statin for non-HDL-C (B).
FIGURE 4.
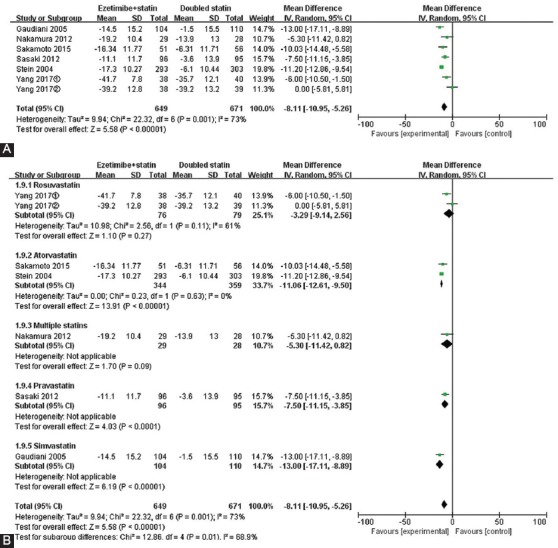
Forest plot of the percentages of changes in total cholesterol (TC) (A). Forest plot of subgroup meta-analysis by the type of statin for TC (B).
For TG levels, as shown in Figure 5A, the point estimation and 95% CI of MD were -5.96 (95% CI -9.12 to -2.80, p = 0.0002) and z-value was 3.70, which indicated that compared with double-dose statin monotherapy, the combination therapy significantly decreased TG levels, by 5.96%. The results obtained from Q-test and I2-test showed that there was moderate heterogeneity for TG (I2 = 42%, p = 0.08). Notably, the results from atorvastatin subgroup showed a statistical significance (MD = -5.74, 95% CI -10.28 to -1.19, z = 2.47, p = 0.01) and lower heterogeneity between atorvastatin in combination with ezetimibe and double-dose atorvastatin (I2 = 0%, p = 0.59, Figure 5B). However, there was great heterogeneity in rosuvastatin subgroup (I2 = 42%), indicating that rosuvastatin subgroup was the single source of heterogeneity for TG.
FIGURE 5.

Forest plot of the percentages of changes in triglyceride (TG) (A). Forest plot of subgroup meta-analysis by the type of statin for TG (B).
In terms of the percentages of changes in HDL, the point estimation of combined MD was 0.49 (95% CI -0.73 to 1.72), suggesting that there was no statistical significance. Also, there was no heterogeneity between the studies (I2 = 0%, Figure 6A).
FIGURE 6.
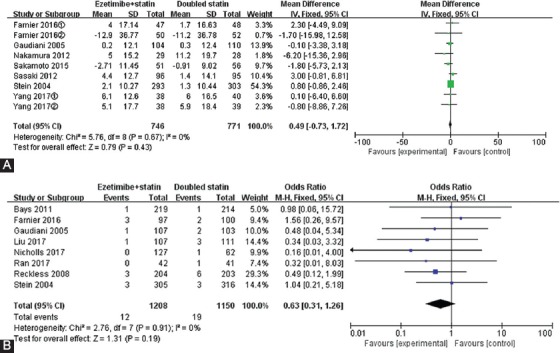
The percentages of changes in high-density lipoprotein cholesterol (HDL-C) (A) and the rate of adverse events associated with statin therapies (B).
Safety and tolerability assessment
Indicators of AEs include: elevated levels of alanine aminotransferase (ALT) or aspartate aminotransferase (AST), i.e., more than threefold of the upper limit of normal [ULN] for two successive measurements; elevated levels of transaminases, i.e., more than threefold of the ULN during the final laboratory examination (considered as “presumed consecutive”); or elevated creatine phosphokinase (CPK) levels, i.e., more than tenfold of the ULN. In this study, there was no significant difference in the incidence of laboratory-related AEs between the two therapies (I2 = 0%, p = 0.91, RR = 0.63, 95% CI 0.31 to 1.26, z = 1.31, p = 0.19) (Figure 6B).
