Abstract
Gastric cancer (GC) is one of the foremost causes of cancer-related death around the world. The P2X7 receptor (P2X7R), a member of the P2X7R subfamily of P2 receptors, is a unique molecule that has been shown to affect tumor growth and progression as well as various inflammatory processes, including proliferation of T lymphocytes, release of cytokines, and production of free oxygen radicals. P2X7R has been established as a prognostic parameter in some cancers, and recently, it has been investigated in the development of new targeted therapies. In the present study, we aimed to investigate the prognostic value of P2X7R expression in GC. The expression profile of P2X7R was evaluated immunohistochemically in 156 paraffin-embedded human GC specimens. P2X7R expression was higher in patients with lymph node metastasis than in those without (p < 0.001). P2X7R overexpression was closely related with tumor-infiltrating lymphocytes (TILs) (p = 0.001), vascular invasion (p = 0.006), depth of invasion (p < 0.001), distant metastasis (p < 0.001), and advanced tumor, node, metastasis stage (p < 0.001). Moreover, univariate (hazard ratio [HR] 3.98; 95% confidence interval (CI) 1.89-11.82; p < 0.001) and multivariate (HR 2.24; 95% CI 3.53-12.50; p < 0.001) Cox regression analysis showed that upregulated P2X7R expression clearly correlated with worsened overall survival. In summary, our data revealed that P2X7R may serve as a reliable prognostic parameter and promising therapeutic target for GC.
Keywords: Gastric cancer, P2X7 receptor, survival, tumor-infiltrating lymphocytes, prognosis
INTRODUCTION
Gastric cancer (GC), caused roughly 780,000 deaths in 2018, is one of the most common cancers in developed countries and is the second cause of cancer-related mortality in addition to lung cancer [1-3]. GCs are histologically highly heterogeneous tumors. There are several classification systems such as Lauren’s and World Health Organization (WHO) classifications for this cancer [4-6]. GCs are classified into three main histological groups as intestinal, diffuse, and indeterminate types according to Lauren’s design [5]. Intestinal type predominantly affects men and occurs in older ages. Distal (non-cardiac) involvement and intestinal metaplasia are common in this type. However, diffuse GC occurs in younger patients, prefers proximal localization, and is associated with unfavorable prognosis [4]. Surgical modalities are the most appropriate treatment option for patients with GC who want long-term survival. However, the prognosis is very poor, as the majority of patients are diagnosed at advanced stages characterized by invasion or metastasis rather than asymptomatic early stage [7]. Therefore, new biomarkers are needed to predict prognosis in patients with GC and to discover target treatment options.
The P2X receptors (P2XR) identified as seven subunits (P2X1-7) are ATP-gated calcium-permeable cationic channels. Of these, in particular, the P2X7R is of increasing attention as a possible therapeutic target for the treatment of some diseases, including certain cancers [8-11]. P2X7R plays an important role in regulating inflammatory events. This exclusive molecule that is contribute both adaptive and innate immunity is expressed from almost all inflammatory cell types, including lymphocytes, macrophages, dendritic cells, monocytes, neutrophils, basophils, eosinophils, and mast cells [12]. In addition, the P2X7R has a key regulatory ability on aerobic glycolysis. The cells expressing P2X7R upregulate glucose transporter 1 as well as various glycolytic enzymes [13]. P2X7R localized to the cytoplasmic membrane is considered to be an important mediator for tumor invasion and metastasis as well as its role in inflammation and aerobic glycolysis [12]. In a few recent studies, it is claimed that P2X7R expression in tumor cells associated with aggressive clinical features and poor prognosis in patients with colorectal cancer (CRC) [14,15]. In another study, it was reported that P2X7R regulates the cell survival and cell migration in ductal pancreatic adenocarcinoma cells, and P2X7R allosteric inhibitor reduced cell proliferation [16]. Therefore, P2X7R may be a new biomarker for various cancers and a new option for targeted therapies. To the best of our knowledge, however, there is no large-scale clinical study investigating the association of P2X7R expression with GC prognosis. In our opinion, this study is the first clinical research to this issue.
In this study, we aimed mainly to investigate whether P2X7R expression adversely effects on overall and disease-free survival of patients with GC. We also aimed to evaluate the effects of classical clinical and pathological parameters such as tumor localization, Goseki histological grade, and CD8+ tumor-infiltrating lymphocytes (TILs) on prognosis.
MATERIALS AND METHODS
Patients and pathological review
This study included 156 patients who underwent surgery for GC in Fırat University Faculty of Medicine between 2010 and 2015. Patients who received adjuvant chemotherapy were excluded from the study. Clinical information of patients was obtained from the electronic data center of our hospital. Pathological materials of the cases were reevaluated by two pathologists in terms of tumor localization, histopathological type, histological grade, Goseki grade, lymphovascular invasion, perineural invasion, TILs, depth of invasion (pT), lymph node metastasis (pN), and tumor, node, metastasis (TNM) stage. Histological tumor types were specified according to the criteria of the WHO classification system and the Lauren’s classification. Patients were divided into four groups according to Goseki histological grading system. The TNM stages of the cases were determined according to the American Joint Committee on Cancer, 7th edition [17]. The patients have been subdivided into four groups according to the pT category: T1 (tumor invades lamina propria, muscularis mucosa, or submucosa), T2 (tumor invades muscularis propria), T3 (tumor penetrates subserosal connective tissue without invasion of visceral peritoneum or adjacent structures), and T4 (tumor invades serosa or adjacent structures). While evaluating vascular invasion, tumor embolus was investigated in lymphatic and blood vessels in tumor areas in routine preparations with hematoxylin-eosin staining. The cases were divided into two groups with and without vascular invasion. Survival data of the patients were collected from the hospital medical data processing record. Overall survival (OS) was determined as the interval between the dates of surgery and death.
