Abstract
Knee osteoarthritis is a degenerative “wear and tear” disorder affecting mainly population over 50 years old. It can also present in younger people, especially after an injury or as a part of other diseases. While many therapeutic options exist for knee osteoarthritis, none of them has the potential to cure this condition. Cellular Matrix represents a combination of natural non-crosslinked hyaluronic acid (HA), thixotropic cell separation gel, and sodium citrate anticoagulant solution. A combination of Cellular Matrix with autologous platelet-rich plasma (A-PRP) is a novel therapeutic approach to the management of knee osteoarthritis. It is assumed that the active components HA and PRP have a synergistic effect contributing to a better therapeutic outcome in patients with knee osteoarthritis. Physiotherapy could provide an additional benefit. This is a retrospective pilot study assessing the potential benefit of Cellular Matrix and A-PRP combined with physiotherapy in the management of chronic knee osteoarthritis. Twenty-five patients were enrolled in the study and injected with three doses of Cellular Matrix combined with A-PRP with a time span of 2 weeks between each injection. All patients received standardized physiotherapy. The results showed that 68% of patients achieved more than 50% improvement in pain, stiffness, and function of the knee joints. There were no adverse reactions. This retrospective pilot study confirmed the positive effect of PRP and HA combination in the management of mild and moderate knee osteoarthritis. These preliminary results need to be verified in randomized control trials.
Keywords: Knee osteoarthritis, Cellular Matrix, Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), hyaluronic acid, HA, autologous platelet-rich plasma, A-PRP
INTRODUCTION
Osteoarthritis (OA) is a common disease of the aged population. It is a progressive joint disease frequently affecting large weight-bearing joints such as hips and knees. Mechanical stress in combination with biochemical factors contributes to the development of symptoms.
The prevalence of knee OA is higher compared to other types of OA, and younger obese women are a particularly vulnerable population [1]. A longer life span and higher body weight will probably increase the incidence of knee OA in the future [2].
Persistent knee pain, restricted function, sometimes followed by swelling and morning stiffness, are the main features of the disease. Physical stress and joint deformities (genu varum and valgum) usually aggravate the condition [3].
Treatment options include lifestyle modifications, pain management, and corticosteroid injections, with joint replacement reserved for those who have exhausted non-surgical procedures. Physical therapy has proved to be useful in helping patients with pain and mobility [4,5]. Analgesics and nonsteroidal anti-inflammatory drugs (NSAIDs) are most commonly prescribed for pain management, but their use can lead to many side effects [6,7].
High-quality studies confirmed the superiority of hyaluronic acid (HA) to placebo in patients with knee OA [8]. Moreover, multiple studies have indicated that platelet-rich plasma (PRP) is superior to HA and corticosteroids in terms of improving patient-reported pain and functionality scores. The current studies show a promising potential of Cellular Matrix in pain reduction and improvement of joint functions [9,10].
We conducted a retrospective, pilot study to test the hypothesis that Cellular Matrix together with autologous PRP (A-PRP) and standard physical therapy (including hot packs, transcutaneous electrical nerve stimulation [TENS], and quadriceps strengthening exercises) is a safe procedure that improves pain and the functionality scores compared to baseline values in patients with primary knee OA.
MATERIALS AND METHODS
This is a retrospective, uncontrolled, case study. The study was conducted on a group of 25 patients diagnosed with primary knee OA. Medical records were surveyed for inclusion and exclusion criteria and proceeded for analysis.
We included patients with grade 1-3 knee OA using the Kellgren and Lawrence system for the classification of OA of the knee [11]. Patients with grade 4 OA and a history of trauma were excluded from the study. We also excluded patients with inflammatory rheumatologic disorders and patients who continued the regular use of nonsteroidal anti-inflammatory medications and pain killers. The patients were referred from primary health centers and from specialty clinics located in two hospitals.
Cellular Matrix is described as a medical device containing natural, non-crosslinked HA, thixotropic separation gel, and sodium citrate anticoagulant solution. The concentration of HA is 20 mg/mL (40 mg total) and the quantity is 2 mL [12]. It is combined with A-PRP prepared by drawing 10 mL blood from the cubital vein and processing in a single-spin centrifuge machine. Intra-articular injections included three doses with a time span of 2 weeks between each dose. All patients received 10 sessions of standardized physiotherapy including hot packs, TENS, and quadriceps strengthening exercise. The treatment was conducted 2 times per week for 5 weeks. This study was approved by Dubai Scientific Research Ethics Committee (Reference number DSREC- 01/2019_05).