Publication bias
For TG, HDL-C, and laboratory-related AEs, the Egger’s test showed no statistically significant publication bias (p > 0.05), while the funnel plots were symmetrically distributed, suggesting that publication bias could not be excluded (Figure S3-S5). For TC, LDL-C, and non-HDL-C the Egger’s test showed statistically significant publication bias (p < 0.05), and the funnel plots were unsymmetrically distributed, suggesting that there was publication bias (Figure S6-S8).
FIGURE S3.
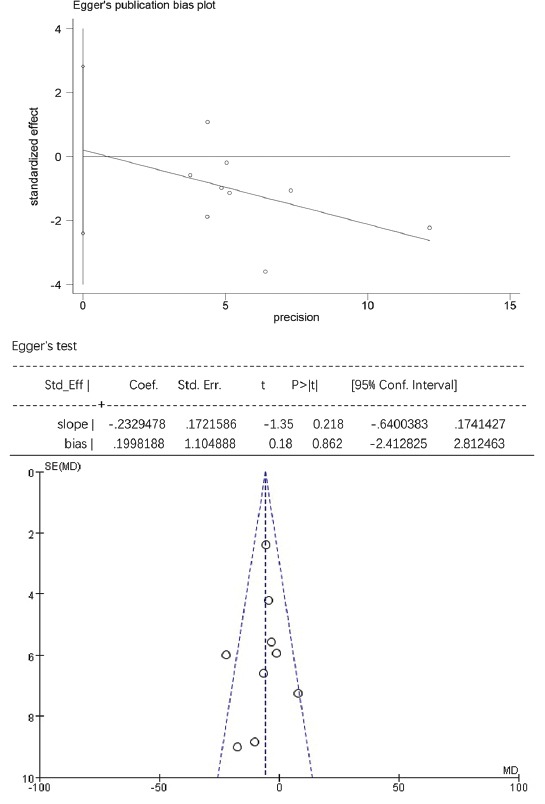
The Egger’s publication bias plot and funnel plot for triglyceride (TG).
FIGURE S5.
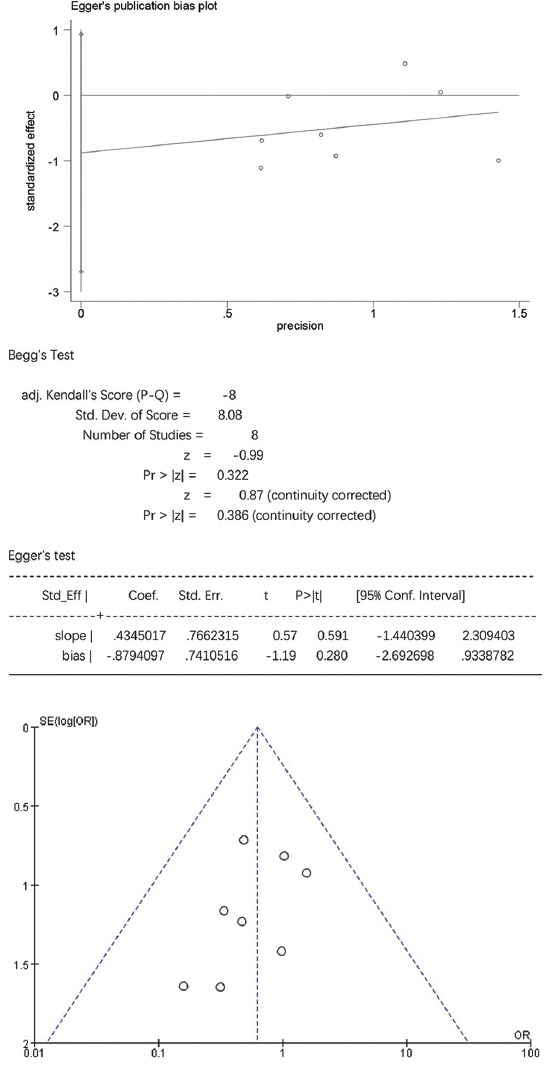
The Egger’s publication bias plot and funnel plot for adverse events (AE).