Immunohistochemistry (IHC)
For immunohistochemical examination, 5-µm thick sections from paraffinized tumor tissues were used. In the immunophenotypic staining process, the following antibodies were applied: Anti-P2X7R (ab48871, ABCAM, Cambridge CB2 0AX, UK) and anti-CD8 (SP57, Ventana, Arizona, USA). The prepared paraffinized sections were stained using the Ventana BenchMark ULTRA coater (Ventana, Tucson, AZ-85755, USA) and the ultraView Universal DAB kit (Ventana, Tucson, AZ-85755, USA) following the manufacturer’s instructions. The intensity and distribution of P2X7R expression in cancer cells were evaluated immunohistochemically. In addition, the TILs density was assessed by CD8 staining. Immunostaining of each sample was examined by two objective investigators unaware of the clinical features, survival data, and pathological features of the patients.
Scoring of P2X7R expression
The expression of P2X7R in tumor cells was evaluated by IHC-score which is obtained multiplying the staining intensity (cytoplasmic and membranous) (0: Negative, 1: Weak staining, 2: Medium staining, and 3: Strong staining) by the extent of staining (0: <10%; 1: 10% −25%; 2: 25% −70%; and 3: >70%.). IHC-score ranged from 0 to 9. The median value of the IHC score (2.89±3.32) was accepted as the cutoff criterion. The cases with IHC-score below this value were considered as a low-P2X7R group and those above were characterized as a high-P2X7R group. These criteria had previously been validated in various studies [14,15].
Evaluating of CD8+ TILs density
The density of CD8 + TILs was evaluated considering previous studies [18,19]. Shortly, five fields with the intense infiltration of GC were selected from each slide and the percentages of CD8+ TILs were calculated. The average of five fields was used as the density of CD8+ TILs. First, a semi-quantitative staining score was determined according to the following scale: 1 (<1% cells); 2 (1-10% cells); 3 (11-33% cells); 4 (34-66% cells); and 5 (67-100% cells). The cells with positive immunoreactivity were described as those showing partial or complete positivity in the cytoplasm and/or plasma membrane. Second, staining density was scored as follows: 0 (absent), 1+ (slight), 2+ (moderate), and 3+ (intense). Finally, scores (ranging from 1 to 8) were calculated by adding the percentage staining scores and the density scores for each slide. The cases were divided into two groups as a low and high density of CD8 + TILs using median value (2.37±2.12).
Statistical analysis
All of the data were analyzed statistically using SPSS v.26 software (IBM Corporation, Armonk, NY, USA). Results were expressed as percentages, means, and standard deviations. Normality was checked using the Shapiro-Wilk test. Furthermore, the kurtosis and skewness values between −0.5 and +1.5 were considered to indicate normality. While independent samples t test and ANOVA were used to determine the differences between the groups showing normal distribution, Mann–Whitney U test was used to determine the differences between groups without normal distribution. The relationship between survival and prognostic parameters was evaluated using the Kaplan–Meier method (log-rank test). For univariate and multivariate models, Cox regression analysis was performed to estimate the hazard ratio (HR) and 95% confidence interval (CI). p < 0.05 was considered statistically significant for all data.
RESULTS
Many basic pathological parameters were closely related to overall and disease-free survival
Of the patients included in this study, 95 (60.9%) were male and 61 (39.1%) were female. The minimum and maximum ages were 25 and 86, respectively. The mean age was 63.92±1.08 years. Since most patients were diagnosed at an advanced stage, the mean survival time was 37.98±1.15 months and the 5-year survival rate was only 21.8%, unfortunately. The majority of our cases consisted of Stage III (n=58; 37.2%) and Stage IV (n = 49; 31.4%) patients. Other clinical and pathological data are shown in Table 1 in detail. According to univariate Cox regression analysis, tumor site, histopathologic tumor type, histological grade, Goseki grade, vascular invasion, depth of invasion (pT), lymph node metastasis (pN), and advanced TNM stage were found to be significantly correlated with poor prognosis (Table 1). Among these, pN (p < 0.001) and TNM stage (p < 0.001) was found to be more closely related to survival. While the mean OS was 49.14±1.09 month and DFS was 42.23±2.16 month in TNM Stage II patients, they were significantly lower in Stage IV patients (24.77±1.04 month and 16.67±1.04 month, respectively) (Figure 1A and B). Similarly, the mean OS and DFS (56.83±1.34 month and 51.83±2.01 month, respectively) in cases without lymph node metastasis were significantly higher than in patients with metastases to more than 7 lymph nodes (30.0±1.37 month and 22.27±1.40 month, respectively). According to our data, other clinicopathological parameters such as gender, age, tumor size, and perineural invasion did not have a statistically significant effect on OS (Table 1).
TABLE 1.