Outcome measures
To provide an accurate result, we applied the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) test, Likert version 3.1 [13]. The test demonstrates the severity of the patient’s condition in a range from none to extreme (none, mild, moderate, severe, and extreme). It has a scoring system from 0–4. The maximal score that can be obtained is 96 and the measurement includes pain, stiffness, and function. A lower score represents a better status, i.e., score 0 is the best and score 96 is the worst [14].
Interventions
Cellular Matrix intra-articular injections for all patients were conducted by a physician in the following way: the injection site was marked and cleaned with Betadine (povidone-iodine) solution for disinfection. After that, blood was drawn from the cubital vein into a syringe of 10 mL. The blood was processed using a centrifuge machine to get a high concentration of platelets in plasma (PRP). A nurse prepared the centrifuged Cellular Matrix for injection, then lidocaine injection (local anesthetic) was used to numb the injection site, and the physician injected the Cellular Matrix combined with PRP into the knee joint. The same procedure was repeated two more times with a 2-week duration between the previous dose and the following one. Patients were recommended to cease the use of NSAIDs 1 week before starting the injections and during the whole treatment.
Statistical analysis
Statistical analysis was performed using GraphPad InStat version 3.10, 32 bit for Windows (GraphPad Software, San Diego, California, USA, www.graphpad.com). A value of p < 0.0001 was considered statistically highly significant.
RESULTS
There were a total of 25 patients, 19 females (76%) and 6 males (24%), aged 39–76 years (mean: 57 years) (Table 1).
TABLE 1.
Demographic data of patients with primary knee osteoarthritis

The patients were diagnosed with primary knee OA grade 1-3 before starting the treatment with Cellular Matrix combined with PRP. Twenty-four patients (96%) reported improvement in pain, stiffness, and function from the baseline, measured by the WOMAC score after 6 months. One patient (4%) showed a deterioration in pain, stiffness, and function after 6 months. A total of 17 out of 25 (68%) patients showed more than 50% improvement in pain, stiffness, and function (Table 2). The statistical analysis confirmed a significant improvement from the baseline at the level of p < 0.001 (Table 3).
TABLE 2.
Therapeutic results of combined Cellular Matrix treatment and physiotherapy
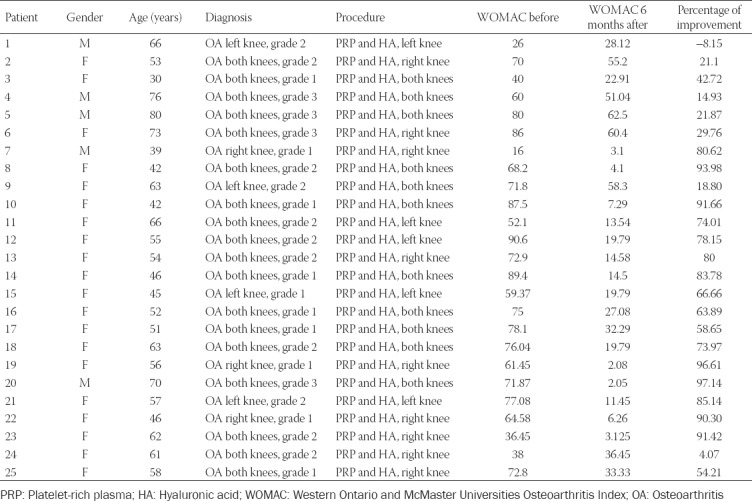
TABLE 3.
Statistical analysis of the treatment results

DISCUSSION
Numerous studies investigated separately HA and A-PRP in the treatment of knee OA [15-18]. However, studies including a combination of HA and PRP are very scarce. In one case study, the authors found that HA and PRP applied together provide a statistically significant improvement in pain reduction, function, and the quality of life of patients 6 months after the treatment [19]. Our study showed similar results. However, a study published in 2015 compared Cellular Matrix and PRP with PRP alone in the treatment of mild and moderate knee OA and showed that there is no difference in the treatment outcome between those two procedures [20].