FIGURE S6.
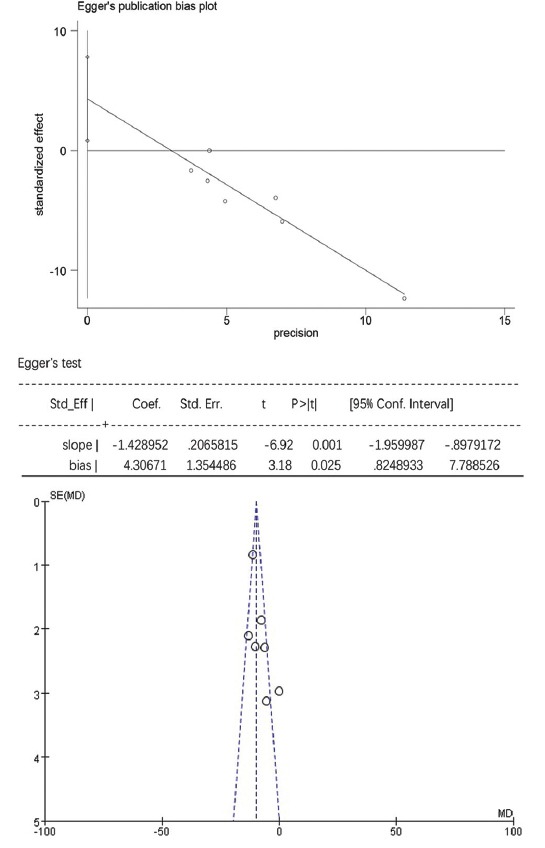
The Egger’s publication bias plot and funnel plot for total cholesterol (TC).
FIGURE S8.
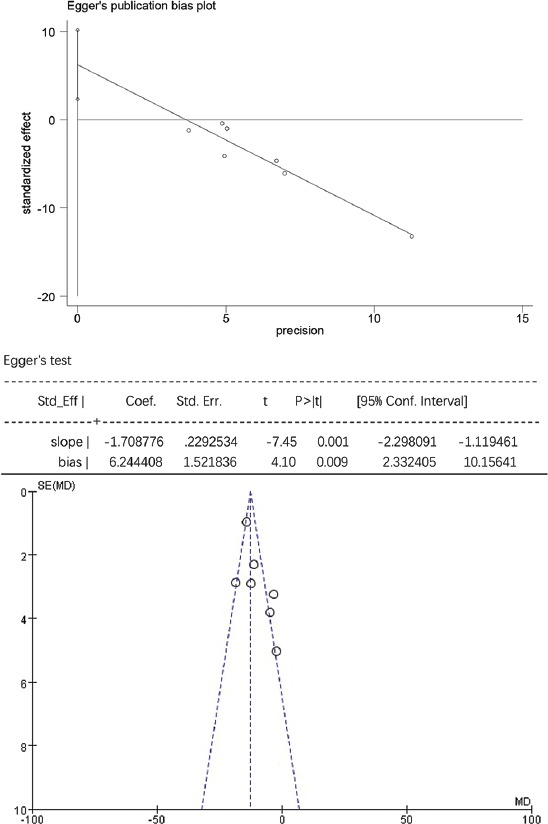
The Egger’s publication bias plot and funnel plot for non-high-density lipoprotein cholesterol (non-HDL-C).
FIGURE S4.