The effect of classical clinical and pathological parameters of gastric cancer on disease‑free survival (n=156)
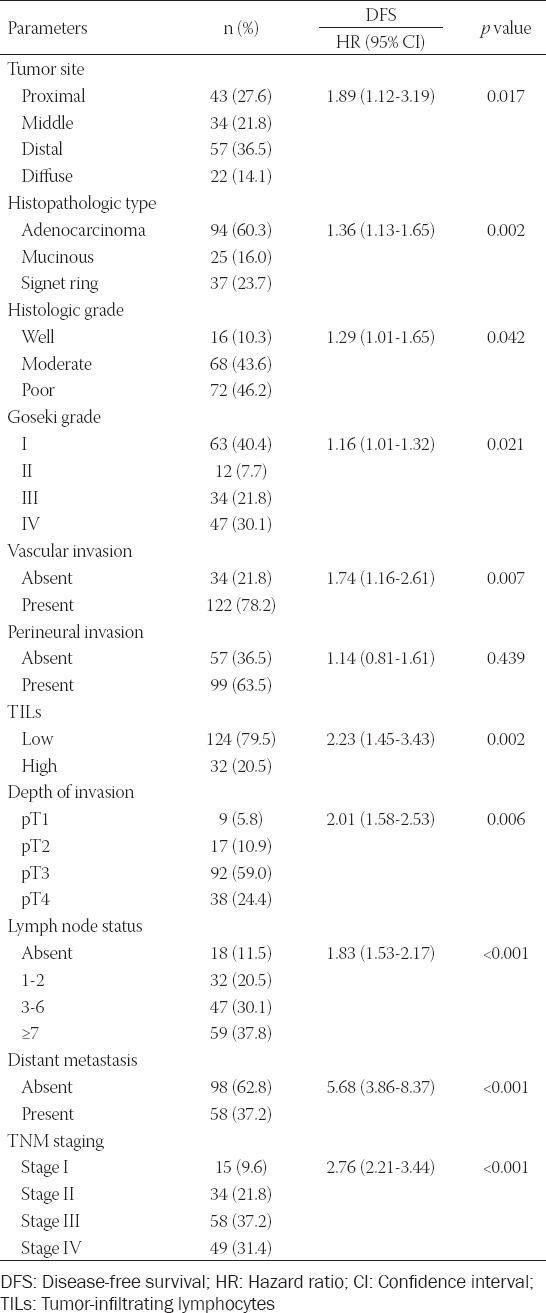
FIGURE 1.
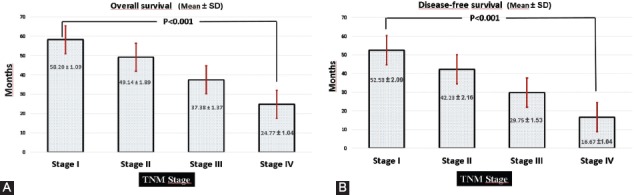
Overall and disease-free survival times of patients according to the tumor, node, metastasis (TNM) stage. (A) In advanced TNM stage patients, survival times were significantly lower than TNM Stage I ones (p < 0.001, Log Rank). (B) Likewise, disease-free survival time was significantly shorter in patients with TNM Stage IV than those in Stage I (p < 0.001, Log Rank). Quantitative data were expressed as mean ± standard deviation.
High expression of P2X7R was correlated with aggressive clinical and pathobiological behavior
P2X7R expression was not determined in non-tumoral colonic mucosa, immunohistochemically (Figure 2A). We specified the interrelationship between P2X7R expression and various classic pathological parameters of patients with CRC. In accordance with the IHC scores, 156 patients with GC were split into two groups as low-P2X7R expressing (n = 96, 61.5%) (Figure 2B) and high-P2X7R expressing (n = 60, 38.5%) (Figure 2C) ones. As indicated in Table 2, high-P2X7R expression was significantly related with vascular invasion (p < 0.001; Figure 3A and B), low TILs-density (p < 0.001), high-pT (p < 0.001; Figure 3C and D), high-pN (p = 0.001; Figure 3E), distant metastasis (Figure 3F), and advanced TNM stage (p < 0.001), but not with other clinicopathological parameters such as gender, age, tumor size, tumor site, histological type, histological grading, and Goseki grade.
FIGURE 2.
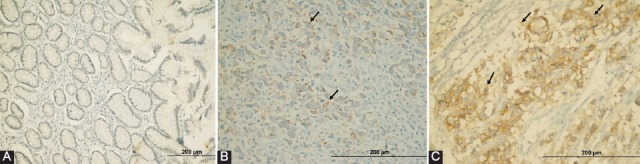
Representative examples of P2X7 receptor (P2X7R) expression in gastric cancer specimens: (A) No staining (normal mucosa adjacent to tumor) (×200); (B) low-P2X7R expression of tumor cells (arrows) in a patient with early-stage (×400); (C) high-expression of P2X7R (×400) in a patient with advanced tumor, node, metastasis (TNM) stage. The high expression group showed intense cytoplasmic and membranous staining.
TABLE 2.