Because we did not find any study exploring the outcome of Cellular Matrix combined with physiotherapy in patients with knee OA, we were unable to directly compare our results with previous studies.
While the mechanisms of action of PRP and HA have been explained [21,22], it is not clear how these two substances act when they are applied together. We speculate that they may have a synergistic effect, but this has to be tested in randomized controlled trials. The key factors that should be taken into consideration are the changes in the concentration of PRP from baseline, presence or absence of leukocytes, and the optimal molecular weight of hyaluronate.
Considering that this is a single-arm pilot study applied on a small sample of patients, currently, it is not possible to recommend Cellular Matrix combined with A-PRP as an advanced therapeutic option in patients with primary knee OA. The main aim of this study was to test the treatment safety profile and therapeutic effect in combination with physiotherapy. Our study shows promising results with this combined treatment, i.e., a very high percentage of pain reduction and functional improvement in the patients without any significant adverse reactions. The patients selected for this study did not undergo regular radiological procedures, so we were not able to verify whether the clinical improvement was followed by any morphological changes detectable by magnetic resonance imaging.
CONCLUSION
Our single-arm pilot study exploring the combination of Cellular Matrix and A-PRP together with physiotherapy showed promising results in the treatment of mild to moderate knee OA. These results have to be verified in randomized controlled trials with larger samples of participants.
Footnotes
Conflict of interest statement: The authors declare no conflict of interests
REFERENCES
- 1.Heidari B. Knee osteoarthritis prevalence, risk factors, pathogenesis and features:Part I. Caspian J Intern Med. 2011;2:205–12. [PMC free article] [PubMed] [Google Scholar]
- 2.Bliddal H, Christensen R. The treatment and prevention of knee osteoarthritis:a tool for clinical decision-making. Expert Opin Pharmacother. 2009;10:1793–804. doi: 10.1517/14656560903018911. https://doi.org/10.1517/14656560903018911. [DOI] [PubMed] [Google Scholar]
- 3.Heidari B. Rheumatic Diseases. 1st ed. Babol: Iran Babol University of Medical Sciences Publication; 2002. [Google Scholar]
- 4.Voelker R. Few adults with knee osteoarthritis meet national guidelines for physical activity. JAMA. 2011;306:1428–1430. doi: 10.1001/jama.2011.1388. https://doi.org/10.1001/jama.2011.1388. [DOI] [PubMed] [Google Scholar]
- 5.Smink AJ, van den Ende CH, Vliet Vliel TP, Swierstra BA, Kortland JH, Bijlsma JW, et al. “Beating osteoarthritis”:development of a stepped care strategy to optimize utilization and timing of non-surgical treatment modalities for patients with hip or knee osteoarthritis. Clin Rheumatol. 2011;30:1623–9. doi: 10.1007/s10067-011-1835-x. https://doi.org/10.1007/s10067-011-1835-x. [DOI] [PubMed] [Google Scholar]
- 6.Kon E, Filardo G, Drobnic M, Madry H, Jelic M, van Dijk N, et al. Non-surgical management of early knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2012;20:436–49. doi: 10.1007/s00167-011-1713-8. https://doi.org/10.1007/s00167-011-1713-8. [DOI] [PubMed] [Google Scholar]
- 7.Pinals RS. Pharmacologic treatment of osteoarthritis. Clin Ther. 1992;14:336–46. [PubMed] [Google Scholar]
- 8.Richette P, Chevalier X, Ea HK, Eymard F, Henrotin Y, Ornetti P, et al. Hyaluronan for knee osteoarthritis:an updated meta-analysis of trials with low risk of bias. RMD Open. 2015;1:e000071. doi: 10.1136/rmdopen-2015-000071. https://doi.org/10.1136/rmdopen-2015-000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Southworth TM, Naveen NB, Tauro TM, Leong NL, Cole BJ. The use of platelet-rich plasma in symptomatic knee osteoarthritis. J Knee Surg. 2019;32:37–45. doi: 10.1055/s-0038-1675170. https://doi.org/10.1055/s-0038-1675170. [DOI] [PubMed] [Google Scholar]