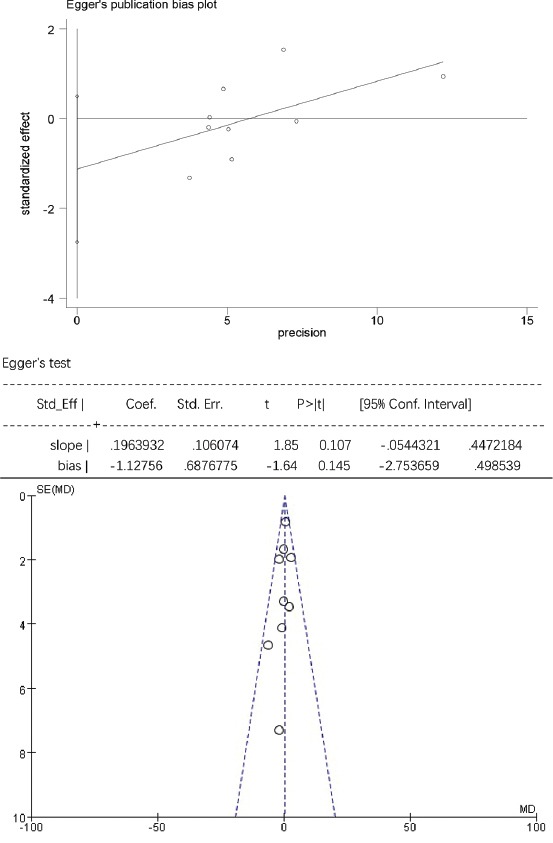
The Egger’s publication bias plot and funnel plot for high-density lipoprotein cholesterol (HDL-C).
FIGURE S7.
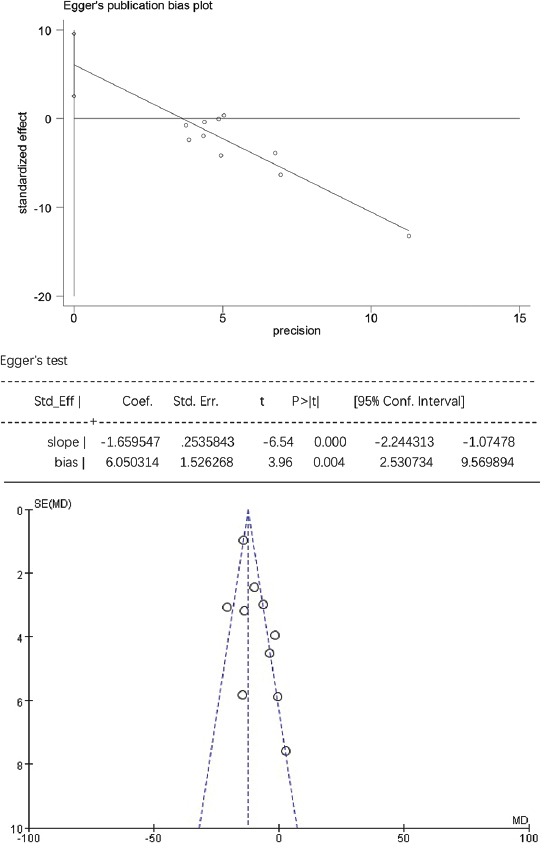
The Egger’s publication bias plot and funnel plot for low-density lipoprotein cholesterol (LDL-C).
DISCUSSION
Multiple studies have investigated the lipid-lowering efficacy and safety of ezetimibe and statin combination therapy and double-dose statin monotherapy in patients with high cardiovascular risk, however, the results of those studies are inconsistent and inconclusive (Table S1). In this study, we reviewed the relevant published studies and performed a meta-analysis to estimate the value of two distinct statin-based therapies. We analyzed the outcomes of 3105 patients from fourteen individual studies. Our results indicated that the efficacy of 10-mg ezetimibe and statin combination therapy to reduce LDL-C, non-HDL-C, TC, and TG levels was significantly better than the efficacy of double-dose statin monotherapy in patients with hypercholesterolemia with high risk of cardiovascular diseases. Notably, the subgroup analyses showed that, compared with double-dose atorvastatin monotherapy, ezetimibe and atorvastatin combination therapy caused significant reductions in the levels of LDL-C, non-HDL-C, TC, and TG, with no heterogeneity between the studies.
TABLE S1.