The interrelationship between P2X7R expression and clinicopathological parameters
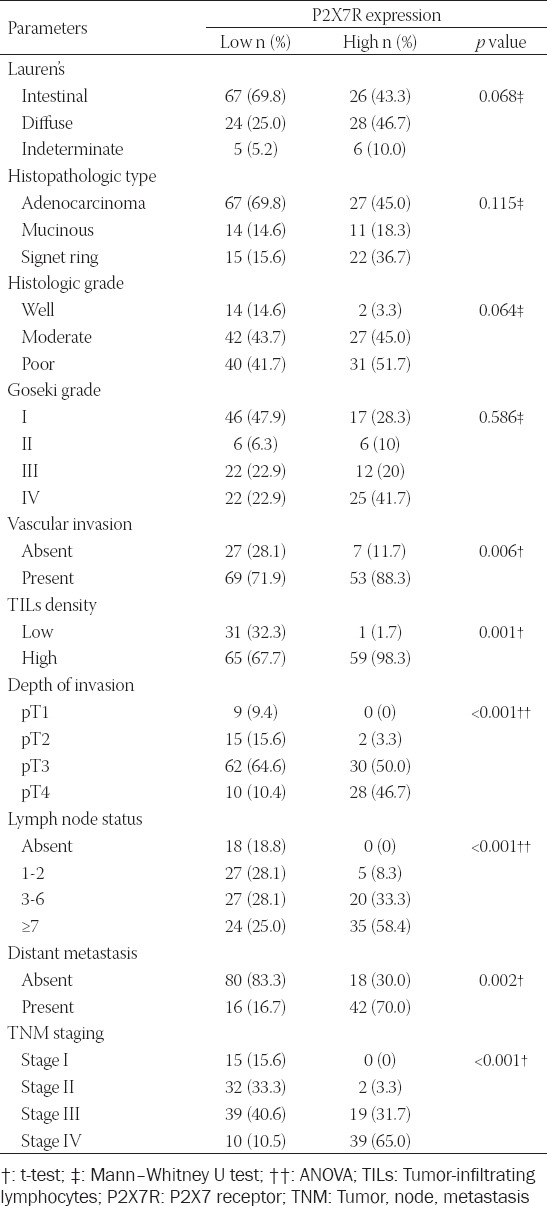
FIGURE 3.
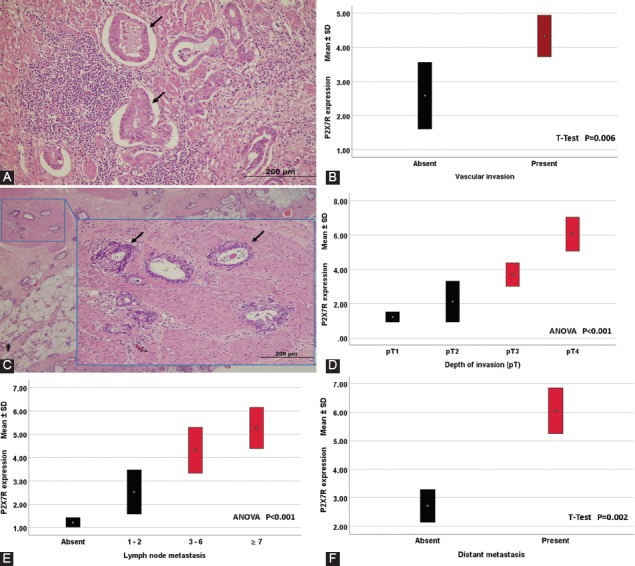
Representative images showing the relationship between P2X7 receptor (P2X7R) expression and classical prognostic parameters such as vascular invasion, depth of tumor invasion, lymph node metastasis, and distant metastasis. (A) Vascular invasions (arrows) are seen in areas adjacent to the tumor (hematoxylin and eosin ×200). (B) Higher expression of P2X7R was observed in patients with vascular invasion (p = 0.006, t-test). (C) The malign glands invading muscularis propria are clearly seen in a case with pathologic Stage 2 (hematoxylin and eosin ×200). (D) As tumor invasion depth increased, P2X7R expression increased (p < 0.001, ANOVA). (E) Expression of P2X7R was significantly upregulated in patients with lymph node metastasis (p < 0.001, ANOVA). (F) High P2X7R expression was significantly associated with an increased distant metastasis rate (p = 0.002, t-test). Quantitative data were expressed as mean ± standard deviation.
Low survival rates were observed in patients with high P2X7R expression
The Kaplan–Meier survival analysis was used to assess the relationship between P2X7R expression and survival times in patients with GC (Figure 4A and B). The group expressing high P2X7R showed a clearly lower mean survival time (23.83±0.63 months) than those with low P2X7R expression (46.83±1.10 months). In addition, univariate and multivariate Cox regression analyses were carried out to determine whether P2X7R expression and other pathological parameters could be used as independent prognostic indicators of GC patients. When evaluated in terms of OS, it was determined that P2X7R expression together with pN and TNM stage could be used as an independent predictor of poor prognosis in patients with GC (Table 3). In addition, when the cases were reviewed for DFS, both univariate and multivariate Cox regression analysis showed a significant correlation between high P2X7R expression and DFS (univariate: HR 4.37, 95% CI 4.13-12.02, p < 0.001 and multivariate: HR 2.51, 95 % CI 6.84-12.90, p < 0.001).
FIGURE 4.
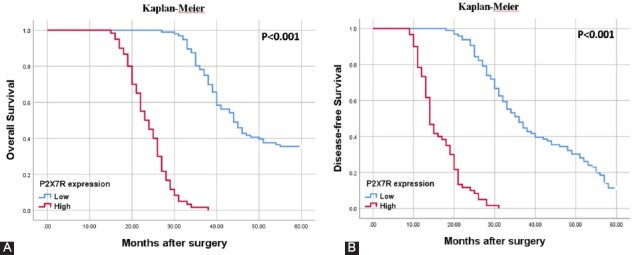
Representative Kaplan–Meier survival curves are seen according to P2X7 receptor (P2X7R) expression. (A) Patients with high-P2X7R expression had a worsened overall survival (p < 0.001, Log Rank). (B) Disease-free survival times were significantly higher in cases with downregulated P2X7R expression (p < 0.001, Log Rank).
TABLE 3.