- 10.Pierelli L. Proceedings of International Biobridge Conference Generation Regeneration 2014. Venice, Italy. San Antonio, United States: Biobridge Foundation; 2015. Sep, Cellular Matrix in Osteoarthritis San Camillo Protocol; pp. 21–23. [Google Scholar]
- 11.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. https://doi.org/10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frizziero A. Proceedings of International Biobridge conference Generation Regeneration. Venice, Italy. San Antonio, United States: Biobridge Foundation; 2014. Sep, Treatment of Knee Osteoarthritis with Cellular Matrix, a Synergistic Association of Platelet-Rich Plasma and Hyaluronic Acid; pp. 21–23. [Google Scholar]
- 13.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC:a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–40. [PubMed] [Google Scholar]
- 14.Faik A, Benbouazza K, Amine B, Maaroufi H, Bahiri R, Lazrak N, et al. Translation and validation of Moroccan Western Ontario and McMaster universities (WOMAC) osteoarthritis index in knee osteoarthritis. Rheumatol Int. 2008;28:677–83. doi: 10.1007/s00296-007-0498-z. https://doi.org/10.1007/s00296-007-0498-z. [DOI] [PubMed] [Google Scholar]
- 15.Xing D, Wang B, Liu Q, Ke Y, Xu Y, Li Z, et al. Intra-articular hyaluronic acid in treating knee osteoarthritis:a PRISMA-compliant systematic review of overlapping meta-analysis. Sci Rep. 2016;6:32790. doi: 10.1038/srep32790. https://doi.org/10.1038/srep32790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strand V, McIntyre LF, Beach WR, Miller LE, Block JE. Safety and efficacy of US-approved viscosupplements for knee osteoarthritis:a systematic review and meta-analysis of randomized, saline-controlled trials. J Pain Res. 2015;8:217–28. doi: 10.2147/JPR.S83076. https://doi.org/10.2147/jpr.s83076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldberg VM, Buckwalter JA. Hyaluronans in the treatment of osteoarthritis of the knee:evidence for disease-modifying activity. Osteoarthritis Cartilage. 2005;13:216–24. doi: 10.1016/j.joca.2004.11.010. https://doi.org/10.1016/j.joca.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 18.Glynn LG, Mustafa A, Casey M, Krawczyk J, Blom J, Galvin R, et al. Platelet-rich plasma (PRP) therapy for knee arthritis:a feasibility study in primary care. Pilot Feasibility Stud. 2018;4:93. doi: 10.1186/s40814-018-0288-2. https://doi.org/10.1186/s40814-018-0288-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurapati K, Tapadia S, Rao M, Anbarasu K, Verma VK, Beevi SS. Efficacy of intra-articular injection of platelet rich plasma and hyaluronic acid in early knee osteoarthritis case series. Eur J Mol Clin Med. 2018;5:30–6. https://doi.org/10.5334/ejmcm.251. [Google Scholar]
- 20.Abate M, Verna S, Schiavone C, Di Gregorio P, Salini V. Efficacy and safety profile of a compound composed of platelet-rich plasma and hyaluronic acid in the treatment for knee osteoarthritis (preliminary results) Eur J Orthop Surg Traumatol. 2015;25:1321–6. doi: 10.1007/s00590-015-1693-3. https://doi.org/10.1007/s00590-015-1693-3. [DOI] [PubMed] [Google Scholar]
- 21.Knighton DR, Hunt TK, Thakral KK, Goodson WH., 3rd Role of platelets and fibrin in the healing sequence:an in vivo study of angiogenesis and collagen synthesis. Ann Surg. 1982;196:379–88. doi: 10.1097/00000658-198210000-00001. https://doi.org/10.1097/00000658-198210000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Altman RD, Manjoo A, Fierlinger A, Niazi F, Nicholls M. The mechanism of action for hyaluronic acid treatment in the osteoarthritic knee:a systematic review. BMC Musculoskelet Disord. 2015;16:321. doi: 10.1186/s12891-015-0775-z. https://doi.org/10.1186/s12891-015-0775-z. [DOI] [PMC free article] [PubMed] [Google Scholar]