The results of the studies included in the meta‑analysis in terms of therapy efficacy and safety
Our results are consistent with the results of previous studies comparing ezetimibe and atorvastatin combination therapy with atorvastatin monotherapy, which showed that statin administration alone could not properly control the LDL-C levels of patients with moderate to high cardiovascular risk [28,29]. Compared with 40-mg atorvastatin therapy, coadministration of 10-mg ezetimibe with 20-mg atorvastatin resulted in more significant decreases in LDL-C levels in hypercholesterolemic patients at moderately high risk for CHD [28]. In hypercholesterolemia patients with high risk of CHD, 10-mg ezetimibe combined with 40-mg atorvastatin had a more potent effect than 80-mg atorvastatin in lowering LDL-C levels and could even reduce LDL-C levels to less than 70 mg/dL [29]. In our analysis, compared with 40-mg atorvastatin monotherapy, atorvastatin and ezetimibe combination therapy caused a higher decrease in non-HDL-C and TC levels. All these results suggest that the lipid-lowering efficacy of ezetimibe plus statin therapy is more potent.
We did not observe a significant difference between double-dose rosuvastatin monotherapy and ezetimibe and rosuvastatin combination therapy in lowering the levels of LDL-C, non-HDL-C, TC, and TG. However, it should be noted that an increase in the basal dose of rosuvastatin in combination therapy could impair the efficacy of this therapy, causing only a mild decrease in LDL-C levels. Moreover, our results are inconsistent with the ACTE study in which 10-mg ezetimibe in combination with stable starting doses of rosuvastatin decreased LDL-C more significantly in patients with hypercholesterolemia compared with rosuvastatin uptitration, which caused a 21% reduction in LDL-C levels from the baseline [13]. The inconsistent results could be associated with the different dosages of rosuvastatin between the studies. The dosage applied in the ACTE study was 5 mg or 10 mg, while the dosage of rosuvastatin in the studies included in this meta-analysis ranged from 5 mg to 40 mg.
When comparing the overall curative effects between ezetimibe and statin combination therapy and double-dose statin monotherapy, we found that the percentages of changes in HDL-C levels were not significantly different.
Notably, our meta-analysis showed no significant difference in the laboratory AEs between double-dose statin-treated patients and ezetimibe-statin-treated patients. ALT, AST, and CPK are sensitive biomarkers for consecutive hepatocyte or muscle cell injury. Compared with ezetimibe and statin combination therapy, there was no significant difference in the levels of these indicators in patients receiving double-dose statin monotherapy. Randomized trials generally involve limited numbers of cases, which poses a limitation on obtaining a realistic overview of patients with poly-medications and comorbidities. Myalgia generally occurs in 5% to 10% of patients and the incidence of severe rhabdomyolysis is <0.07% in clinical practice [30]. In addition, due to the different durations of therapies in the included studies (the duration ranged from 42 days to 365 days), we could not draw a solid conclusion about the tolerability of regimens. Studies involving longer treatment durations are needed for collecting more specific data on drug safety.
The main focus of the current therapeutic guidelines is to reach the recommended LDL-C levels and to ensure drug safety. In our meta-analysis, ezetimibe and statin combination therapy significantly lowered LDL-C, non-HDL-C, and TC levels, especially when ezetimibe was administrated in combination with atorvastatin. Moreover, the application of double-dose statin monotherapy did not increase the risk of ALT, AST, and CPK elevation, compared with ezetimibe and statin combination therapy. The results suggested that ezetimibe and statin combination therapy could be more effective than double-dose statin monotherapy when the classical treatments fail to reduce LDL-C levels. However, more clinical trials are required for specifying the incidence of major cardiac AEs between two groups, i.e., ezetimibe plus statin therapy and double-dose statin monotherapy.
Limitations
There are several limitations to our study. First, significant heterogeneity was observed among the studies due to the different types of HMG-CoA reductase inhibitors used in each trial. The type of HMG-CoA reductase inhibitors was the only significant variable defined by meta-regression. In addition, the dosages of statins varied significantly among these studies, which partially affected the endpoints. Third, this meta-analysis was based on published data. In the future, more real-world data need to be included to draw a more comprehensive conclusion. Finally, although a large proportion of raw data was obtained from original authors in this study, a small portion of related data was incomplete.