Relationship between P2X7R expression and overall survival in univariate and multivariate Cox regression analysis
Low CD8+ TILs density had a negative effect on prognosis in GC patients
The patients were divided into two groups as the low (n = 124, 79.5%) (Figure 5A) and high (n = 32, 20.5%) (Figure 5B) TILs density group according to CD8+ TILs density. Low TILs density was observed more in patients with diffuse-type tumor than in intestinal ones. In addition, low CD8+ TILs density was correlated with lymph node metastasis (p < 0.001), distant metastasis (p = 0.045), and advanced TNM stage (p < 0.001). Moreover, Kaplan–Meier survival curves showed that the OS periods of the low CD8+ TILs density group were significantly shorter than the high-density group (Figure 6A). However, multivariate cox regression analysis showed no significant difference between low CD8+ TILs density group with high ones (HR 1.72, 95% CI 0.89-3.32, p = 0.105). On the other hand, there was an inverse correlation between TILs density and P2X7R expression. Obviously, P2X7R overexpression was observed in cases with low TILs density, whereas the group with high TILs density showed low P2X7R expression (Figure 6B).
FIGURE 5.
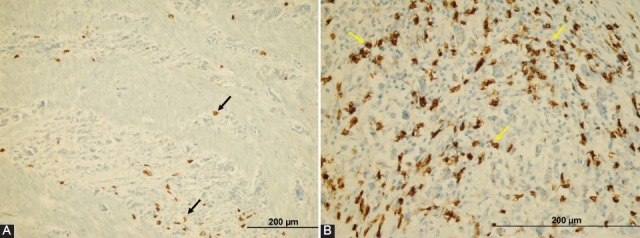
Representative images showing density of CD8+ tumor-infiltrating lymphocytes (TILs) in gastric cancer (×200). (A) Low density of CD8+ TILs. (B) High density of CD8+ TILs.
FIGURE 6.
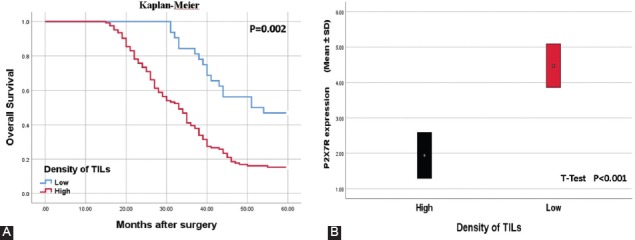
(A) Kaplan–Meier curves of overall survival according to tumor infiltrating lymphocytes (TILs) density. The patients with high TILs density had significantly longer survival time than those with high expression (p < 0.001, Log Rank). (B) The overexpression of P2X7 receptor (P2X7R) appears to reduce the inflammatory reaction to the tumor. TILs density was significantly lower in patients with high P2X7R expression (p < 0.001, t-test).
DISCUSSION
Various studies supporting each other have asserted that chronic inflammation is an important factor caused to cancer progression [14]. P2X7R, a purinergic receptor for ATP, is mainly expressed in various inflammatory cells and plays an essential role in the development of rapidly occurring allergic inflammatory responses by allowing the release of important cytokines that regulate inflammatory events [14,15,20]. In the present study, we first examined the expression profile of P2X7R in GC samples immunohistochemically and found that P2X7R was significantly upregulated in human GC tissues compared to peritumoral gastric mucosa. We revealed that high P2X7R expression was importantly correlated with vascular invasion, pT, pN, distant metastasis, and advanced TNM stage in GC patients. Particularly, both univariate and multivariate Cox regression analysis revealed that P2X7R could serve as an independent predictor of poor prognosis in GC patients. These findings implied that P2X7R plays an important role in GC progression and might be important in determining the prognosis of patients with GC.
Recently, various researches have reported that the presence of P2X7R overexpression in some cancer types [15,21-23]. Liu et al. reported that high P2X7R expression induces worse clinical outcomes in patients with clear cell renal cell carcinoma, including cancer-specific survival and advanced TNM stage [24]. Prolonged activation of P2X7R, which plays a key role in various inflammatory events, may promote tumor inflammatory microenvironment (TIM) and cancer progression by facilitating chronic inflammation [14]. Tafani et al. reported that transcription factors such as HIF 1α and nuclear factor-kappa B (NF-κB) stimulate P2X7R expression in cancer cells, and then activated P2X7R stimulate aggressive biological behavior and tumor invasion by triggering NF-κB activation [25]. In addition, Ryu et al. stated that in vivo inhibition of P2X7R through P2X7R antagonist brilliant blue G weakened inflammatory activity in TIM, leading to a decrease in tumor growth [26]. These data suggested that the effects of P2X7R on tumor progression might be mediated by tumor-associated inflammatory processes. In an interesting study, Jelassi et al. reported that P2X7R is over-expressed in highly aggressive breast cancer cells and contributes greatly to tumor invasiveness [27]. In another study, including 116 cases with CRC, investigated the P2X7R expression profile in CRC tissues [14]. Qian et al. found that P2X7R was significantly upregulated in human CRC tissues. Moreover, they found out that P2X7R might facilitate the activation of Akt and NF-κB signaling pathways to trigger the expression of cyclin D1. And so, they implied that P2X7R plays an important role in promoting CRC cell proliferation. These data suggest that P2X7R may establish a link between chronic inflammation and tumor progression through activation of classical oncogenic pathways.
In a recent study, Qiu et al. claimed that P2X7R contributes to tumor cell invasiveness [28]. In prostate cancer cells, PI3K/AKT and ERK1/2 pathways were enhanced by the activation of P2X7R after ATP addition, with the downriver invasiveness-associated genes Claudin-1, E-cadherin, interleukin-8, and matrix metalloproteinase-3 (MMP-3) which is attend as further inductors [28]. In addition, MMP-3-dependent tumor cell invasion was also showed in mouse-CRC [29]. These data implied that an overexpression of P2X7R may also induce MMP-3-dependent tumor cell invasion in cancer. In another study, Zhang et al. reported that P2X7R expression was higher in CRC patients with metastatic disease than non-metastatic ones [15]. They revealed that P2X7R expression was upregulated in metastatic cancer cells and cell lines. In addition, Zhang et al. stated that the survival time in CRC patients with P2X7R expression was significantly shorter than in those with low expression. Similar to the previous studies on the other cancers, our study showed more intense P2X7R expression in tumoral sites compared to non-tumoral regions. In this study, maybe the first study investigating the effect of P2X7R expression on the prognosis of GC, P2X7R overexpression was closely related with aggressive pathological features, including advanced-pT, increased number of metastatic lymph nodes, high distant metastasis rates, and advanced TNM stage. In addition, there was an interrelation between P2X7R expression and TILs density. Interestingly, the cases with P2X7R overexpression had low TILs density. Moreover, in our study, P2X7R overexpression was shown to be associated with low survival times (OS and DFS) according to both univariate and multivariate Cox regression analysis. When all these data are evaluated together, it can be said that P2X7R overexpression in GC is closely related to tumor progression, invasiveness, and ability of distant metastasis. Furthermore, the low density of TILs in cases with P2X7R overexpression can suggest that P2X7R promotes the inflammatory events at the early period of tumor, but significantly suppresses the host inflammatory response to the tumor as the process progresses. On the other hand, it is considered that inhibition of P2XR7 may be a good strategy for the treatment of various cancers, including GC. Although perhaps too early for human use, a P2X7R inhibitor has recently been tested on pancreatic cancer cells and reported to have anticancer activity [10]. In two different studies supporting this, Blockade of P2X7R decreased growth and metastases of implanted tumors, such as hepatocellular carcinoma and breast cancer [30,31].
Gastric TIM contains a wide range of immunoinflammatory cells that significantly affect cancer development and progression. These cells are involved in the regulation of immune reactions associated with cancer biology and therefore have been a major focus of interest in cancer-related research today. Recent series have investigated the association between TILs density and GC survival. However, in most of these studies, incompatible results were obtained [32,33]. For instance, Fukuda et al. found no significant difference in terms of survival times between groups with low and high TILs density [32]. On the contrary, Lee et al. stated that OS time of GC patients with high CD8+ TILs density tended to be longer than those with low TILs intensity, although they were in the same TNM stage [33]. In a meta-analysis, Yu et al. stated that TILs are prognostic markers for OS in patients with GC. Moreover, they revealed that intratumoral CD8+ and CD3+ lymphocyte density were associated with good prognosis in GC, whereas CD4+ and FOXP3+ lymphocyte density had no significant effect on survival outcomes [34]. In the present study, both OS and DFS times were significantly higher in patients with high CD8+ TILs density than those with low TILs density. These conflicting results in the literature may be caused by differences in the methods used to determine the density of TILs. Furthermore, it should be noted that the function of CD8+ T lymphocytes in TIM is linked to other immune cells, such as macrophages. Therefore, to obtain more reliable results, studies investigating the effect of multiple inflammatory cell types on prognosis should be performed.
CONCLUSION
Consequently, this present study 1st-time revealed that P2X7R overexpression was an independent poor prognostic indicator in patients with GC. The up-regulated P2X7R expression was related to advanced TNM stage and metastasis in GC patients. In addition, CD8 TILs density had a considerable effect on the survival of patients with GC. Especially, patients with high TILs density had higher survival times than those with low. Again, the inverse correlation between P2X7R expression and TILs density revealed that P2X7R is important in regulating the cancer microenvironment. These findings support that P2X7R expression might be an ideal prognostic predictor and biomolecular target for GC.