CONCLUSION
Overall, this meta-analysis indicated that compared with double-dose statin monotherapy, ezetimibe and statin combination therapies, especially ezetimibe and atorvastatin combination therapy, showed higher efficacies in lowering LDL-C, non-HDL-C, TC, and TG levels in patients with high cardiovascular risk. In addition, the safety of ezetimibe and statin combination therapy vs. double-dose statin therapy was no significantly different.
ACKNOWLEDGMENTS
The research is supported by Ningbo Municipal Natural Science Foundation (grant no.2017A610209) and by Medical Health Science and Technology Project of Zhejiang Provincial Health Commission (grant no.2020KY822).
Footnotes
Conflict of interest statement: The authors declare no conflict of interests
REFERENCES
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics-2015 update:a report from the American Heart Association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. https://doi.org/10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Hendrani AD, Adesiyun T, Quispe R, Jones SR, Stone NJ, Blumenthal RS, et al. Dyslipidemia management in primary prevention of cardiovascular disease:current guidelines and strategies. World J Cardiol. 2016;8:201–10. doi: 10.4330/wjc.v8.i2.201. https://doi.org/10.4330/wjc.v8.i2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verschuren WM, Jacobs DR, Bloemberg BP, Kromhout D, Menotti A, Aravanis C, et al. Serum total cholesterol and long-term coronary heart disease mortality in different cultures. Twenty-five-year follow-up of the seven countries study. JAMA. 1995;274:131–6. https://doi.org/10.1001/jama.1995.03530020049031. [PubMed] [Google Scholar]
- 4.Chen Z, Peto R, Collins R, MacMahon S, Lu J, Li W, et al. Serum cholesterol concentration and coronary heart disease in population with low cholesterol concentrations. BMJ. 1991;303:276–82. doi: 10.1136/bmj.303.6797.276. https://doi.org/10.1136/bmj.303.6797.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Keefe JH., Jr Cordain L, Harris WH, Moe RM, Vogel R. Optimal low-density lipoprotein is 50 to 70 mg/dl:lower is better and physiologically normal. J Am Coll Cardiol. 2004;43:2142–6. doi: 10.1016/j.jacc.2004.03.046. https://doi.org/10.1016/j.jacc.2004.03.046. [DOI] [PubMed] [Google Scholar]
- 6.Fulcher J, O'Connell R, Voysey M, Emberson J, Blackwell L, et al. Cholesterol Treatment Trialists'(CTT) Collaboration. Efficacy and safety of LDL-lowering therapy among men and women:meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet. 2015;385:1397–405. doi: 10.1016/S0140-6736(14)61368-4. https://doi.org/10.1016/s0140-6736(14)61368-4. [DOI] [PubMed] [Google Scholar]
- 7.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults:a report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2889–934. doi: 10.1016/j.jacc.2013.11.002. https://doi.org/10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 8.Steg PG, James SK, Atar D, Badano LP, Blömstrom-Lundqvist C, et al. Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC) ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33:2569–619. doi: 10.1093/eurheartj/ehs215. https://doi.org/10.15829/1560-4071-2018-5-103-158. [DOI] [PubMed] [Google Scholar]
- 9.Expert Dyslipidemia Panel of the International Atherosclerosis Society Panel members. An international atherosclerosis society position paper:global recommendations for the management of dyslipidemia full report. J Clin Lipidol. 2014;8:29–60. doi: 10.1016/j.jacl.2013.12.005. https://doi.org/10.1016/j.jacl.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 10.Jacobson TA, Cheeley MK, Jones PH, La Forge R, Maki KC, López JAG, et al. The STatin Adverse Treatment Experience Survey:Experience of patients reporting side effects of statin therapy. J Clin Lipidol. 2019;13:415–24. doi: 10.1016/j.jacl.2019.04.011. https://doi.org/10.1016/j.jacl.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 11.Grundy SM, Stone NJ. Guideline Writing Committee for the 2018 Cholesterol Guidelines. 2018 cholesterol clinical practice guidelines:synopsis of the 2018 American Heart Association/American College of Cardiology/Multisociety Cholesterol Guideline. Ann Intern Med. 2019;170:779–83. doi: 10.7326/M19-0365. https://doi.org/10.7326/m19-0365. [DOI] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses:the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. https://doi.org/10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. https://doi.org/10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bays HE, Davidson MH, Massaad R, Flaim D, Lowe RS, Tershakovec AM, et al. Safety and efficacy of ezetimibe added on to rosuvastatin 5 or 10 mg versus up-titration of rosuvastatin in patients with hypercholesterolemia (the ACTE study) Am J Cardiol. 2011;108:523–30. doi: 10.1016/j.amjcard.2011.03.079. https://doi.org/10.1016/j.amjcard.2011.03.079. [DOI] [PubMed] [Google Scholar]
- 15.Farnier M, Jones P, Severance R, Averna M, Steinhagen-Thiessen E, Colhoun HM, et al. Efficacy and safety of adding alirocumab to rosuvastatin versus adding ezetimibe or doubling the rosuvastatin dose in high cardiovascular-risk patients:the ODYSSEY OPTIONS II randomized trial. Atherosclerosis. 2016;244:138–46. doi: 10.1016/j.atherosclerosis.2015.11.010. https://doi.org/10.1016/j.atherosclerosis.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 16.Gaudiani LM, Lewin A, Meneghini L, Perevozskaya I, Plotkin D, Mitchel Y, et al. Efficacy and safety of ezetimibe co-administered with simvastatin in thiazolidinedione-treated Type 2 diabetic patients. Diabetes Obes Metab. 2005;7:88–97. doi: 10.1111/j.1463-1326.2004.00420.x. https://doi.org/10.1111/j.1463-1326.2004.00420.x. [DOI] [PubMed] [Google Scholar]
- 17.Liu Z, Hao H, Yin C, Chu Y, Li J, Xu D, et al. Therapeutic effects of atorvastatin and ezetimibe compared with double-dose atorvastatin in very elderly patients with acute coronary syndrome. Oncotarget. 2017;8:41582–9. doi: 10.18632/oncotarget.15078. https://doi.org/10.18632/oncotarget.15078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakamura T, Hirano M, Kitta Y, Fujioka D, Saito Y, Kawabata K, et al. Acomparison of the efficacy of combined ezetimibe and statin therapy with doubling of statin dose in patients with remnant lipoproteinemia on previous statin therapy. J Cardiol. 2012;60:12–7. doi: 10.1016/j.jjcc.2012.02.005. https://doi.org/10.1016/j.jjcc.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 19.Nicholls SJ, Ray KK, Ballantyne CM, Beacham LA, Miller DL, Ruotolo G, et al. Comparative effects of cholesteryl ester transfer protein inhibition, statin or ezetimibe on lipid factors:the ACCENTUATE trial. Atherosclerosis. 2017;261:12–8. doi: 10.1016/j.atherosclerosis.2017.04.008. https://doi.org/10.1016/j.atherosclerosis.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 20.Ran D, Nie HJ, Gao YL, Deng SB, Du JL, Liu YJ, et al. Arandomized, controlled comparison of different intensive lipid-lowering therapies in Chinese patients with non-ST-elevation acute coronary syndrome (NSTE-ACS):ezetimibe and rosuvastatin versus high-dose rosuvastatin. Int J Cardiol. 2017;235:49–55. doi: 10.1016/j.ijcard.2017.02.099. https://doi.org/10.1016/j.ijcard.2017.02.099. [DOI] [PubMed] [Google Scholar]