Footnotes
Conflict of interest statement: The authors declare no conflict of interests
REFERENCES
- 1.Radović S, Dorić M, Hukić A, Babić M, Kuskunović S, Spahović N. Immunohistochemical expression and significance of NM23 suppressor protein in primary gastric adenocarcinoma. Bosn J Basic Med Sci. 2013;13(2):72–7. doi: 10.17305/bjbms.2013.2368. https://doi.org/10.17305/bjbms.2013.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kepil N, Batur S, Sonmez Wetherilt C, Erdamar Cetin S. Human epidermal growth factor receptor 2 (HER-2) status evaluation in advanced gastric cancer using immunohistochemistry versus silver in situ hybridization. Bosn J Basic Med Sci. 2017;17(2):109–13. doi: 10.17305/bjbms.2016.1497. https://doi.org/10.17305/bjbms.2016.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018:GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. https://doi.org/10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 4.Kataoka I, Funata S, Nagahama K, Isogaya K, Takeuchi H, Abe N, et al. DNMT3A overexpression is associated with aggressive behavior and enteroblastic differentiation of gastric adenocarcinoma. Ann Diagn Pathol. 2020;44:151456. doi: 10.1016/j.anndiagpath.2019.151456. https://doi.org/10.1016/j.anndiagpath.2019.151456. [DOI] [PubMed] [Google Scholar]
- 5.Calik M, Calik I, Demirci E, Altun E, Gundogdu B, Sipal S, et al. Goseki grade and tumour location influence survival of patients with gastric cancer. Asian Pac J Cancer Prev. 2014;15(3):1429–34. doi: 10.7314/apjcp.2014.15.3.1429. https://doi.org/10.7314/apjcp.2014.15.3.1429. [DOI] [PubMed] [Google Scholar]
- 6.Çalik M, Demirci E, Altun E, Çalik İ, Gündoğdu ÖB, Gürsan N, et al. Clinicopathological importance of Ki-67, p27, and p53. expression in gastric cancer. Turk J Med Sci. 2015;45(1):118–28. doi: 10.3906/sag-1311-107. https://doi.org/10.3906/sag-1311-107. [DOI] [PubMed] [Google Scholar]
- 7.Hu C, Xu Z, Chen S, Lv H, Wang Y, Wang X, et al. Overexpression of B7H5/CD28H is associated with worse survival in human gastric cancer. J Cell Mol Med. 2020;24:1360–9. doi: 10.1111/jcmm.14812. https://doi.org/10.1111/jcmm.14812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scarpellino G, Genova T, Munaron L. Purinergic P2X7 Receptor:A Cation Channel Sensitive to Tumor Microenvironment. Recent Pat Anticancer Drug Discov. 2019;14(1):32–8. doi: 10.2174/1574892814666190116122256. https://doi.org/10.2174/1574892814666190116122256. [DOI] [PubMed] [Google Scholar]
- 9.Vázquez-Cuevas FG, Martínez-Ramírez AS, Robles-Martínez L, Garay E, García-Carrancá A, Pérez-Montiel D, et al. Paracrine stimulation of P2X7 receptor by ATP activates a proliferative pathway in ovarian carcinoma cells. J Cell Biochem. 2014;115(11):1955–66. doi: 10.1002/jcb.24867. https://doi.org/10.1002/jcb.24867. [DOI] [PubMed] [Google Scholar]
- 10.Giannuzzo A, Saccomano M, Napp J, Ellegaard M, Alves F, Novak I. Targeting of the P2X7 receptor in pancreatic cancer and stellate cells. Int J Cancer. 2016;139(11):2540–52. doi: 10.1002/ijc.30380. https://doi.org/10.1002/ijc.30380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amoroso F, Capece M, Rotondo A, Cangelosi D, Ferracin M, Franceschini A, et al. The P2X7 receptor is a key modulator of the PI3K/GSK3β/VEGF signaling network:Evidence in experimental neuroblastoma. Oncogene. 2015;34(41):5240–51. doi: 10.1038/onc.2014.444. https://doi.org/10.1038/onc.2014.444. [DOI] [PubMed] [Google Scholar]
- 12.Burnstock G, Knight GE. The potential of P2X7 receptors as a therapeutic target, including inflammation and tumour progression. Purinergic Signal. 2018;14(1):1–8. doi: 10.1007/s11302-017-9593-0. https://doi.org/10.1007/s11302-017-9593-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amoroso F, Falzoni S, Adinolfi E, Ferrari D, Di Virgilio F. The P2X7 receptor is a key modulator of aerobic glycolysis. Cell Death Dis. 2012;3:e370. doi: 10.1038/cddis.2012.105. https://doi.org/10.1038/cddis.2012.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qian F, Xiao J, Hu B, Sun N, Yin W, Zhu J. High expression of P2X7R is an independent postoperative indicator of poor prognosis in colorectal cancer. Hum Pathol. 2017;64:61–8. doi: 10.1016/j.humpath.2017.03.019. https://doi.org/10.1016/j.humpath.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Ding J, Wang L. The role of P2X7 receptor in prognosis and metastasis of colorectal cancer. Adv Med Sci. 2019;64(2):388–94. doi: 10.1016/j.advms.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Giannuzzo A, Pedersen SF, Novak I. The P2X7 receptor regulates cell survival, migration and invasion of pancreatic ductal adenocarcinoma cells. Mol Cancer. 2015;14:203. doi: 10.1186/s12943-015-0472-4. https://doi.org/10.1186/s12943-015-0472-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Washington K. 7th edition of the AJCC cancer staging manual:Stomach. Ann Surg Oncol. 2010;17(12):3077–9. doi: 10.1245/s10434-010-1362-z. https://doi.org/10.1245/s10434-010-1362-z. [DOI] [PubMed] [Google Scholar]