- 21.Reckless JP, Henry P, Pomykaj T, Lim ST, Massaad R, Vandormael K, et al. Lipid-altering efficacy of ezetimibe/simvastatin 10/40 mg compared with doubling the statin dose in patients admitted to the hospital for a recent coronary event:the INFORCE study. Int J Clin Pract. 2008;62:539–54. doi: 10.1111/j.1742-1241.2008.01697.x. https://doi.org/10.1111/j.1742-1241.2008.01697.x. [DOI] [PubMed] [Google Scholar]
- 22.Rotella CM, Zaninelli A, Le Grazie C, Hanson ME, Gensini GF. Ezetimibe/simvastatin vs simvastatin in coronary heart disease patients with or without diabetes. Lipids Health Dis. 2010;9:80. doi: 10.1186/1476-511X-9-80. https://doi.org/10.1186/1476-511x-9-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakamoto K, Kawamura M, Kohro T, Omura M, Watanabe T, Ashidate K, et al. Effect of ezetimibe on LDL-C lowering and atherogenic lipoprotein profiles in Type 2 diabetic patients poorly controlled by statins. PLoS One. 2015;10:e0138332. doi: 10.1371/journal.pone.0138332. https://doi.org/10.1371/journal.pone.0138332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sasaki J, Otonari T, Sawayama Y, Hata S, Oshima Y, Saikawa T, et al. Double-dose pravastatin versus add-on ezetimibe with low-dose pravastatin - effects on LDL cholesterol, cholesterol absorption, and cholesterol synthesis in Japanese patients with hypercholesterolemia (PEAS study) J Atheroscler Thromb. 2012;19:485–93. doi: 10.5551/jat.12013. https://doi.org/10.5551/jat.12013. [DOI] [PubMed] [Google Scholar]
- 25.Stein E, Stender S, Mata P, Sager P, Ponsonnet D, Melani L, et al. Achieving lipoprotein goals in patients at high risk with severe hypercholesterolemia:efficacy and safety of ezetimibe co-administered with atorvastatin. Am Heart J. 2004;148:447–55. doi: 10.1016/j.ahj.2004.03.052. https://doi.org/10.1016/j.ahj.2004.03.052. [DOI] [PubMed] [Google Scholar]
- 26.Yang YJ, Lee SH, Kim BS, Cho YK, Cho HJ, Cho KI, et al. Combination therapy of rosuvastatin and ezetimibe in patients with high cardiovascular risk. Clin Ther. 2017;39:107–17. doi: 10.1016/j.clinthera.2016.11.014. https://doi.org/10.1016/j.clinthera.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 27.Yu CC, Lai WT, Shih KC, Lin TH, Lu CH, Lai HJ, et al. Efficacy, safety and tolerability of ongoing statin plus ezetimibe versus doubling the ongoing statin dose in hypercholesterolemic Taiwanese patients:an open-label, randomized clinical trial. BMC Res Notes. 2012;5:251. doi: 10.1186/1756-0500-5-251. https://doi.org/10.1186/1756-0500-5-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conard SE, Bays HE, Leiter LA, Bird SR, Rubino J, Lowe RS, et al. Efficacy and safety of ezetimibe added on to atorvastatin (20 mg) versus uptitration of atorvastatin (to 40 mg) in hypercholesterolemic patients at moderately high risk for coronary heart disease. Am J Cardiol. 2008;102:1489–94. doi: 10.1016/j.amjcard.2008.09.075. https://doi.org/10.1016/j.amjcard.2008.09.076. [DOI] [PubMed] [Google Scholar]
- 29.Leiter LA, Bays H, Conard S, Bird S, Rubino J, Hanson ME, et al. Efficacy and safety of ezetimibe added on to atorvastatin (40 mg) compared with uptitration of atorvastatin (to 80 mg) in hypercholesterolemic patients at high risk of coronary heart disease. Am J Cardiol. 2008;102:1495–501. doi: 10.1016/j.amjcard.2008.09.076. https://doi.org/10.1016/j.amjcard.2008.09.076. [DOI] [PubMed] [Google Scholar]
- 30.Deharo P, Pankert M, Quilici J, Grosdidier C, Verdier V, Bonnet G, et al. Safety and effectiveness of the association ezetimibe-statin (E-S) versus high dose rosuvastatin after acute coronary syndrome:the SAFE-ES study. Ann Cardiol Angeiol (Paris) 2014;63:222–7. doi: 10.1016/j.ancard.2014.04.018. https://doi.org/10.1016/j.ancard.2014.04.018. [DOI] [PubMed] [Google Scholar]