- 18.Lu J, Xu Y, Wu Y, Huang XY, Xie JW, Wang JB, et al. Tumor-infiltrating CD8+T cells combined with tumor-associated CD68+macrophages predict postoperative prognosis and adjuvant chemotherapy benefit in resected gastric cancer. BMC Cancer. 2019;19:920. doi: 10.1186/s12885-019-6089-z. https://doi.org/10.1186/s12885-019-6089-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen J, He Q, Liu J, Xiao Y, Xiao C, Chen K, et al. CD8+tumor-infiltrating lymphocytes as a novel prognostic biomarker in lung sarcomatoid carcinoma, a rare subtype of lung cancer. Cancer Manag Res. 2018;10:3505–11. doi: 10.2147/CMAR.S169074. https://doi.org/10.2147/cmar.s169074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Virgilio F, Dal Ben D, Sarti AC, Giuliani AL, Falzoni S. The P2X7 receptor in infection and inflammation. Immunity. 2017;47(1):15–31. doi: 10.1016/j.immuni.2017.06.020. https://doi.org/10.1016/j.immuni.2017.06.020. [DOI] [PubMed] [Google Scholar]
- 21.Chong JH, Zheng GG, Zhu XF, Guo Y, Wang L, Ma CH, et al. Abnormal expression of P2X family receptors in Chinese pediatric acute leukemias. Biochem Biophys Res Commun. 2010;391(1):498–504. doi: 10.1016/j.bbrc.2009.11.087. https://doi.org/10.1016/j.bbrc.2009.11.087. [DOI] [PubMed] [Google Scholar]
- 22.Solini A, Cuccato S, Ferrari D, Santini E, Gulinelli S, Callegari MG, et al. Increased P2X7 receptor expression and function in thyroid papillary cancer:A new potential marker of the disease? Endocrinology. 2008;149(1):389–96. doi: 10.1210/en.2007-1223. https://doi.org/10.1210/en.2007-1223. [DOI] [PubMed] [Google Scholar]
- 23.Raffaghello L, Chiozzi P, Falzoni S, Di Virgilio F, Pistoia V. The P2X7 receptor sustains the growth of human neuroblastoma cells through a substance P-dependent mechanism. Cancer Res. 2006;66(2):907–14. doi: 10.1158/0008-5472.CAN-05-3185. https://doi.org/10.1158/0008-5472.can-05-3185. [DOI] [PubMed] [Google Scholar]
- 24.Liu Z, Liu Y, Xu L, An H, Chang Y, Yang Y, et al. P2X7 receptor predicts postoperative cancer-specific survival of patients with clear-cell renal cell carcinoma. Cancer Sci. 2015;106(9):1224–31. doi: 10.1111/cas.12736. https://doi.org/10.1111/cas.12736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tafani M, Russo A, Di Vito M, Sale P, Pellegrini L, Schito L, et al. Up-regulation of pro-inflammatory genes as adaptation to hypoxia in MCF-7 cells and in human mammary invasive carcinoma microenvironment. Cancer Sci. 2010;101(4):1014–23. doi: 10.1111/j.1349-7006.2010.01493.x. https://doi.org/10.1111/j.1349-7006.2010.01493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ryu JK, Jantaratnotai N, Serrano-Perez MC, McGeer PL, McLarnon JG. Block of purinergic P2X7R inhibits tumor growth in a C6 glioma brain tumor animal model. J Neuropathol Exp Neurol. 2011;70(1):13–22. doi: 10.1097/NEN.0b013e318201d4d4. https://doi.org/10.1097/nen.0b013e318201d4d4. [DOI] [PubMed] [Google Scholar]
- 27.Jelassi B, Chantôme A, Alcaraz-Pérez F, Baroja-Mazo A, Cayuela ML, Pelegrin P, et al. P2X(7) receptor activation enhances SK3 channels- and cystein cathepsin-dependent cancer cells invasiveness. Oncogene. 2011;30(18):2108–22. doi: 10.1038/onc.2010.593. https://doi.org/10.1038/onc.2010.593. [DOI] [PubMed] [Google Scholar]
- 28.Qiu Y, Li WH, Zhang HQ, Liu Y, Tian XX, Fang WG. P2X7 mediates ATP-driven invasiveness in prostate cancer cells. PLoS One. 2014;9(12):e114371. doi: 10.1371/journal.pone.0114371. https://doi.org/10.1371/journal.pone.0114371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ji Y, Li J, Li P, Wang L, Yang H, Jiang G. C/EBPβpromotion of MMP3-dependent tumor cell invasion and association with metastasis in colorectal cancer. Genet Test Mol Biomarkers. 2018;22(1):5–10. doi: 10.1089/gtmb.2017.0113. https://doi.org/10.1089/gtmb.2017.0113. [DOI] [PubMed] [Google Scholar]
- 30.Liu H, Liu W, Liu Z, Liu Y, Zhang W, Xu L, et al. Prognostic value of purinergic P2X7 receptor expression in patients with hepatocellular carcinoma after curative resection. Tumour Biol. 2015;36(7):5039–49. doi: 10.1007/s13277-015-3155-2. https://doi.org/10.1007/s13277-015-3155-2. [DOI] [PubMed] [Google Scholar]
- 31.Zheng L, Zhang X, Yang F, Zhu J, Zhou P, Yu F, et al. Regulation of the P2X7R by microRNA-216b in human breast cancer. Biochem Biophys Res Commun. 2014;452(1):197–204. doi: 10.1016/j.bbrc.2014.07.101. https://doi.org/10.1016/j.bbrc.2014.07.101. [DOI] [PubMed] [Google Scholar]
- 32.Fukuda K, Tsujitani S, Maeta Y, Yamaguchi K, Ikeguchi M, Kaibara N. The expression of RCAS1 and tumor infiltrating lymphocytes in patients with T3 gastric carcinoma. Gastric Cancer. 2002;5(4):220–7. doi: 10.1007/s101200200038. https://doi.org/10.1007/s101200200038. [DOI] [PubMed] [Google Scholar]
- 33.Lee HE, Chae SW, Lee YJ, Kim MA, Lee HS, Lee BL, et al. Prognostic implications of type and density of tumour-infiltrating lymphocytes in gastric cancer. Br J Cancer. 2008;99(10):1704–11. doi: 10.1038/sj.bjc.6604738. https://doi.org/10.1038/sj.bjc.6604738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu PC, Long D, Liao CC, Zhang S. Association between density of tumor-infiltrating lymphocytes and prognoses of patients with gastric cancer. Medicine (Baltimore) 2018;97(27):e11387. doi: 10.1097/MD.0000000000011387. https://doi.org/10.1097/md.0000000000011387. [DOI] [PMC free article] [PubMed] [Google Scholar]


